Abstract
In this study, we characterized the viral determinants of coreceptor usage in relation to susceptibility to antibody-mediated neutralization or enhancement of infectivity by using chimeras of three highly related human immunodeficiency virus type 1 (HIV-1) isolates of different phenotypes. We found that the V3 region was the main determinant of antibody-mediated enhancement and coreceptor specificity but that the overall structure of gp120 was also important for these properties. Constructs susceptible to antibody-mediated enhancement preferentially use CCR5 as a coreceptor, in contrast to constructs that were neutralized or not affected. Using monoclonal antibodies directed against CD4 or CCR5, we were able to show that antibody-mediated enhancement was CD4 dependent. Altogether, our results suggest that the modulation of the interaction of gp120 with CCR5 is the mechanism underlying antibody-mediated enhancement of HIV-1 infectivity.
Human immunodeficiency virus type 1 (HIV-1) entry into CD4+ cells is initiated by the high-affinity interaction between the viral envelope glycoprotein and the cell membrane-associated CD4 molecule. This interaction triggers conformational changes in gp120, allowing the subsequent binding of the gp120-CD4 complex to a chemokine receptor (15, 36, 41, 57). While primary macrophage-tropic HIV-1 isolates use CCR5 as a coreceptor (R5 isolates), T-cell-line-tropic or laboratory-adapted strains of HIV-1 can also use other coreceptors, such as CXCR4 (X4 or R5X4 isolates) (5, 27). Binding to the coreceptor induces additional conformational changes in gp120, demasking the fusion complex of gp41 and allowing fusion between the cellular and viral lipid membranes and entry of the viral capsid into the target cell (17, 22).
HIV-1 variants can also be distinguished by their sensitivity to gp120-specific monoclonal antibodies (37). The infectivity of most primary HIV-1 strains is neutralized or not affected in the presence of soluble CD4 or monoclonal antibodies directed against the V3 loop or the CD4-binding domain of gp120. The mechanism of and the viral determinants involved in HIV-1 neutralization have been studied extensively. HIV-1 neutralization results from the inhibition of virus attachment to the cell, either by disruption of the gp120-gp41 interaction (shedding) or by steric hindrance or direct inhibition of the entry process (37, 55). It has been shown that primary HIV-1 strains are less sensitive to shedding than laboratory-adapted strains (11, 18, 31), and HIV-1 susceptibility to neutralization appears to be mainly determined by the overall structure of the envelope glycoprotein (34, 35, 38).
In contrast, the infectivity of some primary HIV-1 strains is enhanced by gp120-specific monoclonal antibodies or soluble CD4 under the same conditions (45, 51), but little is known about the mechanisms of antibody-mediated enhancement of HIV-1 entry. The process has been shown to be independent of Fc or complement receptors and to be temperature independent, while the involvement of cross-linking between gp120 subunits remains controversial (45, 50). The V3 loop has been suggested as the main viral determinant for antibody-mediated enhancement in cooperation with other domains of gp120 (50). So far, this characteristic has been shown only for one HIV-1 clone, and the level at which the entry process is affected by antibody-mediated enhancement is still unidentified.
Here, we studied three closely related HIV-1 envelopes, 16.1, 16.2, and 16.4, isolated from the same patient (1). Syncytium-inducing (SI) variants 16.1 and 16.2 were unaffected and neutralized, respectively, when preincubated with gp120-specific monoclonal antibodies, whereas the infectivity of non-syncytium-inducing (NSI) variant 16.4 was enhanced under the same conditions (45, 46). Using chimeras of these three envelopes, we studied the viral determinants of antibody-mediated enhancement and the influence of antibodies directed against CD4 and CCR5 on the entry process. We found that antibody-mediated enhancement of infectivity depends on the structure of the gp120 protein and that it involves the modulation of the interaction of gp120 with CCR5 but not with CXCR4.
MATERIALS AND METHODS
Envelope genes.
The parental envelope genes were amplified from three biological clones, 16.1, 16.2, and 16.4, isolated from the same patient and cloned in expression vector pSHRS (1, 2, 14). Chimeric envelope genes were generated by using previously described restriction sites (1) and are shown in Fig. 1. Constructs were checked by restriction analysis and/or automatic sequencing by using custom oligonucleotides and a dye-deoxy terminator sequencing kit (Perkin-Elmer).
FIG. 1.
Antibody-mediated modulation of entry of various chimeric viruses. (Left) Schematic representation of chimeric constructs. S, SpeI; H, HindII; C, CvnI; B, BspLU11I; M, MamI; A, AvrII. (Middle) Efficiency of entry into SupT1 cells for viruses pseudotyped with different constructs after 30 to 45 min of preincubation with 15 μg of V3 loop-specific antibody 391/95-D/ml. The broken line at 100% indicates no effect. Values represent the mean of at least three different experiments. (Right) Basal level of entry of the various chimeras, as indicated by the CAT activity observed for each construct in the absence of monoclonal antibody.
Cells.
SupT1 and COS cells were cultured in RPMI medium supplemented with 10% fetal calf serum (FCS) and antibiotics. A panel of U87.CD4 cells, stably transfected with human CD4 and different chemokine receptors (23) (kindly provided by D. Littman), was cultured in Dulbecco modified Eagle medium (DMEM)-10% FCS. The expression of the transgenes was regularly selected for with 1 mg of Geneticin (Gibco-BRL)/ml and 0.1 μg of puromycin (Calbiochem)/ml. CD4 expression was assessed regularly by FACScan analysis.
Generation of Env-pseudotyped viruses.
Five to 10 million COS cells were electroporated at 220 V and 960 μF in serum-free RPMI medium with 10 μg of pSHRS expressing HIV-1 envelopes and 10 μg of an Env-deleted HIV-1 molecular clone containing the chloramphenicol acetyltransferase (CAT) reporter gene as described previously (2, 21). Three days after transfection, supernatants containing Env-pseudotyped viruses were harvested, stored at −70°C, and used for coreceptor studies or neutralization assays.
Coreceptor usage assay.
One day before infection, U87.CD4 cells expressing chemokine receptors were plated in six-well plates (Costar) at 2 × 105 cells per well. On the next day, medium was removed from the cells and infection was performed in parallel for cells expressing the second receptor for 8 to 16 h with Env-pseudotype viruses in a final volume of 750 μl. Cells were washed once and cultured for 2 to 3 days in DMEM-10% FCS. Cell pellets were collected and analyzed for CAT activity as described previously (2, 48). The relative efficiency of CXCR4 usage was determined for each experiment as the ratio of CAT activity of CXCR4-expressing cells to CAT activity of CCR5-expressing cells in the same experiment. The nomenclature of viruses was based on previously defined criteria (12).
Neutralization enhancement assay.
The neutralization enhancement assay was performed as previously described (45). Briefly, Env-pseudotyped viruses were preincubated with or without 15 μg of V3 loop-specific antibody 391/95-D (a kind gift from S. Zolla-Pazner) for 30 to 45 min at 37°C in a final volume of 500 μl. Two million SupT1 cells were added to the virus-antibody mixture (or to the virus alone) and incubated overnight at 37°C in a final volume of 1 ml. Cells were washed, cultured in 3 ml of RPMI medium-10% FCS for 2 to 3 days, lysed, and analyzed for CAT activity. The change in infectivity caused by the antibody was determined for each experiment as the ratio of CAT activity in the presence of antibody 391/95-D to CAT activity in the absence of antibody.
Cellular receptor requirements.
CD4-specific monoclonal antibodies L120.3, Q425, Q4120, 13B8-2, JAH 7.3F11, and RFT4 (kindly provided by Q. Sattentau) are directed against the CD4 receptor: Q4120, 13B8-2, JAH 7.3F11, and RFT4 bind to domain 1, Q425 is directed against domain 2, and L120.3 is directed against domain 4 of the CD4 molecule (20, 43, 56). All these antibodies, except for L120.3, have been shown to inhibit HIV-1 infection by interfering with gp120-CD4 binding (Q4120, 13B8-2, and JAH 7.3F11) and/or inhibiting syncytium formation (Q425, Q4120, 13B8-2, JAH 7.3F11, and RFT4) (10, 20, 56). Two million SupT1 cells were preincubated with 10 μg of monoclonal antibodies/ml (or 10 μl of ascitic fluid per ml of cells) for 30 to 45 min at 37°C and further processed as described for the neutralization enhancement assay.
The mouse immunoglobulin G3 anti-CCR5 antibody 2D7 was purchased from Pharmingen and was previously shown to inhibit HIV-1 entry by blocking the gp120-CCR5 interaction (16, 59). Infection of U87.CD4 cells expressing CCR5 (U87.CD4-CCR5 cells) was performed for 4 h in the presence of serial dilutions of this antibody in DMEM-10% FCS. Cells were washed and further processed as described for the coreceptor usage assay.
RESULTS
Viral determinants of antibody-mediated enhancement of 16.4.
By use of chimeras of 16.1, 16.2, and 16.4, it was previously shown that antibody-mediated neutralization or enhancement was determined by the gp120 but not the gp41 subunit of the HIV-1 envelope protein (46). Although CCR5 is not efficiently expressed by SupT1 cells, the level of expression is sufficient to sustain infection by R5 isolate 16.4. Here, we generated various chimeric envelopes by exchanging domains of gp120 of 16.4 with those of 16.1 or 16.2 and characterized the sensitivity of the resulting chimeras to antibody-mediated modulation of entry by using the V3 loop-specific antibody 391/95-D. As shown in Fig. 1, the introduction of the V3 region of 16.4 into 16.1 or 16.2 (41HC or 42HC, respectively) resulted in chimeric envelopes that could be enhanced. Separate introduction of the V1-V2 region or the V4-C5 region of 16.4 into 16.2 (42XS or 42CA, respectively) was not sufficient to transfer enhancement, but the chimera containing both regions (24HC) was enhanced under these conditions (Fig. 1). The introduction of any single domain of 16.1 or 16.2 into the 16.4 background was not sufficient to induce antibody-mediated neutralization (14HC, 24XS, 24HC, and 24CA) (Fig. 1). However, the introduction of a single mutation (E439K) from the C4 domain of 16.4 into the background of 16.1 was sufficient to change the phenotype from unaffected to neutralized (41BM) (Fig. 1). These results suggest that antibody-mediated modulation of entry is determined by multiple domains within gp120.
The same experiments performed with monoclonal antibody GP68, directed against the CD4-binding site of gp120 (47), gave similar results (data not shown), indicating that the enhanced or neutralized phenotype is determined by the envelope conformation independently of the specificity of the anti-gp120 antibody.
Cellular receptor used during antibody-mediated enhancement.
We determined whether antibody-mediated enhancement of 16.4 was associated with a CD4-independent pathway of entry, as has been shown for HIV-2 (8, 39, 40). For this purpose, we used a panel of six CD4-specific antibodies, five of which (RFT-4, JAH 7.3F11, Q4120, 13B8-2, and Q425) were shown to efficiently inhibit the interaction of CD4 with HIV-1 gp120 and one of which (L120.3) did not influence HIV-1 entry. We investigated the effect of preincubation of SupT1 cells with these CD4-specific antibodies on the efficiency of entry of 16.4 in the presence or absence of the V3 loop-specific antibody 391/95-D. Figure 2A shows that preincubation of 16.4 with 391/95-D did not overcome the block of entry by the different CD4-specific antibodies. The fact that CD4-specific antibodies block the infectivity of 16.4 to the same extent in the presence or absence of V3 loop-specific antibodies indicates that antibody-mediated enhancement does not modulate the interaction between CD4 and the gp120 glycoprotein.
FIG. 2.
Impact of antibody-mediated enhancement on the usage of cellular receptors by 16.4. (A) Effect of a CD4-specific antibody on antibody-mediated enhancement of infectivity. The values represent the percentage of entry of pseudotyped viruses into SupT1 cells relative to values obtained without preincubation of the cells with the CD4-specific antibody. For preincubation, 391/95-D was used at 15 μg/ml. N.T., not tested. (B) Coreceptor usage of the three parental clones in the absence of gp120-specific monoclonal antibodies. Values are representative of at least three different experiments. (C) Impact of V3 loop-specific antibody 391/95-D on the infectivity of 16.4. Pseudotyped virus was preincubated or not preincubated with 15 μg of 391/95-D/ml prior to infection of U87.CD4 cells. Values are representative of four different experiments.
Antibody-mediated enhancement may be the result of the use of an alternative coreceptor. In agreement with previous reports (5, 27), 16.1 and 16.2 were able to enter U87.CD4-CCR5 cells or U87.CD4 cells expressing CXCR4 (U87.CD4-CXCR4 cells), whereas the NSI clone 16.4 could enter only U87.CD4-CCR5 cells (Fig. 2B). We next compared the coreceptors used by 16.4 in the absence or presence of the V3 loop-specific antibody 391/95-D. As shown in Fig. 2C, the addition of the V3 loop-specific antibody did not result in a change in the spectrum of coreceptor usage by 16.4. A high multiplicity of infection was used to allow the detection of low-efficiency use of an alternative coreceptor(s). This strategy resulted in saturating CAT activities in both the presence and the absence of the V3 loop-specific monoclonal antibody (Fig. 2C). As a result, enhancement was not detected in this experiment, but enhancement was detected at a lower multiplicity of infection (data not shown).
Interaction between gp120 and CCR5 during antibody-mediated enhancement.
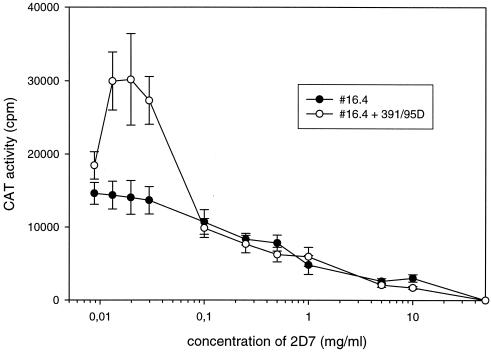
Next, we tested whether antibody-mediated enhancement of 16.4 infectivity could be explained by a difference in the interaction between gp120 and CCR5. Therefore, 16.4 pseudotypes were preincubated with or without the V3 loop-specific antibody 391/95-D before infection of U87.CD4-CCR5 cells in the presence of serial dilutions of the CCR5-specific monoclonal antibody 2D7. Figure 3 shows that the inhibition of infection by 2D7 was concentration dependent at between 0.1 and 50 μg/ml. In this range, the amount of 2D7 needed to inhibit the entry of 16.4 was significantly lower (P = 0.02; Student's t test) when the 16.4 envelope was preincubated with the V3 loop-specific antibody 391/95-D. At lower concentrations of 2D7 (between 0.01 and 0.1 μg/ml), concentration-dependent inhibition of infectivity was still observed for untreated 16.4, while the infectivity of 16.4 preincubated with 391/95-D was enhanced. The finding that the pretreatment of 16.4 gp120 by an anti-envelope antibody modifies its infectivity in the presence of an anti-CCR-5 antibody suggests that the antibody-mediated enhancement of infectivity of 16.4 results from modulation of the interaction between gp120 and CCR5.
FIG. 3.
Impact of a CCR5-specific antibody on antibody-mediated enhancement of 16.4 infectivity. Values represent intracellular CAT activity after infection of U87.CD4-CCR5 cells in the presence of serial dilutions of CCR5-specific antibody 2D7. Values represent the mean for two different experiments; error bars indicate standard deviations. Regression curve determinations and Student's t test were performed by using the tools of SigmaPlot 5.0 software.
Coreceptor usage of chimeric viruses.
We next tested our panel of chimeric viruses with U87.CD4-CCR5 or U87.CD4-CXCR4 cells to determine which domains of 16.1 and 16.2 were involved in the ability to use CXCR4 as a coreceptor. The entry of chimeric viruses into U87.CD4 cells expressing CCR-1, CCR-2b, CCR-3, or no coreceptor was never observed (data not shown). As shown in Fig. 4, the introduction of any domain of an R5X4 clone into the 16.4 (R5 only) background led to entry into U87.CD4-CXCR4 cells, although at different efficiencies. In particular, the introduction of the V3 loop of 16.1 or 16.2 into 16.4 (14HC or 24HC, respectively) (Fig. 4) resulted in an efficient use of CXCR4. In contrast, the introduction of the V3 loop of 16.4 into an R5X4 background (41HC and 42HC) only moderately influenced the usage of CXCR4. Independent transfer of the V1-V2 region or the N-terminal part of 16.2 into 16.4 resulted in chimeras with a very low capacity to enter CXCR4-expressing cells (24XS or 24CA, respectively) (Fig. 4), while the chimera containing both domains (42HC) efficiently used CXCR4. These results suggests that the V3 loop is the main determinant used by clones 16.1 and 16.2 to enter CXCR4-expressing cells but that interactions between other domains outside the V3 loop are also involved in CXCR4 usage.
FIG. 4.
Relative efficiency of CXCR4 usage of various chimeric viruses. (Left) Schematic representation of chimeras. See the legend to Fig. 1 for details. (Middle) Relative efficiency of entry into U87.CD4-CXCR4 cells compared to that for U87.CD4-CCR5 cells. Values represent the mean of at least three different experiments. (Right) Basal level of entry of the various chimeras, as indicated by the CAT activity observed for each construct.
Inverted correlation between antibody-mediated enhancement and CXCR4 usage.
Since the same regions of the envelope are involved in determining the effects of antibodies on the entry of HIV-1 and on coreceptor usage, we compared the different chimeric viruses with respect to these properties. For this purpose, we divided all the constructs into three categories depending on their sensitivity to neutralization or enhancement by the V3 loop-specific antibody. Chimeras were considered to be significantly neutralized when their infectivity was inhibited more than 50% by the V3 loop-specific antibody (13) and to be significantly enhanced when their infectivity was increased by a factor of 2 or more under the same conditions. The third category contained all the chimeras that were not affected or were moderately affected (between 50 and 200% the control). A clear correlation between antibody-mediated enhancement and coreceptor usage existed: chimeras that were enhanced by 391/95-D used CXCR4 less efficiently than chimeras that were neutralized or unaffected (Fig. 5). No significant difference in the relative efficiency of CXCR4 usage was observed between chimeras that were efficiently neutralized by gp120 antibodies and chimeras whose infectivity was not significantly affected.
FIG. 5.
Correlation between antibody-mediated modulation of entry and efficient CXCR4 usage. The box plot was generated by using SigmaPlot 5.0 software and the paired data of Fig. 1 and 4 for each virus or chimera tested. Error bars indicate standard deviations.
DISCUSSION
In this study, we used chimeric viruses of three related HIV-1 variants to identify the viral determinants of coreceptor usage and antibody-mediated modulation of HIV-1 infectivity. Upon interaction with CD4 and the appropriate chemokine receptor, the HIV-1 envelope glycoprotein oligomer undergoes conformational changes that create the optimal structure for the subsequent steps of the fusion process (6, 44). The recent characterization of the gp120 crystal structure (28, 41, 59) has suggested that these conformational changes are dictated by the interaction between the V1-V2 and C4 domains, forming the so-called “bridging sheet.” In this model, the exact involvement of the V3 loop was not determined, but it has been suggested to be the domain that interacts directly with the chemokine receptor (7, 52).
For HIV-2, enhancement of infectivity has been associated with a CD4-independent, CXCR4-dependent pathway of entry (39, 40). However, the mechanism is probably different for HIV-1, since antibody-mediated enhancement of 16.4 entry was CD4 dependent and entry did not occur via an alternative coreceptor. Dose-dependent inhibition of entry of 16.4 in the absence or presence of a V3 loop-specific antibody was observed for concentrations of 2D7 above 0.1 μg/ml, which have been shown to completely inhibit the binding of monomeric gp120-CD4 complexes to CCR5 (58). However, we could still detect infection with 2D7 concentrations of up to 10 μg/ml, suggesting an incomplete inhibition of binding of oligomeric gp120 to CCR5 under these conditions. In this range of 2D7 concentrations, preincubation of 16.4 with an anti-gp120 monoclonal antibody increased susceptibility to inhibition of entry by 2D7. This result suggests that, in contrast to the results of a previous study (50), the binding of a V3 loop-specific monoclonal antibody to an Envelope that is enhanced modifies the subsequent gp120-CCR5 interaction. This apparent discrepancy may be related to experimental conditions, and additional experiments are needed to verify this point.
We observed that infection in the presence of low concentrations of CCR5-specific antibody 2D7 was 30 to 40% more efficient than that in the absence of 2D7 for 16.4 preincubated with a V3 loop-specific antibody. This phenomenon was observed only for 2D7 concentrations of 0.01 to 0.1 μg/ml when the binding of monomeric gp120-CD4 complexes to CCR5-expressing cells was only partially inhibited (58). This phenomenon could be related to the enhancement of infectivity triggered by low concentrations of RANTES protein, a natural ligand for CCR5 (53). The fact that we observed this phenomenon with 16.4 only when it was preincubated with an anti-gp120 antibody suggests that it is specific for a certain conformation of the envelope protein. This mechanism could result from the induction of a conformational change or multimerization of CCR5 and/or the triggering of an intracellular signal (4, 19, 30), but the determination of its molecular basis requires further investigations. In parallel, we could also show that antibody-mediated neutralization of entry was determined by the overall structure of gp120 but was independent of coreceptor usage, in accordance with previous results (29, 33, 54).
In accordance with previous reports (5, 27, 49), our experiments showed that SI clones 16.1 and 16.2 are R5X4 viruses (using both CXCR4 and CCR5 as coreceptors), while NSI clone 16.4 is an R5 virus. Interestingly, the introduction of any domain from an SI, R5X4 clone into NSI, R5 clone 16.4 creates chimeras able to use CXCR4 as a coreceptor, although with various efficacies. That the same exchanges were previously shown to create chimeras that were all able to induce syncytia in vitro (2) substantiates previous reports of a correlation between CXCR4 usage and SI capacity (5). This ability to easily switch from an R5 to an R5X4 phenotype in our constructs can also be related to reports showing that the coreceptor usage of the variants present in infected individuals evolves over time: patients harbor only R5, macrophage-tropic variants at early stages, and progression toward AIDS is associated with the appearance of R5X4 variants (5, 9). Moreover, CCR5 appears to be the most important coreceptor for HIV-1 in vivo (32, 42). Furthermore, strong conservation of amino acid sequences in early NSI, R5, macrophage-tropic isolates can be observed (25). Finally, NSI, R5 viruses harbor a more stable structure for the V3 loop than SI viruses (24).
One model which could fit with our data and the above observations is that the stability of the envelope structure is crucial for determining phenotypic properties such as coreceptor usage or SI capacity. In this view, mutations or domain swapping in an NSI, R5 gp120 protein is likely to perturb its stability, and the resulting relaxed structure would allow CXCR4 usage and syncytium induction. Thus, both properties are unlikely to represent a gain of function for the envelope protein. This view also suggests that the susceptibility of antibody-mediated enhancement of infectivity, although a characteristic of NSI, CCR5 viruses, is not directly dependent on the stability of the envelope protein, since this phenotype is not changed by domain swapping in gp120. The property is therefore more likely to be a consequence of gp120 folding itself, where domains of the envelope protein interacting with cellular receptors are cryptic and exposed only after the binding of monoclonal antibodies.
Because HIV-1 variants susceptible to antibody-mediated enhancement would benefit from the immune response of the patient, this phenomenon may have a role during HIV-1 infection. This notion is corroborated by the observations that up to 28% of combinations of sera and primary HIV-1 isolates obtained directly from patients caused serum-dependent infectivity enhancement and that this phenomenon was specific for the viral isolate (3, 26), as confirmed in the present study. However, the overall infectivity of variants susceptible to antibody-mediated enhancement is probably poor in the absence of gp120-specific antibodies. Only in the presence of Env-specific antibodies did these isolates reach levels of infectivity comparable to those of isolates that were not enhanced (46). This scenario may explain why only a few isolates have been identified that are susceptible to antibody-mediated enhancement, because these viruses will be outgrown rapidly during in vitro culturing in the absence of gp120-specific antibodies. It is therefore relevant to further investigate viruses isolated without in vitro culturing steps for their role in the establishment of a chronic infection during seroconversion.
Acknowledgments
We thank Q. Sattentau for the gift of the CD4-specific antibodies. V3 loop-specific antibody 391/95-D was kindly provided by S. Zolla-Pazner. We are grateful to D. Littman and the NIBSC Centralised Facility for AIDS Reagents, supported by EU Programme EVA (contract BMH4 97/2515) and the UK Medical Research Council, for providing the U87.CD4 cells. We also thank A. Andeweg for stimulating discussions.
This project was supported by European Community Biomed grant PL96-2115 and Dutch AIDS Foundation grant 1022. C.G. was supported by the Biomedicine and Health Research Programme of the European Community (Marie Curie fellowship ERBBMH4CT985079).
REFERENCES
- 1.Andeweg, A. C., M. Groenink, P. Leeflang, R. E. Y. de Goede, A. D. M. E. Osterhaus, M. Tersmette, and M. L. Bosch. 1992. Genetic and functional analysis of a set of HIV-1 envelope genes obtained from biological clones with varying syncytium inducing capacities. AIDS Res. Hum. Retrovir. 8:1803-1813. [DOI] [PubMed]
- 2.Andeweg, A. C., P. Leeflang, A. D. M. E. Osterhaus, and M. L. Bosch. 1993. Both the V2 and V3 regions of the human immunodeficiency virus type 1 surface glycoprotein functionally interact with other envelope regions in syncytium formation. J. Virol. 67:3232-3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Auewarakul, P., S. Louisirirotchanakul, R. Sutthent, T. Taechowisan, C. Kanoksinsombat, and C. Wasi. 1996. Analysis of neutralizing and enhancing antibodies to human immunodeficiency virus type 1 primary isolates in plasma of individuals infected with env Genetic subtype B and E viruses in Thailand. Viral Immunol. 9:175-185. [DOI] [PubMed] [Google Scholar]
- 4.Benkirane, M., D.-Y. Jin, R. F. Chun, R. A. Koup, and K.-J. Jeang. 1997. Mechanism of transdominant inhibition of CCR-5 mediated HIV-1 infection by ccr5Δ32. J. Biol. Chem. 272:30603-30606. [DOI] [PubMed] [Google Scholar]
- 5.Bjorndal, A., H. Deng, M. Jansson, J. R. Fiore, C. Colognesi, A. Karlsson, J. Albert, G. Scarlatti, D. R. Littman, and E. M. Fenyo. 1997. Coreceptor usage of primary human immunodeficiency virus type 1 isolates varies according to biological phenotype. J. Virol. 71:7478-7487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan, D. C., and P. S. Kim. 1998. HIV entry and its inhibition. Cell 93:681-684. [DOI] [PubMed] [Google Scholar]
- 7.Cho, M. W., M. K. Lee, M. C. Carney, J. F. Berson, R. W. Doms, and M. A. Martin. 1998. Identification of determinants on a dualtropic human immunodeficiency virus type 1 envelope glycoprotein that confer usage of CXCR4. J. Virol. 72:2509-2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clapham, P. R., A. McKnight, and R. A. Weiss. 1992. Human immunodeficiency virus type 2 infection and fusion of CD4-negative human cell lines: induction and enhancement by soluble CD4. J. Virol. 66:3531-3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Connor, R. I., K. E. Sheridan, D. Ceradini, S. Choe, and N. R. Landau. 1997. Change in coreceptor use correlates with disease progression in HIV-1 infected individuals. J. Exp. Med. 185:621-628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Corbeau, P., M. Benkirane, R. Weil, C. David, S. Emiliani, D. Olive, C. Mawas, A. Serre, and C. Devaux. 1993. Ig CDR3-like region of the CD4 molecule is involved in HIV-induced syncytia formation but not in viral entry. J. Immunol. 150:290-301. [PubMed] [Google Scholar]
- 11.Dimmock, N. J. 1995. Update on the neutralization of animal viruses. Rev. Med. Virol. 5:165-179. [Google Scholar]
- 12.Doms, R. W., A. L. Edinger, and J. P. Moore. 1998. Coreceptor use by primate lentiviruses, p. III20-III35. In B. Korber, C. L. Kuiken, B. Foley, B. Hahn, F. McCutchan, J. W. Mellors, and J. Sodroski (ed.), Human retroviruses and AIDS 1998: a compilation and analysis of nucleic acid and amino acid sequences. Theoretical Biology and Biophysics Group, Los Alamos National Laboratory, Los Alamos, N.Mex.
- 13.D'Souza, M. P., G. Milman, J. A. Bradac, D. McPhee, C. V. Hanson, and R. M. Hendry. 1995. Neutralization of primary HIV-1 isolates by anti-envelope monoclonal antibodies. AIDS 9:867-874. [DOI] [PubMed] [Google Scholar]
- 14.Dubay, J. W., S. J. Roberts, B. H. Hahn, and E. Hunter. 1992. Truncation of the human immunodeficiency virus type 1 transmembrane glycoprotein cytoplasmic domain blocks virus infectivity. J. Virol. 66:6616-6625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Earl, P. L., R. W. Doms, and B. Moss. 1990. Oligomeric structure of the human immunodeficiency virus type 1 envelope glycoprotein. Proc. Natl. Acad. Sci. USA 87:648-652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Endres, M. J., P. R. Clapham, M. Marsh, M. Ahuja, J. D. Turner, A. McKnight, J. F. Thomas, B. Stoebenau-Haggarty, S. Choe, P. J. Vance, T. N. Wells, C. A. Power, S. S. Sutterwala, R. W. Doms, N. R. Landau, and J. A. Hoxie. 1996. CD4-independent infection by HIV-2 is mediated by fusin/CXCR4. Cell 87:745-756. [DOI] [PubMed] [Google Scholar]
- 17.Freed, E. O., D. J. Myers, and R. Risser. 1990. Characterization of the fusion domain of the human immunodeficiency virus type 1 envelope glycoprotein gp41. Proc. Natl. Acad. Sci. USA 87:4650-4654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Groenink, M., J. P. Moore, S. Broersen, and H. Schuitemaker. 1995. Equal levels of gp120 retention and neutralization resistance of phenotypically distinct primary human immunodeficiency virus type 1 variants upon soluble CD4 treatment. J. Virol. 69:523-527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guntermann, C., B. J. Murphy, R. Zheng, A. Qureshi, P. A. Eagles, and K. E. Nye. 1999. Human immunodeficiency virus type-1 infection requires pertussis toxin sensitive G-protein-coupled signalling and mediates cAMP downregulation. Biochem. Biophys. Res. Commun. 256:429-435. [DOI] [PubMed] [Google Scholar]
- 20.Healey, D., L. Dianda, J. P. Moore, J. S. McDougal, M. J. Moore, P. Estess, D. Buck, P. D. Kwong, P. C. L. Beverley, and Q. J. Sattentau. 1990. Novel anti-CD4 monoclonal antibodies separate human immunodeficiency virus infection and fusion of CD4+ cells from virus binding. J. Exp. Med. 172:1233-1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Helseth, E., M. Kowalski, D. Gabudza, U. Olshevsky, W. Haseltine, and J. Sodroski. 1990. Rapid complementation assays measuring replicative potential of human immunodeficiency virus type 1 envelope glycoprotein mutants. J. Virol. 64:2416-2420. [DOI] [PMC free article] [PubMed]
- 22.Helseth, E., U. Olshevsky, C. Furman, and J. Sodroski. 1991. Human immunodeficiency virus type 1 gp120 envelope glycoprotein regions important for association with the gp41 transmembrane glycoprotein. J. Virol. 65:2119-2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hill, C. M., H. Deng, D. Unutmaz, V. N. Kewalramani, L. Bastiani, M. K. Gorny, S. Zolla-Pazner, and D. R. Littman. 1997. Envelope glycoproteins from human immunodeficiency virus types 1 and 2 and simian immunodeficiency virus can use human CCR5 as a coreceptor for viral entry and make direct CD4-dependent interactions with this chemokine receptor. J. Virol. 71:6296-6304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Innocenti-Francillard, P., K. Brengel, C. Guillon, F. Mallet, P. Morand, R. Gruters, and J. M. Seigneurin. 1994. Blood monocytes infected in vivo by HIV-1 variants with a syncytium-inducing phenotype. AIDS Res. Hum. Retrovir. 10:683-690. [DOI] [PubMed] [Google Scholar]
- 25.Korber, B., C. L. Kuiken, B. Foley, B. Hahn, F. McCutchan, J. W. Mellors, and J. Sodroski (ed.). 1998. Human retroviruses and AIDS 1998: a Compilation and analysis of nucleic acid and amino acid sequences. Theoretical Biology and Biophysics Group, Los Alamos National Laboratory, Los Alamos, N.Mex.
- 26.Kostrikis, L. G., Y. Cao, H. Ngai, J. P. Moore, and D. D. Ho. 1996. Quantitative analysis of serum neutralization of human immunodeficiency virus type 1 from subtypes A, B, C, D, E, F, and I: lack of direct correlation between neutralization serotypes and genetic subtypes and evidence for prevalent serum-dependent infectivity enhancement. J. Virol. 70:445-458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kozak, S. L., E. J. Platt, N. Madani, F. E. Ferro, Jr., K. Peden, and D. Kabat. 1997. CD4, CXCR-4, and CCR-5 dependencies for infections by primary patient and laboratory-adapted isolates of human immunodeficiency virus type 1. J. Virol. 71:873-882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kwong, P. D., R. Wyatt, J. Robinson, R. W. Sweet, J. Sodroski, and W. A. Hendrickson. 1998. Structure of an HIV gp120 envelope glycoprotein in complex with the CD4 receptor and a neutralizing human antibody. Nature 393:648-659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.LaCasse, R. A., K. E. Follis, T. Moudgil, M. Trahey, J. M. Binley, V. Planelles, S. Zolla-Pazner, and J. H. Nunberg. 1998. Coreceptor utilization by human immunodeficiency virus type 1 is not a primary determinant of neutralization sensitivity. J. Virol. 72:2491-2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee, B., M. Sharron, C. Blanpain, B. J. Doranz, J. Vakili, P. Setoh, E. Berg, G. Liu, H. R. Guy, S. R. Durell, M. Parmentier, C. N. Chang, K. Price, M. Tsang, and R. W. Doms. 1999. Epitope mapping of CCR5 reveals multiple conformationnal states and distinct but overlapping structures involved in chemokine and co-receptor function. J. Biol. Chem. 274:9617-9626. [DOI] [PubMed] [Google Scholar]
- 31.McKeating, J. A., A. McKnight, and J. P. Moore. 1991. Differential loss of envelope glycoprotein gp120 from virions of human immunodeficiency virus type 1 isolates: effects on infectivity and neutralization. J. Virol. 65:852-860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Michael, N. L., G. Chang, L. G. Louie, J. R. Mascola, D. Dondero, D. L. Birx, and H. W. Sheppard. 1997. The role of viral phenotype and CCR-5 gene defects in HIV-1 transmission and disease progression. Nat. Med. 3:338-340. [DOI] [PubMed] [Google Scholar]
- 33.Montefiori, D. C., R. G. Collman, T. R. Fouts, J. Y. Zhou, M. Bilska, J. A. Hoxie, J. P. Moore, and D. P. Bolognesi. 1998. Evidence that antibody-mediated neutralization of human immunodeficiency virus type 1 by sera from infected individuals is independent of coreceptor usage. J. Virol. 72:1886-1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nara, P. L., L. Smit, N. Dunlop, W. Hatch, M. Merges, D. Waters, J. Kelliher, R. C. Gallo, P. J. Fischinger, and J. Goudsmit. 1990. Emergence of viruses resistant to neutralization by V3-specific antibodies in experimental human immunodeficiency virus type 1 IIIB infection of chimpanzees. J. Virol. 64:3779-3791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Park, E. J., L. K. Vujcic, R. Anand, T. S. Theodore, and G. V. Quinnan, Jr. 1998. Mutations in both gp120 and gp41 are responsible for the broad neutralization resistance of variant human immunodeficiency virus type 1 MN to antibodies directed at V3 and non-V3 epitopes. J. Virol. 72:7099-7107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pinter, A., W. J. Honnen, S. A. Tilley, C. Bona, H. Zaghouani, M. K. Gorny, and S. Zolla-Pazner. 1989. Oligomeric structure of gp41, the transmembrane protein of human immunodeficiency virus type 1. J. Virol. 63:2674-2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Poignard, P., P. J. Klasse, and Q. J. Sattentau. 1996. Antibody neutralization of HIV-1. Immunol. Today 17:239-246. [DOI] [PubMed] [Google Scholar]
- 38.Poignard, P., R. Sabbe, G. R. Picchio, M. Wang, R. J. Gulizia, H. Katinger, P. W. Parren, D. E. Mosier, and D. R. Burton. 1999. Neutralizing antibodies have limited effects on the control of established HIV-1 infection in vivo. Immunity 10:431-438. [DOI] [PubMed] [Google Scholar]
- 39.Potempa, S., L. Picard, J. D. Reeves, D. Wilkinson, R. A. Weiss, and S. J. Talbot. 1997. CD4-independent infection by human immunodeficiency virus type 2 strain ROD/B: the role of the N-terminal domain of CXCR-4 in fusion and entry. J. Virol. 71:4419-4424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reeves, J. D., A. McKnight, S. Potempa, G. Simmons, P. W. Gray, C. A. Power, T. Wells, R. A. Weiss, and S. J. Talbot. 1997. CD4-independent infection by HIV-2 (ROD/B): use of the 7-transmembrane receptors CXCR-4, CCR-3, and V28 for entry. Virology 231:130-134. [DOI] [PubMed] [Google Scholar]
- 41.Rizzuto, C. D., R. Wyatt, N. Hernandez-Ramos, Y. Sun, P. D. Kwong, W. A. Hendrickson, and J. Sodroski. 1998. A conserved HIV gp120 glycoprotein structure involved in chemokine receptor binding. Science 280:1949-1953. [DOI] [PubMed] [Google Scholar]
- 42.Samson, M., F. Libert, B. J. Doranz, J. Rucker, C. Liesnard, C. M. Farber, S. Saragosti, C. Lapoumeroulie, J. Cognaux, C. Forceille, G. Muyldermans, C. Verhofstede, G. Burtonboy, M. Georges, T. Imai, S. Rana, Y. Yi, R. J. Smyth, R. G. Collman, R. W. Doms, G. Vassart, and M. Parmentier. 1996. Resistance to HIV-1 infection in caucasian individuals bearing mutant alleles of the CCR-5 chemokine receptor gene. Nature 382:722-725. [DOI] [PubMed] [Google Scholar]
- 43.Sattentau, Q. J., J. Arthos, K. Deen, N. Hanna, D. Healey, P. C. L. Beverley, R. Sweet, and A. Truneh. 1989. Structural analysis of the human immunodeficiency virus-binding domain of CD4. Epitope mapping with site-directed mutants and anti-idiotypes. J. Exp. Med. 170:1319-1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sattentau, Q. J., and J. P. Moore. 1991. Conformational changes induced in the human immunodeficiency virus envelope glycoprotein by soluble CD4 binding. J. Exp. Med. 174:407-415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schutten, M., A. C. Andeweg, M. L. Bosch, and A. D. Osterhaus. 1995. Enhancement of infectivity of a non-syncytium inducing HIV-1 by sCD4 and by human antibodies that neutralize syncytium inducing HIV-1. Scand. J. Immunol. 41:18-22. [DOI] [PubMed] [Google Scholar]
- 46.Schutten, M., A. C. Andeweg, G. F. Rimmelzwaan, and A. D. Osterhaus. 1997. Modulation of primary human immunodeficiency virus type 1 envelope glycoprotein-mediated entry by human antibodies. J. Gen. Virol. 78:999-1006. [DOI] [PubMed] [Google Scholar]
- 47.Schutten, M., A. McKnight, R. C. Huisman, M. Thali, J. A. McKeating, J. Sodroski, J. Goudsmit, and A. D. M. E. Osterhaus. 1993. Further characterization of an antigenic site of HIV-1 gp120 recognized by virus neutralizing human monoclonal antibodies. AIDS 7:919-923. [DOI] [PubMed] [Google Scholar]
- 48.Seed, B., and J. Sheen. 1988. A simple phase extraction assay for chloramphenicol acetyltransferase activity. Gene 67:271-277. [DOI] [PubMed] [Google Scholar]
- 49.Simmons, G., D. Wilkinson, J. D. Reeves, M. T. Dittmar, S. Beddows, J. Weber, G. Carnegie, U. Desselberger, P. W. Gray, R. A. Weiss, and P. R. Clapham. 1996. Primary, syncytium-inducing human immunodeficiency virus type 1 isolates are dual-tropic and most can use either Lestr or CCR5 as coreceptors for virus entry. J. Virol. 70:8355-8360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sullivan, N., Y. Sun, J. Binley, J. Lee, C. F. Barbas III, P. W. Parren, D. R. Burton, and J. Sodroski. 1998. Determinants of human immunodeficiency virus type 1 envelope glycoprotein activation by soluble CD4 and monoclonal antibodies. J. Virol. 72:6332-6338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sullivan, N., Y. Sun, J. Li, W. Hofmann, and J. Sodroski. 1995. Replicative function and neutralization sensitivity of envelope glycoproteins from primary and T-cell-line-passaged human immunodeficiency virus type 1 isolates. J. Virol. 69:4413-4422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Trkola, A., T. Dragic, J. Arthos, J. M. Binley, W. C. Olson, G. P. Allaway, C. Cheng-Mayer, J. Robinson, P. J. Maddon, and J. P. Moore. 1996. CD4-dependent, antibody-sensitive interactions between HIV-1 and its co-receptor CCR-5. Nature 384:184-187. [DOI] [PubMed] [Google Scholar]
- 53.Trkola, A., C. Gordon, J. Matthews, E. Maxwell, T. Ketas, L. Czaplewski, A. E. Proudfoot, and J. P. Moore. 1999. The CC-chemokine RANTES increases the attachment of human immunodeficiency virus type 1 to target cells via glycosaminoglycans and also activates a signal transduction pathway that enhances viral infectivity. J. Virol. 73:6370-6379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Trkola, A., T. Ketas, V. N. Kewalramani, F. Endorf, J. M. Binley, H. Katinger, J. Robinson, D. R. Littman, and J. P. Moore. 1998. Neutralization sensitivity of human immunodeficiency virus type 1 primary isolates to antibodies and CD4-based reagents is independent of coreceptor usage. J. Virol. 72:1876-1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ugolini, S., I. Mondor, P. W. Parren, D. R. Burton, S. A. Tilley, P. J. Klasse, and Q. J. Sattentau. 1997. Inhibition of virus attachment to CD4+ target cells is a major mechanism of T-cell line-adapted HIV-1 neutralization. J. Exp. Med. 186:1287-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wilks, D., L. Walker, J. O'Brien, J. Habeshaw, and A. Dalgleish. 1990. Differences in affinity of anti-CD4 monoclonal antibodies predict their effects on syncytium induction by human immunodeficiency virus. Immunology 71:10-15. [PMC free article] [PubMed] [Google Scholar]
- 57.Wu, L., N. P. Gerard, R. Wyatt, H. Choe, C. Parolin, N. Ruffing, A. Borsetti, A. A. Cardoso, E. Desjardin, W. Newman, C. Gerard, and J. Sodroski. 1996. CD4-induced interaction of primary HIV-1 gp120 glycoproteins with the chemokine receptor CCR-5. Nature 384:179-183. [DOI] [PubMed] [Google Scholar]
- 58.Wu, L., G. LaRosa, N. Kassam, C. J. Gordon, H. Heath, N. Ruffing, H. Chen, J. Humblias, M. Samson, M. Parmentier, J. P. Moore, and C. R. Mackay. 1997. Interaction of chemokine receptor CCR5 with its ligands: multiple domains for HIV-1 gp120 binding and a single domain for chemokine binding. J. Exp. Med. 186:1373-1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wyatt, R., P. D. Kwong, E. Desjardins, R. W. Sweet, J. Robinson, W. A. Hendrickson, and J. G. Sodroski. 1998. The antigenic structure of the HIV gp120 envelope glycoprotein. Nature 393:705-711. [DOI] [PubMed] [Google Scholar]