Abstract
Although Nef has been proposed to effect the escape of human immunodeficiency virus type 1 (HIV-1) from cytotoxic T lymphocytes (CTL) through downmodulation of major histocompatibility complex class I molecules, little direct data have been presented previously to support this hypothesis. By comparing nef-competent and nef-deleted HIV-1 strains in an in vitro coculture system, we demonstrate that the presence of this viral accessory gene leads to impairment of the ability of HIV-1-specific CTL clones to suppress viral replication. Furthermore, inhibition by genetically modified CTL that do not require major histocompatibility complex class I-presented antigen (expressing the CD4 T-cell receptor [TCR] ζ-chain hybrid receptor) is similar for both nef-competent and -deleted strains, indicating that Nef does not impair the effector functions of CTL but acts at the level of TCR triggering. In contrast, we note that another accessory gene, vpr, does not induce resistance of HIV-1 to suppression by CTL clones. We conclude that Nef (and not Vpr) contributes to functional HIV-1 immune evasion and that this effect is mediated by diminished antigen presentation to CTL.
Major histocompatibility complex class I (MHC-I)-restricted CD8+ cytotoxic T lymphocytes (CTL) have emerged as an important arm of immunity in human immunodeficiency virus type 1 (HIV-1) infection (reviewed in reference 68). Increasing evidence suggests that HIV-1-specific CTL are involved in the control of viremia in acute (7, 33) and chronic (41) infections, long-term nonprogressing infection (24, 47), and perhaps prevention of infection in some highly exposed yet uninfected individuals (34, 50). Other studies have documented the potent antiviral effects of CTL in lysing infected cells (65) and suppressing viral replication in vitro (66). Although these data suggest that CTL play an important role in antiviral immunity, it is not clear why they fail to clear HIV-1 in vivo and what mechanisms HIV-1 may employ to escape from this immunity (5).
The HIV-1 accessory gene nef was originally thought to be a negative effector of viral transcription (36, 59). Subsequent studies showed this to be a misnomer and identified multiple roles that the Nef protein may play in infected cells. Downregulation of cell surface CD4 has been the most clearly documented function, and this occurs through direct bridging of the cytoplasmic tail of CD4 to an adapter protein which targets it for endocytosis and degradation in lysosomes (2, 3). Other less clearly described effects of Nef are the enhancement of virion infectivity (8, 38, 57) and modulation of cellular activation (4, 6, 15, 23, 51, 54, 56). Finally, downregulation of major histocompatibility complex class I (MHC-I) molecules has been reported as a potentially important function of Nef that may allow for escape from cellular immunity (10, 55). Nef is presumed to interact indirectly with the cytoplasmic tail of MHC-I A and B (but not C) molecules, leading to endocytosis (9). Although the roles of MHC downregulation and other functions of Nef remain unclear, its importance in the development of AIDS in simian immunodeficiency virus (SIV)-infected monkeys (32) and clinically attenuated disease in a patient cohort infected with nef-defective HIV-1 (40) demonstrate its key importance in the immunopathogenesis of HIV-1 infection.
The functional significance of MHC-I downregulation by Nef leading to escape from cellular immunity has been somewhat controversial. To our knowledge, only one published study has evaluated the effects of Nef on the interaction of HIV-1-infected cells with CTL (10). Using HIV-1 reporter viruses and flow cytometric analysis, Collins et al. showed that CD4+ cells acutely infected with nef-deleted virus disappear after exposure to CTL in vitro, in contrast to nef-competent HIV-1-infected cells, which are cleared less efficiently. A caveat to this finding, however, was the late time at which susceptibility to cytolysis of the acutely infected cells was assessed (approximately 2 to 5 days after infection). Given the estimation that the entire replicative cycle of HIV-1 requires only about 2 days in vivo (27, 62), measurement of cytolysis of cells exposed to CTL late after infection (more than 2 days) might not be relevant to the interaction between CTL and infected cells. Depending on the kinetics of Nef-mediated resistance to CTL (presumably through MHC downregulation), CTL could clear infected cells before the effects observed by Collins et al. at the late time points of their assay.
Vpr is another accessory protein whose immunopathogenic impact and functions have become increasingly clear. The ability of Vpr to mediate nuclear import of the preintegration complex in growth-arrested cells (26) and induce cell cycle arrest (25, 31, 46, 49) have been well documented. Like Nef, Vpr is not required for viral replication in most culture systems in vitro. Conservation of Vpr in vivo, however, suggests important function(s) of this gene in the pathogenesis of HIV-1 infection (58). A candidate for an important in vivo role would be evasion of cellular immune responses, particularly antiviral CTL.
In previous studies, we have developed assays to measure the antiviral activities of CTL, as opposed to standard methods measuring lysis or cytokine release in response to target cells infected with recombinant vaccinia or labeled with synthetic peptides (68). We found that HIV-1-specific CTL clones exert potent antiviral effects on T cells acutely infected with HIV-1 (66, 67). Notably, however, these studies almost exclusively utilized the polyclonal HIV-1 strain IIIB, a long-term passaged laboratory isolate of virus containing multiple clones noted to be defective in accessory gene reading frames, including nef (39). Our earlier work therefore examined the function of CTL in the absence of Nef and other accessory proteins.
In this study, we examine the roles of Nef and Vpr in the functional antiviral activity of HIV-1-specific CTL clones. Using the in vitro coculture assay we previously developed (66), we show that the antiviral effect of CTL is markedly diminished by the presence of nef in the infecting virus. Furthermore, this phenomenon is not explained by impaired susceptibility of HIV-1-infected cells to the effector functions of CTL, suggesting that escape is due to diminished recognition by CTL. In contrast to nef, vpr does not interfere with the antiviral function of CTL.
MATERIALS AND METHODS
Virus.
The experiments with Nef utilized HIV-1 strains NL4-3 (1) and NL4-3ΔNef (21), which were kindly provided by R. Desrosiers. Experiments with Vpr utilized NL4-3 constructs from I. S. Y. Chen (NL4-3 Thy and NL4-3 Thy-X [31]) and HXB2 constructs from H. G. Gottlinger (HXBH10 [22] and HXBH10/R+ [14]) which are all additionally nef defective. HIV-1 IIIB was originally obtained from the laboratory of Robert Gallo. Low-passage virus stocks were produced by expansion in H9 cells, harvested, and frozen in aliquots at −80°C until use. Viral titer was determined by endpoint dilution with C8166 indicator cells as previously described (30).
Target cells. (i) Immortalized HIV-1 permissive cell lines.
T1 (53), T2 (52), H9 (43), and H9-B14 (H9 cells stably transfected with class I HLA B14 cDNA [65]) cells were maintained in RPMI 1640 (Sigma, St. Louis, Mo.) supplemented with 20% heat-inactivated fetal calf serum (Sigma), 10 mM HEPES, 2 mM glutamine, 100 U of penicillin/ml, and 10 μg of streptomycin (R20)/ml.
(ii) CD4-positive cell line from HIV-1-seronegative individual.
Polyclonal CD4+ cells (greater than 98% CD3- and CD4-expressing by fluorescence-activated cell sorting; data not shown) were generated from Ficoll gradient-purified peripheral blood mononuclear cells (PBMC) using a CD3- and CD8-bispecific monoclonal antibody as previously described (66). These cells were grown in RPMI 1640 containing 10% heat-inactivated fetal calf serum, 10 mM HEPES, 2 mM glutamine, 100 U of penicillin/ml, 10 μg of streptomycin/ml, and 50 U of interleukin-2 (IL-2) (R10-50)/ml and infected 5 to 7 days after stimulation with the bispecific antibody. MHC haplotyping of the donor was performed by the tissue typing laboratory at Massachusetts General Hospital, Boston, Mass.
Effector cells. (i) CTL clones from HIV-1 infected individuals.
HIV-1-specific CTL clones were obtained by the cloning of stimulated PBMC at limiting dilution and characterized for specificity and MHC restriction as previously described (61). The MHC A2-restricted CTL clones were 18030D23 specific for a Gag p17 epitope (amino acids [aa] 77 to 85 [SLYNTVATL]) and 68A62 specific for a reverse transcriptase epitope (aa 476 to 484 [ILKEPVHGV]). The MHC B14-restricted clone 15160D75 recognized an envelope gp41 epitope (aa 584 to 592 [ERYLKDQQL]). The MHC B60-restricted clone 161JD27 recognized a Gag epitope (aa 92 to 101 [IEIKDTKEAL]). Amino acids are numbered according to the HXB2 sequence. All CTL clones were maintained in R10-50 and restimulated at least 10 days prior to usage with irradiated allogeneic PBMC and the anti-CD3 monoclonal antibody 12F6 (64) or phytohemagglutinin.
(ii) Universal receptor CD8+ T cells.
A clonal cell line of T3F3, a CD8+ cell line from an HIV-1-seronegative donor which bears a hybrid receptor consisting of the external domain of human CD4 and the transmembrane and signaling domains of the T-cell receptor (TCR) ζ-chain (48, 67), was maintained in the same manner as the HIV-1-specific CTL clones above. A control cell line not transduced with the CD4-ζ hybrid receptor, T3 (48, 67), was also used.
Inhibition assays.
Inhibition of viral replication was assessed in a previously established assay system (66). Target cells were infected with the indicated strain of HIV-1 at a multiplicity of infection of 0.01 (0.01 50% tissue culture infective doses [TCID50s] per cell) for 4 h at 37°C, washed twice, and plated at 5 × 105 cells per well in a 24-well plate. To assess inhibition by CTL, effector cells then were added at a ratio of 0.25:1 (unless otherwise indicated) in R10-50. At 2- to 4-day intervals, the cocultures were fed by removing and replacing one-half of the culture supernatant with fresh medium. The removed supernatant was cryopreserved for later p24 antigen quantitation by a standard quantitative enzyme-linked immunosorbent assay (ELISA) (commercial kit; Dupont, Boston, Mass.).
RESULTS
Inhibition of HIV-1 replication by cytotoxic T lymphocytes is antigen dependent.
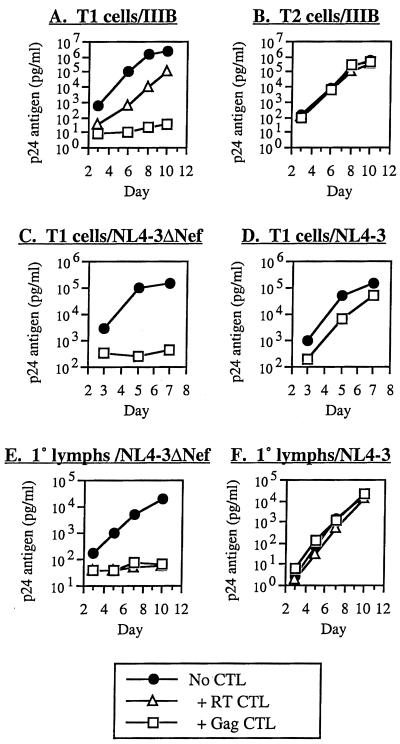
We have previously demonstrated the potent antiviral activity of HIV-1-specific, MHC-I-restricted CTL clones (66). We showed that this activity is MHC restricted, consistent with TCR-mediated triggering of CTL to recognize infected cells. To define further the role of antigen presentation in viral suppression by CTL, we tested the antiviral activity of CTL clones against HIV-1 replicating in T1 versus T2 cells in our coculture system (Fig. 1A and B). T1 cells (53) are a lymphoblastoid cell line that is permissive for T-tropic HIV-1 replication, and T2 cells are a derivative of T1 cells deficient in transporter associated with processing complex (TAP) and therefore are unable to transport antigens through the class I pathway (52) for presentation and recognition by CTL. MHC-matched clones inhibited viral replication in T1 cells, as previously reported (66). In T2 cells, however, viral suppression was ablated. In three experiments with five different CTL clones (restricted by HLA A2 and B60 found on T1 cells), inhibition (at approximately day 7; mean ± standard deviation [SD]) was 2.8 ± 1.5 log10 units for T1 and 0.1 ± 0.2 for T2 cells (P = 0.0014). Although properties of T2 cells other than TAP deletion could account for these differences, these results strongly suggested a pivotal requirement for MHC-I/antigen presentation in triggering the antiviral activity of CTL in this in vitro culture system.
FIG. 1.
Interference with antiviral activity of HIV-1-specific CTL by TAP deletion and HIV-1 Nef. The ability of HIV-1-specific CTL clones to suppress HIV-1 replication in acutely infected cells was compared for normal versus TAP-deleted cells (A versus B), Nef-deleted versus wild-type Nef HIV-1 in immortalized CD4+ lymphocytes (C versus D), and Nef-deleted versus wild-type Nef HIV-1 in primary CD4+ lymphocytes (E versus F). After acute infection of the cells, CTL were added in coculture and viral replication was assessed at the indicated time points by quantitative p24 antigen ELISA. The CTL clones utilized were 68A62 (RT CTL [A, B, E, and F]), 161JD27 (Gag CTL [A and B]), and 18030D23 (Gag CTL [C, D, E, and F]).
Nef reduces the susceptibility of HIV-1 to inhibition by CTL.
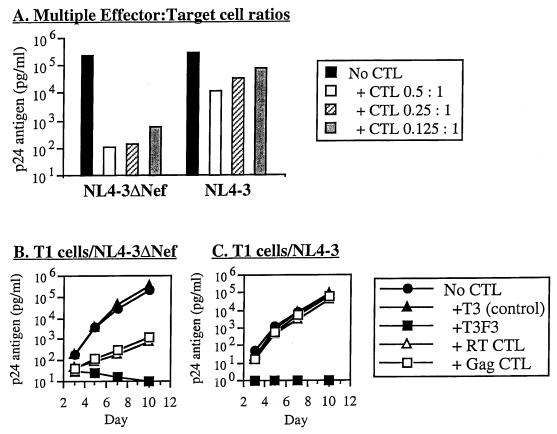
Nef has been reported to downregulate the expression of MHC-I molecules (9, 10, 55), and cells infected with HIV-1 containing competent nef have been shown to be relatively resistant to cytolysis by CTL in comparison to those without (10). To correlate these observations to the antiviral function of CTL, we compared the susceptibility of HIV-1 with or without intact nef to suppression by CTL clones. Viral replication in target cells infected with HIV-1 containing intact nef (NL4-3) was markedly less suppressed than in cells infected with deleted nef (NL4-3ΔNef) (Fig. 1C and D). Both MHC A2- and B14-restricted clones exhibited this effect, in MHC-matched T1 and H9-B14 target cells, respectively (B14 data not shown). In eight experiments using five different CTL clones, inhibition (at approximately day 7; mean ± SD) was 0.6 ± 0.7 log10 units for NL4-3 and 2.7 ± 0.8 log10 units for NL4-3ΔNef (P < 0.00001). This phenomenon was also seen using primary CD4+ T lymphocytes as target cells (Fig. 1E and F). In three experiments using three different CTL clones, mean inhibition was 0.2 ± 0.2 log10 units for NL4-3 and 2.1 ± 0.8 log10 units for NL4-3ΔNef (P = 0.0040). The differential susceptibility of these viruses was furthermore observed over multiple effector-to-target cell ratios (Fig. 2A). Nef therefore appeared to confer relative resistance against the antiviral activity of HIV-1-specific CTL.
FIG. 2.
Maintenance of Nef effect over various concentrations of CTL but loss of Nef effect with use of genetically engineered class I antigen-independent CTL. T1 cells were acutely infected with NL4-3ΔNef or NL4-3 and cocultured with various ratios of a CTL clone (A) or either CTL clones or chimeric receptor T cells that directly bind cell surface gp120 (B). Viral replication was assessed at the indicated time points by quantitative p24 antigen ELISA (day 7 data are shown in panel A). The CTL clones utilized were 18030D23 (Gag CTL [all panels]) and 68A62 (RT CTL [B and C]). The chimeric immune receptor-transduced cells (CD4-ζ receptor transduced) were T3F3, with the nontransduced control T3 (B and C).
Nef does not diminish susceptibility of infected cells to post-TCR signaling effector functions of CTL.
The protective effect of Nef demonstrated by the above studies might occur either through altered triggering of CTL or a change in the susceptibility of infected cells to the effector functions of CTL. To distinguish between these two possibilities, we utilized an HIV-1 gp120-specific, antigen-processing-independent cell line (T3F3). This CD8+ T cell line has been transduced with a CD4-TCR ζ-chain hybrid molecule, which renders it virus specific through the direct interaction of the hybrid receptor with gp120 on the surface of infected cells, bypassing the need for antigen processing (48). We previously demonstrated that this cell line has antiviral activity similar to that of HIV-1-specific CTL clones from infected individuals, but acts without MHC restriction (67). These cells were efficient inhibitors of both wild-type and nef-deleted virus, in contrast to processed-antigen-dependent CTL clones (Fig. 2B and C). Although viral suppression by MHC-I-restricted CTL was blunted in Nef-expressing compared with nef-deleted virus, T3F3 was a potent suppressor of viral replication of both, suggesting Nef does not render cells resistant to CTL effects once the TCR is triggered. T3F3 also inhibited HIV-1 efficiently in T2 cells, in contrast to naturally derived CTL clones (data not shown). Because we have previously demonstrated that CTL act through cytolytic and noncytolytic mechanisms of viral inhibition (66), we also tested these viruses for suppression by supernatant from an activated CTL clone, and we found that nef-expressing CTL remained sensitive to soluble factors released by CTL (data not shown). Thus, Nef did not appear to act by antagonizing the effector functions of CTL.
Viral protein R (Vpr) does not interfere with antiviral function of CTL.
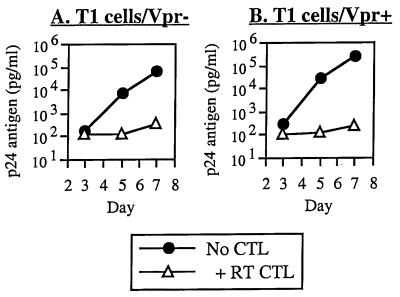
Another HIV-1 accessory gene which has been suggested to contribute to the pathogenesis of infection is viral protein R (vpr), whose functions include cellular changes that could hypothetically impair CTL lysis of infected cells. We therefore tested whether Vpr might affect CTL antiviral infection in the same coculture system using nef-deleted viruses (to allow adequate and consistent measurement of inhibition) discordant for vpr. In contrast to Nef, Vpr did not impair the ability of CTL to suppress viral replication (Fig. 3). In five separate experiments comparing vpr-competent and -deleted viruses (two utilizing NL4-3 Thy and NL4-3 Thy-X [31] and three utilizing HXBH10 [22] and HXBH10/R+ [14]), inhibition (mean ± SD) was 2.6 ± 0.6 log10 units for vpr+ HIV-1 and 2.3 ± 0.5 log10 units for vpr− HIV-1. Thus, there was no detectable effect of Vpr on the antiviral activity of CTL, in contrast to what was observed for Nef.
FIG. 3.
Lack of effect of Vpr on antiviral activity of CTL. T1 cells were infected with nef-deleted HIV-1 containing or lacking vpr and cocultured with the CTL clone 68A62 (RT CTL) as described above.
DISCUSSION
Despite its relatively small genome, HIV-1 contains numerous accessory genes (11, 19, 60). Although most retroviruses require only the three structural proteins Gag, Pol, and Env, HIV-1 contains genes for at least six additional proteins which are usually conserved in vivo. Of these, two are clearly required for viral replication: the LTR transactivator Tat (12) and the viral RNA nuclear export factor Rev (42). The others, however, are nonessential for viral growth in many in vitro culture systems, suggesting that their functions are specialized for viral persistence in vivo (11). The effects and roles of these proteins remain to be fully elucidated, but evasion of host immune responses is one intriguing possibility.
CD8+ CTL require processed antigen presented on MHC-I (reviewed in reference 18). Recognition of the antigen/MHC complex by the CTL TCR then triggers the antiviral activities of CTL through cytolytic and noncytolytic pathways (66). Any interference with antigen presentation on MHC-I might therefore impede the activity of CTL against infected cells. Prompted by the hypothesis that Nef might thus interfere with CTL function, one study has examined the effect of Nef on the ability of CTL to lyse acutely HIV-1-infected cells. Collins et al. devised an assay using HIV-1 with a reporter green fluorescent protein where MHC-matched CTL clones were added to PBMC acutely infected with nef-competent or -deleted virus, and clearance of infected cells was then assessed by flow cytometry for green fluorescent protein-expressing cells (10). Cells infected with nef mutant virus were found to express normal levels of MHC-I and to be cleared by CTL, whereas cells infected with nef+ virus were found to have decreased MHC-I and persistence in the presence of CTL. A potential caveat to this finding, however, was the fact that clearance was measured by adding CTL at 2 to 5 days after infection. Because the viral life cycle is approximately 2 days in vivo (27, 62), and CTL can recognize infected cells early in this cycle (65), Nef might have little effect on CTL function in vivo if its action on MHC-I were late.
The present study examines the influence of viral accessory proteins on the ability of CTL to inhibit viral replication, a more functional assay. Although we have previously found that HIV-1 is well suppressed (66) by CTL, these studies utilized the polyclonal strain IIIB, which contains multiple clones with defective nef reading frames (39). Controlling for functional nef, here we find that whereas nef-deleted virus is potently suppressed by CTL, wild-type virus is clearly less subject to suppression. This confirms and expands the findings of Collins et al. (10) by providing a functional correlate to their results, demonstrating that CTL suppress viral production by acutely infected cells. Furthermore, we show that this phenomenon is not mediated by resistance to the effector functions of CTL post-triggering; CTL triggered independently of processed antigen (T3F3 CD4-ζ universal receptor cells) retain the capacity to suppress viral replication efficiently. This is indicative that Nef interferes with the action of CTL by preventing triggering via recognition of antigen on infected cells.
In contrast, another accessory gene, vpr, has no apparent impact on the antiviral activity of CTL. Similarly to Nef, Vpr is not required for HIV-1 replication in most in vitro culture systems, although its conservation in vivo suggests an important role(s) in the pathogenesis of infection (58). It has been hypothesized to improve the efficiency of viral replication in the host through cell cycle arrest (25, 31, 46, 49). We find that it does not directly alter the susceptibility of infected cells to the action of CTL in our coculture system, suggesting that in vivo selection for vpr function is due to other factors besides CTL pressure.
The action of Nef may contribute to explaining the paradoxical ineffectiveness of CTL at clearing HIV-1 infection in vivo (5). Despite the high levels of activated virus-specific CTL in many infected individuals, the virus persists and ultimately causes progressive disease in the vast majority of persons. Furthermore, studies evaluating for CTL epitope escape mutation have often yielded no evidence for selective pressure by CTL in vivo. Nef-mediated impaired recognition of infected cells could contribute to persistence of infection in the face of a vigorous CTL response, as well as partial shielding of HIV-1 from immune pressure.
Deletion of nef has been shown to attenuate infection of macaques by SIV, suggesting a key role in the immunopathogenesis of infection (13). Macaques immunized with nef-deleted SIV exhibit low level but persistent infection, with immunity to subsequent challenge by wild-type SIV, suggesting enhanced immunogenicity. Further indirect evidence is a cohort of patients all infected by blood transfusion from a single donor who was infected with a nef-defective HIV-1. These patients, including the donor, have exhibited a significantly attenuated disease course, with detectable cellular proliferative responses to viral proteins (16) and vigorous HIV-1-specific CTL responses (17).
The influence of nef on the immunogenicity and pathogenicity of HIV-1 infection suggests that this gene could be a target for enhancing cellular immunity in chronically infected individuals or the design of preventative vaccines. Antagonizing the function of Nef pharmacologically could be a means of increasing the efficiency of protective CTL responses. Because the Nef protein is expressed early in the viral life cycle (45), it is unclear whether Nef-specific CTL could be more effective than other CTL, by recognizing infected cells earlier and before the onset of Nef-mediated effects. Another strategy could be to produce or enhance MHC-I C-restricted CTL responses. Such CTL specificity is uncommon but has been reported (20, 28, 29, 35, 37, 44, 63). Nef selectively downregulates MHC A and B, but not C molecules, by virtue of specific binding to the intracytoplasmic domains of A and B but not C (9). Alternatively, strategies to develop CTL responses independent of antigen presentation, such as T-cell transduction with the CD4-ζ TCR construct (48), could bypass its effect (as shown in Fig. 2). Further studies of the effects of Nef on CTL function may yield insight into future immunotherapeutic options.
Acknowledgments
This work was funded by Public Health Service grant AI43203.
IL-2 was provided by the NIH AIDS Research and Reference Reagent Program.
REFERENCES
- 1.Adachi, A., H. E. Gendelman, S. Koenig, T. Folks, R. Willey, A. Rabson, and M. A. Martin. 1986. Production of acquired immunodeficiency syndrome-associated retrovirus in human and nonhuman cells transfected with an infectious molecular clone. J. Virol. 59:284-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aiken, C., J. Konner, N. R. Landau, M. E. Lenburg, and D. Trono. 1994. Nef induces CD4 endocytosis: requirement for a critical dileucine motif in the membrane-proximal CD4 cytoplasmic domain. Cell 76:853-864. [DOI] [PubMed] [Google Scholar]
- 3.Bandres, J. C., A. S. Shaw, and L. Ratner. 1995. HIV-1 Nef protein downregulation of CD4 surface expression: relevance of the lck binding domain of CD4. Virology 207:338-341. [DOI] [PubMed] [Google Scholar]
- 4.Baur, A. S., E. T. Sawai, P. Dazin, W. J. Fantl, C. Cheng-Mayer, and B. M. Peterlin. 1994. HIV-1 Nef leads to inhibition or activation of T cells depending on its intracellular localization. Immunity 1:373-384. [DOI] [PubMed] [Google Scholar]
- 5.Bevan, M. J., and T. J. Braciale. 1995. Why can't cytotoxic T cells handle HIV? Proc. Natl. Acad. Sci. USA 92:5765-5767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bodeus, M., A. Marie-Cardine, C. Bougeret, F. Ramos-Morales, and R. Benarous. 1995. In vitro binding and phosphorylation of human immunodeficiency virus type 1 Nef protein by serine/threonine protein kinase. J. Gen. Virol. 76:1337-1344. [DOI] [PubMed] [Google Scholar]
- 7.Borrow, P., H. Lewicki, B. H. Hahn, G. M. Shaw, and M. B. Oldstone. 1994. Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J. Virol. 68:6103-6110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chowers, M. Y., M. W. Pandori, C. A. Spina, D. D. Richman, and J. C. Guatelli. 1995. The growth advantage conferred by HIV-1 nef is determined at the level of viral DNA formation and is independent of CD4 downregulation. Virology 212:451-457. [DOI] [PubMed] [Google Scholar]
- 9.Cohen, G. B., R. T. Gandhi, D. M. Davis, O. Mandelboim, B. K. Chen, J. L. Strominger, and D. Baltimore. 1999. The selective downregulation of class I major histocompatibility complex proteins by HIV-1 protects HIV-infected cells from NK cells. Immunity 10:661-671. [DOI] [PubMed] [Google Scholar]
- 10.Collins, K. L., B. K. Chen, S. A. Kalams, B. D. Walker, and D. Baltimore. 1998. HIV-1 Nef protein protects infected primary cells against killing by cytotoxic T lymphocytes. Nature 391:397-401. [DOI] [PubMed] [Google Scholar]
- 11.Cullen, B. R. 1998. HIV-1 auxiliary proteins: making connections in a dying cell. Cell 93:685-692. [DOI] [PubMed] [Google Scholar]
- 12.Cullen, B. R. 1995. Regulation of HIV gene expression. AIDS 9:S19-S32. [PubMed]
- 13.Daniel, M. D., F. Kirchhoff, S. C. Czajak, P. K. Sehgal, and R. C. Desrosiers. 1992. Protective effects of a live attenuated SIV vaccine with a deletion in the nef gene. Science 258:1938-1941. [DOI] [PubMed] [Google Scholar]
- 14.Dorfman, T., and H. G. Göttlinger. 1996. The human immunodeficiency virus type 1 capsid p2 domain confers sensitivity to the cyclophilin-binding drug SDZ NIM 811. J. Virol. 70:5751-5757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Du, Z., S. M. Lang, V. G. Sasseville, A. A. Lackner, P. O. Ilyinskii, M. D. Daniel, J. U. Jung, and R. C. Desrosiers. 1995. Identification of a nef allele that causes lymphocyte activation and acute disease in macaque monkeys. Cell 82:665-674. [DOI] [PubMed] [Google Scholar]
- 16.Dyer, W. B., A. F. Geczy, S. J. Kent, L. B. McIntyre, S. A. Blasdall, J. C. Learmont, and J. S. Sullivan. 1997. Lymphoproliferative immune function in the Sydney Blood Bank Cohort, infected with natural nef/long terminal repeat mutants, and in other long-term survivors of transfusion-acquired HIV-1 infection. AIDS 11:1565-1574. [DOI] [PubMed] [Google Scholar]
- 17.Dyer, W. B., G. S. Ogg, M.-A. Demoitie, X. Jin, A. F. Geczy, S. L. Rowland-Jones, A. J. McMichael, D. F. Nixon, and J. S. Sullivan. 1999. Strong human immunodeficiency virus (HIV)-specific cytotoxic T-lymphocyte activity in Sydney Blood Bank Cohort patients infected with nef-defective HIV type 1. J. Virol. 73:436-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eisen, H. N., Y. Sykulev, and T. J. Tsomides. 1996. Antigen-specific T-cell receptors and their reactions with complexes formed by peptides with major histocompatibility complex proteins. Adv. Protein Chem. 49:1-56. [DOI] [PubMed] [Google Scholar]
- 19.Emerman, M., and M. H. Malim. 1998. HIV-1 regulatory/accessory genes: keys to unraveling viral and host cell biology. Science 280:1880-1884. [DOI] [PubMed] [Google Scholar]
- 20.Falk, K., O. Rotzschke, B. Grahovac, D. Schendel, S. Stevanovic, V. Gnau, G. Jung, J. L. Strominger, and H. G. Rammensee. 1993. Allele-specific peptide ligand motifs of HLA-C molecules. Proc. Natl. Acad. Sci. USA 90:12005-12009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gibbs, J. S., D. A. Regier, and R. C. Desrosiers. 1994. Construction and in vitro properties of HIV-1 mutants with deletions in “nonessential” genes. AIDS Res. Hum. Retrovir. 10:343-350. [DOI] [PubMed] [Google Scholar]
- 22.Gottlinger, H. G., T. Dorfman, E. A. Cohen, and W. A. Haseltine. 1993. Vpu protein of human immunodeficiency virus type 1 enhances the release of capsids produced by gag gene constructs of widely divergent retroviruses. Proc. Natl. Acad. Sci. USA 90:7381-7385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Graziani, A., F. Galimi, E. Medico, E. Cottone, D. Gramaglia, C. Boccaccio, and P. M. Comoglio. 1996. The HIV-1 nef protein interferes with phosphatidylinositol 3-kinase activation 1. J. Biol. Chem. 271:6590-6593. [DOI] [PubMed] [Google Scholar]
- 24.Harrer, T., E. Harrer, S. A. Kalams, T. Elbeik, S. I. Staprans, M. B. Feinberg, Y. Cao, D. D. Ho, T. Yilma, A. M. Caliendo, R. P. Johnson, S. P. Buchbinder, and B. D. Walker. 1996. Strong cytotoxic T cell and weak neutralizing antibody responses in a subset of persons with stable nonprogressing HIV type 1 infection. AIDS Res. Hum. Retrovir. 12:585-592. [DOI] [PubMed] [Google Scholar]
- 25.He, J., S. Choe, R. Walker, P. Di Marzio, D. O. Morgan, and N. R. Landau. 1995. Human immunodeficiency virus type 1 viral protein R (Vpr) arrests cells in the G2 phase of the cell cycle by inhibiting p34cdc2 activity. J. Virol. 69:6705-6711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heinzinger, N. K., M. I. Bukinsky, S. A. Haggerty, A. M. Ragland, V. Kewalramani, M. A. Lee, H. E. Gendelman, L. Ratner, M. Stevenson, and M. Emerman. 1994. The Vpr protein of human immunodeficiency virus type 1 influences nuclear localization of viral nucleic acids in nondividing host cells. Proc. Natl. Acad. Sci. USA 91:7311-7315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ho, D. D., A. U. Neumann, A. S. Perelson, W. Chen, J. M. Leonard, and M. Markowitz. 1995. Rapid turnover of plasma virions and CD4 lymphocytes in HIV-1 infection. Nature 373:123-126. [DOI] [PubMed] [Google Scholar]
- 28.Johnson, R. P., A. Trocha, T. M. Buchanan, and B. D. Walker. 1993. Recognition of a highly conserved region of human immunodeficiency virus type 1 gp120 by an HLA-Cw4-restricted cytotoxic T-lymphocyte clone. J. Virol. 67:438-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnson, R. P., A. Trocha, L. Yang, G. P. Mazzara, D. L. Panicali, T. M. Buchanan, and B. D. Walker. 1991. HIV-1 gag-specific cytotoxic T lymphocytes recognize multiple highly conserved epitopes. Fine specificity of the gag-specific response defined by using unstimulated peripheral blood mononuclear cells and cloned effector cells. J. Immunol. 147:1512-1521. [PubMed] [Google Scholar]
- 30.Johnson, V. A., and B. D. Walker. 1990. HIV-infected cell fusion assay, p. 92-94. In A. Aldovini and B. D. Walker (ed.), Techniques in HIV research. Stockton Press, New York, N.Y.
- 31.Jowett, J. B. M., V. Planelles, B. Poon, N. P. Shah, M.-L. Chen, and I. S. Y. Chen. 1995. The human immunodeficiency virus type 1 vpr gene arrests infected T cells in the G2 + M phase of the cell cycle. J. Virol. 69:6304-6313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kestler, H. W., III, D. J. Ringler, K. Mori, D. L. Panicali, P. K. Sehgal, M. D. Daniel, and R. C. Desrosiers. 1991. Importance of the nef gene for maintenance of high virus loads and for development of AIDS. Cell 65:651-662. [DOI] [PubMed] [Google Scholar]
- 33.Koup, R. A., J. T. Safrit, Y. Cao, C. A. Andrews, G. McLeod, W. Borkowsky, C. Farthing, and D. D. Ho. 1994. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J. Virol. 68:4650-4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Langlade-Demoyen, P., N. Ngo-Giang-Huong, F. Ferchal, and E. Oksenhendler. 1994. Human immunodeficiency virus (HIV) nef-specific cytotoxic T lymphocytes in noninfected heterosexual contact of HIV-infected patients. J. Clin. Investig. 93:1293-1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Littaua, R. A., M. B. A. Oldstone, A. Takeda, C. Debouck, J. T. Wong, C. U. Tuazon, B. Moss, F. Kievits, and F. A. Ennis. 1991. An HLA-C-restricted CD8+ cytotoxic T-lymphocyte clone recognizes a highly conserved epitope on human immunodeficiency virus type 1 gag. J. Virol. 65:4051-4056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Luciw, P. A., C. Cheng-Mayer, and J. A. Levy. 1987. Mutational analysis of the human immunodeficiency virus: the orf-B region down-regulates virus replication. Proc. Natl. Acad. Sci. USA 84:1434-1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McMichael, A. J., and B. D. Walker. 1994. Cytotoxic T lymphocyte epitopes: implications for HIV vaccines. AIDS 8(Suppl.):S155-S173.7857559
- 38.Miller, M. D., M. T. Warmerdam, I. Gaston, W. C. Greene, and M. B. Feinberg. 1994. The human immunodeficiency virus-1 nef gene product: a positive factor for viral infection and replication in primary lymphocytes and macrophages. J. Exp Med. 179:101-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Myers, G., S. Josephs, A. Rabson, T. Smith, and F. Wong-Staal (ed.). 1988. Human retroviruses and AIDS 1999: a compilation and analysis of nucleic acid and amino acid sequences. Los Alamos National Laboratory, Los Alamos, N.M.
- 40.Oelrichs, R., A. Tsykin, D. Rhodes, A. Solomon, A. Ellett, D. McPhee, and N. Deacon. 1998. Genomic sequence of HIV type 1 from four members of the Sydney Blood Bank Cohort of long-term nonprogressors. AIDS Res. Hum. Retrovir. 14:811-814. [DOI] [PubMed] [Google Scholar]
- 41.Ogg, G. S., X. Jin, S. Bonhoeffer, P. R. Dunbar, M. A. Nowak, S. Monard, J. P. Segal, Y. Cao, S. L. Rowland-Jones, V. Cerundolo, A. Hurley, M. Markowitz, D. D. Ho, D. F. Nixon, and A. J. McMichael. 1998. Quantitation of HIV-1-specific cytotoxic T lymphocytes and plasma load of viral RNA. Science 279:2103-2106. [DOI] [PubMed] [Google Scholar]
- 42.Pollard, V. W., and M. H. Malim. 1998. The HIV-1 Rev protein. Annu. Rev. Microbiol. 52:491-532. [DOI] [PubMed] [Google Scholar]
- 43.Popovic, M., M. G. Sarngadharan, E. Read, and R. C. Gallo. 1984. Detection, isolation, and continuous production of cytopathic retroviruses (HTLV-III) from patients with AIDS and pre-AIDS. Science 224:497-500. [DOI] [PubMed] [Google Scholar]
- 44.Rammensee, H. G., T. Friede, and S. Stevanoviic. 1995. MHC ligands and peptide motifs: first listing. Immunogenetics 41:178-228. [DOI] [PubMed] [Google Scholar]
- 45.Ranki, A., A. Lagerstedt, V. Ovod, E. Aavik, and K. J. Krohn. 1994. Expression kinetics and subcellular localization of HIV-1 regulatory proteins Nef, Tat and Rev in acutely and chronically infected lymphoid cell lines. Arch. Virol. 139:365-378. [DOI] [PubMed] [Google Scholar]
- 46.Re, F., D. Braaten, E. K. Franke, and J. Luban. 1995. Human immunodeficiency virus type 1 Vpr arrests the cell cycle in G2 by inhibiting the activation of p34cdc2-cyclin B. J. Virol. 69:6859-6864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rinaldo, C., X. L. Huang, Z. F. Fan, M. Ding, L. Beltz, A. Logar, D. Panicali, G. Mazzara, J. Liebmann, and M. Cottrill. 1995. High levels of anti-human immunodeficiency virus type 1 (HIV-1) memory cytotoxic T-lymphocyte activity and low viral load are associated with lack of disease in HIV-1-infected long-term nonprogressors. J. Virol. 69:5838-5842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roberts, M. R., L. Qin, D. Zhang, D. H. Smith, A. C. Tran, T. J. Dull, J. E. Groopman, D. J. Capon, R. A. Byrn, and M. H. Finer. 1994. Targeting of human immunodeficiency virus-infected cells by CD8+ T lymphocytes armed with universal T-cell receptors. Blood 84:2878-2889. [PubMed] [Google Scholar]
- 49.Rogel, M. E., L. I. Wu, and M. Emerman. 1995. The human immunodeficiency virus type 1 vpr gene prevents cell proliferation during chronic infection. J. Virol. 69:882-888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rowland-Jones, S., J. Sutton, K. Ariyoshi, T. Dong, F. Gotch, S. McAdam, D. Whitby, S. Sabally, A. Gallimore, T. Corrah, et al. 1995. HIV-specific cytotoxic T-cells in HIV-exposed but uninfected Gambian women. Nat. Med. 1:59-64. [DOI] [PubMed] [Google Scholar]
- 51.Saksela, K., G. Cheng, and D. Baltimore. 1995. Proline-rich (PxxP) motifs in HIV-1 Nef bind to SH3 domains of a subset of Src kinases and are required for the enhanced growth of Nef+ viruses but not for down-regulation of CD4. EMBO J. 14:484-491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Salter, R. D., and P. Cresswell. 1986. Impaired assembly and transport of HLA-A and -B antigens in a mutant TxB cell hybrid. EMBO J. 5:943-949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Salter, R. D., D. N. Howell, and P. Cresswell. 1985. Genes regulating HLA class I antigen expression in T-B lymphoblast hybrids. Immunogenetics 21:235-246. [DOI] [PubMed] [Google Scholar]
- 54.Sawai, E. T., A. Baur, H. Struble, B. M. Peterlin, J. A. Levy, and C. Cheng-Mayer. 1994. Human immunodeficiency virus type 1 Nef associates with a cellular serine kinase in T lymphocytes. Proc. Natl. Acad. Sci. USA 91:1539-1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schwartz, O., V. Marechal, S. Le Gall, F. Lemonnier, and J. M. Heard. 1996. Endocytosis of major histocompatibility complex class I molecules is induced by the HIV-1 Nef protein. Nat. Med. 2:338-342. [DOI] [PubMed] [Google Scholar]
- 56.Smith, B. L., B. W. Krushelnycky, D. Mochly-Rosen, and P. Berg. 1996. The HIV nef protein associates with protein kinase C theta. J. Biol. Chem. 271:16753-16757. [DOI] [PubMed] [Google Scholar]
- 57.Spina, C. A., T. J. Kwoh, M. Y. Chowers, J. C. Guatelli, and D. D. Richman. 1994. The importance of nef in the induction of human immunodeficiency virus type 1 replication from primary quiescent CD4 lymphocytes. J. Exp. Med. 179:115-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stivahtis, G. L., M. A. Soares, M. A. Vodicka, B. H. Hahn, and M. Emerman. 1997. Conservation and host specificity of Vpr-mediated cell cycle arrest suggest a fundamental role in primate lentivirus evolution and biology. J. Virol. 71:4331-4338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Terwilliger, E., J. G. Sodroski, C. A. Rosen, and W. A. Haseltine. 1986. Effects of mutations within the 3′ orf open reading frame region of human T-cell lymphotropic virus type III (HTLV-III/LAV) on replication and cytopathogenicity. J. Virol. 60:754-760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Trono, D. 1995. HIV accessory proteins: leading roles for the supporting cast. Cell 82:189-192. [DOI] [PubMed] [Google Scholar]
- 61.Walker, B. D., C. Flexner, K. Birch-Limberger, L. Fisher, T. J. Paradis, A. Aldovini, R. Young, B. Moss, and R. T. Schooley. 1989. Long-term culture and fine specificity of human cytotoxic T-lymphocyte clones reactive with human immunodeficiency virus type 1. Proc. Natl. Acad. Sci. USA 86:9514-9518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wei, X., S. K. Ghosh, M. E. Taylor, V. A. Johnson, E. A. Emini, P. Deutsch, J. D. Lifson, S. Bonhoeffer, M. A. Nowak, B. H. Hahn, et al. 1995. Viral dynamics in human immunodeficiency virus type 1 infection. Nature 373:117-122. [DOI] [PubMed] [Google Scholar]
- 63.Wolinsky, S. M., B. T. Korber, A. U. Neumann, M. Daniels, K. J. Kunstman, A. J. Whetsell, M. R. Furtado, Y. Cao, D. D. Ho, and J. T. Safrit. 1996. Adaptive evolution of human immunodeficiency virus-type 1 during the natural course of infection. Science 272:537-542. [DOI] [PubMed] [Google Scholar]
- 64.Wong, J. T., and R. B. Colvin. 1987. Bi-specific monoclonal antibodies: selective binding and complement fixation to cells that express two different surface antigens. J. Immunol. 139:1369-1374. [PubMed] [Google Scholar]
- 65.Yang, O. O., S. A. Kalams, M. Rosenzweig, A. Trocha, N. Jones, M. Koziel, B. D. Walker, and R. P. Johnson. 1996. Efficient lysis of human immunodeficiency virus type 1-infected cells by cytotoxic T lymphocytes. J. Virol. 70:5799-5806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yang, O. O., S. A. Kalams, A. Trocha, H. Cao, A. Luster, R. P. Johnson, and B. D. Walker. 1997. Suppression of human immunodeficiency virus type 1 replication by CD8+ cells: evidence for HLA class I-restricted triggering of cytolytic and noncytolytic mechanisms. J. Virol. 71:3120-3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yang, O. O., A. C. Tran, S. A. Kalams, R. P. Johnson, M. R. Roberts, and B. D. Walker. 1997. Lysis of HIV-1-infected cells and inhibition of viral replication by universal receptor T cells. Proc. Natl. Acad. Sci. USA 94:11478-11483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yang, O. O., and B. D. Walker. 1997. CD8+ cells in human immunodeficiency virus type I pathogenesis: cytolytic and noncytolytic inhibition of viral replication. Adv. Immunol. 66:273-311. [DOI] [PubMed] [Google Scholar]