Abstract
Human immunodeficiency virus (HIV) continues to be a major public health problem throughout the world, with high levels of mortality and morbidity associated with AIDS. Considerable efforts to develop an effective vaccine for HIV have been directed towards the generation of cellular, humoral, and mucosal immune responses. A major emphasis of our work has been toward the evaluation of oligomeric (o-gp140) forms of the HIV type 1 (HIV-1) envelope protein for their ability to induce neutralizing antibody responses. We have derived stable CHO cell lines expressing o-gp140 envelope protein from the primary non-syncytium-inducing (R5) subtype B strain HIV-1US4. We have developed an efficient purification strategy to purify oligomers to near homogeneity. Using a combination of three detectors measuring intrinsic viscosity, light scattering, and refractive index, we calculated the molecular mass of the oligomer to be 474 kDa, consistent with either a trimer or a tetramer. The hydrodynamic radius (Rh) of o-gp140 was determined to be 8.40 nm, compared with 5.07 nm for the monomer. The relatively smaller Rh of the oligomer suggests that there are indeed differences between the foldings of o-gp140 and gp120. To assess the structural integrity of the purified trimers, we performed a detailed characterization of the glycosylation profile of o-gp140, its ability to bind soluble CD4, and also its ability to bind to a panel of monoclonal antibodies with known epitope specificities for the CD4 binding site, the CD4 inducible site, the V3 loop, and gp41. Immunogenicity studies with rabbits indicated that the purified o-gp140 protein was highly immunogenic and induced high-titer, high-avidity antibodies directed predominantly against conformational epitopes. These observations confirm the structural integrity of purified o-gp140 and its potential as a vaccine antigen.
AIDS continues to be a major health problem throughout the world, with high degrees of mortality and morbidity. The situation is aggravated by an increasing rate of primary infections (9,000 infections every day) and the emergence of drug-resistant viruses. An estimated 40,000 to 80,000 primary infections occur each year in the United States alone. Therefore, there is an urgent need for an effective anti-human immunodeficiency virus (anti-HIV) vaccine. Considerable efforts are being made to develop such a vaccine, using a variety of different vaccine technologies. One common feature of these approaches is the objective of inducing broadly cross-reactive humoral and cellular immune responses at peripheral as well as mucosal sites (6, 9, 10, 31, 36, 39, 42, 45, 49, 56, 61, 63, 69, 73, 77, 80, 93, 109, 117).
For the induction of neutralizing antibody responses, HIV Env glycoprotein is the major target. Historically, it has been difficult to induce neutralizing antibody responses against diverse primary HIV type 1 (HIV-1) strains by utilizing monomeric HIV Env (i.e., gp120) glycoprotein. Furthermore, it has been shown by several groups that the antibody responses induced by gp120 are directed primarily against the V3 loop and other linear epitopes (22, 34, 43, 44, 70, 81, 82, 102, 109, 110, 117) and that limited responses are directed towards the conserved conformational epitopes (9, 41, 75, 88, 95, 107). Due to the high degree of variability in gp120, these nonconformational anti-gp120-specific antibodies were found to be predominantly subtype specific and, to some extent, isolate specific. In addition, the magnitudes of antibody responses induced by gp120-based subunit protein vaccines were initially modest, and multiple boosts were required to induce strong antibody responses. Similarly, during the course of natural infection, antibody responses are slow to develop, and it may take years before antibodies of average affinity and avidity are induced (12, 41, 62, 71, 72, 76, 86, 123). In contrast to the case for HIV, high-avidity antibodies against hepatitis C virus (120), varicella-zoster virus (47), and rubella virus (54) are fairly rapidly induced. Antibodies induced by gp120 immunization appear to have weaker binding affinity to the oligomeric Env than to the monomer (8, 73, 77, 84, 85), and although they neutralized T-cell line-adapted (TCLA) viruses very efficiently and at high serum dilutions (62, 91, 101, 108), they were not able to neutralize primary isolates (2, 7, 9, 62, 73, 75, 77, 84, 85, 115, 123, 124). In contrast, sera from HIV-infected individuals were found to neutralize primary isolates (using CCR5 [R5] as a coreceptor) (12, 106). Upon affinity purification of these antibodies, it was demonstrated that primary-isolate-neutralizing antibodies were directed towards the gp120 (106, 109). Thus, unlike monomeric gp120, HIV Env can, during the course of an active infection, elicit gp120-specific antibodies that neutralize primary isolates. This suggests that there are qualitative differences between the antibodies induced during the natural course of infection and antibodies elicited by the immunization strategies used to date. For example, the primary-isolate-neutralizing antibodies induced during the natural course of infection are directed predominantly against conformational epitopes and generally showed little reactivity towards the V3 loop (36, 37, 40, 74, 78, 79, 108, 109). Moreover, the reactivity of antibodies with gp120 does not predict their neutralizing potential against primary HIV strains, while reactivity of antibodies against oligomeric Env protein is a good predictor of neutralizing activity (30, 73, 92, 96). These observations suggest that there are significant structural and conformational differences between the HIV Env present on the surface of the virion and gp120 (52, 73, 76, 85, 98, 112, 126). These conformational differences may affect the structure of critical neutralizing epitopes on monomer compared to trimer.
Structural studies indicate that HIV Env is present as a trimer on the surface of the virion (13, 38, 113, 121, 127). The most potent neutralizing antibodies generated so far (i.e., IgG1b12, 2G12, and 2F5) have stronger affinity for the native envelope (trimer) than for gp120 or gp41 (11, 30, 92, 96, 98, 112, 114). These data suggest that the epitopes recognized by these monoclonal antibodies (MAbs) are best represented on oligomeric Env. Earlier studies have suggested that HIV Env oligomers (consisting of gp120 and the ectodomain of gp41) might be superior to monomeric gp120 for inducing strong humoral responses towards the conformational epitopes (8, 22, 87, 89, 90, 117). In addition, it has also been shown that oligomeric Env induced antibodies that cross-reacted with HIV envelopes of other subtypes and neutralized both T-cell-adapted and susceptible primary isolates of HIV-1 (26, 117, 130). Therefore, it is reasonable to evaluate oligomeric Env for its capability to induce primary-isolate-neutralizing antibodies of high avidity.
Here we describe an effective strategy to stabilize HIV Env (o-gp140) in its oligomeric conformation and to purify oligomeric protein from a mammalian cell expression system for vaccine applications. Biochemical, structural, and immunological characterizations of o-gp140 indicate that the purified protein is in a stable trimeric conformation with critical neutralizing epitopes exposed. Preliminary vaccine studies with rabbits demonstrate that the o-gp140 protein is highly immunogenic and induces high-avidity and predominantly conformationally directed antibody responses.
MATERIALS AND METHODS
Envelope plasmid construction.
The sequences encoding the open reading frame of the ectodomain of the Env protein (gp140) from the HIV-1US4 strain were codon optimized as described elsewhere (35, 131) and constructed synthetically as a 2.1-kb EcoRI-XbaI DNA fragment (Midland Reagent Company, Midland, Tex.). This gene cassette contained the protein-encoding region of the Env protein fused in frame to the human tissue plasminogen activator signal sequence as previously described (14). In order to stabilize the oligomeric structure of the encoded gp140 protein, the DNA sequence was mutated to introduce an arginine-to-serine change in the primary protease cleavage site (REKR) in the Env polypeptide (23) (Fig. 1A). The resulting Env expression cassette (gp140) was cloned into the EcoRI-XbaI sites of the pCMV3 expression vector for the derivation of stable CHO cell lines. This vector contains the cytomegalovirus enhancer-promoter elements, an ampicillin resistance gene, and sequences encoding a fusion protein composed of dihydrofolate reductase and an attenuated neomycin resistance protein.
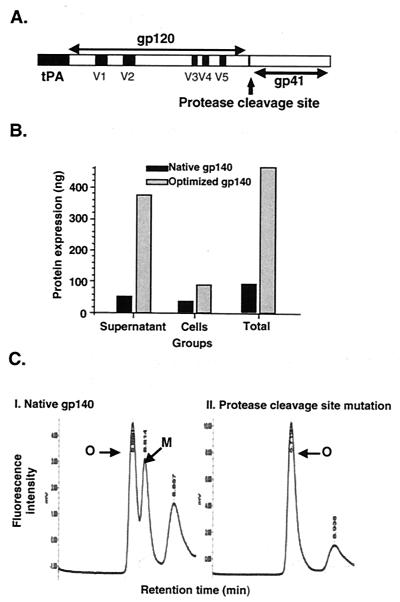
FIG. 1.
Structure, expression, and stabilization of HIV-1 US4 envelope glycoprotein in the oligomeric conformation. (A) Linear map of the HIV-1 gp120 and gp41 envelope glycoproteins. The gp120 variable regions (V1 to V5) are indicated as solid squares, and the mutation of arginine 509 to serine 509 in the protease cleavage site between gp120 and gp41 is indicated by an arrow. The gp41 portion includes the N and C α-helical regions known as oligomerization domains. tPA, human tissue-type plasminogen activator. (B) Effect of codon optimization on expression of o-gp140. Total protein expression obtained from native and codon-optimized constructs by using capture ELISA in different fractions is presented. (C) Effect of protease cleavage site mutation upon oligomer stabilization. 293 cells were transfected with gp140 protease cleavage site-mutated and nonmutated constructs by using the LT-1 transfection reagent as described for CHO cells in Materials and Methods. Supernatants were collected on day 5, and partial purification of the proteins was performed. These partially purified proteins were tested in a CD4 binding assay using a Bio Sil SEC-250 sizing column to separate monomers from oligomers as described in Materials and Methods. Peaks representing oligomer (O), monomer (M), and CD4 are indicated.
Production of US4 o-gp140.
The CHO cell line DG-44 was used as the host cell line for generation of stable cell lines. DG-44 has a double deletion mutation in the dihydrofolate reductase gene, thus making the cell line dependent on the addition of hypoxanthine, glycine, and thymidine to the growth medium. DG-44 cells were plated on 100-mm-diameter dishes in nonselective F12 medium (Bio-Whittaker, Walkersville, Md.) containing 10% fetal bovine serum plus 4 mM glutamine and transfected with the Env plasmid DNA using the LT-1 lipid-based transfection system as described by the manufacturer (Miras, Inc.). At 24 to 48 h posttransfection, depending on the cell density, the cells in each 100-mm-diameter dish were split into four to six 100-mm-diameter dishes, and the medium was changed to complete selective medium (F12 lacking hypoxanthine, glycine, and thymidine; 10% dialyzed fetal bovine serum; 4 mM glutamine). Twenty-four hours later, the medium was changed again to complete selective medium containing 250 μg of Geneticin (G418) per ml. Ten to 14 days later, individual colonies were picked, transferred to 96-well plates, and cultured in complete selective medium without G418. When approximately 80% of the wells were confluent, supernatants were screened by a gp120 capture enzyme-linked immunosorbent assay (ELISA) (see below). Positive clones were transferred to 24-well plates. Methotrexate amplification of the clones was then initiated, and clones were expanded, amplified, and screened at each step for gp140 expression.
The three highest-expressing gp140-CHO cell clones were used to seed 3-liter bioreactors (one for each clone). Bioreactors were monitored for cell density, pH, and CO2 and O2 concentrations, etc., on a daily basis. The structure, conformation, and expression level of secreted gp140 were monitored on a weekly basis. High-expressing clones were adapted to medium containing a very low serum concentration (0.5%) and to a cell density and perfusion rate that facilitated the maximum expression of o-gp140 in its oligomeric conformation. From the three bioreactor-adapted clones, the best clone was used to set up a production run in a 12.5-liter bioreactor. At the end of the run, the collected medium was concentrated 20-fold through a 100-kDa-cutoff membrane filter. The concentrated material was stored at −80°C in the presence of 1 mM EDTA and 1 mM EGTA.
Envelope antigen capture ELISA.
Corning (Corning, N.Y.) 96-well U-bottom plates were coated with 250 ng of purified immunoglobulin G (IgG) (obtained by ammonium sulfate precipitation of goat anti-gp120 SF2 sera) per well by incubation at 4°C overnight. Between steps, the plates were washed in a buffer containing 16% NaCl and 1% Triton X-100. One hundred microliters of tissue culture supernatant (diluted in a buffer containing 100 mM NaPO4, 0.1% casein, 1 mM EDTA, 1% Triton X-100, 0.5 M NaCl, and 0.01% thimerosal, pH 7.5) was added and incubated for 2 h at 37°C. Bound gp140 was reacted against pooled HIV-positive serum (1 h of incubation, 37°C) and detected using goat anti-human peroxidase conjugate (30 min of incubation at 37°C) (TAGO, Burlingame, Calif.). The plates were developed for 15 min at room temperature (RT) using TMB substrate (Pierce, Rockford, Ill.), and optical densities were recorded at 450 nm. The gp140 concentration was calculated from a standard curve derived using serial dilutions of a known concentration of recombinant envelope protein.
Purification of US4 o-gp140.
The concentrated CHO cell supernatant was thawed in the presence of complete protease inhibitor cocktail (Boehringer Mannheim) at 4°C, and after its conductivity was adjusted, it was loaded onto a DEAE (Pharmacia, Uppsala, Sweden) column equilibrated with buffer (20 mM Tris, 100 mM NaCl, pH 8.0). Under these conditions, o-gp140 did not bind to the column, but contaminating proteins were retained on the column. The DEAE flowthrough was adjusted to 10 mM PO4 and pH 6.8 and loaded on to a ceramic hydroxyapatite (CHAP) (Bio-Rad Laboratories, Hercules, Calif.) column equilibrated with buffer (10 mM Na2HPO4, 100 mM NaCl, pH 6.8). gp140 was recovered in the flowthrough, and the pH was adjusted to 7.4 with 2 M Tris (pH 8.8) and loaded on to a protein A-agarose column to remove immunoglobulin contamination. The protein A-agarose flowthrough was loaded onto a Galanthus Nivalis-agarose (GNA) (Vector Laboratories, Burlingame, Calif.) column equilibrated with 20 mM Tris-100 mM NaCl (pH 7.4). Bound gp140 was eluted with 500 mM methyl mannose pyranoside (Sigma Chemical Co., St. Louis, Mo.) in equilibration buffer. All of the fractions were analyzed on polyacrylamide gels (native and reduced and denatured) and also in a CD4 binding assay (see below). The fractions showing strong CD4 binding activity were pooled and fractionated on Suprose-6 and Superdex-200 (Pharmacia) tandem columns equilibrated with 10 mM Na-citrate and 500 mM NaCl. Fractions containing o-gp140 were pooled, concentrated using a Stir cell (Millipore, Inc., Bedford, Mass.), and stored frozen at −80°C until used. During the purification process, fractions were analyzed by polyacrylamide gel electrophoresis (PAGE) both under reducing and denaturing and under native conditions according to standard methods. Gels were stained with Coomassie brilliant blue or processed for immunoblotting.
Immunoblot detection of US4 o-gp140.
Approximately 0.5 to 1 μg of the purified o-gp140 was separated by sodium dodecyl sulfate (SDS)-PAGE. After electrophoresis, the gel was treated with 50 mM Tris-HCl (pH 8.0) containing 20% glycerol for 30 min at room temperature. Proteins were transferred onto a 0.22-μm-pore-size nitrocellulose membrane by using a semidry transfer system (Bio-Rad Laboratories). Immunodetection of o-gp140 was performed as described previously (103) with minor modifications. Briefly, after the transfer, the nitrocellulose membrane was incubated with 3% bovine serum albumin in phosphate-buffered saline (PBS) (pH 7.4) for 1 h at 37°C, washed with 0.1% Tween 20, and incubated with primary antibody (anti-gp120-specific MAb 20-2-C8.5F3, specific for the C4 domain [K. S. Steimer et al., unpublished observations]) at an appropriate dilution (1:1,000) for 1 h at RT. The membrane was washed as described above and incubated with anti-mouse IgG secondary antibody (1:20,000) conjugated to horseradish peroxidase (HRP) (Amersham Life Sciences) for 1 h at RT. At the end of the incubation, the membrane was washed as described above and developed with ECL reagent (Amersham Life Sciences).
CD4 binding assay.
Soluble CD4 (sCD4) was produced and purified as follows. Stable CHO cells expressing a truncated form of CD4 were adapted and grown in the bioreactor as described above. Concentrated medium was thawed in the presence of 1 mM EDTA and 1 mM EGTA and loaded onto an SP (Pharmacia) column preequilibrated with 20 mM acetate, pH 5.0. Bound protein was eluted with a 0 to 1 M continuous NaCl gradient. The fractions were analyzed by SDS-PAGE and anti-CD4 ELISA. All of the fractions showing high CD4 activity in both ELISA and SDS-PAGE analysis were pooled, diluted, adjusted to 10 mM PO4 using NaH2PO4, and then loaded on to a CHAP column equilibrated with 10 mM NaH2PO4-100 mM NaCl, pH 6.8. Under these conditions, sCD4 did not bind to the column and was recovered in the flowthrough. At the final step of purification, the CHAP flowthrough was concentrated and fractionated over a Supredex-75 high-resolution column (Pharmacia) using 2× PBS (pH 7.4). The relevant fractions were pooled, concentrated, and stored at −80°C until used.
To determine the capability of purified o-gp140 and gp120 to bind to CD4, we have developed a high-performance liquid chromatography (HPLC)-based assay using fluorescinated CD4. Purified sCD4 was labeled with amine-reactive succinimidyl esters of carboxyfluorescein according to the instructions provided by the manufacturer (Molecular Probes, Inc., Eugene, Oreg.). Approximately 1 μg of purified HIV Env gp120 was mixed with 0.33 μg of fluorescinated CD4 in a reaction volume of 60 μl using 2× PBS at pH 7.4. After a 15-min incubation at RT, 50 μl of this sample was injected onto a Bio Sil SEC-250 (Bio-Rad Laboratories) gel filtration HPLC column using an Alliance 2690 HPLC system (Waters Corporation, Milford, Calif.). Samples were run in 20 mM NaH2PO4-2 mM Na2HPO4-400 mM ammonium sulfate buffer (pH 6.0) at a flow rate of 1 ml/min. The fluorescence profile was monitored at 490 nm using a 996 fluorescence detector and the Millennium software package (Waters Corporation).
Glycosylation profile analysis.
The glycosylation profile of the purified proteins was assessed by four methods.
First, immunoblot analysis of the purified gp120 or gp140 proteins was performed using an immunoblot kit from Bio-Rad Laboratories (catalog no. 170-6490). Proteins were then biotinylated via their carbohydrate moieties using the periodate oxidation reaction, and the labeled proteins were subsequently detected using streptavidin-HRP and chromogenic reagents according to the directions provided with the kit.
Second, enzymatic deglycosylation of the purified HIV Env proteins was performed using a kit from Bio-Rad Laboratories (catalog no. 170-6500). Briefly, to detect N-linked oligosaccharides, 25 to 100 μg of the purified protein was treated with 2 μl of peptidyl-N-glycosidase F (PNGF) (2.5 U/ml) for 1 h at 37°C. To study O-linked oligosaccharides, 25 μg of the purified protein was treated with 2 μl of neuraminidase (NANase) II (10 U/ml) and 2 μl of O-glycosidase (1 U/ml) at 37°C for 1 h. At the end of the incubation period, samples were diluted in sample buffer containing 0.1% SDS and β-mercaptoethanol (βME) and analyzed by SDS-PAGE. In addition, we have also performed endo-β-N-acetylglucosaminidase H (endo-H) digestion of the purified protein according to the protocol of the manufacturer (Boehringer Mannheim).
Third, N-linked oligosaccharide profiling analysis was performed using a kit from Bio-Rad Laboratories (catalog no. 170-6501) according to the manufacturer's directions. Briefly, this protocol involves (i) enzymatic release and purification of asparagine-linked oligosaccharides from the purified HIV Env proteins by using PNGF, (ii) labeling of the released oligosaccharides with the fluorophore 8-aminonapthalene-1,3,6-trisulfonic acid (ANTS) at their reducing termini by reductive amination, (iii) electrophoretic separation of the fluorescently labeled oligosaccharides, and (iv) imaging of fluorescinated oligosaccharides by long-wave UV. The oligosaccharides of interest were excised and eluted from the gel to perform sequencing analysis.
Finally, sequencing of N-linked oligosaccharides of the HIV Env proteins was performed using a kit from Bio-Rad Laboratories (catalog no. 170-6510), as described by the manufacturer. Briefly, the purified oligosaccharides obtained from the previous analysis were digested with NANase, β-galactosidase (GALase), β-N-acetylhexosaminidase (HEXase), and mannosidase (MANase). These digests resulted in a sequential release of specific oligosaccharides from the intact carbohydrate structure, resulting in a trisaccharide core structure. After enzymatic digestion, the samples were dried, reconstituted in sample buffer containing 0.1% SDS and βME, subjected to gel electrophoresis, and imaged by long-wave UV.
Immunochemical characterization of US4 o-gp140.
The binding of well-characterized HIV Env-specific MAbs was performed by a capture ELISA. gp120 was captured by its C5 domain via the sheep polyclonal antibody 6205 (78), and o-gp140 was captured by its gp41 portion via MAb D50, which recognizes a linear gp41 peptide (21) as described previously in detail (105). Briefly, ELISA plates were coated with these respective antibodies at a concentration of 10 μg/ml, and o-gp140 and gp120 were captured. The captured proteins were incubated with serial dilutions of various MAbs (0.01 to 10 μg/ml) for 2 h at RT. Plates were washed, and specifically bound antibodies were detected using anti-species-specific IgG coupled to alkaline phosphatase as described above.
Immunizations.
Age- and weight-matched New Zealand female rabbits were immunized intramuscularly with 50 μg of the purified o-gp140 at 0, 4, 12, and 24 weeks in the RAS-3C adjuvant (RIBI Immunochemicals, Hamilton, Mont.) at two sites (250 μl each). These rabbits were bled regularly at 2-week intervals until the completion of the study, and serum was collected.
Measurement of antibody responses.
ELISA was performed on serum samples collected every 2 weeks, essentially as described previously (104). Briefly, plates were coated with 100 ng of purified HIV Env protein/well in sodium carbonate-bicarbonate buffer (pH 9.6) at 4°C and then blocked with 3% bovine serum albumin containing 0.3% Tween 20 in PBS (pH 7.4). Serial dilutions of the test sera were incubated for 2 h at RT. At the end of the incubation, the plates were washed with 0.1% Tween 20 in PBS to remove nonspecifically bound antibodies and incubated with species-specific secondary antibody coupled to HRP (anti-mouse, 1/50,000; anti-rabbit, 1/200,000 [Bethyl Laboratories, Montgomery, Tex.]) for 1 h at RT. After washing, the plate was developed with TMB and H2O2 (Pierce) for 30 min at RT in the dark. The reaction was stopped with 4 N H2SO4, absorbance values were recorded at 450 nm using a Power Wave 340 ELISA reader (Bio-Tek Instruments, Inc.) and the data were analyzed using KC Junior software (Bio-Tek Instruments, Inc.).
The antibody avidity index was determined using an ammonium thiocyanate displacement (NH4SCN) ELISA as described elsewhere in detail (4, 15, 58), with some modifications. Microtiter plates were coated with 100 ng of the purified HIV Env protein as described above. After incubation with test sera, the plates were washed three times with 0.1% Tween 20 in PBS (pH 7.4), once with PBS containing 0 to 8 M NH4SCN for 20 min, and then five more times with 0.1% Tween 20 in PBS (pH 7.4). Bound antibodies were detected as described above. All of the samples were tested in duplicate, and results were expressed as the NH4SCN concentration required to displace 50% of the bound antibodies.
RESULTS
Expression and stabilization of US4 o-gp140.
The sequences encoding codon-optimized gp140 were cloned into an expression vector for the evaluation of Env expression in transient-transfection experiments and for protein purification. To facilitate the efficient secretion of recombinant o-gp140 protein, the native HIV signal sequence was replaced by the human tissue-type plasminogen activator signal sequence (Fig. 1A). The effect of codon optimization on gp140 expression was determined by transient transfection of 293 cells with codon-optimized and native (non-codon-optimized) gp140 constructs followed by comparison of expression levels by use of a capture ELISA (Fig. 1B) and immunoblotting (data not shown). It was shown previously that sequence modification of HIV gag dramatically improved the level of expression (131); similarly, codon optimization also improved the expression of gp140 by 4- to 10-fold compared to the native construct (35) (Fig. 1B). Using such sequence-modified constructs, we developed stable CHO cell lines secreting 1 to 15 μg of o-gp140 and gp120 per ml. The antigenicity of oligomeric gp140 with and without a point mutation (R509 to S509) in the gp120/g41 primary protease cleavage site was also evaluated by transiently transfecting the 293 cells. Expression and structural characterization data indicated that the native form of the HIV-1 ectodomain-encoding region did not form gp140 oligomers efficiently (only about 50% of the expressed protein was found to be in the oligomeric conformation) (Fig. 1C). In contrast, the single R-to-S mutation in the protease cleavage site resulted in the expression of stable gp140 protein in its oligomeric conformation. Therefore, the constructs employing the protease cleavage site mutation were used for the derivation of stable CHO cell lines for protein production. Cell lines were also derived for the monomeric US4 gp120.
Purification of US4 o-gp140.
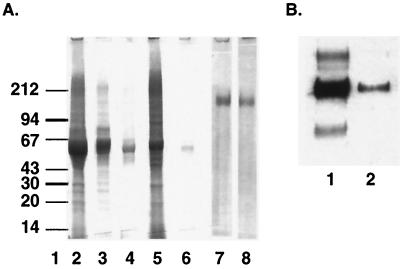
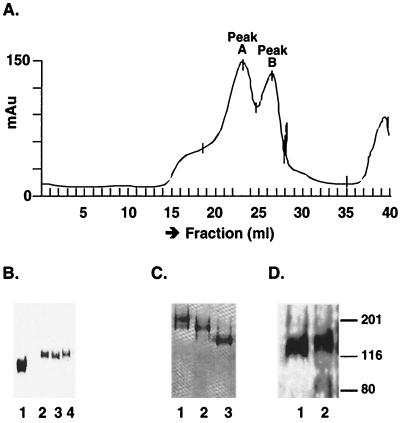
A simple, three-step strategy was designed for efficient purification of o-gp140. First, concentrated cell supernatants were passed over DEAE, CHAP, and protein A columns, where contaminating proteins bound to the columns and gp140 flowed through. Second, o-gp140 was captured using a GNA lectin column. Finally, the oligomeric protein was separated from monomers and dimers by higher-resolution size exclusion chromatography (SEC). The flowthrough from each column was analyzed by SDS-PAGE, immunoblotting, and a CD4 binding assay. Based on SDS-PAGE analysis, a significant reduction in the number and concentration of contaminating proteins was achieved after passage over the DEAE and CHAP columns (Fig. 2A). Immunoprobing analysis indicated that more than 90% of o-gp140 was recovered in the flowthrough. Similar data were obtained using the CD4 binding assay (data not shown). Purification by the GNA lectin column captured >90% of gp140 (Fig. 2B) through the terminal mannose, and bound protein was eluted with 500 mM methyl mannose pyranoside. At the final stage of purification, o-gp140 was separated from gp140 monomers and dimers by using Superose-6 and Superdex-200 columns in tandem. At low salt concentrations, there was no separation of these different structural forms of Env proteins on the sizing column (data not shown), suggesting that strong protein-protein interaction prevented the separation of the oligomer from the monomer. However, the addition of 500 mM NaCl readily allowed separation of the oligomer from the monomer and dimer (Fig 3A). All of the fractions constituting peaks A and B migrated with an apparent molecular mass of 140 kDa in reducing and denaturing SDS-PAGE (Fig. 3B). These fractions were also analyzed under denaturing-only conditions (data not shown), and no apparent change in the electrophoretic mobility of these fractions was observed under denaturing conditions versus reducing and denaturing conditions. This suggests that gp140 monomers are noncovalently associated with each other to give rise to oligomers. To make sure that oligomers were not disulfide-linked aggregates, we analyzed the purified oligomers with increasing concentrations of βME and did not observe any change in the migration profile, indicating that they are not disulfide-linked aggregates (data not shown). Fractions constituting peaks A and B were also analyzed by PAGE under native conditions (Fig. 3C). Based on the relative mobilities of fractions in peaks A and B, it seems that peak A contains predominantly Env protein oligomers that potentially could be trimers. However, it is difficult to determine the trimeric nature of the molecule based on this analysis alone. Purified o-gp140 was recognized as a single species on Western blots using a MAb (20-2-C8.5F3) directed against the conserved (C4) domain of gp120 (Fig. 3D, lane 1). It appears that there is no major degradation or proteolysis of o-gp140 (Fig. 3D, lane 1). However, some degradation of monomeric protein was observed (Fig. 3D, lane 2).
FIG. 2.
Analysis of gp140 purification profile after column purification. (A) SDS-PAGE analysis of various fractions: starting material (lane 2); flowthrough after elution through DEAE (lane 3), CHAP (lane 4), and protein G (lane 6) columns; and elution of CHAP (lane 5) and GNA (lanes 7 and 8) columns. Numbers on the left are molecular masses in kilodaltons. (B) Immunoblot analysis of gp140 before (lane 1) and after (lane 2) incubation with GNA.
FIG. 3.
Purification and analysis of o-gp140. (A) o-gp140 was separated on a high-resolution sizing column from the dimeric and monomeric forms of gp140 in presence of a higher salt concentration (500 mM NaCl). Fractions corresponding to different conformational states of the protein, such as oligomer (peak A), dimer, and monomer (peak B), are indicated. (B) Polyacrylamide gel analysis of the sizing fractions under reducing and denaturing conditions. Lane 1, gp120 SF2; lane 2, oligomer; lane 3, dimer; lane 4, monomer. (C) Polyacrylamide gel analysis of the sizing fractions under native conditions. Lane 1, oligomer; lane 2, dimer; lane 3, monomer. (D) Immunodetection of o-gp140 using a MAb (20-2-C8.5F3) directed against the C4 domain of gp120 SF2. Lane 1, o-gp140; lane 2, gp140 monomer. Numbers on the right are molecular masses in kilodaltons.
Biophysical characterization of US4 o-gp140.
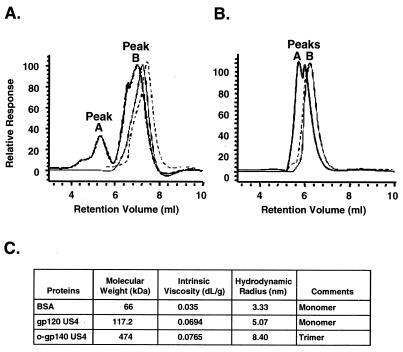
Biophysical characterization of purified US4 o-gp140 was performed by determining the molecular mass, hydrodynamic radius (Rh), and intrinsic viscosity by using a triple-detector array (Viscotek Corp., Houston, Tex.) system in conjunction with a precalibrated gel filtration HPLC column (Bio Sil SEC-250). The resulting data were compared to those obtained with purified US4 gp120; the relative responses obtained for gp120 and o-gp140 are shown in Fig. 4A and B, respectively. The calculated molecular masses of gp120 and o-gp140 are 117 and 474 kDa, respectively, with the molecular mass of purified o-gp140 being slightly more than the expected molecular mass for a trimer (3 × 140 kDa [monomer] = 420 kDa [trimer]). The light-scattering profiles for gp120 and o-gp140 indicated that both of these purified proteins were homogeneous, because the relative responses obtained from all three detectors (light scattering, refractive index, and viscosity) align quite well for peak B. However, a very weak signal or no signal was observed for the refractive index detector and viscometer for peak A, suggesting that only a small fraction of the purified gp120 and o-gp140 proteins were in an aggregated state. The Rh of the purified o-gp140 was determined to be larger than that of gp120 (8.40 versus 5.07). In contrast, the intrinsic viscosity values for both proteins were in a similar range (Fig. 4C), suggesting that the proteins had not become unfolded during the purification.
FIG. 4.
Biophysical characterization of purified o-gp140 using a triple-detector array system. (A and B) Relative responses obtained for light scattering (thick lines), refractive index (thin lines), and viscosity (dashed lines) for gp120 US4 (A) and o-gp140 US4 (B). The majority of the purified protein is homogeneous. Maximum protein is in peak B as indicated by the refractive index detector signal. The lack of a refractive index signal in peak A indicates that a smaller fraction of the protein is in the aggregated state. (C) Summary table of biophysical properties, including molecular mass, intrinsic viscosity, and hydrodynamic radius of o-gp140. BSA, bovine serum albumin.
Biochemical characterization of US4 o-gp140. (i) Immunodetection of the carbohydrate.
After purification of o-gp140, a systematic characterization of the carbohydrates was undertaken. First, the purified oligomeric Env glycoprotein was biotinylated in the aqueous phase through its sialic acid moiety and detected with streptavidin-HRP and a chromogenic substrate on immunoblotting, thereby indicating that o-gp140 was a glycoprotein (Fig. 5A). To determine that the labeling was specifically through the sialic acid moiety, the biotinylated protein was treated with NANase, thereby preventing its recognition by streptavidin-HRP. To ensure that there was no loss of the protein during the NANase treatment, o-gp140 samples were analyzed with and without NANase digestion by SDS-PAGE, and similar levels of o-gp140 were found (data not shown).
FIG. 5.
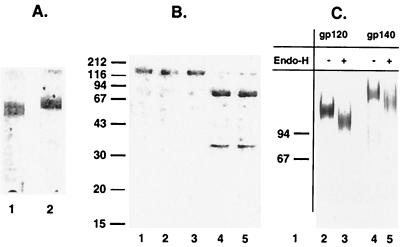
Carbohydrate analysis of purified o-gp140. (A) Immunoblot detection of biotinylated gp120 US4 (lane 1) and o-gp140 US4 (lane 2) using streptavidin-HRP. (B) Carbohydrate linkage analysis of o-gp140 using different enzymes. o-gp140 was digested with NANase (lane 2), O-glycosidase (lane 3), and PNGF (lane 4) separately and together (lane 5). o-gp140 without any enzyme was used as control (lane 1), and molecular mass standards (in kilodaltons) are also indicated. (C) endo-H digestion of gp120 US4 (lanes 2 and 3) and o-gp140 US4 (lanes 4 and 5). + and −, proteins with and without endo-H, respectively.
(ii) Differential deglycosylation studies.
Second, the nature of the oligosaccharide linkage in the purified o-gp140 was also analyzed and compared to that of monomeric gp120. Purified protein was digested with PNGF, which releases asparagine-linked oligosaccharides, and with NANase II and O-glycosidase, which release unsubstituted Gal-(β1,3)-GalNAc-(α1) disaccharides attached to serine or threonine either alone or in combination. It was observed that o-gp140, like gp120, has predominantly N-linked oligosaccharides, since PNGF treatment reduced o-gp140 to its protein backbone (Fig. 5B, lane 6). This is similar to what has been reported previously for gp120 monomeric protein (50). In contrast, NANase and O-glycosidase had no effect on the electrophoretic mobility of o-gp140 (Fig. 5B, lanes 4 and 5). Similar observations were made for gp120 (data not shown). Furthermore, digestion of o-gp140 with a mixture of all three enzymes had no discernible effect compared to digestion with PNGF alone (Fig. 5, lane 7). Therefore, recombinant o-gp140 contained little or no O-linked oligosaccharides, as was observed for both native and recombinant gp120 released from virions (32, 67). To ensure that o-gp140 was secreted by cells after passage through the endoplasmic reticulum and Golgi, rather than being an intracellular protein released by lysed cells, o-gp140 was treated with endo-H, which releases terminal mannose carbohydrates. After endo-H digestion of gp120 and o-gp140, only a 15 to 20% change in the apparent molecular mass of either gp120 or o-gp140 was observed (Fig. 5C).
(iii) N-linked oligosaccharide profiling and sequencing analysis.
N-linked oligosaccharide profiling analysis of o-gp140 was performed. This involved oligosaccharide purification, labeling with a fluorophore (ANTS), gel analysis, and imaging under long-wave UV. The results of such an analysis are shown in Fig. 6A. Both o-gp140 and gp120 (data not shown) have similar N-linked oligosaccharide profiles dominated by two types of oligosaccharides. These oligosaccharides are referred to as oligosaccharides 1 and 2 on the basis of their degrees of polymerization.
FIG. 6.
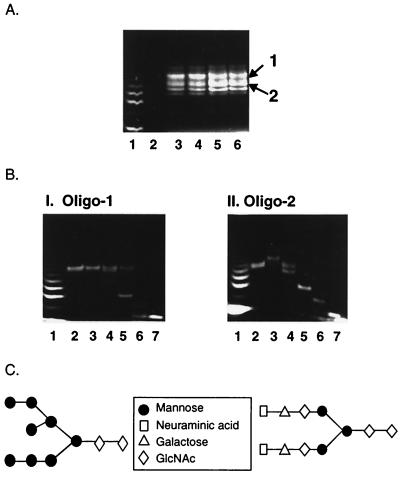
Carbohydrate profiling and sequencing analysis of purified o-gp140. (A) Heterogeneity of carbohydrates associated with gp120 US4 (lanes 3 and 4) and o-gp140 US4 (lanes 5 and 6). Two predominant oligosaccharides are indicated by arrows. (B) Carbohydrate sequencing analysis of oligosaccharide 1 (Oligo-1) and oligosaccharide 2. Purified oligosaccharides 1 and 2 were digested with NANase (lanes 3), GALase (lanes 4), HEXase (lanes 5), and MANase (lanes 6). Glucose ladder and fucosylated trisaccharide core structures were run in lanes 1 and 7, respectively. (C) Structure-based carbohydrate analysis.
Oligosaccharides 1 and 2 were individually eluted from the gels (Fig. 6B) and digested with enzymes specific for neuraminic acid (NANase), galactose (GALase), N-acetylglucosamine (HEXase), and mannose (MANase). After digestion, each sample was analyzed on a polyacrylamide gel and imaged under long-wave UV. Based on sequencing data, oligosaccharide 1 did not contain sialic acid, galactose, or N-acetylglucosamine (Fig. 6B, panel I, lanes 3, 4, and 5). However, in presence of MANase, oligosaccharide 1 was reduced to its trisaccharide core structure (Fig. 6B, panel I, lane 6), indicating that it contained primarily if not exclusively high-terminal-mannose N-linked oligosaccharides. This is consistent with the observed reduction in mobility after endo-H treatment of o-gp140 (Fig. 5C). A similar analysis was performed with oligosaccharide 2 obtained from the o-gp140 (Fig. 6B, panel II). There was a negative shift in mobility when oligosaccharide 2 was treated with NANase, indicating that the terminal residue in oligosaccharide 2 was sialic acid (Fig. 6B, panel II, lane 3). There were sequential shifts in the relative mobility of oligosaccharide 2 after GALase, HEXase, and MANase treatments (Fig. 6B, panel II, lanes 4, 5, and 6, respectively). The deduced carbohydrate sequences present in oligosaccharides 1 and 2 obtained from o-gp140 are shown in Fig. 6C. Based on this analysis, it appears that recombinant o-gp140 contained two kinds of oligosaccharides, a higher-terminal-mannose type (oligosaccharide 1) and a complex-carbohydrate type (oligosaccharide 2).
Binding of US4 o-gp140 to CD4.
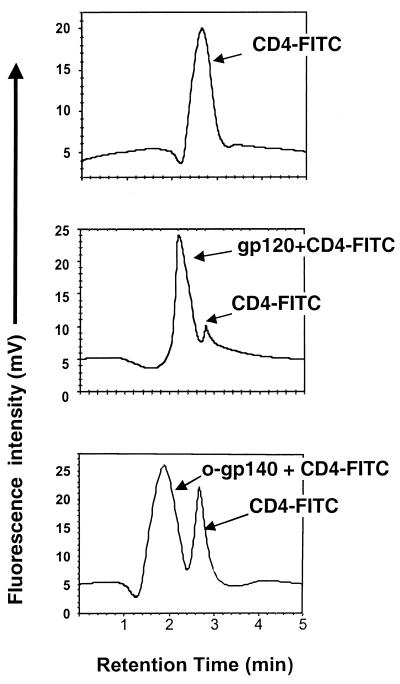
The ability of native HIV Env to bind to CD4 is an essential factor of its biological function and thus can serve as an indicator of the native structure and functionality of recombinant Env (99). Therefore, to demonstrate that purified o-gp140 was in its correct conformation, we developed an HPLC-based receptor binding assay using fluorescently labeled CD4 (CD4-fluorescein isothiocyanate). When the labeled CD4 was run on an HPLC gel filtration column, a fluorescent peak at an expected elution time of 2.67 min was observed (Fig. 7). However, when unlabeled HIV Env protein (either gp120 or o-gp140) was incubated with CD4-fluorescein isothiocyanate and the resulting complex was run on the same column, we observed shorter retention times (2.1 and 1.9 min for gp120 and o-gp140, respectively) as a consequence of the interaction between HIV Env and CD4. However, compared to monomer, o-gp140 binds to CD4 with reduced efficiency as reflected by more free CD4 seen Fig. 7C. Furthermore, reduced and denatured gp120 and deglycosylated gp120 (Env2 and−3) did not bind efficiently to CD4 (data not shown). These observations suggest that purified o-gp140 bound to CD4 and was, therefore, in the native conformation.
FIG. 7.
(B and C) Binding of purified gp120 US4 (B) and o-gp140 US4 (C) to CD4 as determined by an HPLC-based assay. (A) Profile of unbound CD4. FITC, fluorescein isothiocyanate.
Immunochemical characterization of US4 o-gp140.
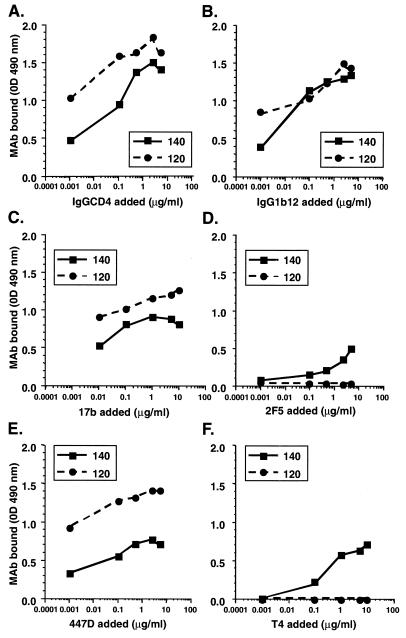
The structural integrity of o-gp140 was further evaluated by immunoprobing in a capture ELISA with a panel of MAbs with known epitope specificities, i.e., 2F5, IgG1b12, 17b, IgGCD4, and 447D. Antibodies directed against the CD4 binding site (IgGCD4 and IgG1b12) reacted slightly more strongly to gp120 monomer than to oligomer (Fig. 8A and B). This was consistent with the relative CD4 binding to gp120 and o-gp140 presented in Fig. 7. Both gp120 monomer and o-gp140 were recognized by a MAb (17b) directed against the CD4-inducible site (Fig. 8C). The purified o-gp140 protein was also recognized by 2F5, a neutralizing MAb that recognizes an epitope in gp41 (Fig. 8D). To determine the relative levels of exposure of the V3 loop, MAb 447D was used. This antibody recognized the gp120 monomer much more readily than the oligomer, suggesting that the V3 loop was less exposed in the oligomer, as it is also on intact virions (Fig. 8E). Finally, the oligomer-specific MAb (T4) also recognized o-gp140 (Fig. 8F). In addition, o-gp140 was also recognized well by sera from HIV-infected individuals and by a V2 loop-specific MAb (G3.4) (data not shown). Based on these observations, it seems that many of the critical immunodominant and neutralizing epitopes are intact and accessible in recombinant o-gp140. However, the V3 loop appears to be less exposed in o-gp140, suggesting that o-gp140 may be effective at directing the immune responses away from variable epitopes, such as those present in the immunodominant V3 loop region.
FIG. 8.
Immunochemical characterization of purified gp120 US4 and o-gp140 US4 by using a panel of MAbs, i.e., IgGCD4 (A), IgG1b12 (B), 17b (C), 2F5 (D), 447D (E), and T4 (F). OD, optical density.
Immunogenicity of US4 o-gp140.
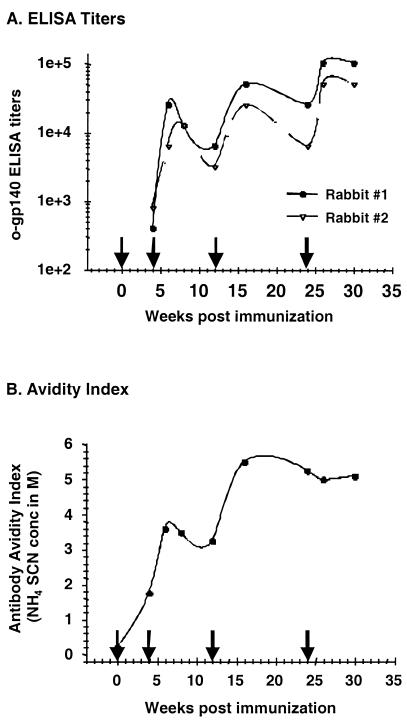
A pilot study to investigate the immunogenicity of o-gp140 was conducted with rabbits. Animals were immunized with purified o-gp140 protein in the RIBI adjuvant system at different time points. Sera were collected at 2-week intervals and tested for the quality and the quantity of the antibody responses induced by o-gp140. As shown in Fig. 9A, o-gp140 induced a robust (ELISA titers of >50,000) and long-lasting antibody response in the immunized rabbits, with detectable antibody responses observed even after a single immunization. Antibodies were directed against both the gp120 and gp41 portions of HIV Env, as judged by the Chiron RIBA analysis (data not shown).
FIG. 9.
Magnitude (A) and quality (B) of antibody responses induced by o-gp140 in rabbits at different time points after immunization. Animals were immunized at 0, 4, 12, and 24 weeks as indicated by the arrows.
The avidity of the antibodies induced by o-gp140 was determined by an NH4SCN displacement ELISA and demonstrated that the nature of the Env-specific antibody changed over time (Fig. 9B). The avidity of anti-o-gp140 antibodies at 4 weeks after the primary immunization appeared to be low, as a relatively lower concentration of NH4SCN (1.3 M) was sufficient to displace 50% of the bound antibody. However, after each immunization there were corresponding increases in the apparent avidity of the antibodies. Strong avidity was reached after the third immunization and was maintained for at least 8 weeks after the fourth immunization.
DISCUSSION
Immunogenicity studies conducted with humans and animals have shown that gp120 monomer is capable of eliciting high-titer antienvelope antibodies (2, 34, 36, 62, 73, 109, 115, 117, 118). However, in contrast to what happens during the course of natural infection, neutralizing antibodies are not readily developed against HIV-1 primary isolates, especially those of the R5 phenotype (2, 7, 9, 62, 73, 75, 85, 115, 117, 124). Therefore, it appears that there are significant qualitative differences between the antibodies induced during the course of natural infection and the antibodies induced by immunization with gp120 monomer (22, 34, 36, 37, 40, 43, 44, 70, 74, 79, 81, 82, 102, 108-110, 115). Several possible reasons may account for these differences, such as the structure and conformation of the immunogen and also poor exposure of neutralizing epitopes on the surface of the gp120 monomer. Furthermore, immunization with gp120 proteins derived from X4 viruses generally induced antibodies that reacted strongly to monomer compared to oligomer (8, 73, 77, 84, 115, 125). Thus, immunization with gp120, even if it maintains its conformation, may lead to the generation of antibodies that react poorly to native Env and therefore may not neutralize primary isolates. To achieve better immunogenicity with Env protein-based vaccines, attention has been focused recently on oligomeric forms of Env derived from R5 isolates (5, 26, 128, 129). This paper describes the derivation, purification, and characterization of o-gp140 from the subtype B R5 primary HIV isolate US4.
Three main strategies have been used by different groups to create stable oligomers: (i) protease cleavage site mutation between gp120 and gp41 (8, 22-25), (ii) intramolecular disulfide bond formation (SOS) between gp120 and gp41 (5, 27, 94), and (iii) extension of the gp41 coiled-coil region by the addition of sequences of GCN4 (a transcription factor that normally forms stable homodimers) (128, 129). We have evaluated both protease site mutation and the SOS approach to create stable trimers. Our data are in agreement with earlier findings that a single mutation in the primary protease cleavage site from R509 to S509 is both necessary and sufficient to allow formation of stable oligomers (22-25). As shown in Fig. 1C, gp140 constructs with the protease cleavage site mutation resulted in nearly 100% of the Env protein being expressed as oligomers, versus only 50% with constructs not containing the mutation in the protease cleavage site. However, the efficiency of this strategy may depend on the specific HIV isolate, since the same R-to-S mutation that was highly efficient for the US4 strain was relatively inefficient for stabilization of oligomers for SF162 Env (I. K. Srivastava, J. B. Ulmer, and S. W. Barnett, unpublished observations). Similar observations were made by Yang et al. (129) using a primary isolate (YU2) and a TCLA isolate (HXBc2). The SOS approach used by Binley et al. (5) and Sanders et al. (94) for creating stable oligomers from both primary (JF-RL) and TCLA isolates has worked reasonably well in transient-transfection experiments. However, the lower oligomerization efficiency (40%) observed by Binley et al. (5) with the SOS approach may be a cause for concern for the large-scale production of o-gp140 in stably transfected CHO cell lines. These observations suggest that strategies for preparations of oligomers from a given isolate will need to be determined experimentally. Sequence variation, glycosylation, size of variable loops, expression levels, and type of protein expression system may all play a role (129).
Most of the Env oligomeric proteins purified to date have been shown to be either dimers or tetramers (8, 22-25, 86, 87, 95, 100, 112, 117), with the possible exception of those most recently described by Binley et al. (5), Sanders et al. (94), and Yang et. al (129). The most common and widely used methods for determining the molecular mass are osmometry, analytical centrifugation, and classical light scattering. Osmometry and analytical ultracentrifugation are labor-intensive and time-consuming. In contrast, classical light scattering can be performed quickly and relatively easily; therefore, it is a reasonable choice for determining the molecular mass (20). This approach has been used extensively to determine molecular masses of proteins (20, 46, 51, 60, 66, 111). To ascertain the quaternary structure of o-gp140 produced in the CHO cell lines, we performed a detailed biophysical characterization of the purified protein using a triple-detector array system (Viscotek Corp.) to determine the molecular mass, Rh, and intrinsic viscosity. By using a classical light-scattering detector coupled to SEC, the molecular mass of the purified HIV envelope oligomer was estimated to be 474 kDa, close to the expected molecular mass of a trimer (420 kDa). However, mass spectrometry data will be required to determine definitively whether the purified US4 oligomer is a trimer or a tetramer.
There are several possible explanations for this difference in molecular mass, such as differences in isolates, differences in approaches to create trimers, and differences in techniques employed to determine the molecular mass. Binley et al. (5) and Yang et al. (129) have used conventional SEC to determine the molecular mass. SEC may not be the most appropriate means to determine the molecular masses of purified proteins due to the lack of a direct relationship between molecular size and molecular mass of proteins even under strongly denaturing conditions (33, 64). In addition, proteins with extensive posttranslational modifications such as glycosylation (122), as is the case with HIV envelope proteins, may further complicate this type of analysis. The gp120 monomer was shown to be a loosely folded globular structure with an Rh value of 5.07 nm. In contrast, the o-gp140 trimer was a tightly folded structure, as indicated by its rather smaller-than-expected Rh value of 8.4 nm. These data are consistent with earlier observations that there are significant differences in the structure and folding of monomers and trimers (73, 76, 126). It has also been proposed by Binley et al. (5) that there are some biochemical differences between the cleaved and uncleaved forms of trimers. However, presently it is not clear what these differences are and how they may affect the immunogenicity of the trimers. Therefore, it will be interesting to perform a comparative biophysical characterization of the cleaved and uncleaved trimers. It is hoped that this will elucidate structural differences, if any, between the cleaved and uncleaved oligomers and help us perform further structural optimization of trimers to induce primary-isolate-neutralizing antibody responses.
HIV envelope protein is highly glycosylated, with approximately 50% of its molecular mass contributed by carbohydrate (55). The binding of HIV envelope protein to CD4, an obligatory step in viral replication (19, 48, 65), is dependent upon conformation (53, 65, 94, 97, 99) and glycosylation (3, 57, 83, 89). The evidence so far suggests that N-linked glycosylation is important for the formation of correct disulfide bonds and for providing functional conformation to the Env protein (28, 29, 55, 57, 59, 119), but it is not directly involved in CD4 binding (57). Significant data on the nature of glycosylation are available for native and recombinant gp120 (32, 55, 67, 68) but not for oligomeric forms of Env. We have demonstrated that the purified recombinant US4 o-gp140 protein contains predominantly N-linked glycosylation, as observed for native and recombinant gp120 (50). Similarly, o-gp140 and gp120 have two kinds of sugar structures: (i) high-terminal-mannose type and (ii) complex type (32, 55, 67, 68). Furthermore, the purified oligomer protein binds to CD4, indicating that the protein is properly folded with the CD4 binding site intact. The efficiency of CD4 binding to the oligomer is reduced compared to that to the monomer. This is in agreement with the predicted structure of native oligomer, where the CD4 binding site is predicted to be somewhat constrained due the interaction between the individual gp120 components of the trimer (73, 92, 96, 126).
The structural integrity of the purified oligomers also was probed with MAbs of known epitope specificity. The results obtained are similar to those observed with native Env derived from virions (30, 73, 76, 77, 95, 98, 105, 108, 111, 114) and for SOS-stabilized oligomers (5, 128, 129). Important epitopes exposed on purified trimers include those near the CD4 binding site (recognized by MAbs IgG1b12 and CD4-IgG2), the CD4-inducible site that overlaps the coreceptor binding site (recognized by MAb 17b), and the V3 loop (recognized by MAb 447). The decreased binding of the anti-V3 loop-specific MAb 447D to trimers, compared to monomers, suggests that the V3 loop is less exposed on trimers than on monomers. This is in agreement with the proposed nature of oligomers based on epitope mapping and structural studies (7, 9, 126). The gp41 carbohydrate-dependent epitope (recognized by MAb 2F5) is accessible on correctly folded trimeric envelope. Like native trimers (76, 98), the purified US4 recombinant oligomer was recognized by 2F5, which is encouraging since this is an important neutralizing epitope. The preservation of critical neutralizing epitopes on the recombinant trimers and the reduced exposure of variable epitopes, such as the ones in the V3 loop, suggest that o-gp140 may be able to direct immune responses away from the variable domains and instead focus them toward the relevant functional epitopes (1, 24).
Early studies suggested that oligomers may be more potent and effective in inducing strong antibody responses to conformational epitopes (8, 22, 87, 89, 115). In addition, the antibodies induced were able to neutralize susceptible primary isolates (30, 84, 92, 96, 110, 116). However, with the exception of a few studies (22, 26, 116, 117, 130), there is a lack of strong immunogenicity data with oligomers, as well as a lack of studies that directly compare immune responses to monomeric and oligomeric R5 Env antigens. Therefore, we assessed the immunogenicity of the purified o-gp140 from US4 in a pilot study with rabbits, and further studies to directly compare the immune responses to monomeric and oligomeric proteins derived from the R5 US4 HIV-1 strain are under way. Cole et al. (17, 18) previously showed that during the course of infection with HIVs or simian immunodeficiency viruses, antienvelope antibody responses mature with time. During this process, although the titers of the binding antibodies remain basically unaltered, an increase in the antibody avidity, an expansion of epitope recognition, and an increase in neutralization potential take place (17, 18). The present study has clearly shown that oligomeric protein administered with an adjuvant primed strong antibody responses directed against conformational epitopes. Furthermore, high-avidity antibodies were induced, requiring 5 M NH4SCN to elute 50% of the bound antibodies. Yang et al., in a pilot immunogenicity study with mice (130), demonstrated that antibodies induced by oligomers were able to neutralize primary isolates. Combined, these data may suggest that immunization with oligomers induces mature antibody responses that are critical for neutralizing activity. Nevertheless, the association between the induction of high-avidity conformationally directed antibodies and virus neutralization has yet to be established in the setting of HIV vaccination with Env immunogens.
A full analysis of the potency of o-gp140 in animal models is ongoing in our laboratory, including analysis of the breadth of neutralization antibody responses induced in rabbits and nonhuman primates. Further evidence regarding the role of oligomers in inducing functional antibodies comes from an immunogenicity and challenge study in rhesus macaques with HIV-1 SF162-derived o-gp140 with the V2 loop deleted (o-gp140ΔV2) prepared and characterized similarly to that described here (Srivastava et al., unpublished data) (16). In those studies, rhesus macaques that were immunized by employing a DNA prime-protein boost immunization regimen with o-gp140ΔV2 showed robust and cross-reactive neutralizing antibody responses against primary HIV-1 isolates (1) and were partially protected against a virulent SHIVSF162P4 challenge (16). Work to further evaluate the immunogenicity and protective efficacy of vaccine regimens employing purified recombinant oligomeric proteins derived from primary HIV-1 strains is in progress.
Acknowledgments
We thank Margaret Liu for her support and encouragement during her tenure as Vice-President for Vaccines and Gene Therapy Research at Chiron. We also thank Patricia Earl, Bernard Moss, Dennis Burton, Susan Zolla-Pazner, Michael Gorny, and James Robinson for providing MAbs for the structural characterization of the purified proteins. We thank Michael Houghton for critically reading the manuscript and Nelle Cronen and Suzanne Stevenson for their help in preparing the manuscript. We thank Karen Matsuoka for excellent technical assistance.
Work in Leonidas Stamatatos' laboratory was supported by investigator-initiated research grants awarded by NIH (AI 44309 and AI 47708).
REFERENCES
- 1.Barnett, S. W., S. Lu, I. Srivastava, S. Cherpelis, A. Gettie, J. Blanchard, S. Wang, I. Mboudjeka, L. Leung, Y. Lian, A. Fong, C. Buckner, A. Ly, S. Hilt, J. Ulmer, C. T. Wild, J. R. Mascola, and L. Stamatatos. 2001. The ability of an oligomeric human immunodeficiency virus type 1 (HIV-1) envelope antigen to elicit neutralizing antibodies against primary HIV-1 isolates is improved following partial deletion of the second hypervariable region. J. Virol. 75:5526-5540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barnett, S. W., S. Rajasekar, H. Legg, B. Doe, D. H. Fuller, J. R. Haynes, C. M. Walker, and K. S. Steimer. 1997. Vaccination with HIV-1 gp120 DNA induces immune responses that are boosted by a recombinant gp120 protein subunit. Vaccine 15:869-873. [DOI] [PubMed] [Google Scholar]
- 3.Barr, P. J., K. S. Steimer, E. A. Sabin, D. Parkes, C. George-Nascimento, J. C. Stephans, M. A. Powers, A. Gyenes, G. A. Van Nest, E. T. Miller, et al. 1987. Antigenicity and immunogenicity of domains of the human immunodeficiency virus (HIV) envelope polypeptide expressed in the yeast Saccharomyces cerevisiae. Vaccine 5:90-101. [DOI] [PubMed] [Google Scholar]
- 4.Binley, J. M., H. Arshad, T. R. Fouts, and J. P. Moore. 1997. An investigation of the high-avidity antibody response to glycoprotein 120 of human immunodeficiency virus type 1 AIDS Res. Hum. Retroviruses 13:1007-1015. [DOI] [PubMed] [Google Scholar]
- 5.Binley, J. M., R. W. Sanders, B. Clas, N. Schuelke, A. Master, Y. Guo, F. Kajumo, D. J. Anselma, P. J. Maddon, W. C. Olson, and J. P. Moore. 2000. A recombinant human immunodeficiency virus type 1 envelope glycoprotein complex stabilized by an intermolecular disulfide bond between the gp120 and gp41 subunits is an antigenic mimic of the trimeric virion-associated structure. J. Virol. 74:627-643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borrow, P., H. Lewicki, B. H. Hahn, G. M. Shaw, and M. B. Oldstone. 1994. Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J. Virol. 68:6103-6110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bou-Habib, D. C., G. Roderiquez, T. Oravecz, P. W. Berman, P. Lusso, and M. A. Norcross. 1994. Cryptic nature of envelope V3 region epitopes protects primary monocytotropic human immunodeficiency virus type 1 from antibody neutralization. J. Virol. 68:6006-6013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Broder, C. C., P. L. Earl, D. Long, S. T. Abedon, B. Moss, and R. W. Doms. 1994. Antigenic implications of human immunodeficiency virus type 1 envelope quaternary structure: oligomer-specific and -sensitive monoclonal antibodies. Proc. Natl. Acad. Sci. USA 91:11699-11703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burton, D. R., and D. C. Montefiori. 1997. The antibody response in HIV-1 infection. AIDS 11(Suppl. A):S87-S98. [PubMed]
- 10.Burton, D. R., and J. P. Moore. 1998. Why do we not have an HIV vaccine and how can we make one? Nat. Med. 4:495-498. [DOI] [PubMed] [Google Scholar]
- 11.Burton, D. R., J. Pyati, R. Koduri, S. J. Sharp, G. B. Thornton, P. W. Parren, L. S. Sawyer, R. M. Hendry, N. Dunlop, P. L. Nara, et al. 1994. Efficient neutralization of primary isolates of HIV-1 by a recombinant human monoclonal antibody. Science 266:1024-1027. [DOI] [PubMed] [Google Scholar]
- 12.Cao, Y., L. Qin, L. Zhang, J. Safrit, and D. D. Ho. 1995. Virologic and immunologic characterization of long-term survivors of human immunodeficiency virus type 1 infection. N. Engl. J. Med. 332:201-208. [DOI] [PubMed] [Google Scholar]
- 13.Chan, D. C., D. Fass, J. M. Berger, and P. S. Kim. 1997. Core structure of gp41 from the HIV envelope glycoprotein. Cell 89:263-273. [DOI] [PubMed] [Google Scholar]
- 14.Chapman, B. S., R. M. Thayer, K. A. Vincent, and N. L. Haigwood. 1991. Effect of intron A from human cytomegalovirus (Towne) immediate-early gene on heterologous expression in mammalian cells. Nucleic Acids Res. 19:3979-3986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Charoenvit, Y., M. Sedegah, L. F. Yuan, M. Gross, C. Cole, R. Bechara, M. F. Leef, F. A. Robey, G. H. Lowell, and R. L. Beaudoin. 1990. Active and passive immunization against Plasmodium yoelii sporozoites. Bull. W. H. O. 68(Suppl):26-32. [PMC free article] [PubMed] [Google Scholar]
- 16.Cherpelis, S., I. Shrivastava, A. Gettie, X. Jin, D. D. Ho, S. W. Barnett, and L. Stamatatos. 2001. DNA vaccination with the human immunodeficiency virus type 1 SF162DeltaV2 envelope elicits immune responses that offer partial protection from simian/human immunodeficiency virus infection to CD8+ T-cell-depleted rhesus macaques. J. Virol. 75:1547-1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cole, K. S., M. Murphey-Corb, O. Narayan, S. V. Joag, G. M. Shaw, and R. C. Montelaro. 1998. Common themes of antibody maturation to simian immunodeficiency virus, simian-human immunodeficiency virus, and human immunodeficiency virus type 1 infections. J. Virol. 72:7852-7859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cole, K. S., J. L. Rowles, B. A. Jagerski, M. Murphey-Corb, T. Unangst, J. E. Clements, J. Robinson, M. S. Wyand, R. C. Desrosiers, and R. C. Montelaro. 1997. Evolution of envelope-specific antibody responses in monkeys experimentally infected or immunized with simian immunodeficiency virus and its association with the development of protective immunity. J. Virol. 71:5069-5079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dalgleish, A. G., P. C. Beverley, P. R. Clapham, D. H. Crawford, M. F. Greaves, and R. A. Weiss. 1984. The CD4 (T4) antigen is an essential component of the receptor for the AIDS retrovirus. Nature 312:763-767. [DOI] [PubMed] [Google Scholar]
- 20.Dollinger, G., B. Cunico, M. Kunitani, D. Johnson, and R. Jones. 1992. Practical on-line determination of biopolymer molecular weights by high-performance liquid chromatography with classical light-scattering detection. J. Chromatogr. 592:215-228. [Google Scholar]
- 21.Earl, P. L., C. C. Broder, R. W. Doms, and B. Moss. 1997. Epitope map of human immunodeficiency virus type 1 gp41 derived from 47 monoclonal antibodies produced by immunization with oligomeric envelope protein. J. Virol. 71:2674-2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Earl, P. L., C. C. Broder, D. Long, S. A. Lee, J. Peterson, S. Chakrabarti, R. W. Doms, and B. Moss. 1994. Native oligomeric human immunodeficiency virus type 1 envelope glycoprotein elicits diverse monoclonal antibody reactivities. J. Virol. 68:3015-3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Earl, P. L., R. W. Doms, and B. Moss. 1990. Oligomeric structure of the human immunodeficiency virus type 1 envelope glycoprotein. Proc. Natl. Acad. Sci. USA 87:648-652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Earl, P. L., S. Koenig, and B. Moss. 1991. Biological and immunological properties of human immunodeficiency virus type 1 envelope glycoprotein: analysis of proteins with truncations and deletions expressed by recombinant vaccinia viruses. J. Virol. 65:31-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Earl, P. L., and B. Moss. 1993. Mutational analysis of the assembly domain of the HIV-1 envelope glycoprotein. AIDS Res. Hum. Retroviruses 9:589-594. [DOI] [PubMed] [Google Scholar]
- 26.Earl, P. L., W. Sugiura, D. C. Montefiori, C. C. Broder, S. A. Lee, C. Wild, J. Lifson, and B. Moss. 2001. Immunogenicity and protective efficacy of oligomeric human immunodeficiency virus type 1 gp140. J. Virol. 75:645-653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Farzan, M., H. Choe, E. Desjardins, Y. Sun, J. Kuhn, J. Cao, D. Archambault, P. Kolchinsky, M. Koch, R. Wyatt, and J. Sodroski. 1998. Stabilization of human immunodeficiency virus type 1 envelope glycoprotein trimers by disulfide bonds introduced into the gp41 glycoprotein ectodomain. J. Virol. 72:7620-7625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fennie, C., and L. A. Lasky. 1989. Model for intracellular folding of the human immunodeficiency virus type 1 gp120 J. Virol. 63:639-646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fenouillet, E., B. Clerget-Raslain, J. C. Gluckman, D. Guetard, L. Montagnier, and E. Bahraoui. 1989. Role of N-linked glycans in the interaction between the envelope glycoprotein of human immunodeficiency virus and its CD4 cellular receptor. Structural enzymatic analysis. J. Exp. Med. 169:807-822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fouts, T. R., J. M. Binley, A. Trkola, J. E. Robinson, and J. P. Moore. 1997. Interaction of polyclonal and monoclonal anti-glycoprotein 120 antibodies with oligomeric glycoprotein 120-glycoprotein 41 complexes of a primary HIV type 1 isolate: relationship to neutralization. AIDS Res. Hum. Retroviruses 14:591-597. [DOI] [PubMed] [Google Scholar]
- 31.Gauduin, M. C., P. W. Parren, R. Weir, C. F. Barbas, D. R. Burton, and R. A. Koup. 1997. Passive immunization with a human monoclonal antibody protects hu-PBL-SCID mice against challenge by primary isolates of HIV-1. Nat. Med. 3:1389-1393. [DOI] [PubMed] [Google Scholar]
- 32.Geyer, H., C. Holschbach, G. Hunsmann, and J. Schneider. 1988. Carbohydrates of human immunodeficiency virus. Structures of oligosaccharides linked to the envelope glycoprotein 120. J. Biol. Chem. 263:11760-11767. [PubMed] [Google Scholar]
- 33.Gooding, K. M., and F. E. Regnier. 1990. In HPLC of biological macromolecules. Marcel Dekker, New York, N.Y.
- 34.Gorny, M. K., J. Y. Xu, S. Karwowska, A. Buchbinder, and S. Zolla-Pazner. 1993. Repertoire of neutralizing human monoclonal antibodies specific for the V3 domain of HIV-1 gp120 J. Immunol. 150:635-643. [PubMed] [Google Scholar]
- 35.Haas, J., E. C. Park, and B. Seed. 1996. Codon usage limitation in the expression of HIV-1 envelope glycoprotein. Curr. Biol. 6:315-324. [DOI] [PubMed] [Google Scholar]
- 36.Haigwood, N. L., P. L. Nara, E. Brooks, G. A. Van Nest, G. Ott, K. W. Higgins, N. Dunlop, C. J. Scandella, J. W. Eichberg, and K. S. Steimer. 1992. Native but not denatured recombinant human immunodeficiency virus type 1 gp120 generates broad-spectrum neutralizing antibodies in baboons. J. Virol. 66:172-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hariharan, K., P. L. Nara, V. M. Caralli, F. L. Norton, N. Haigwood, and C. Y. Kang. 1993. Analysis of the cross-reactive anti-gp120 antibody population in human immunodeficiency virus-infected asymptomatic individuals. J. Virol. 67:953-960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hill, C. P., D. Worthylake, D. P. Bancroft, A. M. Christensen, and W. I. Sundquist. 1996. Crystal structures of the trimeric human immunodeficiency virus type 1 matrix protein: implications for membrane association and assembly. Proc. Natl. Acad. Sci. USA 93:3099-3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hilleman, M. R. 1998. A simplified vaccinologists' vaccinology and the pursuit of a vaccine against AIDS. Vaccine 16:778-793. [DOI] [PubMed] [Google Scholar]
- 40.Ho, D. D., M. S. Fung, H. Yoshiyama, Y. Cao, and J. E. Robinson. 1992. Discontinuous epitopes on gp120 important in HIV-1 neutralization. AIDS Res. Hum. Retroviruses 8:1337-1339. [DOI] [PubMed] [Google Scholar]
- 41.Ho, D. D., M. G. Sarngadharan, M. S. Hirsch, R. T. Schooley, T. R. Rota, R. C. Kennedy, T. C. Chanh, and V. L. Sato. 1987. Human immunodeficiency virus neutralizing antibodies recognize several conserved domains on the envelope glycoproteins. J. Virol. 61:2024-2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Igarashi, T., Y. Endo, G. Englund, R. Sadjadpour, T. Matano, C. Buckler, A. Buckler-White, R. Plishka, T. Theodore, R. Shibata, and M. Martin. 1999. Emergence of a highly pathogenic simian/human immunodeficiency virus in a rhesus macaque treated with anti-CD8 mAb during a primary infection with a nonpathogenic virus. Proc. Natl. Acad. Sci. USA 96:14049-14054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Javaherian, K., A. J. Langlois, G. J. LaRosa, A. T. Profy, D. P. Bolognesi, W. C. Herlihy, S. D. Putney, and T. J. Matthews. 1990. Broadly neutralizing antibodies elicited by the hypervariable neutralizing determinant of HIV-1. Science 250:1590-1593. [DOI] [PubMed] [Google Scholar]
- 44.Javaherian, K., A. J. Langlois, C. McDanal, K. L. Ross, L. I. Eckler, C. L. Jellis, A. T. Profy, J. R. Rusche, D. P. Bolognesi, S. D. Putney, et al. 1989. Principal neutralizing domain of the human immunodeficiency virus type 1 envelope protein. Proc. Natl. Acad. Sci. USA 86:6768-6772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Joag, S. V., Z. Li, C. Wang, L. Foresman, F. Jia, E. B. Stephens, W. Zhuge, and O. Narayan. 1999. Passively administered neutralizing serum that protected macaques against infection with parenterally inoculated pathogenic simian-human immunodeficiency virus failed to protect against mucosally inoculated virus. AIDS Res. Hum. Retroviruses 15:391-394. [DOI] [PubMed] [Google Scholar]
- 46.Kameyama, K., T. Nakae, and T. Takagi. 1982. Estimation of molecular weights of membrane proteins in the presence of SDS by low-angle laser light scattering combined with high-performance porous silica gel chromatography. Confirmation of the trimer structure of porin of the E. coli outer membrane. Biochim. Biophys. Acta 706:19-26. [DOI] [PubMed] [Google Scholar]
- 47.Kangro, H. O., S. Manzoor, and D. R. Harper. 1991. Antibody avidity following varicella-zoster virus infections. J. Med. Virol. 33:100-105. [DOI] [PubMed] [Google Scholar]
- 48.Klatzmann, D., E. Champagne, S. Chamaret, J. Gruest, D. Guetard, T. Hercend, J. C. Gluckman, and L. Montagnier. 1984. T-lymphocyte T4 molecule behaves as the receptor for human retrovirus LAV. Nature 312:767-768. [DOI] [PubMed] [Google Scholar]
- 49.Koup, R. A., J. T. Safrit, Y. Cao, C. A. Andrews, G. McLeod, W. Borkowsky, C. Farthing, and D. D. Ho. 1994. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J. Virol. 68:4650-4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kozarsky, K., M. Penman, L. Basiripour, W. Haseltine, J. Sodroski, and M. Krieger. 1989. Glycosylation and processing of the human immunodeficiency virus type 1 envelope protein. J. Acquir. Immune Defic. Syndr. 2:163-169. [PubMed] [Google Scholar]
- 51.Krull, I. S., H. H. Stuting, and S. C. Krzysko. 1988. Conformational studies of bovine alkaline phosphatase in hydrophobic interaction and size-exclusion chromatography with linear diode array and low-angle laser light scattering detection. J. Chromatogr. 442:29-52. [DOI] [PubMed] [Google Scholar]
- 52.Kwong, P. D., R. Wyatt, J. Robinson, R. W. Sweet, J. Sodroski, and W. A. Hendrickson. 1998. Structure of an HIV gp120 envelope glycoprotein in complex with the CD4 receptor and a neutralizing human antibody. Nature 393:648-659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lasky, L. A., G. Nakamura, D. H. Smith, C. Fennie, C. Shimasaki, E. Patzer, P. Berman, T. Gregory, and D. J. Capon. 1987. Delineation of a region of the human immunodeficiency virus type 1 gp120 glycoprotein critical for interaction with the CD4 receptor. Cell 50:975-985. [DOI] [PubMed] [Google Scholar]
- 54.Leinikki, P. O., I. Shekarchi, P. Dorsett, and J. L. Sever. 1978. Enzyme-linked immunosorbent assay determination of specific rubella antibody levels in micrograms of immunoglobulin G per milliliter of serum in clinical samples. J. Clin. Microbiol. 8:419-423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Leonard, C. K., M. W. Spellman, L. Riddle, R. J. Harris, J. N. Thomas, and T. J. Gregory. 1990. Assignment of intrachain disulfide bonds and characterization of potential glycosylation sites of the type 1 recombinant human immunodeficiency virus envelope glycoprotein (gp120) expressed in Chinese hamster ovary cells. J. Biol. Chem. 265:10373-10382. [PubMed] [Google Scholar]
- 56.Letvin, N. L. 1998. Progress in the development of an HIV-1 vaccine. Science 280:1875-1880. [DOI] [PubMed] [Google Scholar]
- 57.Li, Y., L. Luo, N. Rasool, and C. Y. Kang. 1993. Glycosylation is necessary for the correct folding of human immunodeficiency virus gp120 in CD4 binding. J. Virol. 67:584-588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Luxton, R. W., and E. J. Thompson. 1990. Affinity distributions of antigen-specific IgG in patients with multiple sclerosis and in patients with viral encephalitis. J. Immunol. Methods 131:277-282. [DOI] [PubMed] [Google Scholar]
- 59.Machamer, C. E., and J. K. Rose. 1988. Vesicular stomatitis virus G proteins with altered glycosylation sites display temperature-sensitive intracellular transport and are subject to aberrant intermolecular disulfide bonding. J. Biol. Chem. 263:5955-5960. [PubMed] [Google Scholar]
- 60.Maezawa, S., Y. Hayashi, T. Nakae, J. Ishii, K. Kameyama, and T. Takagi. 1983. Determination of molecular weight of membrane proteins by the use of low-angle laser light scattering combined with high-performance gel chromatography in the presence of a non-ionic surfactant. Biochim. Biophys. Acta 747:291-297. [DOI] [PubMed] [Google Scholar]
- 61.Mascola, J. R., M. G. Lewis, G. Stiegler, D. Harris, T. C. VanCott, D. Hayes, M. K. Louder, C. R. Brown, C. V. Sapan, S. S. Frankel, Y. Lu, M. L. Robb, H. Katinger, and D. L. Birx. 1999. Protection of macaques against pathogenic simian/human immunodeficiency virus 89.6PD by passive transfer of neutralizing antibodies. J. Virol. 73:4009-4018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mascola, J. R., S. W. Snyder, O. S. Weislow, S. M. Belay, R. B. Belshe, D. H. Schwartz, M. L. Clements, R. Dolin, B. S. Graham, G. J. Gorse, M. C. Keefer, M. J. McElrath, M. C. Walker, K. F. Wagner, J. G. McNeil, F. E. McCutchan, and D. S. Burke. 1996. Immunization with envelope subunit vaccine products elicits neutralizing antibodies against laboratory-adapted but not primary isolates of human immunodeficiency virus type 1. The National Institute of Allergy and Infectious Diseases AIDS Vaccine Evaluation Group. J. Infect. Dis. 173:340-348. [DOI] [PubMed] [Google Scholar]
- 63.Mascola, J. R., G. Stiegler, T. C. VanCott, H. Katinger, C. B. Carpenter, C. E. Hanson, H. Beary, D. Hayes, S. S. Frankel, D. L. Birx, and M. G. Lewis. 2000. Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat. Med. 6:207-210. [DOI] [PubMed] [Google Scholar]
- 64.McDonnell, M. E., and A. M. Jamieson. 1976. Rapid characterization of protein molecular weights and hydrodynamic structures by quasielastic laser-light scattering. Biopolymers 15:1283-1299. [DOI] [PubMed] [Google Scholar]
- 65.McDougal, J. S., J. K. Nicholson, G. D. Cross, S. P. Cort, M. S. Kennedy, and A. C. Mawle. 1986. Binding of the human retrovirus HTLV-III/LAV/ARV/HIV to the CD4 (T4) molecule: conformation dependence, epitope mapping, antibody inhibition, and potential for idiotypic mimicry. J. Immunol. 137:2937-2944. [PubMed] [Google Scholar]
- 66.Mhatre, R., I. S. Krull, and H. H. Stuting. 1990. Determination of biopolymer (protein) molecular weights by gradient elution, reversed-phase high-performance liquid chromatography with low-angle laser light scattering detection. J. Chromatogr. 502:21-46. [DOI] [PubMed] [Google Scholar]
- 67.Mizuochi, T., M. W. Spellman, M. Larkin, J. Solomon, L. J. Basa, and T. Feizi. 1988. Carbohydrate structures of the human-immunodeficiency-virus (HIV) recombinant envelope glycoprotein gp120 produced in Chinese-hamster ovary cells. Biochem. J. 254:599-603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mizuochi, T., M. W. Spellman, M. Larkin, J. Solomon, L. J. Basa, and T. Feizi. 1988. Structural characterization by chromatographic profiling of the oligosaccharides of human immunodeficiency virus (HIV) recombinant envelope glycoprotein gp120 produced in Chinese hamster ovary cells. Biomed. Chromatogr. 2:260-270. [DOI] [PubMed] [Google Scholar]
- 69.Montefiori, D. C., and T. G. Evans. 1999. Toward an HIV type 1 vaccine that generates potent, broadly cross-reactive neutralizing antibodies. AIDS Res. Hum. Retroviruses 15:689-698. [DOI] [PubMed] [Google Scholar]
- 70.Montefiori, D. C., B. S. Graham, J. Zhou, R. A. Bucco, D. H. Schwartz, L. A. Cavacini, and M. R. Posner. 1993. V3-specific neutralizing antibodies in sera from HIV-1 gp160-immunized volunteers block virus fusion and act synergistically with human monoclonal antibody to the conformation-dependent CD4 binding site of gp120. NIH-NIAID AIDS Vaccine Clinical Trials Network. J. Clin. Investig. 92:840-847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Montefiori, D. C., G. Pantaleo, L. M. Fink, J. T. Zhou, J. Y. Zhou, M. Bilska, G. D. Miralles, and A. S. Fauci. 1996. Neutralizing and infection-enhancing antibody responses to human immunodeficiency virus type 1 in long-term nonprogressors. J. Infect. Dis. 173:60-67. [DOI] [PubMed] [Google Scholar]
- 72.Moog, C., H. J. Fleury, I. Pellegrin, A. Kirn, and A. M. Aubertin. 1997. Autologous and heterologous neutralizing antibody responses following initial seroconversion in human immunodeficiency virus type 1-infected individuals. J. Virol. 71:3734-3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Moore, J. P., Y. Cao, L. Qing, Q. J. Sattentau, J. Pyati, R. Koduri, J. Robinson, C. F. Barbas, D. R. Burton, and D. D. Ho. 1995. Primary isolates of human immunodeficiency virus type 1 are relatively resistant to neutralization by monoclonal antibodies to gp120, and their neutralization is not predicted by studies with monomeric gp120. J. Virol. 69:101-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Moore, J. P., and D. D. Ho. 1993. Antibodies to discontinuous or conformationally sensitive epitopes on the gp120 glycoprotein of human immunodeficiency virus type 1 are highly prevalent in sera of infected humans. J. Virol. 67:863-875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Moore, J. P., and D. D. Ho. 1995. HIV-1 neutralization: the consequences of viral adaptation to growth on transformed T cells. AIDS 9(Suppl. A):S117-S136. [PubMed]
- 76.Moore, J. P., Q. J. Sattentau, R. Wyatt, and J. Sodroski. 1994. Probing the structure of the human immunodeficiency virus surface glycoprotein gp120 with a panel of monoclonal antibodies. J. Virol. 68:469-484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Moore, J. P., and J. Sodroski. 1996. Antibody cross-competition analysis of the human immunodeficiency virus type 1 gp120 exterior envelope glycoprotein. J. Virol. 70:1863-1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Moore, J. P., L. A. Wallace, E. A. Follett, and J. A. Mckeating. 1989. An enzyme-linked immunosorbent assay for antibodies to the envelope glycoproteins of divergent strains of HIV-1. AIDS 3:155-163. [DOI] [PubMed] [Google Scholar]
- 79.Moore, J. P., H. Yoshiyama, D. D. Ho, J. E. Robinson, and J. Sodroski. 1993. Antigenic variation in gp120s from molecular clones of HIV-1 LAI. AIDS Res. Hum. Retroviruses 9:1185-1193. [DOI] [PubMed] [Google Scholar]
- 80.Ogg, G. S., X. Jin, S. Bonhoeffer, P. R. Dunbar, M. A. Nowak, S. Monard, J. P. Segal, Y. Cao, S. L. Rowland-Jones, V. Cerundolo, A. Hurley, M. Markowitz, D. D. Ho, D. F. Nixon, and A. J. McMichael. 1998. Quantitation of HIV-1-specific cytotoxic T lymphocytes and plasma load of viral RNA. Science 279:2103-2106. [DOI] [PubMed] [Google Scholar]
- 81.Ohno, T., M. Terada, Y. Yoneda, K. W. Shea, R. F. Chambers, D. M. Stroka, M. Nakamura, and D. W. Kufe. 1991. A broadly neutralizing monoclonal antibody that recognizes the V3 region of human immunodeficiency virus type 1 glycoprotein gp120. Proc. Natl. Acad. Sci. USA 88:10726-10729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Page, M., C. Vella, T. Corcoran, P. Dilger, C. Ling, A. Heath, and R. Thorpe. 1992. Restriction of serum antibody reactivity to the V3 neutralizing domain of HIV gp120 with progression to AIDS. AIDS 6:441-446. [DOI] [PubMed] [Google Scholar]
- 83.Papandreou, M. J., T. Idziorek, R. Miquelis, and E. Fenouillet. 1996. Glycosylation and stability of mature HIV envelope glycoprotein conformation under various conditions. FEBS Lett. 379:171-176. [DOI] [PubMed] [Google Scholar]
- 84.Parren, P. W., D. R. Burton, and Q. J. Sattentau. 1997. HIV-1 antibody--debris or virion? Nat. Med. 3:366-367. [DOI] [PubMed] [Google Scholar]
- 85.Parren, P. W., J. P. Moore, D. R. Burton, and Q. J. Sattentau. 1999. The neutralizing antibody response to HIV-1: viral evasion and escape from humoral immunity. AIDS 13(Suppl. A):S137-S162. [PubMed]
- 86.Pilgrim, A. K., G. Pantaleo, O. J. Cohen, L. M. Fink, J. Y. Zhou, J. T. Zhou, D. P. Bolognesi, A. S. Fauci, and D. C. Montefiori. 1997. Neutralizing antibody responses to human immunodeficiency virus type 1 in primary infection and long-term-nonprogressive infection. J. Infect. Dis. 176:924-932. [DOI] [PubMed] [Google Scholar]
- 87.Pinter, A., W. J. Honnen, S. A. Tilley, C. Bona, H. Zaghouani, M. K. Gorny, and S. Zolla-Pazner. 1989. Oligomeric structure of gp41, the transmembrane protein of human immunodeficiency virus type 1. J. Virol. 63:2674-2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Poignard, P., T. Fouts, D. Naniche, J. P. Moore, and Q. J. Sattentau. 1996. Neutralizing antibodies to human immunodeficiency virus type-1 gp120 induce envelope glycoprotein subunit dissociation. J. Exp. Med. 183:473-484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Putney, S. D., T. J. Matthews, W. G. Robey, D. L. Lynn, M. Robert-Guroff, W. T. Mueller, A. J. Langlois, J. Ghrayeb, S. R. Petteway, K. J. Weinhold, et al. 1986. HTLV-III/LAV-neutralizing antibodies to an E. coli-produced fragment of the virus envelope. Science 234:1392-1395. [DOI] [PubMed] [Google Scholar]
- 90.Richardson, T. M., B. L. Stryjewski, C. C. Broder, J. A. Hoxie, J. R. Mascola, P. L. Earl, and R. W. Doms. 1996. Humoral response to oligomeric human immunodeficiency virus type 1 envelope protein. J. Virol. 70:753-762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Richmond, J. F., S. Lu, J. C. Santoro, J. Weng, S. L. Hu, D. C. Montefiori, and H. L. Robinson. 1998. Studies of the neutralizing activity and avidity of anti-human immunodeficiency virus type 1 Env antibody elicited by DNA priming and protein boosting. J. Virol. 72:9092-9100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Roben, P., J. P. Moore, M. Thali, J. Sodroski, C. F. Barbas, and D. R. Burton. 1994. Recognition properties of a panel of human recombinant Fab fragments to the CD4 binding site of gp120 that show differing abilities to neutralize human immunodeficiency virus type 1. J. Virol. 68:4821-4828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rowland-Jones, S., R. Tan, and A. McMichael. 1997. Role of cellular immunity in protection against HIV infection. Adv. Immunol. 65:277-346. [PubMed] [Google Scholar]
- 94.Sanders, R. W., L. Schiffner, A. Master, F. Kajumo, Y. Guo, T. Dragic, J. P. Moore, and J. M. Binley. 2000. Variable-loop-deleted variants of the human immunodeficiency virus type 1 envelope glycoprotein can be stabilized by an intermolecular disulfide bond between the gp120 and gp41 subunits. J. Virol. 74:5091-5100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sattentau, Q. J. 1996. Neutralization of HIV-1 by antibody. Curr. Opin. Immunol. 8:540-545. [DOI] [PubMed] [Google Scholar]
- 96.Sattentau, Q. J., and J. P. Moore. 1995. Human immunodeficiency virus type 1 neutralization is determined by epitope exposure on the gp120 oligomer. J. Exp. Med. 182:185-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sattentau, Q. J., and R. A. Weiss. 1988. The CD4 antigen: physiological ligand and HIV receptor. Cell 52:631-633. [DOI] [PubMed] [Google Scholar]
- 98.Sattentau, Q. J., S. Zolla-Pazner, and P. Poignard. 1995. Epitope exposure on functional, oligomeric HIV-1 gp41 molecules. Virology 206:713-717. [DOI] [PubMed] [Google Scholar]
- 99.Scandella, C. J., J. Kilpatrick, W. Lidster, C. Parker, J. P. Moore, G. K. Moore, K. A. Mann, P. Brown, S. Coates, B. Chapman, et al. 1993. Nonaffinity purification of recombinant gp120 for use in AIDS vaccine development. AIDS Res. Hum. Retroviruses 9:1233-1244. [DOI] [PubMed] [Google Scholar]
- 100.Schawaller, M., G. E. Smith, J. J. Skehel, and D. C. Wiley. 1989. Studies with crosslinking reagents on the oligomeric structure of the env glycoprotein of HIV. Virology 172:367-369. [DOI] [PubMed] [Google Scholar]
- 101.Schwartz, D. H., G. Gorse, M. L. Clements, R. Belshe, A. Izu, A. M. Duliege, P. Berman, T. Twaddell, D. Stablein, R. Sposto, et al. 1993. Induction of HIV-1-neutralising and syncytium-inhibiting antibodies in uninfected recipients of HIV-1IIIB rgp120 subunit vaccine. Lancet 342:69-73. [DOI] [PubMed] [Google Scholar]
- 102.Skinner, M. A., A. J. Langlois, C. B. McDanal, J. S. McDougal, D. P. Bolognesi, and T. J. Matthews. 1988. Neutralizing antibodies to an immunodominant envelope sequence do not prevent gp120 binding to CD4. J. Virol. 62:4195-4200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Srivastava, I. K., M. Schmidt, U. Certa, H. Dobeli, and L. H. Perrin. 1990. Specificity and inhibitory activity of antibodies to Plasmodium falciparum aldolase. J. Immunol. 144:1497-1503. [PubMed] [Google Scholar]
- 104.Srivastava, I. K., B. Takacs, P. Caspers, U. Certa, I. A. McGregor, J. Scaife, and L. H. Perrin. 1989. Recombinant polypeptides for serology of malaria. Trans. R. Soc. Trop. Med. Hyg. 83:317-321. [DOI] [PubMed] [Google Scholar]
- 105.Stamatatos, L., and C. Cheng-Mayer. 1995. Structural modulations of the envelope gp120 glycoprotein of human immunodeficiency virus type 1 upon oligomerization and differential V3 loop epitope exposure of isolates displaying distinct tropism upon virion-soluble receptor binding. J. Virol. 69:6191-6198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Stamatos, N. M., J. R. Mascola, V. S. Kalyanaraman, M. K. Louder, L. M. Frampton, D. L. Birx, and T. C. VanCott. 1998. Neutralizing antibodies from the sera of human immunodeficiency virus type 1-infected individuals bind to monomeric gp120 and oligomeric gp140. J. Virol. 72:9656-9667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Steimer, K. S., and N. L. Haigwood. 1992. Immunization of primates with native, recombinant HIV-SF2 gp120 generates broadly effective neutralizing antibodies directed to conformational epitopes. AIDS Res. Hum. Retroviruses 8:1391.. [DOI] [PubMed] [Google Scholar]
- 108.Steimer, K. S., and N. L. Haigwood. 1991. Importance of conformation on the neutralizing antibody response to HIV-1 gp120. Biotechnol. Ther. 2:63-89. [PubMed] [Google Scholar]
- 109.Steimer, K. S., C. J. Scandella, P. V. Skiles, and N. L. Haigwood. 1991. Neutralization of divergent HIV-1 isolates by conformation-dependent human antibodies to Gp120. Science 254:105-108. [DOI] [PubMed] [Google Scholar]
- 110.Stephens, D. M., J. W. Eichberg, N. L. Haigwood, K. S. Steimer, D. Davis, and P. J. Lachmann. 1992. Antibodies are produced to the variable regions of the external envelope glycoprotein of human immunodeficiency virus type 1 in chimpanzees infected with the virus and baboons immunized with a candidate recombinant vaccine. J. Gen. Virol. 73:1099-1106. [DOI] [PubMed] [Google Scholar]
- 111.Stuting, H. H., and I. S. Krull. 1990. Complete on-line determination of biopolymer molecular weight via high-performance liquid chromatography coupled to low-angle laser light scattering, ultraviolet, and differential refractive index detection. Anal. Chem. 62:2107-2114. [DOI] [PubMed] [Google Scholar]
- 112.Sullivan, N., Y. Sun, J. Li, W. Hofmann, and J. Sodroski. 1995. Replicative function and neutralization sensitivity of envelope glycoproteins from primary and T-cell line-passaged human immunodeficiency virus type 1 isolates. J. Virol. 69:4413-4422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tan, K., J. Liu, J. Wang, S. Shen, and M. Lu. 1997. Atomic structure of a thermostable subdomain of HIV-1 gp41. Proc. Natl. Acad. Sci. USA 94:12303-12308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Trkola, A., M. Purtscher, T. Muster, C. Ballaun, A. Buchacher, N. Sullivan, K. Srinivasan, J. Sodroski, J. P. Moore, and H. Katinger. 1996. Human monoclonal antibody 2G12 defines a distinctive neutralization epitope on the gp120 glycoprotein of human immunodeficiency virus type 1. J. Virol. 70:1100-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.VanCott, T. C., F. R. Bethke, D. S. Burke, R. R. Redfield, and D. L. Birx. 1995. Lack of induction of antibodies specific for conserved, discontinuous epitopes of HIV-1 envelope glycoprotein by candidate AIDS vaccines. J. Immunol. 155:4100-4110. [PubMed] [Google Scholar]
- 116.VanCott, T. C., R. W. Kaminski, J. R. Mascola, V. S. Kalyanaraman, N. M. Wassef, C. R. Alving, J. T. Ulrich, G. H. Lowell, and D. L. Birx. 1998. HIV-1 neutralizing antibodies in the genital and respiratory tracts of mice intranasally immunized with oligomeric gp160. J. Immunol. 160:2000-2012. [PubMed] [Google Scholar]
- 117.VanCott, T. C., J. R. Mascola, R. W. Kaminski, V. Kalyanaraman, P. L. Hallberg, P. R. Burnett, J. T. Ulrich, D. J. Rechtman, and D. L. Birx. 1997. Antibodies with specificity to native gp120 and neutralization activity against primary human immunodeficiency virus type 1 isolates elicited by immunization with oligomeric gp160. J. Virol. 71:4319-4330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.VanCott, T. C., J. R. Mascola, L. D. Loomis-Price, F. Sinangil, N. Zitomersky, J. McNeil, M. L. Robb, D. L. Birx, and S. Barnett. 1999. Cross-subtype neutralizing antibodies induced in baboons by a subtype E gp120 immunogen based on an R5 primary human immunodeficiency virus type 1 envelope. J. Virol. 73:4640-4650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Vidal, S., G. Mottet, D. Kolakofsky, and L. Roux. 1989. Addition of high-mannose sugars must precede disulfide bond formation for proper folding of Sendai virus glycoproteins. J. Virol. 63:892-900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ward, K. N., W. Dhaliwal, K. L. Ashworth, E. J. Clutterbuck, and C. G. Teo. 1994. Measurement of antibody avidity for hepatitis C virus distinguishes primary antibody responses from passively acquired antibody. J. Med Virol. 43:367-372. [DOI] [PubMed] [Google Scholar]
- 121.Weissenhorn, W., A. Dessen, S. C. Harrison, J. J. Skehel, and D. C. Wiley. 1997. Atomic structure of the ectodomain from HIV-1 gp41. Nature 387:426-430. [DOI] [PubMed] [Google Scholar]
- 122.Wen, J., T. Arakawa, and J. S. Philo. 1996. Size-exclusion chromatography with on-line light-scattering, absorbance, and refractive index detectors for studying proteins and their interactions. Anal. Biochem. 240:155-166. [DOI] [PubMed] [Google Scholar]
- 123.Wrin, T., L. Crawford, L. Sawyer, P. Weber, H. W. Sheppard, and C. V. Hanson. 1994. Neutralizing antibody responses to autologous and heterologous isolates of human immunodeficiency virus. J. Acquir. Immune Defic. Syndr. 7:211-219. [PubMed] [Google Scholar]
- 124.Wrin, T., and J. H. Nunberg. 1994. HIV-1MN recombinant gp120 vaccine serum, which fails to neutralize primary isolates of HIV-1, does not antagonize neutralization by antibodies from infected individuals. AIDS 8:1622-1623. [DOI] [PubMed] [Google Scholar]
- 125.Wyatt, R., E. Desjardin, U. Olshevsky, C. Nixon, J. Binley, V. Olshevsky, and J. Sodroski. 1997. Analysis of the interaction of the human immunodeficiency virus type 1 gp120 envelope glycoprotein with the gp41 transmembrane glycoprotein. J. Virol. 71:9722-9731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wyatt, R., P. D. Kwong, E. Desjardins, R. W. Sweet, J. Robinson, W. A. Hendrickson, and J. G. Sodroski. 1998. The antigenic structure of the HIV gp120 envelope glycoprotein. Nature 393:705-711. [DOI] [PubMed] [Google Scholar]
- 127.Wyatt, R., and J. Sodroski. 1998. The HIV-1 envelope glycoproteins: fusogens, antigens, and immunogens. Science 280:1884-1888. [DOI] [PubMed] [Google Scholar]
- 128.Yang, X., M. Farzan, R. Wyatt, and J. Sodroski. 2000. Characterization of stable, soluble trimers containing complete ectodomains of human immunodeficiency virus type 1 envelope glycoproteins. J. Virol. 74:5716-5725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Yang, X., L. Florin, M. Farzan, P. Kolchinsky, P. D. Kwong, J. Sodroski, and R. Wyatt. 2000. Modifications that stabilize human immunodeficiency virus envelope glycoprotein trimers in solution. J. Virol. 74:4746-4754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Yang, X., R. Wyatt, and J. Sodroski. 2001. Improved elicitation of neutralizing antibodies against primary human immunodeficiency viruses by soluble stabilized envelope glycoprotein trimers. J. Virol. 75:1165-1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.zur Megede, J., M. C. Chen, B. Doe, M. Schaefer, C. E. Greer, M. Selby, G. R. Otten, and S. W. Barnett. 2000. Increased expression and immunogenicity of sequence-modified human immunodeficiency virus type 1 gag gene. J. Virol. 74:2628-2635. [DOI] [PMC free article] [PubMed] [Google Scholar]