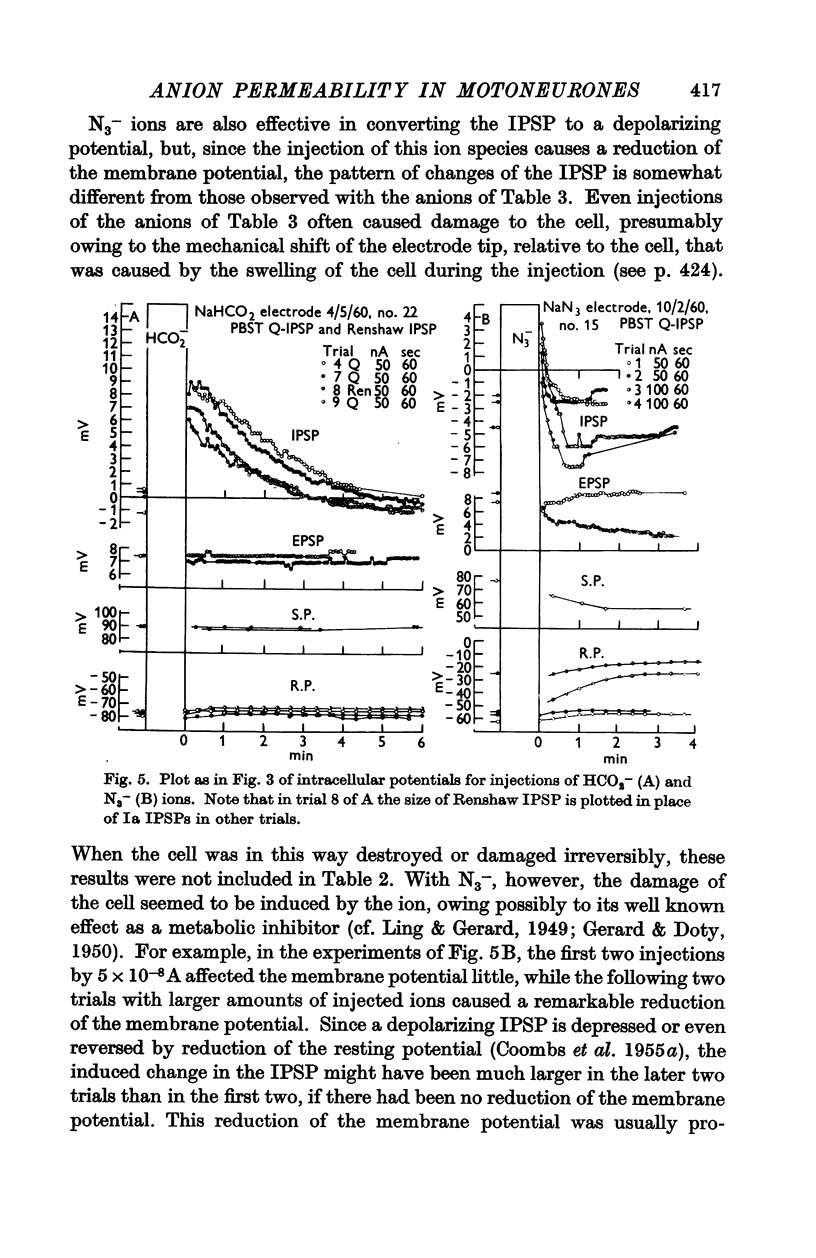
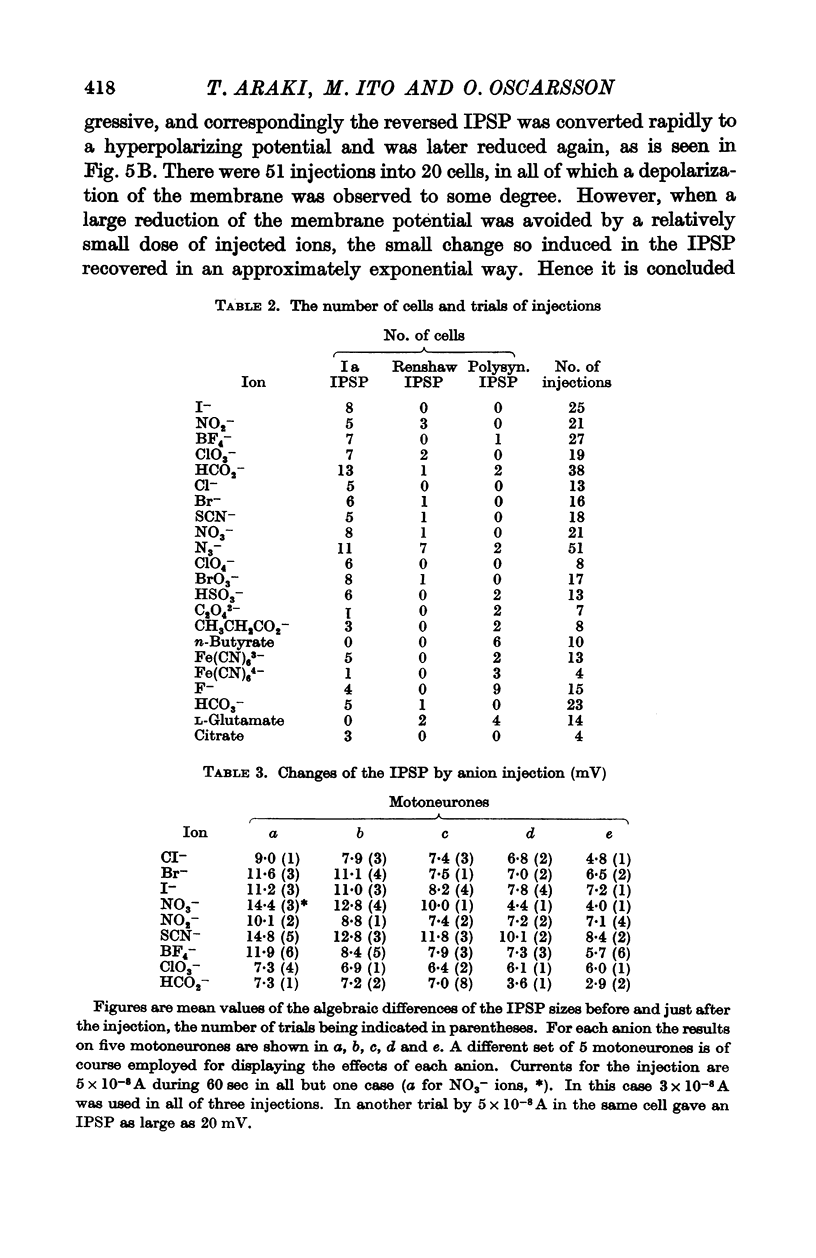
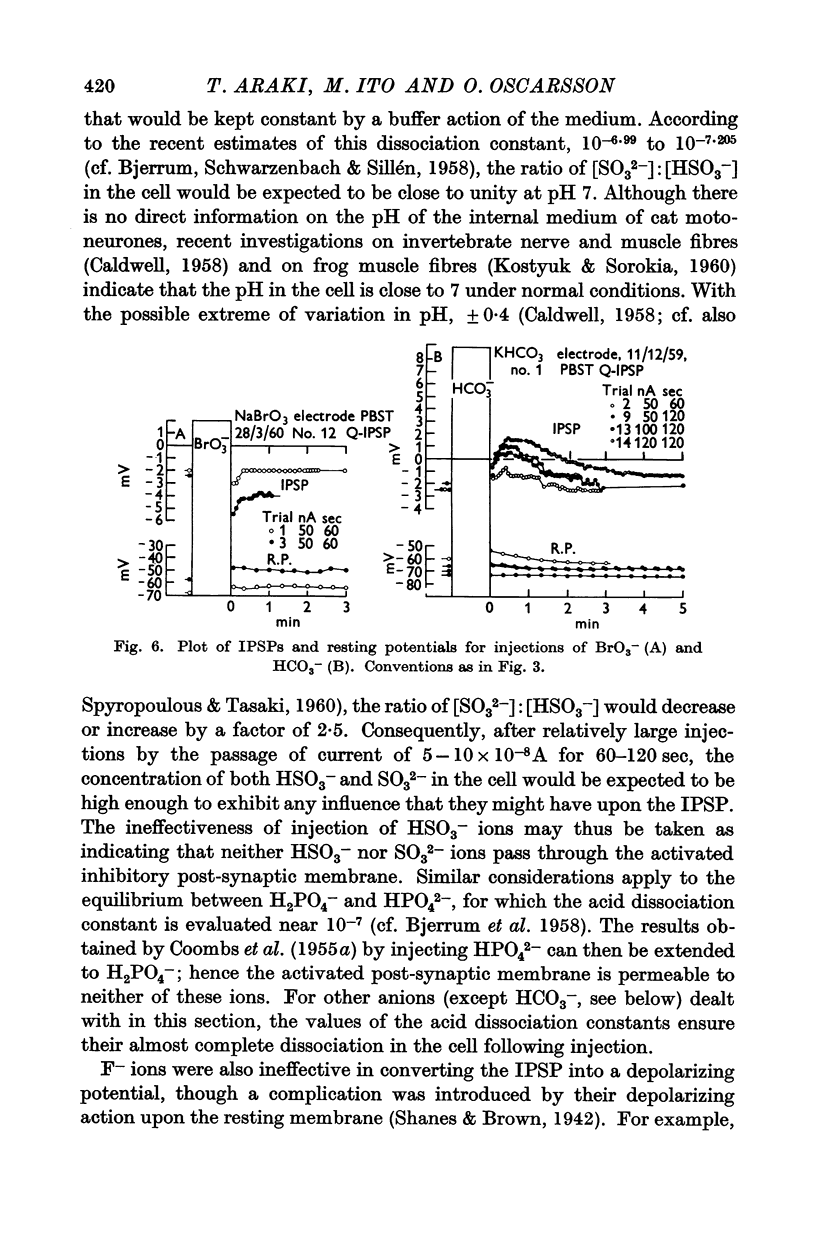
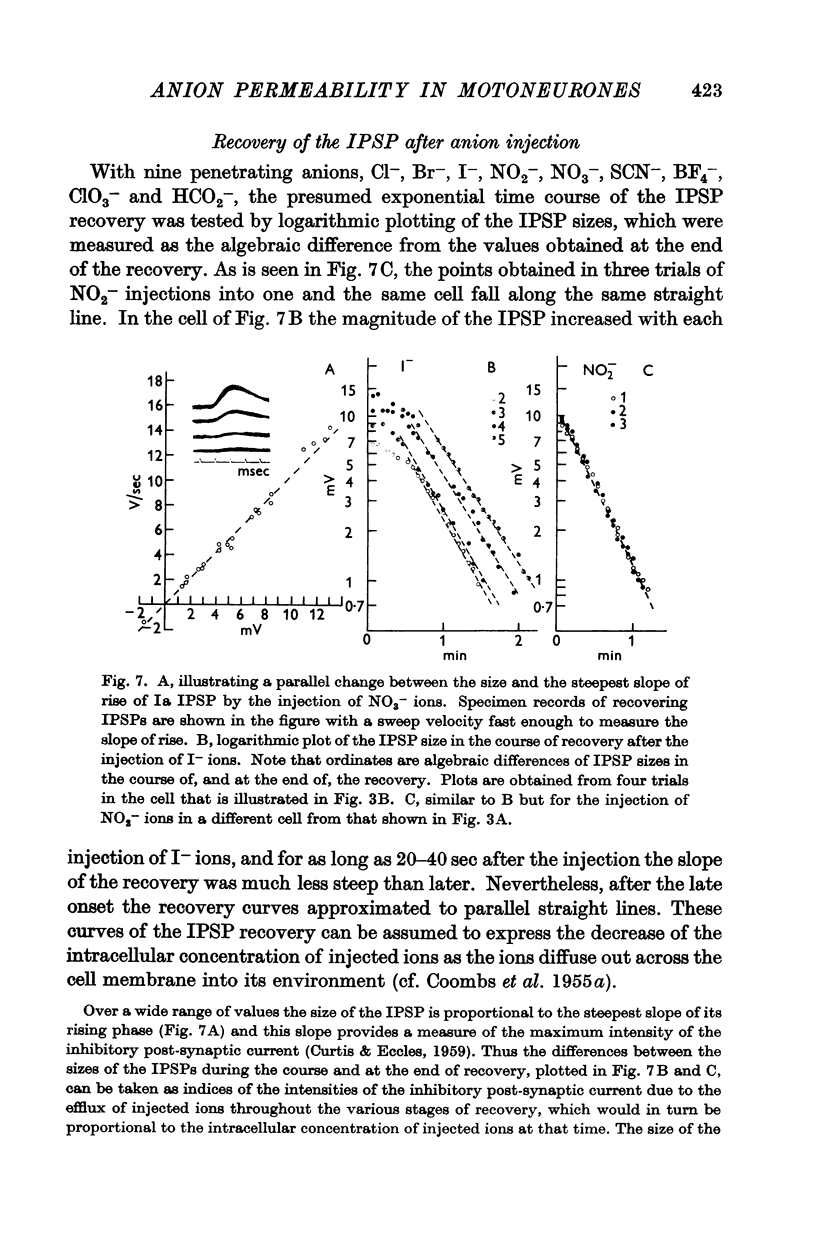
Full text
PDF














Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARAKI T., EOCLES J. C., ITO M. Correlation of the inhibitory post-synaptic potential of motoneurones with the latency and time course of inhibition of monosynaptic reflexes. J Physiol. 1960 Dec;154:354–377. doi: 10.1113/jphysiol.1960.sp006584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ARAKI T., ITO M., OSCARSSON O. Anionic permeability of the inhibitory postsynaptic membrane of motoneurones. Nature. 1961 Jan 7;189:65–65. doi: 10.1038/189065a0. [DOI] [PubMed] [Google Scholar]
- BOISTEL J., FATT P. Membrane permeability change during inhibitory transmitter action in crustacean muscle. J Physiol. 1958 Nov 10;144(1):176–191. doi: 10.1113/jphysiol.1958.sp006094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRADLEY K., ECCLES J. C. Analysis of the fast afferent impulses from thigh muscles. J Physiol. 1953 Dec 29;122(3):462–473. doi: 10.1113/jphysiol.1953.sp005014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROCK L. G., COOMBS J. S., ECCLES J. C. The recording of potentials from motoneurones with an intracellular electrode. J Physiol. 1952 Aug;117(4):431–460. doi: 10.1113/jphysiol.1952.sp004759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle P. J., Conway E. J. Potassium accumulation in muscle and associated changes. J Physiol. 1941 Aug 11;100(1):1–63. doi: 10.1113/jphysiol.1941.sp003922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CALDWELL P. C. Studies on the internal pH of large muscle and nerve fibres. J Physiol. 1958 Jun 18;142(1):22–62. doi: 10.1113/jphysiol.1958.sp005998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COOMBS J. S., CURTIS D. R., ECCLES J. C. The electrical constants of the motoneurone membrane. J Physiol. 1959 Mar 12;145(3):505–528. doi: 10.1113/jphysiol.1959.sp006158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COOMBS J. S., ECCLES J. C., FATT P. Excitatory synaptic action in motoneurones. J Physiol. 1955 Nov 28;130(2):374–395. doi: 10.1113/jphysiol.1955.sp005413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COOMBS J. S., ECCLES J. C., FATT P. The specific ionic conductances and the ionic movements across the motoneuronal membrane that produce the inhibitory post-synaptic potential. J Physiol. 1955 Nov 28;130(2):326–374. doi: 10.1113/jphysiol.1955.sp005412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CURTIS D. R., ECCLES J. C. The time courses of excitatory and inhibitory synaptic actions. J Physiol. 1959 Mar 12;145(3):529–546. doi: 10.1113/jphysiol.1959.sp006159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES J. C., ECCLES R. M., LUNDBERG A. Synaptic actions on motoneurones caused by impulses in Golgi tendon organ afferents. J Physiol. 1957 Sep 30;138(2):227–252. doi: 10.1113/jphysiol.1957.sp005849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES J. C., ECCLES R. M., LUNDBERG A. The convergence of monosynaptic excitatory afferents on to many different species of alpha motoneurones. J Physiol. 1957 Jun 18;137(1):22–50. doi: 10.1113/jphysiol.1957.sp005794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES J. C., FATT P., KOKETSU K. Cholinergic and inhibitory synapses in a pathway from motor-axon collaterals to motoneurones. J Physiol. 1954 Dec 10;126(3):524–562. doi: 10.1113/jphysiol.1954.sp005226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES J. C., FATT P., LANDGREN S., WINSBURY G. J. Spinal cord potentials generated by volleys in the large muscle afferents. J Physiol. 1954 Sep 28;125(3):590–606. doi: 10.1113/jphysiol.1954.sp005183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES R. M., LUNDBERG A. Supraspinal control of interneurones mediating spinal reflexes. J Physiol. 1959 Oct;147:565–584. doi: 10.1113/jphysiol.1959.sp006262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GERARD R. W., DOTY R. W. Nerve conduction without increased oxygen consumption; the action of azide and fluoroacetate. Biochim Biophys Acta. 1950 Jan;4(1-3):115–117. doi: 10.1016/0006-3002(50)90013-8. [DOI] [PubMed] [Google Scholar]
- HAGIWARA S., KUSANO K., SAITO S. Membrane changes in crayfish stretch receptor neutron during synaptic inhibition and under action of gamma-aminobutyric acid. J Neurophysiol. 1960 Sep;23:505–515. doi: 10.1152/jn.1960.23.5.505. [DOI] [PubMed] [Google Scholar]
- HARRIS E. J. Anion interaction in frog muscle. J Physiol. 1958 Apr 30;141(2):351–365. doi: 10.1113/jphysiol.1958.sp005979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HILL A. V., MACPHERSON L. The effect of nitrate, iodide and bromide on the duration of the active state in skeletal muscle. Proc R Soc Lond B Biol Sci. 1954 Dec 15;143(910):81–102. doi: 10.1098/rspb.1954.0055. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., KATZ B. The effect of sodium ions on the electrical activity of giant axon of the squid. J Physiol. 1949 Mar 1;108(1):37–77. doi: 10.1113/jphysiol.1949.sp004310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUTTER O. F., NOBLE D. The chloride conductance of frog skeletal muscle. J Physiol. 1960 Apr;151:89–102. [PMC free article] [PubMed] [Google Scholar]
- HUTTER O. F., PADSHA S. M. Effect of nitrate and other anions on the membrane resistance of frog skeletal muscle. J Physiol. 1959 Apr 23;146(1):117–132. doi: 10.1113/jphysiol.1959.sp006182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUNO M. Excitability following antidromic activation in spinal motoneurones supplying red muscles. J Physiol. 1959 Dec;149:374–393. doi: 10.1113/jphysiol.1959.sp006345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LING G., GERARD R. W. The membrane potential and metabolism of muscle fibers. J Cell Physiol. 1949 Dec;34(3):413–438. doi: 10.1002/jcp.1030340307. [DOI] [PubMed] [Google Scholar]
- LUFT J. H. Permanganate; a new fixative for electron microscopy. J Biophys Biochem Cytol. 1956 Nov 25;2(6):799–802. doi: 10.1083/jcb.2.6.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MULLINS L. J., MOORE R. D. The movement of thallium ions in muscle. J Gen Physiol. 1960 Mar;43:759–773. doi: 10.1085/jgp.43.4.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MULLINS Lj. The penetration of some cations into muscle. J Gen Physiol. 1959 Mar 20;42(4):817–829. doi: 10.1085/jgp.42.4.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHANES A. M. Electrochemical aspects of physiological and pharmacological action in excitable cells. I. The resting cell and its alteration by extrinsic factors. Pharmacol Rev. 1958 Mar;10(1):59–164. [PubMed] [Google Scholar]
- SIMON S. E., JOHNSTONE B. M., SHANKLY K. H., SHAW F. H. Muscle: a three phase system. The partition of monovalent ions across the cell membrane. J Gen Physiol. 1959 Sep;43:55–79. doi: 10.1085/jgp.43.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SJODIN R. A. Rubidium and cesium fluxes in muscle as related to the membrane potential. J Gen Physiol. 1959 May 20;42(5):983–1003. doi: 10.1085/jgp.42.5.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOLOMON A. K. Red cell membrane structure and ion transport. J Gen Physiol. 1960 May;43:1–15. doi: 10.1085/jgp.43.5.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPYROPOULOS C. S., TASAKI I. Nerve excitiation and synaptic transmission. Annu Rev Physiol. 1960;22:407–432. doi: 10.1146/annurev.ph.22.030160.002203. [DOI] [PubMed] [Google Scholar]


