Abstract
Objective:
Purposes of this study were: 1) to compare mortality and postoperative morbidities (intra-abdominal abscess, wound dehiscence, and intestinal stricture) in extremely low birth weight (ELBW) infants who underwent initial laparotomy or drainage for necrotizing enterocolitis (NEC) or isolated intestinal perforation (IP); 2) to determine the ability to distinguish NEC from IP preoperatively and the importance of this distinction on outcome measures; and 3) to evaluate the association between extent of intestinal disease determined at operation and outcome measures.
Background:
ELBW infants who undergo operation for NEC or IP have a postoperative, in-hospital mortality rate of approximately 50%. Whether to perform laparotomy or drainage initially is controversial. Also unknown is the importance of distinguishing NEC from IP and the current ability to make this distinction based on objective data available prior to operation.
Methods:
A prospective, multicenter cohort study of 156 ELBW infants at 16 neonatal intensive care units (NICU) within the NICHD Neonatal Research Network.
Results:
Among the 156 enrolled infants, 80 underwent initial peritoneal drainage and 76 initial laparotomy. Mortality rate was 49% (76 of 156). Ninety-six patients had a preoperative diagnosis of NEC and 60 had presumed IP. There was a high level of agreement between the presumed preoperative diagnosis and intraoperative diagnosis in patients undergoing initial laparotomy (kappa = 0.85). The relative risk for death with a preoperative diagnosis of NEC (versus IP) was 1.4 (95% confidence interval, 0.99–2.1, P = 0.052). The overall incidence of postoperative intestinal stricture was 10.3%, wound dehiscence 4.4%, and intra-abdominal abscess 5.8%, and did not significantly differ between groups undergoing initial laparotomy versus initial drainage.
Conclusions:
Survival to hospital discharge after operation for NEC or IP in ELBW neonates remains poor (51%). Patients with a preoperative diagnosis of NEC have a relative risk for death of 1.4 compared with those with a preoperative diagnosis of IP. A distinction can be made preoperatively between NEC and IP based on abdominal radiographic findings and the patient's age at operation. Future randomized trials that compare laparotomy versus drainage would likely benefit from stratification of treatment assignment based on preoperative diagnosis.
This prospective, multicenter observational study reports mortality and morbidity outcomes after initial laparotomy or initial peritoneal drainage in premature infants with necrotizing enterocolitis or isolated intestinal perforation. Postoperative mortality did not differ between the 2 treatment groups. A preoperative diagnosis of necrotizing enterocolitis was an important prognostic factor indicating an increased likelihood of death.
Extremely low birth-weight (ELBW), premature infants who develop necrotizing enterocolitis (NEC) or isolated intestinal perforation (IP) represent a group of patients with extremely high morbidity and mortality. ELBW refers specifically to infants with birth weight <1000 g. Multiple case series report the mortality of ELBW infants that undergo operation for NEC to be approximately 50%.1–5
There are several controversial aspects of the surgical management of these infants. Most surgeons now believe that the population of ELBW infants with intestinal perforation (IP) is heterogeneous with some infants having true NEC and others having a different and distinct pathology, termed isolated intestinal perforation (IP).6–8 The ability to distinguish these 2 conditions preoperatively, based on perinatal characteristics, physical examination findings, and findings on abdominal plain film imaging, remains unknown. If these 2 entities can be distinguished preoperatively, the impact on prognosis is also unclear. However, if a distinction could be made, we may be better able to predict prognosis and to develop different therapies for the 2 conditions.
It is not known whether initial laparotomy or peritoneal drain placement is more effective. Despite the widespread use of these 2 surgical options, there have been no prospective studies to date addressing this question.9,10
This study was a prospective, multicenter observational study. The purpose of this report is to describe surgical outcomes (mortality, postoperative intestinal stricture, intra-abdominal abscess formation, and wound dehiscence) in ELBW infants with either NEC or IP who underwent initial laparotomy or peritoneal drainage. We also evaluated the ability to distinguish NEC and IP preoperatively and the relevance of this distinction on outcome. Finally, an analysis of the impact of extent of intestinal involvement with NEC on outcome measures is reported.
METHODS
Study Design
This was a prospective cohort study conducted at 16 neonatal intensive care units that are associated with the NIH, NICHD Neonatal Research Network. A major objective of this study was to gather data needed to design a future randomized trial comparing initial laparotomy versus drainage in ELBW infants with either NEC or IP. Results from this study related to future trial design are being reported separately.11 This report includes a planned analysis of major clinical outcomes after either laparotomy or drainage, as well as a detailed evaluation of the ability to distinguish NEC and IP preoperatively. Formal institutional review board approval at all participating institutions was obtained prior to enrolling infants.
All ELBW infants born at participating Network NICUs were screened by research coordinators for the presence of NEC or IP that was thought by the pediatric surgeon and neonatologist to require surgical intervention. At this point, infants were enrolled, and both the research coordinators and the participating surgeons collected data prospectively. Data collected included demographic variables, patient characteristics at time of enrollment, and intraoperative findings recorded by the surgeon. Postoperative data were collected until time of death, discharge, or 120 days after birth. Specific postoperative complications that were screened for prospectively included intestinal stricture that was documented by contrast imaging, intra-abdominal abscess requiring drainage, and wound dehiscence documented by the surgeon.
Sample Size and Enrollment Period
A predetermined sample size of 150 infants was selected to provide adequate data to facilitate the design of a future randomized trial and the estimated enrollment period was 18 months. Between February 2001 and August 2002, ELBW infants were screened and 156 infants were enrolled and underwent at least one operation for NEC or IP.
Statistical Analysis
Univariate analyses were performed to assess baseline differences between treatment groups (laparotomy and drainage) and diagnosis subgroups (NEC and IP), the relationship of patient characteristics to outcome, the agreement between preoperative and intraoperative diagnosis among patients receiving laparotomy, and the impact of extent of disease determined at operation on outcome. Mean values were analyzed with t tests, proportions with Fisher exact test, or χ2 analyses, and correlations with the Cochran-Mantel-Haenzel statistic and kappa coefficient. Multivariable logistic regression analyses were performed to evaluate variables known at enrollment that would most accurately predict the intraoperative diagnosis (NEC or IP). Survival to hospital discharge was evaluated by Cox survival analysis, adjusting for potentially influential covariates.
RESULTS
Overall Cohort
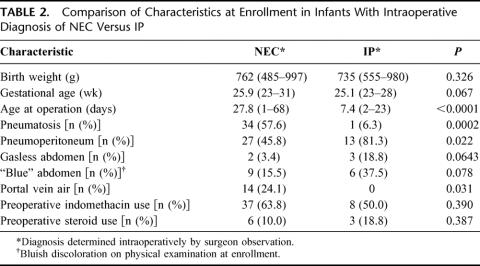
All 156 infants enrolled in the study were diagnosed with either NEC (Bell's stage III) or IP. The indications for surgical intervention were: pneumoperitoneum (n = 99, 63.9%), clinical deterioration despite maximal medical therapy (n = 101, 65.6%), portal vein air (n = 21, 13.8%), infected ascites (n = 8, 5.2%), abdominal wall erythema (n = 43, 28.1%), and other factors (n = 41, 26.3%) [infants may have multiple indications]. Perinatal, preoperative, and operative characteristics are shown in Table 1. Among the 80 infants undergoing initial drainage, 18 (23%) underwent a subsequent laparotomy. The indication for subsequent laparotomy in these patients was further clinical deterioration (n= 11, 61.1%), persistent pneumoperitoneum (n = 4, 22.2%), infected ascites (n = 2, 11.1%), abdominal wall erythema (n= 3, 16.7%), and other reasons (n = 11, 61.1%).
TABLE 1. Characteristics of Study Cohort
NEC Versus IP
At the time of enrollment, all patients had a presumptive diagnosis (NEC or IP) recorded by the surgeon based on physical examination, abdominal radiographic data, demographic information, and other factors. Ninety-six patients had a preoperative diagnosis of NEC and 60 had a diagnosis of IP. Patients diagnosed with NEC were more likely to have initial laparotomy (58 of 96, 60%) and IP patients were more likely to have initial drainage (42 of 60, 70%).
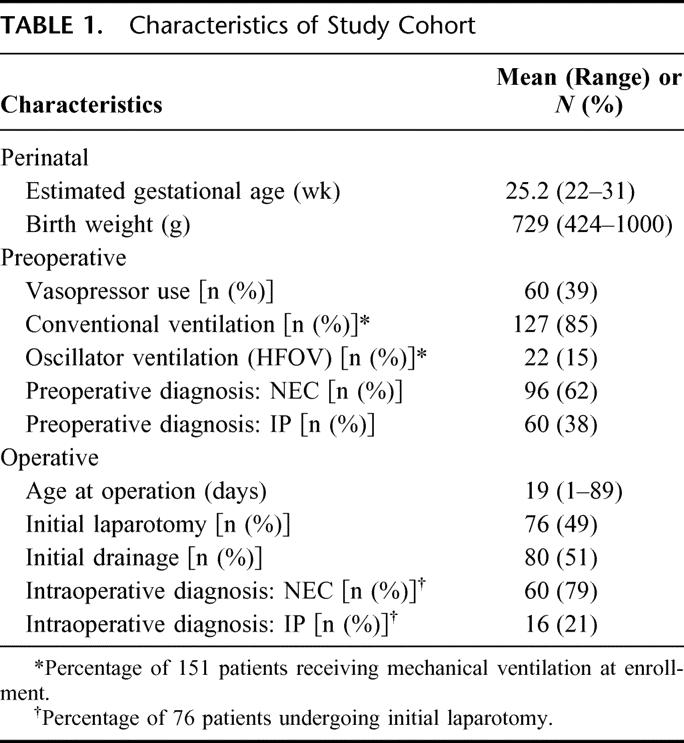
A comparison of characteristics at enrollment in infants with a confirmed (intraoperative) diagnosis of NEC and IP is shown in Table 2. NEC patients more commonly had pneumatosis and portal vein air on preoperative abdominal radiographs, were older at time of initial operation, and less commonly had pneumoperitoneum.
TABLE 2. Comparison of Characteristics at Enrollment in Infants With Intraoperative Diagnosis of NEC Versus IP
An intraoperative diagnosis was recorded for the 76 patients undergoing an initial laparotomy (this was not possible for patients undergoing initial drainage). Among the 58 patients with presumptive NEC who had initial laparotomy, 57 also had an intraoperative diagnosis of NEC; for the 18 presumptive IP infants, 15 had this diagnosis confirmed at operation. Thus, 95% of cases were correctly classified; kappa statistic = 0.85. Because the same observer often made the preoperative and intraoperative diagnoses, thus allowing potential bias, multiple objective variables available preoperatively were examined in a multivariable logistic regression to attempt to use a combination of patient characteristics to achieve the same accuracy in distinguishing NEC from IP preoperatively. The group of variables that most accurately predicted the intraoperative diagnosis included pneumatosis, age at operation, and gasless abdomen on preoperative radiographs. The R-square value for this model was 0.53. Specifically, positive pneumatosis and older age at operation predicted NEC. Variables that did not significantly predict the intraoperative diagnosis included: pneumoperitoneum (Y/N), blue abdomen on physical examination (Y/N), preoperative indomethacin or postnatal steroid exposure, gestational age, and birth weight.
There was not a significant survival difference in the overall cohort between initial laparotomy and initial drainage.11 Among patients with a preoperative diagnosis of NEC, the relative risk (RR) for death (initial laparotomy versus drainage) was 0.79 (95% confidence inverval [CI], 0.6–1.1). For IP infants, the RR for death (laparotomy versus drainage) was 0.49 (95% CI, 0.19–1.2). These relative risk figures are not adjusted for differences between groups with regard to characteristics other than initial treatment.
Mortality
The overall in-hospital mortality was 49% (76 of 156). The median time to death was 8.5 days (range, 0–249 days). The recorded causes of death were: NEC (45), respiratory distress syndrome or other pulmonary pathology (13), infection (7), and other (11). Patient characteristics and their association with the likelihood of death are shown in Table 3. Significant poor prognostic factors include: decreasing birth weight and gestational age, vasopressor use at time of enrollment (preoperatively), and use of high-frequency oscillator ventilation at time of enrollment. A preoperative diagnosis of NEC was associated with a relative risk for death of 1.4 (95% CI, 0.99–2.1, P = 0.052). The mortality rate in patients with a preoperative diagnosis of NEC was 55.2% (53 of 96) versus 38.3% (23 of 60) among patients with a preoperative diagnosis of isolated perforation (P = 0.049, Fisher exact test). Table 4 summarizes mortality data according to preoperative diagnosis (NEC or IP) and also by initial treatment received (laparotomy or drainage). The Cox-adjusted survival analysis comparing survival in patients with a preoperative diagnosis of NEC versus IP is shown in Figure 1. This survival analysis was adjusted for treatment group (laparotomy versus drainage), level of ventilation (PIP > 30 mm Hg or HFOV versus PIP ≤ 30 mm Hg), vasopressor use versus none, FiO2 (continuous variable), pH ≤ 7.28 versus >7.28, birthweight, and age at operation (continuous). Only preoperative diagnosis was a significant predictor of survival (P = 0.0498).
TABLE 3. Association of Patient and Disease Characteristics and Mortality
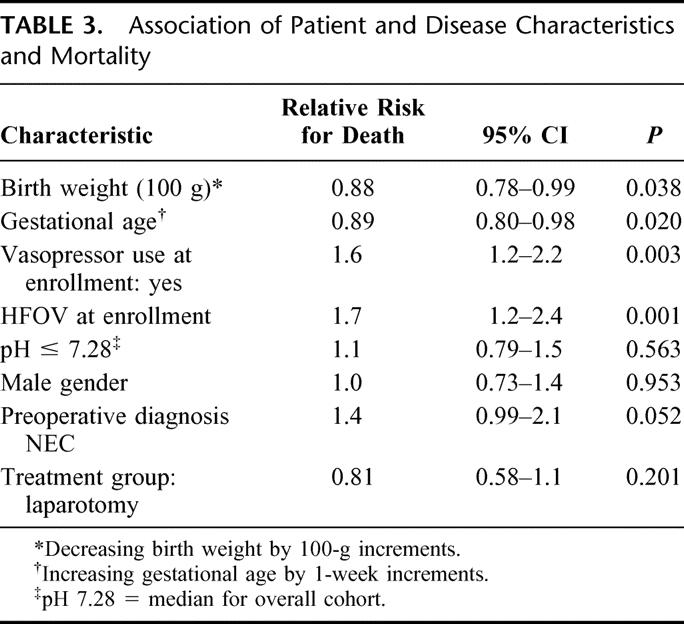
TABLE 4. Mortality Rates by Preoperative Diagnosis and Treatment Group
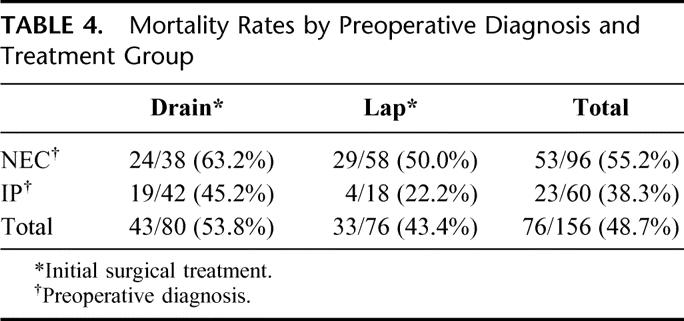
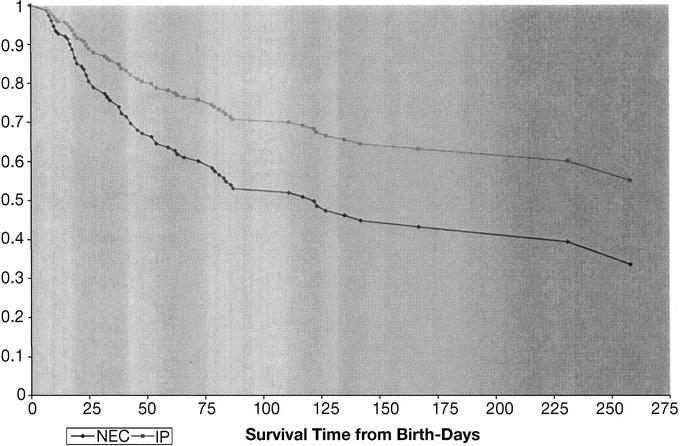
FIGURE 1. Cox-adjusted survival analysis for overall cohort: preoperative diagnosis of NEC versus IP.
The extent of disease was determined at operation for patients undergoing initial laparotomy (n = 76) by measuring the length of normal intestine, diseased bowel, and/or resected bowel. These measurements were available for between 34% and 72% of patients, indicating the difficulty of obtaining such measurements in critically ill neonates. The mean length of normal small bowel was greater in survivors (81 cm) compared with nonsurvivors (23 cm, P = 0.003). Survival was 75% for infants with >80 cm normal bowel at operation (n = 20), 46% in infants with 10 to 80 cm normal bowel (n = 11), and 0% for 11 infants with <10 cm normal bowel (P = 0.0001).
Morbidity
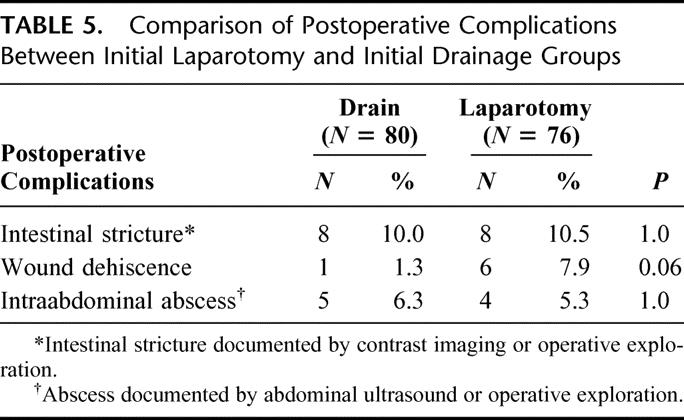
A comparison of the frequency of postoperative complications in the initial drainage versus the initial laparotomy subgroups is shown in Table 5. Although there was a trend toward an increased incidence of wound dehiscence in the laparotomy group compared with the initial drainage group, this did not achieve statistical significance and the other complications occurred equally in each group. Among the 80 patients undergoing initial drainage, 28 survived without a subsequent laparotomy (35% of initial drain patients). In this small subgroup of patients, the incidence of intra-abdominal abscess was 7%, wound dehiscence 2%, and intestinal stricture 7%.
TABLE 5. Comparison of Postoperative Complications Between Initial Laparotomy and Initial Drainage Groups
DISCUSSION
The overall mortality of our study cohort of ELBW infants with NEC or IP of 49% supports the catastrophic nature of this illness and the fragility of these premature neonates. Despite many advances in neonatal, anesthetic, and surgical care of these infants, the overall mortality rate for those requiring surgery has not decreased over the past 30 years. This emphasizes the fact that, although appropriate medical and surgical care is critical, the true advance in outcomes will likely only occur with prevention of NEC or IP.
Our data support the idea that NEC and IP can be distinguished preoperatively in the majority of patients. While our data point to a very high level of agreement between the preoperative and intraoperative diagnoses (95% correct classification), this was not a primary objective of the study, and a cautious interpretation of these data is recommended. We did not have independent blinded observers making each diagnosis, thus allowing for bias. Also, there was no attempt at formal central review of radiographic findings (eg, pneumatosis, pneumoperitoneum, gasless abdomen), and there is known to be interobserver variability in the radiologic diagnosis of NEC.12 The strength of the correlation, however, does appear to indicate a reasonable ability to distinguish NEC from IP preoperatively. Because of the potential bias in the surgeon's prerecorded presumptive diagnosis, a separate analysis of objective patient characteristics that are available prior to operation was also performed. This showed that the presence of pneumatosis on the preoperative abdominal radiograph and an increased age at operation predicted intraoperative NEC. Previously, radiographic findings alone were shown to have limited ability to discriminate NEC and IP.13 Other factors are likely important in accurately distinguishing these 2 conditions, given the modest predictive ability of the multivariable logistic model (R2 = 0.53).
The preoperative distinction of NEC from IP did have significant prognostic importance, with an increased likelihood for death for patients diagnosed as having NEC. Our data indicate that this is a clinically important distinction, although statistically the difference was equivocal with P values from multiple statistical tests (χ2, Fisher exact test, relative risk with 95% CI) being just above or below P = 0.05. Other patient characteristics associated with an increased likelihood of death were lower gestational age, lower birth weight, use of vasopressors at the time of enrollment preoperatively, as well as use of high-frequency oscillator ventilation (also at enrollment). Initial treatment (laparotomy or drainage) was not a significant variable in predicting mortality in this cohort of patients.
The ability to accurately distinguish NEC from IP is likely important for several reasons. First, it is possible that the most effective surgical therapy will differ in the 2 patient subsets. Second, for future randomized trials comparing laparotomy versus drainage, it is thought to be potentially important to stratify randomized treatment assignments based on the preoperative diagnosis, since these patients appear to have different risks for death.
There is considerable attention to the controversy regarding laparotomy versus drainage, with 2 ongoing randomized trials evaluating this question. It may be as important to distinguish NEC from IP preoperatively, as the preexisting condition may impact survival in a more significant manner than initial treatment performed.14,15
Overall, postoperative complications were less frequent than expected based on prospective screening and data collection. Previous reports have cited higher postoperative complication rates after NEC surgery, at times in excess of 50%.16,17 In our study cohort, postoperative intestinal stricture was the most common complication and occurred equally in infants after initial drainage or laparotomy.
These data support the need for stratification by preoperative diagnosis (NEC or IP) in randomized trials comparing laparotomy versus drainage (and perhaps other important prognostic variables) to ensure as equal treatment groups with respect to baseline risk as possible. Although it is important to apply different surgical options in the most efficacious manner, the largest impact in the outcome of these infants is likely to come only with prevention of NEC and IP. A major limitation in the development of preventive strategies is the limitations of the available animal models for NEC and the paucity of human tissue to examine.18 For future randomized clinical trials involving these infants, correlative biology studies to investigate pathways in the etiology of these diseases via resected intestinal tissue or blood samples might help to identify potential targets to be used in prevention and treatment.19
ACKNOWLEDGMENTS
NICHD Neonatal Research Network, Principal Investigators: Waldemar A. Carlo, MD (University of Alabama at Birmingham), Edward F. Donovan, MD (University of Cincinnati), Shahnaz Duara, MD (University of Miami), Richard A. Ehrenkranz, MD (Yale University), Avroy A. Fanaroff, MD, BCh (Case Western Reserve University), Neil N. Finer, MD (University of California at San Diego), Ronald N. Goldberg, MD (Duke University), Abbot R. Laptook, MD (University of Texas Southwestern Medical Center, Dallas), James A. Lemons, MD (Indiana University), William Oh, MD (Women and Infants’ Hospital of Rhode Island), T. Michael O'Shea, MD (Wake Forest University), Dale L. Phelps, MD (University of Rochester), W. Kenneth Poole, PhD (Research Triangle Institute), Seetha Shankaran, MD (Wayne State University), David K. Stevenson, MD (Stanford University), Barbara J. Stoll, MD (Emory University), Jon E. Tyson, MD, MPH (University of Texas Health Science Center, Houston), and Rosemary D. Higgins, MD (National Institute of Child Health and Human Development).
Discussions
Dr. Max R. Langham, Jr. (Gainesville, Florida): This baby is a 32-week baby who had been born at 22 weeks gestation, weaned off the ventilator, fed, who developed abdominal distention, billious vomiting, bloody stools, and after this x-ray was referred to the surgical service for emergency operation, IV antibiotics, and subsequently got better.
The treatment and diagnosis of this condition haven't changed a bit since I was a third-year student on Dr. Haller's service, where I became fascinated by pediatric surgery. No animal model exists of the condition that is relevant to the epidemiology or precise pathology, so you can imagine the treatment and the results have not changed very much.
It is hard to convey how exciting it is, therefore, to have the opportunity to read an NIH-funded prospective multi-institutional study, which is done as a precursor to a randomized hypothesized-driven multi-institutional study.
Dr. Blakely and Dr. Lally and the co-authors are to be congratulated for bringing the first report of a study, which has the opportunity to become a landmark in clinical investigation in surgery in infants.
There are several limitations to this study. First, it is a secondary analysis of a larger study by the neonatal network and as such was not designed, I believe and I would like the authors to clarify this for us with the primary question in mind. It focuses on patients only requiring operation, and we hope that the primary analysis of the data by the neonatal network will address the pressing questions of etiology, prevalence of the disease, and potential for prevention of the disease, which is really needed badly.
The central question of this paper is: which operation is better for these infants? The authors do not include infants that are above 1000 g at birth. And I wonder if they believe that those infants don't need to be randomized? Do we know what the best operation is for larger children with NEC? How many babies died without surgery in this series of patients of 6000-some-odd extremely low birth infants? Is there a way to capture those patients and to look at them? In designing the future study, is there a way to capture those patients and to look at them?
Patients with isolated intestinal perforation, as Dr. Lally pointed out to us, look healthier, and yet in these data they have lower blood pressures, higher pressor requirements, and higher ventilator support, and still had a reasonable mortality. Could it be that the lower blood pressure and other statistically significant variables were because these patients were more premature and that the higher ventilator requirements had to do with this prematurity?
We know that ventilator pressures with mean or peak inspiratory pressures of 31 are toxic. We and others have shown before this group and others that in patients with diaphragmatic hernia, high ventilator pressures are associated with increased mortality. That is certainly true in other diseases as well. Can the authors separate for us or can they stratify in their prospective randomized trial toxicity of ventilators and mortality in these infants from ventilator therapies and from other co-morbidities?
Finally, are they sure they have the right variables to stratify their prospective randomized study? Should lactic acid, acidemia, renal failure, liver dysfunction, or other clinical variables be used? Have they analyzed these data and could they share those with us?
And finally, the major question, the really exciting part of this. What is the evidence of based hypothesis to be tested in the properly designed, prospective randomized multicenter trial, which the authors propose and which I look forward to with a great deal of interest?
Dr. Wallace W. Neblett, III (Nashville, Tennessee): I would like to offer my congratulations to Drs. Lally, Blakely and co-authors for this carefully prepared and beautifully presented paper dealing with a difficult and increasingly common clinical problem in pediatric surgery.
Their study depicts all too accurately the dismal outcome in so many of these tiny babies with co-morbid heart and lung disease who develop intestinal necrosis with a 49% mortality rate when cared for in some of the very best neonatal intensive care units available in the world.
I congratulate the authors on the purpose and design of this study, an observational prospective multi-institutional study designed to gather data to confirm the feasibility and improve the study design of a subsequent prospective randomized treatment trial designed to provide evidence-based recommendations for optimum treatment of these infants.
Many of the findings from the current study are confirmatory of previous work and the findings or feelings that most pediatric surgeons have. We learned important information regarding the accuracy of pretreatment diagnosis in this group of patients, the presence of hematosis, intestinal and portal venoarya in a somewhat older infant correlates very well with necrotizing enterocolitis with a pretreatment diagnostic accuracy of 98%. However, the pretreatment diagnostic accuracy for isolated intestinal perforation was less at 83% in a small group of patients.
As expected, this study does not adequately clarify outcomes according to treatment by laparotomy versus drainage and supports the need for further prospective randomized studies as planned by the authors. I would be interested in Dr. Blakely's comments regarding several factors in this patient population that may be important in achieving proper stratification of treatment groups in the next study.
First, how will that study achieve reliable accuracy in treatment groups regarding the preoperative diagnosis, necrotizing enterocolitis versus isolated intestinal perforation? Specifically, I would be concerned about the 83% successful preoperative diagnosis of intestinal perforation in babies that are not operated on initially.
Second, how will you achieve uniformity of treatment groups regarding operative indications? We know that pneumoperitoneum correlates highly with the presence of full-thickness intestinal necrosis, whereas other preoperative indicators such as abdominal wall erythema, clinical deterioration, and other indications for intervention have substantial lower specificity.
Third, the presence of total or near-total intestinal necrosis in most series, including this one, predicts a uniformly fatal outcome. Should these infants be identified and excluded from study groups?
Fourth, pretreatment physiologic parameters have been shown to be highly reliable predictors of outcome in many neonatal diseases. In this series, vasopressor use and use of high frequency oscillation or ventilator support correlated with a poor outcome. Have the authors utilized a physiologic scoring system to predict mortality in comparison of the treatment groups in the current study, and will that be of benefit in the prospective study yet to come?
Dr. Joseph P. Tepas, III (Jacksonville, Florida): My comment is that, as Dr. Lally stated, this disease is beginning to supplant congenital anomaly as the most common reason for urgent operation on very small birth weight infants. It therefore becomes an absolute imperative that we look at this from 3 perspectives because it is, in fact, becoming an increasingly problematic social as well as surgical disease.
The 3 imperatives are: first, the welfare of the baby. Our decisions affect whether the baby will survive with a quality of life that we would wish for any child. Secondly, this is an extraordinarily resource-intensive disease, as you have heard. Thus, it becomes important for us to become good stewards to the resources that our hospitals are quickly running out of, as we all know, from the evolving healthcare finance situation. And finally, we must assure that these babies grow up as societal contributors rather than as long-term dependents for custodial care. We have been struggling with this because, like many other institutions that also serve as safety net hospitals, this becomes increasingly important.
We have looked at our experience, with our 14-year prospective database, to try to identify objective criteria that would help us make appropriate clinical decisions. In the process of doing that, we have analyzed odds ratios comparing peritoneal drainage versus laparotomy and have come to the conclusion that, in fact, this disease may not really be 2 diseases, as some authors have stated. The disease of IP or intestinal perforation is actually a benign variant of necrotizing enterocolitis. Our odds ratio analysis suggests to us that the babies who are treated with laparotomy, lavage, and debridement do significantly better if they have prodromo of necrotizing enterocolitis.
On the other hand, in the absence of findings commonly associated with NEC, those numbers are reversed. This has led us to believe that this disease is, in fact, a spectrum of neonatal gut injury syndrome, the most benign variant of course being IP and the most malignant being the often fatal metabolic disease that results in sepsis, organ system failure, and death, all related to necrotic intestine.
Having said that, we have attempted to identify through analysis of our data, a logistic regression model to provide weight and identity of specific factors to help us make the decision as to which way the patient should be treated, IP or lavage and laparotomy.
Dr. Lally was kind enough to share with me his manuscript. I have reviewed it, and I have taken the liberty of looking at odds ratios of his data. I note that, if you assume that laparotomy is the gold standard, babies who are identified with necrotizing enterocolitis who receive peritoneal drainage instead of laparotomy had mortality odds ratio of 1.7.
Interestingly enough, if they were identified as IP, they had an odds ratio of mortality with drainage even higher, which leads me to wonder how exactly the decision to drain versus operate was made. Was it protocolized? Or was it the result of individual surgeons having specific or subjective assessment as to what they thought was the appropriate way to go?
The next question is the issue of the logistic regression model and specific weights. Have you looked at your data with the perspective maybe of being able to identify a model that would develop a protocol that would, in fact, identify which patient would benefit from reduction of the septic load, if you will, by laparotomy, debridement, and lavage?
Finally, in the IP group who did not require operative intervention, there was a significant mortality. What was the cause of death for those children? Was it from sepsis? Was it, in fact, disease that was not operated on? Or was it some other issue?
Dr. James A. O'Neill, Jr. (Nashville, Tennessee): My comments will be much shorter on this paper, which I think reflects an idealistic approach to determining how to treat infants better from a study of outcomes.
As I listened carefully to the remarks of Dr. Lally, it came to my mind that the one firm conclusion that might be reached from the data is that it is possible to tell the difference between isolated perforation and more generalized necrotizing enterocolitis.
Now, there are certain questions that have to do with the validity of other conclusions with regard to this study. I will not comment on what the authors should do in the future, but rather to talk about what they actually did.
I think it is questionable whether it is reasonable to include in any comparison, isolated intestinal perforation and necrotizing enterocolitis, which involves widespread areas of intestinal gangrene. It seems to me that those groups should be studied separately.
The second thing is that there is a comparison of intestinal drainage as an initial procedure for intestinal perforation for these disparate disorders with initial laparotomy. It may very well be that there are indications of one or the other in each of these diseases. There needs to be, and I could not gather from the presentation that there was any standardization of approach with regard to selection of patients for intestinal drainage.
So this potentially leads to a very serious, at the very least, type 1 error in the interpretation of data. At least in my mind, there is serious question about whether the 2 therapeutic approaches are indeed equal. So the question is: what were the indications for peritoneal drainage, and were they standardized across the multicenter study? The nice part about a multicenter study is that you get a large number of patients. The difficult part is that people treat things differently.
Another question is: were the cases distributed equally, or close to equally, among the 15 centers? Or did you have one with 3 cases and another one with 15? There was an average of 10 per center.
Were any patients relegated to no treatment or were all patients operated on, for example, patients with intracranial hemorrhage grade 3 or grade 4? Were these patients operated on or were they excluded? If so, did this make a difference in the interpretation of data?
Additionally, why was central nervous system disability not considered an outcome measure, something we think may be every bit as bad as mortality?
So there are a number of issues related to study design and execution that I think need to be carefully analyzed before we can make firm conclusions, with the exception that perhaps you can tell the diseases apart. I would appreciate your comments and enjoyed the way you have tried to put this together.
Dr. J. Alex Haller, Jr. (Baltimore, Maryland): I want to make one comment to clarify this drainage for this very sick group of patients because for the nonpediatric surgeons present you may not realize that many of us have not accepted this as a form of treatment for a disease, which requires at least 50% of the time another operation than drainage in order to correct the surgical problem within the abdomen of the micropreemie with necrotizing enterocolitis.
This concept came to us from the Hospital for Sick Children in Toronto some 15 years ago and was based upon the fact that as they looked at their patients who were so sick, their micropreemies with perforation, when they took them to the operating room they had a higher mortality if they operated on them than if they just drained them in the nursery. But when closely questioned, our pediatric surgery colleagues said that the reason for that was that they developed hypothermia during transport from the nursery to the operating room through the cold corridors of the Hospital for Sick Children in Toronto and that that was the reason for changing the treatment.
Now, it seems to me in the protocol that is now being proposed, we must be careful we don't incorporate in it that heresy. Because no drainage can be the treatment for dead bowel or for perforated bowel, it can be a temporizing approach. And it seems to me, and in speaking to my colleagues who are going to be on this committee, that you must be very careful as to what do you believe is the cause of death in children with isolated perforation and those with necrotizing enterocolitis.
As I have tried to figure out over these last 15 years why our Canadian colleagues just drain those patients, it seems to me the only reason they got better survival was because they decompressed the abdomen. If that is the main factor, then laparotomy as an alternative to drainage does the same thing. And it is going to be very difficult, I think, Dr. Lally, to separate that. If that is the major factor in survival, that you decompress and therefore allow for better circulation because you don't have an increased pressure within the abdomen, it is going to be difficult to separate those groups of patients in terms of trying to decide which is the better modality for treatment.
So as Dr. O'Neill said, caution is certainly in order, and this design must be very carefully looked at in terms of what outcome can be expected.
Dr. Charles E. Bagwell (Richmond, Virginia): The treatment by drainage alone, as proposed by Siggie Einback in the 1970s, treats the perforation like an abscess. It has been controversial since first proposed, but has become well accepted simply because it works. These tiny infants are just awfully sick, and one can imagine that laparotomy for this group, especially when septic, carries an inordinate surgical risk.
One question for the authors involves the use of indomethicin, which has been shown to be associated with isolated small bowel perforations. Accordingly, I wonder how many of these babies with perforations were given Indocin for patent ductus arteriosus or other conditions?
The value of this paper is to establish at last a cooperative group to look at these treatment options and provide some guidelines for treatment based on data and results. Hopefully, this is the beginning on accumulating such data for us.
Dr. Martin L. Blakely (Memphis, Tennessee): I would like to thank you for that vigorous discussion of this paper.
Indocin use didn't really matter in any of the comparisons we made. There was no difference in those babies that were diagnosed with NEC or isolated perforation, which goes against some of our biases, and Indocin use was not important in the outcome of the patients. When we sat down 5 years ago in the Neonatal Research Network to study this question, we wanted to pick the most rigorous study design and felt like a randomized control trial would be the appropriate method. There are 2 widely disparate treatment options for this patient population. Most pediatric surgeons think that the relevant population is birth weight less than 1000 g. But when we started looking at the data that were available, including a meta-analysis done by Larry Moss in 2001, it was very difficult, and we felt impossible, to adequately design a randomized trial.
What I see as one of the positives of this study is that rather than just guess based on the previous data, we felt like we needed to acquire the data in order to help us design a trial. We felt then, and we feel now, that the best way to answer this question is with a randomized trial; however, we felt like we just couldn't do that with the available background data.
Dr. Langham appropriately touched on kind of a “so what” question. We have been looking at NEC. Now we think there is maybe a new disease called isolated intestinal perforation. But we still don't know how to diagnose NEC earlier. There is no animal model that accurately mimics NEC. The etiology of the disease remains unknown.
One of the things that the NIH Neonatal Research Network is planning to do is to use a randomized trial evaluating the surgical options as a vehicle to collect biologic specimens, blood spots, and resected tissue. And I think that may be one of the most important parts of the trial.
There is a lot of interest to perform such a trial so that we can figure out hopefully what causes NEC, what are some of the molecular pathways, is there a difference between NEC and isolated perforation, and hopefully to identify some targets where we can diagnosis this earlier and have some more effective treatments.
So my thought is that a clinical trial evaluating the surgical therapies available to date will be very important for us to figure out how to treat these children. But it may be that the biologic exploration of these diseases may be more important.
Several people asked about patients who were identified as having NEC or isolated intestinal perforation and then died before surgery. We had none of those infants in this series. The starting point for enrollment in the study was the neonatologist and pediatric surgeon felt like that the child had neither NEC nor isolated perforation and required surgery. All of those babies received either a drain or a laparotomy.
Regarding stratification variables to be used in a future trial, obviously, that is very important. Neither of the ongoing trials stratifies based on preoperative dianogis (NEC or IP). Obviously, you can't use too many stratification variables. In all of the analysis we did, the preoperative diagnosis of NEC versus isolated perforation was a very important characteristic.
What is the evidence-based hypothesis to be tested? That also was kind of the $20 million question. This will have to be worked out with all of the pediatric surgeons at the participating centers. But I think we will certainly include a follow-up measure, which is one of the other questions that Dr. O'Neill asked.
We now have over 90% complete follow-up at 18 to 22 months (neurodevelopmental and growth outcome). Very early indications are that the drainage group has a significantly worse developmental assessment at that follow-up period. So, the hypothesis thus far is that infants with initial laparotomy would have equivalent survival but improved longer-term outcome.
We have some thoughts about why that may be. One is that drainage, while it doesn't change the mortality, may allow for some ongoing sepsis or cytokine imbalances, which have been shown to translate into worse neurodevelopmental impairment in these babies in other studies.
Dr. Neblett asked about the accuracy of preoperative diagnosis and how you would use an 83% accuracy rate in isolated perforation and how would you have the ability to stratify based on that. I think that is a valid concern. But I think you have to just take one step at a time. There are 2 trials going on now that don't consider there to be any difference in the starting population. We don't feel like that is the most accurate approach.
And while the study was not designed to specifically test the ability to diagnose NEC versus isolated perforation, there was such a tight correlation that I think we have at least a reasonable ability to do that and that that would be important in stratifying our treatment assignments in a trial so that we are starting with more equivalent groups.
The operative indications in this study were not standardized. In fact, it is going to be very difficult to do. It will be important to do this in a trial. But what we were trying to do in this study was to see what surgeons are actually doing currently. Obviously, many pediatric surgeons are diametrically opposed on this topic. Some think drainage is the way to go. Others think that makes no sense. This study was designed to specifically find that data and try to figure out who is doing what and why they are doing it. So before we start this trial, all of the surgeons participating in this will have to come together and have some agreement in order for a trial to go forward.
The question about excluding NEC totalis or near-total NEC, I think, is very difficult because you don't know this information on the front end. So I don't think that is going to be possible. It is true that basically none of those babies survives. But since you can't tell that at the time of randomization, then I don't think you can exclude those babies, and you would just have to assume that, with a randomization procedure, there would be equivalent numbers of such infants between groups and that could be measured.
We did not use a scoring system, although we did discuss this. Our statisticians warned us away from this. If there are 10 to 12 variables that go into the scoring system, which is then used in a multivariable logistic regression, then pretty soon you have more variables than patients. And they felt like that was not appropriate. For the same reason, we did not stratify by center, which could be a criticism.
Dr. Tepas asked about 2 diseases or not. There are some surgeons who think that this is just different ends of the spectrum of the same disease. Again, I think that is a very important reason why we need a trial with a biology component. I think our surgical oncology colleagues have made great strides because not only have they done randomized trials of surgical therapies or medical therapies but they always collect tissue, and they have learned what are the targets and they develop better therapeutics. So I think now is a good time to do a randomized trial, including a biology study so that we can start to explore this. The treatments were decided based purely on the surgeons’ wishes during this observational study, as I mentioned.
The modeling you mentioned, I think, is another method to try to get at this. We did not do this and felt, and still feel, that a true prospective randomized trial between hopefully fairly equivalent groups will be the best way to determine this.
As for cause of death in isolated perforation in those babies who didn't get a subsequent laparotomy, most died due to the perforation or sepsis. But as you saw on that adjusted Kaplan-Meier survival curve, some died really late, perhaps due to comorbidities.
And I think that is one of the problems; we put a drain in and the baby becomes somewhat of a black box. If it gets better, does that mean you should operate on him? Well, that is somewhat counterintuitive. Usually, if our therapy is getting someone better, that means you don't change what you are doing. But obviously, people take the opposite strategy that, if you put a drain in, and they are doing better, of course, you should operate on them. Some of these babies with isolated perforation do die of chronic lung disease and some of other morbidities of prematurity very late.
One of the things we did not present today was the morbidity data. Obviously, we looked at mortality and also morbidity. And there was a trend toward lower rates of prolonged parental nutrition in the babies with laparotomy versus drainage. So we feel that, although mortality is not really different, if anything, the morbidity may be higher with the drainage patients.
One last comment on Dr. O'Neill's question about the CNS disability. We agree with him wholeheartedly that neurologic outcome is the most important thing, and we hopefully will have a separate analysis on the follow-up to present soon.
Footnotes
Supported in part by the following grants: K23HD001473 (M.L.B.), U10HD21373 (J.E.T.), K24RR17050 (K.P.L.), U10HD27851 (B.J.S.), U10HD27880 (D.K.S.), U01HD36790 (W.K.P.), as well as U10HD34216 (W. Carlo), U10HD21397 (S. Duara), U10HD27853 (E. Donovan), U10HD27871 (R. Ehrenkranz), U10HD21364 (A. Fanaroff), U10HD40461 (N. Finer), U10HD40492 (R. Goldberg), U10HD40689 (A. Laptook), U10HD27856 (J. Lemons), U10HD27904 (W. Oh), U10HD40498 (T.M. O'Shea), U10HD40521 (D. Phelps), and U10HD21385 (S. Shankaran).
*NEC Subcommittee of NICHD Network: Martin L. Blakely, MD, Jon E. Tyson, MD, MPH, Kevin P. Lally, MD, Barbara J. Stoll, MD, David K. Stevenson, MD, W. Kenneth Poole, PhD, Alan H. Jobe, MD, Linda L. Wright, MD, and Rosemary D. Higgins, MD.
Reprints: Martin L. Blakely, MD, University of Tennessee, Health Science Center, 777 Washington Avenue, Suite P220, Memphis, TN 38105. E-mail: mblakely@utmem.edu.
REFERENCES
- 1.Ricketts RR, Jerles ML. Neonatal necrotizing enterocolitis: experience with 100 consecutive surgical patients. World J Surg. 1990;14:600–605. [DOI] [PubMed] [Google Scholar]
- 2.Ketzer de Souza JC, da Motta UIC, Ketzer CR. Prognostic factors of mortality in newborns with necrotizing enterocolitis submitted to exploratory laparotomy. J Pediatr Surg. 2001;36:482–486. [DOI] [PubMed] [Google Scholar]
- 3.Dzakovic A, Notrica DM, O'Brian S, et al. Primary peritoneal drainage for increasing ventilatory requirements in critically ill neonates with necrotizing enterocolitis. J Pediatr Surg. 2001;36:730–732. [DOI] [PubMed] [Google Scholar]
- 4.Holman RC, Stoll BJ, Clarke MJ, et al. The epidemiology of necrotizing enterocolitis infant mortality in the United States. Am J Public Health. 1997;87:2026–2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheu HW, Sukarochana K, Lloyd DA. Peritoneal drainage for necrotizing enterocolitis. J Pediatr Surg. 1988;23:557–561. [DOI] [PubMed] [Google Scholar]
- 6.Buchheit JQ, Stewart DL. Clinical comparison of localized intestinal perforation and necrotizing enterocolitis in neonates. Pediatrics. 1994;93:32–36. [PubMed] [Google Scholar]
- 7.Adderson EE, Pappin A, Pavia AT. Spontaneous intestinal perforation in premature infants: a distinct clinical entity associated with systemic candidiasis. J Pediatr Surg. 1998;33:1463–1467. [DOI] [PubMed] [Google Scholar]
- 8.Pumberger W, Mayr M, Kohlhauser C, et al. Spontaneous localized intestinal perforation in very-low-birth-weight infants: a distinct clinical entity different from necrotizing enterocolitis. J Am Coll Surg. 2002;195:796–803. [DOI] [PubMed] [Google Scholar]
- 9.Ahmed T, Ein S, Moore A. The role of peritoneal drains in treatment of perforated necrotizing enterocolitis: recommendations from recent experience. J Pediatr Surg. 1998;33:1468–1470. [DOI] [PubMed] [Google Scholar]
- 10.Moss RL, Dimmitt RA, Henry MCW, et al. A meta-analysis of peritoneal drainage versus laparotomy for perforated necrotizing enterocolitis. J Pediatr Surg. 2001;36:1210–1213. [DOI] [PubMed] [Google Scholar]
- 11.Blakely ML, Tyson JE, Lally KP, et al. Outcome of extremely low birth weight premature infants with necrotizing enterocolitis or isolated intestinal perforation treated with initial laparotomy or peritoneal drainage. Pediatrics. submitted 2005. [DOI] [PubMed]
- 12.Mata AG, Rosengart RM. Interobserver variability in the radiographic diagnosis of necrotizing enterocolitis. Pediatrics. 1980;66:68–71. [PubMed] [Google Scholar]
- 13.Tam AL, Camberos A, Applebaum H. Surgical decision making in necrotizing enterocolitis and focal intestinal perforation: predictive value of radiologic findings. J Pediatr Surg. 2002;37:1688–1691. [DOI] [PubMed] [Google Scholar]
- 14.Ehrlich PF, Sato TT, Short BL, et al. Outcome of perforated necrotizing enterocolitis in the very low-birth weight neonate may be independent of the type of surgical treatment. Am Surg. 2001;67:752–756. [PubMed] [Google Scholar]
- 15.Camberos A, Patel K, Applebaum H. Laparotomy in very small premature infants with necrotizing enterocolitis or focal intestinal perforation: postoperative outcome. J Pediatr Surg. 2002;37:1692–1695. [DOI] [PubMed] [Google Scholar]
- 16.Janik JS, Ein SH, Mancer K. Intestinal stricture after necrotizing enterocolitis. J Pediatr Surg. 1981;16:438–443. [DOI] [PubMed] [Google Scholar]
- 17.Horwitz JR, Lally KP, Cheu HW, et al. Complications after surgical intervention for necrotizing enterocolitis: a multicenter review. J Pediatr Surg. 1995;30:994–999. [DOI] [PubMed] [Google Scholar]
- 18.Chung DH, Ethridge RT, Kim S, et al. Molecular mechanisms contributing to necrotizing enterocolitis. Ann Surg. 2001;233:835–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Caplan MS, Jilling T. New concepts in necrotizing enterocolitis. Curr Opin Pediatr. 2001;13:111–115. [DOI] [PubMed] [Google Scholar]