Abstract
The importance of CD8+ T-cell responses in the control of human immunodeficiency virus type 1 (HIV-1) infection has been demonstrated, yet few studies have been able to correlate these responses with markers of HIV-1 disease progression. This study measured cell-mediated immune responses using peripheral blood mononuclear cells (PBMC) obtained from 27 patients with chronic HIV-1 infection, the majority of whom were off antiretroviral therapy. The ELISPOT assay was used to detect gamma interferon-secreting PBMC after stimulation with overlapping HIV-1 peptides spanning the Gag, Pol, Env, and Nef proteins in addition to the baculovirus-derived p24 and gp160 proteins. All volunteers had responses to at least one HIV-1-specific peptide. All but one of the subjects (96%) responded to the Gag peptide pool, and 86% responded to the Pol and/or Nef peptide pools. The magnitude and the breadth of T-cell responses directed to either the Gag or p24 peptide pools correlated inversely with viral load in plasma (r = −0.60, P < 0.001 and r = −0.52, P < 0.005, respectively) and directly with absolute CD4+ T-cell counts (r = 0.54, P < 0.01 and r = 0.39, P < 0.05, respectively) using the Spearman rank correlation test. Responses to the Pol and integrase peptide pools also correlated with absolute CD4+ T-cell counts (r = 0.45, P < 0.05 and r = 0.49, P < 0.01, respectively). No correlation with markers of disease progression was seen with specific T-cell responses directed toward the Env or Nef peptides. These data serve as strong evidence that major histocompatibility complex class I presentation of Gag peptides is an essential feature for any HIV-1 vaccine designed to elicit optimal CD8+ T-cell responses.
Numerous advances have been made in the treatment of human immunodeficiency virus type 1 (HIV-1); however, ultimate control of this virus will rely on an effective vaccine. In the current global context where antiretroviral therapy (ART) is not available for the majority of infected patients, and patients who do have access to ART can experience significant side effects (7, 9, 12, 33, 39, 40, 52), a vaccine is essential. Indeed, the ultimate control of HIV-1 disease depends on it; ART, as it is currently given, is unlikely to eradicate the infection, resulting in the need for chronic suppressive treatment (16-18, 53).
An immune correlate of protection against infection or disease does not exist for an HIV-1 vaccine; however, numerous data suggest that CD8+ T-lymphocyte responses are important in controlling viral replication in HIV-1-infected individuals. Their appearance is temporally associated with the initial decline in viral RNA (vRNA) in plasma seen shortly after acute infection (6, 22, 35, 45). Additionally, the documentation of CD8+ cytotoxic T-lymphocyte (CTL) escape mutants suggests that selective pressure is placed on the virus by this arm of the immune system (5, 14, 20, 47).
Several groups have demonstrated that certain class I human leukocyte antigen (HLA) alleles correlated with disease-free survival or progression (10, 26). The mechanism of this correlation is most likely due to differences in CD8+ CTL (43). An observed correlation between certain “favorable” class I alleles and CD8+ CTL responses to HIV-1 vaccines in uninfected human volunteers is consistent with this proposed mechanism (27). HIV-1-specific CTL responses were also demonstrated in several cohorts of individuals who were heavily exposed to HIV-1 yet were clinically uninfected (28, 29, 49, 50), suggesting that CTL responses may also play a role in protection against chronic HIV-1 infection.
Recent data using macaque models of AIDS have demonstrated that the control of simian immunodeficiency virus (SIV) infection directly correlated with the presence of CD8+ T lymphocytes (25, 51). Several groups have also shown that vaccine-induced CD8+ T lymphocytes can protect against the development of AIDS when macaques were challenged with an SIV-HIV chimera (1, 3, 4).
Taken together, these data convincingly demonstrate the role of CD8+ T cells in combating HIV-1 infection and have been a driving force in strategies aimed at eliciting these responses with HIV-1 vaccines (41). Determining which HIV-1 proteins should be included in such vaccines is a topic of considerable debate. One strategy is to target vaccines to HIV-1 proteins associated with CTL that are protective against the development of AIDS in chronically infected patients. If such responses are elicited prior to HIV-1 challenge, they could protect against infection or disease progression.
Several studies have been able to correlate HIV-1-specific CTL responses in chronically infected patients with disease progression; however, these immune responses were based on indirect measurements and qualitative assessments relying on the expansion of cultured peripheral blood mononuclear cells (PBMC) in vitro for extended periods of time (8, 31, 48). A study by Greenough et al. correlated unstimulated CTL responses directed towards Gag with proviral DNA but not vRNA (23). More recent studies correlated HIV-1-specific CD8+ T-cell responses ex vivo with disease progression or vRNA (34, 45); nevertheless, these reports examined only a few immunodominant epitopes and did not demonstrate correlations with functional CD8+ T-cell responses.
We screened a cohort of chronically infected patients using peptides that spanned the Gag, Pol, Env, and Nef proteins of HIV-1 and demonstrate that responses to the Gag and Pol regions correlate with markers of progression to the AIDS.
(This study was presented at the AIDS 2001 vaccine meeting in Philadelphia, Pa., 5 to 8 September 2001.)
MATERIALS AND METHODS
Human subjects.
Twenty-seven HIV-1-infected individuals were recruited from the AIDS clinic at the University of Alabama at Birmingham. Patients were only enrolled if they were off therapy for at least 6 months or had stable and detectable vRNA while on therapy. Patients with active opportunistic infections were excluded from participation in this study. The laboratory personnel were blinded as to the clinical status of the patients in the cohort.
vRNA measurements in plasma and absolute CD4+ T-cell counts.
HIV-1 vRNA in plasma was measured on frozen EDTA-plasma samples using the Amplicor HIV-1 Monitor 1.0 (Roche Diagnostic Systems, Branchburg, N.J.). The limit of detection of the assay is from 50 to 75,000 copies/ml. Samples with measurements of >75,000 copies/ml were diluted and retested, enabling an increase in the upper limit of detection of the assay to 750,000 copies/ml.
Absolute CD4+ T-cell counts were measured by flow cytometry using the flow count method with a FACScan/FACSort instrument and analyzed using MultiSET software (Becton Dickinson and Company, San Jose, Calif.). Whole blood was incubated with monoclonal antibodies (MAb) directed against CD3, CD4, CD8, and CD45 (Caltag Laboratories, Burlingame, Calif.). Absolute counts of CD3+ CD4+ T cells were then calculated using the results from the complete blood cell counts and lymphocyte differential.
Cell preparation and culture medium.
PBMC were obtained by standard Histopaque density centrifugation (Sigma, St. Louis, Mo.). Both fresh and frozen PBMC were used for gamma interferon (IFN-γ) detection. PBMC were frozen in 90% heat-inactivated fetal bovine serum and 10% dimethyl sulfoxide (Sigma) and stored at −140°C. After thawing at 37°C, cells were centrifuged at 400 × g for 10 min at 4°C and then washed once again before being counted using trypan blue. Culture medium consisted of RPMI 1640 medium with 25 mM HEPES (Gibco, Grand Island, N.Y.), 2 mM l-glutamine (Gibco), 50 U of pencillin-streptomycin per ml, and 10% human AB plasma (Sigma).
ELISPOT assay.
Fresh or freshly thawed PBMC were plated in 96-well nitrocellulose plates (Millipore, Bedford, Mass.) that had been coated with 5 μg of anti-IFN-γ monoclonal antibody 1-DIK (Mabtech, Nacka, Sweden) per ml. PBMC were added at 100,000 cells per well in duplicate in a volume of 50 μl, and the peptides were added in a volume of 50 μl. The plates were incubated overnight at 37°C in 5% CO2 and washed with phosphate-buffered saline (PBS), 1% fetal bovine serum (Gibco), and 0.05% Tween 20 before addition of the biotinylated anti-IFN-γ MAb 7-B6-1 (Mabtech) at 1 μg/ml and incubation at room temperature for 2 h. After a wash, streptavidin-conjugated alkaline phosphatase (Southern Biotechnologies, Birmingham, Ala.) was added at room temperature for 1 h and washed.
Individual cytokine-producing cells were detected as dark spots after a 15-min reaction with 5-bromo-4-chloro-3-indolylphosphate-nitroblue tetrazolium (Sigma). All experiments contained a subset of cells that were unstimulated (medium) for a negative control or stimulated with phytohemagglutinin for a positive control. The number of IFN-γ-secreting cells was calculated by subtracting the negative (medium) control values. The median background medium response was 25 spot-forming units (SFU) per 106 PBMC. Our definition of a positive response is greater than 50 SFU/106 PBMC and greater than three times the background medium control for each individual. The amino acid (AA) adjusted score is defined as the median magnitude of responses (SFU/106 PBMC) divided by the number of AA in the peptides used to measure the response.
Peptide pools and protein.
Peptides used to identify CD8+ T-cell responses spanned the HIV-1 Gag (HXB2), Pol (MN), Env (HXB2), and Nef (BRU) gene products (AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, National Institutes of Health). Lyophilized peptides derived from HIV-1 (20 AA in length, overlapping by 10 AA) were resuspended in dimethyl sulfoxide or distilled water at a concentration of 2 mg/ml. For gene product screening, HIV-1 peptide pools (5 Gag, 10 Pol, 8 Env, and 2 Nef) consisted of 10 peptides per pool. The final concentration of each peptide was 2 μg/ml in all experiments. HIV-1 p24, gp160, and control proteins (Protein Sciences, Meriden, Conn.) were used at a concentration of 10 μg/ml.
T-cell depletions.
Anti-CD8 or anti-CD4 MAb-coated magnetic beads (Dynal, Lake Sucess, N.Y.) were used to selectively deplete PBMC from effector CTLs as described in the manufacturer's instructions. Briefly, PBMC were incubated with the Dynabeads at a 7:1 ratio (beads to cells) for 30 min at 4°C in a rocker. After incubation, tubes containing cells were placed on a magnet for 3 min, and the depleted supernatant, containing ≥98% CD4+ or CD8+ T cells, was removed and added to a fresh tube. Depleted cells were washed twice with RPMI-10% AB serum, counted, and used directly in an ELISPOT assay.
Statistical analysis.
All statistics were performed using the Analyze-It software (version 1.61) (Analyze-It Software Ltd., Leeds, England). Comparisons were made using the Spearman rank correlation test, and linear regression analysis was used to determine the slope.
RESULTS
Detection of HIV-1-specific IFN-γ-secreting cells.
Subjects at all stages of HIV-1 infection were represented in the current cohort (Table 1). The majority of patients (78%) in the cohort were not taking ART. Because of the scarcity of patients with advanced HIV-1 infection who are not taking ART, we included six patients on ART in the current study. These latter individuals all had detectable vRNA despite ART. All patients had measurable responses to the HIV-1-specific peptides, though there were obvious quantitative and qualitative differences among the subjects (Fig. 1A).
TABLE 1.
Demographics of study patient cohort
| Demographic parameter | Mean (range) | No./27 (%) |
|---|---|---|
| Age (yr) | 38 (22-59) | |
| Duration of infection (yr) | 6 (1-17) | |
| Absolute CD4+ T cells/μl | 573 (49-1,356) | |
| HIV RNA copies/ml of plasma | 40,806 (<50->750,000) | |
| Males | 23 (85) | |
| Caucasian | 19 (70) | |
| African-American | 8 (30) |
FIG. 1.
HIV-1-specific immune responses as measured by the IFN-γ ELISPOT assay. (A) Responses to Gag, Pol, Env, and Nef by measuring IFN-γ secretion using PBMC stimulated with overlapping 20-mer peptide pools are represented for each individual subject. (B) HIV-1-specific responses using the 20-mer peptides that correspond to the listed proteins are demonstrated for each subject. The black bar represents the median response among the cohort for each of the protein responses. The AA adjusted score is defined as the magnitude of responses (median SFU/106 PBMC) divided by the number of AA in the respective protein sequences. The 20-mer peptide pools exactly represent the Gag, Pol, Env, and Nef proteins. The 20-mer peptide pools represent the smaller proteins as follows. Gag: matrix protein p17 (AA 1 to 110), capsid protein p24 (AA 140 to 361), nucleocapsid protein NC (AA 399 to 500); Pol: protease Pr (AA 57 to 155), reverse transcriptase RT (AA 155 to 593), RNase H (AA 601 to 710), and integrase IN (AA 711 to 1003); Env: surface protein gp120 (AA 1 to 431) and transmembrane protein gp41 (AA 513 to 856). ∗, FOJA3818 had measurements performed at two separate time points.
An average of five peptide pools were recognized per patient (range, 1 to 10), with a median response of 2,213 SFU/106 PBMC (range, 70 to 6,075). The highest rate of positive responses was directed against the Gag peptide pool (96%, with a median of 782 SFU/106 PBMC), followed by the Nef and Pol peptide pools (85% for both, median of 505 and 358 SFU/106 PBMC for Nef and Pol, respectively) (Fig. 1B). However, when these responses were adjusted to the number of AA in the protein (AA adjusted score), the Nef peptide pools scored highest for the subjects in our cohort (1.8).
Reactivity to smaller sets of peptide pools representing the mature subunit proteins of HIV-1 was also analyzed. The greatest number of CD8+ T cells were specific for the capsid protein (p24) even when adjusted for the number of AA in the protein (1.8). In contrast to the IFN-γ response elicited by the peptide pools, the average response elicited by the p24 whole protein was 85 SFU/106 PBMC, and less than 50% had a detectable response to this HIV-1 capsid protein (not shown). No responses to the Env whole protein gp160 were detected in this cohort (not shown).
In one of the subjects (FOJA3818), we measured responses at a second time point when we observed a marked decline in his CD4+ T-cell counts (501 to 266/μl) and rise in his vRNA (3.64 to 4.14 log copies/ml). This change was associated with a decrease in the total number of HIV-1-specific IFN-γ-secreting PBMC (2,850 to 2,070 SFU/106 PBMC).
HIV-1-specific IFN-γ responses correlate with markers of disease progression.
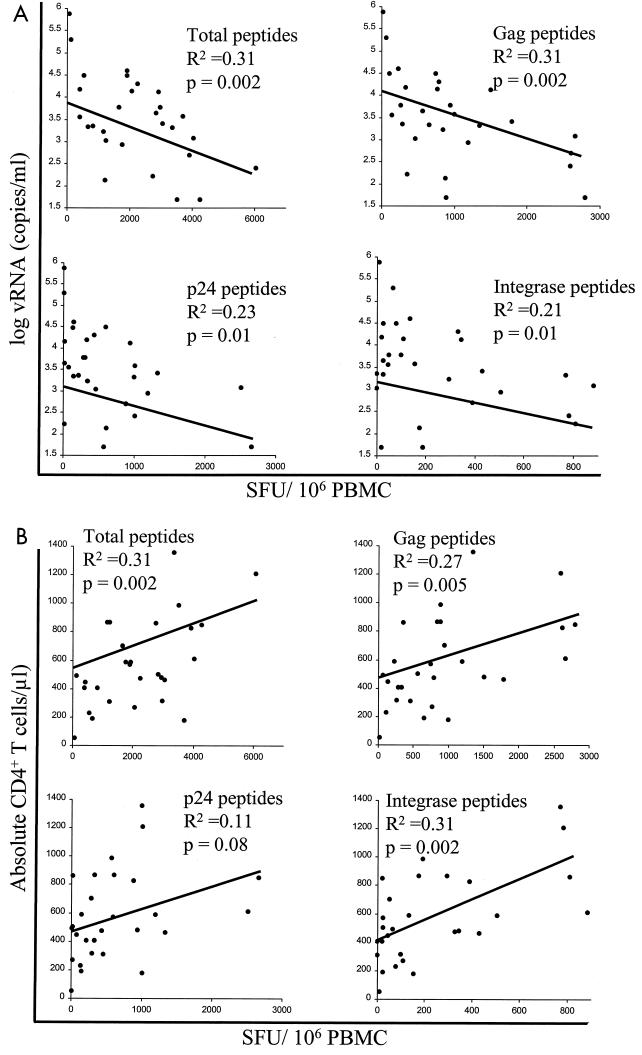
Next, we wanted to determine whether any of these responses correlated with markers of HIV-1 disease progression. Using the Spearman rank correlation test, we compared HIV-1-specific T-cell responses to both vRNA and absolute CD4+ T cell counts, as both of these were shown in numerous studies to be markers of HIV-1 disease progression (2, 15, 21, 37, 38, 44, 46). Consistent with the earlier studies, the absolute CD4+ T-cell count correlated inversely with vRNA in the current cohort (r = −0.62, P = 0.005). The total number of IFN-γ responses to HIV-1-specific peptide pools (SFU/106 PBMC) correlated inversely with vRNA and directly with absolute CD4+ T cells (r = −0.50 and 0.46, respectively) (Fig. 2A and B).
FIG. 2.
HIV-1-specific immune responses correlate with vRNA and absolute CD4+ T-cell counts. HIV-1-specific immune responses as measured by the 20-mer IFN-γ ELISPOT assay were compared with either vRNA (A) or absolute CD4+ T-cell counts (B) using linear regression analysis. The P values are shown for the slope of each data set.
When comparing markers of disease progression with the individual proteins that comprise the total response, only Gag correlated with vRNA (r = −0.60) and absolute CD4+ T-cell counts (r = 0.54), while Pol correlated exclusively with the latter marker (r = 0.45) (Table 2). Correlations could be further mapped to the p24 and integrase (IN) peptide pools of the Gag and Pol precursor proteins, respectively (Fig. 2A and B).
TABLE 2.
Comparison of the magnitude of HIV-1-specific immune responses with markers of disease progressiona
| Protein | Absolute CD4+ T cells
|
vRNA in plasma
|
||
|---|---|---|---|---|
| Rb (95% CI) | P | r (95% CI) | P | |
| p24 | 0.29 (−0.11, 0.61) | 0.1537 | −0.44 (−0.71, −0.06) | 0.0244 |
| gp160 | 0.07 (0.46 to 0.34) | 0.7376 | 0.34 (−0.08, 0.65) | 0.1066 |
| 20-mer peptide pools | ||||
| Total | 0.46 (0.11, 0.71) | 0.0135 | −0.50 (−0.74, −0.15) | 0.0069 |
| Gag | 0.54 (0.20, 0.76) | 0.0033 | −0.60 (−0.79, −0.29) | 0.0008 |
| p17 | 0.50 (0.16, 0.74) | 0.0065 | −0.14 (−0.49, 0.25) | 0.4853 |
| p24 | 0.39 (0.03, 0.67) | 0.0376 | −0.52 (−0.75, −0.18) | 0.0047 |
| NC | 0.27 (−0.12, 0.58) | 0.1697 | −0.36 (−0.65, 0.02) | 0.0605 |
| Pol | 0.45 (0.09, 0.70) | 0.0163 | −0.23 (−0.56, 0.15) | 0.2359 |
| Pr | 0.09 (−0.29, 0.45) | 0.6555 | −0.17 (−0.51, 0.22) | 0.3983 |
| RT | 0.24 (−0.15, 0.56) | 0.2173 | 0.03 (−0.34, 0.40) | 0.8644 |
| RNase H | 0.04 (−0.33, 0.41) | 0.8219 | −0.05 (−0.42, 0.33) | 0.7927 |
| IN | 0.49 (0.15, 0.73) | 0.0078 | −0.36 (−0.65, 0.01) | 0.0588 |
| Env | −0.03 (−0.40, 0.35) | 0.8911 | −0.26 (−0.58, 0.13) | 0.1828 |
| gp120 | −0.08 (−0.44, 0.30) | 0.6690 | −0.04 (−0.41, 0.34) | 0.8364 |
| gp41 | 0.14 (−0.25, 0.49) | 0.4864 | −0.17 (−0.51, 0.21) | 0.3803 |
| Nef | 0.00 (−0.37, 0.38) | 0.9879 | 0.04 (−0.34, 0.41) | 0.8442 |
The magnitude of responses was defined as the sum total of IFN-γ responses (SFU/106 PBMC) minus the medium background.
R, Spearman rank correlation coefficient. CI, confidence interval. Statistically significant values are represented in boldface type.
Next, we examined the breadth of the HIV-1-specific immune reactivity. The breadth of a subject's response was defined as the number of peptide pools (10 peptides per pool) for which positive ELISPOT responses were observed. The breadth of the total HIV-1-specific responses for each individual subject correlated with both vRNA (r = −0.38) and absolute CD4+ T-cell count (r = 0.62) (Table 3). Additionally, the breadth of responses to the Gag peptide pools demonstrated significant correlations with disease marker parameters (r = −0.43 and 0.53 for correlations with vRNA and absolute CD4+ T-cell counts, respectively), whereas the breadth of responses to the Pol pool of peptides only correlated with absolute CD4+ T-cell counts (r = 0.38). Neither the breadth nor the magnitude of responses to any other HIV-1-specific peptide pool (including Env and Nef) correlated with either marker of disease progression (Tables 2 and 3).
TABLE 3.
Comparison of the breadth of HIV-1-specific responses with markers of disease progressiona
| Protein | Absolute CD4 count
|
Viral load in plasma
|
||
|---|---|---|---|---|
| Rb (95% CI) | P | r (95% CI) | P | |
| Total | 0.62 (0.32, 0.81) | 0.0004 | −0.38 (−0.66, −0.01) | 0.0474 |
| Gag | 0.53 (0.02, 0.76) | 0.0034 | −0.43 (−0.69, −0.07) | 0.0230 |
| Pol | 0.38 (0.01, 0.66) | 0.0433 | −0.32 (−0.2, 0.06) | 0.0998 |
| Env | 0.13 (−0.25, 0.48) | 0.5064 | −0.24 (−0.56, 0.15) | 0.2253 |
| Nef | −0.03 (−0.40, 0.35) | 0.8926 | 0.18 (−0.20, 0.52) | 0.3515 |
Breadth was defined as the number of peptide pools (10 peptides per pool) for which positive ELISPOT responses were observed. An ELISPOT response was considered positive if ≥50 SFU/106 PBMC and ≥3 times the value for the subject's medium control.
Spearman rank correlation coefficient. CI, confidence interval. Statistically significant values are represented in boldface type.
The majority of responses to Gag peptides are due to HIV-1-specific CD8+ T cells.
Because responses to the p24 whole protein also correlated inversely with vRNA (Table 2), we wanted to substantiate that the responses seen with the 20-mer stimulations (presumably representing CD8+ T-cell responses) were not due to MHC class II presentation of the 20-mer peptides, thereby measuring IFN-γ secreted by CD4+ T helper cells. We therefore performed CD8+ and CD4+ T-cell depletion experiments using the frozen PBMC from eight individuals with the greatest magnitude of responses to the Gag peptide pool. Unfractionated, CD8-depleted, and CD4-depleted PBMC were stimulated with a Gag-specific peptide pool (50 peptides), and IFN-γ was measured using the ELISPOT assay (19).
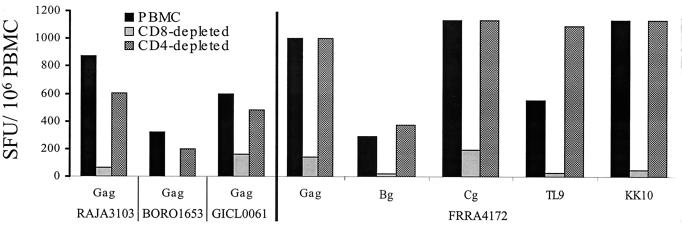
All peptide-stimulated PBMC derived from the eight subjects had HIV-1-specific responses that were decreased by greater than 70% when the CD8+ T cells were depleted. The data for four of the eight subjects are shown (Fig. 3). Although some decrease in the IFN-γ response was seen when CD4+ T cells were depleted, the majority of the HIV-1-specific IFN-γ responses remained.
FIG. 3.
IFN-γ secretion of PBMC stimulated with Gag peptides is due primarily to CD8+ T cells. The number of cells secreting IFN-γ was measured after unfractionated, CD8-depleted or CD4-depleted PBMC obtained from four subjects were stimulated with the Gag peptide pool overnight in an ELISPOT plate. For subject FRRA4172, the different cell subsets were also stimulated with peptides in the p24 protein (Bg and Cg) in addition to the optimized CD8+ T-cell peptides TL9 (TPQDLNTML) and KK10 (KRWIILGLNK), restricted by his HLA-B42 and B27 HLA class I proteins, respectively.
We also mapped the epitope-specific responses to the p24 peptides in six of these eight individuals. Five of these subjects had responses that mapped to HLA class I-restricted epitopes, and only one of them had p24-specific responses that were HLA class II restricted (GICL0061; data not shown). This latter individual also had responses directed at the C terminus of the Gag protein that were due exclusively to CD8+ T cells. Only 11 of 27 (41%) of the subjects in our cohort had IFN-γ responses to the p24 whole protein. Additionally, these responses were weak (mean, 85 SFU/106 PBMC) compared to those directed against Gag (mean, 962 SFU/106 PBMC) or the p24 peptides (mean, 620 SFU/106 PBMC). Even if we excluded the 11 subjects with responses to the p24 whole protein, responses to the Gag peptides still correlated inversely with vRNA (r = −0.59, P = 0.01).
As further evidence that the IFN-γ responses elicited by the peptide pools and the p24 whole protein were not measuring similar effects, we compared the magnitude of these responses to each other. No significant correlation was observed for comparison of the p24 whole protein with the total 20-mer (r = 0.09), Gag (r = 0.27), or p24 (r = 0.28) peptide pool responses. Taken together, these data demonstrated that the great majority of the HIV-1-specific responses measured after stimulation with 20-mer peptides were CD8+ T-cell specific and that they correlated independently with markers of disease progression.
DISCUSSION
In the current study we demonstrated that HIV-1-specific CD8+ T-cell responses as measured by the IFN-γ ELISPOT assay correlated inversely with vRNA. Moreover, this correlation was largely due to responses directed against Gag and specifically the p24 protein. Responses to Gag also correlated directly with absolute CD4+ T-cell counts, as did responses directed against the Pol precursor and IN. Our data demonstrate two separate and independent immune functions that correlate with vRNA: response to the p24 whole protein (likely due to CD4+ T cells) and response to 20-mer peptide pools representing Gag (due to CD8+ T cells, as demonstrated above). In view of the known significant correlations of vRNA and CD4+ T-cell counts with HIV-1 disease progression (2, 15, 21, 37, 38, 44, 46), these immune responses to Gag (as detected by the ELISPOT assay) would likely correlate with progression to AIDS as well.
Our study is the first to demonstrate correlations of HIV-1-specific T cells with both vRNA and absolute CD4+ T cells. It is also the first to see correlations of markers of disease progression with more than one protein (p24 and IN). Although at least two groups have correlated the frequency of HIV-1-specific cells with vRNA using the tetramer assay (34, 45), the results have not been duplicated by other groups (4a). The reasons that our study demonstrated correlations between immune responses and markers of HIV-1 disease progression could be due to a number of factors. In this study, we used a total of 250 peptides spanning all of the Gag, Pol, Env, and Nef proteins, thereby studying a much greater number of potential epitopes. We purposely quantified the IFN-γ responses, vRNA measurements, and absolute CD4+ T-cell counts from the same blood specimen for each subject. Additionally, the majority of patients were off medications for at least 6 months prior to sampling or had detectable vRNA despite ART. Furthermore, our cohort included patients in all stages of chronic HIV-1 infection, as evidenced by the broad range in their vRNA and absolute CD4+ T-cell counts (Table 1). Finally, assays used to measure the HIV-1-specific CD8+ T-cell responses differ in their methods of optimization. As an example, the intracellular cytokine staining technique utilizes costimulatory molecules (4a, 11, 30) that are not routinely used in the ELISPOT assay, and it is possible that certain assay optimization techniques may negate in vivo immune response differences that may exist among HIV-1-infected individuals.
Our ability to correlate responses to p24 with markers of disease progression is novel, and it is likely that these responses are due to CD8+ rather than CD4+ T-cell responses. We demonstrated that CD8+ T cells were being measured by using our ELISPOT technique in eight of eight subjects tested. The responses were due exclusively to CD8+ T cells in seven of these subjects and were mapped to HLA class I-restricted epitopes in all six of the individuals tested. Additionally, responses to the p24 whole protein did not correlate with p24 peptide responses, indicating that they measured separate effects. Therefore, HIV-1-specific CD8+ T cells were responsible for the majority of peptide-stimulated IFN-γ ELISPOT responses and correlated with markers of disease progression independently of CD4+ T-cell responses.
The reasons for the observed correlations seen with p24 peptide responses are unclear, but may be due to a number of factors. The p24 protein was one of the most frequently recognized subunit proteins, and the greatest magnitude of responses were directed to this protein when adjusted for the AA score (Fig. 1B). The reasons for this high frequency and magnitude of response are likely due to AA sequence conservation. Indeed, the two regions demonstrating the strongest correlations with markers of disease progression (p24 and IN) are also the most highly conserved proteins among the clade B viruses (36).
The inability to correlate Env-specific responses with risk factors for HIV-1 disease progression is likely due to the low magnitude of these responses in our cohort (median, 88 SFU/106 PBMC). Musey et al. were able to demonstrate a correlation between freshly isolated Env-specific CTL and vRNA (42). However, unlike our study, this group looked at HIV-1-specific CD8+ T cells in early infection where responses to Env were immunodominant; being present in up to 80% of their subjects. The inability to correlate vRNA with Nef-specific responses cannot be explained by the low magnitude of reactivity directed towards this protein, as the median response was 388 SFU/106 PBMC (Fig. 1B). In fact, Nef-specific responses were immunodominant (i.e., had the greatest magnitude) in 6 of the 27 subjects.
The relative lack of genetic sequence conservation in the Env and Nef regions in clade B viruses could explain the inability to correlate IFN-γ responses specific to these proteins with markers of disease progression. Among the different proteins of clade B viruses (36), Env and Nef are the most variable of the proteins tested in this study. A possible consequence of this genetic variability is that the measured immune responses to the Env and Nef proteins no longer serve to control viral replication due to the presence of CTL escape mutants (5, 14, 20, 47). This hypothesis may be particularly pertinent to the Nef-specific responses, as several studies have demonstrated the presence of CTL escape mutants to this protein (13, 24, 32, 47).
One important limitation of this study is the use of peptides derived from HIV clones that may not actually represent autologous virus. It is therefore safe to assume that the T-cell responses seen in our study underrepresent the true response. Use of autologous viral peptides not only would increase the frequency and magnitude of responses, but may also change some of the correlations seen in our study. Clearly sequencing autologous virus in order to design individual immune reagents should be performed in the future to more accurately measure HIV-1-specific CD8+ T-cell responses.
The goal of many of the newer candidate HIV-1 vaccines is to elicit strong CD8+ T-cell responses in an attempt to prevent HIV-1 disease in both infected and uninfected individuals. The current study presents further evidence that the HIV-1 Gag protein, particularly its p24 capsid subunit, should be included in any vaccine designed to elicit cell-mediated immune responses. Studies currently in progress, which aim to refine the specificity of CD8+ T-cell responses to HIV-1 and examine these responses longitudinally, will likely elucidate other proteins to be included in an optimal CTL-based vaccine.
Acknowledgments
This study was supported by a developmental grant to Paul A. Goepfert from the Center for AIDS Research (NIH award P30-AI-27767) at the University of Alabama at Birmingham.
We thank the patients of the 1917 Clinic at the University of Alabama at Birmingham, Susan Duncan and Greg Sfakianos for recruitment of subjects, G. Douglas Ritter for technical assistance, Vickie Barron for preparation of the manuscript, and Feng Gao and Heidi Weiss for their assistance with HIV-1 sequence and statistical analyses, respectively.
REFERENCES
- 1.Amara, R. R., F. Villinger, J. D. Altman, S. L. Lydy, S. P. O'Neil, S. I. Staprans, D. C. Montefiori, Y. Xu, J. G. Herndon, L. S. Wyatt, M. A. Candido, N. L. Kozyr, P. L. Earl, J. M. Smith, H. L. Ma, B. D. Grimm, M. L. Hulsey, J. Miller, H. M. McClure, J. M. McNicholl, B. Moss, and H. L. Robinson. 2001. Control of a mucosal challenge and prevention of AIDS by a multiprotein DNA/MVA vaccine. Science 292:69-74. [DOI] [PubMed] [Google Scholar]
- 2.Anastos, K., L. A. Kalish, N. Hessol, B. Weiser, S. Melnick, D. Burns, R. Delapenha, J. DeHovitz, M. Cohen, W. Meyer, J. Bremer, and A. Kovacs. 1999. The relative value of CD4 cell count and quantitative HIV-1 RNA in predicting survival in HIV-1-infected women: results of the women's interagency HIV study. AIDS 13:1717-1726. [DOI] [PubMed] [Google Scholar]
- 3.Barouch, D. H., S. Santra, M. J. Kuroda, J. E. Schmitz, R. Plishka, A. Buckler-White, A. E. Gaitan, R. Zin, J. H. Nam, L. S. Wyatt, M. A. Lifton, C. E. Nickerson, B. Moss, D. C. Montefiori, V. M. Hirsch, and N. L. Letvin. 2001. Reduction of simian-human immunodeficiency virus 89.6p viremia in rhesus monkeys by recombinant modified vaccinia virus Ankara vaccination. J. Virol. 75:5151-5158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barouch, D. H., S. Santra, J. E. Schmitz, M. J. Kuroda, T. M. Fu, W. Wagner, M. Bilska, A. Craiu, X. X. Zheng, G. R. Krivulka, K. Beaudry, M. A. Lifton, C. E. Nickerson, W. L. Trigona, K. Punt, D. C. Freed, L. Guan, S. Dubey, D. Casimiro, A. Simon, M. E. Davies, M. Chastain, T. B. Strom, R. S. Gelman, D. C. Montefiori, and M. G. Lewis. 2000. Control of viremia and prevention of clinical AIDS in rhesus monkeys by cytokine-augmented DNA vaccination. Science 290:486-492. [DOI] [PubMed] [Google Scholar]
- 4a.Betts, M. R., D. R. Ambrozak, D. C. Douek, S. Bonhoeffer, J. M. Benchley, J. P. Casazza, R. A. Koup, and L. J. Picker. 2001. Analysis of total human immunodeficiency virus (HIV)-specific CD4+ and CD8+ T-cell responses: relationship to viral load in untreated HIV infection. J. Virol. 75:11983-11991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borrow, P., L. Hanna, Z. Wei, M. S. Horowitz, N. Peffer, H. Meyers, J. A. Nelson, J. E. Gairin, B. H. Hahn, M. B. A. Oldstone, and G. M. Shaw. 1997. Antiviral pressure exerted by HIV-1-specific cytotoxic T lymphocytes (CTLs) during primary infection demonstrated by rapid selection of CTL escape virus. Nat. Med. 3:205-211. [DOI] [PubMed] [Google Scholar]
- 6.Borrow, P., H. Lewicki, B. H. Hahn, G. M. Shaw, and M. B. Oldstone. 1994. Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J. Virol. 68:6103-6110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bouscarat, F., M. H. Prevot, and S. Matheron. 1999. Alopecia associated with indinavir therapy. N. Engl. J. Med. 341:618.. [DOI] [PubMed] [Google Scholar]
- 8.Cao, U., Q. Limo, Z. Linqu, J. Safrit, and D. H. Ho. 1995. Virologic and immunologic characterization of long-term survivors of human immunodeficiency virus type 1 infection. N. Engl. J. Med. 332:201-208. [DOI] [PubMed] [Google Scholar]
- 9.Carr, A., K. Samaras, S. Burton, M. Law, J. Freund, D. J. Chisholm, and D. A. Cooper. 1998. A syndrome of peripheral lipodystrophy, hyperlipidaemia and insulin resistance in patients receiving HIV protease inhibitors. AIDS 12:F51-F58. [DOI] [PubMed] [Google Scholar]
- 10.Carrington, M., G. W. Nelson, M. P. Martin, T. Kissner, D. Vlahov, J. J. Goedert, R. Kaslow, S. Buchbinder, K. Hoots, and S. J. O'Brien. 1999. HLA and HIV-1: heterozygote advantage and B*35-Cw*04 disadvantage. Science 283:1748-1752. [DOI] [PubMed] [Google Scholar]
- 11.Casazza, J. P., M. R. Betts, L. J. Picker, and R. A. Koup. 2001. Decay kinetics of human immunodeficiency virus-specific CD8+ T cells in peripheral blood after initiation of highly active antiretroviral therapy. J. Virol. 75:6508-6516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chariot, P., I. Drogou, I. de Lacroix-Szmania, M. C. Eliezer-Vanerot, B. Chazaud, A. Lombes, A. Schaeffer, and E. S. Zafrani. 1999. Zidovudine-induced mitochondrial disorder with massive liver steatosis, myopathy, lactic acidosis, and mitochondrial DNA depletion. J. Hepatol. 30:156-160. [DOI] [PubMed] [Google Scholar]
- 13.Couillin, I., B. Culmann-Penciolelli, E. Gomard, J. Choppin, J. P. Levy, J. G. Guillet, and S. Saragosti. 1994. Impaired cytotoxic T lymphocyte recognition due to genetic variations in the main immunogenic region of the human immunodeficiency virus 1 NEF protein. J. Exp. Med. 180:1129-1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Evans, D. T., D. H. O'Connor, P. Jing, J. L. Dzuris, J. Sidney, J. da Silva, T. M. Allen, H. Horton, J. E. Venham, R. A. Rudersdorf, T. Vogel, C. D. Pauza, R. E. Bontrop, R. DeMars, A. Sette, A. L. Hughes, and D. I. Watkins. 1999. Virus-specific cytotoxic T-lymphocyte responses select for amino-acid variation in simian immunodeficiency virus Env and Nef. Nat. Med. 5:1270-1276. [DOI] [PubMed] [Google Scholar]
- 15.Fahey, J. L., J. M. Taylor, R. Detels, B. Hofmann, R. Melmed, P. Nishanian, and J. V. Giorgi. 1990. The prognostic value of cellular and serologic markers in infection with human immunodeficiency virus type 1. N. Engl. J. Med. 322:166-172. [DOI] [PubMed] [Google Scholar]
- 16.Finzi, D., J. Blankson, J. D. Siliciano, J. B. Margolick, K. Chadwick, T. Pierson, K. Smith, J. Lisziewicz, F. Lori, C. Flexner, T. C. Quinn, R. E. Chaisson, E. Rosenberg, B. Walker, S. Gange, J. Gallant, and R. F. Siliciano. 1999. Latent infection of CD4+ T-cells provides a mechanism for lifelong persistence of HIV-1, even in patients on effective combination therapy. Nat. Med. 5:512-517. [DOI] [PubMed] [Google Scholar]
- 17.Finzi, D., M. Hermankova, T. Pierson, L. M. Carruth, C. Buck, R. E. Chaisson, T. C. Quinn, K. Chadwick, J. Margolick, R. Brookmeyer, J. Gallant, M. Markowitz, D. D. Ho, D. D. Richman, and R. F. Siliciano. 1997. Identification of a reserovir for HIV-1 in patients on highly active antiretroviral therapy. Science 278:1295-1300. [DOI] [PubMed] [Google Scholar]
- 18.Furtado, M. R., D. S. Callaway, J. P. Phair, K. J. Kunstman, J. L. Stanton, C. A. Macken, A. S. Perlson, and S. M. Wolinsky. 1999. Persistence of HIV-1 transcription in peripheral-blood mononuclear cells in patients receiving potent antiretroviral therapy. N. Engl. J. Med. 340:1614-1622. [DOI] [PubMed] [Google Scholar]
- 19.Goepfert, P. A., A. Bansal, B. H. Edwards, G. D. Ritter, Jr., I. Tellez, S. A. McPherson, S. Sabbaj, and M. J. Mulligan. 2000. A significant number of human immunodeficiency virus epitope-specific cytotoxic T lymphocytes detected by tetramer binding do not produce gamma interferon. J. Virol. 74:10249-10255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goulder, P. J. R., R. E. Phillips, R. A. Colbert, S. McAdam, G. Ogg, M. A. Nowak, P. Giangrande, G. Luzzi, B. Morgan, A. Edwards, A. J. McMichael, and S. Rowland-Jones. 1997. Late escape from an immunodominant cytotoxic T-lymphocyte response associated with progression to AIDS. Nat. Med. 3:212-217. [DOI] [PubMed] [Google Scholar]
- 21.Grant, M. D., F. M. Smaill, D. P. Singal, and K. L. Rosenthal. 1992. The influence of lymphocyte counts and disease progression on circulating and inducible anti-HIV-1 cytotoxic T-cell activity in HIV-1-infected subjects. AIDS 6:1085-1094. [DOI] [PubMed] [Google Scholar]
- 22.Gray, C. M., J. Lawrence, J. M. Schapiro, J. D. Altman, M. A. Winters, M. Crompton, M. Loi, S. K. Kundu, M. M. Davis, and T. C. Merigan. 1999. Frequency of class I HLA-restricted anti-HIV CD8+ T-cells in individuals receiving highly active antiretroviral therapy (HAART). J. Immunol. 162:1780-1788. [PubMed] [Google Scholar]
- 23.Greenough, T. C., D. B. Brettler, M. Somasundaran, D. L. Panicali, and J. L. Sullivan. 1997. Human immunodeficiency virus type 1-specific cytotoxic T lymphocytes (CTL), virus load, and CD4 T-cell loss: evidence supporting a protective role for CTL in vivo. J. Infect. Dis. 176:118-125. [DOI] [PubMed] [Google Scholar]
- 24.Haas, G., U. Plikat, P. Debre, M. Lucchiari, C. Katlama, Y. Dudoit, O. Bonduelle, M. Bauer, H. G. Ihlenfeldt, G. Jung, B. Maier, A. Meyerhans, and B. Autran. 1996. Dynamics of viral variants in HIV-1 Nef and specific cytotoxic T lymphocytes in vivo. J. Immunol. 157:4212-4221. [PubMed] [Google Scholar]
- 25.Jin, X., D. E. Bauer, S. E. Tuttleton, S. Lewin, A. Gettie, J. Blanchard, C. E. Irwin, J. T. Safrit, J. Mittler, L. Weinberger, L. G. Kostrikis, L. Zhang, A. S. Perelson, and D. D. Ho. 1999. Dramatic rise in plasma viremia after CD8+ T-cell depletion in simian immunodeficiency virus-infected macaques. J. Exp. Med. 189:991-998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaslow, R. A., M. Carrington, R. Apploe, L. Park, A. Munoz, A. J. Saah, J. J. Goedert, C. Winkler, S. J. O'Brien, C. Rinaldo, R. Detels, W. Blattner, J. Phair, H. Erlich, and D. L. Mann. 1996. Influence of combinations of human major histocompatibility complex genes on the course of HIV-1 infection. Nat. Med. 2:405-411. [DOI] [PubMed] [Google Scholar]
- 27.Kaslow, R. A., C. Rivers, J. Tang, P. A. Goepfert, T. J. Bender, R. E. Habib, K. Weinhold, and M. J. Mulligan. 2001. Polymorphisms in HLA class I genes associated with both favorable prognosis of human immunodeficiency virus type 1 (HIV-1) infection and positive cytotoxic T-lymphocyte response to ALVAC-HIV recombinant canarypox vaccines. J. Virol. 75:8681-8689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaul, R., T. Dong, F. A. Plummer, J. Kimani, T. Rostron, P. Kiama, E. Njagi, E. Irungu, B. Farah, J. Oyugi, R. Chakraborty, K. S. MacDonald, J. J. Bwayo, A. McMichael, and S. L. Rowland-Jones. 2001. CD8+ lymphocytes respond to different HIV epitopes in seronegative and infected subjects. J. Clin. Investig. 107:1303-1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaul, R., F. A. Plummer, J. Kimani, T. Dong, P. Kiama, T. Rostron, E. Njagi, K. S. Macdonald, J. J. Bwayo, A. J. McMichael, and S. Rowland-Jones. 2000. HIV-1-specific mucosal CD8+ lymphocyte responses in the cervix of HIV-1-resistant prostitutes in Nairobi. J. Immunol. 164:1602-1611. [DOI] [PubMed] [Google Scholar]
- 30.Kern, F., I. P. Surel, C. Brock, B. Freistedt, H. Radtke, A. Scheffold, R. Blasczyk, P. Reinke, J. Schneider-Mergener, A. Radbruch, P. Walden, and H. D. Volk. 1998. T-cell epitope mapping by flow cytometry. Nat. Med. 4:975-978. [DOI] [PubMed] [Google Scholar]
- 31.Klein, M. R., C. A. van Baalen, A. M. Holwerda, S. R. Kerkhof Garde, R. J. Bende, I. P. Keet, J. K. Eeftinck-Schattenkerk, A. D. Osterhaus, H. Schuitemaker, and F. Miedema. 1995. Kinetics of Gag-specific cytotoxic T lymphocyte responses during the clinical course of HIV-1 infection: a longitudinal analysis of rapid progressors and long-term asymptomatics. J. Exp. Med. 181:1365-1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koenig, S., A. J. Conley, Y. A. Brewah, G. M. Jones, S. Leath, L. J. Boots, V. Davey, G. Pantaleo, J. F. Demarest, C. Carter, et al. 1995. Transfer of HIV-1-specific cytotoxic T lymphocytes to an AIDS patient leads to selection for mutant HIV variants and subsequent disease progression. Nat. Med. 1:330-336. [DOI] [PubMed] [Google Scholar]
- 33.Kopp, J. B., K. D. Miller, J. A. Mican, I. M. Feuerstein, E. Vaughan, C. Baker, L. K. Pannell, and J. Falloon. 1997. Crystalluria and urinary tract abnormalities associated with indinavir. Ann. Intern. Med. 127:119-125. [DOI] [PubMed] [Google Scholar]
- 34.Kostense, S., G. S. Ogg, E. H. Manting, G. Gillespie, J. Joling, K. Vandenberghe, E. Z. Veenhof, D. van Baarle, S. Jurriaans, M. R. Klein, and F. Miedema. 2001. High viral burden in the presence of major HIV-specific CD8+ T-cell expansions: evidence for impaired CTL effector function. Eur. J. Immunol. 31:677-686. [DOI] [PubMed] [Google Scholar]
- 35.Koup, R. A., J. T. Safrit, Y. Cao, C. A. Andrews, G. McLeod, W. Borkowsky, C. Farthing, and D. D. Ho. 1994. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J. Virol. 68:4650-4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kuiken, C. L. (ed.). 1999. Human retroviruses and AIDS 1999: a compilation and analysis of nucleic acid and amino acid sequences . Theoretical Biology and Biophysics Group, Los Alamos National Laboratory, Los Alamos, N.Mex.
- 37.Mellors, J. W., A. Munoz, J. V. Giorgi, J. B. Margolick, C. J. Tassoni, P. Gupta, L. A. Kingsley, J. A. Todd, A. J. Saah, R. Detels, J. P. Phair, and C. R. Rinaldo, Jr. 1997. Plasma viral load and CD4+ lymphocytes as prognostic markers of HIV-1 infection. Ann. Intern. Med. 126:946-954. [DOI] [PubMed] [Google Scholar]
- 38.Mellors, J. W., C. R. Rinaldo, Jr., P. Gupta, R. M. White, J. A. Todd, and L. A. Kingsley. 1996. Prognosis in HIV-1 infection predicted by the quantity of virus in plasma. Science 272:1167-1170. [DOI] [PubMed] [Google Scholar]
- 39.Miller, K. D., E. Jones, J. A. Yanovski, R. Shankar, I. Feuerstein, and J. Falloon. 1998. Visceral abdominal-fat accumulation associated with use of indinavir. Lancet 351:871-875. [DOI] [PubMed] [Google Scholar]
- 40.Mokrzycki, M. H., C. Harris, H. May, J. Laut, and J. Palmisano. 2000. Lactic acidosis associated with stavudine administration: a report of five cases. Clin. Infect. Dis. 30:198-200. [DOI] [PubMed] [Google Scholar]
- 41.Mulligan, M. J., and J. Weber. 1999. Human trials of HIV-1 vaccines. AIDS 13(Suppl. A):S1-S8. [PubMed] [Google Scholar]
- 42.Musey, L., J. Hughes, T. Schacker, T. Shea, L. Corey, and M. J. McElrath. 1997. Cytotoxic-T-cell responses, viral load, and disease progression in early human immunodeficiency virus type 1 infection. N. Engl. J. Med. 337:1267-1274. [DOI] [PubMed] [Google Scholar]
- 43.Nelson, G. W., R. Kaslow, and D. L. Mann. 1997. Frequency of HLA allele-specific peptide motifs in HIV-1 proteins correlates with the allele's association with relative rates of disease progression after HIV-1 infection. Proc. Natl. Acad. Sci. USA 94:9802-9807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.O'Brien, W. A., P. M. Hartigan, D. Martin, J. Esinhart, A. Hill, S. Benoit, M. Rubin, M. S. Simberkoff, and J. D. Hamilton. 1996. Changes in plasma HIV-1 RNA and CD4+ lymphocyte counts and the risk of progression to AIDS. Veterans Affairs Cooperative Study Group on AIDS. N. Engl. J. Med. 334:426-431. [DOI] [PubMed] [Google Scholar]
- 45.Ogg, G. S., X. Jin, S. Bonhoeffer, P. R. Dunbar, M. A. Nowak, S. Monard, J. P. Segal, Y. Cao, S. L. Rowland-Jones, V. Cerundolo, A. Hurley, M. Markowitz, D. D. Ho, D. F. Nixon, and A. J. McMichael. 1998. Quantitation of HIV-1-specific cytotoxic T lymphocytes and plasma load of viral RNA. Science 279:2103-2106. [DOI] [PubMed] [Google Scholar]
- 46.Polk, B. F., R. Fox, R. Brookmeyer, S. Kanchanaraksa, R. Kaslow, B. Visscher, C. Rinaldo, and J. Phair. 1987. Predictors of the acquired immunodeficiency syndrome developing in a cohort of seropositive homosexual men. N. Engl. J. Med. 316:61-66. [DOI] [PubMed] [Google Scholar]
- 47.Price, D. A., P. J. Goulder, P. Klenerman, A. K. Sewell, P. J. Easterbrook, M. Troop, C. R. Bangham, and R. E. Phillips. 1997. Positive selection of HIV-1 cytotoxic T lymphocyte escape variants during primary infection. Proc. Natl. Acad. Sci. USA 94:1890-1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rinaldo, C., X. L. Huang, Z. F. Fan, M. Ding, L. Beltz, A. Logar, D. Panicali, G. Mazzara, J. Liebmann, M. Cottrill, et al. 1995. High levels of anti-human immunodeficiency virus type 1 (HIV-1) memory cytotoxic T-lymphocyte activity and low viral load are associated with lack of disease in HIV-1-infected long-term nonprogressors. J. Virol. 69:5838.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rowland-Jones, S., R. Tan, and A. McMichael. 1997. Role of cellular immunity in protection against HIV infection. Adv. Immunol. 65:277-346. [PubMed] [Google Scholar]
- 50.Rowland-Jones, S. L., T. Dong, K. R. Fowke, J. Kimani, P. Krausa, H. Newell, T. Blanchard, K. Ariyoshi, J. Oyugi, E. Ngugi, J. Bwayo, K. S. MacDonald, A. J. McMichael, and F. A. Plummer. 1998. Cytotoxic T-cell responses to multiple conserved HIV epitopes in HIV-resistant prostitutes in Nairobi. J. Clin. Investig. 102:1758-1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schmitz, J. E., M. J. Kuroda, S. Santra, V. G. Sasseville, M. A. Simon, M. A. Lifton, P. Racz, K. Racz-Tanner, M. Dalesandro, B. Scallon, J., J. Ghrayeb, M. A. Forman, D. C. Montefiori, E. P. Rieber, N. L. Letvin, and K. A. Reimann. 1999. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science 283:857-860. [DOI] [PubMed] [Google Scholar]
- 52.Viraben, R., and C. Aquilina. 1998. Indinavir-associated lipodystrophy. AIDS 12:F37-F39. [DOI] [PubMed] [Google Scholar]
- 53.Zhang, L., B. Ramratnam, K. Tenner-Racz, Y. He, M. Vesanen, S. Lewin, A. Talal, P. Racz, A. S. Perelson, B. T. Korber, M. Markowitz, and D. D. Ho. 1999. Quantifying residual HIV-1 replication in patients receiving combination antiretroviral therapy. N. Engl. J. Med. 340:1605-1613. [DOI] [PubMed] [Google Scholar]