Abstract
Expression of human herpesvirus 8 viral Bcl-2 protein was demonstrated in spindle cells of late-stage Kaposi's sarcoma lesions but not in primary effusion lymphoma cell lines. In contrast, strong expression of human Bcl-2 was found in stimulated primary effusion lymphoma cells, whereas in Kaposi's sarcoma lesions preferential mononuclear cells, and to a lesser extent spindle cells, stained positive.
The Kaposi's sarcoma (KS)-associated herpesvirus (KSHV), also called human herpesvirus 8, is associated with KS, primary effusion lymphoma (PEL), and multicentric Castleman's disease (4-6, 14). Viral Bcl-2 homologues are found in all sequenced members of the gamma herpesvirus family, which includes KSHV, herpesvirus saimiri, murine herpesvirus 68, and Epstein-Barr virus (7). KSHV viral Bcl-2 (KSBcl-2) transcripts have been detected in stimulated PEL cells and in KS lesions (11). Transfection of yeast and human cells demonstrated that KSBcl-2 suppresses Bax toxicity and heterodimerizes with human Bcl-2 (huBcl-2) in a yeast two-hybrid system (11). In addition, huBcl-2 oncoprotein is also expressed in KS, with the strongest expression being reported in late-stage KS lesions (9). It has been suggested that KSBcl-2, like huBcl-2, may prevent early apoptotic cell death in virus-infected cells (2, 7, 11) and, in addition, may play a role in the development and growth of transformed cells (12). Thus, KSBcl-2 expression may be of importance for the development and maintenance of KS and PEL. However, so far KSBcl-2 has not been demonstrated at the protein level and thus, nothing is known about its expression pattern in KSHV-associated diseases. Using a specific antibody (Ab) made against a full-length KSBcl-2 fusion protein, we analyzed KSBcl-2 protein expression in PEL cell lines and KS and compared this pattern with huBcl-2 protein expression.
The polyclonal anti-KSBcl-2 Ab was generated against prokaryotically expressed full-length glutathione S-transferase (GST)-KSBcl-2 fusion protein. The episomally located KSHV genome was extracted from BC3 cells using the Wizard Plus SV MiniPrep kit (Promega, Catalys AG, Wallisellen, Switzerland). The 528-bp KSBcl-2 (open reading frame 16) gene was amplified in a PCR with primers 5" T G GG ATC CCC ATG GAC GAG GAC GTT TTG CCT (M1801) and 3" C GAA TTC TTA TCT CCT GCT CAT CGC GAC (M1802). Using appropriate restriction sites introduced into the primers, the 545-bp fragment was ligated into the PGEX-5X-1 vector (Amersham Pharmacia Biotech Europe, Dübendorf, Switzerland) for expression purposes. Purification of the resulting GST fusion protein was done according to the manufacturer's protocol. After protein identity was confirmed by mass spectrometry, the fusion protein was used for the immunization of a New Zealand White rabbit according to standard procedures. Rabbit serum was preadsorbed on a Sepharose 4B column to which GST from GST-expressing HB101 had been coupled and subsequently affinity purified on activated glutathione-Sepharose 4B (Pharmacia) coupled to GST-KSBcl-2 protein. Preimmune serum was identically treated and used as a negative control.
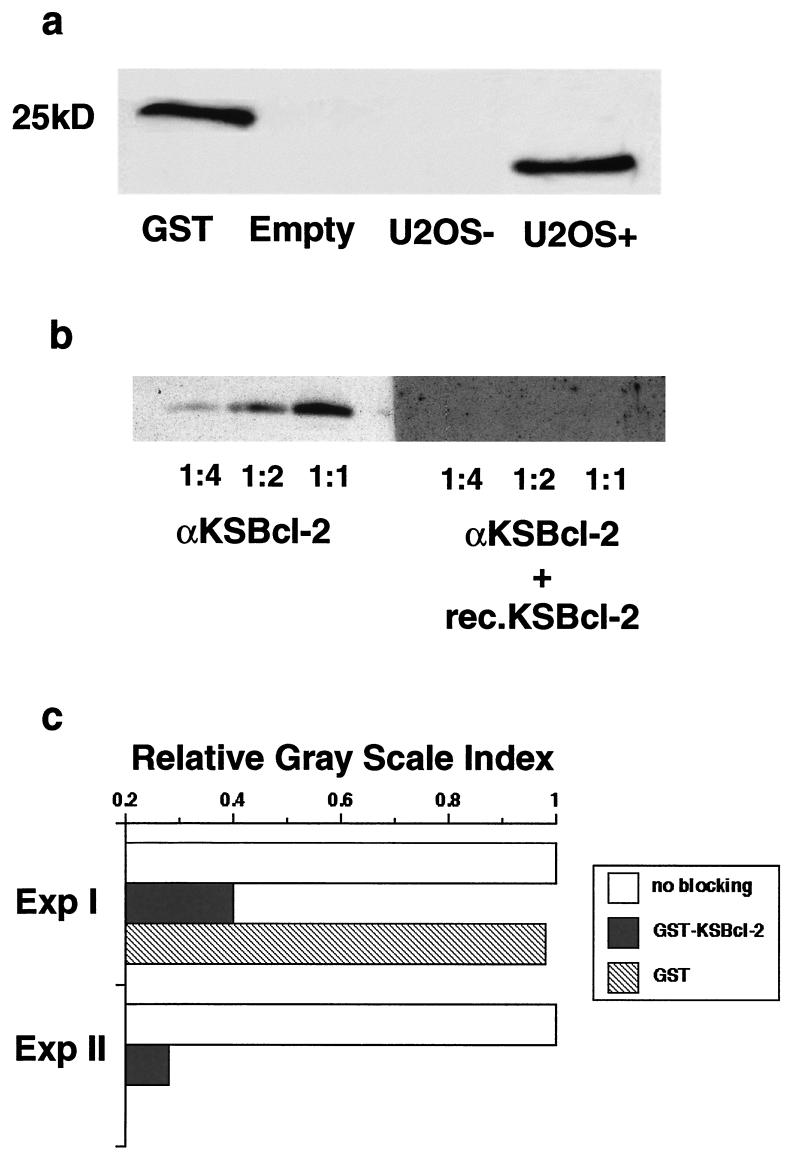
In Western blots, the KSBcl-2 Ab recognized eukaryotically expressed hemagglutinin (HA)-tagged KSBcl-2 and prokaryotically expressed GST-KSBcl-2 antigen (Fig. 1a). The specificity of the KSBcl-2 Ab was tested in competition experiments by running HA-KSBcl-2 protein containing cell lysates on Western blots and preincubating the KSBcl-2 Ab with GST-KSBcl-2 prior to staining the Western blots. Figure 1b demonstrates that preincubation of the Ab with GST-KSBcl-2 completely abolished its immunoreactivity. In addition, preadsorption of the KSBcl-2 antibody with GST-KSBcl-2, but not with GST-Sepharose beads, abolished the staining activity of the antibody in paraffin sections of nodular KS (Fig. 1c), further confirming the high specificity of the antibody. Moreover, cross-reactivity to Epstein-Barr virus BHRF1 (KSBcl-2 homologue) or to huBcl-2 was ruled out by immunofluorescence on Burkitt lymphoma cells and by immunohistochemistry on lymph node sections which all tested negative (data not shown). In addition, no staining with KSBcl-2 Ab was detected in paraffin sections of tissues of tonsils, hemangioma, dermatitis, a seborrheic wart, and a histiocytoma.
FIG. 1.
Specificity of anti-KSBcl-2. (a) Cell lysates from pSG5-KSBcl-2 transfected human osteosarcoma cells (U2OS+) (a generous gift from Paivi Ojala, Helsinki, Finland) expressing a 24-kDa HA-tagged KSBcl-2 fusion protein or pCIneo-transfected U2OS control cells (U2OS−) were run on a Tricine gel, and proteins were transferred to nitrocellulose membranes. The Western blots were incubated overnight at 4°C with KSBcl-2 Ab at a dilution of 1:200 in blocking buffer. After washing, the membranes were incubated with goat anti-rabbit peroxidase at a dilution of 1:2,000 (P 0448; Dako) in blocking buffer at room temperature for 1 h. Protein bands were tagged with enhanced chemiluminescence (Pharmacia) and visualized using X-ray film. A 25-kDa GST standard was used as a size marker. Empty, no cell lysate. (b) Three dilutions of U2OS+ cell lysate (1:1, 1:2, and 1:4, corresponding to 8, 4, and 2 μl) were run on a Tricine gel and transferred to nitrocellulose membranes. Anti-KSBcl-2 (αKSBcl-2) was preincubated for 2 h at room temperature with the prokaryotically produced GST-KSBcl-2 protein in 20 mM Tris HCl, pH 7.4, and subsequently used for staining in the Western blots. The KSBcl-2 Ab alone resulted in increasing concentration-dependent bands, but no bands resulted if preadsorbed with the fusion protein (rec.KSBcl-2). (c) Preadsorption of the KSBcl-2 antibody with GST-KSBcl-2, but not with GST-Sepharose beads, abolishes the staining activity of the antibody. One hundred microliters of KSBcl-2 Ab diluted in 100 μl of 20 mM Tris buffer (pH 7.4) was incubated with 50 μl of KSBcl-2-Sepharose or GST-Sepharose beads for 2 h at room temperature. The supernatants were then used for staining (final concentration, 1:40). Quantification of the staining was done by computer-assisted image analysis as described in detail elsewhere (1). In brief, the immunostainings, done without counterstaining, were photographed on a Nikon Eclipse TE200 inverted microscope using a Hamamatsu digital camera and controller (Bucher AG, Basel, Switzerland). For scientific imaging and quantification, the Openlab program was used (Improvision, Coventry, United Kingdom). Regions of interest were arbitrarily defined by choosing at least three different sections of 150,000 to 400,000 pixels, and the gray-level intensity within the region of interest was calculated. The gray intensity level of the staining without blocking reagent was set at 1.
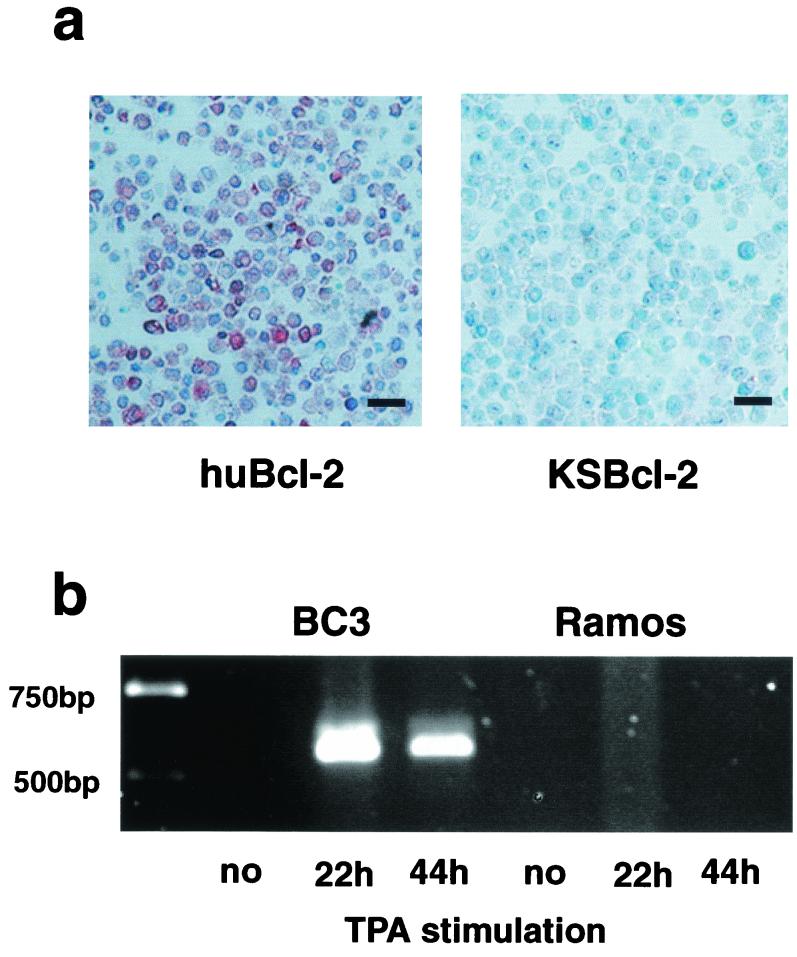
It has been previously shown that KSBcl-2 mRNA is only expressed in KSHV-positive PEL cell lines, such as BC3, upon 12-O-tetradecanoylphorbol-13-acetate (TPA) treatment for 20 to 30 h (11, 16). Thus, BC3 and the KSHV-negative control cell line Ramos (American Type Culture Collection, Rockville, Md.) were not stimulated or were stimulated with 20 ng of TPA (Sigma, Fluka Chemie AG, Buchs, Switzerland)/ml for 22 or 44 h and tested for expression of KSBcl-2 and huBcl-2. huBcl-2 protein was expressed in TPA-stimulated BC3 cells as analyzed by immunohistochemical staining of paraffin sections (Fig. 2a) or by Western blots (data not shown) with monoclonal Ab (MAb) clone 124 (Dako, Zug, Switzerland), but it was not detectable in Ramos cells or in unstimulated BC3 cells (data not shown). In contrast, KSBcl-2 protein was neither found in Western blots (data not shown) nor demonstrated in immunohistochemical staining (Fig. 2a) independent of the amount of time of TPA stimulation of the BC3 cells. However, KSBcl-2 mRNA expression was clearly detectable in TPA-stimulated BC3 cells by reverse transcription PCR (RT-PCR) (Fig. 2b), but not in Ramos cells, as it has been previously described by others (16). Identical results were obtained with another PEL cell line, BCBL (data not shown). Thus, KSBcl-2 is either not translated in PEL cells or translated in such a low concentration that it is not detectable by immunohistochemistry or Western blots. Alternatively, the protein may be unstable, which is unlikely in view of the fact that it can be easily expressed in eukaryotic cells (7).
FIG. 2.
huBcl-2, but not KSBcl-2, is expressed in TPA-stimulated PEL cells. BC3 and the KSHV-negative Ramos cell line were either not stimulated or stimulated with TPA (20 ng/ml) for 22 and 44 h, respectively. (a) Immunohistochemistry of paraffin sections of BC3 cells stimulated with TPA for 44 h and stained with KSBcl-2 Ab (diluted 1:40 in phosphate-buffered saline containing 0.1% goat serum) overnight at 4°C or with huBcl-2 MAb (clone 124; Dako). The slides were then incubated for 30 min at room temperature with anti-rabbit biotin (Dako) (diluted 1:300 in phosphate-buffered saline containing 0.1% goat serum) or anti-mouse biotin (diluted 1:300) (Dako), respectively. Signal amplification was performed using the ABC kit according to the manufacturer's instructions; subsequently, 3-amino-9-ethylcarbazole substrate (Dako) was added, and slides were developed for 30 min. The BC3 cell line is shown at 44 h following TPA stimulation. Unstimulated BC3 and Ramos cells did not stain with the antibodies nor did the preimmune or isotype controls. BC3 cells stimulated with TPA for 22 h stained somewhat more weakly (data not shown). Bars, 25 μm. (b) KSBcl-2 mRNA expression analyzed by RT-PCR. RNA was purified from BC3 or Ramos cells, a DNase digest was performed, and the RNA was used for RT-PCR. The cDNA was amplified by PCR. RT-PCR and PCR were performed with primers M1801 and M1802.
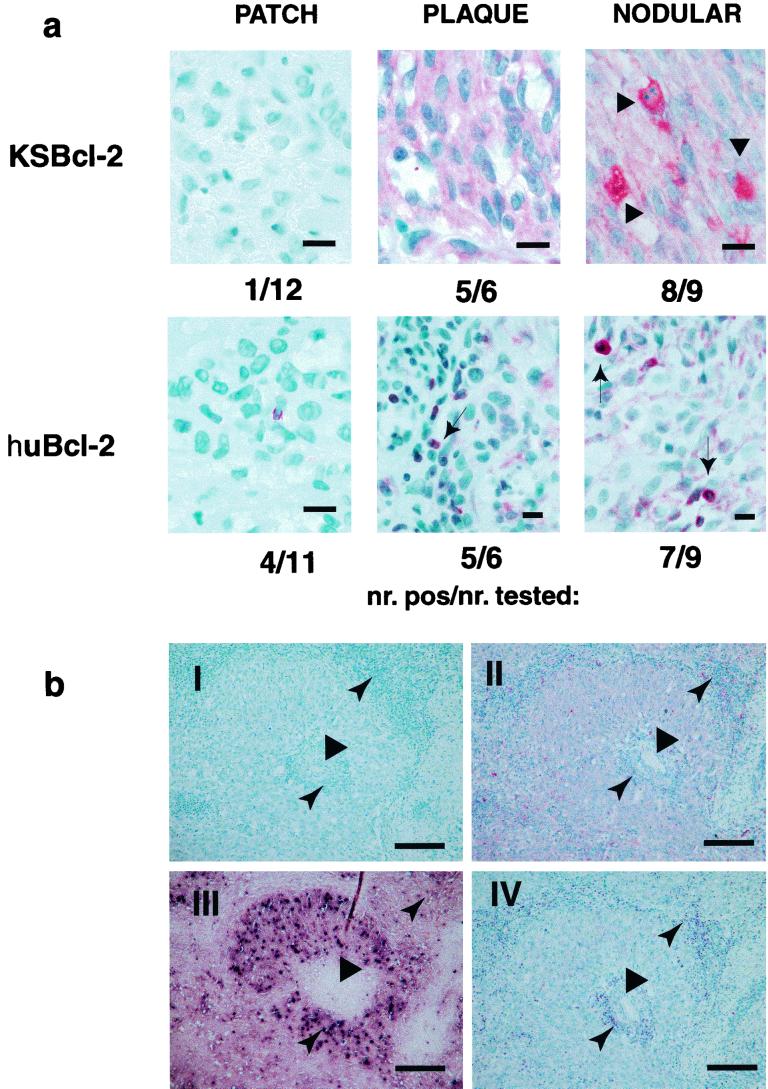
KS lesions of various stages (12 patch, 6 plaque, 10 nodular) were investigated for KSBcl-2 and huBcl-2 expression by immunohistochemistry of paraffin sections. As Fig. 3a demonstrates, both KSBcl-2 and huBcl-2 protein expression levels increased relative to the tumor stage. Only 1 of the 12 patch-stage lesions investigated tested weakly positive for KSBcl-2, whereas 5 of the 6 plaque- and 8 of the 9 nodular-stage lesions analyzed tested strongly positive. The plaque and nodular lesions demonstrated strong cytoplasmic staining of the majority of spindle cells and rarely stained endothelial cells of atypical blood vessels (Fig. 3a and b, section II), whereas huBcl-2 Ab variably stained spindle cells (very weakly to moderately) but strongly stained mononuclear cells, e.g., lymphocytes (Fig. 3a and b, section IV). Staining for latency-associated nuclear antigen (ABI, Columbia, Md.) demonstrated immunoreactivity in the same region as for KSBcl-2 staining (Fig. 3b, section III), although fewer spindle cells stained positive. Sun et al. (16) reported that 1 to 3% of spindle cells tested positive for KSBcl-2 by in situ hybridization analysis. However, this group only investigated one KS lesion of an unspecified stage. KSBcl-2 belongs to the primary lytic genes, whose expression becomes detectable in PEL cell lines within 10 h after stimulation with TPA (8). The proportion of lytically infected cells in KS lesions varies but is believed to be less than 10% (3, 13, 15). This contrasts with the widespread KSBcl-2 detection in late-stage KS biopsies as shown here, indicating that KSBcl-2 may be the exception to the rule. This makes sense as KSBcl-2 may prevent spindle cell destruction when virus progeny is produced. In addition, the fact that KSBcl-2 protein can be detected in late-stage KS lesions but not in stimulated PEL cell lines despite the identification of mRNA strongly supports the view that viral gene expression in cell lines does not accurately reflect what occurs in diseased tissues (10) and indicates that certain genes are expressed at different time points and levels depending on the host tissue.
FIG.3.
Late-stage lesions of KS express KSBcl-2. (a) Lesions in the patch, plaque, and nodular stages of KS were stained with KSBcl-2 Ab and huBcl-2 MAb as described in the legend to Fig. 2. Preimmune and isotype control stains were completely negative (data not shown). Some spindle cells within the nodular lesions stained particularly strongly with KSBcl-2 Ab (large arrowhead), indicating different levels of expression. Lymphocytes stained strongly with huBcl-2 (small arrow). The numbers below each sample represent the number of positive KS lesions per number of KS lesions tested. Bars, 10 μm. (b) Staining of consecutive sections of a nodular KS lesion with preimmune control (I), KSBcl-2 Ab (II), open reading frame 73 (latency-associated nuclear antigen 1) MAb (III), and huBcl-2 MAb (IV). The spindle cell region surrounds a central blood vessel (wide arrowhead). The lymphocyte infiltration (narrow arrowhead) is near the vessel lumen and in the periphery of the spindle cells. Bars, 375 μm.
Our study demonstrates for the first time KSBcl-2 protein expression in spindle cells of late-stage KS lesions. Due to its antiapoptotic effect it may, in conjunction with huBcl-2, support virus production by preventing premature cell death, and additionally, it may also support tumor progression by prolonging cell survival. The failure to demonstrate KSBcl-2 in PEL cell lines might be due to the fact that high huBcl-2 expression makes the expression of KSBcl-2 unnecessary.
Acknowledgments
This work was supported by Swiss National Science Fund grant no. 31-064233.00.
The protein analysis by mass spectrometry was kindly performed by P. Hunziker and R. Sack, Biochemistry Institute, University of Zurich.
REFERENCES
- 1.Bachmann, F., S. A. Buechner, M. Wernli, S. Strebel, and P. Erb. 2001. Ultraviolet light downregulates CD95 ligand and trail receptor expression facilitating actinic keratosis and squamous cell carcinoma formation. J. Investig. Dermatol. 117:59-66. [DOI] [PubMed] [Google Scholar]
- 2.Bellows, D. S., B. N. Chau, P. Lee, Y. Lazebnik, W. H. Burns, and J. M. Hardwick. 2000. Antiapoptotic herpesvirus Bcl-2 homologs escape caspase-mediated conversion to proapoptotic proteins. J. Virol. 74:5024-5031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blasig, C., C. Zietz, B. Haar, F. Neipel, S. Esser, N. H. Brockmeyer, E. Tschachler, S. Colombini, B. Ensoli, and M. Sturzl. 1997. Monocytes in Kaposi's sarcoma lesions are productively infected by human herpesvirus 8. J. Virol. 71:7963-7968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cathomas, G. 2000. Human herpes virus 8: a new virus discloses its face. Virchows Arch. 436:195-206. [DOI] [PubMed] [Google Scholar]
- 5.Cesarman, E., Y. Chang, P. S. Moore, J. W. Said, and D. M. Knowles. 1995. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N. Engl. J. Med. 332:1186-1191. [DOI] [PubMed] [Google Scholar]
- 6.Chang, Y., E. Cesarman, M. S. Pessin, F. Lee, J. Culpepper, D. M. Knowles, and P. S. Moore. 1994. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science 266:1865-1869. [DOI] [PubMed] [Google Scholar]
- 7.Cheng, E. H., J. Nicholas, D. S. Bellows, G. S. Hayward, H. G. Guo, M. S. Reitz, and J. M. Hardwick. 1997. A Bcl-2 homolog encoded by Kaposi sarcoma-associated virus, human herpesvirus 8, inhibits apoptosis but does not heterodimerize with Bax or Bak. Proc. Natl. Acad. Sci. USA 94:690-694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jenner, R. G., M. M. Alba, C. Boshoff, and P. Kellam. 2001. Kaposi's sarcoma-associated herpesvirus latent and lytic gene expression as revealed by DNA arrays. J. Virol. 75:891-902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morris, C. B., R. Gendelman, A. J. Marrogi, M. Lu, J. M. Lockyer, W. Alperin-Lea, and B. Ensoli. 1996. Immunohistochemical detection of Bcl-2 in AIDS-associated and classical Kaposi's sarcoma. Am. J. Pathol. 148:1055-1063. [PMC free article] [PubMed] [Google Scholar]
- 10.Parravicini, C., B. Chandran, M. Corbellino, E. Berti, M. Paulli, P. S. Moore, and Y. Chang. 2000. Differential viral protein expression in Kaposi's sarcoma-associated herpesvirus-infected diseases—Kaposi's sarcoma, primary effusion lymphoma, and multicentric Castleman's disease. Am. J. Pathol. 156:743-749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sarid, R., T. Sato, R. A. Bohenzky, J. J. Russo, and Y. Chang. 1997. Kaposi's sarcoma-associated herpesvirus encodes a functional bcl-2 homologue. Nat. Med. 3:293-298. [DOI] [PubMed] [Google Scholar]
- 12.Sarid, R., J. S. Wiezorek, P. S. Moore, and Y. Chang. 1999. Characterization and cell cycle regulation of the major Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) latent genes and their promoter. J. Virol. 73:1438-1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schulz, T. F. 2000. Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8): epidemiology and pathogenesis. J. Antimicrob. Chemother. 45:15-27. [DOI] [PubMed] [Google Scholar]
- 14.Soulier, J., L. Grollet, E. Oksenhendler, P. Cacoub, D. Cazals-Hatem, P. Babinet, M. F. d'Agay, J. P. Clauvel, M. Raphael, L. Degos, et al. 1995. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in multicentric Castleman's disease. Blood 86:1276-1280. [PubMed] [Google Scholar]
- 15.Staskus, K. A., W. Zhong, K. Gebhard, B. Herndier, H. Wang, R. Renne, J. Beneke, J. Pudney, D. J. Anderson, D. Ganem, and A. T. Haase. 1997. Kaposi's sarcoma-associated herpesvirus gene expression in endothelial (spindle) tumor cells. J. Virol. 71:715-719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun, R., S. F. Lin, K. Staskus, L. Gradoville, E. Grogan, A. Haase, and G. Miller. 1999. Kinetics of Kaposi's sarcoma-associated herpesvirus gene expression. J. Virol. 73:2232-2242. [DOI] [PMC free article] [PubMed] [Google Scholar]