Abstract
A heteroduplex mobility assay was used to identify variants of varicella-zoster virus circulating in the United Kingdom and elsewhere. Within the United Kingdom, 58 segregating sites were found out of the 23,266 examined (0.25%), and nucleotide diversity was estimated to be 0.00063. These are an order of magnitude smaller than comparable estimates from herpes simplex virus type 1. Sixteen substitutions were nonsynonymous, the majority of which were clustered within surface-expressed proteins. Extensive genetic correlation between widely spaced sites indicated that recombination has been rare. Phylogenetic analysis of varicella-zoster viruses from four continents distinguished at least three major genetic clades. Most geographical regions contained only one of these three strains, apart from the United Kingdom and Brazil, where two or more strains were found. There was minimal genetic differentiation (one or fewer substitutions in 1,895 bases surveyed) between the samples collected from Africa (Guinea Bissau, Zambia) and the Indian subcontinent (Bangladesh, South India), suggesting recent rapid spread and/or low mutation rates. The geographic pattern of strain distribution would favor a major influence of the former. The genetic uniformity of most virus populations makes recombination difficult to detect. However, at least one probable recombinant between two of the major strains was found among the samples originating from Brazil, where mixtures of genotypes co-occur.
Genetic variation among strains of human herpesviruses has been used to distinguish viral genotypes and to conduct epidemiological studies (7, 14, 33, 40, 45-51). Variation results predominantly from single nucleotide polymorphisms (SNPs) or from alteration in the composition and number of repeat elements present either within the internal and terminal repeat regions or within tandem direct reiterations scattered throughout the genome (7, 8, 27, 35). Genetic variation in varicella-zoster virus (VZV) has been defined by the presence or absence of restriction sites, such as a PstI site in gene 38 (25) and a BglI site in gene 54 (1), or differences in the number of repeat elements within the five repeat regions in the VZV genome (5, 20, 24, 25, 26, 28, 43, 44). These methods were found to distinguish viruses from different geographic regions and have been used to differentiate between the live attenuated Japanese Oka vaccine strain and wild-type viruses circulating in the United States and United Kingdom (17, 21, 23, 29, 47). Distinctive restriction enzyme patterns have also allowed differentiation between epidemiologically unrelated viruses, while viruses occurring in a single outbreak have been shown to be identical (20, 41). The establishment of latency by the virus in the dorsal root ganglion does not appear to affect the genotype, as evidenced by the identical restriction enzyme profiles of the infecting and reactivating strain from a single individual (42). Restriction enzyme profiles have also been shown to remain stable on serial passage in tissue culture (20).
Using these established methods, we have previously shown a mixture of genetically distinct strains to be circulating in the United Kingdom, most particularly in the East End of London, where the proportion of VZV strains carrying the BglI restriction endonuclease site in gene 38 increased from 10% in the early 1980s to more than 30% in the 1990s (22). Sampling of viruses from outside the United Kingdom showed a 90 to 100% prevalence of BglI-positive viruses in Asian and African countries, while the United States was similar to the United Kingdom (4). More recently several groups, including ours, have identified additional SNPs which can be used to genotype VZV strains (3, 12; V. N. Loparev and D. S. Schmid, Abstr. 26th Int. Herpesvirus Workshop, abstr. 3.15., 2001). Using the SNP map we have generated, estimates of VZV variation have been derived. In this report we use phylogenetic and population genetic analysis, which has enabled us, for the first time, to examine the evolutionary relationships between genotypes and to analyze the putative mechanisms by which VZV evolution and spread are likely to occur in the future.
MATERIALS AND METHODS
Samples.
The polymorphic map was developed using 10 VZV isolates randomly selected from around the United Kingdom. These data were used to estimate the time since the common ancestor of the major clades (see below). This calculation does not require a large sample size, because of the redundancy of information from similar sequences of the same clade, but it does benefit from the extensive sequence survey. The most informative sites were selected for further analysis (Fig. 1). We proceededto test further 10 United Kingdom samples for the whole set of primers to verify this choice and to establish that these were SNPs and not rare variants or PCR/sequencing artifacts. A larger sample, made up by an additional 67 strains typed at the phylogenetically informative loci, was used to survey the global distribution of genetic diversity. These comprised an additional 25 United Kingdom samples, collected from patients with varicella and zoster in East London, and 42 non-United Kingdom samples (Fig. 1).
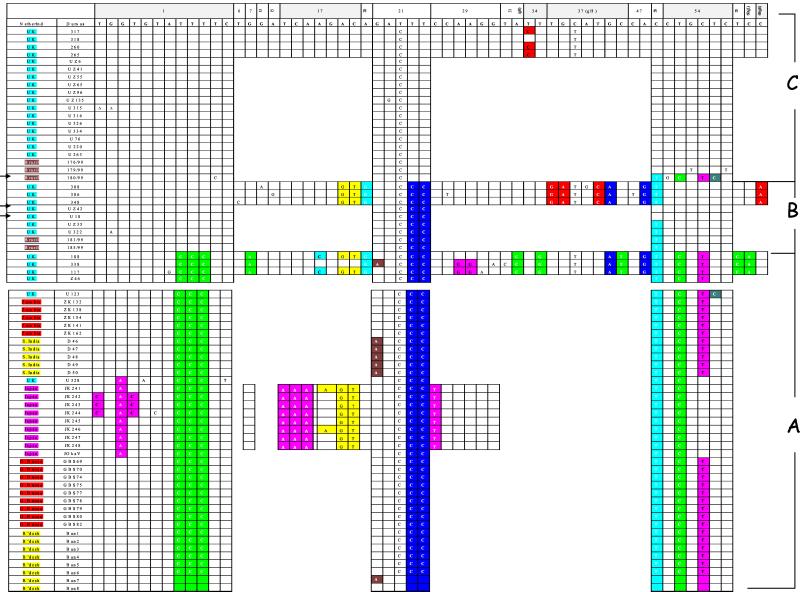
FIG. 1.
Alignment of nucleotide polymorphisms in strains of VZV from around the world. The country of origin of the virus is shown in the first column (Netherlnd, The Netherlands; G. Bissau, Guinea Bissau; B'desh, Bangladesh; S. India, South India; UK, United Kingdom). The regions which contained SNPs among the original 10 United Kingdom viruses typed are shown. Shaded boxes represent ORFs in which nucleotide changes were nonsynonymous. Regions within ORFs 1, 21, 50, and 54 which show fixed differences between genotypes were used to type additional strains from the United Kingdom and around the world; ▸ indicates recombinant virus. Letters A, B, and C refer to different genotypes as defined by phylogenetic analysis (see Fig. 2). Virus originating in the United Kingdom (blue), Brazil (brown), Zambia or Guinea Bissau (orange), South India or Bangladesh (yellow), and Japan (magenta) are shown.
DNA extraction and amplification.
DNA was extracted from 200 μl of each vesicle fluid sample using the QIAamp Blood Mini Kit (Qiagen Ltd., Crawley, United Kingdom). Viral DNA from each sample was initially genotyped at four loci by methods previously established (3). The 56 sets of primers shown in Table 1 This result suggested that recombination had little effect on the evolution of the genotypes, and so phylogenetic methods were used to represent the relationships between the genotypes. The United Kingdom genotypes fell into three major clades (Fig. 2), designated A, B, and C.
TABLE 1.
Primers used to amplify and sequence viruses
| Primer name | Position (5" → 3") | Size (bp) | Sequence (5" → 3") |
|---|---|---|---|
| VZV-1 F | 427-450 | 506 | TCAGCTGGCTTTTCTAAGAATTCG |
| VZV-1R | 932-911 | 506 | TATTTTTGGGATCCGCAATGTC |
| VZV-4 F | 3270-3291 | 458 | GCAGACTCCAACGCTTCAATCA |
| VZV-4 R | 3727-3706 | 458 | AATCGAAGACGGCGTCCTACAA |
| VZV-6 F | 6066-6087 | 455 | CAACAAATCCCCGTTCCAGCAA |
| VZV-6 R | 6520-6500 | 455 | CATGCGAGCATTTCGTCATGG |
| VZV-7 F | 8723-8743 | 487 | AGAGGCTTTAACCGCTGTGAA |
| VZV-7 R | 9208-9189 | 487 | CGCCTCCGATTTAATAGGTGA |
| VZV-10 F | 12423-12447 | 493 | CTGGAACGAGGATTTATTCTCATGT |
| VZV-10 R | 12915-12894 | 493 | TGCAGATTGACTGGCGTACAAA |
| VZV-11 F | 15898-15919 | 500 | TGAACTTCCGTTTTGTGGGATT |
| VZV-12 R | 16397-16378 | 500 | CGGGCGTTGGAATAAGACAT |
| VZV-13 F | 19017-19039 | 476 | AACGGTGAATTATCCTGCCAAGT |
| VZV-13 R | 19492-19470 | 476 | TCCTTGGGACATTGGGTCTTATC |
| VZV-14a F gC | 19434-19454 | 514 | TGAACAGCAACGGATGCATAG |
| VZV-14a R | 19947-19925 | 514 | AATTCCACAGCAAAAACAAATGG |
| VZV-14b F gC | 19925-19947 | 523 | CCATTTGTTTTTGCTGTGGAATT |
| VZV-14b R | 20447-20425 | 523 | TGGTTTACGCGTCACCTTATAGA |
| VZV-15 F | 21709-21730 | 522 | CGTTTAAGGGTCCGGGAACTTT |
| VZV-15 R | 22230-22211 | 522 | TTCGGGGTTATGGCCAACAT |
| VZV-17 F | 24402-24424 | 487 | GTTGTACAGACGGCCCATTATCA |
| VZV-17 R | 24888-24865 | 487 | TCACAGCCCATGAAGAGTAAATCA |
| VZV-19 F | 27209-27232 | 497 | TCCATGCTTGTTTTCATTGTCTCA |
| VZV-19 R | 27705-27682 | 497 | TCTTCTGTTCCCATTCAAGATATC |
| VZV-20 F | 30027-30049 | 496 | TCAGATGGATGACGAAGGATTAA |
| VZV-20 R | 30522-30500 | 496 | GATAGTACCACGTCACGATTGCA |
| VZV-21 FOR | 33497-33518 | 503 | TAATGAATTGAGGCGCGGTTTA |
| VZV-21 R | 33999-33976 | 503 | CACGTGTAGCTCCAAAAACCTAGG |
| VZV-22a F | 37007-37027 | 509 | GGTAACCGGTTTTGTGGGACT |
| VZV-22a R | 37515-37489 | 509 | GTAATTCAGTCAACTTATGGGTATTCG |
| VZV22b F | 40507-40528 | 492 | AGGCCAATGTCGATGCAGTTAC |
| VZV-22b R | 40998-40973 | 492 | ATATACCCGAATCTGTAGCCATATGC |
| VZV-24 F | 43985-44006 | 511 | TCCTGCGTTCACTCCGTACATA |
| VZV-25 R | 44495-44476 | 511 | AGGGCCCGCGATTTAATTAC |
| VZV-28 F | 47506-47525 | 509 | GGGGCCACAATAACATAAGG |
| VZV-28 R | 48014-47992 | 509 | GCACATATAATTTCAACGGCTCT |
| VZV-29a F | 51043-51060 | 507 | GCGGTGGTTTCTGGAGCA |
| VZV-29aR | 51549-51526 | 507 | GGTGTTATAAAAGGGTGTTGGGTA |
| VZV-29 F | 52795-52814 | 542 | TTCGAAGCCACCCATTCAAT |
| VZV-29 R | 53336-53315 | 542 | CGAGGAAATAACAGGCCGTGAA |
| VZV-30 F | 56314-56335 | 513 | CGGCCTTTGATGCACATAGATT |
| VZV-30 R | 56826-56804 | 513 | GGGGACATGTTTCTTCGTACGTT |
| VZV-31a F gB | 56988-57008 | 513 | GCTTTTTATGCATATTTTCTA |
| VZV-31a R | 57500-57480 | 513 | CTTCAACTTTGTGGTTATTTC |
| VZV-31b F gB | 57480-57500 | 507 | GAAATAACCACAAAGTTGAAG |
| VZV-31b R | 57986-57967 | 507 | GAAAATTGTGTGCATACTCA |
| VZV-31c F gB | 57967-57986 | 514 | TGAGTATGCACACAATTTTC |
| VZV-31c R | 58480-58463 | 514 | AAATAGTCCGCTCCAAAG |
| VZV-31d F gB | 58463-58482 | 515 | CTTTGGAGCGGACTATTTCC |
| VZV-31d R | 58977-58959 | 515 | CGGCGTTGAATTTCACTGT |
| VZV-31e F gB | 58935-58956 | 355 | TGCGGGATACAGGATTACTAGA |
| VZV-31e R | 59289-59268 | 355 | AGTGGATATAATGCCTTCATCG |
| VZV-31f F gB | 59268-59289 | 413 | CGATGAAGGCATTATATCCACT |
| VZV-31f R | 59680-59657 | 413 | AATGGAGACACATGAGTAACGTAA |
| VZV-32 F | 59798-59819 | 503 | ACCGTCGTCTATCGCACATCAT |
| VZV-32 R | 60300-60278 | 503 | ATTCATAAAAGGCGTGTTTGGTT |
| VZV-34 F | 63291-63312 | 509 | TTCATAAACGTACGGGAAATGC |
| VZV-34 R | 63799-63780 | 509 | GCTCCCGCGTTTGAGTTAAC |
| VZV-37 F | 66787-66806 | 516 | CGGTTCGGTGCTTCTCACAA |
| VZV-37 R | 67302-67277 | 516 | ATCCGCATTAAAATGTAATTAAGGAA |
| VZV-37a F gH | 66036-66055 | 508 | AACGTTGCGGTGATATTGTA |
| VZV-37a R | 66543-66526 | 508 | AGGGGGTTTGGTGGGAAC |
| VZV-37b F gH | 66527-66544 | 511 | TTCCCACCAAACCCCCTT |
| VZV-37b R | 67037-67014 | 511 | CATGCTTTGAAAAATTCATATCCA |
| VZV-37c F gH | 67007-67028 | 514 | CGTGGGTTGGATATGAATTTTT |
| VZV-37c R | 67520-67502 | 514 | GCCTGGCGAGAGAAAGTGC |
| VZV-37d F gH | 67440-67460 | 430 | ATGAAGCCCGTGATCAACTAA |
| VZV-37d R | 67869-67851 | 430 | CGTGTTGGTATTGCCGACA |
| VZV-37e F gH | 67851-67869 | 438 | TGTCGGCAATACCAACACG |
| VZV-37e R | 68288-68268 | 438 | CCCCCGTGGTTAGATACCTAA |
| VZV-37f F gH | 68252-68272 | 327 | TGCGGAAGTGTTTTTCTTAGG |
| VZV37f R | 68578-68558 | 327 | ATATTCTCGAAGGCGGGAATT |
| VZV-38 F | 70295-70320 | 490 | TTAAAGCGTTTATCGGAACATTAACC |
| VZV-39 R | 70784-70761 | 490 | TCACCCCCATACGATAACATAACC |
| VZV-40 F | 73771-73793 | 512 | GGATGACACGTTTATTGCACCTA |
| VZV-40 R | 74282-74260 | 512 | CTTGTAGTGTGAGCAATGCATCA |
| VZV-42 F | 77260-77283 | 500 | AACTGTTGTGCTATAGCCAACGAT |
| VZV-42 R | 77759-77741 | 500 | AAGCCGGTTTTCATCACAA |
| VZV-44 F | 80747-80765 | 513 | AAGAACCCGATCGCGTACC |
| VZV-44 R | 81259-81240 | 513 | TGCGGTAATGCGAGGATTTT |
| VZV-47 F | 84247-84266 | 513 | GATTGATCTTTATGCATTGG |
| VZV-48 R | 84759-84741 | 513 | TTTTACAAAAGGGTGCTGT |
| VZV-50 F | 87736-87756 | 515 | CGCACCCAAAGTGAACATCAT |
| VZV-51 R | 88250-88227 | 515 | TCTCGGATGTCAAATATGTTACGA |
| VZV-52 F | 91226-91247 | 495 | GCTTGGTTCTTAGTGGCAATCG |
| VZV-52 R | 91720-91699 | 495 | CAGGCTGTTTGAAGACGGTAGA |
| VZV-54 F | 94172-94194 | 505 | GGGTGGCCCGTAATATGAATGAA |
| VZV-54 R | 94676-94654 | 505 | TCCGGAACAACAAACGTTCAAAG |
| VZV-54BgII F+ | 95005-95027 | 497 | CGTAATGCATAACAGGCCAACAC |
| VZV-54BgII R+ | 95501-95479 | 497 | GAAACCTGGCGTCAAACATTACA |
| VZV-55 F | 98505-98523 | 496 | CCATGTCGCGTACGGTCTCTC |
| VZV-56 R | 99000-98982 | 496 | TTGCGTGGGGTTTGTCCTG |
| VZV-60 F gL | 101156-101176 | 522 | CCGATAGTTTCATTTCATTGG |
| VZV-60 R | 1011677-101656 | 522 | GTCGTAGTGAAGGGAAAACACA |
| VZV-62a F | 105497-105514 | 502 | CCGCCTGGGTTTCTGACG |
| VZV-62a R | 105998-105982 | 502 | ACCCGCGTCCCCCTGTC |
| VZV-62 F | 107299-107320 | 527 | CGGTCGTATCGACGGCTCATAG |
| VZV-62 R | 107825-107804 | 527 | GGGCGCCAGAGACAGAAATCAT |
| VZV63 F-O* | 110512-110529 | 515 | TTCGTCCGATTCATAACG |
| VZV63 RI* | 111026-111008 | 515 | CTATGCAAAGGAACATTCG |
| VZV64 F | 111512-111531 | 661 | GCTGGTTTGAACGTCTCCAT |
| VZV64 R | 112172-112151 | 661 | CAGTACGCTTTTATCCGGTGTA |
| VZV-67NC F gI | 114480-114502 | 508 | GCCTCATTTAATCGCGATGTTTT |
| VZV-67 R | 114987-114963 | 508 | ATTTACACCAAGAATGAAACCATCG |
| VZV-67b F gl | 114930-114954 | 522 | ACTTCTTGTTCGGTTAGACCATAGC |
| VZV-67b R | 115451-115430 | 522 | TGTATGCCCCTTCTTGTTTTTG |
| VZV-68NC F gE | 115798-115819 | 515 | CGCCTGTAATATGGGGACAGTT |
| VZV-68 R | 116312-116292 | 515 | GGCCTTGGGGTTTTGGATTAA |
| VZV-68b F gE | 116245-116268 | 511 | TGTAAATGTGGACCAACGTCAATA |
| VZV-68b R | 116755-116735 | 511 | CAAAAACGTGGCGTAGGTAGA |
| VZV-68c F gE | 116717-116732 | 507 | CGCGGCTCCGATGGTA |
| VZV-68c R | 117223-117199 | 507 | CAAACTCTCGGGTGTATCTACAAAC |
| VZV-68d F gE | 117174-117191 | 522 | TACACGACGGGGGCACCA |
| VZV-68d R | 117695-117680 | 522 | TCGCCCGGTTCGGTGA |
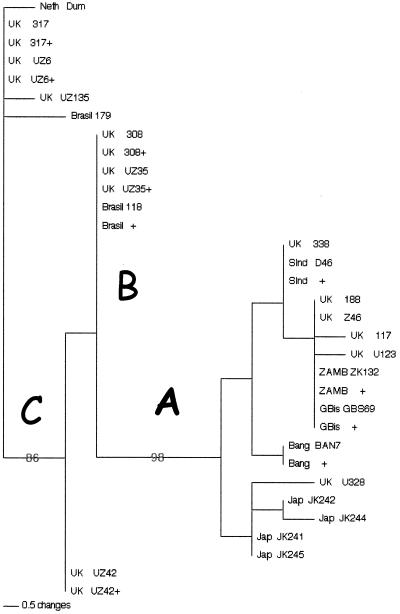
FIG. 2.
Phylogenetic tree of varicella-zoster virus strains by the neighbor-joining method. The countries from which specimens were obtained are shown: Bang, Bangladesh; Brasil, Brazil; Jap, Japan; GB, Guinea Bissau; Neth, The Netherlands; Sind, South India; ZAMB, Zambia. Bootstrap values separating clades A, B, and C are shown. + denotes additional viruses with identical sequence.
To map the worldwide distribution of genotypes, we used a subset of informative markers. Four regions, located in ORFs 1, 21, 50, and 54, which contained fixed differences between the major clades were selected manually and used to analyze a further 25 United Kingdom viruses (Fig. 1). This restricted marker set gave distinct genotypes, consistent with the previously identified clades. Genotyping additional informative markers located in ORFs 55, 20, and 47 did not reveal further genotypes (data not shown). Additional genotyping of 42 viruses from Africa, Asia, Europe, and South America (Fig. 1 and 2) using the four informative regions was therefore undertaken. Phylogenetic analysis of all of the viruses at the above four regions, representing 1,895 bp, is shown in Fig. 2. A bootstrap analysis using the maximum parsimony criterion was used to assess the support for the major clades. The number of inter- and intrapopulation nucleotide differences at these sites ranged from 0 to 13 and 1 to 12, respectively. These values were standardized as gross and net nucleotide diversities, using the methods set out by Nei (34), and are shown in Table 2.
TABLE 2.
Nucleotide diversities estimated from the four regions of 1.9 kb typed worldwidea
| Origin of virus | Nucleotide diversity in comparison with populations from:
|
||||||
|---|---|---|---|---|---|---|---|
| UK | Brazil | G. Bissau | Zambia | Bangladesh | S. India | Japan | |
| UK | 0.0005 | 0.0001 | 0.0007 | 0.0007 | 0.0007 | 0.0008 | 0.0008 |
| Brasil | 0.0006 | 0.0005 | 0.0008 | 0.0007 | 0.0008 | 0.0009 | 0.0009 |
| G. Bissau | 0.0009 | 0.0011 | 0.0000 | 0.0000 | 0.0000 | 0.0001 | 0.0003 |
| Zambia | 0.0009 | 0.0011 | 0.0000 | 0.0000 | 0.0000 | 0.0001 | 0.0003 |
| Bangladesh | 0.0009 | 0.0011 | 0.0000 | 0.0000 | 0.0000 | 0.0001 | 0.0003 |
| S. India | 0.0011 | 0.0012 | 0.0001 | 0.0001 | 0.0001 | 0.0000 | 0.0005 |
| Japan | 0.0011 | 0.0012 | 0.0004 | 0.0004 | 0.0004 | 0.0006 | 0.0002 |
Diagonal (bold) values, within-population diversities (px); above diagonal values, net nucleotide diversity between populations (pA); below diagonal values, mean proportion of pairwise differences between populations (pxy). The 1.9-kb subset had higher than average diversity, so the values in Table 2 are adjusted accordingly (by a factor of 0.277). UK, United Kingdom; G. Bissau, Guinea Bissau; S. India, South India.
The estimated time since the common ancestor of the major clades was 3,000 to 19,000 years, assuming the mutation rate derived from other alphaherpesviruses. The upper limit on the mutation rate that remains consistent with a divergence time of 100,000 years was one-seventh of the value for herpes simplex virus (HSV) type 1 and alphaherpesviruses (1.4 × 10−8).
DISCUSSION
Evolutionary inference from the phylogenetic data.
Our estimate of genetic diversity of VZV is 0.00063, which is 10 times lower than that obtained for HSV and 40 times less than estimated for cytomegalovirus (CMV) (36, 51). This difference could be due to a lower mutation rate, a more recent common ancestry, or a combination of the two. Most striking is the genetic uniformity within and between samples from Guinea Bissau, Zambia, Bangladesh, and Southern India. In pairwise comparisons between genotypes from these localities, a maximum of one substitution was found in the 1.9 kb surveyed. Although small samples were obtained from each locality, the broad geographical scope precludes a sampling artifact. The human populations in these countries are not thought to have a particularly recent common ancestry (6), so the low differentiation of VZV indicates possible spread subsequent to the initial colonization of these areas. The contrasting higher level of diversity in the United Kingdom and Brazil may be explained by the history of recent human migration. There is clear evidence from the United Kingdom in support of the hypothesis of viral spread associated with migration. In East London, the frequency of clade A viruses among cases of varicella has increased with time, during a period when immigrants from the Indian and African subcontinents have come to make up more than 25% of the population (22). Clade A viruses are characterized by a BglI restriction site in gene 54 and are ubiquitous in Asia, Africa, and the Far East but generally make up fewer than 10% of British strains (22).
One way of interpreting the differentiation between populations is to make use of it to estimate the time of divergence. This estimate is conventionally obtained from the net accumulation of nucleotide diversity (πa) after subtracting an estimate of the diversity within the ancestral populations, which is obtained from the current diversity (34). Unfortunately, this calculation is not appropriate for VZV, as we have evidence that the high diversity of current populations may be a consequence of mixing, which has not yet reached equilibrium. We instead obtain an alternative estimate of the time (t) since the splitting of the major clades rather than populations, from the number of synonymous substitutions that have accumulated between them. This calculation requires an estimate of the mutation rate. One possible approach is to make use of estimates of mutation rates obtained from related viruses. For example, a synonymous mutation rate of 10−7 (per year) has been obtained from differentiation between HSV type 1 strains from different parts of the world. It would imply that differentiation was initiated at the time of human dispersal out of Africa around 100 kya and is also consistent with rates estimated from other alphaherpesviruses (31, 32, 36). When this rate is applied to the VZV data, it leads to a much more recent estimate, implying that the currently circulating strains of VZV have spread in the past 3,000 to 19,000 years.
This interpretation is somewhat at odds with established estimates of varicella-zoster dispersal (19, 31, 32). Although the calculation allowed for some error on the estimate of the mutation rate (standard deviation, 20%), an alternative explanation is that the mutation rate of VZV is substantially lower than that of other alphaherpesviruses. Our calculations showed that if the rate were one-seventh of that of other alphaherpesviruses, then the different clades could date back 100,000 years. Is such a low rate plausible? Given the similar biology of the alphaherpesviruses, a lower mutation rate per division seems unlikely.
Is it then possible that VZV has a smaller number of divisions per year than other herpesviruses? The natural history of varicella is of primary infection in childhood following which the virus remains quiescent before reactivating once in adulthood, although recent evidence has confirmed observations that subclinical reactivation of VZV is not uncommon (30). The number of replication cycles before latency is established could be as low as 20 (19), and viral shedding is limited to the duration of lesions (approximately 5 to 7 days). By contrast, herpes simplex virus is known to have multiple reactivation episodes, which give rise to both symptomatic and asymptomatic viral shedding. Similarly CMV is 40 times more variable than VZV (51). This virus appears to be more distantly related to VZV than the alphaherpesviruses (32), but it is not sufficiently different that it is likely to be subject to a higher mutation rate per division. Instead, the same two explanations that we discussed for HSV may explain its higher diversity: a more ancient ancestry or a higher number of divisions since the common ancestor. The more ancient ancestry could be again explained by the mode of infection, which typically involves close contact. Furthermore, although CMV may be latent in mononuclear cells, there are long periods of asymptomatic shedding which suggest a high rate of division per unit time.
While the activity of VZV between varicella and zoster is not quantified, it is instructive to calculate the effect on the number of divisions per year under the assumption that this is an essentially quiescent period. The relevant transmission dynamics can be assessed using the parameters estimated by Schuette and Hethcote (39), which indicate that the majority of infections along any evolutionary lineage will be from varicella case to varicella case. The proportion of zoster episodes in the evolutionary history will be lower, first because of the lower relative infectivity (0.07) and second because only around 15% of cases reactivate as zoster. The latent period for varicella is only around 14 days, whereas the mean quiescent period before zoster activation is around 40 years. The ratio of time spent quiescent along an evolutionary lineage is given by (nz vtz pz + nv tv pz)/(nz vtz + nv tv), where nz and nv are the proportion of the population with zoster and varicella, respectively, tz and tv are the periods between initial infection and transmission, and pz and pv are the proportion of time spent quiescent between infection and transmission (pz ≈ 1 and pv ≈ 0). The infectivity of zoster (relative to varicella) is given by v. The values of n depend on the population demographics and viral epidemiology of the population, but we estimate that the proportion of time spent quiescent is of the order of 96%. HSV may spend less time quiescent, and since the infectivity and interval of each episode are relatively constant, the proportion of quiescent time along an evolutionary lineage will correspond to that within an individual. It is plausible that the proportion of time quiescent could be several times smaller than for VZV. Although this explanation of low diversity in VZV may be credible, the issue is most likely to be resolved by a more accurate calibration of the difference in mutation rate between VZV and other herpesviruses.
Although rapid spread and a low mutation rate could both explain the low level of genetic diversity of VZV, the geographical distribution and relatedness of strains does suggest that the spread of VZV subsequent to the major human migrations has made a strong contribution to its geographic epidemiology. The validity of this hypothesis is underlined by the recent spread in London of clade A strains, which appears to coincide with the major period of immigration from the Indian subcontinent and Africa to the United Kingdom. Such rapid spread is possible because VZV, uniquely among herpesviruses, is transmitted by aerosolization of virus, resulting in airborne epidemic infection. Geographic spread may be particularly rapid in warmer regions, where varicella occurs on average 10 years later in life (16). In this scenario, the disease is transmitted between individuals who are more mobile and who mix more freely than the children who account for the majority of infectious cases in more temperate climates.
Significant associations (linkage disequilibrium) between SNPs that are widely spaced within the genome suggest low recombination rates. Recombination rates, however, cannot be calculated from population genetic data because there is evidence of population subdivision, so that the different haplotypes may not come into contact. This study revealed that two geographic areas, London in the United Kingdom and Rio de Janeiro in Brazil, are populated with mixtures of strains. One haplotype originating with Brazil (Fig. 1) is most parsimoniously explained as a recombinant between A and C strains which coexist in the region. At least two other strains, UZ 42 and UZ 42+ (Fig. 1 and 2) from London, are also likely to be recombinants. Although in vitro recombination of the vaccine vOka strain with a wild-type U.S. strain has been described (10), this is the first evidence of recombination between strains occurring in vivo.
Recombination of wild-type strains may have consequences for virulence and selection. For example, the MSP VZV strain, in which a single nucleotide substitution results in an amino acid change from aspartate to asparagine in glycoprotein E, appears to be more virulent in the skin SCID-hu mouse model (37, 38). Epidemic spread of VZV affords the opportunity for repeated and widespread exposure of immune and na&ıuml;ve populations to circulating virus, and reinfection is well described (2, 18). In these circumstances, cocirculation of distinct wild-type genotypes, such as occurs in East London, will allow measurement of the rate of recombination and assessment of its contribution to VZV evolution. Such data may prove useful in understanding the spread and pathogenesis of VZV, especially against a background of mass immunization with the attenuated Oka vaccine.
Acknowledgments
This work was supported by grants from North Thames Health Authority Research & Development and the Special Trustees of St. Bartholomew's Hospital.
We thank colleagues who provided samples for this study, including Yamima Talukdar (Bangladesh), Marilda Siqueira (Brazil), Peter Aaby (Guinea Bissau), Koichi Yamanashi (Japan), Jacob John (South India), and Patrick Mtondo (Zambia).
REFERENCES
- 1.Adams, S. G., D. E. Dohner, and L. D. Gelb. 1989. Restriction fragment differences between the genomes of the Oka varicella vaccine virus and American wild-type varicella-zoster virus. J. Med. Virol. 29:38-45. [DOI] [PubMed] [Google Scholar]
- 2.Arvin, A. M., C. M. Koropchak, and A. E. Wittek. 1983. Immunologic evidence of reinfection with varicella-zoster virus. J. Infect. Dis. 148:200-205. [DOI] [PubMed] [Google Scholar]
- 3.Barrett-Muir, W., K. Hawrami, J. Clarke, and J. Breuer. 2001. Investigation of varicella-zoster virus variation by heteroduplex mobility assay. Arch. Virol. 17(Suppl. 1):17-25. [DOI] [PubMed] [Google Scholar]
- 4.Breuer, J. 1997. Oka vaccine for VZV. Herpes 4:62-67. [Google Scholar]
- 5.Casey, T. A., W. T. Ruyechan, M. N. Flora, W. Reinhold, S. E. Straus, and J. Hay. 1985. Fine mapping and sequencing of a variable segment in the inverted repeat region of varicella-zoster virus DNA. J. Virol. 54:639-642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cavalli-Sforza, L. L., P. Menozzi, and A. Piazza. 1994. The history and geography of human genes. Princeton University Press, Princeton, N.J.
- 7.Chou, S., and K. M. Dennison. 1991. Analysis of interstrain variation in cytomegalovirus glycoprotein B sequences encoding neutralizing epitopes. J. Infect. Dis. 163:1229-1234. [DOI] [PubMed] [Google Scholar]
- 8.Davis, C. L., D. Field, D. Metzgar, R. Saiz, P. A. Morin, I. L. Smith, S. A. Spector, and C. Wills. 1999. Numerous length polymorphisms at short tandem repeats in human cytomegalovirus. J. Virol. 73:6265-6270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davison, A. J., and J. E. Scott. 1986. The complete DNA sequence of varicella-zoster virus. J. Gen. Virol. 67:1759-1816. [DOI] [PubMed] [Google Scholar]
- 10.Dohner, D. E., S. G. Adams, and L. D. Gelb. 1988. Recombination in tissue culture between varicella-zoster virus strains. J. Med. Virol. 24:329-341. [DOI] [PubMed] [Google Scholar]
- 11.Dumas, A. M., J. L. Geelen, M. W. Weststrate, P. Wertheim, and J. van der Noordaa. 1981. XbaI, PstI, and BglI restriction enzyme maps of the two orientations of the varicella-zoster virus genome. J. Virol. 39:390-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Faga, B., W. Maury, D. A. Bruckner, and C. Grose. 2001. Identification and mapping of single nucleotide polymorphisms in the varicella-zoster virus genome. Virology 280:1-6. [DOI] [PubMed] [Google Scholar]
- 13.Felsenstein, J. 1993. PHYLIP inference package, version 3.5. Department of Genetics, University of Washington, Seattle.
- 14.Franti, M., J.-T. Aubin, L. Poirel, A. Gautheret-Dejean, D. Candotti, J.-M. Huraux, and H. Agut. 1998. Definition and distribution analysis of glycoprotein B gene alleles of human herpesvirus 7. J. Virol. 72:8725-8730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ganguly, A., M. J. Rock, and D. J. Prockop. 1993. Conformation-sensitive gel electrophoresis for rapid detection of single-base differences in double stranded PCR products and DNA fragments: evidence for solvent-induced bends in DNA heteroduplexes. Proc. Natl. Acad. Sci. USA 90:10325-10329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garnett, G. P., M. J. Cox, D. A. P. Bundy, J. M. Didier, and J. St. Catharine. 1993. The age of infection with varicella-zoster virus in St-Lucia, West-Indies. Epidemiol. Infect. 110:361-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gelb, L. D., D. E. Dohner, A. A. Gershon, et al. 1987. Molecular epidemiology of live, attenuated varicella virus vaccine in children and in normal adults. J. Infect. Dis. 155:633-640. [DOI] [PubMed] [Google Scholar]
- 18.Gershon, A. A., S. P. Steinberg, and L. Gelb. 1984. Clinical reinfection with varicella-zoster virus. J. Infect. Dis. 149:137-142. [DOI] [PubMed] [Google Scholar]
- 19.Grose, C. 1999. Varicella-zoster virus: less immutable than once thought. Pediatrics 103:1027-1028. [DOI] [PubMed] [Google Scholar]
- 20.Hawrami, K., D. Harper, and J. Breuer. 1996. Typing of varicella zoster virus by amplification of DNA polymorphisms. J. Virol. Methods 57:169-174. [DOI] [PubMed] [Google Scholar]
- 21.Hawrami, K., and J. Breuer. 1997. Analysis of United Kingdom wild-type strains of varicella-zoster virus: differentiation from the Oka vaccine strain. J. Med. Virol. 53:60-62. [DOI] [PubMed] [Google Scholar]
- 22.Hawrami, K., I. J. Hart, F. Pereira, S. Argent, B. Bannister, B. Bovill, D. Carrington, M. Ogilvie, S. Rawstorne, Y. Tryhorn, and J. Breuer. 1997. Molecular epidemiology of varicella-zoster virus in East London, England, between 1971 and 1995. J. Clin. Microbiol. 35:2807-2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hayakawa, Y., S. Torigoe, K. Shiraki, K. Yamanishi, and M. Takahashi. 1984. Biologic and biophysical markers of a live varicella vaccine strain (Oka): identification of clinical isolates from vaccine recipients. J. Infect. Dis. 149:956-963. [DOI] [PubMed] [Google Scholar]
- 24.Hondo, R., and Y. Yogo. 1988. Strain variation of R5 direct repeats in the right-hand portion of the long unique segment of varicella-zoster virus DNA. J. Virol. 62:2916-2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hondo, R., Y. Yogo, M. Yoshida, A. Fujima, and S. Itoh. 1989. Distribution of varicella-zoster virus strains carrying a PstI-site-less mutation in Japan and DNA change responsible for the mutation. Jpn. J. Exp. Med. 59:233-237. [PubMed] [Google Scholar]
- 26.Kinchington, P. R., J. Remenick, J. M. Ostrove, S. Straus, W. D. Ruyechan, and J. Hay. 1986. Putative glycoprotein gene of varicella-zoster virus with variable copy numbers of a 42-base-pair repeat sequence has homology to herpes simplex virus glycoprotein C. J. Virol. 59:660-668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kinchington, P. R., and S. E. Turse. 1995. Molecular basis for a geographic variation of varicella-zoster virus recognized by a peptide antibody. Neurology 45(Suppl. 8):S13-S14. [DOI] [PubMed]
- 28.Kinoshita, H., R. Hondo, F. Taguchi, and Y. Yogo. 1988. Variation of R1 repeated sequence present in open reading frame 11 of varicella-zoster virus strains. J. Virol. 62:1097-1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.La Russa, P., O. Lungu, I. Hardy, A. Gershon, S. P. Steinberg, and S. Silverstein. 1992. Restriction fragment length polymorphism of polymerase chain reaction products from vaccine and wild-type varicella-zoster virus isolates. J. Virol. 66:1016-1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mainka, C., B. Fuss, H. Geiger, H. Hofelmayr, and M. H. Wolff. 1998. Characterization of viremia at different stages of varicella-zoster virus infection. J. Med. Virol. 56:91-98. [DOI] [PubMed] [Google Scholar]
- 31.McGeoch, D. J., and S. Cook. 1994. Molecular phylogeny of the Alphaherpesvirinae subfamily and a proposed evolutionary timescale. J. Mol. Biol. 238:9-22. [DOI] [PubMed] [Google Scholar]
- 32.McGeoch, D. J., S. Cook, A. Dolan, F. E. Jamieson, and E. A. Telford. 1995. Molecular phylogeny and evolutionary timescale for the family of mammalian herpesviruses. J. Mol. Biol. 247:443-458. [DOI] [PubMed] [Google Scholar]
- 33.Meng, Y. X., T. J. Spira, G. J. Bhat, C. J. Birch, J. D. Druce, B. R. Edlin, R. Edwards, C. Gunthel, R. Newton, F. R. Stamey, C. Wood, and P. E. Pellett. 1999. Individuals from North America, Australasia, and Africa are infected with four different genotypes of human herpesvirus 8. Virology 261:106-119. [DOI] [PubMed] [Google Scholar]
- 34.Nei, M. 1987. Molecular evolutionary genetics. Columbia University Press, New York, N.Y.
- 35.Rixon, F. J., and D. J. McGeoch. 1985. Detailed analysis of the mRNAs mapping in the short unique region of herpes simplex virus type 1. Nucleic Acids Res. 13:953-973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sakaoka, H., K. Kurita, Y. Lida, S. Takada, K. Umene, Y. Tae Kim, C. Shang Ren, and A. J. Nahmias. 1994. Quantitative analysis of genomic polymorphism of herpes simplex virus type 1 strains from six countries: studies of molecular evolution and molecular epidemiology of the virus. J. Gen. Virol. 75:513-527. [DOI] [PubMed] [Google Scholar]
- 37.Santos, R. A., J. A. Padilla, C. Hatfield, and C. Grose. 1998. Antigenic variation of varicella zoster virus Fc receptor gE: loss of a major B cell epitope in the ectodomain. Virology 249:21-31. [DOI] [PubMed] [Google Scholar]
- 38.Santos, R. A., C. C. Hatfield, N. L. Cole, J. A. Padilla, J. F. Moffat, A. M. Arvin, W. T. Ruyechan, J. Hay, and C. Grose. 2000. Varicella-zoster virus gE escape mutant VZV-MSP exhibits an accelerated cell-to-cell spread phenotype in both infected cell cultures and SCID-hu mice. Virology 275:306-317. [DOI] [PubMed] [Google Scholar]
- 39.Schuette, M. C., and H. W. Hethcote. 1999. Modelling the effects of varicella vaccination programs on the incidence of chickenpox and shingles. Bull. Math. Biol. 61:1031-1064. [DOI] [PubMed] [Google Scholar]
- 40.Shepp, D. H., M. Match, S. M. Lipson, and R. G. Pergolizzi. 1998. A fifth human cytomegalovirus glycoprotein B genotype. Res. Virol. 149:109-114. [DOI] [PubMed] [Google Scholar]
- 41.Straus, S. E., J. Hay, H. Smith, and J. Owens. 1983. Genome differences among varicella-zoster virus isolates. J. Gen. Virol. 64:1031-1041. [DOI] [PubMed] [Google Scholar]
- 42.Straus, S. E., W. Reinhold, H. A. Smith, W. T. Ruyechan, D. K. Henderson, R. M. Blaese, and J. Hay. 1984. Endonuclease analysis of viral DNA from varicella and subsequent zoster infections in the same patient. N. Engl. J. Med. 311:1362-1364. [DOI] [PubMed] [Google Scholar]
- 43.Takada, M., T. Suzutani, I. Yoshida, M. Matoba, and M. Azuma. 1995. Identification of varicella-zoster virus strains by PCR analysis of three repeat elements and a PstI-site-less region. J. Clin. Microbiol. 33:658-660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Takayama, M., N. Takayama, N. Inoue, and Y. Kameoka. 1996. Application of long PCR method to identification of variations in nucleotide sequences among varicella-zoster isolates. J. Clin. Microbiol. 34:2869-2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Umene, K., and M. Yoshida. 1993. Genomic characterization of two predominant genotypes of herpes simplex virus type 1. Arch. Virol. 131:29-46. [DOI] [PubMed] [Google Scholar]
- 46.Umene, K., and H. Sakaoka. 1997. Populations of two eastern countries of Japan and Korea and with a related history share a predominant genotype of herpes simplex virus type 1. Arch. Virol. 142:1953-1961. [DOI] [PubMed] [Google Scholar]
- 47.Williams, D. L., A. Gershon, L. D. Gelb, M. K. Spraker, S. Steinberg, and A. H. Ragab. 1985. Herpes zoster following varicella vaccine in a child with acute lymphocytic leukaemia. J. Paediatr. 106:259-261. [DOI] [PubMed] [Google Scholar]
- 48.Zimber, U., H. K. Adldinger, G. M. Lenoir, M. Vuillaume, M. V. Knebel-Doeberitz, G. Laux, C. Desgranges, P. Wittman, U. K. Freese, U. Schneider, and G. Bornkamm. 1986. Geographical prevalence of two types of Epstein-Barr virus. Virology 154:56-66. [DOI] [PubMed] [Google Scholar]
- 49.Zipeto, D., C. Hong, G. Gerna, M. Zavattoni, D. Katzenstein, T. C. Merigan, and L. Rasmussen. 1998. Geographic and demographic differences in the frequency of human cytomegalovirus gB genotypes 1-4 in immunocompromised patients. AIDS Res. Hum. Retrovir. 14:533-536. [DOI] [PubMed] [Google Scholar]
- 50.Zong, J.-C., D. M. Ciufo, D. J. Alcendor, X. Wan, J. Nicholas, P. J. Browning, P. L. Rady, S. K. Tyring, J. M. Orenstein, C. S. Rabkin, I.-J. Su, K. F. Powell, M. Croxson, K. E. Foreman, B. J. Nickoloff, S. Alkan, and G. S. Hayward. 1999. High-level variability in the ORF-K1 membrane protein gene at the left end of the Kaposi's sarcoma-associated herpesvirus genome defines four major subtypes and multiple variants or clades in different human populations. J. Virol. 73:4156-4170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zweygberg, L., B. Wirgart, M. Brytting, A. Linde, B. Wahren, and L. Grillner. 1998. Sequence variation within three important cytomegalovirus gene regions in isolates from four different patient populations. J. Clin. Microbiol. 36:3662-3669. [DOI] [PMC free article] [PubMed] [Google Scholar]