Abstract
Mucous hypersecretion is a major cause of airway obstruction in asthma, chronic obstructive pulmonary disease, and cystic fibrosis. EGFR ligands and IL-13 are known to stimulate mucous induction, but the detailed mechanisms of epithelial mucous regulation have not been well defined. In this issue of the JCI, Tyner et al. show, in a mouse model of chronic mucous hypersecretion, that ciliated epithelial cell apoptosis is inhibited by EGFR activation, allowing IL-13 to stimulate the differentiation of these cells into goblet cells, which secrete mucus. In defining this coordinated, 2-step process, we can consider the therapeutic effects of blocking mucous production. This begs the question, Is it possible to reduce airway obstruction in chronic lung disease by inhibiting EGFR activation and/or by inhibiting IL-13?
In the respiratory tract, mucus is a critical component of the innate host defense system. On the airway epithelial cell surface, the sticky gel layer traps particles and the sol layer, which is predominantly water, contacts the surface of ciliated cells and permits moving of the gel out of the lower airways like an escalator so that it can ultimately be cleared by coughing or swallowing. Mucus also contains antibacterial agents to aid in its defense function. Pathogens and harmless proteins we inhale are thus removed from the respiratory tract and have a limited encounter with other immune components. In the bronchial airways, mucus is produced by surface epithelial cells with secretory features and a classical goblet shape, called goblet cells. Goblet cells produce mucins that are complexed with water in secretory granules and are released into the airway lumen. In the large airways, mucus is also produced by mucous glands. Under basal conditions, the columnar epithelial surface comprises a small percentage of goblet cells and a majority of ciliated cells. This structure provides adequate mucus to capture particles and remove them in the huge volumes of air we breathe. After infection or toxic exposure, the airway epithelium upregulates its mucous secretory ability, and we cough and bring up sputum. Subsequently, the airway epithelium recovers and returns to its normal state, goblet cells disappear, and coughing abates.
Mucous hypersecretion is a hallmark of chronic airway diseases, including asthma, chronic obstructive pulmonary disease (COPD), and cystic fibrosis, and goblet cell hyperplasia and persistence are characteristic pathologic features. In asthmatics, 20–25% of airway epithelial cells are goblet cells, even in mild disease (1, 2). All of these diseases have distinct etiologies and different inflammatory responses that drive mucous hypersecretion. In asthma, inflammation appears to be mediated by allergen-specific Th2 cells, leading to eosinophilia, while in COPD, the inflammatory response is neutrophilic and may be induced by infection or components in cigarette smoke (3). Controlling inflammation is at the root of treatment through the use of corticosteroids and/or antibiotics, yet despite therapy, airway obstruction remains the cause of morbidity and mortality. Mucous secretions in the airways in asthma and COPD appear to be a major cause of airway obstruction, ventilation-perfusion mismatching, and hypoxemia, leading to wheezing and dyspnea (4–6). Can and should we be doing more to control mucus?
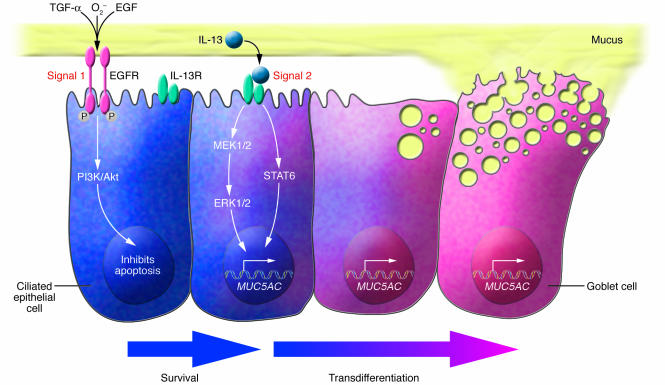
Two-step model that leads to chronic mucous production
In this issue of the JCI, Tyner et al. present a novel mechanism of chronic mucous hypersecretion in an asthma-like model and show a coordinated sequence of events that controls a conversion of normal epithelium into a chronic mucous secretory organ (7). This progression clarifies how blockade of certain pathways might affect mucous production. Past studies by other groups identified 2 cytokine-mediated mechanisms of mucous induction, 1 activated by engagement of EGFR and the other dependent on IL-13 and STAT6 signaling (8–12). Tyner et al. (7) show for the first time that both of these signals are necessary for mucous production to occur. EGFR phosphorylation on ciliated cells inhibits apoptosis, and this allows the second signal, IL-13, to stimulate ciliated cells to differentiate into goblet cells (Figure 1). This logical sequence of events ensures that barrier function is maintained after an insult to the airway. Multiple ligands released after airway damage, including EGF, TGF-α, and reactive oxygen species (13), activate the promiscuous EGFR. If an appropriate signal, such as IL-13, is provided, the epithelium can be converted to a mucus-producing organ that will sweep away pathogens and debris. Goblet cell differentiation does not occur in all immune responses, particularly those rich in IFN-γ (14), so it appears to be closely regulated, yet the need for regulating mucous secretion is not clear.
Figure 1.
Ciliated cell differentiation into goblet cells requires 2 signals. Tyner et al. (7), using a mouse model of Sendai virus infection, propose that the induction of a chronic mucus-producing airway epithelium requires 2 signals. Signal 1 activates the EGFR on ciliated cells and induces EGFR phosphorylation and activation of PI3K/Akt. This pathway leads to inhibition of ciliated cell apoptosis. Ciliated cells that survive can respond to signal 2: IL-13 binding to its receptor. Upon IL-13 receptor (IL-13R) activation and STAT6 signaling, ciliated cells begin to produce mucins (including those encoded by MUC5AC), which are contained within mucous secretions, and lose their ciliated cell surface, taking on features of mucus-producing goblet cells. It also appears that other epithelial cells, such as Clara cells, can differentiate into goblet cells. Thus, the airway epithelium is driven to become a mucus-producing organ, presumably to enhance host defense. In some diseases, such as asthma, this response may be misdirected. Airway tissue from human asthmatics exhibits EGFR activation on ciliated cells, and mucus appears to be induced by IL-13, suggesting that this may also be an important pathway for mucous induction in humans, yet it remains unclear whether other pathways of mucous induction are active in chronic airway diseases.
In the model presented here (7), chronic mucous production follows Sendai virus infection in mice after viral clearance due to constitutive activation of EGFR in the absence of obvious inflammation. This effect is unique to 1 strain of mice and suggests a strain-specific response to the virus that leads to constitutive EGFR phosphorylation. After Sendai virus infection, mice exhibit an “abnormally programmed” epithelium, while in human airway diseases, mucous hypersecretion is most likely a normal response of the epithelium to inflammation. In these patients, airway inflammation provides an abundance of EGFR ligands to turn on EGFR (15, 16).
IL-13 stimulates mucus in asthma and possibly other airway diseases
IL-13 is a potent stimulator of mucus in vivo, and its effects extend beyond the classical Th2 lymphocyte response. After Sendai virus infection, mucous hyperproduction is driven by IL-13, and this occurs in the absence of a visible airway inflammatory response. Likewise, mucous production is IL-13 dependent in non–Th2 driven airway inflammation when IL-13 levels are very low in the airways (17). Furthermore, many cytokine-driven models of airway inflammation result in mucous hypersecretion, yet each has been shown to do this through the production of IL-13 (18). Other factors can also induce mucus. For example, LPS can stimulate low-level mucous production in IL-13–deficient mice (17), and many studies have shown a role for human neutrophil elastase in mucous production (19, 20). Even in light of the seminal studies of mucin gene expression by Carol Basbaum (21, 22), there is only a rudimentary understanding of how these mediators stimulate mucins, the contribution of different mucins to the protective response, and which pathways are active in human disease. IL-13 is likely to play a critical role in mucous induction in asthma, and it may prove to be an important stimulus for mucous production in other chronic airway diseases, despite their diverse inflammation profiles, as IL-13 levels in the respiratory tract are often elevated (23, 24). In addition to its role in mucin gene expression, IL-13 induces other components of the secretory machinery, further supporting its identification as a master regulator of the goblet cell (25). Other candidates for the second stimulus have not been studied in such depth. They are lined up, and it is hoped they will be tested in this model by the Tyner and Holtzman laboratory.
Does inhibition of mucus lead to clinical improvement in airway diseases?
Inflammation in asthma results from an exuberant inflammatory response to allergens that pose no threat to the individual; therefore, reducing mucous production in response to these agents should improve patient symptoms. Blockade of mucus may be problematic if its overproduction facilitates removal of damaged cells. Inhaled steroids are effective in quelling both inflammation and mucous production in asthma, but even during this treatment, some inflammation persists, and when disease exacerbations occur, there is a swift increase in mucus. Clinical trials of inhibitors of IL-13, TGF-α, and EGFR offer potential solutions to control airway obstruction and are on the horizon. Blocking the production of mucus in COPD has more potential to be detrimental since normal mucus helps to eliminate bacteria from the airways. It is unknown whether the mucus in these distorted airways inhibits or even promotes bacterial growth. Given the high prevalence of COPD, it may be worth defining a balance point that separates effective and pathologic mucous production. Since inhaled steroids have limited effectiveness in chronic bronchitis, therapeutic targeting of IL-13, TGF-α, and EGFR offers a potential way to limit mucous production and improve lung function.
Acknowledgments
The author thanks Robert Homer and Donna Farber for helpful discussion. This work is supported by NIH grant NHLBI-64040.
Footnotes
See the related article beginning on page 309.
Nonstandard abbreviations used: COPD, chronic obstructive pulmonary disease.
Conflict of interest: The author has declared that no conflict of interest exists.
References
- 1.Shimura S, Andoh Y, Haraguchi M, Shirato K. Continuity of airway goblet cells and intraluminal mucus in the airways of patients with bronchial asthma. Eur. Respir. J. 1996;9:1395–1401. doi: 10.1183/09031936.96.09071395. [DOI] [PubMed] [Google Scholar]
- 2.Rogers DF. Airway goblet cells: responsive and adaptable front-line defenders. Eur. Respir. J. 1994;7:1690–1706. [PubMed] [Google Scholar]
- 3.Barnes PJ, Shapiro SD, Pauwels RA. Chronic obstructive pulmonary disease: molecular and cellular mechanisms. Eur. Respir. J. 2003;22:672–688. doi: 10.1183/09031936.03.00040703. [DOI] [PubMed] [Google Scholar]
- 4.Hogg JC, Macklem PT, Thurlbeck WM. Site and nature of airway obstruction in chronic obstructive lung disease. N. Engl. J. Med. 1968;278:1355–1360. doi: 10.1056/NEJM196806202782501. [DOI] [PubMed] [Google Scholar]
- 5.Moreno RH, Hogg JC, Pare PD. Mechanics of airway narrowing. Am. Rev. Respir. Dis. 1986;133:1171–1180. doi: 10.1164/arrd.1986.133.6.1171. [DOI] [PubMed] [Google Scholar]
- 6.James A, Carroll N. Theoretical effects of mucus gland discharge on airway resistance in asthma. Chest. 1995;107:110S. doi: 10.1378/chest.107.3_supplement.110s. [DOI] [PubMed] [Google Scholar]
- 7.Tyner JW, et al. Blocking airway mucous cell metaplasia by inhibiting EGFR antiapoptosis and IL-13 transdifferentiation signals. J. Clin. Invest. 2006;116:309–321. doi:10.1172/JCI25167. doi: 10.1172/JCI25167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guzman K, Randell SH, Nettesheim P. Epidermal growth factor regulates expression of the mucous phenotype of rat tracheal epithelial cells. Biochem. Biophys. Res. Commun. 1995;217:412–418. doi: 10.1006/bbrc.1995.2792. [DOI] [PubMed] [Google Scholar]
- 9.Takeyama K, et al. Epidermal growth factor system regulates mucin production in airways. Proc. Natl. Acad. Sci. U. S. A. 1999;96:3081–3086. doi: 10.1073/pnas.96.6.3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wills-Karp M, et al. Interleukin-13: central mediator of allergic asthma. Science. 1998;282:2258–2261. doi: 10.1126/science.282.5397.2258. [DOI] [PubMed] [Google Scholar]
- 11.Grunig G, et al. Requirement for IL-13 independently of IL-4 in experimental asthma. Science. 1998;282:2261–2263. doi: 10.1126/science.282.5397.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cohn L, et al. Th2-induced airway mucus production is dependent on IL-4Ralpha, but not on eosinophils. J. Immunol. 1999;162:6178–6183. [PubMed] [Google Scholar]
- 13.Burgel PR, Nadel JA. Roles of epidermal growth factor receptor activation in epithelial cell repair and mucin production in airway epithelium. Thorax. 2004;59:992–996. doi: 10.1136/thx.2003.018879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cohn L, Homer RJ, Niu N, Bottomly K. T helper 1 cells and interferon gamma regulate allergic airway inflammation and mucus production. J. Exp. Med. 1999;190:1309–1318. doi: 10.1084/jem.190.9.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chung KF. Cytokines in chronic obstructive pulmonary disease. Eur. Respir. J. Suppl. 2001;34:50s–59s. [PubMed] [Google Scholar]
- 16.Holgate ST, Lackie PM, Davies DE, Roche WR, Walls AF. The bronchial epithelium as a key regulator of airway inflammation and remodelling in asthma. Clin. Exp. Allergy. 1999;29(Suppl. 2):90–95. doi: 10.1046/j.1365-2222.1999.00016.x. [DOI] [PubMed] [Google Scholar]
- 17.Whittaker L, et al. Interleukin-13 mediates a fundamental pathway for airway epithelial mucus induced by CD4 T cells and interleukin-9. Am. J. Respir. Cell Mol. Biol. 2002;27:593–602. doi: 10.1165/rcmb.4838. [DOI] [PubMed] [Google Scholar]
- 18.Cohn L, Elias JA, Chupp GL. Asthma: mechanisms of disease persistence and progression. Annu. Rev. Immunol. 2004;22:789–815. doi: 10.1146/annurev.immunol.22.012703.104716. [DOI] [PubMed] [Google Scholar]
- 19.Park JA, et al. Human neutrophil elastase induces hypersecretion of mucin from well-differentiated human bronchial epithelial cells in vitro via a protein kinase Cdelta-mediated mechanism. Am. J. Pathol. 2005;167:651–661. doi: 10.1016/s0002-9440(10)62040-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Agusti C, et al. Goblet cell degranulation after antigen challenge in sensitized guinea pigs. Role of neutrophils. Am. J. Respir. Crit. Care Med. 1998;158:1253–1258. doi: 10.1164/ajrccm.158.4.9801041. [DOI] [PubMed] [Google Scholar]
- 21.Longphre M, et al. Allergen-induced IL-9 directly stimulates mucin transcription in respiratory epithelial cells. J. Clin. Invest. 1999;104:1375–1382. doi: 10.1172/JCI6097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li JD, et al. Activation of NF-kappaB via a Src-dependent Ras-MAPK-pp90rsk pathway is required for Pseudomonas aeruginosa-induced mucin overproduction in epithelial cells. Proc. Natl. Acad. Sci. U. S. A. 1998;95:5718–5723. doi: 10.1073/pnas.95.10.5718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hauber HP, et al. Increased expression of interleukin-13 but not interleukin-4 in cystic fibrosis patients. J. Cyst. Fibros. 2003;2:189–194. doi: 10.1016/S1569-1993(03)00091-2. [DOI] [PubMed] [Google Scholar]
- 24.Miotto D, et al. Interleukin-13 and -4 expression in the central airways of smokers with chronic bronchitis. Eur. Respir. J. 2003;22:602–608. doi: 10.1183/09031936.03.00046402. [DOI] [PubMed] [Google Scholar]
- 25.Kuperman DA, et al. Dissecting asthma using focused transgenic modeling and functional genomics. J. Allergy Clin. Immunol. 2005;116:305–311. doi: 10.1016/j.jaci.2005.03.024. [DOI] [PubMed] [Google Scholar]