Abstract
In order to boost immune responses in persons in whom highly active antiretroviral therapy (HAART) was initiated within 120 days of the onset of symptoms of newly acquired human immunodeficiency virus type 1 (HIV-1) infection, we administered vaccines containing a canarypox virus vector, vCP1452, with HIV-1 genes encoding multiple HIV-1 proteins, and recombinant gp160. Fifteen HIV-1-infected subjects who achieved sustained suppression of plasma viremia for at least 2 years were enrolled. While continuing antiretroviral therapy, each subject received at least four intramuscular injections of the vaccines on days 0, 30, 90, and 180. Adverse events were mild, with the most common being transient tenderness at the vCP1452 injection site. Of the 14 patients who completed vaccination, 13 had significant increases in anti-gp120 or anti-p24 antibody titers, and 9 had transient augmentation of their T-cell proliferation responses to gp160 and/or p24. HIV-1-specific CD8+ T cells were quantified using an intracellular gamma interferon staining assay. Among 11 patients who had increased CD8+ T-cell responses, seven had responses to more than one HIV-1 antigen. In summary, vaccination with vCP1452 and recombinant gp160 appears safe and immunogenic in newly HIV-1-infected patients on HAART.
Administering highly active antiretroviral therapy (HAART) to human immunodeficiency virus type 1 (HIV-1)-infected individuals results in a rapid, sustained, and highly significant reduction of plasma viremia in most patients (23, 58). The virologic and immunologic consequences of HAART have resulted in a dramatic reduction in HIV-1 infection-related morbidity and mortality (39). However, the existence of latently infected resting memory CD4+ T cells has made the eradication of HIV-1 infection with HAART alone problematic (6, 15, 59). The goal of eradication may be even more difficult to attain due to the presence of residual viral replication during therapy (14, 19, 41, 61). As a result, irrespective of the time of initiation of therapy, cessation of HAART is accompanied by a rebound in viremia in days to weeks in most if not all treated patients (11, 20, 21, 36).
These findings are clear indicators that current HAART regimens alone are unable to reduce total body viral burden to levels controllable by host immune responses in the absence of drug. Given the long-term toxicities of HIV-1 therapies, the risk of the emergence of drug resistance, and the cost of life-long HAART, the need to define treatment strategies to limit drug exposure has become critical.
To achieve durable viral suppression after a finite course of HAART, alternative treatment strategies are needed. Several lines of evidence suggest that strong cellular immune responses contribute to the control of retroviral replication in the absence of antiretroviral treatment (5, 24, 27, 37, 44, 47, 49). Therefore, we hypothesized that the use of adjunctive vaccination, if capable of augmenting HIV-1-specific immune responses, may provide a beneficial virologic outcome in HIV-1-infected persons treated with HAART who elect to discontinue therapy.
Studies suggest that an effective HIV-1 vaccine, either therapeutic or preventative, should stimulate broadly reactive humoral and cellular immunity, in particular cytotoxic T-lymphocyte (CTL) responses. A number of experimental vaccines have conferred protective immunity against intracellular pathogens, such as malaria, by stimulating strong immune responses in animal models (50, 52). Vaccine strategies directed against HIV-1 include the use of recombinant proteins, peptides, recombinant bacterial or viral vectors, and DNA (9; NIH AIDS Vaccine Evaluation Group, posting date 9 September 1999).
Recombinant protein and peptide vaccines are single-component vaccines that stimulate either humoral or cellular immune responses, but not both, and thus are not ideal candidates in a therapeutic setting. The bacterial vectors and DNA vaccines in development were not available for use in seronegative or seropositive individuals when we initiated this trial. However, at the time this trial was developed, limited studies using earlier versions of the recombinant canarypox viruses (ALVAC) in combination with HIV-1 envelope proteins had been performed. It had been demonstrated that this strategy was safe in uninfected (12) as well as HIV-1-infected individuals (40). Furthermore, these earlier constructs were also capable of generating some degree of humoral and cellular immunity in seronegative individuals (1, 7, 16).
The excellent safety record of ALVAC vaccines is attributed to their virologic properties. Canarypox viruses belong to the Avipox genus of the Orthopoxviridae family of DNA viruses. Although replicating well in avian cells, they do not replicate productively in mammalian cells (53-56). Despite a self-limiting abortive replication cycle, canarypox vaccine vectors can efficiently infect antigen-presenting cells and express proteins encoded by inserted genes under the control of early promoters (13). This may result in sufficient antigenic stimulation to elicit cellular immune responses.
Several generations of ALVAC vaccines have been designed and constructed over time to include single or multiple HIV-1 genes (35). The vector used in the current study, vCP1452, is a third-generation ALVAC vaccine, which includes not only multiple HIV-1 genes and a number of HLA-A2-restricted CTL epitopes from both the Pol and Nef regions of HIV-1, but also two vaccinia virus-derived antiapoptosis genes to enhance protein expression in mammalian cells. We therefore selected this recombinant vaccine to be the cornerstone of our vaccine strategy.
Here, we describe the first safety and immunogenicity study of the novel recombinant vCP1452 in combination with recombinant gp160 in 15 newly HIV-1-infected patients who were treated with HAART and achieved durable viral suppression for a minimum of 2 years. To determine levels of vaccine-induced CD8+ T-cell-mediated HIV-1-specific immune responses, we developed an assay that used both the property of cytokine production from stimulated T cells and an objective gauging instrument, fluorescence-activated cell sorting (FACS), as a read-out. This assay is detailed below. We believe this pilot study establishes the scientific rationale for future investigations combining therapeutic vaccines and HAART as a strategy to treat HIV-1 infection.
MATERIALS AND METHODS
Patients.
Individuals included in the vaccine study were diagnosed and treated within 120 days of the onset of symptoms of acute HIV-1 infection. Treatment included zidovudine and lamivudine in combination with ritonavir, indinavir, ritonavir/saquinavir, or abacavir/amprenavir (Table 1). Inclusion criteria required participants to have sustained levels of plasma HIV-1 RNA below the detection level of 50 copies/ml (less than one detectable HIV-1 RNA determination per treatment year). All vaccine recipients were born prior to 1972 (latest, 1967) and had received previous vaccination with vaccinia virus. Subjects were treated for 983 days on average (range, 807 to 1,225 days); their CD4 counts were 779 on average (range, 449 to 1,311/μl) (Table 1). In addition, their HLA-A, -B, and -C loci were determined by using sequence-specific primers according to published methods (10, 38) (Table 1). All studies were approved by the Rockefeller University Institutional Review Board and conducted in accordance with good clinical practices. Written informed consent was obtained from all study participants.
TABLE 1.
Patient characteristics
| Subject no. | Time from onset of symptoms to treatment (days) | Baseline findings
|
Treatmenta | Duration of HAART (days) | HLA types
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| HIV-1 RNA (log10) | CD4+ T cells (cells/mm3) | A1 | A2 | B1 | B2 | C1 | C2 | ||||
| 313-2 | 90 | 3.9 | 564 | A | 1,182 | 2 | 2 | 15 | 39 | 3 | 12 |
| 313-7 | 90 | 5.0 | 534 | A | 1,070 | 33 | 70 | 14 | 56 | 3 | 8 |
| 900 | 120 | 4.1 | 609 | D | 974 | 1 | 4 | 7 | 9 | 7 | 7 |
| 904 | 60 | 4.4 | 689 | D | 1,066 | 11 | 23 | 15 | 59 | 3 | 3 |
| 905 | 60 | 5.1 | 954 | D | 927 | 3 | 31 | 13 | 36 | 6 | 8 |
| 908 | 80 | 4.4 | 307 | D | 962 | 16 | 69 | 39 | 50 | 7 | 7 |
| 918 | 9 | 6.2 | 1,121 | D | 867 | 1 | 33 | 8 | 36 | 4 | 8 |
| 921 | 30 | 5.6 | 311 | D | 825 | 1 | 1 | 8 | 8 | 7 | 7 |
| 1306 | 30 | 5.3 | 546 | B | 817 | 2 | 2 | 13 | 56 | 3 | 7 |
| 1308 | 9 | 6.2 | 432 | B | 1,038 | 2 | 32 | 40 | 42 | 2 | 18 |
| 1309 | 90 | 4.2 | 500 | B | 807 | 2 | 30 | 18 | 45 | 5 | 5 |
| 1310 | 120 | 4.0 | 387 | B | 1,020 | 3 | 24 | 56 | 58 | 7 | 19 |
| 2001 | 60 | 4.6 | 290 | C | 1,225 | 1 | 3 | 35 | 52 | 4 | 16 |
| 3002 | 60 | 5.0 | 459 | C | 992 | 1 | 2 | 15 | 38 | 3 | 7 |
| Mean ± SD | 65 ± 36 | 4.9 ± 0.8 | 550 ± 240 | 984 ± 129 | |||||||
A, zidovudine (ZDV), lamivudine (3TC), and ritonavir (RTV); B, ZDV/3TC/indinavir (IDV); C, ZDV/3TC/RTV/saquinavir; D, ZDV/3TC/abacavir/amprenavir.
Vaccines and vaccination schedule.
Each patient received at least four vaccinations at days 0, 30, 90, and 180. Each vaccination included 107 50% tissue culture infective doses of ALVAC vCP1452 and 50 μg of recombinant gp160 (rgp160), both kindly provided by Aventis Pasteur (Lyon, France). The recombinant canarypox virus ALVAC vCP1452 expresses the products of several HIV-1 genes, including gp120, expressed by a part of the env gene of the HIV-1 MN strain, and the anchoring transmembrane region of gp41 of the HIV-1 LAI strain; the p55 polyprotein expressed by the gag gene of the HIV-1 LAI strain: a portion of the pol gene sufficient to express protease activity from the HIV-1 LAI strain in order to process the p55gag polyprotein; and genes expressing peptides from pol and nef known to be HLA-A2-restricted cytotoxic T-cell lymphocyte epitopes. Two vaccinia virus-derived coding sequences are also incorporated in the recombinant virus to improve RNA translation and the expression of HIV-1 gene products.
The recombinant vaccine virus was grown on pathogen-free chicken embryo fibroblasts, and the vaccine was suspended in a solution of serum-free, antibiotic-free culture medium containing virus stabilizers and lyophilized. The recombinant gp160 is a hybrid glycoprotein consisting of gp120 from the HIV-1 strain MN and gp41 from the HIV-1 strain LAI.
Vaccines were given intramuscularly in the forearm (ALVAC on the left, rgp160 on the right). After each vaccination, patients were observed in the clinic for 30 min for potential side effects. In addition, either a physician or a nurse contacted all vaccine recipients within 72 h of each vaccination to document any adverse events. Subjects were followed weekly for 2 weeks following the first vaccine and 1 week after each subsequent vaccination. HAART was continued throughout the vaccination period. Patient diaries were also provided to record adverse events.
Virologic determinations.
Longitudinal plasma HIV-1 RNA levels were measured using a reverse transcription PCR (Amplicor HIV-1 Monitor Ultra Sensitive; Roche Molecular Systems, Inc., Alameda, Calif.) that has a lower limit of detection of 50 HIV-1 RNA copies/ml.
Detection of antibody responses.
Levels of plasma antibody to HIV-1 gp120JRFL (Progenics, Tarrytown, N.Y.) and p24SF2 (Chiron, Emeryville, Calif.) proteins were detected using a standard enzyme-linked immunosorbent assay (ELISA) protocol as described previously (4).
Lymphocyte proliferation assay.
The proliferative responses of peripheral blood mononuclear cells (PBMC) were measured using a standard [3H]thymidine (DuPont NEN, Boston, Mass.) incorporation assay as described (48). The concentration of HIV-1 antigens used for stimulation was 5 μg/ml for both HIV-1 gp160LAV and p24NY5 (Protein Sciences, Meriden, Conn.).
ICS.
For intracellular cytokine staining, aliquots of 0.5 × 106 to 1 × 106 cryopreserved PBMC from patients were infected with recombinant vaccinia viruses expressing HIV-1 Env, Gag, Pol, Nef, or control Eco antigens (Virogenetics, Troy, N.Y.) at a multiplicity of infection (MOI) of 2.0 for 18 to 20 h at 37°C. Then 10 μg/ml of brefeldin A (Golgiplug; PharMingen, San Diego, Calif.) was added during the last 5 h of incubation. The cells were stained with anti-CD3PE, -CD4APC, and -CD8PerCP (Becton-Dickson, San Jose, Calif.) antibodies for 30 min at 4°C. After washing, cells were permeablized with CytoFix/Cytoperm solution (PharMingen, San Diego, Calif.), then stained intracellularly by an anti-gamma interferon (IFN-γ)-fluorescein isothiocyanate antibody (PharMingen, San Diego, Calif.) before being analyzed using a FACScalibur flow cytometer.
The FACS data were analyzed with CellQuest (Becton Dickinson, San Jose, Calif.) software by first gating on small CD3+ T cells followed by analysis of the CD8+- and IFN-γ-staining cell populations. The results were expressed as the percentage of CD8+ T cells producing IFN-γ. In the initial 195 consecutive assays in 43 HIV-1-infected individuals, the negative control antigen Eco stimulated 0.02 ± 0.03% of CD8+ T cells to produce IFN-γ, whereas 5 μg of of the positive control superantigen, staphylococcal enterotoxin B (SEB), per ml stimulated between 1 and 20% of the CD8+ T cells to produce IFN-γ. Based on these preliminary experiments, 0.05% of IFN-γ-producing cells was determined to be significantly above the background and considered a positive value.
RESULTS
Safety of vCP1452.
Fifteen subjects who satisfied the entry criteria and gave informed consent were consecutively enrolled from various studies of HAART during early and acute HIV-1 infection conducted at the Aaron Diamond AIDS Research Center. A total of 62 vaccinations were administered to 15 subjects. One subject was lost to follow-up after two vaccinations and was excluded from all subsequent analyses except for safety assessments. The vaccine recipients were mainly Caucasian males, infected sexually, and symptomatic for 9 to 120 days before initiating treatment.
On average, CD4+ T cells increased by 246 cells/μl, and levels of HIV-1 RNA fell 3.2 logs to undetectable levels during a mean of 984 days of treatment with HAART (range, 807 to 1,225 days) (Table 1). Each patient received at least four vaccinations on days 0, 30, 90, and 180. ALVAC vCP1452 and rgp160 vaccines as described above were given intramuscularly in the left and right upper forearms, respectively. Two subjects received a fifth vaccination (313-2 and 1306) on day 270. Subject 313-7 received additional vaccinations on days 270 and 340. All vaccine recipients were given the option of continuing HAART with or without additional vaccinations at 90-day intervals as well as elective discontinuation of antiviral therapy at least 7 days following their fourth or final vaccination.
Transient tenderness in the left arm at the ALVAC 1452 injection site, without swelling or redness, was the most common adverse event reported, occurred in 36 of 62 (58%) vaccinations, and was reported at least once in all 15 subjects. One episode of low-grade fever between 99 and 100°F occurred in 4 of 62 (6.4%) vaccinations in four subjects. Mild transient headache occurred in 4 of 62 vaccinations in four subjects. Headache and low-grade fever elevation did not occur concurrently. All adverse events were mild and of short duration and resolved either without therapy or after treatment with ibuprofen or acetaminophen.
Virologic parameters and levels of CD4+ T cells changed minimally during vaccination.
Prevaccination plasma viremia levels in all study subjects fell below the detection limit of 50 copies/ml within 6 months of initiating HAART. All subjects experienced less than one episode of measurable viremia per treatment year during the subsequent 20 to 40 months before receiving the first vaccination.
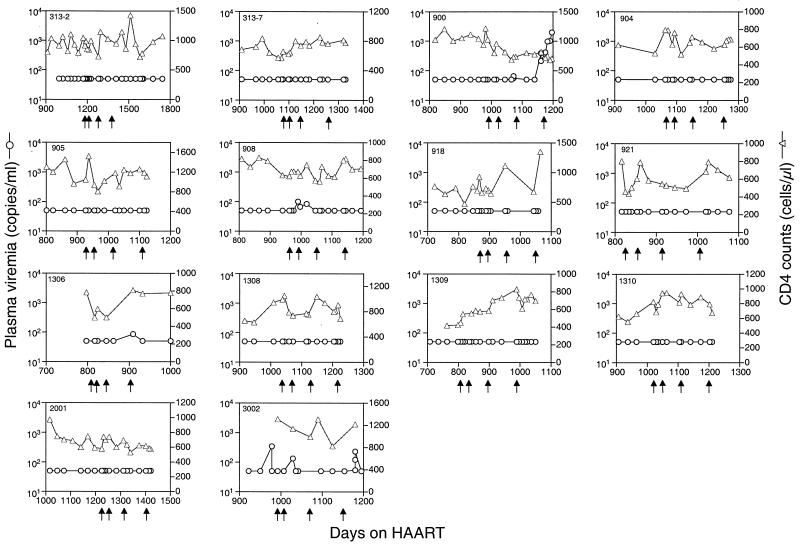
During vaccination, occasional episodes of low-level viremia were observed in four patients (900, 908, 1306, and 3002) (Fig. 1). Viremia in subject 900 was due to admitted nonadherence to the HAART regimen and occurred late in the course of vaccination. An unexplained period of plasma viremia of 100 copies or less appeared 4 weeks after subject 908 received the first vaccination. Despite receiving subsequent vaccines, plasma viremia returned to undetectable levels within 1 month and remained so for the duration of the vaccine study. Subject 3002 had low-level viremia temporally associated with the second vaccination. Subject 1306 had one isolated viremic episode of 85 copies/ml during the 3-month interval between the third and fourth vaccinations that was temporally unrelated to observed vaccine-induced immune responses.
FIG. 1.
Virological and immunological changes observed during vaccination. The plasma HIV-1 RNA levels (circles) and CD4 counts (triangles) of each patient were assessed longitudinally. Each panel represents results for one patient, and the arrows indicate days of vaccination.
There was no apparent detrimental effect on CD4+ T-cell counts associated with vaccination. In fact, levels of CD4+ T cells were generally maintained in all vaccine recipients (Fig. 1). There were no significant changes in lymphocyte subsets or markers of activation during the study period. Both CD45-RA+ and -RO+ cells increased from a baseline of 261 and 491 cells to 329 and 542 cells/μl, respectively. The activation status of CD4+ T cells, as measured by HLA-DR and Ki67 expression, varied little pre- and postvaccination (data not shown).
Vaccination boosted HIV-1 binding antibody production.
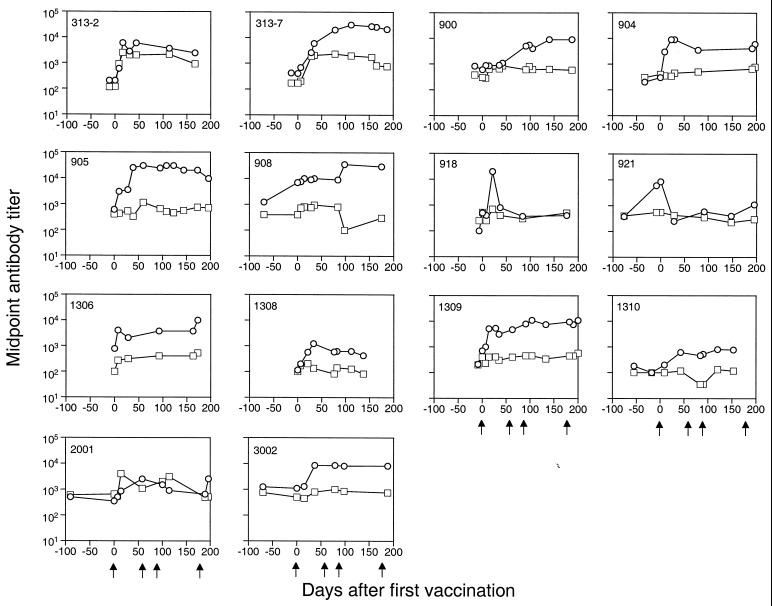
The binding antibody responses to HIV-1 Gag-p24 and Env-gp120 were monitored longitudinally. All except one patient (921) had a 0.5 to 2 log increase in anti-gp120 antibody levels postvaccination. In addition, three patients (313-2, 313-7, and 2001) had a marked increase (1.0 ± 0.3 log) in anti-p24 response (Fig. 2, Table 2). The heightened anti-p24 response in these subjects was temporally associated with a significant augmentation of the T-cell proliferative response to the same antigen (Fig. 3). Of note, the antibody levels generally reached a plateau after the second or third vaccination (Fig. 2). Subject 921 not only failed to mount a humoral response to vaccination but exhibited an unexplained precipitous drop in the level of anti-gp120 antibody postvaccination (Fig. 2). Levels of antibody to gp120 in subject 918 increased more than 2.0 log shortly after the first vaccination but also exhibited a rapid decline. This individual did not develop a humoral response to subsequent vaccinations (Fig. 2).
FIG. 2.
Vaccination boosted binding antibody responses to HIV-1 antigens. The levels of anti-gp120 (circles) and anti-p24 (squares) antibodies in plasma were quantified using a standard ELISA and are expressed as a midpoint antibody titer.
TABLE 2.
Immune responses after vaccination
| Subject no. | Antibodiesa
|
Stimulation indexb
|
Specific CD8+ T cellsc
|
|||||
|---|---|---|---|---|---|---|---|---|
| Anti-p24 | Anti-gp120 | Anti-p24 | Anti-gp160 | Gag | Pol | Env | Pol/Nef | |
| 313-2 | ++ | ++ | ++ | ++ | − | − | − | − |
| 313-7 | + | ++ | ++ | ++ | + | − | − | − |
| 900 | − | ++ | ++ | ++ | +++ | ++ | +++ | +++ |
| 904 | − | ++ | ++ | − | ++ | + | − | − |
| 905 | − | ++ | − | + | − | + | − | − |
| 908 | − | ++ | ++ | ++ | + | + | − | ++ |
| 918 | − | +++ | − | − | − | − | − | ++ |
| 921 | − | − | − | − | + | − | + | + |
| 1306 | − | + | − | − | + | − | − | + |
| 1308 | − | ++ | + | + | − | − | − | − |
| 1309 | − | ++ | + | + | − | ++ | − | ++ |
| 1310 | − | + | − | − | ++ | − | − | − |
| 2001 | + | + | − | − | ++ | − | − | + |
| 3002 | − | + | ++ | + | − | − | − | − |
Antibody titer increase: −, <0.5 log; +, between 0.5 and 1.0 log; ++, between 1.0 and 2.0 logs; +++, m more than 2.0 logs.
T-cell proliferation stimulation index increase: −, <5; +, between 5 and 20; ++, between 20 and 100; +++, more than 100.
IFN-γ+ CD8+ cell increase: −, <0.05%; +, between 0.05 and 0.10%; ++, between 0.1 and 0.5%; +++, more than 0.5%.
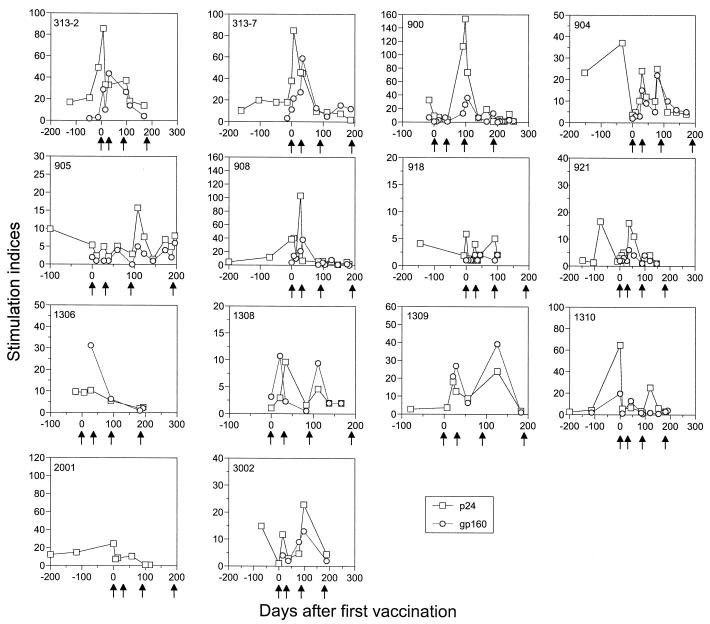
FIG. 3.
Transient elevation of T helper cell responses was achieved by vaccination. T-cell proliferative responses to HIV-1 gp160 (circles) and p24 (squares) were measured using freshly isolated PBMC from each patient. The results are expressed as stimulation indexes, where control antigen stimulation was given a value of 1.
To determine whether neutralizing antibodies to laboratory strains of HIV-1 were induced by vaccination, five plasma samples from two subjects (313-2 and 1309) were used in neutralization assays against a CCR-5-using virus, JR-CSF, and a CXCR-4-using virus, NL4-3. Neither subject exhibited neutralizing activity at any time point postvaccination (data not shown).
Transient T helper cell responses were induced by vaccination.
HIV-1-specific T helper cell activity was examined using a standard lymphocyte proliferation assay with freshly isolated PBMC (48). Eight vaccinated subjects (313-2, 313-7, 900, 904, 908, 1308, 1309, and 3002) had early, transient elevations of anti-gp160 responses that returned to baseline levels soon after the initial peak. Gag-p24-specific T helper cell responses were briefly augmented in eight vaccine recipients (313-2, 313-7, 900, 905, 908, 1308, 1309, and 3002) (Fig. 3, Table 2). Seven of these eight patients also had Env-gp160-specific T-cell proliferative responses, and two of them (313-2 and 313-7) had strong anti-p24 antibody responses. Of note, the p24- and gp160-specific responses were boosted for a second time in three subjects (904, 1308, and 1309) with further vaccination, but were not sustained (Fig. 3).
Quantifying the number of virus-specific IFN-γ-producing CD8+ T cells using intracellular cytokine staining.
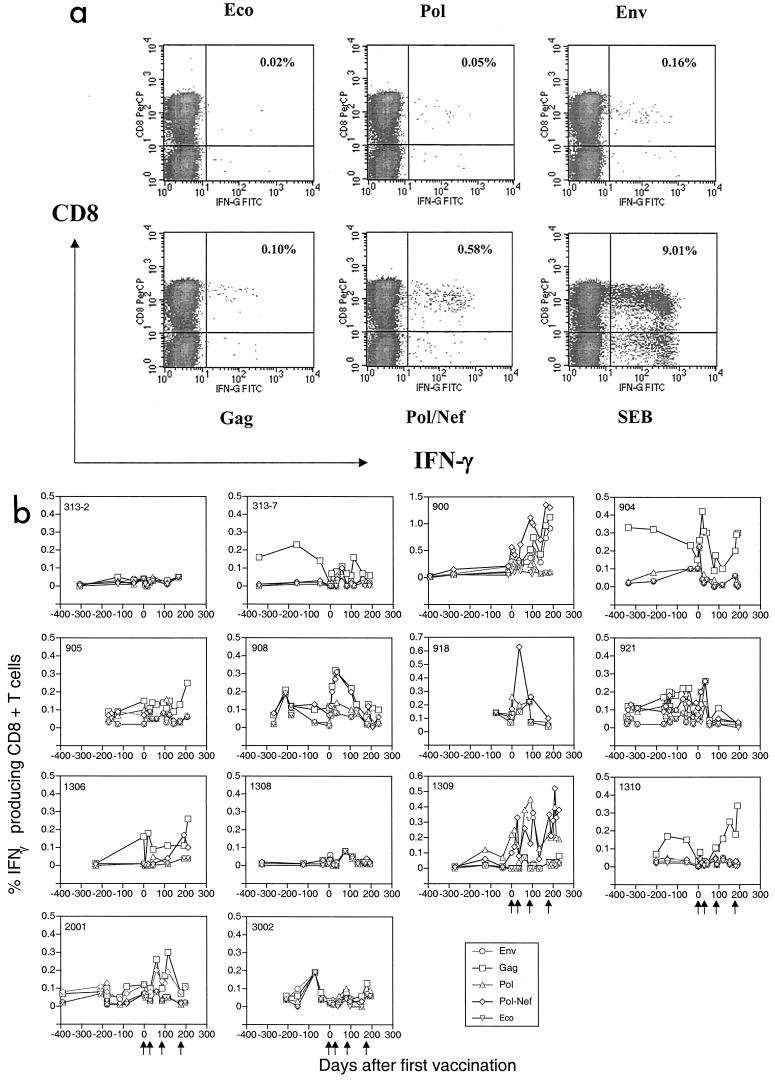
At the time of study initiation in 1998, we decided to explore the possibility of developing an assay that used both the property of cytokine production from stimulated T cells and an objective gauging instrument, FACS, as a read-out. This assay, called intracellular cytokine staining, is illustrated by the following example. Cryopreserved PBMC from an HIV-infected patient were stimulated with recombinant vaccinia virus expressing various HIV-1 or control antigens for 18 to 20 h at 37°C. Then 10 μg/ml BFA was added for the last 6 h of incubation. The cells were first stained with anti-CD3, -4, and -8 antibodies and then stained with an anti-IFN-γ antibody and subjected to analysis by FACS. During the analysis, we gate on the CD3+ small lymphocyte and display a two-dimensional density plot of CD8 versus IFN-γ.
As shown in a typical experimental result (Fig. 4a), only 0.02% of CD8+ T cells produced IFN-γ in response to stimulation with a non-HIV antigen (Eco). In the positive control samples stimulated with SEB, 9% of the total CD8+ T cells produced IFN-γ. The range of IFN-γ produced by HIV-1 antigenic stimulation lies between the positive and negative controls. Env, Gag, and Pol/Nef stimulated 0.16%, 0.10%, and 0.58% CD8+ T cells, respectively, while Pol stimulated minimal IFN-γ production in this patient (Fig. 4a).
FIG.4.
(a) Precise quantitation of HIV-specific CD8+ T-cell responses using the intracellular cytokine staining assay. PBMC from one HIV-infected individual were stimulated by vaccinia virus expression negative control Eco, HIV antigens (Env, Gag, Pol, and Nef) and positive control SEB first, following by staining with anti-CD3, -CD4, -CD8 and -IFN-γ antibodies. The percentages of antigen-specific CD8+ T cells were enumerated and are expressed as a percentage on the upper right quadrant of each plot. (b) Persistent increase in HIV-1-specific CD8+ T cells during vaccination. Longitudinal PBMC samples from each vaccine recipient were used for assessing HIV-1 or control antigen-specific CD8+ T-cell responses. The results were summarized as the percentage of IFN-γ-producing CD8+ T cells to Env (circles), Gag (squares), Pol (triangles), and Pol/Nef (diamonds) after the value for background Eco (inverted diamonds) was subtracted.
To determine if this newly developed intracellular cytokine staining assay was suitable for the study of HIV-1-specific CD8+ T-cell responses in HAART-treated patients, we performed this assay on samples from a large number of such individuals. We performed 195 assays for 43 patients and found that the negative control antigen Eco stimulated 0.02 ± 0.03% of CD8+ T cells to produce IFN-γ, whereas 5 μg/ml of the positive control superantigen, SEB, stimulated between 1 and 20% of the CD8+ T cells to produce IFN-γ (data not shown). Based on these preliminary experiments, 0.05% (mean + 1 standard deviation) of IFN-γ-producing cells was determined to be significantly above the background and considered a positive value.
Persistent HIV-1-specific CD8+ T-cell responses were induced by vaccination in the majority of patients.
The CD8+ T-cell responses to vaccination were quantified longitudinally (Fig. 4b). Seven subjects (1306, 1309, 900, 904, 908, 921, and 2001) had an elevation in CD8+ T-cell responses to more than one HIV-1 antigen. Four subjects (313-7, 905, 918, and 1310) had a measurable response to a single antigen, and the remaining three patients (313-2, 1308, and 3002) had no appreciable response to any antigen (Table 2). Cumulatively, 11 of 14 (78%) subjects had an increase in CD8+ T-cell responses to at least one HIV-1 antigen.
Most patients who had a response recognized Gag (7 of 14) or Pol (5 of 14), and less frequently Env (2 of 14). Although more patients (8 of 14) responded to Pol/Nef, we cannot determine whether Pol or Nef was recognized. However, two patients (1310 and 918) recognized Pol/Nef but not Pol, suggesting that Nef epitopes in the ALVAC vaccine stimulated CD8+ T-cell responses in at least a small number of participants. It is important to observe that these vaccine-induced HIV-1-specific CD8+ T-cell responses persisted throughout the vaccine study period.
HIV-1-specific CD8+ T-cell responses in these vaccine recipients were also analyzed using a major histocompatibility complex class I tetramer containing the A2/Gag and A2/Pol peptides as described (36) in five HLA-A2-positive individuals (313-2, 1306, 1308, 1309, and 3002) included in this study. In two patients (1306 and 3002), whose total number of Gag-specific CD8+ T cells increased after the vaccinations, the A2/Gag tetramer-staining cells increased twofold (0.04 to 0.08%). There were similar increases in the number of A2/Pol tetramer-staining cells (0.04 to 0.09%) in another patient (1309) whose Pol-specific CD8+ T cells were augmented after vaccination (data not shown).
DISCUSSION
In this therapeutic vaccination study, we evaluated the safety and immunogenicity of vCP1452 plus recombinant gp160 in 15 HIV-1-infected patients who were diagnosed and treated within 120 days after the onset of symptoms of newly acquired HIV-1 infection. Fourteen subjects completed the vaccination trial and have been assessed for virologic and immunologic changes pre- and postvaccination. The lack of significant (moderate or severe) adverse events establishes the safety of this vaccine regimen in this cohort of HIV-1-infected and HAART-treated patients. Importantly, these vaccines are also immunogenic. Specifically, all except one patient (93%) had increases in anti-gp120 or anti-p24 antibody titers. Nine out of 14 (64%) patients had an early and transient increase in CD4+ T-cell responses to either Env or Gag, and 11 out of 14 (78%) vaccinated subjects had an augmentation of HIV-1-specific CD8+ T-cell responses postvaccination.
We used a novel FACS-based intracellular cytokine staining assay to assess CD8+ T-cell-specific responses. We believe that this assay represents major advantages over standard assays that were available at the time we initiated this trial. Specifically, we elected to avoid the laborious limiting-dilution assay based on chromium release (26) and the very restrictive and costly tetramer binding assay (3, 37). It is important that FACS-based intracellular cytokine staining assays using overlapping HIV-1 peptides and vaccinia virus constructs to more accurately assess immune responses have become commonplace (2). Importantly, the assay we used was highly reproducible and standardized, and the vaccinia virus vectors contained the same HIV-1 proteins as those in the vaccine being tested in this clinical trial.
Previous therapeutic vaccine studies have been done in the setting of nonsuppressive antiretroviral therapy and used monovalent vaccines. The diversity of immune responses generated by these vaccines was limited (28, 30, 34, 42, 51, 57). In contrast, the vaccine regimen that we used stimulated both humoral and cellular immunity in a majority of patients. Furthermore, CD8+ T-cell responses to all four major HIV-1 gene products included in the canarypox virus vector, Env, Gag, Pol, and Nef, were boosted. It is remarkable to note that over 50% of the vaccine recipients had CD8+ T-cell responses to more than one HIV-1 antigen.
Previous studies using a similar vaccine regimen, albeit an earlier generation of ALVAC vaccine, have shown this strategy to be capable of eliciting both humoral and cellular immune responses in a proportion of vaccinated seronegative persons (7, 12). The strategy of priming with ALVAC containing a single HIV env gene and boosting with rgp120 elicited cumulative envelope-specific CTL responses in over one third of seronegative vaccine recipients (7, 16). When a multivalent canarypox vector was used, 61% of the seronegative vaccine recipients had a CTL response detected at least one time point postvaccination (12). The cellular immune responses, however, were usually transient.
In the current study, we have demonstrated a more sustained CD8+ T-cell response to multiple HIV-1 antigens in a larger proportion of vaccine recipients. Our current vaccine regimen enhanced HIV-1-specific CD8+ T-cell responses in 78% of vaccine recipients. Furthermore, these CD8+ T-cell responses persisted over the study period once they became detectable. Similar to the results observed in the uninfected individuals, the most dominant response tended to be specific to the Gag protein, although every antigen included in the vCP1452 elicited a CD8+ T-cell response. In most vaccine recipients, the magnitude of increase in HIV-specific CD8+ T cells was between 0.1 and 0.5%, which is equivalent to a frequency of 1,000 to 5,000 per 106 CD8+ T cells. This level of antigen-specific CD8+ T cells is similar to levels observed in acutely infected patients by others (2), but less than the magnitude of HIV-1-specific CD8+ T cells in long-term nonprogressors (25, 43).
The size of the cohort limits our ability to dissect factors that would predict a favorable CD8+ T-cell-mediated immune response to vaccination. However, all three subjects without a measurable CD8+ T-cell response had at least one HLA-A2 allele. This is an unexpected finding, considering that the Pol/Nef portion of ALVAC 1452 is based on known HLA-A2-restricted epitopes. However, it has been noted recently that responses to HIV-1 in the acute setting may be quite different from the “immunodominant” epitopes identified in chronically infected individuals (2). Duration of infection pretreatment, duration of treatment, and HLA class I homozygosity at the A, B, or C locus did not predict a more or less robust CD8+ T-cell-mediated immune response, either quantitative or qualitative, to vaccination. As the size of this pilot phase II study was limited, we do not believe it is possible to draw conclusions regarding positive or negative predictors of response until substantially larger cohorts are similarly studied in controlled randomized trials.
The HIV-1-specific CD4+ T-cell responses, as measured by lymphocyte proliferation to Gag, were transiently elevated in 64% of the vaccine recipients. In one third of the patients who had a response, the initial response was boosted for a second time but not sustained. The preponderance and kinetics of these responses are similar to those observed in seronegative individuals receiving similar vaccines (7). It remains unclear why these responses do not persist. One explanation may be that these memory HIV-1-specific CD4+ T cells are both limited in repertoire and more prone to activation-induced cell death, or apoptosis. Therefore, subsequent stimuli would be met by a limited CD4+ T-cell-mediated memory response. Whether this is vaccine specific or global for all HIV-1 antigens remains unanswered.
In agreement with previous studies using a different formulation of rgp160 (28, 42, 57), we observed anti-gp120 antibody responses in all except one patient. However, few subjects demonstrated an induced antibody response to Gag (p24). These data suggest that the rgp160 component was capable of eliciting a humoral response to Env and that the viral vector, ALVAC 1452, is a poor inducer of a humoral response. Alternatively, and less likely, is that ALVAC 1452 does induce an Env antibody response but no Gag antibodies due to differences in antigen expression and processing. However, the kinetics of antibody response in our subjects were similar to that seen in seronegative subjects receiving gp120 vaccination, suggesting that we are indeed seeing a response to the recombinant protein.
The binding antibody titers reached peak levels after the third vaccination and remained at plateau levels during subsequent vaccination (12, 31). Induced anti-gp120 antibody levels exhibited apparently accelerated decay in subject 918, as did prevaccination anti-gp120 levels in subject 921. Neither subject had evidence of conditions associated with accelerated loss of serum proteins (i.e., nephrosis) or rapid extravasation to the extravascular space. The underlying mechanism of this effect in these subjects remains unclear.
In the limited number of subjects studied, we were unable to demonstrate that the antibodies induced by vaccination neutralized CCR5- and CXCR4-using laboratory strains in vitro. Compared to primary isolates, these viruses are generally more neutralizable (33) and have envelopes more analogous to the exogenous immunogens in the vaccines (32). That the induced antibodies did not neutralize these strains discouraged further such studies. With further improvement in the formulation of immunogens, such as using trimeric gp140 capable of eliciting HIV-specific neutralizing antibodies in mice (60), it is possible that neutralizing antibody responses may be induced by vaccination in human subjects. Though antibody-associated cellular cytotoxicity has recently been described as a potential mechanism of virologic control during primary HIV-1 infection (18), we have not yet assessed whether the antibodies induced by vaccination with ALVAC 1452 and rgp160 have similar properties and believe these experiments are beyond the scope of the original study.
We occasionally observed discordance between vaccine-induced CD4+ T-cell immune responses as measured by lymphocyte proliferation, CD8+ T-cell-mediated immune responses as measured by intracellular IFN-γ staining, and humoral responses to vaccination. We hypothesize that a minority of subjects, such as 313-2, responded to vaccination with a dominant Th2 response, accounting for a robust humoral (Gag) and T-cell proliferative response (Gag and Env) without a CD8+ T-cell response (8, 17, 45). On the other hand, if we assume that the Env humoral response was due to the rgp160 component of the vaccine regimen, then most of the subjects appeared to respond in a Th1-dominant pattern of various degrees, with a CD8+ T-cell induced immune response, no humoral response to Gag, and a lymphocyte proliferative response, likely mediated by interleukin-2 or other Th1 cytokines (8, 17, 45).
In assessing the immunogenicity of the vaccine, it is important to determine whether the immune responses observed were due to occasional bursts of viremia, i.e., autovaccination, instead of induction by the exogenous immunogens. Three subjects had one episode of measurable plasma viremia of 50 to 100 copies/ml during the period of vaccination. A fourth patient (900) was admittedly nonadherent to HAART and appeared to have augmented already induced HIV-1-specific CD4+ and CD8+ T-cell responses after the third and fourth vaccinations. In this subject, the first detectable CD8+ T-cell response appeared immediately after the first injections of vaccines, about 60 days prior to a detectable viremic episode. In subject 908, the initial CD4+ and CD8+ T-cell responses appeared to correlate with the timing of his viremic episode, but the second augmentation of CD8+ T-cell responses was observed in the absence of any detectable plasma viremia. In contrast, subject 3002 never had detectable CD8+ T-cell responses despite also having a viremic episode during vaccination. The isolated viremic episode in subject 1306 had little impact on the kinetics of the immune responses measured in this subject. Furthermore, viremia in these three subjects had little influence on the anti-p24 antibody or the HIV-1 specific CD4+ T-cell mediated immune responses observed.
More importantly, eight patients had augmentation of HIV-1-specific CD8+ T-cell responses in the absence of any detectable viremia. Thus, in most vaccinated individuals, the elevated immune responses postvaccination appear to be induced by the exogenously provided immunogens instead of autologous viruses, and the occasional viremic episodes are unlikely to have been the major determinants of immunogenicity. It is also difficult to determine whether these occasional isolated viremic episodes were directly related to immune stimulation caused by the vaccines or reflect the well-documented occurrence of low-level viremia due to incomplete suppression of viral replication during HAART.
Although we observed significant immunogenicity of the vaccine regimen, the unique features of this cohort of vaccine recipients should not be overlooked. All subjects included in the study were treated early, within 120 days of the onset of symptoms of acute HIV-1 infection. Early treatment may have preserved HIV-1-specific immunity, which allowed boosting by vaccination. Indeed, immunological manipulations, such as vaccination and structured treatment interruption, have been shown to be effective in the setting of acute lentivirus infection (22, 29, 46). It is yet to be demonstrated whether such immune-based strategies will be successful in chronic HIV-1 infection.
Although the ALVAC vCP1452 plus rgp160 regimen can induce both humoral and cellular immune responses in HIV-1-infected individuals on HAART, the limited magnitude of the CD8+ T-cell response and the lack of persistence of T helper cell responses may limit the utility of this particular immunogen in this clinical setting. Nevertheless, we have demonstrated the feasibility of using multivalent vaccines as therapeutic modalities in HIV-1-infected patients receiving HAART. The promising safety and immunogenicity data generated from this preliminary study support further controlled studies in a variety of HIV-1-infected patient cohorts to better assess the efficacy of this adjunctive vaccine strategy.
Acknowledgments
This work was supported by NIH grants M01-RR00102, AI-47033, AI-42848, AI-41534, and RR06555 and the ANRS (French Agency for AIDS Research).
We acknowledge Michael Louie, Bharat Ramratnam, and Rhonda Kost for clinical assistance. We thank Roche Molecular Systems for providing materials for viral load measurements; Paul Maddon of Progenics Pharmaceutical Inc. for providing recombinant gp120 (JRFL); John Moore for input into the design, execution, and analysis of the antibody and T-proliferative assays; Wen Chen for assistance with graphics; Abbott Laboratories, Merck Research Laboratories, Hoffmann-La Roche, and Glaxo/SmithKline for supporting our clinical trials; and our study subjects for their cooperation and persistence.
Footnotes
Present address: University of Rochester Medical Center, Rochester, NY 14642.
Present address: The Scripps Research Institute, La Jolla, CA 92037.
REFERENCES
- 1.AIDS Vaccine Evaluation Group. 2001. Cellular and humoral immune responses to a canarypox vaccine containing human immunodeficiency virus type 1 Env, Gag, and Pro in combination with rgp120. J. Infect. Dis. 183:563-570. [DOI] [PubMed] [Google Scholar]
- 2.Altfeld, M., E. S. Rosenberg, R. Shankarappa, J. S. Mukherjee, F. M. Hecht, R. L. Eldridge, M. M. Addo, S. H. Poon, M. N. Phillips, G. K. Robbins, P. E. Sax, S. Boswell, J. O. Kahn, C. Brander, P. J. Goulder, J. A. Levy, J. I. Mullins, and B. D. Walker. 2001. Cellular immune responses and viral diversity in individuals treated during acute and early HIV-1 infection. J. Exp. Med. 193:169-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Altman, J. D., P. A. Moss, P. J. Goulder, D. H. Barouch, M. G. McHeyzer-Williams, J. I. Bell, A. J. McMichael, and M. M. Davis. 1996. Phenotypic analysis of antigen-specific T lymphocytes. Science 274:94-96. [DOI] [PubMed] [Google Scholar]
- 4.Binley, J. M., P. J. Klasse, Y. Cao, I. Jones, M. Markowitz, D. D. Ho, and J. P. Moore. 1997. Differential regulation of the antibody responses to Gag and Env proteins of human immunodeficiency virus type 1. J. Virol. 71:2799-2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borrow, P., H. Lewicki, B. H. Hahn, G. M. Shaw, and M. B. Oldstone. 1994. Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J. Virol. 68:6103-6110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chun, T. W., L. Stuyver, S. B. Mizell, L. A. Ehler, J. A. Mican, M. Baseler, A. L. Lloyd, M. A. Nowak, and A. S. Fauci. 1997. Presence of an inducible HIV-1 latent reservoir during highly active antiretroviral therapy. Proc. Natl. Acad. Sci. USA 94:13193-13197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clements-Mann, M. L., K. Weinhold, T. J. Matthews, B. S. Graham, G. J. Gorse, M. C. Keefer, M. J. McElrath, R. H. Hsieh, J. Mestecky, S. Zolla-Pazner, J. Mascola, D. Schwartz, R. Siliciano, L. Corey, P. F. Wright, R. Belshe, R. Dolin, S. Jackson, S. Xu, P. Fast, M. C. Walker, D. Stablein, J. L. Excler, J. Tartaglia, E. Paoletti, and the NIAID AIDS Vaccine Evaluation Group. 1998. Immune responses to human immunodeficiency virus (HIV) type 1 induced by canarypox expressing HIV-1MN gp120, HIV-1SF2 recombinant gp120, or both vaccines in seronegative adults. J. Infect. Dis. 177:1230-1246. [DOI] [PubMed] [Google Scholar]
- 8.Clerici, M., and G. M. Shearer. 1994. The Th1-Th2 hypothesis of HIV infection: new insights. Immunol. Today 15:575-581. [DOI] [PubMed] [Google Scholar]
- 9.Cohen, J. 1999. Glimmerings of hope from the bottom of the well. Science 285:656-657. [DOI] [PubMed] [Google Scholar]
- 10.Cros, P., P. Allibert, B. Mandrand, J. M. Tiercy, and B. Mach. 1992. Oligonucleotide genotyping of HLA polymorphism on microtitre plates. Lancet 340:870-873. [DOI] [PubMed] [Google Scholar]
- 11.Davey, R. T., Jr., N. Bhat, C. Yoder, T. W. Chun, J. A. Metcalf, R. Dewar, V. Natarajan, R. A. Lempicki, J. W. Adelsberger, K. D. Miller, J. A. Kovacs, M. A. Polis, R. E. Walker, J. Falloon, H. Masur, D. Gee, M. Baseler, D. S. Dimitrov, A. S. Fauci, and H. C. Lane. 1999. HIV-1 and T-cell dynamics after interruption of highly active antiretroviral therapy (HAART) in patients with a history of sustained viral suppression. Proc. Natl. Acad. Sci. USA 96:15109-15114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Evans, T. G., M. C. Keefer, K. J. Weinhold, M. Wolff, D. Montefiori, G. J. Gorse, B. S. Graham, M. J. McElrath, M. L. Clements-Mann, M. J. Mulligan, P. Fast, M. C. Walker, J. L. Excler, A. M. Duliege, and J. Tartaglia. 1999. A canarypox vaccine expressing multiple human immunodeficiency virus type 1 genes given alone or with rgp120 elicits broad and durable CD8+ cytotoxic T lymphocyte responses in seronegative volunteers. J. Infect. Dis. 180:290-298. [DOI] [PubMed] [Google Scholar]
- 13.Fang, Z. Y., I. Kuli-Zade, and P. Spearman. 1999. Efficient human immunodeficiency virus (HIV)-1 Gag-Env pseudovirion formation elicited from mammalian cells by a canarypox HIV vaccine candidate. J. Infect. Dis. 180:1122-1132. [DOI] [PubMed] [Google Scholar]
- 14.Finzi, D., J. Blankson, J. D. Siliciano, J. B. Margolick, K. Chadwick, T. Pierson, K. Smith, J. Lisziewicz, F. Lori, C. Flexner, T. C. Quinn, R. E. Chaisson, E. Rosenberg, B. Walker, S. Gange, J. Gallant, and R. F. Siliciano. 1999. Latent infection of CD4+ T cells provides a mechanism for lifelong persistence of HIV-1, even in patients on effective combination therapy. Nat. Med. 5:512-517. [DOI] [PubMed] [Google Scholar]
- 15.Finzi, D., M. Hermankova, T. Pierson, L. M. Carruth, C. Buck, R. E. Chaisson, T. C. Quinn, K. Chadwick, J. Margolick, R. Brookmeyer, J. Gallant, M. Markowitz, D. D. Ho, D. D. Richman, and R. F. Siliciano. 1997. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science 278:1295-1300. [DOI] [PubMed] [Google Scholar]
- 16.Fleury, B., G. Janvier, G. Pialoux, F. Buseyne, M. Robertson, J. Tartaglia, E. Paoletti, M. Kieny, J. Excler, and Y. Riviere. 1996. Memory cytotoxic T lymphocyte responses in human immunodeficiency virus type 1 (HIV-1)-negative volunteers immunized with a recombinant canarypox expressing gp160 of HIV-1 and boosted with a recombinant gp160. J. Infect. Dis. 174:734-738. [DOI] [PubMed] [Google Scholar]
- 17.Foli, A., M. W. Saville, M. W. Baseler, and R. Yarchoan. 1995. Effects of the Th1 and Th2 stimulatory cytokines interleukin-12 and interleukin-4 on human immunodeficiency virus replication. Blood 85:2114-2123. [PubMed] [Google Scholar]
- 18.Forthal, D. N., G. Landucci, and E. S. Daar. 2001. Antibody from patients with acute human immunodeficiency virus (HIV) infection inhibits primary strains of HIV type 1 in the presence of natural-killer effector cells. J. Virol. 75:6953-6961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Furtado, M. R., D. S. Callaway, J. P. Phair, K. J. Kunstman, J. L. Stanton, C. A. Macken, A. S. Perelson, and S. M. Wolinsky. 1999. Persistence of HIV-1 transcription in peripheral-blood mononuclear cells in patients receiving potent antiretroviral therapy. N. Engl. J. Med. 340:1614-1622. [DOI] [PubMed] [Google Scholar]
- 20.Garcia, F., M. Plana, C. Vidal, A. Cruceta, W. A. O'Brien, G. Pantaleo, T. Pumarola, T. Gallart, J. M. Miro, and J. M. Gatell. 1999. Dynamics of viral load rebound and immunological changes after stopping effective antiretroviral therapy. AIDS 13:F79-F86. [DOI] [PubMed] [Google Scholar]
- 21.Harrigan, P. R., M. Whaley, and J. S. Montaner. 1999. Rate of HIV-1 RNA rebound upon stopping antiretroviral therapy. AIDS 13:F59-F62. [DOI] [PubMed] [Google Scholar]
- 22.Hel, Z., D. Venzon, M. Poudyal, W. P. Tsai, L. Giuliani, R. Woodward, C. Chougnet, G. Shearer, J. D. Altman, D. Watkins, N. Bischofberger, A. Abimiku, P. Markham, J. Tartaglia, and G. Franchini. 2000. Viremia control following antiretroviral treatment and therapeutic immunization during primary SIV251 infection of macaques. Nat. Med. 6:1140-1146. [DOI] [PubMed] [Google Scholar]
- 23.Ho, D. D., A. U. Neumann, A. S. Perelson, W. Chen, J. M. Leonard, and M. Markowitz. 1995. Rapid turnover of plasma virions and CD4 lymphocytes in HIV-1 infection. Nature 373:123-126. [DOI] [PubMed] [Google Scholar]
- 24.Jin, X., D. E. Bauer, S. E. Tuttleton, S. Lewin, A. Gettie, J. Blanchard, C. E. Irwin, J. T. Safrit, J. Mittler, L. Weinberger, L. G. Kostrikis, L. Zhang, A. S. Perelson, and D. D. Ho. 1999. Dramatic rise in plasma viremia after CD8+ T-cell depletion in SIV-infected macaques. J. Exp. Med. 189:991-998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jin, X., G. Ogg, S. Bonhoeffer, J. Safrit, M. Vesanen, D. Bauer, D. Chen, Y. Cao, M. A. Demoitie, L. Zhang, M. Markowitz, D. Nixon, A. McMichael, and D. D. Ho. 2000. An antigenic threshold for maintaining human immunodeficiency virus type 1-specific cytotoxic T lymphocytes. Mol. Med. 6:803-809. [PMC free article] [PubMed] [Google Scholar]
- 26.Koup, R. A., C. A. Pikora, K. Luzuriaga, D. B. Brettler, E. S. Day, G. P. Mazzara, and J. L. Sullivan. 1991. Limiting dilution analysis of cytotoxic T lymphocytes to human immunodeficiency virus gag antigens in infected persons: in vitro quantitation of effector cell populations with p17 and p24 specificities. J. Exp. Med. 174:1593-1600. [DOI] [PMC free article] [PubMed]
- 27.Koup, R. A., J. T. Safrit, Y. Cao, C. A. Andrews, Y. Wu, G. McLeod, W. Borkowsky, C. Farthing, and D. D. Ho. 1994. Temporal association of cellular immune response with the initial control of viremia in primary HIV-1 syndrome. J. Virol. 68:4650-4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kundu, S. K., D. Katzenstein, L. E. Moses, and T. C. Merigan. 1992. Enhancement of human immunodeficiency virus (HIV)-specific CD4+ and CD8+ cytotoxic T-lymphocyte activities in HIV-infected asymptomatic patients given recombinant gp160 vaccine. Proc. Natl. Acad. Sci. USA 89:11204-11208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lori, F., M. G. Lewis, J. Xu, G. Varga, D. E. Zinn, C. Crabbs, W. Wagner, J. Greenhouse, P. Silvera, J. Yalley-Ogunro, C. Tinelli, and J. Lisziewicz. 2000. Control of SIV rebound through structured treatment interruptions during early infection. Science 290:1591-1593. [DOI] [PubMed] [Google Scholar]
- 30.MacGregor, R. R., J. D. Boyer, K. E. Ugen, K. E. Lacy, S. J. Gluckman, M. L. Bagarazzi, M. A. Chattergoon, Y. Baine, T. J. Higgins, R. B. Ciccarelli, L. R. Coney, R. S. Ginsberg, and D. B. Weiner. 1998. First human trial of a DNA-based vaccine for treatment of human immunodeficiency virus type 1 infection: safety and host response. J. Infect. Dis. 178:92-100. [DOI] [PubMed] [Google Scholar]
- 31.McElrath, M. J., L. Corey, D. Montefiori, M. Wolff, D. Schwartz, M. Keefer, R. Belshe, B. S. Graham, T. Matthews, P. Wright, G. Gorse, R. Dolin, P. Berman, D. Francis, A. M. Duliege, D. Bolognesi, D. Stablein, N. Ketter, and P. Fast. 2000. A phase II study of two HIV type 1 envelope vaccines, comparing their immunogenicity in populations at risk for acquiring HIV type 1 infection. AIDS Res. Hum. Retroviruses 16:907-919. [DOI] [PubMed] [Google Scholar]
- 32.Moore, J., and A. Trkola. 1997. HIV-1 coreceptors, neutralization serotypes and vaccine development. AIDS Res. Hum. Retroviruses 3:733-736. [DOI] [PubMed] [Google Scholar]
- 33.Moore, J. P., Y. Cao, L. Qing, Q. J. Sattentau, J. Pyati, R. Koduri, J. Robinson, C. F. I. Barbas, D. R. Burton, and D. D. Ho. 1995. Primary isolates of human immunodeficiency virus type 1 are relatively resistant to neutralization by monoclonal antibodies to gp120 and their neutralization is not predicted by studies with monomeric gp120. J. Virol. 69:101-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moss, R. B., W. K. Giermakowska, M. R. Wallace, J. R. Savary, F. C. Jensen, and D. J. Carlo. 2000. Cell-mediated immune responses to autologous virus in HIV-1-seropositive individuals after treatment with an HIV-1 immunogen. AIDS 14:2475-2478. [DOI] [PubMed] [Google Scholar]
- 35.Mulligan, M. J., and J. Weber. 1999. Human trials of HIV-1 vaccines. AIDS 13(Suppl. A):S105-S112. [PubMed] [Google Scholar]
- 36.Neumann, A. U., R. Tubiana, V. Calvez, C. Robert, T.-S. Li, H. Agut, B. Autran, C. Katlama, and the Comet Study Group. 1999. HIV-1 rebound during interruption of highly active antiretroviral therapy has no deleterious effect on reinitiated treatment. AIDS 13:677-683. [DOI] [PubMed] [Google Scholar]
- 37.Ogg, G. S., X. Jin, S. Bonhoeffe, R. P. Dunbar, M. A. Nowak, S. Monard, J. P. Segal, Y. Cao, S. L. Rowland-Jones, V. Cerundolo, A. Hurley, M. Markowitz, D. D. Ho, D. F. Nixon, and A. J. McMichael. 1998. Quantitation of HIV-1-specific cytotoxic T lymphocytes and plasma load of viral RNA. Science 279:2103-2106. [DOI] [PubMed] [Google Scholar]
- 38.Olerup, O., and H. Zetterquist. 1992. HLA-DR typing by PCR amplification with sequence-specific primers (PCR-SSP) in 2 hours: an alternative to serological DR typing in clinical practice including donor-recipient matching in cadaveric transplantation. Tissue Antigens 39:225-235. [DOI] [PubMed] [Google Scholar]
- 39.Palella, F. J., K. M. Delaney, A. C. Moorman, M. O. Loveless, J. Fuhrer, G. A. Satten, D. J. Aschman, and S. D. Holmberg. 1998. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. N. Engl. J. Med. 338:853-860. [DOI] [PubMed] [Google Scholar]
- 40.Plotkin, S. A., M. Cadoz, B. Meignier, C. Meric, O. Leroy, J. L. Excler, J. Tartaglia, E. Paoletti, E. Gonczol, and G. Chappuis. 1995. The safety and use of canarypox vectored vaccines. Dev. Biol. Stand. 84:165-170. [PubMed] [Google Scholar]
- 41.Ramratnam, B., J. E. Mittler, L. Zhang, D. Boden, A. Hurley, F. Fang, C. A. Macken, A. S. Perelson, M. Markowitz, and D. D. Ho. 2000. The decay of the latent reservoir of replication-competent HIV-1 is inversely correlated with the extent of residual viral replication during prolonged anti-retroviral therapy. Nat. Med. 6:82-85. [DOI] [PubMed] [Google Scholar]
- 42.Redfield, R. R., D. L. Birx, N. Ketter, E. Tramont, V. Polonis, C. Davis, J. F. Brundage, G. Smith, S. Johnson, A. Fowler, et al. 1991. A phase I evaluation of the safety and immunogenicity of vaccination with recombinant gp160 in patients with early human immunodeficiency virus infection. Military Medical Consortium for Applied Retroviral Research. N. Engl. J. Med. 324:1677-1684. [DOI] [PubMed] [Google Scholar]
- 43.Rinaldo, C. R., Jr., X.-L. Huang, Z. Fan, M. Ding, L. Beltz, A. Logar, D. Panicali, G. Mazzara, J. Liebmann, M. Cottrill, and P. Gupta. 1995. High levels of anti-human immunodeficiency virus type 1 (HIV-1) memory cytotoxic T-lymphocyte activity and low viral load are associated with lack of disease in HIV-1-infected long-term nonprogressors. J. Virol. 69:5838-5842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rinaldo, C. R., Jr., L. A. Beltz, X. L. Huang, P. Gupta, Z. Fan, and D. J. Torpey III. 1995. Anti-HIV type 1 cytotoxic T lymphocyte effector activity and disease progression in the first 8 years of HIV type 1 infection of homosexual men. AIDS Res. Hum. Retrovirol. 11:481-489. [DOI] [PubMed] [Google Scholar]
- 45.Romagnani, S., G. Del Prete, R. Manetti, A. Ravina, F. Annunziata, M. De Carli, M. Mazzetti, M.-P. Piccinni, M. M. D'Elios, P. Parronchi, S. Sampognaro, and E. Maggi. 1994. Role of TH1/TH2 cytokines in HIV infection. Immunol. Rev. 140:73-92. [DOI] [PubMed] [Google Scholar]
- 46.Rosenberg, E. S., M. Altfeld, S. H. Poon, M. N. Phillips, B. M. Wilkes, R. L. Eldridge, G. K. Robbins, R. T. D'Aquila, P. J. Goulder, and B. D. Walker. 2000. Immune control of HIV-1 after early treatment of acute infection. Nature 407:523-526. [DOI] [PubMed] [Google Scholar]
- 47.Rosenberg, E. S., J. M. Billingsley, A. M. Caliendo, S. L. Boswell, P. E. Sax, S. A. Kalams, and B. D. Walker. 1997. Vigorous HIV-1-specific CD4+ T-cell responses associated with control of viremia. Science 278:1447-1450. [DOI] [PubMed] [Google Scholar]
- 48.Schiller, D. S., J. M. Binley, K. H. Roux, C. S. Adamson, I. M. Jones, H. G. Krausslich, A. Hurley, M. Markowitz, and J. P. Moore. 2000. Parameters influencing measurement of the Gag antigen-specific T-proliferative response to HIV type 1 infection. AIDS Res. Hum. Retroviruses 16:259-271. [DOI] [PubMed] [Google Scholar]
- 49.Schmitz, J. E., M. J. Kuroda, S. Santra, M. Dalesandro, J. Ghrayeb, D. C. Montefiori, K. Tenner-Racz, P. Racz, E. Rieber, N. L. Letvin, and K. A. Reimann. 1999. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science 283:857-860. [DOI] [PubMed] [Google Scholar]
- 50.Schneider, J., S. C. Gilbert, T. J. Blanchard, T. Hanke, K. J. Robson, C. M. Hannan, M. Becker, R. Sinden, G. L. Smith, and A. V. Hill. 1998. Enhanced immunogenicity for CD8+ T-cell induction and complete protective efficacy of malaria DNA vaccination by boosting with modified vaccinia virus virus Ankara. Nat. Med. 4:397-402. [DOI] [PubMed] [Google Scholar]
- 51.Schooley, R. T., C. Spino, D. Kuritzkes, B. D. Walker, F. A. Valentine, M. S. Hirsch, E. Cooney, G. Friedland, S. Kundu, T. C. Merigan, Jr, M. J. McElrath, A. Collier, S. Plaeger, R. Mitsuyasu, J. Kahn, P. Haslett, P. Uherova, V. deGruttola, S. Chiu, B. Zhang, G. Jones, D. Bell, N. Ketter, T. Twadell, D. Chernoff, and M. Rosandich. 2000. Two double-blinded, randomized, comparative trials of 4 human immunodeficiency virus type 1 (HIV-1) envelope vaccines in HIV-1-infected individuals across a spectrum of disease severity: AIDS Clinical Trials Groups 209 and 214. J. Infect. Dis. 182:1357-1364. [DOI] [PubMed] [Google Scholar]
- 52.Sedegah, M., T. R. Jones, M. Kaur, R. Hedstrom, P. Hobart, J. A. Tine, and S. L. Hoffman. 1998. Boosting with recombinant vaccinia virus increases immunogenicity and protective efficacy of malaria DNA vaccine. Proc. Natl. Acad. Sci. USA 95:7648-7653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tartaglia, J., W. I. Cox, J. Taylor, M. Perkus, M. Riviere, B. Meignier, and E. Paoletti. 1992. Highly attenuated poxvirus vectors. AIDS Res. Hum. Retroviruses 8:1445-1447. [DOI] [PubMed] [Google Scholar]
- 54.Tartaglia, J., M. Perkus, J. Taylor, E. Norton, J. Audonnet, W. Cox, S. Davis, J. van der Hoeven, B. Meignier, and M. Riviere. 1992. NYVAC: a highly attenuated strain of vaccinia virus virus. Virology 188:217-232. [DOI] [PubMed] [Google Scholar]
- 55.Taylor, J., B. Meignier, J. Tartaglia, B. Languet, J. VanderHoeven, G. Franchini, C. Trimarchi, and E. Paoletti. 1995. Biological and immunogenic properties of a canarypox-rabies recombinant, ALVAC-RG (vCP65), in non-avian species. Vaccine 13:539-549. [DOI] [PubMed] [Google Scholar]
- 56.Taylor, J., R. Weinberg, J. Tartaglia, C. Richardson, G. Alkhatib, D. Briedis, E. Norton, and E. Paoletti. 1992. Nonreplicating viral vectors as potential vaccines: recombinant canarypox expressing measles virus fusion (F) and hemagglutinin (HA) glycoproteins. Virology 187:321-328. [DOI] [PubMed] [Google Scholar]
- 57.Valentine, F. T., S. Kundu, P. A. Haslett, D. Katzenstein, L. Beckett, C. Spino, B. Borucki, M. Vasquez, G. Smith, J. Korvick, J. Kagan, and T. C. Merigan. 1996. A randomized, placebo-controlled study of the immunogenicity of human immunodeficiency virus (HIV) rgp160 vaccine in HIV-infected subjects with ≧400/mm3 CD4 T lymphocytes. J. Infect. Dis. 173:1336-1346. [DOI] [PubMed] [Google Scholar]
- 58.Wei, X., S. K. Ghosh, M. E. Taylor, V. A. Johnson, E. A. Emini, P. Deutsch, J. D. Lifson, S. Bonhoeffer, M. A. Nowak, B. H. Hahn, et al. 1995. Viral dynamics in human immunodeficiency virus type 1 infection. Nature 373:117-122. [DOI] [PubMed] [Google Scholar]
- 59.Wong, J. K., M. Hezareh, H. F. Gunthard, D. V. Havlir, C. C. Ignacio, C. A. Spina, and D. D. Richman. 1997. Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. Science 278:1291-1295. [DOI] [PubMed] [Google Scholar]
- 60.Yang, X., R. Wyatt, and J. Sodroski. 2001. Improved elicitation of neutralizing antibodies against primary human immunodeficiency viruses by soluble stabilized envelope glycoprotein trimers. J. Virol. 75:1165-1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang, L., B. Ramratnam, K. Tenner-Racz, Y. He, M. Vesanen, S. Lewin, A. Talal, P. Racz, A. S. Perelson, B. T. Korber, M. Markowitz, and D. D. Ho. 1999. Quantifying residual HIV-1 replication in patients receiving combination antiretroviral therapy. N. Engl. J. Med. 340:1605-1613. [DOI] [PubMed] [Google Scholar]