Abstract
Bovine leukemia virus (BLV) is the etiologic agent of enzootic bovine leukosis. We previously identified several mutants of the BLV Tax protein with an ability to transactivate transcription via the BLV enhancer that is significantly greater than that of the wild-type Tax protein. Moreover, the mutant proteins also activated other viral enhancers, such as the enhancer of human T-cell leukemia virus type 1, which cannot be activated by wild-type BLV Tax. In this study, we demonstrated that the mutant proteins but not wild-type protein activate the upstream sequence of the human c-fos gene, which contains two major cis-acting elements, the CArG box and cyclic AMP-responsive element (CRE) motif. The mutant protein also strongly increased levels of endogenous c-fos mRNA in both human and bovine cell lines. On the other hand, the wild-type Tax protein has no activity to activate the expression of human c-fos, indicating that wild-type BLV Tax might discriminate between human and bovine c-fos promoter sequences. Deletion and point-mutational analysis of the cis-acting elements revealed that both the CArG box and the CRE motif were indispensable for the activation of c-fos by the mutant BLV Tax protein. Our results suggest that the mutant BLV Tax proteins might not only have the ability to enhance the production of virus particles but might also have increased ability to induce leukemia.
Bovine leukemia virus (BLV) is associated with enzootic bovine leukosis, a disease characterized by a very extended course that often involves persistent lymphocytosis and culminates in B-cell lymphoma and is closely related to human T-cell leukemia virus (HTLV) (2, 3).
The Tax proteins of these viruses were identified originally as factors that activated transcription from the respective long terminal repeat (LTR). Tax proteins recognize a triplicate motif, the Tax-responsive element (which contains a cyclic AMP-responsive element [CRE]) in the U3 region of the 5" LTR, and both stimulate transactivation of the proviral genome (4, 5, 8, 20). It has been proposed, moreover, that Tax contributes to BLV-induced and HTLV type 1 (HTLV-1)-induced leukemogenesis. The Tax proteins of BLV can act in cooperation with the Ha-Ras oncoprotein to induce the full transformation of primary rat embryo fibroblasts (11, 21). Established lines of rodent fibroblasts (Rat-1 cells) transfected with the tax gene of HTLV-1 form colonies in soft agar (17). Transgenic mice carrying the same gene develop fibroma (13). In addition, the HTLV-1 Tax protein immortalizes primary T cells in the presence of interleukin 2 (1, 9). In addition to its role in the activation of the viral enhancer, the Tax protein of HTLV-1 modulates the expression of a variety of cellular genes that are related to the regulation of cell growth, via interaction with distinct promoter/enhancer sequences, which include the NF-κB-binding sequence and the CArG box (22). Examination of HTLV-1 Tax mutants demonstrated that activation of the pathway mediated by the CArG box is essential for the transformation of rat embryo fibroblasts. By contrast, activation of the NF-κB-binding sequence is correlated with the transformation of Rat-1 cells by HTLV-1 Tax (11). These findings support the hypothesis that disturbance of the normal expression of cellular genes by HTLV-1 Tax plays a critical role in the Tax-dependent transformation and immortalization steps in HTLV-1-induced lymphoma.
In the case of BLV, no cellular genes whose expression is modulated by BLV Tax have been identified to date apart from the c-fos gene (10). There are several cis-acting elements upstream of the human c-fos gene (Fig. 1A). Sequences that are responsive to the Tax proteins of HTLV-1 and BLV are located between positions −276 and −362 and between positions −225 and −361, respectively (6, 10). These two regions both contain specific cis-acting elements, for example, a CArG box, an AP-1-binding site, and a v -sis-conditioned medium-inducible element (SIE). Studies of HTLV-1 Tax demonstrated that the protein binds indirectly to the CArG box through a CArG box-binding factor, namely, serum-responsive factor (7). However, it remains to be determined whether the Tax protein of BLV activates the c-fos promoter in the same way as does HTLV-1 Tax.
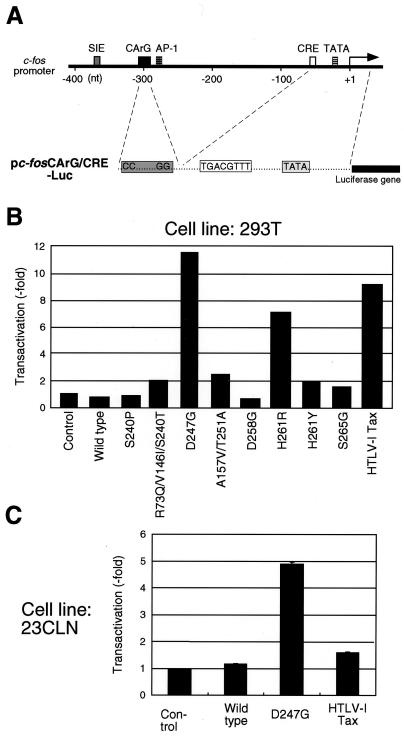
FIG. 1.
BLV Tax mutants with increased transactivational activity activated transcription that was mediated by the promoter of the c-fos gene. (A) Schematic illustration of the upstream elements of the human c-fos gene and the promoter region of pc-fosCArG/CRE-Luc, which was used for the analysis. Nucleotide positions are given relative to the site of initiation of transcription. Five cis-acting elements are indicated by boxes, as follows: SIE, v-sis-conditioned medium inducible element; CArG, CArG box; AP-1, AP-1-binding site; CRE; and TATA, TATA box. (B and C) Transactivation of the c-fos promoter by BLV Tax mutants in human embryonic kidney 293T cells and bovine lymph node 23CLN cells. 293T cells (105 cells/well of a 12-well culture plate) (B) and 23CLN cells (0.5 × 105 cells/well of a 12-well culture plate) (C) were transfected with 0.5 μg of pc -fosCArG/CRE-Luc, 1 μg of an individual Tax-expressing plasmid (pME18Neo encoding wild-type BLV Tax [15], S240P, R73Q/V146I/S240T, D247G, A157V/T251A, D258G, H261R, H261Y, S265G, or HTLV-1 Tax protein) or the control plasmid (pME18Neo), plus 0.3 μg of the reference plasmid pRL-SV40 encoding Renilla luciferase (Promega, Madison, Wis.) as described previously (16). Sixty hours after transfection, cells were harvested and the activities of firefly and Renilla luciferases were measured in lysates. For each sample, the firefly luciferase activity (pc-fosCArG/CRE-Luc) was normalized by reference to Renilla luciferase activity (pRL-SV40). The extent of transactivation (-fold) was calculated by dividing the transactivational activity of the Tax protein by that of the cell lysate transfected with the control plasmid. Each value represents the average obtained from two (B) and three (C) independent transfections.
We recently identified a series of mutants of the BLV Tax proteins, all of which exhibit strikingly more ability to stimulate viral LTR-directed transcription than does the wild-type protein (16). All the mutants have at least one amino acid substitution between amino acids 240 and 265, and a CRE motif in the BLV LTR is sufficient for transactivation by the Tax mutants. Transient expression analysis revealed that mutant Tax protein was better able to increase the production of viral protein and particles from a defective recombinant proviral clone of BLV than was wild-type Tax. Moreover, some of the mutant proteins were also found to activate other retroviral enhancers that are not or are only very slightly activated by the wild-type protein. These findings raised the possibility that the BLV Tax mutants might have the ability to activate various cellular genes that might be barely activated, if at all, by wild-type Tax. In this study, we examined whether these mutant Tax proteins could activate the expression of human and bovine c-fos and whether the activity of the mutants might be significantly higher than that of wild-type Tax. We also attempted to identify the cis element in the c-fos promoter that was responsible for transactivation by the mutant Tax proteins from BLV.
We first tested the ability of wild-type and mutant BLV Tax proteins to activate the c-fos promoter (Fig. 1). The plasmid pc-fosCArG/CRE-Luc, which contains part of the human c-fos promoter that includes the CArG box, the CRE motif, and a TATA box, was used to cotransfect human embryonic kidney 293T cells with plasmids that encoded wild-type BLV Tax (15), one low-activity Tax mutant (S240P), seven high-activity Tax mutants (R73Q/V146I/S240T, D247G, A157V/T251A, D258G, H261R, H261Y, and S265G) (16), and wild-type HTLV-1 Tax as a positive control. Then we performed luciferase assays (Fig. 1B). The D247G, H261R, and HTLV-1 Tax strongly transactivated the c-fos promoter, whereas the R73Q/V146I/S240T, A157V/T251A, and H261Y mutants were weak transactivators. By contrast, we failed to detect the activation of the c-fos promoter by the wild-type Tax from BLV (Fig. 1B, lane 2). A similar result was obtained when bovine lymph node 23CLN cells were transfected with the plasmid pc-fosCArG/CRE-Luc and plasmids that encoded wild-type BLV Tax and mutant D247G (Fig. 1C). On the other hand, HTLV-1 Tax activated the human c-fos promoter in the bovine cells, although it was considerably weaker than in 293T cells (Fig. 1C). These results and previous findings by Katoh et al. (10) suggest that, in addition to a CArG box and CRE motif, wild-type BLV Tax may require some other promoter regions, for example, the AP-1 and SIE sites, for activation of the promoter of c-fos, whereas the mutant Tax activates the c-fos promoter by a mechanism independent of the AP-1 and SIE sites.
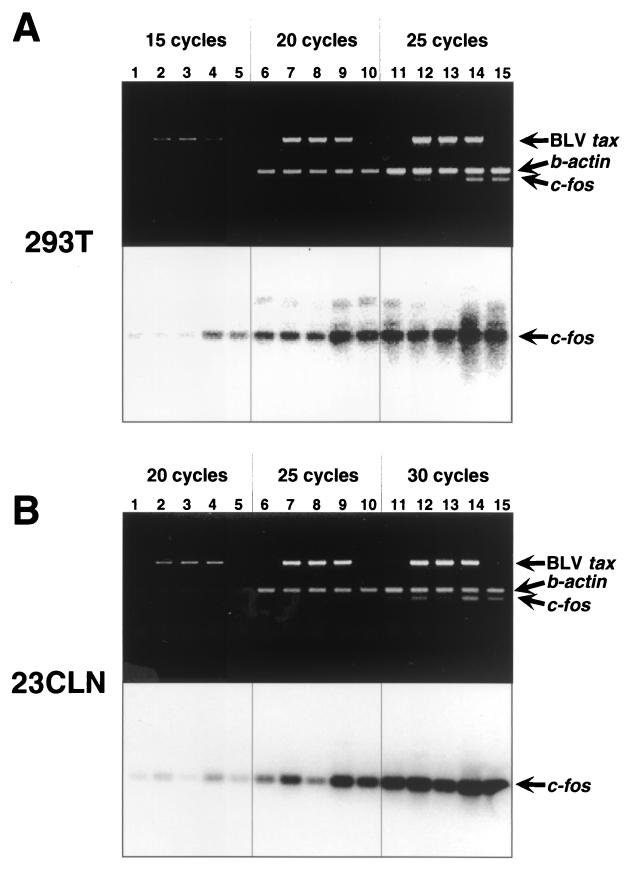
Two of seven Tax mutants with elevated transactivational activity, but not wild-type BLV Tax, strongly activated the human c-fos promoter. However, it was unclear whether the mutants caused an increase in the level of the transcript of the endogenous c-fos gene, because the promoter sequence that we used for the reporter assay included only part of the upstream region of the endogenous human c-fos gene. To investigate the possibility that the wild-type Tax protein and the mutant D247G protein might activate the expression of the endogenous c-fos gene, we transfected 293T cells with the effector plasmid pME18Neo, with pME18Neo that encoded wild-type, S240P, or D247G Tax, and with pME18Neo that encoded HTLV-1 Tax as a positive control (Fig. 2A). Sixty hours after transfections, we isolated total RNA from each transfectant and measured the levels of transcripts of c-fos, β-actin, and BLV tax by reverse transcriptase PCR. The level of c-fos mRNA was markedly elevated in cells that expressed D247G and HTLV-1 Tax, while there were no apparent differences in levels of c-fos mRNA among cells transfected with pME18Neo, with pME18Neo that encoded wild-type Tax, and with pME18Neo that encoded S240P Tax. These results were consistent with the results of the reporter gene assay shown in Fig. 1B. Next, to examine whether these Tax proteins activate the expression of the bovine endogenous c-fos gene, we also transfected bovine 23CLN cells with the effector plasmids and then measured the levels of transcripts of c-fos, β-actin, and BLV tax (Fig. 2B). Similar to the result obtained by using human cells in Fig. 2A, the level of c-fos mRNA was considerably increased in 23CLN cells that expressed D247G. Wild-type BLV and HTLV-1 Tax also exhibited transactivational activity for the bovine endogenous c-fos gene, although these activities were significantly lower than that of D247G. By contrast, the levels of β-actin and BLV tax transcripts were similar among the cells transfected with plasmids that encoded wild-type and mutant BLV Tax. Our results proved that mutant D247G Tax might acquire an enhanced ability to activate both human and bovine c-fos promoters, while the wild-type Tax protein has no activity to activate the expression of the human c-fos gene. Thus, wild-type BLV Tax might discriminate between human and bovine c-fos promoter sequences and activate specifically to the bovine c-fos promoter.
FIG. 2.
BLV Tax mutant protein D247G exhibited stronger transactivation activity for the endogenous c-fos gene than did wild-type Tax. 293T cells (n = 107) (A) and 23CLN cells (n = 4 × 106) (B) were transfected with 30 μg of pME18Neo (lanes 1, 6, and 11), and pME18Neo encoding wild-type Tax (lanes 2, 7, and 12), low-activity Tax mutant protein S240P (lanes 3, 8, and 13), high-activity Tax mutant proteins D247G (lanes 4, 9, and 14), or wild-type HTLV-1 Tax (lanes 5, 10, and 15) by electroporation, as described previously (16). Sixty hours after transfection, cells were lysed and total RNA was extracted with TRIZOL solution (Invitrogen, Carlsbad, Calif.) according to the manufacturer's protocol. Four micrograms of total RNA was used for synthesis of cDNA with the SUPERSCRIPT Preamplification sytem (Invitrogen). Eight primers for amplification of (i) part of human c-fos (c-fosRT1, 5"-GCGTTGTGAAGACCATGACAG-3"; and c-fosRT2, 5"-CTTCTGGGAAGCCCAGGTCATC-3", the length of the expected product of PCR was 313 bp), (ii) part of bovine c-fos (Bc-fosF2, 5"-AGGCGGAGACAGATCAACTAGA-3"; and Bc-fosR1, 5"-CTCAGCCTTCAGCTCCATGCTG-3"; product, 305 bp), (iii) human and bovine β-actin (actinF, 5"-CGTCGCCTTGGACTTCGAGCAGGA-3"; and actinR, 5"-GCTGGAAGGTGGACAGGGAGGCCAGGA-3"; product, 407 bp), and (iv) full-length BLV tax (BtaxF and BtaxR; product, 1,010 bp) (16) cDNA were synthesized. The cDNA in 1 μl of the solution of cDNA that had been generated by reverse transcription was amplified in 30 μl of reaction buffer that contained 10 mM Tris-HCl (pH 8.3), 50 mM KCl, 0.1% Triton X-100, a 0.2 mM concentration of each deoxynucleoside triphosphate, 2.5 mM MgCl2, a 0.33 μM concentration of each of the six primers together, and 0.5 U of rTaq polymerase (Toyobo, Kyoto, Japan). All three kinds of cDNA (c -fos, β-actin, and BLV tax) were amplified in one reaction tube at the same time. The reaction mix was heated to 94°C for 2 min, and then amplification was allowed to proceed for 15 (lanes 1 to 5), 20 (lanes 6 to 10), or 25 (lanes 11 to 15) cycles for 293T cDNA (A) or for 20 (lanes 1 to 5), 25 (lanes 6 to 10), or 30 (lanes 11 to 15) cycles for 23CLN cDNA (B) of 94°C for 30 s, 60°C for 30 s, and 72°C for 1 min. Products of PCR were subjected to electrophoresis on a 0.8% agarose gel. The gel was stained with ethidium bromide, and then the gel was photographed (upper photo). For Southern blotting analysis, the resolved products of PCR were transferred to a nylon membrane (BIODYNE; PALL, Port Washington, N.Y.) and then cross-linked to the membrane by UV light. A 32P-labeled human c-fos probe was prepared with a Random Primed DNA Labeling Kit (Roche Molecular Biochemicals, Mannheim, Germany) according to the manufacturer's protocol. Hybridization was allowed to proceed at 42°C overnight with 106 cpm of c-fos probe per ml in 50% formamide, 5× SSPE (1× SSPE is 0.18 M NaCl, 10 mM NaH2PO4, and 1 mM EDTA, pH 7.4), 1× Denhardt's solution (0.02% bovine serum albumin, 0.02% Ficoll, and 0.02% polyvinylpyrrolidone), 10% dextran sulfate, 0.3% sodium dodecyl sulfate, and 100 μg of heat-denatured salmon sperm DNA/ml. The filter was then washed twice at room temperature in 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) plus 0.1% sodium dodecyl sulfate for 20 min and then twice at 65°C in 0.1× SSC plus 0.2% sodium dodecyl sulfate for 15 min and autoradiographed (lower photo). Arrows indicate the products derived from transcripts of each gene.
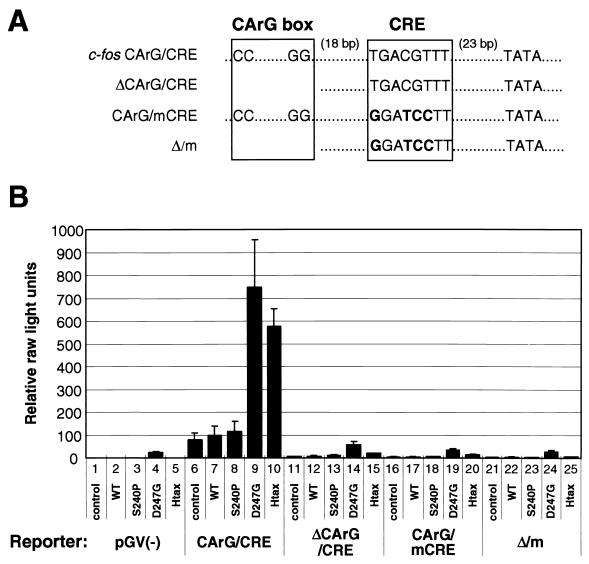
We attempted next to identify the target of the Tax mutant proteins within the c-fos promoter. We introduced deletions and point mutations into the CArG box and the CRE motif, respectively, within the promoter/enhancer region in pc-fosCArG/CRE-Luc (Fig. 3A). The mutant reporter plasmids were used to cotransfect 293T cells with various Tax-expressing plasmids, and the activity of the mutant promoters was determined (Fig. 3B). With all three mutant promoters, the relative luciferase activity fell dramatically to close to the level obtained with the control reporter plasmid pGV(−). No D247G Tax-dependent or HTLV-1 Tax-dependent activation of the mutant promoters was observed. These results indicate that both the CArG box and the CRE motif are indispensable for the mutant BLV Tax-mediated activation of the c-fos promoter.
FIG. 3.
Both a CArG box and a CRE were indispensable for the activation of the c-fos promoter by the BLV Tax mutant protein with elevated transactivational activity. (A) Schematic representation of the three mutant c-fos promoter sequences used for the analysis. Deletion and single-site mutations were introduced into the CArG box (ΔCArG) and the CRE (mCRE), respectively. Bases different from the wild-type sequence are shown in boldface type. (B) 293T cells (105 cells/well of 12-well culture plates) were transfected with the reporter plasmid pGV(−) (bars 1 to 5), pc-fosCArG/CRE-Luc (bars 6 to 10), pc-fosΔCArG/CRE-Luc (bars 11 to 15), pc-fosCArG/mCRE-Luc (bars 16 to 20), and pc-fosΔCArG/mCRE-Luc (bars 21 to 25); the effector plasmid pME18Neo (control; lanes 1, 6, 11, 16, and 21) and pME18Neo encoding wild-type Tax protein (WT; lanes 2, 7, 12, 17, and 22); the low-activity Tax mutant protein (S240P; lanes 3, 8, 13, 18, and 23); the high-activity mutant protein (D247G; lanes 4, 9, 14, 19, and 24); and wild-type HTLV-1 Tax protein (Htax; lanes 5, 10, 15, 20, and 25) and 0.3 μg of pRL-SV40. Sixty hours after transfection, cells were recovered and the activities of firefly and Renilla luciferases were measured. For each sample, the firefly luciferase activity was normalized by reference to the Renilla luciferase activity (relative raw light units). Average values from triplicate transfections with standard deviations (error bars) are shown. Reporters correspond to those in panel A.
In this study, we demonstrated that the Tax mutant proteins with increased transactivation activity via BLV LTR activated the promoter of human c-fos and increased levels of endogenous c-fos mRNA in both human and bovine cell lines. The ability of the mutant D247G Tax protein to activate the promoter was higher than that of wild-type BLV and HTLV-1 Tax, a known activator of the c-fos promoter. Our analysis also suggests that both the CArG box and the CRE motif in the c-fos promoter might be essential for transactivation of the promoter by BLV Tax mutant proteins.
Previous reports have shown that both HTLV-1 Tax and BLV Tax can activate c-fos promoter sequences (6, 10). Fujii et al. examined the details of the activation of the c-fos promoter by HTLV-1 Tax and demonstrated that HTLV-1 Tax can bind indirectly to the CArG box and that this binding is mediated by the transcription factor SRF (7). Although the c-fos promoter contains a single CRE between the CArG box and the TATA box, the CRE is not sufficient for activation of the promoter (6). In contrast to previous findings, we failed to detect the transactivation of the c-fos promoter by wild-type BLV Tax. This discrepancy might be attributable to the promoter region used for the reporter assay. We used the reporter plasmid pc-fosCArG/CRE-Luc, which lacks certain cis-acting elements, such as AP-1 and SIE in the promoter region of human c-fos, whereas Katoh et al. used a reporter plasmid in which the complete promoter region of human c-fos was linked to the upstream region of a reporter gene (10). Moreover, Katoh et al. also reported that a deletion from position −361 to position −225 of the c-fos promoter, including SIE, the CArG box, and AP-1, abolished the induction of transcription by wild-type BLV Tax (10). These previous findings suggest that wild-type BLV Tax-mediated activation of the human c-fos promoter might require SIE and/or AP-1, in addition to the CArG box and the CRE motif. However, we also failed to detect any increase in levels of endogenous c-fos mRNA in human 293T cells, but not bovine 23CLN cells, that expressed wild-type BLV Tax, while 293T and 23CLN cells that expressed mutant D247G Tax produced elevated levels of c-fos mRNA (Fig. 2). These results indicate that, besides the increased ability to stimulate the expression of the endogenous bovine c-fos gene, D247G Tax might acquire the ability to activate the human c-fos promoter, which was not activated by wild-type BLV Tax.
The BLV Tax mutant proteins with increased transactivational activity activated a promoter sequence that contained a CArG box and a CRE. Several genes have been identified with both one or more CArG boxes and CREs in their upstream promoter/enhancer regions, for example, the early growth response genes egr-1 (18) and egr-2 (14), a gene for urokinase-type plasminogen activator (12), and a gene for cysteine dioxygenase (19). Moreover, HTLV-1 Tax can activate the promoter sequences of erg-1 and erg-2 (7), and it seems likely that the BLV mutant Tax proteins might also activate these genes in addition to c-fos. Matsumoto et al. (11) reported that activation of the CArG box-mediated pathway is essential for the transformation of rat embryo fibroblasts by HTLV-1 Tax and, on the other hand, that activation of the κB element is correlated with the transformation of Rat-1 cells by Tax. Thus, both CArG box-mediated and κB element-mediated activation of cellular genes by HTLV-1 Tax might contribute independently to the progression of leukemia induced by HTLV-1. The BLV Tax mutant proteins might not only have the ability to reinforce the production of virus particles but might also have enhanced ability to induce leukemia, compared to wild-type Tax.
As discussed above, certain mutant derivatives of the BLV Tax protein have two distinctive features: (i) markedly increased transactivational activity and (ii) the ability to activate a promoter sequence that is not activated by wild-type BLV Tax. It is now important to clarify the effects of clones of mutant BLV virus that encode the mutant Tax proteins on the replication and propagation of BLV in vitro and in vivo and on leukemogenesis induced by BLV.
Acknowledgments
We thank J. Fujisawa (Kansai Medical School, Osaka, Japan) and M. Fujii (Niigata University, Niigata, Japan) for kindly providing reporter plasmids.
This study was supported by Grants-in-Aid for Scientific Research and for Encouragement of Young Scientists from the Ministry of Education, Science, and Culture of Japan and by a grant for special postdoctoral researcher and a grant for the promotion of research from RIKEN.
REFERENCES
- 1.Akagi, T., and K. Shimotohno. 1993. Proliferative response of Tax1-transduced primary human T cells to anti-CD3 antibody stimulation by an interleukin-2-independent pathway. J. Virol. 67:1211-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burny, A., Y. Cleuter, R. Kettmann, M. Mammerickx, G. Marbaix, D. Portetelle, A. van den Broeke, L. Willems, and R. Thomas. 1988. Bovine leukaemia: facts and hypotheses derived from the study of an infectious cancer. Vet. Microbiol. 17:197-218. [DOI] [PubMed] [Google Scholar]
- 3.Cann, A. J., and I. S. Y. Chen. 1996. Human T-cell leukemia virus types I and II, p. 1849-1880. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Fields virology, 3rd ed. Lippincott-Raven Publishers, Philadelphia, Pa.
- 4.Derse, D. 1987. Bovine leukemia virus transcription is controlled by a virus-encoded trans-acting factor and by cis-acting response elements. J. Virol. 61:2462-2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Felber, B. K., H. Paskalis, C. Kleinman-Ewing, F. Wong-Staal, and G. N. Pavlakis. 1985. The pX protein of HTLV-I is a transcriptional activator of its long terminal repeats. Science 229:675-679. [DOI] [PubMed] [Google Scholar]
- 6.Fujii, M., P. Sassone-Corsi, and I. M. Verma. 1988. c-fos promoter trans-activation by the tax1 protein of human T-cell leukemia virus type I. Proc. Natl. Acad. Sci. USA 85:8526-8530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fujii, M., H. Tsuchiya, T. Chuhjo, T. Akizawa, and M. Seiki. 1992. Interaction of HTLV-1 Tax1 with p67SRF causes the aberrant induction of cellular immediate early genes through CArG boxes. Genes Dev. 6:2066-2076. [DOI] [PubMed] [Google Scholar]
- 8.Fujisawa, J., M. Seiki, T. Kiyokawa, and M. Yoshida. 1985. Functional activation of the long terminal repeat of human T-cell leukemia virus type I by a trans-acting factor. Proc. Natl. Acad. Sci. USA 82:2277-2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grassmann, R., S. Berchtold, I. Radant, M. Alt, B. Fleckenstein, J. G. Sodroski, W. A. Haseltine, and U. Ramstedt. 1992. Role of human T-cell leukemia virus type 1 X region proteins in immortalization of primary human lymphocytes in culture. J. Virol. 66:4570-4575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Katoh, I., Y. Yoshinaka, and Y. Ikawa. 1989. Bovine leukemia virus trans-activator p38tax activates heterologous promoters with a common sequence known as a cAMP-responsive element or the binding site of a cellular transcription factor ATF. EMBO J. 8:497-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matsumoto, K., H. Shibata, J. I. Fujisawa, H. Inoue, A. Hakura, T. Tsukahara, and M. Fujii. 1997. Human T-cell leukemia virus type 1 Tax protein transforms rat fibroblasts via two distinct pathways. J. Virol. 71:4445-4451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miralles, F., I. Ibanez-Tallon, M. Parra, M. Crippa, F. Blasi, D. Besser, Y. Nagamine, and P. Munoz-Canoves. 1999. Transcriptional regulation of the murine urokinase-type plasminogen activator gene in skeletal myoblasts. Thromb. Haemostasis 81:767-774. [PubMed] [Google Scholar]
- 13.Nerenberg, M., S. H. Hinrichs, R. K. Reynolds, G. Khoury, and G. Jay. 1987. The tat gene of human T-lymphotropic virus type 1 induces mesenchymal tumors in transgenic mice. Science 237:1324-1329. [DOI] [PubMed] [Google Scholar]
- 14.Rangnekar, V. M., A. C. Aplin, and V. P. Sukhatme. 1990. The serum and TPA responsive promoter and intron-exon structure of EGR2, a human early growth response gene encoding a zinc finger protein. Nucleic Acids Res. 18:2749-2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sagata, N., T. Yasunaga, J. Tsuzuku-Kawamura, K. Ohishi, Y. Ogawa, and Y. Ikawa. 1985. Complete nucleotide sequence of the genome of bovine leukemia virus: its evolutionary relationship to other retroviruses. Proc. Natl. Acad. Sci. USA 82:677-681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tajima, S., and Y. Aida. 2000. The region between amino acids 245 and 265 of the bovine leukemia virus (BLV) Tax protein restricts transactivation not only via the BLV enhancer but also via other retrovirus enhancers. J. Virol. 74:10939-10949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tanaka, A., C. Takahashi, S. Yamaoka, T. Nosaka, M. Maki, and M. Hatanaka. 1990. Oncogenic transformation by the tax gene of human T-cell leukemia virus type I in vitro. Proc. Natl. Acad. Sci. USA 87:1071-1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsai-Morris, C. H., X. M. Cao, and V. P. Sukhatme. 1988. 5" flanking sequence and genomic structure of Egr-1, a murine mitogen inducible zinc finger encoding gene. Nucleic Acids Res. 16:8835-8846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsuboyama-Kasaoka, N., Y. Hosokawa, H. Kodama, A. Matsumoto, J. Oka, and M. Totani. 1999. Human cysteine dioxygenase gene: structural organization, tissue-specific expression and downregulation by phorbol 12-myristate 13-acetate. Biosci. Biotechnol. Biochem. 63:1017-1024. [DOI] [PubMed] [Google Scholar]
- 20.Willems, L., A. Gegonne, G. Chen, A. Burny, R. Kettmann, and J. Ghysdael. 1987. The bovine leukemia virus p34 is a transactivator protein. EMBO J. 6:3385-3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Willems, L., H. Heremans, G. Chen, D. Portetelle, A. Billiau, A. Burny, and R. Kettmann. 1990. Cooperation between bovine leukaemia virus transactivator protein and Ha-ras oncogene product in cellular transformation. EMBO J. 9:1577-1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yoshida, M. 1996. Molecular biology of HTLV-I: recent progress. J. Acquir. Immune. Defic. Syndr. Hum. Retrovirol. 13(Suppl. 1):S63-S68. [DOI] [PubMed] [Google Scholar]