Abstract
The herpes simplex virus type 1 γ134.5 gene product precludes the host-mediated protein shutoff response induced by activated protein kinase R (PKR). Earlier studies demonstrated that recombinant viruses lacking the γ134.5 gene (Δγ134.5) developed secondary mutations that allowed earlier US11 expression and enabled continued protein synthesis. Further, in vitro studies demonstrated that a recombinant expressed US11 protein binds PKR, blocks the phosphorylation of the α subunit of eukaryotic initiation factor 2 (eIF-2α) by activated PKR, and, if provided prior to PKR activation, precluded PKR autophosphorylation. The present study furthers the hypothesis that early US11 production precludes PKR-mediated host protein shutoff by demonstrating that (i) US11 and PKR interact in the context of viral infection, (ii) this interaction is RNA dependent and requires a 30-amino-acid domain (amino acids 91 to 121) in the carboxyl domain of the US11 protein, (iii) the proteins biochemically colocalize in the S100 ribosomal fraction, and (iv) there is a PKR substrate domain immediately adjacent to the binding domain. The results suggest that the US11 interaction with PKR at the ribosome is RNA dependent and that the US11 protein contains a substrate domain with homology to eIF-2α in close proximity to an essential binding domain.
Most viruses produce double-stranded RNA (dsRNA) during infection, activating interferon-inducible host protein protein kinase R (PKR). The activated PKR precludes protein synthesis in virus-infected cells by phosphorylating the α subunit of eukaryotic initiation factor 2 (eIF-2α) (18). Protein synthesis is integral to productive viral infection, and viruses have evolved diverse strategies to counter PKR-mediated host protein shutoff (1-3, 10, 21, 22). Herpes simplex virus type 1 (HSV-1) is particularly susceptible to PKR-mediated protein shutoff as it encodes genes on both strands of the genome. Previous studies showed that dsRNA is readily produced in the course of HSV-1 infection and that half of the HSV genome is represented in dsRNA (17, 19). As a consequence HSV-1 carries a gene, γ134.5, present in two copies flanking the unique long domain of the genome that subverts the PKR protein shutoff response (6). In HSV-1 infection, the γ134.5 gene product recruits a host phosphatase and dephosphorylates eIF-2α, thus maintaining protein synthesis (16). In contrast, viruses carrying γ134.5 genes with deletions (Δγ134.5) are incapable of maintaining late protein synthesis in human cells following PKR activation and thus display a Δγ134.5 protein shutoff phenotype (7, 8).
Previous studies showed that serially passaged Δγ134.5 viruses incur secondary mutations in the unique short (US) domain that resurrect a cryptic mechanism to evade PKR (23). Studies using a recombinant Δγ134.5 virus (R5104) demonstrated that this change in protein synthesis phenotype is associated with earlier kinetic expression of the US11 gene product and that a viral gene product precludes eIF-2α phosphorylation by activated PKR (4). In vitro assays further demonstrated that recombinant US11 protein physically interacted with PKR, was phosphorylated by activated PKR, and precluded eIF-2α phosphorylation (5). Further biochemical assays also revealed that recombinant US11 protein was especially effective at blocking PKR activation and subsequent eIF-2α phosphorylation when added prior to dsRNA activation (5).
The goals of the present study were threefold: (i) to demonstrate a PKR-US11 interaction in the context of viral infection, (ii) to define the binding and substrate domains of US11 involved in PKR interaction, and (iii) to demonstrate colocalization of US11 and PKR in infected-cell cultures.
MATERIALS AND METHODS
Cells and viruses.
The Vero, HeLa, rabbit skin cell, and SK-N-SH cell lines were obtained from the American Type Culture Collection. The cell lines were propagated in Dulbecco's modified essential medium supplemented with 5% newborn calf serum (Vero and rabbit skin cells) or 10 (SK-N-SH) or 5% (HeLa) fetal bovine serum. HSV-1 (F) is the prototype strain used in this laboratory (12). Recombinant viruses R5103 and R5104 are described elsewhere in detail (4). Briefly, recombinant virus R5103 lacks the native US8 to US12 genes and the γ134.5, ORF O, and ORF P genes and maintains the remainder of the S component frozen in an inverted orientation (Is and Isl). The R5104 virus, derived from R5103, contains the sequence encoding the native US10 gene and the US11 gene fused to the α47 promoter inserted within and disrupting the UL23 locus.
Immunoblotting and generation of a US11 polyclonal serum.
Nitrocellulose sheets containing the electrophoretically separated proteins were incubated in blocking solution-5% skim milk in phosphate-buffered saline lacking Ca and Mg (PBS-A) for at least 1 h, reacted with an antibody diluted in blocking solution for at least 4 h, and then washed five times with PBS-A containing 0.05% Tween. The nitrocellulose filter was next incubated with an appropriate alkaline phosphatase- or peroxidase-conjugated antibody diluted in blocking solution for approximately 90 min. The filter was next washed once in PBS-A-0.05% Tween and then four times in PBS-A. The alkaline phosphatase-stained immunoblots were developed with 150 μg of 5-bromo-4-chloro-3-indolylphosphate (BCIP)/ml and 300 μg of nitroblue tetrazolium/ml in AP buffer (100 mM Tris-HCl [pH 9.5], 5 mM MgCl2, 100 mM NaCl), whereas the peroxidase-stained immunoblots were developed using enhanced chemiluminescence as recommended by the manufacturer (Pierce) (31).
The antibodies used in these studies and their sources are as follows. The rabbit polyclonal antibody to PKR (K-17) was purchased from Santa Cruz Biotechnology, and the monoclonal antibody against US11 was described previously (28). To generate polyclonal antibodies to full-length US11, purified glutathione S-transferase (GST)-US11 was generated from Escherichia coli transformed with plasmid pRB4776 (28). The antibody to full-length US11 was generated using a standard protocol at Galena Biotechnology, Incorporated (Edmonton, Alberta, Canada) in accordance with National Institutes of Health (NIH) guidelines. Specifically, hens were initially immunized by using 100 to 125 μg of purified recombinant GST-US11 with Freund's complete adjuvant followed 3 weeks later by three inoculations with GST-US11 and Freund's incomplete adjuvant at 2-week intervals. The immunoglobulin Y antibody was purified from the eggs of the immunized hens 2 weeks after the last immunization.
Coimmunoprecipitation of S10 fraction PKR with and without RNase treatment.
Replicate 25-cm2 flask cultures of HeLa cells were incubated with 1 ml of 199V or 1 ml of 199V containing 10 PFU of HSV-1 (F), R5103, or R5104/cell. After a 2-h incubation, the inoculum was replaced with 5% newborn calf serum. At 6 h postinfection, medium was removed from mock- and virus-infected cells, ice-cold PBS-A was added and the cells were scraped, transferred to centrifuge tubes, and pelleted. Cytoplasmic S10 fractions were then generated as previously described (9, 15). After the addition of 1 μl of mammalian cell protease inhibitor cocktail (104 mM 4-[2-aminoethyl]benzenesulfonyl fluoride [AEBSF], 80 μM aprotinin, 2.5 mM leupeptin, 36 mM bestatin, 1.5 mM pepstatin A, 1.4 mM E-64) (Sigma P-8340), the lysates were shaken for 30 min at 4°C with 25 μl of protein A conjugated to Sepharose beads and centrifuged, and the supernatants were transferred to new tubes. The samples were then either mock treated or incubated with RNase A (40 μg/ml, final concentration) and RNase T1 (300,000 U/ml, final concentration) for 30 min at 37°C. The mock- and RNase-treated samples were then reacted at 4°C with 1 μg of rabbit polyclonal immunoglobulin anti-PKR (K-17; Santa Cruz Biotechnology) overnight. The immunoprecipitate was incubated with protein A-Sepharose for 2 h, centrifuged, and washed three times with cold PBS-A buffer containing 0.25% NP-40. Samples were resuspended in disruption buffer (50 mM Tris-HCl [pH 7.0], 2% sodium dodecyl sulfate [SDS], 700 mM β-mercaptoethanol, 2.75% sucrose), boiled for 5 min, electrophoretically separated on 13% (vol/vol) polyacrylamide gels cross-linked with N,N"-diallyl-l-tartardiamide containing 0.1% SDS, and electrically transferred to a nitrocellulose sheet, and immunoblotting was performed.
Cosedimentation in S100 pellets and high-salt buffer elutions.
Replicate HeLa cells were mock infected or infected with 10 PFU of HSV-1 (F), R5103, and R5104 virus/cell and harvested at 6 h postinfection. S10 fractions were prepared as previously described using diethyl pyrocarbonate-treated reagents.
The S100 fractions and ribosomal pellet were prepared as described previously (20, 30). Briefly, the S10 equivalent from a 75-cm2 culture was layered on a 3-ml isotonic S100 buffer solution (25 mM HEPES [pH 7.6], 115 mM KCl, 0.3 mM magnesium acetate {Mg[OAc]2}, 10% glycerol, 5 mM spermidine) and centrifuged using a TH-641 rotor in a Sorvall RC-60 ultracentrifuge at 150,000 × g for 2.5 h at 4°C. The ribosomal salt wash fraction, comprising the upper two-thirds of the ultracentrifuge supernatant fraction, was removed, passed over a Sephadex G-25 (Pharmacia) column, and stored. The lower one-third of the supernatant was discarded, and the S100 pellet was resuspended in 100 μl of isotonic buffer (25 mM HEPES, 115 mM KCl, 1 mM spermidine, 10% glycerol); 50 μl of threefold-concentrated 0.3 M salt wash buffer (25 mM HEPES, 785 mM KCl, 15 mM Mg[OAc]2, 1 mM spermidine, 3 mM dithiothreitol [DTT], 10% glycerol) was added, and the mixture was incubated with a stir bar overnight at 4°C. The sample was then loaded on 3 ml of isotonic S100 buffer solution and subjected to ultracentrifugation at 100,000 × g for 4 h at 4°C using a TH-641 rotor. Again the pellet and supernatant portions were divided. The supernatant salt wash fractions were dialyzed (1 ml:4,000 ml) with two buffer changes using 8,000-molecular-weight cutoff dialysis tubing at 4°C overnight. The volume of each dialysate was reduced using a rotary concentrator. The samples were then boiled in protein disruption buffer and stored at −20°C until all samples were prepared. The isotonic S100 pellet was resuspended in 100 μl of isotonic buffer (25 mM HEPES, 115 mM KCl, 1 mM spermidine, 10% glycerol), 50 μl of three-times-concentrated 0.8 M salt wash buffer (25 mM HEPES, 2.17 M KCl, 15 mM Mg[Oac]2, 1 mM spermidine, 3 mM DTT, 10% glycerol) was added, and the mixture was again incubated overnight and then subjected to centrifugation, separation, and concentration as described above for the isotonic fractions. The isotonic, 0.8 M salt wash, and pellet protein samples were then loaded and electrophoretically separated on denaturing polyacrylamide gels and electrically transferred to nitrocellulose filter, and immunoblotting was performed.
Isolation of the tryptic peptide in GST-US11 phosphorylated by PKR.
GST-US11 (40 μg) was phosphorylated by incubation with 48 μg of partially purified, activated PKR as previously described in 30 mM Tris-HCl, pH 7.5-0.07 mM [γ-32P]ATP (13,000 Cerenkov cpm/pmol)-2.5 mM MgCl2 in a final volume of 240 μl at 34°C (13). After 5 h of incubation, the reaction was stopped by the addition of SDS loading buffer, and the reaction mixture was boiled for 1.5 min, loaded into a 5-cm-wide lane of a 7% polyacrylamide-SDS gel, and electrophoretically separated for 20 h at 45 V (3 V/cm). After brief autoradiography of the wet gel, the major phosphorylated band, GST-US11, was excised with reference to the autoradiogram, minced into 1-mm fragments, and digested with 4 μg of trypsin/ml in a final volume of 0.6 ml of gel plus 0.6 ml of 5 mM Tris-HCl, pH 8.0, for 18 h at 34°C. The liquid (containing 70% of the total 32P radioactivity) was removed from the gel fragments and received trifluoroacetic acid at a final concentration of 0.05%. The gel fragments were extracted with 150 μl of 0.05% trifluoroacetic acid, and this extract (containing another 15% of the total 32P radioactivity) was removed and combined with the initial liquid fraction. This pool was chromatographed on an Aquapore RP-300 reverse-phase column (4.6 by 250 mm; Brownlee Laboratories) as previously described (14). The 32P-labeled material was initially eluted with the polypeptide material (as judged by the absorbance at 215 nm) at an acetonitrile concentration (43%) corresponding to partially digested or undigested protein (data not shown). This fraction was pooled, evaporated to dryness, and then redigested with 9 μg of trypsin/ml in 160 μl of 40 mM Tris-HCl, pH 8.1, for 18 h at 34°C. It was then rechromatographed on the reverse-phase column as described above. Most of the 32P that bound to the column was recovered in a single phosphopeptide peak that eluted at 21% acetonitrile. The two (0.5-ml) fractions containing this phosphopeptide were subjected separately to automated amino acid sequencing in an Applied Biosystems sequencer with Cerenkov counting of the eluates from each cycle (14).
RESULTS
US11 binds PKR in R5104-infected cells.
Previous studies have demonstrated that a recombinant expressed PKR protein binds US11 from HSV-infected HeLa cell extracts and that a recombinant expressed US11 protein binds PKR (5, 25). The goal of the first experiment was to determine if US11 and PKR interact in the context of viral infection. To test this hypothesis, HeLa cell cytoplasmic S10 fractions were collected at 6 h postinfection from HeLa cells mock infected or infected with HSV-1 (F), R5103, and R5104 and immunoprecipitation of PKR was performed as described in Materials and Methods. The immunoprecipitated proteins were electrophoretically separated, transferred to nitrocellulose membranes, and probed with a monoclonal antibody to the HSV-1 US11 protein as previously described (28, 31).
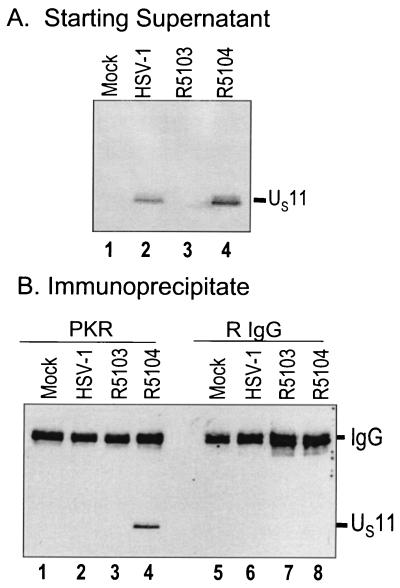
As demonstrated in Fig. 1A, immunoblotting of the starting supernatant samples with anti-US11 serum demonstrated that equivalent quantities of US11 were present in the R5104-infected cell lysate starting solutions used in the coimmunoprecipitation experiments. As expected, the monoclonal antibody to US11 did not react with either the mock- or R5103-infected cell lysates. The R5104-infected cells produced greater amounts of US11 protein than the wild-type virus at 6 h postinfection, consistent with the earlier kinetic expression of US11 in the R5104 virus. The new and significant results of this experiment (Fig. 1B) indicate that US11 and PKR coimmunoprecipitate in the R5104-infected-cell lysates with polyclonal serum against PKR (Santa Cruz Biotechnology; K-17) but not in the samples with rabbit preimmune serum.
FIG. 1.
US11 protein coimmunoprecipitates with PKR in infected HeLa cell cytoplasmic fractions. Replicate cell cultures of HeLa cells were either mock infected or infected with HSV-1 (F), R5103, or R5104 virus, and S10 cytoplasmic fractions were generated. (A) Photograph of an immunoblot of the starting supernatant material. The electrophoretically separated proteins from mock-, HSV-1 (F)-, R5103-, and R5104-infected-HeLa cell S10 fractions (lanes 1 to 4, respectively) were probed with an anti-US11 monoclonal antibody, and the relative amounts of US11 in the starting lysates are shown. (B) Photographic image of electrophoretically separated proteins after immunoprecipitation from the above S10 fractions using 1 μg of anti-PKR antiserum (K-17; Santa Cruz Biotechnology) or rabbit preimmune serum (R IgG; Southern Research Institute). S10 cytoplasmic fractions were precleared with protein A and then incubated either with 1 μg of anti-PKR antibody (K-17; Santa Cruz Biotechnology) or with 1 μg of the rabbit preimmune serum antibody overnight. The complexes were precipitated with protein A-Sepharose and washed three times, and the immunoprecipitated proteins were electrophoretically separated, transferred to nitrocellulose filters, and probed with an antibody to US11.
The carboxyl-terminal US11 RNA binding domain is required for high-level PKR binding in infected cells, and residues 91 to 121 are critical for PKR-US11 interaction.
The previous experiments demonstrated that US11 and PKR interact in infected-cell lysates. The purpose of the next series of experiments was to determine the essential US11 protein domain required for PKR interaction. To identify the minimum domain enabling interaction of PKR and US11 in the context of HSV-1 infection, PKR was immunoprecipitated from cytoplasmic lysates prepared from mock- or recombinant-virus-infected HeLa cells. The recombinant viruses, created by Roller et al., contain deletions within the US11 protein coding region (Fig. 2A) (29). The starting cytoplasmic lysate and immunoprecipitated proteins were then electrophoretically separated, transferred to nitrocellulose membranes, and stained using first a monoclonal antibody against US11 and then a polyclonal serum against US11.
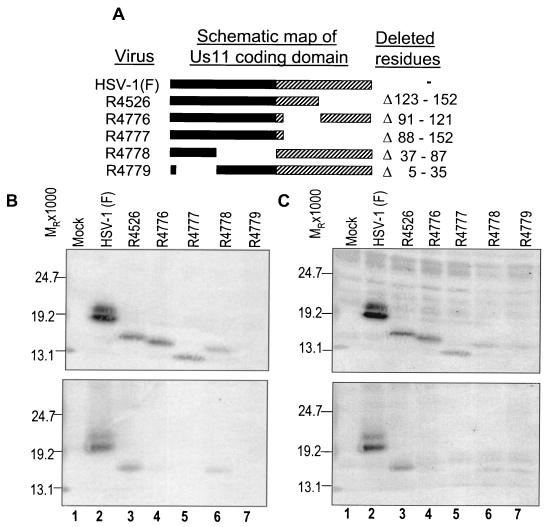
FIG. 2.
Fine mapping of the US11 protein domain required for PKR interaction using recombinant US11 truncation virus-infected HeLa cell cytoplasmic S10 fractions and PKR immunoprecipitation. (A) Redrawing of Roller's schematic depiction of the US11 truncation viruses and the amino acid deletions (29). Solid bars, N-terminal domains of the protein; hatched bars, C-terminal domains comprising tandemly repeated P-R-X amino acids. (B) Photographic depiction of immunoblots. (Top) Photograph of mock- and virus-infected HeLa cell cytoplasmic proteins after electrophoretic separation, transfer to a nitrocellulose membrane, and staining with a mouse anti-US11 monoclonal antibody. (Bottom) Immunoblot of the wild-type and mutant US11 proteins coimmunoprecipitated with 1 μg of rabbit anti-PKR antibody and then stained with an anti-US11 mouse monoclonal antibody. (C) Photograph of the immunoblots from panel B after they were reprobed with a chicken polyclonal antibody against US11 and after the proteins were detected using the alkaline phosphatase-conjugated secondary antibody used for panel B. The new antiserum is capable of detecting the mutant US11 from R4779 in which amino acids 5 to 35 have been deleted.
Figure 2B, top, is an immunoblot of the starting supernatant S10 fraction and demonstrates the relative electrophoretic migration and quantities of the US11 protein from the different recombinant viruses after probing with a mouse US11 monoclonal antibody (27). The US11 monoclonal antibody reacts with an epitope within amino acids 5 to 35 of the US11 protein and therefore cannot detect the US11 protein produced by the R4779 recombinant viruses (Fig. : lane 7). Figure 2B, bottom, shows that the US11 protein coimmunoprecipitated with PKR in the R4526- and R4778-infected cells and was easily detected (Fig. 2B, lanes 3 to 6). However, in the R4776- and R4777-infected cells little or no US11 protein was seen in the immunoprecipitate. These data indicate that deletion of amino acids 88 to 152 of the US11 protein completely eliminated the US11-PKR interaction while deletion of amino acids 91 to 121 largely eliminated the US11-PKR interaction. Disruption of amino acids 121 to 152 or amino acids 37 to 87 of the US11 protein does not alter the interaction of US11 with PKR (Fig. 2B, lanes 3 to 6).
To identify if deletion mutations in the amino-terminal region of US11 interrupted US11 coimmunoprecipitation with PKR, polyclonal serum against full-length US11 was used to reprobe the membranes. Figure 2C, top, is an immunoblot of the starting cytoplasmic proteins and demonstrates that the new antiserum detected the US11 protein in R4779-infected-cell lysates. Figure 2C, bottom, is an immunoblot of the immunoprecipitated proteins and shows that in the R4779-infected cells the US11 protein coimmunoprecipitated with PKR, indicating that elimination of amino acids 5 to 35 of US11 does not disrupt the US11-PKR interaction (Fig. 2C, lane 7). These data indicate that amino acids 91 to 121 within the carboxyl-terminal RNA binding domain of US11 are critical for coimmunoprecipitation with PKR in infected cells and that the amino domain of US11 is not required for this interaction.
RNase A and T1 treatment of R5104-infected-cell S10 fractions interrupts the US11-PKR interaction.
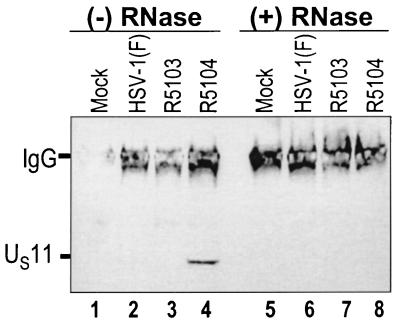
Based on the previous experiments, we next examined whether PKR and US11 interact directly or require an RNA intermediate. To address this question, the coimmunoprecipitation experiment was repeated using mock-infected, wild-type virus-infected, and recombinant R5103- and R5104-infected-HeLa cell lysates which were either mock treated or treated with RNases A and T1 for 30 min at 37°C. The immunoprecipitated proteins were electrophoretically separated, electrically transferred to nitrocellulose membranes, and probed with the mouse monoclonal antibody to US11. The results (Fig. 3) show that, in mock-treated R5104-infected-cell lysates, US11 and PKR coimmunoprecipitated (lane 4) whereas RNase treatment disrupted the US11-PKR interaction in R5104-infected cells (lane 8). These data indicate that PKR and US11 binding in R5104-infected cells is RNase sensitive and support the hypothesis that the US11-PKR interaction is RNA dependent.
FIG. 3.
RNase A and T1 incubation disrupts PKR-US11 interaction. Photograph of an immunoblot probed with an anti-US11 monoclonal antibody. PKR was immunoprecipitated from mock- and virus-infected-cell cytoplasmic lysates after either mock or RNase A and T1 treatment. The immunoprecipitated proteins were washed, disrupted, electrophoretically separated by SDS-PAGE, and transferred to nitrocellulose membranes. The membrane was then developed using an anti-US11 monoclonal antibody and an antimouse peroxidase-conjugated secondary antibody and developed by enhanced chemiluminescence. IgG, immunoglobulin G.
US11 and PKR from infected-HeLa cell cytoplasmic fractions cosediment in the S100 ribosomal fraction and coelute when incubated at a high salt concentration
Previous studies using cytoplasmic fractionation showed that PKR localizes at the ribosome and is present in the S100 pellet portion of disrupted cells (20, 30). Further biochemical analyses showed that PKR eluted from the ribosomal S100 pellet fraction in the presence of high-salt buffer (0.8 M KCl) but remained associated with the ribosomal pellet following incubation in less-stringent salt buffer (isotonic buffer) (20, 30). Additional studies demonstrated that US11 localized to the ribosome (11, 28). To determine the stringency of the US11-PKR interaction at the ribosome, biochemical analysis of the US11-PKR interaction in the ribosome was performed using cosedimentation and high-salt-elution studies. The proteins present in the S100 pellet and salt wash samples were electrophoretically separated, transferred to nitrocellulose membranes, and immunoblotted using antibodies directed against US11 and PKR.
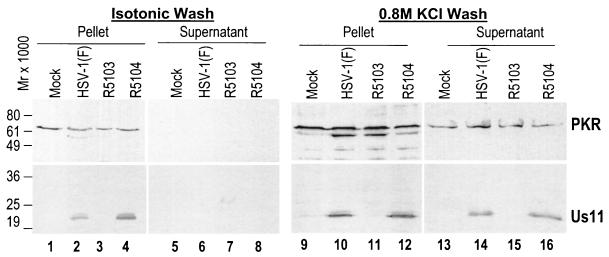
The results shown in Fig. 4, lanes 1 to 8, demonstrate that PKR (top) and US11 (bottom) remained associated with the pellet fraction and that neither protein dissociated into the isotonic salt buffer fraction. However, when the remaining proteins present in the pellet were incubated with 0.8 M KCl buffer, PKR dissociated from the ribosomal fractions and was detectable in the supernatant fraction (top, lanes 13 to 16) of the 0.8 M salt wash fraction of mock-, HSV-1 (F)-, R5103-, and R5104-infected-cell samples, consistent with data reported in the literature (20, 30). When the experiment was repeated and membranes were probed with an antibody directed against the HSV-1 US11 protein, the US11 protein in the HSV-1 (F)- and R5104-infected-cell S100 pellet fraction also eluted (bottom, lanes 14 and 16). These data demonstrate that the US11 protein and PKR protein cosedimented in the S100 ribosomal fraction and coeluted only after incubation at high salt concentration, a characteristic of biochemically colocalizing proteins.
FIG. 4.
Composite photograph of immunoblots of electrophoretically separated S100 ribosomal proteins from mock- or virus-infected HeLa cell after isotonic or high-salt (0.8 M KCl) washes. (Top) Demonstration of PKR in the S100 protein pellet following incubation in isotonic buffer (lanes 1 to 4) and the presence of PKR in the supernatant (lanes 13 to 16) as well as the ribosomal pellet (lanes 9 to 12) following incubation in high-salt-concentration buffer. The US11 protein is detectable only in the S100 ribosomal pellet (lanes 1 to 4) after incubation in isotonic buffer; however, following incubation in high-salt-concentration buffer Us11 is present in both the pellet (lanes 9 to 12) and supernatant (lanes 13 to 16).
US11 protein is phosphorylated by activated PKR on three serine/threonine amino acids adjacent to the PKR binding site.
In R5104-infected cells, PKR is activated and an HSV gene precludes the activated PKR from phosphorylating eIF-2α (4). Further in vitro experiments showed that the US11 protein was phosphorylated by activated PKR in an inverse relationship to eIF-2α phosphorylation (5). These findings suggested that US11 inhibited activated PKR phosphorylation of eIF-2α by providing an alternative substrate site.
To identify the amino acid domain in US11 phosphorylated by activated PKR, a recombinant US11 protein was incubated with purified activated PKR as well as nonactivated PKR in the presence of [γ-32P]ATP and isolated by SDS-polyacrylamide gel electrophoresis (PAGE). Only the US11 incubated with activated PKR was radiolabeled. Following phosphorylation of a preparative amount of GST-US11 by activated PKR in the presence of [γ-32P]ATP, the radiolabeled GST-US11 was isolated by SDS-PAGE, digested with trypsin, run on a reverse-phase column, redigested with trypsin, and then refractionated by reverse-phase chromatography as described in Materials and Methods. Almost all of the 32P that bound to the column was recovered in a single phosphopeptide peak and collected in two fractions, which were subjected separately to automated amino acid sequencing and Cerenkov counting of each Edman cycle. Each analysis gave the sequence VGADTTISKPSEAVRPPTI-R (the dash indicates an unidentified amino acid), demonstrating that the phosphopeptide corresponds to residues 66 to 86 in US11 and that the site of phosphorylation occurs just before a long series of triplet (PRX) repeats that constitutes the carboxyl-terminal region (45%) of the protein. In addition, there were peaks of radioactivity associated with threonine 71, serine 76, and threonine 83. Serine 76 was the most prominently labeled, while threonine 70 and serine 73 were not radiolabeled (Fig. 5). The results indicate that activated PKR selectively phosphorylates 3 amino acids of the US11 protein immediately adjacent to the 30-amino-acid PKR binding domain of PKR.
FIG. 5.
Schematic representation of the HSV-1 (F) US11 amino acid sequence in single-letter code. The sequenced tryptic peptide constituting the site of phosphorylation by activated PKR is in boldface, and the residues phosphorylated by activated PKR are under-lined. The boxed residues represent the required 30-amino-acid PKR binding domain (amino acids 91 to 121).
DISCUSSION
The γ134.5 gene encodes two distinct functions in HSV-1-infected cells. First, it enables efficient virus replication and spread in neuronal tissue. (7). Second, it precludes the PKR-mediated shutoff of protein synthesis in infected human cells (8). During serial passage in infected human cells, secondary compensatory mutations occur; these enable Δγ134.5 recombinants to regain control over protein synthesis in the infected cells (23). These studies are extensions of previous work exploring the mechanism by which secondary mutations enable evasion of the host-mediated protein shutoff response. Prior studies have demonstrated that a viral factor precludes the protein shutoff response by inhibiting the phosphorylation of eIF-2α by activated PKR in Δγ134.5 virus-infected cells (4). Additional studies indicated that early expression of the US11 protein in cells infected with a Δγ134.5 recombinant virus enables viral evasion of the host protein shutoff response (5). Further biochemical studies showed that recombinant US11 can bind PKR and prevent the phosphorylation of eIF-2α by PKR (5).
In this report we determined that US11 and PKR interact and colocalize in the context of virus-infected cells and characterized the binding and substrate domains of US11 instrumental in the PKR interaction.
We first characterized the interaction of US11 and PKR in virus-infected cells. Prior studies using recombinant expressed proteins and infected-cell lysates have shown that PKR and US11 can bind one another. In this report we demonstrated that US11 and PKR interact in the context of virus-infected cells and that the PKR-US11 interaction is dependent on RNA inasmuch as preincubation with RNase disrupts their coimmunoprecipitation. Finally, the proteins colocalized in the S100 ribosomal fraction and coeluted from the ribosomal fraction after incubation at a high salt concentration (0.8 M), indicating biochemical colocalization.
We next defined the binding and substrate domains of US11 involved in the PKR interaction. Using a series of recombinant viruses carrying deletions and premature stop codons in the sequence encoding the US11 protein, we identified a critical 30-amino-acid epitope at the beginning of the tandemly repeated 3-amino-acid arginine-X-proline domain of US11 that enables the interaction with PKR. Further biochemical studies using recombinant US11 and activated PKR demonstrated that a specific kinase substrate region (amino acids 66 to 92) with 22% amino acid identity and 44% amino acid homology with residues 41 to 66 of eIF-2α (Fig. 6) exists adjacent to the PKR binding domain. Fine mapping of the adjacent binding and substrate domains introduces some interesting possibilities with regard to US11-PKR functional interaction.
FIG. 6.
Homology between the substrate site of the HSV-1 US11 protein and eIF-2α. Solid line, identical residues; dots, semiconservative changes. Residues phosphorylated by PKR are in boldface.
Previous work has demonstrated two possible mechanisms for US11 inhibition of PKR. First, biochemical assays using a recombinant US11 protein indicated that the protein can inhibit the phosphorylation of eIF-2α by activated PKR (5). Second, similar biochemical assays demonstrated that when recombinant US11 protein was added prior to poly(I) · poly(C), fractional amounts of the HSV protein blocked PKR activation and phosphorylation of eIF-2α (5). The goal of this work was to extend the previous in vitro studies and analyze the structural and functional basis of US11 in the preclusion of protein shutoff in virus-infected cells.
While prior in vitro data demonstrated that US11 could preclude PKR activation, in HSV-1-infected cells, PKR is activated (4, 9, 15). In R5104-infected cells, early US11 expression only partially restores the wild-type protein synthesis phenotype. The level of protein synthesis, while dramatically greater than that in cells infected with virus carrying Δγ134.5, is still reduced relative to that in wild-type virus-infected cells. The in vitro assays demonstrate that higher levels of recombinant US11 were required to preclude the activated-PKR phosphorylation of eIF-2α. In contrast, when US11 was added prior to PKR activation, the inhibition of eIF-2α phosphorylation was complete and extremely sensitive to small amounts of US11. In the event that inhibition of PKR activation was the principal mechanism, one would suspect a more prominent protein synthesis in the second-site mutant viruses. Furthermore, in the Δγ134.5 suppressor mutant viruses, repair of the γ134.5 locus restores the higher wild-type protein synthesis levels (24). This indicates that some eIF-2α must be phosphorylated in the second-site mutant viruses.
The RNA used in the biochemical studies, poly(I) · poly(C) creates dsRNA of sufficient length to bind and activate PKR. Possible interpretations of the in vitro data are that the recombinant US11 protein sequesters the poly(I) · poly(C) and that US11 acts in a dominant-negative manner precluding PKR dimerization. Based on the data in this report, amino acids 91 to 121 of US11 (within the RNA binding domain) and RNA are required for PKR binding, suggesting that the US11 protein either sequesters RNA or binds PKR and precludes PKR dimerization. What is uncertain, however, is if the poly(I) · poly(C) used in an in vitro experiment reflects the heterogeneity of dsRNA in virus-infected cells. Unlike PKR, the US11 protein does not contain a classic dsRNA binding motif. It binds RNA in a site- and conformation-specific manner and has only been demonstrated to bind two specific viral transcripts, UL34 and a synthetic transcript running antisense to the US11 coding domain, despite >40% of the HSV genome being represented in dsRNA. The poly(I) · poly(C) used in biochemical assays, however, may not reflect the levels of RNA with the correct sequence and conformation compatibility for US11 binding in virus-infected cells.
The alternative mechanism is that US11 inhibits the activated PKR from selectively phosphorylating eIF-2α. While both mechanisms have been demonstrated in vitro, studies using cells infected with a recombinant virus that expresses US11 earlier in infection have demonstrated that a viral factor precludes the phosphorylation of eIF-2α by activated PKR. This suggests that the inhibition of activated PKR is important in cellular infection. Recently a recombinant virus that lacks amino acids 5 to 87 (Δ5-87) of US11 was tested, and reduced levels of late viral protein synthesis were found (26). It was proposed that a reduced quantity of Δ5-87 US11 protein in infected cells was responsible for the significantly lower level of protein synthesis. However, based on in vitro studies extremely low levels (0.8 pmol) of US11 inhibit PKR activation and enable continued protein synthesis (5). Based on the data in this report it is equally plausible that, by deleting the kinase substrate domain, the investigators created a US11 mutant incapable of precluding host protein shutoff effectively. Based on the previous data as well as the data in this report, US11 preclusion of activated PKR from phosphorylating eIF-2α appears a tenable hypothesis.
One of the puzzles regarding the role of US11 in restoring the wild-type protein synthesis phenotype is that the protein is carried in with the virus but appears incapable of precluding host protein shutoff. The US11 protein has been demonstrated by SDS-PAGE analysis as well as two-dimensional electrophoresis to exist in phosphorylated and unphosphorylated forms with different electrophoretic mobilities. Interestingly, in infection with wild-type virus, the virion-associated US11 exists in the phosphorylated form and localizes to the ribosome early in infection (11). Phosphorylation may alter the conformation of US11, making the protein a poor substrate, or may reduce its ability to interact with PKR. Another possibility is that the US11 protein requires a specific RNA for PKR association and that the virion-associated US11 lacks this moiety. The data in this report show that RNase treatment disrupts the PKR-US11 interaction in infected-cell lysates but do not determine if the proteins interact through an RNA bridge. As discussed above US11 binds RNA in a conformation-and sequence-specific manner. It is possible that the RNase preincubation cleaves single-stranded domains and disrupts a complementary stem-loop conformation. These changes could disrupt a specific RNA bridge tethering US11 and PKR or could alter the structure of US11, reducing its ability to bind PKR. Prior studies, however, showed that activated PKR could phosphorylate US11 and that recombinant US11 could preclude eIF-2α phosphorylation independent of infected-cell RNA (5). Ongoing experiments examining specific RNA species in recombinant virus infected cells should clarify these issues.
In summary this work provides additional evidence supporting the hypothesis that US11 inhibits the PKR-mediated host protein shutoff response by demonstrating protein interaction and biochemical colocalization in the context of cellular infection. In addition, fine mapping of the US11 protein demonstrates that RNA and a 30-amino-acid epitope (residues 91 to 121) are necessary for PKR interaction in infected cells. Finally, a PKR kinase substrate site adjacent to the PKR binding site is defined; this site may be important for dissecting the mechanism by which US11 precludes host protein shutoff in cells infected with virus carrying Δγ134.5. Future work will explore the mechanism by which US11 precludes protein shutoff in infected cells, further defining the protein complex and ascribing functional relevance in a cell-based system.
Acknowledgments
We thank Giri Reddy at the University of Chicago for performing the amino acid sequencing and Giang Tong, Na Li, and Suzanne Hessefort for technical assistance.
These studies were aided by grants from the NIH (AI01680, HD28831, and HL30121) and the March of Dimes (FY99-763).
REFERENCES
- 1.Black, T. L., G. N. Barber, and M. G. Katze. 1993. Degradation of the interferon-induced 68,000-Mr protein kinase by poliovirus requires RNA. J. Virol. 67:791-800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brand, S. R., R. Kobayashi, and M. B. Mathews. 1997. The tat protein in human immunodeficiency virus type 1 is a substrate and inhibitor of the interferon-induced, virally activated protein kinase, PKR. J. Biol. Chem. 272:8388-8395. [DOI] [PubMed] [Google Scholar]
- 3.Carroll, K., O. Elroy-Stein, B. Moss, and R. Jagus. 1993. Recombinant vaccinia virus K3L gene product prevents activation of double-stranded RNA-dependent, initiation factor 2α-specific protein kinase. J. Biol. Chem. 268:12837-12842. [PubMed] [Google Scholar]
- 4.Cassady, K. A., M. Gross, and B. Roizman. 1998. The second-site mutation in the herpes simplex virus recombinants lacking the γ134.5 genes precludes shutoff of protein synthesis by blocking phosphorylation of eIF-2α. J. Virol. 72:7005-7011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cassady, K. A., M. Gross, and B. Roizman. 1998. The herpes simplex virus US11 protein effectively compensates for the γ134.5 gene if present before activation of protein kinase R by precluding its phosphorylation and that of the α subunit of eukaryotic translation initiation factor 2. J. Virol. 72:8620-8626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chou, J., E. R. Kern, R. J. Whitley, and B. Roizman. 1990. Mapping of herpes simplex virus-1 neurovirulence to γ134.5, a gene nonessential for growth in cell culture. Science 250:1262-1266. [DOI] [PubMed] [Google Scholar]
- 7.Chou, J., and B. Roizman. 1992. The γ134.5 gene of herpes simplex virus 1 precludes neuroblastoma cells from triggering the total shutoff of protein synthesis characteristic of programmed cell death in neuronal cells. Proc. Natl. Acad. Sci. USA 89:3266-3270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chou, J., and B. Roizman. 1994. Herpes simplex virus 1 γ134.5 gene function, which blocks the host response to infection, maps in the homologous domain of the genes expressed during growth arrest and DNA damage. Proc. Natl. Acad. Sci. USA 91:5247-5251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chou, J., J.-J. Chen, M. Gross, and B. Roizman. 1995. Association of a Mr 90,000 phosphoprotein with protein kinase PKR in cells exhibiting enhanced phosphorylation of translation initiation factor eIF-2α and premature shutoff of protein synthesis after infection with γ134.5− mutants of herpes simplex virus 1. Proc. Natl. Acad. Sci. USA 92:10516-10520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davies, M. V., H. W. Chang, B. L. Jacobs, and R. J. Kaufman. 1993. The E3L and K3L vaccinia virus gene products stimulate translation through inhibition of the double-stranded RNA-dependent protein kinase by different mechanisms. J. Virol. 67:1688-1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diaz, J.-J., D. Simonin, T. Massé, P. Deviller, K. Kindbeiter, L. Denoroy, and J.-J. Madjar. 1993. The herpes simplex virus type 1 US11 gene is a phosphorylated protein found to be non-specifically associated with both ribosomal subunits. J. Gen. Virol. 74:397-406. [DOI] [PubMed] [Google Scholar]
- 12.Ejercito, P. M., E. D. Kieff, and B. Roizman. 1968. Characterization of herpes simplex virus strains differing in their effect on social behavior of cells. J. Gen. Virol. 2:357-364. [DOI] [PubMed] [Google Scholar]
- 13.Gross, M., and D. A. Kaplansky. 1983. Differential effect of Mn2+ on the hemin-controlled translational repressor and the double-stranded RNA-activated inhibitor. Biochim. Biophys. Acta 740:255-263. [DOI] [PubMed] [Google Scholar]
- 14.Gross, M., S. Hessefort, A. Olin, and G. Reddy. 1996. Extensive sequencing of tryptic peptides of a rabbit reticulocyte 66-kDa protein that promotes recycling of hsp 70: homology to stress-related proteins. J. Biol. Chem. 271:16842-16849. [DOI] [PubMed] [Google Scholar]
- 15.He, B., J. Chou, R. Brandimarti, I. Mohr, Y. Gluzman, and B. Roizman. 1997. Suppression of the phenotype of γ134.5− herpes simplex virus 1: failure of activated RNA-dependent protein kinase to shut off protein synthesis is associated with a deletion in the domain of the α47 gene. J. Virol. 71:6049-6054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.He, B., M. Gross, and M. Roizman. 1997. The γ134.5 protein of herpes simplex virus 1 complexes with protein phosphatase 1α to dephosphorylate the α subunit of eukaryotic translation initiation factor 2 and preclude the shutoff of protein synthesis by double stranded RNA-activated protein kinase. Proc. Natl. Acad. Sci. USA 94:843-848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jacquemont, B., and B. Roizman. 1975. RNA synthesis in cells infected with herpes simplex virus. X. Properties of viral symmetric transcripts and double-stranded RNA prepared from them. J. Virol. 15:707-713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Katze, M. 1995. Regulation of interferon-induced PKR: can viruses cope? Trends Microbiol. 3:75-78. [DOI] [PubMed] [Google Scholar]
- 19.Kozak, M., and B. Roizman. 1975. RNA synthesis in cells infected with herpes simplex virus. IX. Evidence for accumulation of abundant symmetric transcripts in nuclei. J. Virol. 15:36-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Langland, J. O., and B. L. Jacobs. 1992. Cytosolic double-stranded RNA-dependent protein kinase is likely a dimer of partially phosphorylated Mr = 66,000 subunits. J. Biol. Chem. 267:10729-10736. [PubMed] [Google Scholar]
- 21.Lu, Y., M. Wambach, M. G. Katze, and R. M. Krug. 1995. Binding of the influenza virus NS1 protein to the protein kinase that phosphorylates the eIF-2 translation initiation factor. Virology 214:222-228. [DOI] [PubMed] [Google Scholar]
- 22.Mathews, M. B., and T. Shenk. 1991. Adenovirus virus-associated RNA and translation control. J. Virol. 65:5657-5662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mohr, I., and Y. Gluzman. 1996. A herpesvirus genetic element which affects translation in the absence of the viral GADD34 function. EMBO J. 15:4759-4766. [PMC free article] [PubMed] [Google Scholar]
- 24.Mohr, I., D. Sternberg, S. Ward, D. Leib, M. Mulvey, and Y. Gluzman. 2001. A herpes simplex virus type 1 γ134.5 second-site suppressor mutant that exhibits enhanced growth in cultured glioblastoma cells is severely attenuated in animals. J. Virol. 75:5189-5196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mulvey, M., J. Poppers, A. Ladd, and I. Mohr. 1999. A herpesvirus ribosome-associated RNA-binding protein confers a growth advantage upon mutants deficient in a GADD34-related function. J. Virol. 73:3375-3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Poppers, J., M. Mulvey, D. Khoo, and I. Mohr. 2000. Inhibition of PKR activation by the proline-rich RNA binding domain of the herpes simplex virus type 1 US11 protein. J. Virol. 74:11215-11221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roller, R. J., and B. Roizman. 1991. Herpes simplex virus 1 RNA binding protein US11 negatively regulates the accumulation of a truncated viral mRNA. J. Virol. 65:5873-5879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roller, R. J., and B. Roizman. 1992. Herpes simplex virus 1 RNA binding protein US11 is a virion component and associates with ribosomal 60S subunits. J. Virol. 66:3624-3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roller, R. J., L. L. Monk, D. Stuart, and B. Roizman. 1996. Structure and function in the herpes simplex virus 1 RNA-binding protein US11: mapping of the domain required for ribosomal and nucleolar association and RNA binding in vitro. J. Virol. 70:2842-2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Samuels, C. E. 1981. Procedures for measurement of phosphorylation of ribosome associated proteins in interferon treated cells. Methods Enzymol. 79:168-178. [DOI] [PubMed] [Google Scholar]
- 31.Ward, P. L., G. Campadelli-Fiume, E. Avitable, and B. Roizman. 1994. Localization and putative function of the UL20 membrane protein in cells infected with herpes simplex virus 1. J. Virol. 68:7406-7417. [DOI] [PMC free article] [PubMed] [Google Scholar]