Herpesvirus particles consist of four morphologically distinct structures, the core, capsid, tegument, and envelope. The inner nucleoprotein core comprising the linear double-stranded DNA genome is included in an icosahedral (T=16) capsid shell of 150 hexons and 12 pentons. The capsid is surrounded by a layer of proteinaceous material designated the tegument which, in turn, is enclosed in an envelope of host cell-derived lipids containing virus-encoded (glyco)proteins. Whereas capsid formation in the nuclei of infected cells is understood in some detail, the mechanisms of tegumentation and envelopment and the intracellular compartments involved have long been disputed. This review focuses on recent findings that demonstrate a rather complex process of herpesvirus maturation including primary envelopment of capsids by budding at the inner leaflet of the nuclear membrane and translocation of capsids into the cytoplasm after loss of the primary envelope by fusion with the outer leaflet of the nuclear membrane. Subsequently, final tegumentation occurs in the cytoplasm and tegumented capsids obtain their final envelope by budding into vesicles of the trans-Golgi network. Tegumentation and envelopment are driven by specific protein-protein interactions that appear, at least in cultured cells, to exhibit a remarkable redundancy.
NUCLEAR EGRESS: PRIMARY ENVELOPMENT
During herpesvirus infection, viral transcription, DNA replication, formation of capsids (Fig. 1A and 2A), and packaging of viral DNA occur in the nucleus (32, 69, 72, 84). Subsequently, intranuclear capsids have to leave the nucleus to gain access to the extracellular environment. An early step in nuclear egress of herpesviruses is a budding process at the inner nuclear membrane whereby capsids acquire an envelope derived from the inner leaflet of the nuclear membrane (Fig. 1B and C and 2B and C). Two conserved herpesvirus proteins, the products of the UL31 and UL34 genes (gene designations in accordance with the herpes simplex virus type 1 [HSV-1] genomic sequence are used throughout to avoid confusion due to differences in protein and gene nomenclature among the viruses covered in this review), have recently been demonstrated to be involved in this process. The UL34 gene encodes a type II C-terminally anchored membrane protein (43, 63) that is located in pseudorabies virus (PrV)-infected cells in both leaflets of the nuclear membrane (43). The UL31 gene codes for a nuclear phosphoprotein (11) that is also present in the nuclear membrane of infected cells (30, 68). The UL31 protein requires UL34 for nuclear membrane targeting (30, 68), whereas the UL34 gene product appears to possess an intrinsic property for targeting to the nuclear membrane (43). However, nuclear membrane localization of the UL34 protein is augmented in the presence of the UL31 gene product (30, 68). A physical interaction between the two proteins has recently been demonstrated for the HSV-1 and PrV homologs by using glutathione S-transferase pulldown or yeast two-hybrid analysis (30, 68). These results indicate that complex formation between the UL31 and UL34 proteins is necessary for proper targeting of the UL31 protein and increases nuclear membrane localization of the UL34 protein. In addition, presence of the UL34 protein may augment the stability of the UL31 protein (98). Analysis of deletion mutants demonstrated that absence of either of the two proteins results in drastic impairment of primary envelopment, and capsids were essentially trapped in the nucleus (12, 30, 43, 68, 73). Thus, the UL31 and UL34 proteins are necessary for efficient egress of capsids from the nucleus (Fig. 3, step 1). In murine cytomegalovirus, the UL34 protein has been proposed to interact with protein kinase C which, in turn, should soften the nuclear lamina by phosphorylation (W. Muranyi, M. Wagner, G. Krohne, and U. H. Koszinowski, 26th Int. Herpesvirus Workshop, abstr. 8.06, 2001). A partial dismantling of the nuclear lamina has also been observed after HSV-1 infection (77) and may be a prerequisite for intranuclear capsids to gain access to the inner nuclear membrane. Interestingly, both the UL31 and UL34 proteins are components of primary enveloped perinuclear PrV virions but are absent from mature virions (see below). Thus, the UL34 protein may represent a primary envelope protein whereas the UL31 protein may constitute a primary tegument protein. It is reasonable to assume that the observed interaction between the two proteins plays a direct role in the budding process at the inner nuclear membrane. However, it is unclear whether additional primary envelope and tegument components exist and whether they are also required for primary envelopment, as has been suggested for the HSV-1 UL11 protein (3). In this context, it is notable that, according to electron microscopic examinations, the primary envelope and primary tegument in perinuclear virions clearly differ in ultrastructure from the final envelope and tegument in intracytoplasmic and extracellular virions (31, 34; Fig. 1 and 2) and that glycoproteins present in the mature envelope that are responsible for fusion during entry are not required for primary envelopment (34, 84).
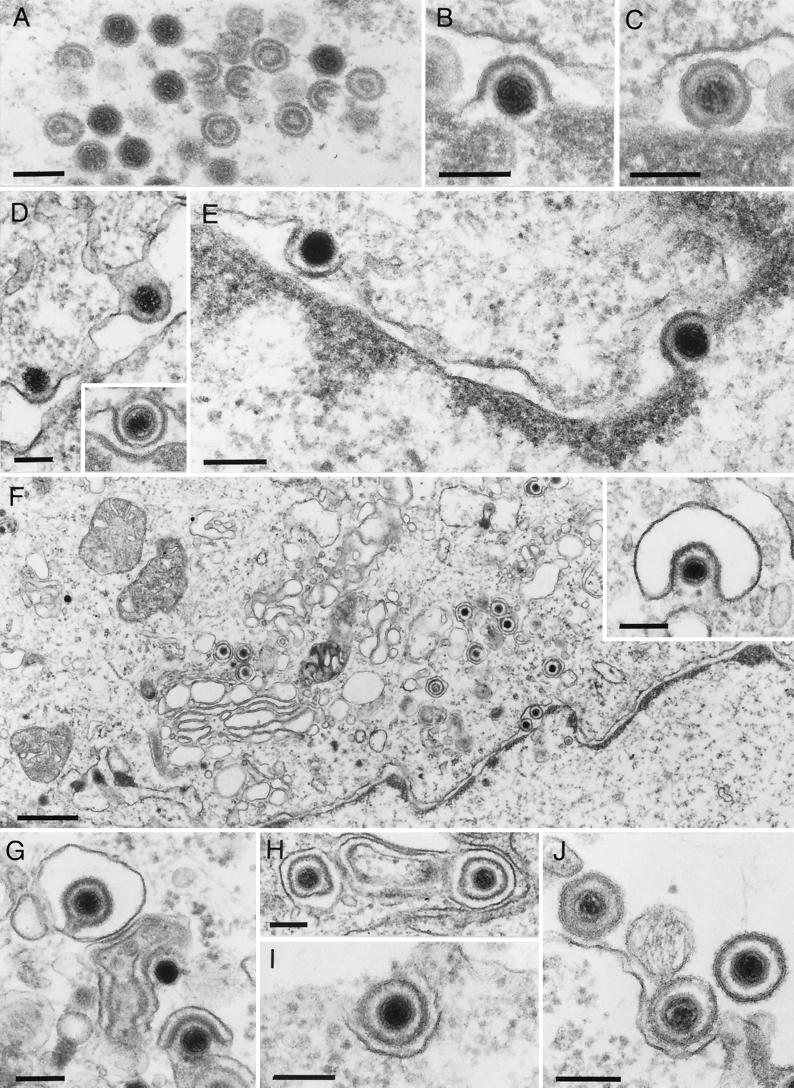
FIG. 1.
Assembly and egress of HSV-1. Electron microscopic illustration of intranuclear capsid assembly (A), budding of capsid at the inner nuclear membrane (B), perinuclear primary enveloped virion in the perinuclear space (C), and fusion of primary envelope with the outer nuclear membrane (D). In a rare picture, budding at the inner nuclear membrane and fusion at the outer nuclear membrane of HSV-1 capsids has been observed in the same section (E; taken, with permission, from reference 34). (F) Overview of HSV-1 morphogenesis events in the cytoplasm. Secondary envelopment of intracytoplasmic capsids by budding into vesicles (F inset, G) results in complete enveloped virions inside exocytic vesicles (H). Fusion of the vesicle membrane with the plasma membrane then leads to release of mature virions (I and J). Bars, 150 nm in panels A to E, panel F inset, and panels G and H and 750 nm in panel F.
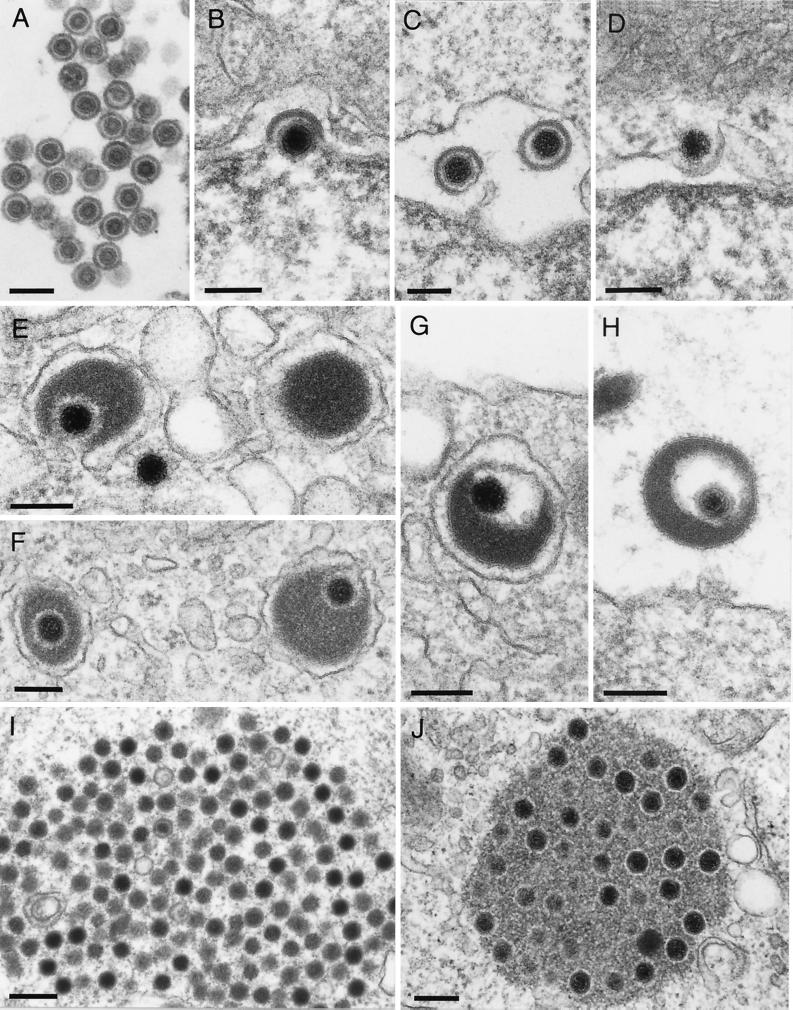
FIG. 2.
Assembly and egress of ILTV and mutant PrV. Intranuclear assembly (A), formation of perinuclear virions (B and C), and fusion with the outer nuclear membrane (D) do not differ morphologically from those of the other alphaherpesviruses examined (34). However, large amounts of tegument material may be incorporated into virions (G and H), which is clearly observed to occur in the cytoplasm only (E to F; see also reference 34). (G) An enveloped ILTV virion inside a vesicle. (H) An extracellular released virion. In panel E on the right, a capsidless L particle is demonstrated. (I) Intracytoplasmic PrV capsid accumulations without apparent tegument in the absence of the UL37 protein (45). (J) Accumulations of tegumented PrV capsids in the absence of gE/gI and gM (6). Bars, 150 nm in panels A to H and 250 nm in panels I and J.
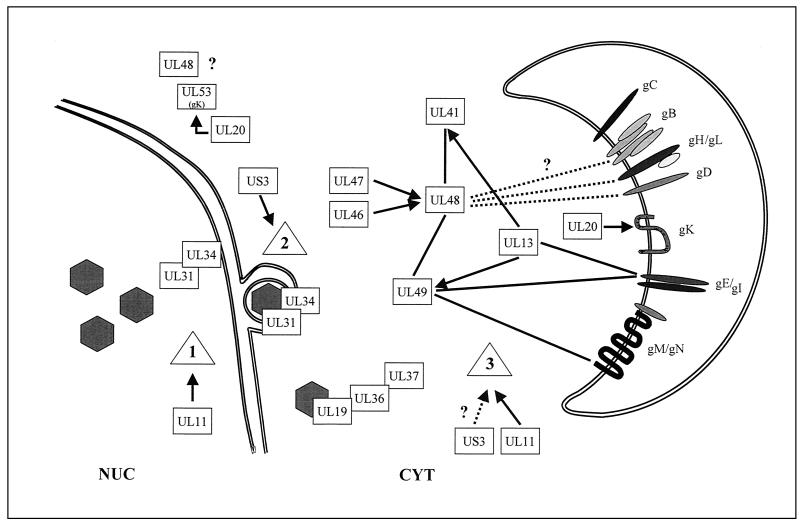
FIG. 3.
Diagram of the interactions of gene products of HSV-1 and PrV involved in virion formation. Solid lines or direct contacts between the rectangles representing the designated gene products indicate physical interaction, whereas arrows indicate functional effects. Dotted lines and question marks denote suggested, but not firmly established, interactions or involvement of proteins. Between glycoproteins, only direct contacts resulting in complex formation are depicted. Steps: 1, primary envelopment; 2, de-envelopment; 3, secondary (final) envelopment. NUC, nucleus; CYT, cytoplasm.
EGRESS FROM THE PERINUCLEAR SPACE: DE-ENVELOPMENT
The subsequent steps in herpesvirus maturation have been disputed for some time. One model, originally proposed for HSV-1, suggested that perinuclear virions retain their integrity and leave the cell via the secretory pathway (10, 17, 38, 89). During this transit, viral envelope glycoproteins are modified in situ. In this model, perinuclear virions already contain the full complement of tegument and envelope proteins characteristic of mature extracellular virions. An alternative model proposed that the primary envelope fuses with the outer leaflet of the nuclear membrane (or the endoplasmic reticulum [ER] membrane with which it is contiguous), resulting in loss of the primary envelope and, presumably, the primary tegument and translocation of capsids into the cytoplasm (reviewed in references 25 and 51; Fig. 3, step 2). Final tegumentation and envelopment then occur in cytoplasmic compartments. In this model, perinuclear and intracytoplasmic/extracellular enveloped virions may differ in composition. Cytoplasmic envelopment of herpesvirus virions was first suggested by Siminoff and Menefee (78) as a regular feature in HSV morphogenesis, whereas Stackpole (83) presented electron microscopic evidence for de-envelopment at the outer nuclear membrane and final envelopment in the cytoplasm during morphogenesis of frog herpesvirus. Data from researchers working on human cytomegalovirus (HCMV), human herpesvirus 6, PrV, and varicella-zoster virus (VZV) (31, 33, 41, 65, 71, 74, 94) supported the de-envelopment/re-envelopment model, which has recently also gained credence for HSV-1 based on results using genetically manipulated HSV-1 glycoproteins that are specifically targeted to different intracellular compartments (9, 79, 95). Electron microscopic analyses indeed demonstrated fusion of primary enveloped virions with the outer nuclear membrane in several alphaherpesviruses, including HSV-1 (33, 34; Fig. 1D and E), as well as budding of intracytoplasmic capsids into cytoplasmic vesicles (Fig. 1F and G). This issue was difficult to resolve since perinuclear virions cannot be purified to homogeneity and therefore are not easily amenable to biochemical analysis. However, recent results from different laboratories using immunoelectron microscopy yielded proof for a difference in composition between perinuclear and mature virions. Whereas the former contain the UL31 and UL34 gene products, as well as a modified form of the envelope glycoprotein D (gD) that was engineered to contain an ER retention signal (79), the UL31 and UL34 proteins, as well as the ER-retained gD are absent from intracytoplasmic or extracellular enveloped virions. In contrast, the major tegument proteins UL49 (43) and UL46 (M. Kopp et al., unpublished data) are present in intracytoplasmic and extracellular but absent from perinuclear enveloped virions. Moreover, the phospholipid composition differs between the envelope of extracellular HSV-1 virions and the host cell nuclear membrane (91). This striking difference in biochemical composition can only be explained by the two-step envelopment model. Studies of a different animal alphaherpesvirus, infectious laryngotracheitis virus (ILTV), support this pathway (Fig. 2) (34). Primary enveloped ILTV virions in the perinuclear space do not differ in morphology from other primary enveloped alphaherpesvirus virions (Fig. 2C and D). However, mature ILTV virions can contain enormous amounts of tegument (Fig. 2G and H) whose addition during budding into cytoplasmic vesicles can easily be observed by electron microscopy (Fig. 2E and F) (34). Thus, the two-step envelopment model is congruent with morphological and biochemical data and constitutes a unified model for morphogenesis of herpesviruses. The molecular details and the proteins involved in de-envelopment at the outer nuclear membrane are totally unclear. None of the known herpesvirus proteins that play essential roles in fusion of the virion envelope and the cellular plasma membrane during entry (81) is required for virus egress (34, 84). These observations demonstrate that fusion during entry and fusion during egress may be fundamentally different.
In several viral mutants, accumulation of perinuclear enveloped virions has been observed, which may indicate involvement of the corresponding gene products in subsequent steps after primary envelopment. These include mutations in PrV glycoprotein B (gB; 58); the HSV-1 UL53 gene product, glycoprotein K (gK; 26); HSV-1 membrane protein UL20 (2); HSV-1 tegument protein UL48 (52; see also below); and the product of the PrV US3 gene (44, 92). The findings on PrV gB could not be reproduced (34), and a role for UL48 in nuclear egress remains to be established unequivocally (52; Fig. 3, step 2). Interestingly, expression of the PrV UL20 protein is required for proper processing of PrV gK (21; see also below), which may be relevant in this context. However, only the involvement of the US3 protein, which possesses protein kinase activity, has been analyzed in more detail. The HSV-1 US3 kinase has been proposed to phosphorylate the UL34 protein, thereby modulating UL34 function (63). Interestingly, the PrV UL34 protein is phosphorylated to similar extents in the presence or absence of the US3 protein, indicating that the observed phenotype of US3 deletion mutants is not due to a difference in UL34 phosphorylation (44). However, PrV and HSV-1 US3 proteins are involved in efficient targeting of the UL34 protein to the nuclear membrane (44, 68). Thus, presence of the US3 protein increases perinuclear localization of the UL34 protein and a high density of UL34 in the nuclear membrane may be favorable for subsequent steps in virion egress (44). It is noteworthy that UL31 and UL34 homologs are present in all herpesvirus subfamilies, indicating that they may be central to the primary envelopment process, whereas only alphaherpesviruses specify US3 homologs. Correlating with this assumption, deletion of UL31 or UL34 in PrV or HSV-1 leads to drastic impairment of viral replication (12, 30, 43, 73), whereas deletion of US3 has, at best, only moderate effects on viral replication (44, 92). It is notable that the US3 protein is also part of the tegument of mature virions (Table 1) and may, therefore, also participate in virion formation in the cytoplasm (see below; Fig. 3, step 3).
TABLE 1.
HSV-1 virion componentsa
| Capsid | Tegument | Envelope |
|---|---|---|
| UL18 (VP23) | UL4 (?) | UL1 (gL) |
| UL19 (VP5) | UL11 | UL10 (gM) |
| UL35 (VP26) | UL13 (PK; VP18.8) | UL20 (?) |
| UL38 (VP19c) | UL14 | UL22 (gH) |
| UL21 | UL27 (gB) | |
| UL6 | UL36 (VP1/2) | UL43 (?) |
| UL25 | UL37 | UL44 (gC) |
| UL41 (vhs) | UL45 | |
| UL46 (VP11/12) | UL49.5 (gN) | |
| UL47 (VP13/14) | UL53 (gK) | |
| UL48 (VP16, α-TIF) | US4 (gG) | |
| UL49 (VP22) | US5 (gJ) (?) | |
| UL51 | US6 (gD) | |
| UL56 (?) | US7 (gI) | |
| ICP0 | US8 (gE) | |
| ICP4 | US9 | |
| US3 (PK) | ||
| US10 | ||
| US11 |
Protein designations as established for HSV-1. (?), sugggested but not firmly established; PK, protein kinase; vhs, viral host cell shutoff; α-TIF, alpha transinducing factor.
TEGUMENTATION IN THE CYTOPLASM
Whereas the complexity of the herpesvirus capsid (Table 1) is not principally different from that of other icosahedral capsids, such as, e.g., the picornavirus capsid (64), the complexity of the herpesvirus tegument, which may be considered equivalent to the matrix of other viruses, is drastically increased. More than 15 proteins have been demonstrated or suggested to be part of the HSV-1 tegument, as outlined in Table 1. Even more (or different) proteins may be found in the tegument of VZV (82) or cytomegalovirus (32). How is this complex structure assembled? Recent data indicate that tegumentation follows an intricate network of protein-protein interactions with significant built-in redundancy, at least in cultured cells. The data also show that the herpesvirus tegument fulfills the roles of other viral matrix proteins by interacting with the capsid on one side and the cytoplasmic tails of envelope glycoproteins on the other side to link these structural components for the final envelopment process and to secure the integrity of the virus particle (Fig. 3, step 3). The herpesvirus tegument has for a long time been considered unstructured. However, cryoelectron microscopic analyses showed that at least the innermost part of the tegument that is located adjacent to the capsid may also exhibit icosahedral symmetry (101). This is due to the interaction of a large tegument protein, presumably the UL36 gene product, the largest herpesvirus protein of more than 2,000 amino acids, with the pentons of the capsid. The UL36 protein has indeed been shown to physically interact with the major capsid protein (50), the product of the UL19 gene, which forms both pentons and hexons (53). Thus, it appears that the first layer of tegument around the capsid is icosahedrally structured and composed of the UL36 protein. We recently showed that in the absence of another tegument protein, the UL37 gene product, characteristic intracytoplasmic aggregates of capsids were formed (Fig. 2I) (45). These capsids did not contact each other directly but seemed to interact by way of proteinaceous protrusions from the vertices. We hypothesized that these interactions are mediated by UL36 proteins emanating from the capsid shells and that the UL37 protein would normally cover these sites. In fact, in coimmunoprecipitation assays, as well as in yeast two-hybrid analyses, the PrV UL37 protein has been shown to physically interact with the UL36 protein (B. G. Klupp, W. Fuchs, H. Granzow, R. Nixdorf, and T. C. Mettenleiter, submitted for publication) and the capsids that accumulate in the absence of the UL37 protein were shown to carry the UL36 protein. Therefore, we propose that the second layer of tegument is composed of the UL37 gene product (Fig. 3). It is interesting that the UL36 and UL37 gene products are the only tegument proteins that appear to be conserved, at least to a certain extent, in all herpesvirus subfamilies and the homologous HCMV gene products have also been proposed to interact (M. E. Harmon and W. Gibson, Proc. Am. Soc. Virol., abstr. W35-4, 1996). Correlating with this conservation, absence of the HSV-1 UL36 and UL37 proteins (18, 19) or the PrV UL37 protein (45) drastically inhibits or abolishes virus maturation. However, from studies of HCMV and simian cytomegalovirus, it has been suggested that additional tegument-capsid interactions may occur (13, 90). The basic phosphoprotein pUL32, a major tegument protein of HCMV, has been shown to bind to HCMV capsids in vitro (5). Since this protein is only conserved in the betaherpesviruses, there may be additional subfamily- or even species-specific interactions. This may also apply to the following steps in tegumentation, which primarily involve proteins that are not conserved between herpesvirus subfamilies.
Unfortunately, these subsequent steps in tegumentation are still largely undefined. Virus morphogenesis has been shown to proceed even in the absence of several other tegument proteins, such as UL13, US3 (62), UL41, UL46, UL47 (reviewed in reference 72), and UL49 (61; G. Elliott and A. Whiteley, 26th Int. Herpesvirus Workshop, abstr. 8.09, 2001). So far, the most dramatic effect on virion formation has been produced by deletion of UL48. The UL48 gene encodes a protein that has primarily been characterized for its transactivating activity on immediate-early gene promoters and has been designated alpha trans-inducing factor (4). However, besides this role in transcriptional regulation, the UL48 protein constitutes a major component of the HSV-1 tegument (36) and its absence interferes with a step in virion formation downstream from primary envelopment, presumably by impairing virus assembly in the cytoplasm (52; Fig. 3, step 3). This indicates that UL48 may constitute a protein whose presence is critical for tegument formation. Interestingly, the HSV-1 UL48 protein interacts with other tegument components, e.g., the UL49 protein (23) and the UL41 gene product, the virion host cell shutoff factor vhs (80). A mutant form of UL41 that does not bind UL48 is not incorporated into HSV-1 virions, demonstrating the importance of the UL41-UL48 interaction for inclusion of UL41 into the nascent virus particle (66). Moreover, the influence of the UL46 and UL47 tegument proteins on UL48 activity (100) may be indicative of a physical interaction, although this has not been demonstrated. It has also been suggested that UL48 may interact directly or indirectly with viral gB, gD, and glycoprotein H (102), although these cross-linking data need to be interpreted with caution. However, taken together, the data suggest an important structural role for the UL48 protein in tegumentation.
FINAL ENVELOPMENT: THE ROLE OF “NONESSENTIAL” GLYCOPROTEINS
Although the molecular basis for secondary envelopment, i.e., the combination of capsid, tegument, and the final envelope, is not clear, analysis of mutants with mutations in so-called nonessential glycoproteins has shed new light on the requirements for the final envelopment process. It had been observed that deletion of glycoproteins E and I (gE/I), which are conserved in the alphaherpesviruses and form a physical complex (reviewed in reference 25) may impair plaque formation of HSV-1 and PrV to a certain extent, as did deletion of glycoprotein M (gM), which complexes with glycoprotein N. The latter two are conserved in members of the family Herpesviridae. However, neither gE/I nor gM is required for productive replication of either HSV-1 or PrV. Surprisingly, simultaneous deletion of gE/I and gM drastically inhibits plaque formation and replication of PrV (6). Ultrastructural analyses showed that in the absence of gE/I and gM, intracytoplasmic aggregates were formed that consisted of capsids surrounded by electron-dense proteinaceous material (Fig. 2J). This material was labeled with anti-tegument protein antibodies (7). Apparently, in the absence of gE/I and gM, tegument formation around capsids still occurred but envelopment was blocked, indicating a prominent role of gE/I and gM in the envelopment process. A similar phenotype was also observed when only the gE cytoplasmic tail was deleted in addition to gM, leaving the remainder of the gE/I complex intact (7). Reversion analysis proved that it was indeed the gE cytoplasmic tail that was relevant for this phenotype since a mutant virus expressing a hybrid protein in which the gE tail had been fused behind the transmembrane region of gD had reverted to the level of a gM-only deletion mutant (7). During re-envelopment, tegumented capsids bud into trans-Golgi vesicles and the orientation of glycoproteins in the vesicle membrane is such that the cytoplasmic tails may make contact with tegument proteins for driving the final budding process. Yeast two-hybrid analysis showed that the gE cytoplasmic tail, indeed, interacts with the UL49 tegument protein and vice versa (W. Fuchs et al., unpublished data; L. W. Enquist, personal communication), and the gM cytoplasmic tail also interacts with the UL49 tegument protein (Fuchs et al., unpublished data). Therefore, in the proposed egress pathway, the cytoplasmic tails of both gE and gM are suggested to interact with UL49 and, due to this redundancy, a prominent effect can only be observed in the simultaneous absence of both interacting glycoproteins. Moreover, in VZV, gI has also been proposed to interact with tegument structures via its C-terminal domain, although this interaction has not been characterized in any detail and the putative interacting tegument protein has not been identified (93). However, there is one caveat. The UL49 tegument protein is not required for virion formation in either HSV-1 (Elliott and Whiteley, 26th Int. Herpesvirus Workshop) or PrV (Fuchs et al., unpublished data; Enquist, personal communication), which indicates that there have to be additional redundancies also on the part of the tegument proteins. Interestingly, the UL49 protein as well as gE and gI seem to be essential for replication of Marek's disease virus, an alphaherpesvirus that, like VZV, is highly cell associated (22, 76). Moreover, gE and gI may also be essential for VZV replication, at least in certain cells (14, 47). Whether this correlates with an essential role of these proteins in envelopment or may reflect other functions is unclear. In summary, molecular interactions between the capsid and UL36, and the UL36 and UL37 proteins start tegumentation at the capsid and interaction between UL49 and gE/I and gM, besides other, hitherto unknown, virion components is likely involved in driving envelopment. It is interesting in this context that in the absence of the UL37 protein, the UL49 gene product can be detected by immunogold labeling at intracytoplasmic membranes but not in the capsid aggregates (45).
Interestingly, gE, in particular the gE cytoplasmic domain, has been implicated in directional cell-to-cell spread of HSV-1 and PrV, both in cultured polarized cells and in neurons (25, 49, 85). This is correlated with a preferential association of the gE/gI complex at sites of cell-cell contact, and it has been speculated that the gE/gI complex may bind a hitherto unknown cellular receptor located in these contact areas (39). Although this scenario is completely speculative, the above data may fit into this hypothesis in that gE/gI directs tegumented capsids to the budding site. Specific presence of gE/gI at certain intracellular membranes, possibly including the plasma membrane in areas that are in close contact with the plasma membrane of an adjacent cell, would then result in directional budding of virions into the intercellular space, thereby securing directional transfer to the neighboring cell (40).
Endocytosis from the plasma membrane has been shown for several herpesvirus glycoproteins, including, e.g., gB and gE. It has been proposed that endocytosis helps to locate viral glycoproteins in a trans-Golgi vesicular compartment for envelopment (65). However, analysis of mutant glycoproteins deficient in endocytosis showed that endocytosis is not required for virion incorporation of PrV gB (55) or gE (86) and therefore is not required for inclusion of viral glycoproteins in the final envelope (8).
ADDITIONAL PROTEINS AFFECTING VIRION MORPHOGENESIS
A number of other proteins have also been shown to play a role in herpesvirus assembly and egress. The HSV-1 UL20 membrane protein has been demonstrated to interfere with the integrity of the Golgi apparatus late in infection in a cell type-specific manner (1). In the absence of the PrV UL20 protein, enveloped virions accumulated in huge intracytoplasmic vacuoles, indicating either fusion of transport vesicles or a high number of budding processes into a limited number of trans-Golgi vesicles (29). Moreover, the PrV UL20 protein is required for proper processing of the gK envelope protein (21), which may indicate a physical interaction between these two transmembrane proteins. Whether the effects of UL20 on the secretory pathway during HSV-1 infection and on virion egress and gK processing in PrV are related is unclear. It also remains to be shown whether the observed accumulation of perinuclear virions in the absence of HSV-1 UL20 (2; see above) and the effects the protein has on cytoplasmic events are related.
An additional open reading frame, UL3.5, which is absent in HSV-1, has been described in several alphaherpesviruses. In the absence of the UL3.5 protein, tegumentation and envelopment of intracytoplasmic PrV capsids are blocked (27), a defect which can be compensated by the homologous bovine herpesvirus 1 (BHV-1) protein (28). Surprisingly, the PrV protein has not been detected in purified virions, whereas the BHV-1 protein is a virion constituent (75) and interacts with the BHV-1 UL48 protein (46). The exact role the UL3.5 protein plays in virion morphogenesis is therefore unclear. However, since HSV-1 matures without this protein, the observed effects may reflect species-specific requirements for virion formation. Species-specific differences may also account for the different relative contributions of proteins to the tegument. In VZV, for example, the IE62 protein, which is homologous to immediate-early protein ICP4 of HSV-1, represents a major component of the tegument with affinity for the UL49 homolog (82), whereas, like the ICP0 homolog (97), it represents a minor component in HSV-1 (96).
In summary, due to the multitude of proteins that are involved in the formation of a secondary tegument and envelope, as well as their sometimes apparent redundancy, the contributions of individual proteins to the overall process are difficult to assess in most cases, and it requires the study of mutants with multiple genes deleted to gain a more detailed insight into the complex network-like interactions. A schematic representation of several of the known and suggested protein-protein interactions during virus egress is shown in Fig. 3. In Fig. 4, the described egress pathway is schematically depicted.
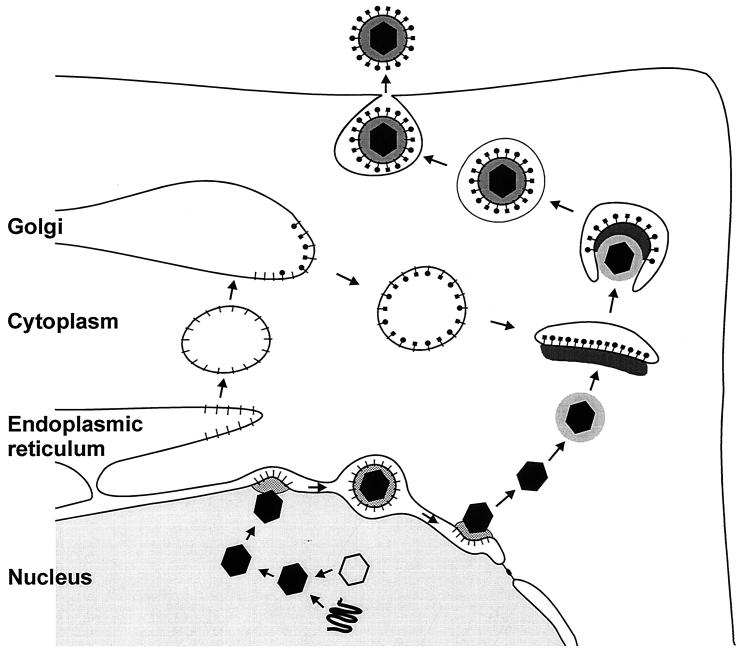
FIG. 4.
Summary diagram of the proposed pathway of herpesvirus egress.
ROLE OF PHOSPHORYLATION IN TEGUMENTATION
Intracellular distribution of tegument proteins has been analyzed primarily by immunofluorescence and confocal laser scanning microscopy with the aim to pinpoint the subcellular compartment in which tegumentation takes place by assessing the localization of tegument proteins. However, these analyses have been hampered by the complexity, and sometimes discrepancy, of the experimental findings. Different intracellular localizations may reflect different functions of tegument proteins, e.g., the transactivating function of UL48 in the nucleus and its structural function as a tegument protein in the cytoplasm. They may also vary at different times after infection (24, 60). Moreover, homologous proteins may differ in intracellular localization in different herpesviruses (35). It is interesting in this context that many tegument proteins are phosphorylated. Moreover, two virus-encoded protein kinases, the product of the UL13 gene, which is conserved in all herpesviruses, and the product of the US3 gene, which is present only in alphaherpesviruses, have been shown to represent virion components, presumably located in the tegument (16, 42, 56, 62, 99). The HSV-1 UL13 protein complexes with and phosphorylates gE (54), phosphorylates UL49 (15), and modulates UL41 function (57). Therefore, protein phosphorylation may play an important role in determining tegument assembly and/or the function of tegument proteins. In support of this hypothesis, incorporation of tegument proteins, e.g., the BHV-1 UL49 homolog (67) and the VZV ICP4 homolog IE62 (42), into virus particles has been shown to be modulated by phosphorylation. Interestingly, virus replication proceeds with only moderately reduced efficiency in the absence of either US3 or UL13 (20, 44, 57, 62), but simultaneous deletion of both results in a severe growth defect, at least in PrV (20).
FORMATION OF L PARTICLES
The presence of capsids in the cytoplasm is not essential for tegument assembly and subsequent envelopment. The occurrence and biochemical composition of extracellular herpesvirus light (L) particles that lack capsids, consist only of tegument and envelope, and are formed independently of normal virus maturation (70; Fig. 2E) have been most thoroughly analyzed in HCMV and HSV-1 (37, 48). These particles appear to contain a bona fide tegument with all known constituents, as well as an authentic envelope with the appropriate glycoprotein content. Our studies of PrV indicate that L particles continue to be formed in the absence of tegument proteins that are important for tegumentation of capsids, i.e., UL36 and UL37. Interestingly, a virus mutant that does not exhibit L-particle formation is the gE/I/M triple mutant, which is drastically impaired in envelopment but is still able to assemble tegument proteins around capsids (6, 7; Fig. 2J). Thus, the interaction between tegument protein(s) and specific envelope glycoproteins is required for envelopment independent of the presence of capsids. It is suggested, as outlined in Fig. 3 (step 3) and 4, that normally tegumentation may be nucleated on the capsid via UL36-UL37 and at the future budding site by interaction of UL49 with gM and/or gE/I. In the absence of capsids, as in L-particle formation, tegument assembly may proceed anchored on UL49, which itself contacts the glycoproteins. In the reverse situation, i.e., in the absence of the tegument-envelope glycoprotein interaction, such as in the gE/I/M triple mutant, tegument assembly only occurs on the capsid starting with the UL36-UL37 interaction. Either way seems to be possible, and both ways may cooperate during morphogenesis of wild-type virus.
WHAT IS ALL THIS GOOD FOR?
In order to find a biological explanation for this highly complex process of herpesvirus assembly, it is necessary to evaluate the natural situation. In particular, this applies to the neurotropic alphaherpesviruses. These viruses infect nerve endings in the periphery and are transported to the cell body, where transcription, DNA replication, and capsid assembly occur. For transneuronal spread, the virus then has to be transported to axon termini, cross the synapse, and infect the transsynaptic neuron. The process will then continue (reviewed in reference 25). As originally proposed for HSV-1 (59) and recently supported by studies on PrV (87), viral subassemblies, i.e., capsids on the one hand and envelope proteins on the other hand, are transported separately in the axon and virus assembly only occurs at the axon terminus. In military terms, the explosive hardware is assembled at the site where it is needed, i.e., the axon terminus. Two proteins, US9 of PrV (88) and HSV-1 US11 (R. J. Diefenbach, E. Szabados, M. Miranda, P. Armatt, and A. L. Cunningham, Abstr. 24th Int. Herpesvirus Workshop, abstr. 7.001, 1999), have been demonstrated to play a role in these distinct transport processes. In this scenario, the two-step envelopment model is required for safe transport of innocuous subassemblies over long distances. Moreover, the multitude of protein-protein interactions may serve to fine-tune the maturation process in accordance with the given circumstances (e.g., the host cell environment). However, to substantiate these theoretical considerations, a number of mutant viruses have to be tested for neuroinvasion capacity beyond their phenotype in a normal cell culture. It is notable that US9 is conserved in the alphaherpesviruses, whereas US11, which has been proposed to interact with the UL48 protein (Diefenbach et al., Abstr. 24th Int. Herpesvirus Workshop), is not.
In Fig. 3, several of the reported interactions between different viral proteins that play a role in egress of HSV-1 and PrV (outlined in Fig. 4) are summarized. They may not all be equally valid, and the picture is certainly incomplete but it gives an indication of the complexity of the molecular events during herpesvirus morphogenesis and egress. Although we are far from understanding all of the molecular details that lead to assembly of an infectious herpesvirus virion, the described results lay the basis for more thorough studies and imply a number of easily testable predictions. This should ultimately lead to a more complete picture of how a herpesvirus virion is formed.
Acknowledgments
I thank Harald Granzow for generously providing the electron micrographs in Fig. 1 and 2, B. G. Klupp and W. Fuchs for help in preparation of the diagrams, and N. Osterrieder and B. G. Klupp for critical reading of the manuscript. I am particularly indebted to A. Minson, Cambridge, United Kingdom, for valuable comments on the manuscript.
Work done in my laboratory was supported by the Deutsche Forschungsgemeinschaft (Me 854/5-1).
REFERENCES
- 1.Avitabile, E., P. Ward, C. Di Lazzaro, M. Torrisi, B. Roizman, and G. Campadelli-Fiume. 1994. The herpes simplex virus UL20 protein compensates for the differential disruption of exocytosis of virions and viral membrane glycoproteins associated with fragmentation of the Golgi apparatus. J. Virol. 68:7397-7405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baines, J. D., P. L. Ward, G. Campadelli-Fiume, and B. Roizman. 1991. The UL20 gene of herpes simplex virus 1 encodes a function necessary for viral egress. J. Virol. 65:6414-6424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baines, J. D., and B. Roizman. 1992. The UL11 gene of herpes simplex virus 1 encodes a function that facilitates nucleocapsid envelopment and egress from cells. J. Virol. 66:5168-5174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Batterson, W., and B. Roizman. 1983. Characterization of the herpes simplex virion-associated factor responsible for the induction of α genes. J. Virol. 46:371-377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baxter, M. K., and W. Gibson. 2001. Cytomegalovirus basic phosphoprotein (pUL32) binds to capsids in vitro through its amino one-third. J. Virol. 75:6865-6873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brack, A. R., J. Dijkstra, H. Granzow, B. G. Klupp, and T. C. Mettenleiter. 1999. Inhibition of virion maturation by simultaneous deletion of glycoproteins E, I, and M of pseudorabies virus. J. Virol. 73:5364-5372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brack, A. R., B. G. Klupp, H. Granzow, R. Tirabassi, L. W. Enquist, and T. C. Mettenleiter. 2000. Role of the cytoplasmic tail of pseudorabies virus glycoprotein E in virion formation. J. Virol. 74:4004-4016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brideau, A., L. W. Enquist, and R. S. Tirabassi. 2000. The role of virion membrane protein endocytosis in the herpesvirus life cycle. J. Clin. Virol. 17:69-82. [DOI] [PubMed] [Google Scholar]
- 9.Browne, H., S. Bell, T. Minson, and D. W. Wilson. 1996. An endoplasmic reticulum-retained herpes simplex virus glycoprotein H is absent from secreted virions: evidence for reenvelopment during egress. J. Virol. 70:4311-4316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Campadelli-Fiume, G., F. Farabegoli, S. Di Gaeta, and B. Roizman. 1991. Origin of unenveloped capsids in the cytoplasm of cells infected with herpes simplex virus 1. J. Virol. 65:1589-1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang, Y. E., and B. Roizman. 1993. The product of the UL31 gene of herpes simplex virus 1 is a nuclear phosphoprotein, which partitions with the nuclear matrix. J. Virol. 67:6348-6356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang, Y. E., C. Van Sant, P. W. Krug, A. E. Sears, and B. Roizman. 1997. The null mutant of the UL31 gene of herpes simplex virus 1: construction and phenotype in infected cells. J. Virol. 71:8307-8315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen, D., H. Jiang, M. Lee, F. Liu, and Z. H. Zhou. 1999. Three-dimensional visualization of tegument/capsid interactions in the intact human cytomegalovirus. Virology 260:10-16. [DOI] [PubMed] [Google Scholar]
- 14.Cohen, J., and H. Nguyen. 1997. Varicella-zoster virus glycoprotein I is essential for growth of virus in Vero cells. J. Virol. 71:6913-6920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coulter, L., H. Moss, J. Lang, and D. J. McGeoch. 1993. A mutant of herpes simplex virus type 1 in which the UL13 protein kinase gene is disrupted. J. Gen. Virol. 74:387-395. [DOI] [PubMed] [Google Scholar]
- 16.Cunningham, C., A. J. Davison, A. Dolan, M. C. Frame, D. J. McGeoch, D. M. Meredith, H. W. M. Moss, and A. C. Orr. 1992. The UL13 virion protein of herpes simplex virus type 1 is phosphorylated by a novel virus-induced protein kinase. J. Gen. Virol. 73:303-311. [DOI] [PubMed] [Google Scholar]
- 17.Darlington, R., and L. Moss. 1968. Herpesvirus envelopment. J. Virol. 2:48-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Desai, P. 2000. A null mutation in the UL36 gene of herpes simplex virus type 1 results in accumulation of unenveloped DNA-filled capsids in the cytoplasm of infected cells. J. Virol. 74:11608-11618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Desai, P., G. Sexton, J. McCaffery, and S. Person. 2001. A null mutation in the gene encoding the UL37 polypeptide of herpes simplex virus type 1 abrogates virus maturation. J. Virol. 75:10259-10271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Wind, N., J. Domen, and A. Berns. 1992. Herpesviruses encode an unusual protein-serine/threonine kinase which is nonessential for growth in cultured cells. J. Virol. 66:5200-5209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dietz, P., B. G. Klupp, W. Fuchs, B. Köllner, E. Weiland, and T. C. Mettenleiter. 2000. Pseudorabies virus glycoprotein K requires the UL20 gene product for processing. J. Virol. 74:5083-5090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dorange, F., B. K. Tischer, J.-F. Vautherot, and N. Osterrieder. 2002. Characterization of Marek's disease virus serotype 1 (MDV-1) deletion mutants that lack UL46 to UL49 genes: MDV-1 UL49, encoding VP22, is indispensable for virus growth. J. Virol. 76:1959-1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Elliott, G., G. Mouzakitis, and P. O'Hare. 1995. VP16 interacts via its activation domain with VP22, a tegument protein of herpes simplex virus, and is relocated to a novel macromolecular assembly in coexpressing cells. J. Virol. 69:7932-7941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Elliott, G., and P. O'Hare. 2000. Cytoplasm-to-nucleus translocation of a herpesvirus tegument protein during cell division. J. Virol. 74:2131-2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Enquist, L. W., P. J. Husak, B. W. Banfield, and G. A. Smith. 1999. Infection and spread of alphaherpesviruses in the nervous system. Adv. Virus Res. 51:237-347. [DOI] [PubMed] [Google Scholar]
- 26.Foster, T. P., and G. Kousoulas. 1999. Genetic analysis of the role of herpes simplex virus type 1 glycoprotein K in infectious virus production and egress. J. Virol. 73:8457-8468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fuchs, W., B. G. Klupp, H. Granzow, H.-J. Rziha, and T. C. Mettenleiter. 1996. Identification and characterization of the pseudorabies virus UL3.5 protein, which is involved in virus egress. J. Virol. 70:3517-3527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fuchs, W., H. Granzow, and T. C. Mettenleiter. 1997. Functional complementation of UL3.5-negative pseudorabies virus by the bovine herpesvirus 1 UL3.5 homolog. J. Virol. 71:8886-8892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fuchs, W., B. G. Klupp, H. Granzow, and T. C. Mettenleiter. 1997. The UL20 gene product of pseudorabies virus functions in virus egress. J. Virol. 71:5639-5646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fuchs, W., B. G. Klupp, H. Granzow, N. Osterrieder, and T. C. Mettenleiter. 2002. The interacting UL31 and UL34 gene products of pseudorabies virus are involved in egress from the host-cell nucleus and represent components of primary enveloped but not of mature virions. J. Virol. 71:364-378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gershon, A. A., D. L. Sherman, Z. Zhu, C. A. Gabel, R. T. Ambron, and M. D. Gershon. 1994. Intracellular transport of newly synthesized varicella-zoster virus: final envelopment in the trans-Golgi network. J. Virol. 68:6372-6390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gibson, W. 1996. Structure and assembly of the virion. Intervirology 39:389-400. [DOI] [PubMed] [Google Scholar]
- 33.Granzow, H., F. Weiland, A. Jöns, B. G. Klupp, A. Karger, and T. C. Mettenleiter. 1997. Ultrastructural analysis of the replication cycle of pseudorabies virus in cell culture: a reassessment. J. Virol. 71:2072-2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Granzow, H., B. G. Klupp, W. Fuchs, J. Veits, N. Osterrieder, and T. C. Mettenleiter. 2001. Egress of alphaherpesviruses: comparative ultrastructural study. J. Virol. 75:3675-3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harms, J., X. Ren, S. Oliveira, and G. Splitter. 2000. Distinctions between bovine herpesvirus 1 and herpes simplex virus type 1 tegument protein subcellular associations. J. Virol. 74:3301-3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heine, J., R. W. Honess, E. Cassai, and B. Roizman. 1974. Proteins specified by herpes simplex virus. XII. The virion polypeptides of type 1 strains. J. Virol. 14:640-651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Irmiere, A., and W. Gibson. 1983. Isolation and characterization of a non-infectious virion-like particle released from cells infected with human strains of cytomegalovirus. Virology 130:118-133. [DOI] [PubMed] [Google Scholar]
- 38.Johnson, D. C., and P. G. Spear. 1982. Monensin inhibits the processing of herpes simplex virus glycoproteins, their transport to the cell surface, and the egress of virions from infected cells. J. Virol. 43:1102-1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Johnson, D. C., M. Webb, T. Wisner, and C. Brunetti. 2001. Herpes simplex virus gE/gI sorts nascent virions to epithelial cell junctions promoting virus spread. J. Virol. 75:821-833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Johnson, D. C., and M. T. Huber. 2002. Directed egress of animal viruses promotes cell-to-cell spread. J. Virol. 76:1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jones, F., and C. Grose. 1988. Role of cytoplasmic vacuoles in varicella-zoster virus glycoprotein trafficking and virion envelopment. J. Virol. 62:2701-2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kinchington, P., K. Fite, A. Seman, and S. Turse. 2001. Virion association of IE62, the varicella-zoster virus (VZV) major transcriptional regulatory protein, requires expression of the VZV open reading frame 66 protein kinase. J. Virol. 75:9106-9113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Klupp, B. G., H. Granzow, and T. C. Mettenleiter. 2000. Primary envelopment of pseudorabies virus at the nuclear membrane requires the UL34 gene product. J. Virol. 74:10063-10071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Klupp, B. G., H. Granzow, and T. C. Mettenleiter. 2001. Effect of the pseudorabies virus US3 protein on nuclear membrane localization of the UL34 protein and virus egress from the nucleus. J. Gen. Virol. 82:2363-2371. [DOI] [PubMed] [Google Scholar]
- 45.Klupp, B. G., H. Granzow, E. Mundt, and T. C. Mettenleiter. 2001. Pseudorabies virus UL37 gene product is involved in secondary envelopment. J. Virol. 75:8927-8936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lam, N., and G. Letchworth. 2000. Bovine herpesvirus 1 UL3.5 interacts with bovine herpesvirus 1 α-transinducing factor. J. Virol. 74:2876-2884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mallory, S., M. Sommer, and A. Arvin. 1997. Mutational analysis of the role of glycoprotein I in varicella-zoster virus replication and its effects on glycoprotein E conformation and trafficking. J. Virol. 71:8279-8288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McLauchlan, J., and F. J. Rixon. 1992. Characterization of enveloped tegument structures (L-particles) produced by alphaherpesviruses: integrity of the tegument does not depend on the presence of capsid or envelope. J. Gen. Virol. 73:269-276. [DOI] [PubMed] [Google Scholar]
- 49.McMillan, T., and D. C. Johnson. 2001. Cytoplasmic domain of herpes simplex virus gE causes accumulation in the trans-Golgi network, a site of virus envelopment and sorting of virions to cell junctions. J. Virol. 75:1928-1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McNabb, D., and R. Courtney. 1992. Characterization of the large tegument protein (ICP1/2) of herpes simplex virus type 1. Virology 190:221-232. [DOI] [PubMed] [Google Scholar]
- 51.Mettenleiter, T. C. 2000. Aujeszky's disease (pseudorabies) virus: the virus and molecular pathogenesis—state of the art, June 1999. Vet. Res. 31:99-115. [DOI] [PubMed] [Google Scholar]
- 52.Mossman, K., R. Sherburne, C. Lavery, J. Duncan, and J. Smiley. 2000. Evidence that herpes simplex virus VP16 is required for viral egress downstream of the initial envelopment event. J. Virol. 74:6287-6299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Newcomb, W. W., B. L. Trus, F. P. Booy, A. C. Steven, J. S. Wall, and J. C. Brown. 1993. Structure of the herpes simplex virus capsid: molecular composition of the pentons and triplexes. J. Mol. Biol. 232:499-511. [DOI] [PubMed] [Google Scholar]
- 54.Ng, T., W. Ogle, and B. Roizman. 1998. UL13 protein kinase of herpes simplex virus 1 complexes with glycoprotein E and mediates the phosphorylation of the viral Fc receptor: glycoproteins E and I. Virology 241:37-48. [DOI] [PubMed] [Google Scholar]
- 55.Nixdorf, R., B. G. Klupp, A. Karger, and T. C. Mettenleiter. 2000. Effects of truncation of the carboxy terminus of pseudorabies virus glycoprotein B on infectivity. J. Virol. 74:7137-7145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Overton, H., D. McMillan, L. Klavinskis, L. Hope, A. Ritchie, and P. Wong-Kai-In. 1992. Herpes simplex virus type 1 gene UL13 encodes a phosphoprotein that is a component of the virion. Virology 190:184-192. [DOI] [PubMed] [Google Scholar]
- 57.Overton, H., D. McMillan, L. Hope, and P. Wong-Kai-In. 1994. Production of host shutoff-defective mutants of herpes simplex virus type 1 by inactivation of the UL13 gene. Virology 202:97-106. [DOI] [PubMed] [Google Scholar]
- 58.Peeters, B., N. de Wind, M. Hooisma, F. Wagenaar, A. Gielkens, and R. Moormann. 1992. Pseudorabies virus envelope glycoproteins gp50 and gII are essential for virus penetration, but only gII is involved in membrane fusion. J. Virol. 66:894-905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Penfold, M. E., P. Armati, and A. L. Cunningham. 1994. Axonal transport of herpes simplex virions to epidermal cells: evidence for a specialized mode of virus transport and assembly. Proc. Natl. Acad. Sci. USA 91:6529-6533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pomeranz, L., and J. Blaho. 1999. Modified VP22 localizes to the cell nucleus during synchronized herpes simplex virus type 1 infection. J. Virol. 73:6769-6781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pomeranz, L., and J. Blaho. 2000. Assembly of infectious herpes simplex virus type 1 virions in the absence of full-length VP22. J. Virol. 74:10041-10054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Purves, F. C., R. M. Longnecker, D. P. Leader, and B. Roizman. 1987. Herpes simplex virus 1 protein kinase is encoded by open reading frame US3 which is not essential for virus growth in cell culture. J. Virol. 61:2896-2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Purves, F., D. Spector, and B. Roizman. 1992. UL34, the target of the herpes simplex virus Us3 protein kinase, is a membrane protein which in its unphosphorylated state associates with novel phosphoproteins. J. Virol. 66:4295-4303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Racaniello, V. 2001. Picornaviridae: the viruses and their replication, p. 2399-2460. In D. Knipe and P. M. Howley (ed.), Fields virology, 4th ed. Lippincott-Raven Publishers, Philadelphia, Pa.
- 65.Radsak, K., M. Eickmann, T. Mockenhaupt, E. Bogner, H. Kern, A. Eis-Hübinger, and M. Reschke. 1996. Retrieval of human cytomegalovirus glycoprotein B from infected cell surface for virus envelopment. Arch. Virol. 141:557-572. [DOI] [PubMed] [Google Scholar]
- 66.Read, G. S., B. M. Karr, and K. Knight. 1993. Isolation of a herpes simplex virus type 1 mutant with a deletion in the virion host shutoff gene and identification of multiple forms of the vhs (UL41) polypeptides. J. Virol. 67:7149-7160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ren, X., J. Harms, and G. A. Splitter. 2001. Tyrosine phosphorylation of bovine herpesvirus 1 tegument protein VP22 correlates with the incorporation of VP22 into virions. J. Virol. 75:9010-9017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Reynolds, A., B. Ryckman, J. Baines, Y. Zhou, L. Liang, and R. Roller. 2001. UL31 and UL34 proteins of herpes simplex virus type 1 form a complex that accumulates at the nuclear rim and is required for envelopment of nucleocapsids. J. Virol. 75:8803-8817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rixon, F. J. 1993. Structure and assembly of herpesviruses. Semin. Virol. 4:135-144. [Google Scholar]
- 70.Rixon, F. J., C. Addison, and J. McLauchlan. 1992. Assembly of enveloped tegument structures (L particles) can occur independently of virion maturation in herpes simplex virus type 1-infected cells. J. Gen. Virol. 73:277-284. [DOI] [PubMed] [Google Scholar]
- 71.Roffman, E., J. P. Albert, J. P. Goff, and N. Frenkel. 1990. Putative site for the acquisition of human herpesvirus 6 tegument. J. Virol. 64:6308-6313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Roizman, B., and D. Knipe. 2001. Herpes simplex viruses and their replication, p. 2399-2460. In D. Knipe and P. M. Howley (ed.), Fields virology, 4th ed. Lippincott-Raven Publishers, Philadelphia, Pa.
- 73.Roller, R., Y. Zhou, R. Schnetzer, J. Ferguson, and D. DeSalvo. 2000. Herpes simplex virus type 1 UL34 gene product is required for viral envelopment. J. Virol. 74:117-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sanchez, V., E. Sztul, and W. Britt. 2000. Accumulation of virion tegument and envelope proteins in a stable cytoplasmic compartment during human cytomegalovirus replication: characterization of a potential site of virus assembly. J. Virol. 74:975-986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schikora, B., Z. Lu, G. F. Kutish, D. Rock, G. Magyar, and G. J. Letchworth. 1998. The bovine herpesvirus type 1 UL3.5 open reading frame encodes a virion structural protein. Virology 240:76-82. [DOI] [PubMed] [Google Scholar]
- 76.Schumacher, D., B. K. Tischer, S. Reddy, and N. Osterrieder. 2001. Glycoproteins E and I of Marek's disease virus serotype 1 are essential for virus growth in cultured cells. J. Virol. 75:11307-11318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Scott, E. S., and P. O'Hare. 2001. Fate of the inner nuclear membrane protein lamin B receptor and nuclear lamins in herpes simplex virus type 1 infection. J. Virol. 75:8818-8830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Siminoff, P., and M. G. Menefee. 1966. Normal and 5-bromodeoxyuridine-inhibited development of herpes simplex virus. Exp. Cell Res. 44:241-255. [DOI] [PubMed] [Google Scholar]
- 79.Skepper, J., A. Whiteley, H. Browne, and A. Minson. 2001. Herpes simplex virus nucleocapsids mature to progeny virions by an envelopment-deenvelopment-reenvelopment pathway. J. Virol. 75:5697-5702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Smibert, C. A., B. Popova, P. Xiao, J. P. Capone, and J. R. Smiley. 1994. Herpes simplex virus VP16 forms a complex with the virion host shutoff protein vhs. J. Virol. 68:2339-2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Spear, P. G. 1993. Entry of alphaherpesviruses into cells. Semin. Virol. 4:167-180. [Google Scholar]
- 82.Spengler, M., N. Niesen, C. Grose, W. T. Ruyechan, and J. Hay. 2001. Interactions among structural proteins of varicella-zoster virus. Arch. Virol. 17(Suppl.):71-79. [DOI] [PubMed] [Google Scholar]
- 83.Stackpole, C. W. 1969. Herpes-type virus of the frog renal adenocarcinoma. I. Virus development in tumor transplants maintained at low temperature. J. Virol. 4:75-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Steven, A. C., and P. G. Spear. 1997. Herpesvirus capsid assembly and envelopment, p. 312-351. In W. Chiu, R. M. Burnett, and R. Garcea (ed.), Structural biology of viruses. Oxford University Press, New York, N.Y.
- 85.Tirabassi, R. S., R. A. Townley, M. G. Eldridge, and L. W. Enquist. 1997. Characterization of pseudorabies virus mutants expressing carboxy-terminal truncations of gE: evidence for envelope incorporation, virulence, and neurotropism domains. J. Virol. 71:6455-6464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tirabassi, R. S., and L. W. Enquist. 1998. Role of envelope glycoprotein gE endocytosis in the pseudorabies virus life cycle. J. Virol. 72:4571-4579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tomishima, M., G. A. Smith, and L. W. Enquist. 2001. Sorting and transport of alpha herpesviruses in axons. Traffic 2:429-436. [DOI] [PubMed] [Google Scholar]
- 88.Tomishima, M., and L. W. Enquist. 2001. A conserved α-herpesvirus protein necessary for axonal localization of viral membrane proteins. J. Cell Biol. 154:741-752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Torrisi, R. M., C. DiLazzaro, A. Pavan, L. Pereira, and G. Campadelli-Fiume. 1992. Herpes simplex virus envelopment and maturation studied by fracture label. J. Virol. 66:554-561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Trus, B., W. Gibson, N. Cheng, and A. C. Steven. 1999. Capsid structure of simian cytomegalovirus from cryoelectron microscopy: evidence for tegument attachment sites. J. Virol. 73:2181-2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Van Genderen, I. L., R. Brandimarti, M. Torrisi, G. Campadelli, and G. van Meer. 1994. The phospholipid composition of extracellular herpes simplex virions differs from that of the host cell nuclei. Virology 200:831-836. [DOI] [PubMed] [Google Scholar]
- 92.Wagenaar, F., J. M. Pol, B. Peeters, A. L. J. Gielkens, N. de Wind, and T. G. Kimman. 1995. The US3-encoded protein kinase from pseudorabies virus affects egress of virions from the nucleus. J. Gen. Virol. 76:1851-1859. [DOI] [PubMed] [Google Scholar]
- 93.Wang, Z.-H., M. Gershon, O. Lungu, Z. Zhu, S. Mallory, A. Arvin, and A. Gershon. 2001. Essential role played by the C-terminal domain of glycoprotein I in envelopment of varicella-zoster virus in the trans-Golgi network: interactions of glycoproteins with tegument. J. Virol. 75:323-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Whealy, M. E., J. P. Card, R. P. Meade, A. K. Robbins, and L. W. Enquist. 1991. Effect of brefeldin A on alphaherpesvirus membrane protein glycosylation and virus egress. J. Virol. 65:1066-1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Whiteley, A., B. Bruun, T. Minson, and H. Browne. 1999. Effects of targeting herpes simplex virus type 1 gD to the endoplasmic reticulum and trans-Golgi network. J. Virol. 73:9515-9520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yao, F., and R. J. Courtney. 1989. A major transcriptional regulatory protein (ICP4) of herpes simplex virus type 1 is associated with purified virions. J. Virol. 63:3338-3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yao, F., and R. Courtney. 1992. Association of ICP0 but not ICP27 with purified virions of herpes simplex virus type 1. J. Virol. 66:2709-2716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ye, G.-J., and B. Roizman. 2000. The essential protein encoded by the UL31 gene of herpes simplex virus 1 depends for its stability on the presence of the UL34 protein. Proc. Natl. Acad. Sci. USA 97:11002-11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhang, G., R. Stevens, and D. P. Leader. 1990. The protein kinase encoded in the short unique region of pseudorabies virus: description of the gene and identification of its product in virions and in infected cells. J. Gen. Virol. 71:1757-1765. [DOI] [PubMed] [Google Scholar]
- 100.Zhang, Y., D. A. Sirko, and J. L. C. McKnight. 1991. Role of herpes simplex virus type 1 UL46 and UL47 in αTIF-mediated transcriptional induction: characterization of three viral deletion mutants. J. Virol. 65:829-841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhou, Z., D. Chen, J. Jakana, F. J. Rixon, and W. Chiu. 1999. Visualization of tegument-capsid interactions and DNA in intact herpes simplex virus type 1 virions. J. Virol. 73:3210-3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhu, W., and R. Courtney. 1994. Chemical cross-linking of virion envelope and tegument proteins of herpes simplex virus type 1. Virology 204:590-599. [DOI] [PubMed] [Google Scholar]