Abstract
Summary Background Data:
Previous studies have suggested a variety of factors that may affect the false negative (FN) rate for sentinel lymph node (SLN) biopsy in breast cancer. Because FN results are relatively rare, no prior studies have had sufficient sample size to allow detailed statistical analysis of factors predicting FN results.
Methods:
Patients with clinical stage T1-2, N0 invasive breast cancer were enrolled in a prospective, multicenter study. All patients underwent SLN biopsy, followed by planned completion axillary dissection regardless of the SLN results, to assess the FN rate. SLN biopsy was performed using radioactive colloid injection in combination with isosulfan blue dye in 94% of cases. Dermal, subdermal, peritumoral, or subareolar radioactive colloid injection techniques were used at the discretion of each institution. Univariate and multivariate analyses were performed to identify factors associated with a FN result.
Results:
SLNs were identified in 3870 of 4117 patients (94%). There were 1243 true positive, 2521 true negative, and 106 FN results. Age, histologic subtype, the number of non-SLN removed, tumor palpability, type of breast biopsy, and SLN injection technique were not significant factors. On multivariate analysis, tumor size <2.5 cm, upper outer quadrant tumor location, removal of only a single SLN, minimal surgeon experience, presence of a single positive axillary LN, and use of immunohistochemistry (IHC) for SLN analysis were independently associated with an increased risk of FN results.
Conclusions:
Surgeon experience, tumor size and location, and the number of SLN removed are preoperative and intraoperative factors that independently predict the risk of a FN result. In contrast to suggestions from other smaller studies, age does not affect the likelihood of a FN result; a lesser, rather than greater, number of positive axillary nodes was associated with an increased likelihood of a FN result; and IHC analysis of the SLN increases, rather than decreases, the risk of FN results.
Although sentinel lymph node (SLN) biopsy has become widely accepted as an alternative to routine axillary dissection for breast cancer, the reported false negative (FN) rates have varied widely, from 0% to as high as 19%.1 A FN result could be detrimental to the patient because it results in inaccurate staging, with important implications for adjuvant therapy and the possibility of persistent axillary nodal disease. Previous studies have suggested a variety of factors that may affect the FN rate. However, because FN results are uncommon, no prior study has had a large enough sample size to systematically evaluate factors that are independently associated with FN results. Thus, the purpose of this analysis was to determine, in a large multi-institutional study, the factors that predict FN SLN biopsy results.
METHODS
The University of Louisville Breast Cancer Sentinel Lymph Node Study is a prospective, multi-institutional study involving over 300 surgeons, mostly from community general surgery practices. The study was approved by the institutional review board of each participating center. For most participating surgeons, this study represented their initial learning experience with SLN biopsy. Patients were enrolled between August 1997 and May 2004. Informed consent was obtained from all patients. Patients with biopsy-proven clinical stage T1-2, N0 breast cancers were eligible. Some patients who were found to have T3 tumors on final pathology were also included in this analysis.
SLN biopsy was performed using isosulfan blue dye alone, radioactive colloid alone, or both agents in combination at the discretion of the operating surgeon as described previously.2 Preoperative radioactive colloid injections were performed with 0.5-mCi technetium sulfur colloid in the peritumoral, dermal, subdermal, periareolar or subareolar locations. Preoperative lymphoscintigraphy (nuclear medicine scanning) was optional. At the time of surgery, 5 mL of isosulfan blue was injected peritumorally in the majority of cases (94%).
All patients underwent attempted SLN biopsy followed by completion level I/II axillary lymph node dissection. Removal of nonaxillary nodes (eg, internal mammary nodes) was not required as part of the study. SLN were examined histologically by hematoxylin and eosin (H&E)-stained sections at no greater than 2-mm intervals. Evaluation of the SLN by cytokeratin immunohistochemistry (IHC) was not required as part of the standard protocol but was performed at each institution's discretion. Nonsentinel axillary lymph nodes were subjected to routine H&E examination. We categorized the results as FN, true positive (TP), or true negative (TN). Statistical comparison was performed using FN and TP results as the response variable and the following explanatory variables: surgeon case number (ordered chronologically), age, size of invasive cancer, tumor palpability, histologic subtype, biopsy type, surgery type, tumor location, number of SLNs removed, number of positive SLNs, use of IHC and injection type. The FN rate was calculated as the number of FN results ÷ (number of TP + the number of FN).3 Statistical analysis was performed by univariate χ2 tests and multivariate logistic regression for odds ratios to identify independent factors predicting FN results compared with TP results. Significance was determined at a P < 0.05.
RESULTS
A total of 4116 patients were entered into the study. The SLN was identified in 3869 (94%) of these patients. There were 1243 TP, 2520 TN, and 106 FN results.
The age distribution of this study is shown in Table 1. There was an even distribution of nodal positivity across all age groups, except in the small group of patients less than 30 years of age. Age was not a significant predictor of FN results, with an even distribution of FN, TN, and TP results in this study (Fig. 1). Similarly, comparisons of histologic subtype (infiltrating ductal versus infiltrating lobular versus other), palpable tumors versus nonpalpable tumors, breast-biopsy technique or definitive surgical technique (lumpectomy versus mastectomy), or injection method did not demonstrate any statistically significant differences in FN rates (Table 1).
TABLE 1. Nonsignificant Factors Predicting FN Results
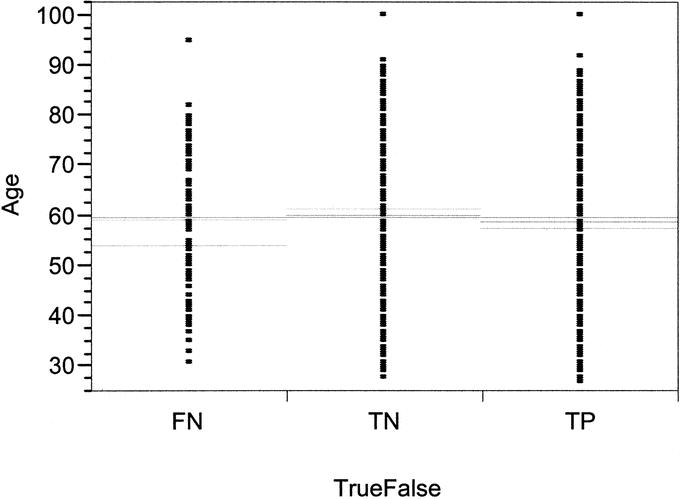
FIGURE 1. Scatter plot of age distribution of FN, TN, and TP in successful SLN.
A significant difference in FN rate was observed for tumors in the upper outer quadrant (UOQ) of the breast, with nearly a 2-fold increase in FN rate compared with lesions located in other locations (P = 0.0007; Table 2). The FN rate was also demonstrated to be significantly greater in smaller tumors (T1) compared with larger tumors (Table 2). When size was treated as a continuous variable, tumors less than 2.5 cm also were associated with a higher FN rate compared with larger tumors (P = 0.0076). The 2.5-cm size was determined based on the distribution of FN results across all tumor sizes as the most significant cut point. A scatter plot of tumor size with FN, TP, and TN results is shown in Figure 2. A 2-fold increase in FN rate was also seen in patients who had only 1 SLN removed compared with patients who had 2 or more SLNs removed (P < 0.0001; Table 2, Fig. 3). The FN rate varied inversely with the number of SLNs removed, with a FN rate of 4.2% of patients who had >2 SLNs removed (Table 2, Fig. 2). An evaluation of the number of cases performed by individual surgeons (case number) was also found to be predictive of a higher FN rate among surgeons performing <4 cases compared with ≥4 cases (P = 0.02; Table 2). There was a steady reduction in FN rate with increasing case number (Fig. 4). Univariate and multivariate analyses utilizing other cutoffs for surgeon case number (5, 10, 15, or 20 cases) were also statistically significant on both univariate and multivariate analyses.
TABLE 2. Significant Factors Predicting FN Results
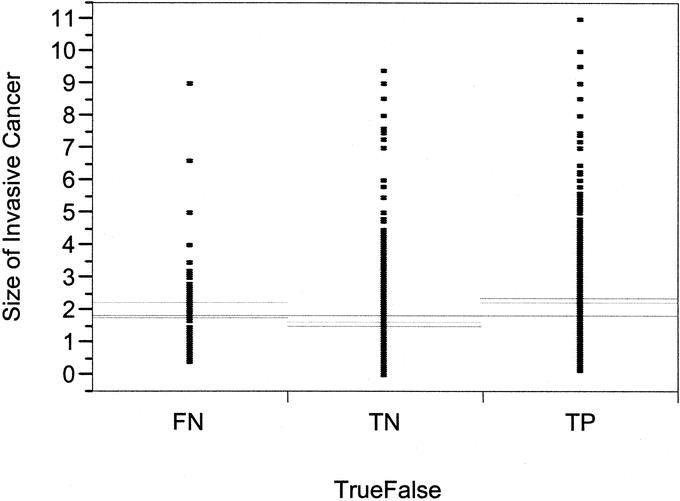
FIGURE 2. Scatter plot distribution of significant difference in FN in patients.
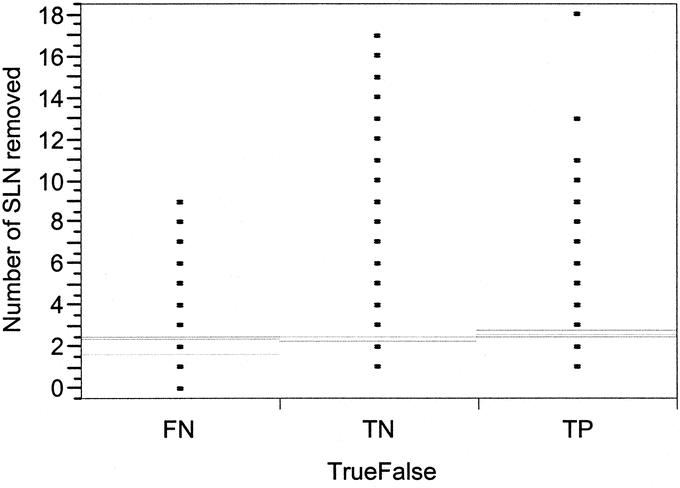
FIGURE 3. Scatter plot distribution showing a significant difference in FN rate by number of SLN removed.
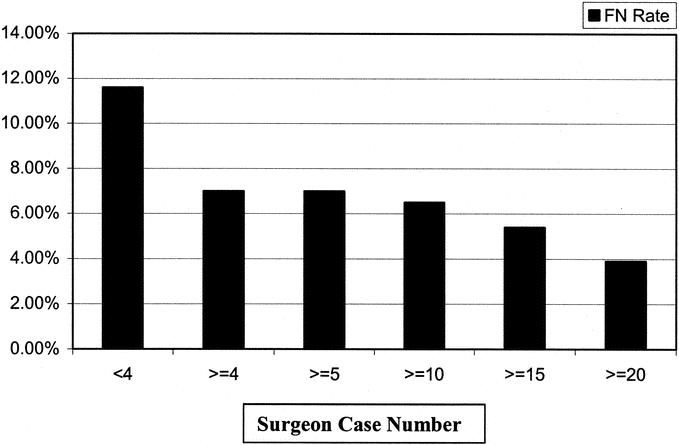
FIGURE 4. Reducion in FN rate by increasing surgeon experience.
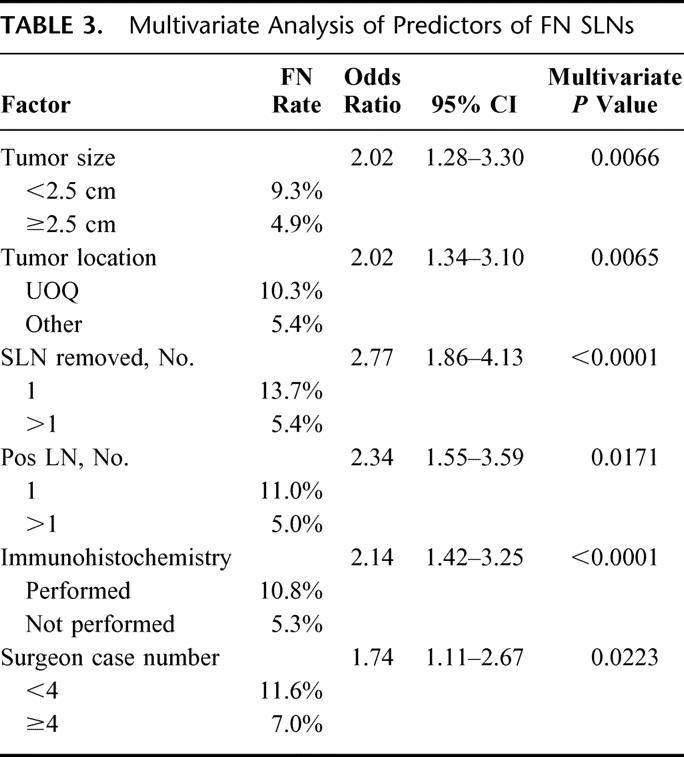
A multivariate logistic regression model confirmed that tumor size, tumor location, the number of SLNs removed, the number of positive lymph nodes, surgeon case number, and the use of IHC for SLN analysis were all independent factors predicting FN results (Table 3).
TABLE 3. Multivariate Analysis of Predictors of FN SLNs
DISCUSSION
Despite refinements in injection techniques that have led to near 100% SLN identification in some studies,1,4,5 variable FN rates have raised concern about the staging accuracy of this procedure. As with most diagnostic tests, SLN biopsy is not 100% sensitive: it does not infallibly detect nodal metastasis. There is a tradeoff between a less invasive, less morbid staging procedure and the possibility of a FN result. Therefore, it is of considerable interest to identify factors that might minimize the incidence of FN results and improve the overall staging accuracy of SLN biopsy.
To assess the FN rate, studies must have been designed (as in the present study) such that all patients underwent SLN biopsy followed by planned completion axillary dissection, regardless of the SLN result. Such studies have become uncommon in the past few years because most centers have adopted SLN biopsy without automatically performing backup axillary dissection. Because FN results are relatively rare, few studies have been able to perform meaningful statistical analyses of factors predicting FN results. As the present study is by far the largest such multi-institutional study to assess FN results, this provides a unique opportunity to evaluate factors that are independently associated with inaccurate SLN results.
We have identified 5 key factors that can be considered preoperatively or intraoperatively that put a patient at risk for a FN result: small tumor size, UOQ tumor location, minimal surgeon experience, and removal of only a single SLN. Use of IHC for SLN analysis is a postoperative consideration that is associated with a higher risk of FN results. Patients having a single positive axillary LN are also at somewhat greater risk for a FN result, although this consideration cannot be assessed prior to surgical intervention. Of these, small tumor size, use of IHC, and a single positive axillary node represent novel findings not reported in other studies. UOQ tumor location6 and surgeon experience2,7,8 and the number of SLNs removed9–12 have been suggested in other studies.
Previous smaller studies have suggested other factors that might be associated with FN results that were not confirmed in the present study. It has been suggested that older patients may have a higher FN rate.8,13 In our study, there was no significant impact of patient age on the FN rate. Others have suggested that excisional biopsy is associated with worse SLN results compared with patients who have had a needle biopsy.14–19 Our results do not support any association with biopsy technique and FN results, indicating that SLN biopsy is equally accurate in patients who have undergone needle or excisional biopsy. Others have suggested that more positive nodes, or increased tumor burden within the axilla, results in a higher FN rate.8,20–24 This is based on the supposition that, in some cases, tumor in the lymph nodes can obstruct the normal lymphatic drainage and result in inaccurate SLN results. This has led to the suggestion that palpation of the axillary nodes should always be performed to identify any abnormal nodes that may be replaced with tumor. While this is a prudent suggestion, the data from the present study and from a previous analysis25 do not support the notion that more positive axillary nodes are associated with an increased FN rate. In fact, the present study suggests the opposite. Although it is possible that, in our study, some surgeons removed palpably suspicious nodes that were neither blue nor radioactive, a previous analysis indicated that nearly all positive SLNs contained either blue dye or radioactivity in excess of 10% of the radioactive count of the most radioactive, or “hottest,” SLN.26
The results of the present study strengthen the previous demonstration that the removal of greater than 1 SLN significantly reduces the FN rate.9–12 It has been well established that lymphatic draining patterns may occur with multiple lymphatic channels, leading to more than 1 SLN in the axilla. The results of the present study confirm that the number of SLNs removed is a powerful independent predictor of FN results. While it is often straightforward to identify the first SLN, which typically is located in level I of the axilla, the results of the present study and others suggest that a diligent search for additional SLN will result in more accurate nodal staging.
Others have suggested that the FN rate may be higher for larger tumors (T2 and T3) compared with T2 tumors.20,27 Others have refuted this idea.28–31 The present analysis indicates that, rather than in larger tumors, the FN rate is greater among patients with tumors <2.5 cm in size. This may reflect the fact that the number of positive nodes increases directly with increasing tumor size and that it may be more likely to identify a positive SLN in an axilla that harbors multiple positive nodes. Alternatively, this may suggest that accurate injection of radioactive colloid and blue dye in the region of the tumor may be more challenging for small, nonpalpable tumors. This seems less likely, given the growing belief that the entire breast may drain via the same few lymphatic channels to the axilla and that injection location may be of little importance.5,32,33
In an earlier analysis with fewer patients, we reported that the FN rate was decreased among patients who underwent dual-agent (blue dye plus radioactive colloid) injection as compared with blue dye alone.34 This finding has been supported by others.35–38 While the use of blue dye alone is associated with a lower identification rate,1,2 the present analysis of over 4000 patients indicates that injection technique is not associated with significant differences in the FN rate. In the absence of clear superiority of one injection technique over another in terms of the FN rate, the choice of injection technique should be made based on the greatest identification rate and the experience and expertise of each institution. We have previously found that dermal or subareolar radioactive colloid injection techniques result in close to 100% SLN identification.5,39
Others have stated that FN results ultimately have little to do with the injection technique or preoperative clinical factors but ultimately rest with a determined pathologic evaluation. Others have previously reported that detailed IHC analysis can reduce the FN rate.40–42 However, the present multi-institutional study strongly indicates that cytokeratin IHC analysis of SLN offers no advantage in terms of reducing the FN rate; in fact, the FN rate was significantly greater among patients who underwent IHC analysis. The reasons for this are unclear and certainly not intuitive. It may reflect the variability of interpretation of cytokeratin IHC stains of the SLN in a multi-institutional setting of largely community hospitals, as opposed to single large-volume centers at which IHC analysis has been studied intensively. IHC analysis has become quite controversial, especially given the high percentage of patients with pure ductal carcinoma in situ who are found to have cytokeratin-positive SLN (12%,43 13%,44 and 23%45), a disease which historically is associated with a minimal risk of axillary nodal metastases.46 Routine cytokeratin IHC for SLN evaluation is not recommended by the College of American Pathology.47 The clinical significance of IHC-detected micrometastases is the subject of 2 large national studies, the National Breast and Bowel Project (NSABP) B-32 study and the American College of Surgeons Oncology Group (ACOSOG) Z11 study. Until or unless the results of these studies indicate that IHC analysis of SLN provides clinically significant information, routine IHC analysis of SLNs should not be performed.
Interestingly, even with concern over FN SLN biopsy results, there have been few reports of axillary lymph node recurrences in patients with negative SLN who did not undergo completion axillary dissection.48,49 While this may suggest that FN results are very uncommon in experienced centers, it also may simply reflect the possibility that persistent axillary nodal metastasis may take many years to become clinically apparent and that longer follow-up is needed. Others have suggested that FN results have little impact on patient therapy and outcome.50 However, if we rely on SLN biopsy as an alternative to axillary dissection, it is important to do everything possible to assure the accuracy of the procedure.
Surgeon experience has been shown to be perhaps the most important factor in reducing FN results. In the present study, the FN rates was 11.6% and 7.0% for surgeons who performed <4 or ≥4 cases, respectively. Tafra et al8 suggested, based on their multi-institutional study of SLN biopsy, that surgeons should perform 30 cases to reduce the FN rate. In a previous analysis of 2148 patients, we determined that the FN rates for surgeons who performed ≥20 cases had a substantial improvement in FN rate to well within the <5% range that is usually considered acceptable.2 In the present study, it appears that significant improvement in FN rate occurs after as little as 3 cases have been performed. This updated analysis from a large patient database likely reflects improvements in injection techniques and overall experience of the multidisciplinary teams (surgeons, nuclear medicine, and pathology) at many institutions that has allowed surgeons to perform SLN biopsy accurately with less than 20 to 30 case experiences.
Consideration of combinations of factors identified in this analysis results in significant reduction of the FN rate, even for the more difficult cases of UOQ tumors, tumors <2.5 cm, or patients with a single positive axillary node. For example, if the surgeon had performed 4 or more cases, removed more than 1 SLN, and did not use IHC for SLN analysis, the FN rates would be 3.1% overall, 1.1% for UOQ tumors, 3.4% for tumors <2.5 cm, and 5.2% for patients with a single positive axillary node.
In conclusion, we have identified a number of factors in this large multi-institutional study that predict a greater risk of FN results. Surgeon experience, tumor size, and tumor location should be considered preoperatively when counseling patients regarding the relative benefit of a minimally invasive staging procedure versus the risk of inaccurate nodal staging. The number of SLNs removed is an intraoperative factor that can reduce the risk of a FN result. Use of IHC for SLN evaluation is an important postoperative consideration.
ACKNOWLEDGMENTS
The authors would like to thank Kristy Greenwell, Ivan Deyahs, Sherri Matthews, and Deborah Hulsewede for their dedication and hard work in managing the study and the database. The authors would also like to thank the University of Louisville Breast Cancer Sentinel Lymph Node Study Group for participation in the study. A complete listing of study members is available.51
Discussions
Dr. Edward M. Copeland, III (Gainesville, Florida): Drs. McMasters, Martin, and their colleagues, especially Dr. Michael Edwards, are to be congratulated for conducting a study that has community practitioners as its primary participants. This surgical study is somewhat unique in this regard and, consequently, the data generated become even more important since the majority of breast-cancer patients are taken care of in the private environment. The study also provides the much-needed quality control of the procedure for all of the participants. All surgeons who participated should be congratulated for allowing their results to be scrutinized, and this study can be used as a model for community hospital participation in the National Surgical Quality Improvement Program initiated by the Veterans Administration System and now being extended into the private sector through the auspices of the American College of Surgeons.
I will focus on only 1 significant factor identified by the investigators. Several studies, including one of ours, have shown that the “hottest” sentinel node is not always the positive one. Therefore, diligence in identifying all either hot or blue nodes must be the rule. This can be time consuming and somewhat frustrating but must not be ignored if the proper staging of the axilla is to be done. In my experience, an axillary dissection is sometimes quicker than a thorough search for nodes that meet the criteria for being sentinel. And those in the audience who do the procedure are smiling and snickering, because they know this to be true.
Dr. Martin, you found factors related to a high false-negative rate were number of sentinel nodes identified, upper outer quadrant location, and surgeon experience. Consequently, a question leaps out of the study to me from those 3 factors. Are you now recommending that a surgeon with minimal experience who finds a single negative sentinel node in a patient with an upper outer quadrant lesion do an axillary dissection? If so, define “minimal experience” since you indicate that a reasonable success rate can be expected with as few as 4 sentinel node procedures under your belt.
Dr. Marshall M. Urist (Birmingham, Alabama): Drs. Martin, McMasters, and their colleagues have presented important information on a major obstacle to sentinel lymph-node biopsy for breast cancer, namely, the risk of a false-positive report. Their analysis is derived from a database developed to assist surgeons in their efforts to learn a technique and to verify their newly acquired skills. It is very commendable. The design of the study facilitated surgeon participation and therefore provided a large number of patients for this analysis, but the nonuniformity of the procedures has limited the scope of their conclusions.
Overall, the results show that sentinel lymph node biopsy is a very accurate and reproducible procedure with a low incidence of false negatives. Several factors were found to be associated with the risk of finding a false-negative sentinel lymph node: surgeon experience, tumor size and location, number of sentinel lymph nodes removed. These results are in concordance with some previous reports and in conflict with others. As with all excellent studies, it provides a number of questions:
The risk of a false-negative biopsy was reduced by removal of more than 1 sentinel lymph node. Irrespective of when or where or by what technique the biopsy is performed, do you recommend removal of additional lymph nodes when only 1 radioactive or blue lymph node is found? If so, which ones, and how many?
Tumors in the upper outer quadrant had a higher false-negative rate. Could this have been related to the site of injection of the radioisotope? A peritumoral injection in the upper outer quadrant may mask the detection of a sentinel node in the tail of the breast or in the low axilla. I would also like to ask whether or not any positive nodes were found in intramammary nodes.
The results of immunohistochemistry are not logical, and the technique was not applied to the entire patient population. Since most centers do not use this technique, why have you included it in your analysis today?
In the final analysis, the most important question is whether or not any patients would have been harmed by a false-negative biopsy. Resection of a low number of axillary lymph nodes has not been shown to result in a reduction of overall survival in breast-cancer patients. A false-negative sentinel lymph node biopsy does potentially have an adverse effect on survival in the case where it prevents a patient from receiving systemic adjuvant therapy. How many patients with a false-negative node would have received additional therapy if this node metastasis had been detected? I suspect that it is a very low number.
Dr. Kirby I. Bland (Birmingham, Alabama): Dr. Martinand Dr. McMasters are to be congratulated for bringing a very important study to the Association, and I commend Dr. McMasters and Dr. Edwards for this large database theyhave created with over 300 surgeons. My questions are really very focused in following with the 2 previous discussants.
Basically, if a patient has the tumor metastatic to a nodal group and the afferent lymphatics carry volumes of tumor cells, which obstruct the efferent lymphatics, you often see a node in which same is not hot and it is not blue. These are often obviously replaced with tumor.
Sometimes it does not light up with radioactivity at all. Therefore, my question focuses upon the issue if you looked at tumor burden in the axilla and, specifically, extracapsular invasion of that nodal group because that is clearly an indication where you truly will get a false-negative nodal evaluation. And once you do an axillary dissection where the rest of them find those nodes, you clearly are going to find that 1 or 2 or 3 of those are positive and often a node replaced with significant volume of tumor.
The second question I have relates to the upper outer quadrant lesion. Would you expand on that and how you manage this? There is a way to do this that we often utilize with 1 incision, but there has got to be adequate exposure to approach the axilla. We always do a subareolar or periareolar injection rather than a paratumoral injection in all lesions in the upper outer quadrant.
Dr. Kelly K. Hunt (Houston, Texas): I would also like to congratulate the group at the University of Louisville for the work that they have done with this large database. They have contributed significantly to understanding the success of sentinel lymph-node surgery for breast cancer but now have raised a number of questions in what to do to reduce the false-negative rate. Although the false-negative rate appears to be relatively low in a large number of patients, many of the patients in this series had negative nodes, and so the overall incidence is relatively low.
I had the opportunity to review the manuscript prior to the meeting, so I have a couple of questions for the authors. One is similar to Dr. Bland's comment in that what methods could you use or could you suggest surgeons use in order to improve visibility of the sentinel nodes when you have an upper outer quadrant tumor? Is this the type of case where surgeons should definitely use 2 methods for identifying the sentinel node? You suggested that only 1 method, such as radioisotope, is effective. But perhaps in the upper outer quadrant, blue dye and radioisotopes should be used to reduce the incidence of leaving behind some of the sentinel nodes.
The authors have previously stated in some of their work that lymphoscintigraphy is not useful in sentinel lymph node surgery and should not be performed. But perhaps for upper outer quadrant tumors this is a case where lymphoscintigraphy would be useful, because it often gives us the number of hot spots in the axilla and that can help guide the surgeon to understand how many nodes they need to identify before they can feel secure that they have adequately completed the procedure.
We have used the technique of injecting a higher dose of radioisotope the day prior to surgery so that, based on the half-life of the isotope, the sentinel nodes still have the same amount of radioactivity in them the following day as they would if isotope was injected on the morning of surgery with a lower dose. And we have examined the lymphoscintigraphy the day of the injection and delayed lymphoscintigraphy and find the same number of sentinel lymph nodes using this technique. So I suggest that this might be of use and would like to hear the authors' comments. With the results of the IHC in mind, do you think that many pathologists may use IHC as a crutch instead of actually an additional tool in sentinel lymph-node identification? What I am suggesting is that perhaps they just do an IHC and they don't do serial sectioning of the paraffin blocks. You stated that the nodes were sectioned at 2-mm intervals. But were the paraffin blocks actually serial sectioned? If so, how many serial sections were performed on each of the blocks? So perhaps the pathologists were doing one quick IHC, and if they didn't see anything there they weren't evaluating further. So more detailed examination of the sentinel node might reveal that some of these were actually node positive.
Finally, when the surgeon has limited experience with breast-cancer cases in their practice, what would you suggest that they do for sentinel lymph-node surgery? Do you think 4 cases is enough if all the nodes are negative for T-1 early-stage breast-cancer patients? Or do they need to continue to perform the procedure until they have a certain number of patients who actually have positive nodes identified in the sentinel lymph node?
Dr. Kelly M. McMasters (Louisville, Kentucky): Dr. Copeland asked whether a surgeon with minimal experience who finds a single negative sentinel lymph node in a patient with an upper outer quadrant tumor should perform an axillary dissection. My answer would be that if the surgeon has minimal experience, he should do an axillary dissection anyway because we do recommend that a certain amount of surgeon experience is probably necessary before you can quit doing axillary dissection. Exactly how many cases that requires is subject to interpretation. You can see from the numbers that, as surgeon experience increases from 4 cases to 10 cases to 15 cases or more, 15 to 20 cases appears to be adequate to get a false-negative rate in the 5% or lower range, in our experience. Yet the false-negative rate drops off significantly after 4 cases.
Dr. Urist asked about the timing of lymphoscintigraphy and the number of radioactive sentinel nodes. We previously have analyzed our data and have not found that the timing of the injection of radioactive colloid made a big difference in the number of lymph nodes removed, the ability to find the lymph nodes, and the false-negative rate.
Another question Dr. Urist asked was if a surgeon only finds a single sentinel node in the axilla, irrespective of the type of procedure or injection that the patient had, should an additional nonsentinel lymph node be removed or should we remove some other lymph nodes? And my answer is that we have previously described the 10% rule for both breast cancer and in our large sentinel lymph-node study in melanoma, the Sunbelt Melanoma Trial. I think that adequately gives you guidelines, very practical guidelines, to know when to stop removing sentinel nodes. This rule states that any lymph node that is blue, any node that is the hottest (most radioactive) lymph node, any lymph node that is 10% or greater of the radioactive count of the hottest lymph node, and any node that is palpably enlarged should be removed. If you do that, you will reduce the false-negative rate significantly to a very low rate. Even if you do this, sometimes there is just a single sentinel node. But I think that if you diligently search, make sure the background radioactivity is very low, [that you]have no blue or palpable nodes, you have done an adequate sentinel lymph node biopsy. We can't always make a second sentinel node. After you have removed 4 sentinel nodes, our data would suggest you are not going to get a lot of yield for finding additional positive sentinel nodes.
Upper outer quadrant tumors Dr. Urist and others have commented upon as associated with an increased false-negative rate: It is especially a problematic area when you do a peritumoral injection because you now inject 6 or 8 ccs of radioactive colloid in the upper outer quadrant of the breast, which diffuses like plutonium throughout that area. Trying to find a mildly radioactive lymph node in the axilla next door to this high background can be very difficult. Other injection techniques are definitely preferable in terms of identification of sentinel nodes, such as subareolar injection or dermal injection of the radioactive tracer; this will help you find those sentinel nodes.
We did not capture data on intramammary lymph nodes in the database. Although there were some identified, I can't tell you any specific data about intramammary lymph nodes.
Dr. Urist asked why we included immunohistochemistry in our analysis. It turns out a lot of people did use immunohistochemistry in the study. And around the country people continue to use cytokeratin immunohistochemical stains for sentinel node analysis despite recommendations from the College of American Pathology, the American College of Surgeons, and others that we shouldn't do so until we know the clinical significance of micrometastases detected by immunohistochemical stains. Nevertheless, there has been an argument in the literature and several publications that suggests that immunostains will allow us to find small micrometastases and will reduce the false-negative rate.
I can't explain why immunohistochemistry in this study significantly increased the false negative rate. But the data are the data. It is the largest study available to look at. And it is pretty compelling to say that there is no real value here for immunostains to reduce the false-negative rate, at least in a broad experience of community surgeons in different hospitals across the United States. Maybe it is different in specialized centers.
Would anybody be harmed by having a false-negative result? Dr. Urist asked a very good question. Others have suggested that very few patients would be harmed by a false-negative result. But an example would be the first patient on whom I performed sentinel node biopsy and didn't complete the axillary dissection: 38 years old, 8-mm well-differentiated cancer; she had 3 positive sentinel nodes. Had I missed those, she would have gotten no chemotherapy. Since I found them, she got very aggressive chemotherapy. For patients with T1a and T1b tumors, a false-negative result probably makes the biggest difference.
Dr. Bland asked whether a patient who has metastatic tumor to a lymph node and obstruction of lymphatic channels will have alteration in the ability to find the sentinel node and be accurate with it. It has been postulated that that is true, and I think we have all seen cases like that.
The way we could look at tumor burden in the lymph node in the present study was by the number of positive nodes. We didn't really have data on the size of the lymph nodes or extracapsular extension to be able to analyze it that way. But when we look at the number of positive nodes, it is not significant. It is likely that there are some cases like that, however, where there is obstruction of the lymphatic channels and lymph nodes by tumor.
Upper outer quadrant tumors: We have talked about how to manage those. Subareolar or intradermal injection help with sentinel-node identification.
Lymphoscintigraphy we have not found in a previous analysis and in an updated analysis to be helpful in finding the sentinel nodes or in reducing the false-negative rate. Dr. Hunt uses a technique where the patients are injected the day before. Does that improve things? We have not found timing of injection to be a significant factor in our study. I look forward to seeing results from others.
My recommendation about IHC is that you do not perform it, very simply. It may be a crutch for pathologists who are too lazy to look at multiple sections of H&E-stained sentinel nodes. All I can say is that my recommendation would be multiple sections from the lymph node—how many exactly is open to interpretation—but good pathologists looking at H&E sections of the lymph nodes is all that is required.
What do I recommend for number of cases a surgeon should perform? Again, I think that 4 cases are probably not enough if you look at our data. But the previous recommendation of 30 cases before you abandon axillary dissection is probably too high. It was reduced to 20 cases based on a previous analysis, and I think we can reduce it down further to 10 to 15 cases. And as surgeons and institutions have gotten better at this, injection techniques have gotten better, it is now possible to identify the sentinel nodes 100% of the time. Those of us who do this a lot never fail to find the sentinel nodes. So that hurdle has been overcome. Now the issue is reducing the false-negative rate. And I think this paper helps us to identify some of the factors that go into making a false-negative result.
Footnotes
Supported by the Center for Advanced Surgical Technologies (CAST) of Norton Hospital.
Reprints: Kelly M. McMasters, MD, PhD, Division of Surgical Oncology, Department of Surgery, University of Louisville, J. Graham Brown Cancer Center, 315 East Broadway, Room 308, Louisville, KY 40202. E-mail: kelly.mcmasters@nortonhealthcare.org.
REFERENCES
- 1.Kelley MC, Hansen N, McMasters KM. Lymphatic mapping and sentinel lymphadenectomy for breast cancer. Am J Surg. 2004;188:49–61. [DOI] [PubMed] [Google Scholar]
- 2.McMasters KM, Wong SL, Chao C, et al. Defining the optimal surgeon experience for breast cancer sentinel lymph node biopsy: a model for implementation of new surgical techniques. Ann Surg. 2001;234:292–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McMasters KM, Giuliano AE, Ross MI, et al. Sentinel-lymph-node biopsy for breast cancer: not yet the standard of care. N Engl J Med. 1998;339:990–995. [DOI] [PubMed] [Google Scholar]
- 4.Derossis AM, Fey J, Yeung H, et al. A trend analysis of the relative value of blue dye and isotope localization in 2,000 consecutive cases of sentinel node biopsy for breast cancer. J Am Coll Surg. 2001;193:473–478. [DOI] [PubMed] [Google Scholar]
- 5.Chagpar A, Martin RC III, Chao C, et al. Validation of subareolar and periareolar injection techniques for breast sentinel lymph node biopsy. Arch Surg. 2004;139:614–618. [DOI] [PubMed] [Google Scholar]
- 6.Chao C, Wong SL, Woo C, et al. Reliable lymphatic drainage to axillary sentinel lymph nodes regardless of tumor location within the breast. Am J Surg. 2001;182:307–311. [DOI] [PubMed] [Google Scholar]
- 7.Krag D, Weaver D, Ashikaga T, et al. The sentinel node in breast cancer: a multicenter validation study. N Engl J Med. 1998;339:941–946. [DOI] [PubMed] [Google Scholar]
- 8.Tafra L, Lannin DR, Swanson MS, et al. Multicenter trial of sentinel node biopsy for breast cancer using both technetium sulfur colloid and isosulfan blue dye. Ann Surg. 2001;233:51–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wong SL, Edwards MJ, Chao C, et al. Sentinel lymph node biopsy for breast cancer: impact of the number of sentinel nodes removed on the false-negative rate. J Am Coll Surg. 2001;192:684–689. [DOI] [PubMed] [Google Scholar]
- 10.Kennedy RJ, Kollias J, Gill PG, et al. Removal of two sentinel nodes accurately stages the axilla in breast cancer. Br J Surg. 2003;90:1349–1353. [DOI] [PubMed] [Google Scholar]
- 11.Duncan M, Cech A, Wechter D, et al. Criteria for establishing the adequacy of a sentinel lymphadenectomy. Am J Surg. 2004;187:639–642. [DOI] [PubMed] [Google Scholar]
- 12.Martin RC, Fey J, Yeung H, et al. Highest isotope count does not predict sentinel node positivity in all breast cancer patients. Ann Surg Oncol. 2001;8:592–597. [DOI] [PubMed] [Google Scholar]
- 13.Chua B, Olivotto IA, Donald JC, et al. Outcomes of sentinel node biopsy for breast cancer in British Columbia, 1996 to 2001. Am J Surg. 2003;185:118–126. [DOI] [PubMed] [Google Scholar]
- 14.Albertini JJ, Lyman GH, Cox C, et al. Lymphatic mapping and sentinel node biopsy in the patient with breast cancer. JAMA. 1996;276:1818–1822. [PubMed] [Google Scholar]
- 15.Veronesi U, Paganelli G, Galimberi V, et al. Sentinel-node biopsy to avoid axillary dissection in breast cancer with clinically negative lymph-nodes. Lancet. 1997;349:1864–1867. [DOI] [PubMed] [Google Scholar]
- 16.Borgstein PJ, Pijpers R, Comans EF, et al. Sentinel lymph node biopsy in breast cancer: guidelines and pitfalls of lymphoscintigraphy and gamma probe detection. J Am Coll Surg. 1998;186:275–283. [DOI] [PubMed] [Google Scholar]
- 17.Feldman SM, Krag DN, McNally RK, et al. Limitation in gamma probe localization of the sentinel node in breast cancer patients with large excisional biopsy. J Am Coll Surg. 1999;188:248–254. [DOI] [PubMed] [Google Scholar]
- 18.Ollila DW, Guilano AE. Intraoperative lymphatic mapping and sentinel lymphadenectomy using isosulfan blue dye. Breast Dis. 1998;8:297–300. [Google Scholar]
- 19.Cody HS. Sentinel lymph node mapping in breast cancer. Oncology. 1999;13:25–34. [PubMed] [Google Scholar]
- 20.O'Hea BJ, Hill ADK, El-Shirbiny AM, et al. Sentinel lymph node biopsy in breast cancer: initial experience at Memorial Sloan-Kettering Cancer Center. J Am Coll Surg. 1998;186:423–427. [DOI] [PubMed] [Google Scholar]
- 21.Huseh EC, Turner RR, Glass EC, et al. Sentinel node biopsy in breast cancer. J Am Coll Surg. 1999;189:207–213. [DOI] [PubMed] [Google Scholar]
- 22.Guenther JM. Axillary dissection after unsuccessful sentinel lymphadenectomy for breast cancer. Am Surg. 1997;65:991–993. [PubMed] [Google Scholar]
- 23.Estourgie SH, Nieweg OE, Valdes Olmos RA, et al. Eight false negative sentinel node procedures in breast cancer: what went wrong? Eur J Surg Oncol. 2003;29:336–340. [DOI] [PubMed] [Google Scholar]
- 24.Estourgie SH, Nieweg OE, Rutgers EJ, et al. What is a false-negative result for sentinel node procedures in breast cancer? J Surg Oncol. 2003;82:141–142. [DOI] [PubMed] [Google Scholar]
- 25.Wong SL, Edwards MJ, Chao C, et al. The effect of lymphatic tumor burden on sentinel lymph node biopsy results. Breast J. 2002;8:192–198. [DOI] [PubMed] [Google Scholar]
- 26.Martin RC, Edwards MJ, Wong SL, et al. Practical guidelines for optimal gamma probe detection of sentinel lymph nodes in breast cancer: results of a multi-institutional study. Surgery. 2000;128:139–144. [DOI] [PubMed] [Google Scholar]
- 27.Noguchi M, Motomura K, Imoto S, et al. A multicenter validation study of sentinel lymph node biopsy by the Japanese Breast Cancer Society. Breast Cancer Res Treat. 2000;63:31–40. [DOI] [PubMed] [Google Scholar]
- 28.Olson JA Jr, Fey J, Winawer J, et al. Sentinel lymphadenectomy accurately predicts nodal status in T2 breast cancer. J Am Coll Surg. 2000;191:593–599. [DOI] [PubMed] [Google Scholar]
- 29.Chung MH, Ye W, Giuliano AE. Role for sentinel lymph node dissection in the management of large (> or = 5 cm) invasive breast cancer. Ann Surg Oncol. 2001;8:688–692. [DOI] [PubMed] [Google Scholar]
- 30.Wong SL, Chao C, Edwards MJ, et al. Accuracy of sentinel lymph node biopsy for patients with T2 and T3 breast cancers. Am Surg. 2001;67:522–526. [PubMed] [Google Scholar]
- 31.Bedrosian I, Reynolds C, Mick R, et al. Accuracy of sentinel lymph node biopsy in patients with large primary breast tumors. Cancer. 2000;88:2540–2545. [DOI] [PubMed] [Google Scholar]
- 32.Kern KA. Lymphoscintigraphic anatomy of sentinel lymphatic channels after subareolar injection of Technetium 99m sulfur colloid. J Am Coll Surg. 2001;193:601–608. [DOI] [PubMed] [Google Scholar]
- 33.Smith LF, Cross MJ, Klimberg VS. Subareolar injection is a better technique for sentinel lymph node biopsy. Am J Surg. 2000;180:434–437. [DOI] [PubMed] [Google Scholar]
- 34.McMasters KM, Tuttle TM, Carlson DJ, et al. Sentinel lymph node biopsy for breast cancer: a suitable alternative to routine axillary dissection in multi-institutional practice when optimal technique is used. J Clin Oncol. 2000;18:2560–2566. [DOI] [PubMed] [Google Scholar]
- 35.Radovanovic Z, Golubovic A, Plzak A, et al. Blue dye versus combined blue dye-radioactive tracer technique in detection of sentinel lymph node in breast cancer. Eur J Surg Oncol. 2004;30:913–917. [DOI] [PubMed] [Google Scholar]
- 36.Sardi A, Spiegler E, Colandrea J, et al. The benefit of using two techniques for sentinel lymph node mapping in breast cancer. Am Surg. 2002;68:24–28. [PubMed] [Google Scholar]
- 37.Cserni G, Rajtar M, Boross G, et al. Comparison of vital dye-guided lymphatic mapping and dye plus gamma probe-guided sentinel node biopsy in breast cancer. World J Surg. 2002;26:592–597. [DOI] [PubMed] [Google Scholar]
- 38.Motomura K, Inaji H, Komoike Y, et al. Combination technique is superior to dye alone in identification of the sentinel node in breast cancer patients. J Surg Oncol. 2001;76:95–99. [DOI] [PubMed] [Google Scholar]
- 39.McMasters KM, Wong SL, Martin RC, et al. Dermal injection of radioactive colloid is superior to peritumoral injection for breast cancer sentinel lymph node biopsy: results of a multi-institutional study. Ann Surg. 2001;233:676–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jakub JW, Diaz NM, Ebert MD, et al. Completion axillary lymph node dissection minimizes the likelihood of false negatives for patients with invasive breast carcinoma and cytokeratin positive only sentinel lymph nodes. Am J Surg. 2002;184:302–306. [DOI] [PubMed] [Google Scholar]
- 41.Chu KU, Turner RR, Hansen NM, et al. Sentinel node metastasis in patients with breast carcinoma accurately predicts immunohistochemically detectable nonsentinel node metastasis. Ann Surg Oncol. 1999;6:756–761. [DOI] [PubMed] [Google Scholar]
- 42.Nos C, Freneaux P, Louis-Sylvestre C, et al. Macroscopic quality control improves the reliability of blue dye-only sentinel lymph node biopsy in breast cancer. Ann Surg Oncol. 2003;10:525–530. [DOI] [PubMed] [Google Scholar]
- 43.Klauber-DeMore N, Tan LK, Liberman L, et al. Sentinel lymph node biopsy: is it indicated in patients with high-risk ductal carcinoma-in-situ and ductal carcinoma-in-situ with microinvasion? Ann Surg Oncol. 2000;7:636–642. [DOI] [PubMed] [Google Scholar]
- 44.Cox CE, Nguyen K, Gray RJ, et al. Importance of lymphatic mapping in ductal carcinoma in situ (DCIS): why map DCIS? Am Surg. 2001;67:513–521. [PubMed] [Google Scholar]
- 45.Tamhane R, Dahlstrom JE, McCallum DD, et al. The clinical significance of cytokeratin-positive cells in lymph nodes at the time of mastectomy from patients with ductal carcinoma-in-situ. Ann Surg Oncol. 2002;9:999–1003. [DOI] [PubMed] [Google Scholar]
- 46.McMasters KM, Chao C, Wong SL, et al. Sentinel lymph node biopsy in patients with ductal carcinoma in situ: a proposal. Cancer. 2002;95:15–20. [DOI] [PubMed] [Google Scholar]
- 47.Hammond MEH, Fitzgibbons PL, Compton CC, et al. College of American Pathologists Conference XXXV: solid tumor prognostic factors: which, how, and so what? Arch Pathol Lab Med. 2000;124:958–965. [DOI] [PubMed] [Google Scholar]
- 48.Pendas S, Giuliano R, Swor G, et al. Worldwide experience with lymphatic mapping for invasive breast cancer. Semin Oncol. 2004;31:318–323. [DOI] [PubMed] [Google Scholar]
- 49.Giuliano AE, Haigh PI, Brennan MB, et al. Prospective observational study of sentinel lymphadenectomy without further axillary dissection in patients with sentinel node-negative breast cancer. J Clin Oncol. 2000;18:2553–2559. [DOI] [PubMed] [Google Scholar]
- 50.Nano MT, Kollias J, Farshid G, et al. Clinical impact of false-negative sentinel node biopsy in primary breast cancer. Br J Surg. 2002;89:1430–1434. [DOI] [PubMed] [Google Scholar]
- 51.Wong SL, Chao C, Edwards MJ, et al, for the University of Louisville Breast Cancer Study Group. Frequency of sentinel lymph node metastases in patients with favorable breast cancer histologic subtypes. Am J Surg. 2002;184:492–498. [DOI] [PubMed] [Google Scholar]