Abstract
To investigate the basis for envelope (Env) determinants influencing simian immunodeficiency virus (SIV) tropism, we studied a number of Envs that are closely related to that of SIVmac239, a pathogenic, T-tropic virus that is neutralization resistant. The Envs from macrophage-tropic (M-tropic) virus strains SIVmac316, 1A11, 17E-Fr, and 1100 facilitated infection of CCR5-positive, CD4-negative cells. In contrast, the SIVmac239 Env was strictly dependent upon the presence of CD4 for membrane fusion. We also found that the Envs from M-tropic virus strains, which are less pathogenic in vivo, were very sensitive to antibody-mediated neutralization. Antibodies to the V3-loop, as well as antibodies that block SIV gp120 binding to CCR5, efficiently neutralized CD4-independent, M-tropic Envs but not the 239 Env. However, triggering the 239 Env with soluble CD4, presumably resulting in exposure of the CCR5 binding site, made it as neutralization sensitive as the M-tropic Envs. In addition, mutations of N-linked glycosylation sites in the V1/V2 region, previously shown to enhance antigenicity and immunogenicity, made the 239 Env partially CD4 independent. These findings indicate that Env-based determinants of M tropism of these strains are generally associated with decreased dependence on CD4 for entry into cells. Furthermore, CD4 independence and M tropism are also associated with neutralization sensitivity and reduced pathogenicity, suggesting that the humoral immune response may exert strong selective pressure against CD4-independent M-tropic SIVmac strains. Finally, genetic modification of viral Envs to enhance CD4 independence may also result in improved humoral immune responses.
The entry of primate immunodeficiency viruses into target cells is achieved through activities of the virus-encoded envelope (Env) glycoprotein, a trimeric structure composed of three gp120 surface and three gp41 transmembrane subunits (6, 25, 37, 56, 59, 60). Binding of the gp120 subunit to CD4 induces changes in Env that enable it to efficiently interact with a coreceptor (35, 54, 57). The major human immunodeficiency virus type 1 (HIV-1) coreceptors are the CCR5 and CXCR4 chemokine receptors, while CCR5 is the primary coreceptor for simian immunodeficiency virus SIVmac (16, 22, 29). Coreceptor binding then enables Env to undergo the final conformational changes needed to elicit fusion between the viral and cellular membranes (7, 56, 58).
Several CD4-independent HIV-1 strains have been obtained by passaging virus on CD4-negative, coreceptor-positive cells in vitro (18, 27, 32, 34). The resulting viruses can use either CCR5 or CXCR4 to infect cells in the absence of CD4, although infection is more efficient in its presence. In the cases examined thus far, relatively few changes are needed to render HIV-1 Env proteins CD4 independent, although a common theme appears to be enhanced exposure of a highly conserved region in gp120 that is important for CCR5 binding (17, 27, 31, 49). This binding site, located largely in the bridging sheet region of gp120, is normally induced or exposed as a consequence of CD4 binding (33). Thus, CD4-independent Env proteins may exist in a partially triggered state in which this conserved region is constitutively exposed and therefore available to interact directly with coreceptors (27). CD4-independent viruses that use CXCR4 would be expected to exhibit a much broader tropism in vivo since CXCR4 is expressed on numerous CD4-negative cell types. Despite this, naturally occurring CD4-independent HIV-1 isolates have not yet been identified, perhaps because CD4 independence of HIV is associated with markedly enhanced sensitivity to antibody mediated neutralization (24, 27, 31).
Many SIVmac strains exhibit at least some degree of CD4 independence, being able to infect CD4-negative, CCR5-positive cell types (21, 23). An exception to this is SIVmac239, a consistently pathogenic, neutralization-resistant, T-cell-tropic(T-tropic) SIV strain that is dependent on a threshold level of CD4 for entry into CCR5-expressing cells (4, 5, 23, 28, 44). In this study, we show that Envs from four independently generated macrophage-tropic (M-tropic) viruses that are close relatives of SIVmac239 exhibit variable degrees of CD4 independence on both human and rhesus CCR5 (RhCCR5), thus extending our previous studies (21, 23). In addition, these Envs exhibited enhanced susceptibility to neutralization by sera from SIVmac239-infected animals and to monoclonal antibodies (MAbs) directed against the V3 loop and CCR5 binding site. Interestingly, the M-tropic virus strains whose Envs we have studied here are less pathogenic than SIVmac239 in vivo: SIVmac1A11 infection of rhesus macaques results in a transient viremia that fails to persist (38), while 17E-Fr infection of macaques leads to disseminated infection of tissues but does not result in immunosuppression (39). In contrast, SIVmac239 reproducibly induces T-cell decline and leads to death of the animal due to immunosuppression by approximately 1 year postinfection (28). Taken together, our results show a relationship between CD4 independence, macrophage tropism, neutralization sensitivity of the viral Env protein, and reduced pathogenicity in the SIVmac system. Finally, we found that the loss of only two N-linked glycosylation sites in the V1/V2 region of SIVmac239 Env resulted in gain of CD4-independent function. Partial deglycosylation of the V1/V2 region of SIVmac239 Env has also been shown to enhance antigenicity and immunogenicity, eliciting antibodies that can neutralize the parental SIVmac239 (48), raising the possibility that the structural changes in Env associated with CD4 independence may result in either enhanced immunogenicity or exposure of neutralizing determinants in Env.
MATERIALS AND METHODS
Plasmids.
The SIVmac239, 316, g4,5 g4,6, and g5,6 Env sequences were PCR amplified with primers 239f5 (5′-CGGGATCCGCCGCCACCATGGGATGTCTTGGGAATCAGC-3′) and 239f3 (5′-CCGAATTCCTCACAAGAGAGTGAGCTCAAGCCC-3′) and cloned into the pcDNA3 expression vector by using the BamHI and EcoRI sites. The 1A11 and 1100 expression plasmids have been described previously (20, 55). All gp160 expression clones were sequenced entirely prior to fusion assay analysis.
Selection of stable cell lines.
Stable cell lines expressing either the RhCCR5 protein with or without RhCD4 were generated for this study. Human 293 cells were calcium phosphate transfected with pcDNA3.1+Zeo carrying the RhCD4 gene and/or pcDNA3 carrying the RhCCR5 gene. At 2 days posttransfection, selective medium was added containing 300 μg of Zeocin (Invitrogen)/ml and/or 600 μg of Geneticin (Life Technologies)/ml. Cells surviving selection were pooled and used for further studies. Inducible expression of RhCCR5 was achieved by using the T-Rex system (Invitrogen). Stable T-Rex cells expressing RhCCR5 were generated by transfection of T-Rex 293 cells with the pcDNA4 RhCCR5 plasmid. At 48 h posttransfection, cells were selected with 5 μg of Blasticidin/ml and 200 μg of Zeocin (both from Invitrogen)/ml. RhCCR5 expression was induced with the designated concentration of doxycycline 24 h prior to use in experiments.
Cell-cell fusion assay.
This assay has been described previously (50). Briefly, effector QT6 cells were infected with vaccinia virus expressing T7 polymerase (vTF1.1 [1]) and then transfected with a pcDNA3 plasmid containing the designated Env gene via the calcium phosphate transfection method. Target QT6 quail cells were transfected with plasmids expressing CD4, coreceptor, and T7 luciferase. At 24 h posttransfection, effector cells were added to the target cells and fusion quantitated at 8 h postmixing by lysing the cells with 0.5% Triton X-100. An aliquot of lysate was then mixed with an equal volume of luciferase assay reagent (Promega) and luminescence read in a luminometer (Wallac).
Pseudotype infection assays.
Pseudotyped virus particles were produced by transiently transfecting 293T cells with the HIV pNL-Luc-R−-E− backbone (8, 13) and Env expression plasmids. HIV-1 cores have previously been used for pseudotyping SIV Envs, and the results obtained with them agree with data obtained by other assays that examine the functionality of the Env (9, 19-21, 23, 46, 53). At 4 h posttransfection, fresh medium was added and the cells were incubated overnight at 37°C. At ca. 30 h posttransfection, 10 mM sodium butyrate was added to boost virus production. Virus was harvested ca. 14 h afterward, immediately mixed with serial dilutions of the designated sera in a final volume of 250 μl for ca. 30 min at 37°C, and then spinoculated onto target cells stably expressing RhCCR5 (in 48-well plates) by centrifugation at room temperature for 2 h at ca. 1,200 × g (45). All infections were performed in the presence of 8 μg of DEAE-dextran/ml. Target cells were transfected with CD4 or green fluorescent protein (GFP) expression plasmids 48 h prior to infection. CCR5 expression was induced with doxycycline 24 h prior to infection. At 48 h postinfection the cell monolayers were lysed in 0.5% Triton X-100, and an aliquot was mixed with an equal volume of luciferase substrate and analyzed in a luminometer.
FACS analysis of doxycycline-induced cells.
At 24 h postinduction, cells were lifted from the plate with phosphate-buffered saline (PBS) and washed with fluorescence-activated cell sorting (FACS) staining buffer (3% calf serum, 0.02% azide, in PBS). MAb 182 anti-CCR5 (R&D Systems) was added at a concentration of 10 μg/ml for 1 h (36). Cells were briefly washed in FACS staining buffer, and then secondary antibody (phycoerythrin-conjugated anti-mouse antibody; Vector Labs) was added for 1 h. Washing was repeated, and the cells were resuspended in FACS staining buffer and fixed with 2% paraformaldehyde. Cell staining was analyzed on a FACScanner (Becton Dickinson) with CellQuest software.
RESULTS
Relative CD4 independence of SIVmac239-related Envs.
SIVmac239 is a pathogenic, T-tropic virus that requires CD4 and CCR5 to infect cells (14, 23, 44). A number of Envs that are closely related to SIVmac239 and yet differ in characteristics such as tropism and pathogenesis have been described (Table 1). We have previously shown that Env proteins derived from SIVmac316, 1A11, and 17E-Fr can mediate infection of cells expressing human CCR5 independently of CD4 (21, 23, 42, 51) and that 1A11 and 17E-Fr can also utilize RhCCR5 for CD4-independent infection. To determine whether CD4 independence is typically associated with alterations in tropism and pathogenesis and to directly compare the extent of CD4 independence exhibited by each Env on RhCCR5, we examined the ability of Env proteins derived from the macrophage-tropic SIVmac316, 1A11, and 17E-Fr, as well as a fourth Env protein, derived from the M-tropic strain 1100 (55) to mediate CD4-independent fusion on cells expressing human or rhesus CCR5. The derivation of each of these Envs is described here briefly. The pathogenic, biologically cloned virus 251 was generated by culture of frozen splenocytes from Macaca mulatta 251 (Mm251) with Hut78 cells (14). The nonpathogenic molecular clones 251 and 1A11 (M tropic) were generated from subsequent passages of this culture (14, 38). Tissues from Mm251 were also inoculated into additional rhesus macaques to generate the pathogenic, T-tropic, neutralization-resistant SIVmac239 molecular clone (14). The SIVmac316 Env (M tropic) was cloned from the lung tissue at the terminal stage of disease of animal Mm316 that had been infected with the SIVmac239 molecular clone (43). Passage of SIVmac239 or uncloned SIVmac251 through brain tissue or isolated brain microglia generated the M-tropic 17E-Fr and 1100 viruses which exhibit various degrees of neurotropism and neuroinvasiveness (26, 55).
TABLE.
1. Envs examined in this study
| Env | Tropism | Disease description and other relevant information |
|---|---|---|
| 239 | T | CD4 decline; death due to opportunistic infections occurred 1 yr postinfection |
| 316 | M | Disseminated viral infection in tissues; neutralizing antibody; death occurred several years postinfection |
| 1A11 | M | Transient viremia that fails to persist; neutralizing antibody; 1A11 was used as an attenuated viral vaccine |
| 17E-Fr | M | Disseminated viral infection in tissues; neutralizing antibody; 17E-Fr was used as an attenuated viral vaccine |
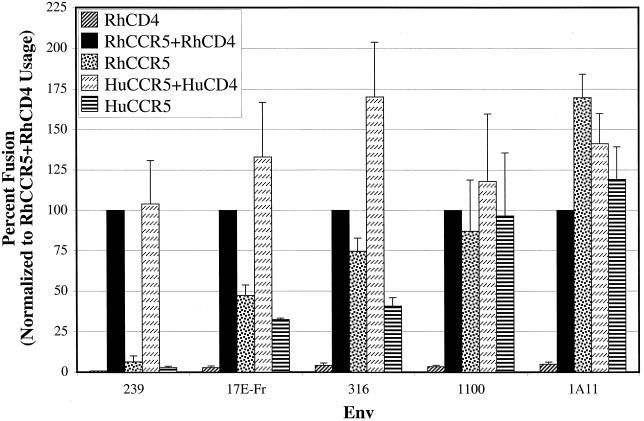
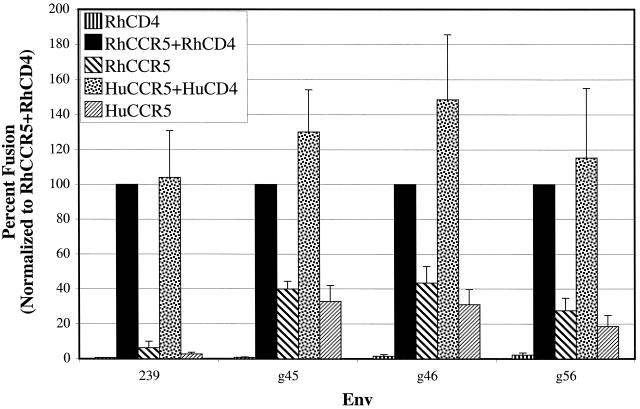
The Envs were analyzed for their relative ability to support fusion with target cells expressing either rhesus or human CD4 and/or CCR5 (Fig. 1). Each Env supported fusion of cells expressing human or rhesus CCR5 and CD4. When CCR5 was expressed alone, the 239 Env did not mediate fusion efficiently as previously demonstrated (23). In contrast, the M-tropic Env proteins 17E-Fr, 316, 1100, and 1A11 elicited fusion in the absence of CD4 (23). The 17E-Fr and 316 Env proteins elicited fusion more efficiently in the presence of CD4 than in its absence, while Envs 1100 and 1A11 caused cell-cell fusion equally well in the presence or absence of CD4. We observed here that CD4-independent fusion of the 316 and 1100 Envs was more efficient with RhCCR5 than human CCR5, a finding consistent with earlier studies of the 17E-Fr and 1A11 Envs which showed that a single amino acid difference at position 13 in the CCR5 N-terminal domain impacts the efficiency of SIV gp120 binding to CCR5, as well as CD4-independent fusion and infection (21, 41). In conclusion, four independently derived M-tropic relatives of the SIVmac239 Env protein exhibited considerable degrees of CD4 independence in stark contrast to the CD4-dependent SIVmac239.
FIG. 1.
CD4-independent CCR5 use by M-tropic SIVmac Envs. A fusion assay was performed by using quail QT6 target cells transiently expressing rhesus or human CD4 and CCR5 as indicated. These cells were also transfected with a plasmid encoding luciferase under control of the T7 promoter. At 24 h posttransfection, effector cells expressing the designated Env and T7 polymerase were added to the target cells. Approximately 8 h after mixing, cells were lysed and assayed for luciferase activity. Data from at least three experiments were averaged, and the standard error of the mean was calculated. All values were normalized to levels of fusion detected when effector cells were mixed with target cells transfected with RhCCR5 and RhCD4.
CD4 independence is supported at low levels of coreceptor expression.
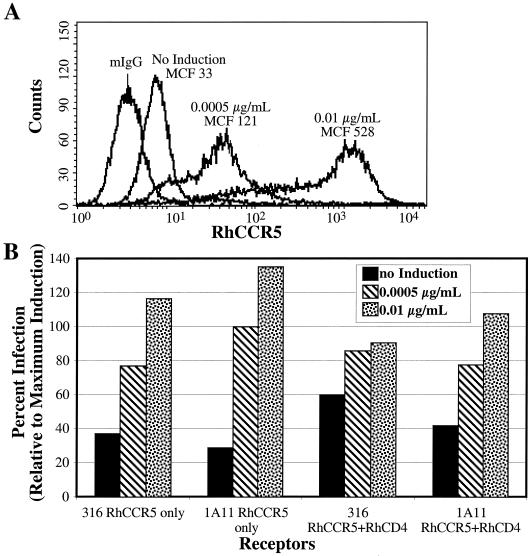
The cell-cell fusion assay used to screen the M-tropic SIVmac Envs for CD4 independence typically results in the overexpression of both Env and coreceptor in order to maximize assay sensitivity (50). To determine whether the CD4-independent fusion depicted in Fig. 1 was an artifact of this overexpression, we generated virus pseudotypes bearing either the SIVmac239 Env protein or one of the M-tropic Envs. These viruses were used to infect a stably transfected human 293 cell line in which RhCCR5 expression can be induced to various degrees with increasing concentrations of doxycycline, either in the presence or absence (Fig. 2B) of RhCD4. RhCCR5 expression was monitored in parallel by FACS analysis (Fig. 2A). As shown in Fig. 2B, each of the Envs examined was able to support infection of CD4-positive cells even at very low levels of coreceptor expression. The same pattern was observed upon infection of CD4-negative cells (Fig. 2B). Thus, the M-tropic SIVmac Env proteins studied here mediated CD4-independent cell-cell fusion and virus infection even at low levels of CCR5 expression.
FIG. 2.
RhCCR5 receptor expression correlates with ability to support infection. (A) RhCCR5 stable T-Rex cells were transfected with either GFP or CD4. At 24 h posttransfection, cells were induced with the designated concentration of doxycycline overnight, fixed, stained with the anti-CCR5 MAb182 (R&D Systems), and analyzed by FACS to determine the mean channel fluorescence (MCF). (B) Doxycycline-induced cells transfected with a CD4 expression plasmid where indicated were infected with the designated pseudotype virus by spinoculation in the presence of dextran. At 48 h postinfection, cells were lysed and an aliquot of the lysate was analyzed for luciferase activity.
CD4-independent infection is facilitated more efficiently by CCR5 than alternate coreceptors.
HIV-1 tropism can be governed by differential use of CCR5 and CXCR4 (3). In contrast, both M- and T-tropic SIVmac strains use CCR5, whereas CXCR4 is rarely used (9, 20, 22, 40). Some M- and T-tropic SIV Envs have been shown to use the GPR1, GPR15, STRL33, and ChemR23 receptors when CD4 is present. However, only the M-tropic 316 Env has been shown to use both CCR5 and GPR15 for entry of CD4-negative cells (51). It is possible that CD4-independent use of alternate coreceptors may play a role in expanded SIVmac cell tropism. Recently, rhesus macaque macrophages have been shown to express low levels of CD4 (2, 44). This, coupled with the relative CD4 independence of the four M-tropic SIVmac Envs studied here suggests that M tropism and CD4 independence are linked.
To determine whether M tropism might be correlated with the use of alternative coreceptors either in the presence or absence of CD4, we examined the ability of the M-tropic Envs to elicit fusion with cells expressing the human or rhesus receptors GPR1, GPR15, and STRL33 either with or without CD4. Only human STRL33 and GPR15, and RhGPR15, supported CD4-independent fusion (data not shown). As previously described, rhesus STRL33 did not support fusion with any of the tested Envs due to the presence of an arginine at position 31 in the STRL33 N terminus rather than the serine present at this position in human STRL33 (46). Thus, while human STRL33 can be used CD4 independently by the 316 and 1A11 Envs, the rhesus homolog does not support virus infection (46).
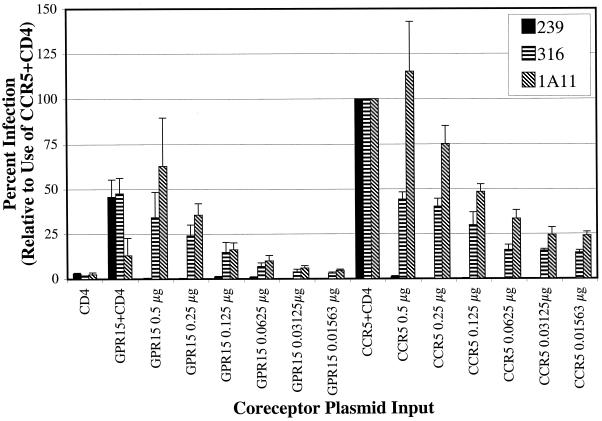
We further examined the ability of the M-tropic SIVmac Envs to utilize RhGPR15 in a CD4-independent manner. We have previously shown that use of alternative receptors such as STRL33 by SIVmac strains sometimes occurs only when the receptor is expressed at artificially high levels (53). We therefore tested the ability of these Envs to utilize limiting levels of RhGPR15 on CD4-negative cells to support virus infection. As shown in Fig. 3, CD4-independent infection of RhGPR15-expressing cells was less efficient than CD4-independent use of CCR5. Infection of RhGPR15-expressing cells declined rapidly as the level of coreceptor decreased, while infection of CCR5-expressing cells declined more slowly. Thus, while RhGPR15 could be used CD4 independently, this was only observed at high levels of receptor expression. In contrast, RhCCR5 was used more efficiently in the absence of CD4 at low levels of receptor expression. Therefore, it is more likely that CCR5 is the relevant receptor utilized for entry of macrophages that express limiting levels of CD4.
FIG. 3.
Analysis of CD4-independent infection of RhGPR15- and RhCCR5-expressing cells. Target cells were transfected with the designated amount of coreceptor plasmid. GFP plasmid was added to maintain a constant level of total input DNA. At 24 h posttransfection, target cells were infected with the designated virus pseudotype by spinoculation in the presence of dextran. At 48 h postinfection the cells were lysed with PBS-Triton X-100, and an aliquot of lysate was analyzed for luciferase expression.
Sensitivity of CD4-independent Envs to neutralization by immune serum.
The CD4-independent HIV-1 Env proteins examined to date are notable for being far more sensitive to antibody-mediated neutralization than their closely related CD4-dependent counterparts (24, 31). In addition, SIVmac316 is more sensitive to neutralization than SIVmac239 (42). To determine whether neutralization sensitivity, CD4 independence, pathogenicity, and M tropism are associated in the SIV system, we examined the ability of sera from SIVmac239-infected rhesus macaques to neutralize pseudotypes bearing the parental 239 Env or any of its M-tropic, CD4-independent relatives. Assays were performed with 293T cells expressing both RhCD4 and RhCCR5 or with 293T cells expressing RhCCR5 alone. As shown in Fig. 4A, virus pseudotypes bearing the CD4-dependent 239 Env were neutralization resistant, a finding consistent with previous reports (4). In contrast, the M-tropic, CD4-independent Envs 17E-Fr, 316, 1A11, and 1100 were easily neutralized by both of the sera examined. We observed that the least CD4-independent Env, 17E-Fr, was less sensitive to neutralization than the most CD4-independent Envs (1100 and 1A11), demonstrating a correlation between level of CD4 independence and neutralization sensitivity. Furthermore, neutralization assays performed with target cells expressing only RhCCR5 demonstrated that CD4-independent infection was more easily neutralized than CD4-assisted entry (Fig. 4B). These experiments demonstrated that antibodies are generated in animals infected with SIVmac239 that, while being unable to neutralize SIVmac239, neutralize closely related CD4-independent, M-tropic viruses. Furthermore, entry is more easily neutralized in the absence of CD4 than in its presence.
FIG. 4.
Neutralization of SIVmac239-related Envs. Virus pseudotypes were generated by cotransfection of 293T cells with the pNL-Luc-R−-E− backbone and the designated Env. At 24 h posttransfection the cells were treated with 10 mM sodium butyrate, and virus was harvested at 48 h posttransfection. Supernatant containing pseudotyped virus was incubated with the designated dilution of SIVmac239-infected rhesus macaque serum for 1 h. Panels on the left are neutralizations performed with serum from Mm258-95, and panels on the right are neutralizations performed with serum from Mm259-95, each obtained at 24 weeks postinfection. Virus and serum were then added to target cells stably transfected with RhCCR5 and RhCD4 (A) or RhCCR5 and GFP (B). Cells were lysed 48 h posttransfection, and the lysate was analyzed for luciferase activity.
sCD4 triggering of SIVmac239 Env renders it neutralization sensitive.
Our previous work with a CD4-independent HIV-1 strain showed that the Env protein from this virus exists in a partially triggered conformation in which the conserved coreceptor binding site exhibits enhanced exposure (27). As a result, MAbs that bind to epitopes within the highly conserved bridging sheet of gp120 efficiently neutralize this CD4-independent Env (24, 27). Binding soluble CD4 (sCD4) to the closely related, CD4-dependent parental Env enabled CD4-induced antibodies to bind to this gp120 protein equally well (27). We reasoned that binding sCD4 to the 239 Env might trigger conformational changes that make it resemble CD4-independent Env proteins, thus making it sensitive to antibody-mediated neutralization. Previous studies have shown that addition of sCD4 to SIVmac239 enables it to fuse with CD4-negative, CCR5-positive cells (51). Therefore, we performed cell-cell fusion assays by using either the CD4-independent SIVmac316 or 1A11 Envs or the SIVmac239 Env protein in the presence or absence of sCD4 to expose the CCR5 binding site. The Env-expressing cells were then incubated with normal macaque sera or sera from one of eight different SIVmac239-infected animals at a 1:50 dilution for 1 h prior to addition of target cells. The extent of cell-cell fusion was assayed 8 h later. When target cells expressed RhCD4 and RhCCR5, the 239 Env protein was neutralization resistant, while the SIVmac316 and 1A11 Envs were more easily neutralized by most of the serum samples (Fig. 5). When target cells expressed CCR5 alone, the 316 and 1A11 Env proteins elicited membrane fusion, while SIVmac239 Env elicited fusion only when incubated with sCD4 as expected. Under these conditions, all three Env proteins were easily neutralized by all eight of the SIVmac239-infected macaque sera (Fig. 5). These results indicate that the M-tropic, CD4-independent Env proteins studied here are more sensitive to antibody-mediated neutralization in the absence of CD4 than in its presence. In addition, the neutralization-resistant, CD4-dependent 239 Env protein was made as neutralization sensitive as the closely related M-tropic Envs upon incubation with sCD4, indicating that conformational changes induced by CD4 binding, likely including exposure of the CCR5 binding site, lead to enhanced neutralization sensitivity.
FIG. 5.
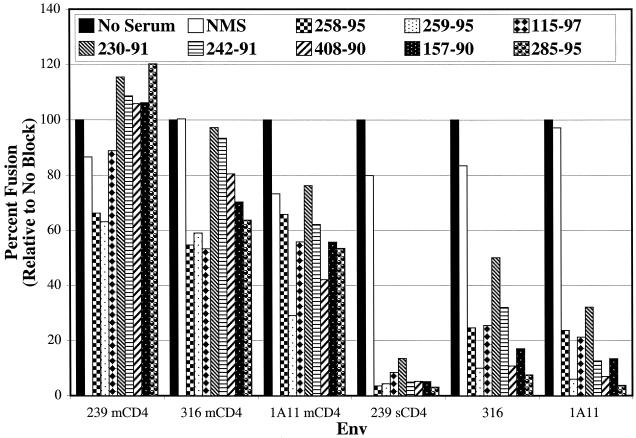
“Triggered” Env neutralization by SIVmac239-infected rhesus macaque sera. Fusion assays were performed essentially as described in the legend to Fig. 1. Effector cells expressing the indicated Env protein were incubated for 1 h with SIVmac239-infected rhesus macaque serum at a 1:50 dilution for 1 h prior to the addition of target cells expressing CCR5. Sera from eight different animals were tested. All macaque sera were isolated 24 weeks postinfection. In addition, normal macaque serum (NMS) was used to control for nonspecific virus neutralization. Membrane-bound rhesus macaque CD4 (mCD4) was expressed in CCR5 target cells where indicated. SIVmac239 Env was triggered with sCD4 at a concentration of 2 μg/ml at the time of serum addition. At 8 h postmixing, cells were lysed and an aliquot of lysate was analyzed for luciferase activity.
Analysis of SIVmac239 V1/V2 region glycosylation mutants.
While SIVmac239-infected animals typically fail to elicit antibodies that are competent to neutralize the homologous 239 Env, they do produce antibodies that can neutralize closely related, M-tropic SIVmac strains that are CD4 independent (61). In addition, sera from these animals can effectively neutralize fusion mediated by the SIVmac239 Env protein if it is first incubated with sCD4 (Fig. 5). Recent studies have shown that the removal of N-linked glycosylation sites in gp120 can also contribute to neutralization sensitivity (30, 48). Reitter et al. have shown that removal of glycosylation sites from the V1/V2 region of SIVmac239 Env results in a neutralization- sensitive virus that, when used to infect rhesus macaques, elicits an enhanced neutralizing antibody response, including antibodies that neutralize the fully glycosylated SIVmac239 virus (48). The structure of the HIV-1 gp120 molecule shows that the stems of the V1/V2 and V3 loops flank the conserved CCR5 binding site region, and it has been proposed that the variable loops, as well as carbohydrate chains, occlude this region in the native protein (33). Removal of glycosylation sites in this region could result in exposure of the CCR5 binding site and facilitate CD4-independent infection. To test this hypothesis, SIVmac239 Env proteins lacking pairs of N-linked glycosylation sites in the V1/V2 region of gp120 were expressed on QT6 cells and examined for their ability to elicit fusion with CCR5-positive cells either in the absence or presence of CD4. As shown in Fig. 6, elimination of the 4th and 5th, 4th and 6th, or 5th and 6th N-linked glycosylation sites in SIVmac239 gp120 enabled these proteins to elicit fusion in the absence of CD4. Generally, fusion activity was ca. 40% of the fusion levels seen in the presence of CD4 (Fig. 6). Thus, partial deglycosylation of SIVmac239 gp120 can impart CD4 independence and neutralization sensitivity, reduce pathogenicity, and, at least under some conditions, result in a more robust humoral immune response.
FIG. 6.
Fusion assay analysis of 239 Envs with deleted glycosylation sites. Fusion assays were performed as described in the legend to Fig. 1, with rhesus macaque and human CCR5 and CD4 being expressed where indicated. Cells expressing the wild-type SIVmac239 Env or mutants lacking the indicated N-linked glycosylation sites were used as effector cells. The amount of fusion for each Env was normalized to the amount of fusion obtained when target cells expressed both rhesus macaque CD4 and CCR5.
Identification of neutralizing epitopes exposed in CD4-induced and CD4-independent Envs.
The results shown above indicate that relatively subtle modifications in the SIVmac Env protein can have profound effects on tropism, pathogenicity, and neutralization sensitivity and that these traits can be associated with a CD4-independent phenotype. To study the mechanism by which these modifications render Env neutralization sensitive, we tested the ability of MAbs to inhibit fusion elicited by SIVmac239 Env, the M-tropic relatives of SIVmac239, and the glycosylation mutants described above.
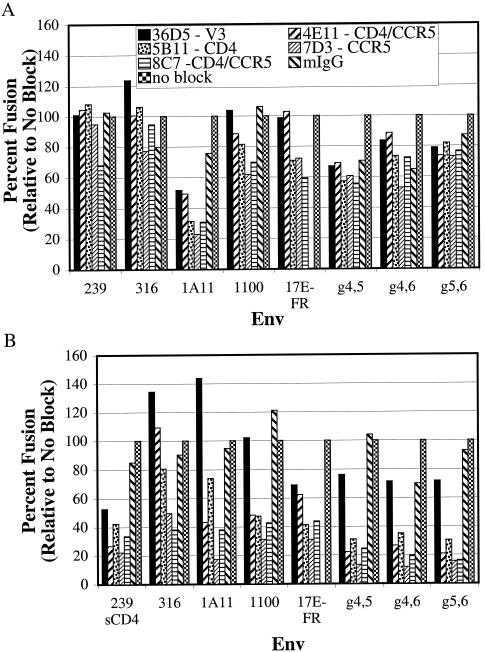
Env-expressing cells were incubated with MAbs prior to mixing with target cells expressing RhCCR5 in the presence or absence of RhCD4. When target cells expressed both CD4 and CCR5, neutralization was either not observed or was relatively modest (Fig. 7B). Only the 1A11 Env was readily inhibited by several of the antibodies. Fusion elicited by some of the other Envs was typically inhibited by MAbs 7D3 and 8C7, which block CCR5 binding, by 25 to 50% under the conditions tested. SIVmac239 Env was resistant to all of the MAbs except for 8C7, which inhibited fusion by ca. 30% (Fig. 7B).
FIG. 7.
Identification of exposed structural domains on “triggered” Envs. Fusion assays were performed as described in the legend to Fig. 5, with the indicated MAbs being added to a final concentration of 2 μg/ml in place of the macaque sera. (A) Target cells expressed rhesus macaque CCR5 alone. (B) Target cells expressed both CCR5 and CD4. SIVmac239 Env was triggered with sCD4 as described in the legend to Fig. 5. At least three independent experiments were performed for each Env. Data were normalized to the level of fusion observed in the absence of blocking antibody.
While the MAbs inhibited fusion with CD4-positive, CCR5-positive cells inefficiently, much greater inhibition was obtained when target cells expressed CCR5 alone. Neutralization of the CD4-independent Env proteins was obtained with MAbs that recognized either the V3 loop (36D5) or conformational epitopes that block CD4 binding (5B11), CCR5 binding (7D3), or both (4E11 and 8C7) (Fig. 7A) (19). MAbs to other determinants, including the V1/V2 region, did not neutralize fusion under the conditions examined (data not shown). Results with the V3-loop MAb were variable, perhaps due to differences in binding of this MAb to the different Env proteins. To obtain fusion with the CD4-dependent SIVmac239 under these conditions, sCD4 was added. Triggering with sCD4 made the 239 Env more susceptible to neutralization by an MAb to the V3 loop (36D5), as well as by MAbs that blocked CCR5 and/or CD4 binding. These results indicate that fusion in the absence of membrane expressed CD4 is more susceptible to inhibition by antibodies that block receptor binding.
DISCUSSION
SIVmac239 is a pathogenic, neutralization-resistant, CD4-dependent, T-tropic virus. Several macrophage-tropic relatives of SIVmac239 have been described, including SIVmac316, 1A11, 1100, and 17E-Fr. The 316 virus was derived by cloning novel Env sequences into the SIVmac239 provirus; thus, all viral genes other than Env are identical between these viruses, and differences in tropism, pathogenicity, and neutralization sensitivity can be attributed directly to the Env protein. The Env proteins from the M-tropic viruses share on average 97% amino acid homology with SIVmac239 Env and 95% amino acid identity with each other. The V3 loop does not appear to be a determinant of M versus T tropism since the 316 and 239 Envs have identical V3 sequences. Other M-tropic viruses do encode amino acid changes in the V3 region, but none of these changes are consistently observed. Therefore, M tropism evolved in these viruses without requiring V3-loop sequence modifications. Only a lysine-to-threonine substitution at position 573 in gp41, which has been shown to enhance macrophage tropism (44), was consistently observed in the M-tropic Envs. Similarly located changes have been identified in the gp41 proteins of CD4-independent HIV-2 and have been hypothesized to lower the activation threshold required for gp120 triggering (47).
While SIVmac strains rarely use CXCR4, it is possible that use of an alternative coreceptor could contribute to SIVmac tropism. We found that coreceptor choice was not responsible for M tropism in this panel of virus Env proteins. All of the viruses used RhCCR5 and RhGPR15; none used RhCXCR4 or RhSTRL33. In addition, the efficient use of alternative coreceptors such as STRL33 often requires levels of receptor expression that far exceed those found on primary cell types (53). We found that all of the M-tropic Envs that are closely related to SIVmac239 studied here were able to infect CCR5-positive cells independently of CD4. Recent studies by Bannert et al. and Mori et al. have demonstrated that rhesus macrophages express very low levels of surface CD4 (2, 44). Previous studies with purified 316 gp120 indicate that this Env has evolved an increased affinity for CD4 relative to that of 239 Env (51), perhaps compensating for the low level of CD4 expression. These studies indicate that decreased dependence on CD4 is an adaptation of the virus to allow replication in rhesus tissue macrophages. It remains to be determined whether enhanced affinity for CD4 is a general property of M-tropic SIVmac strains and to what extent it may be seen in M-tropic strains of HIV-1.
In addition to being associated with M tropism, CD4 independence was also associated with greatly increased sensitivity to neutralizing antibodies. Two CD4-independent HIV-1 strains have been shown to be far more sensitive to neutralizing antibodies than their CD4-dependent parental viruses (24, 27, 31). The CD4-independent HIV-1 strain that we studied, for example, has been efficiently neutralized by every HIV-1-positive human serum sample we examined (24, 27). Therefore, the humoral immune response may provide strong selective pressure against the emergence of CD4-independent HIV-1 strains that might otherwise exhibit broadened cellular tropism. In the case of SIVmac, SIVmac316 was recently shown to exhibit a neutralization-sensitive phenotype (42). In this study, we found that all of the M-tropic, CD4-independent strains we studied were easily neutralized by a panel of SIVmac239-infected macaque sera, even though SIVmac239 itself was neutralization resistant. It is interesting that several of the CD4-independent SIVmac Envs studied here were isolated for their neurotropism (1100 and 17E-Fr) or have been detected in brain tissue (SIVmac316) (43, 52, 55). As an immune-privileged site, the brain may represent a reservoir in which CD4-independent viruses can evolve in the absence of a strong humoral immune response. It will be interesting to determine whether CD4-independent HIV-1 strains can be isolated from the central nervous system, especially at the end stage of disease.
Why are CD4-independent viruses more easily neutralized? In the case of the CD4-independent HIV-1 strain 8x, the conserved CCR5 binding site in the bridging sheet region of gp120 exhibits enhanced exposure, as judged by the ability of 8x gp120 to bind directly to coreceptor, as well as to CD4-induced antibodies that bind to this region (27). Normally, this region only becomes exposed after CD4 binding. Thus, CD4-independent HIV-1 strains may exist in a partially triggered conformation. We have previously shown that SIVmac17E-Fr gp120 binds directly to CCR5 in the absence of CD4 (21). While SIVmac239 gp120 can also bind directly to CCR5 (41), it does so less efficiently. These observations suggest that the bridging sheet region is more greatly exposed in CD4-independent SIVmac Env proteins as well. Since this domain is important for coreceptor interactions, which in turn are critical for virus entry, binding of antibodies to this normally sequestered region would be expected to neutralize virus. Indeed, we found that several MAbs that prevent SIVmac gp120 binding to CCR5 exhibited enhanced neutralizing activity against CD4-independent SIVmac strains. Finally, studies performed by Means et al. with the 239 and 316 viruses indicate that sequence changes leading to CD4 independence alter the conformation of the V3 loop, resulting in sensitivity to sera that fails to neutralize the identical V3 sequence in the context of 239 gp120 (42).
We have found that the efficiency of SIVmac neutralization is related in part to the types of receptors expressed on the target cell. For example, it is easier to prevent SIVmac infection of CD4+ STRL33+ cells than infection of CD4+ CCR5+ cells (19). These differences may be related to the affinity with which Env binds to coreceptors: while SIVmac gp120 binding to CCR5 is readily detected, we have been unable to detect binding of monomeric SIVmac gp120 to other coreceptors, including STRL33 (21). Increased affinity (or receptor density) may result in more rapid membrane fusion and virus entry, making virus more difficult to neutralize (15). In this study, we found that the presence of CD4 on the target cell had a significant impact on neutralization sensitivity. Even CD4-independent viruses were more difficult to neutralize when target cells expressed both CD4 and CCR5. The reason for this is not clear but could reflect the fact that if SIVmac can attach to the cell surface via high-affinity interactions with CD4, the chances of encountering a coreceptor may be increased. Consequently, the ability of low-affinity antibodies that prevent coreceptor binding to neutralize virus may be decreased. In the absence of CD4, direct binding to coreceptors becomes more important, and antibodies that prevent coreceptor binding, such as those directed against the V3 loop, might neutralize virus more efficiently as we observed in this study. A complete understanding of why CD4-independent SIVmac strains are more easily neutralized than CD4-dependent viruses will require a greater understanding of the structural determinants of CD4 independence.
The ability of CD4-independent SIVmac strains to be easily neutralized may provide insights into how Env can be modified so as to enhance immunogenicity. Several vaccine studies with what we have demonstrated here to be CD4-independent viruses have been shown to prime the humoral immune response. Studies with 1A11, which was the most neutralization-sensitive and CD4-independent Env of the panel examined, demonstrated that 1A11 elicits a neutralizing antibody response that increases for several months postinfection (38). Prior infection with 1A11 has been shown to either completely protect animals subsequently challenged with the acutely pathogenic SIVmac251 strain or to significantly delay the onset of clinical symptoms infection (38). Studies with 17E-Cl, bearing an Env that differs from 17E-Fr only by expression of a full-length gp41, have shown it to protect macaques from a stringent B670 challenge (10). In this case, protection of macaques required maturation of the humoral immune response to recognize conformational epitopes with an increase in avidity for 6 to 8 months postimmunization (11, 12). Finally, infection of macaques with SIVmac239 with genetic deletions of glycosylation sites in the V1/V2 region results in a strong neutralizing antibody response in the infected animals that is also capable of neutralizing the fully glycosylated parental 239 Env (48). We have shown here that these Envs have gained CD4-independent function and hypothesize that it is exposure of the coreceptor binding site in these Envs that elicited the strong humoral response. These studies support the hypothesis that CD4-independent Envs could be utilized as immunogens to generate neutralizing antibodies that recognize the coreceptor binding site, which is a highly conserved site among heterologous SIVmac Envs. It will be important to characterize the diverse structural determinants in this panel of M-tropic Env proteins that result in CD4 independence, neutralization sensitivity, and enhanced immunogenicity in order to determine ways in which genetic modification of Env can result in better immunogens.
Acknowledgments
B.A.P. was supported by NIH Public Health Service grant F32 AI 10577. H.S.F was supported by MH61224 and MH62261. S.P. was supported by a fellowship from the Deutsche Forschungsgemeinschaft. M.D.S. was supported by NIH F31 RR05074. R.C.D. was supported by NIH Public Health Service grants RR00168, AI 35365, and AI 25338. R.W.D. was supported by NIH R01 35383 and 40880, a Burroughs Wellcome Fund Translational Research Award, and an Elizabeth Glaser Scientist Award from the Pediatric AIDS Foundation.
REFERENCES
- 1.Alexander, W. A., B. Moss, and T. R. Fuerst. 1992. Regulated expression of foreign genes in vaccinia virus under the control of bacteriophage T7 RNA polymerase and the Escherichia coli lac repressor. J. Virol. 66:2934-2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bannert, N., D. Schenten, S. Craig, and J. Sodroski. 2000. The level of CD4 expression limits infection of primary rhesus monkey macrophages by a T-tropic simian immunodeficiency virus and macrophage tropic human immunodeficiency viruses. J. Virol. 74:10984-10993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berger, E. A., R. W. Doms, E. M. Fenyo, B. T. Korber, D. R. Littman, J. P. Moore, Q. J. Sattentau, H. Schuitemaker, J. Sodroski, and R. A. Weiss. 1998. A new classification for HIV-1. Nature 391:240.. [DOI] [PubMed] [Google Scholar]
- 4.Burns, D. P., C. Collignon, and R. C. Desrosiers. 1993. Simian immunodeficiency virus mutants resistant to serum neutralization arise during persistent infection of rhesus monkeys. J. Virol. 67:4104-4113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burns, D. P., and R. C. Desrosiers. 1994. Envelope sequence variation, neutralizing antibodies, and primate lentivirus persistence. Curr. Top. Microbiol. Immunol. 188:185-219. [DOI] [PubMed] [Google Scholar]
- 6.Chan, D. C., D. Fass, J. M. Berger, and P. S. Kim. 1997. Core structure of gp41 from the HIV envelope glycoprotein. Cell 89:263-273. [DOI] [PubMed] [Google Scholar]
- 7.Chan, D. C., and P. S. Kim. 1998. HIV entry and its inhibition. Cell 93:681-684. [DOI] [PubMed] [Google Scholar]
- 8.Chen, B. K., K. Saksela, R. Andino, and D. Baltimore. 1994. Distinct modes of human immunodeficiency virus type 1 proviral latency revealed by superinfection of nonproductively infected cell lines with recombinant luciferase-encoding viruses. J. Virol. 68:654-660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen, Z., P. Zhou, D. D. Ho, N. R. Landau, and P. A. Marx. 1997. Genetically divergent strains of simian immunodeficiency virus use CCR5 as a coreceptor for entry. J. Virol. 71:2705-2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clements, J. E., R. C. Montelaro, M. C. Zink, A. M. Amedee, S. Miller, A. M. Trichel, B. Jagerski, D. Hauer, L. N. Martin, and R. P. Bohm. 1995. Cross-protective immune responses induced in rhesus macaques by immunization with attenuated macrophage-tropic simian immunodeficiency virus. J. Virol. 69:2737-2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cole, K. S., M. Murphey-Corb, O. Narayan, S. V. Joag, G. M. Shaw, and R. C. Montelaro. 1998. Common themes of antibody maturation to simian immunodeficiency virus, simian-human immunodeficiency virus, and human immunodeficiency virus type 1 infections. J. Virol. 72:7852-7859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cole, K. S., J. L. Rowles, B. A. Jagerski, M. Murphey-Corb, T. Unangst, J. E. Clements, J. Robinson, M. S. Wyand, R. C. Desrosiers, and R. C. Montelaro. 1997. Evolution of envelope-specific antibody responses in monkeys experimentally infected or immunized with simian immunodeficiency virus and its association with the development of protective immunity. J. Virol. 71:5069-5079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Connor, R. I., B. K. Chen, S. Choe, and N. R. Landau. 1995. Vpr is required for efficient replication of human immunodeficiency virus type-1 in mononuclear phagocytes. Virology 206:935-944. [DOI] [PubMed] [Google Scholar]
- 14.Daniel, M., N. Letvin, N. King, M. Kannagi, P. Sehgal, R. Hunt, P. Kanki, M. Essex, and R. Desrosiers. 1985. Isolation of T-cell tropic HTLV-III-like retrovirus from macaques. Science 228:1201-1204. [DOI] [PubMed] [Google Scholar]
- 15.Doms, R. W., and J. Moore. 2000. HIV-1 membrane fusion: targets of opportunity. J. Cell Biol. 16:F9-F14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Doms, R. W., A. L. Edinger, and J. P. Moore (ed.). 1998. Coreceptor use by primate lentiviruses. Los Alamos National HIV Sequence Database, pt. 3, p. 1-12. Los Alamos National Laboratory, Los Alamos, N.Mex.
- 17.Dumonceaux, J., C. Goujon, V. Joliot, P. Briand, and U. Hazan. 2001. Determination of essential amino acids involved in the CD4-independent tropism of the X4 human immunodeficiency virus type 1 m7NDK isolate: role of potential N glycosylations in the C2 and V3 regions of gp120. J. Virol. 75:5425-5428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dumonceaux, J., S. Nisole, C. Chanel, L. Quivet, A. Amara, F. Baleux, P. Briand, and U. Hazan. 1998. Spontaneous mutations in the env gene of the human immunodeficiency virus type 1 NDK isolate are associated with a CD4-independent entry phenotype. J. Virol. 72:512-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Edinger, A. L., M. Ahuja, T. Sung, K. C. Baxter, B. Haggarty, R. W. Doms, and J. A. Hoxie. 2000. Characterization and epitope mapping of neutralizing monoclonal antibodies produced by immunization with oligomeric simian immunodeficiency virus envelope protein. J. Virol. 74:7922-7935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Edinger, A. L., A. Amedee, K. Miller, B. J. Doranz, M. Endres, M. Sharron, M. Samson, Z.-H. Lu, J. E. Clements, M. Murphey-Corb, S. C. Peipper, M. Parmentier, C. C. Broder, and R. W. Doms. 1997. Differential utilization of CCR5 by macrophage and T cell tropic simian immunodeficiency virus strains. Proc. Natl. Acad. Sci. USA 94:4005-4010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Edinger, A. L., C. Blanpain, K. J. Kunstman, S. M. Wolinsky, M. Parmentier, and R. W. Doms. 1999. Functional dissection of CCR5 coreceptor function through the use of CD4-independent simian immunodeficiency virus strains. J. Virol. 73:4062-4073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Edinger, A. L., J. E. Clements, and R. W. Doms. 1999. Chemokine and orphan receptors in HIV-2 and SIV tropism and pathogenesis. Virology 260:211-221. [DOI] [PubMed] [Google Scholar]
- 23.Edinger, A. L., J. L. Mankowski, B. J. Doranz, B. J. Margulies, B. Lee, J. Rucker, M. Sharron, T. L. Hoffman, J. F. Berson, M. C. Zink, V. M. Hirsch, J. E. Clements, and R. W. Doms. 1997. CD4-independent, CCR5-dependent infection of brain capillary endothelial cells by a neurovirulent simian immunodeficiency virus strain. Proc. Natl. Acad. Sci. USA 94:14742-14747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Edwards, T., T. Hoffman, F. Baribaud, S. Wyss, C. LaBranche, J. Romano, J. Adkinson, M. Sharron, J. Hoxie, and R. Doms. 2001. Relationships between CD4 independence, neutralization sensitivity, and exposure of a CD4-induced epitope in a human immunodeficiency virus type 1 envelope protein. J. Virol. 75:5230-5239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Farzan, M., H. Choe, E. Desjardins, Y. Sun, J. Kuhn, J. Cao, D. Archambault, P. Kolchinsky, M. Koch, R. Wyatt, and J. Sodroski. 1998. Stabilization of human immunodeficiency virus type 1 envelope glycoprotein trimers by disulfide bonds introduced into the gp41 glycoprotein ectodomain. J. Virol. 72:7620-7625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Flaherty, M. T., D. A. Hauer, J. L. Mankowski, M. C. Zink, and J. E. Clements. 1997. Molecular and biological characterization of a neurovirulent molecular clone of simian immunodeficiency virus. J. Virol. 71:5790-5798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoffman, T. L., C. C. LaBranche, W. Ahang, G. Canziani, J. Robinson, I. Chaiken, J. A. Hoxie, and R. W. Doms. 1999. Stable exposure of the coreceptor-binding site in a CD4-independent HIV-1 envelope protein. Proc. Natl. Acad. Sci. USA 96:6359-6364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kestler, H. W., III, T. Kodama, D. Ringler, M. Marthas, N. Pederson, A. Lackner, D. Regier, P. Sehgal, M. Daniel, N. King, and R. C. Desrosiers. 1990. Induction of AIDS in rhesus monkeys by molecularly cloned simian immunodeficiency virus. Science 248:1109-1112. [DOI] [PubMed] [Google Scholar]
- 29.Kirchhoff, F., S. Pohlmann, M. Hamacher, R. E. Means, T. Kraus, K. Uberla, and P. D. Marzio. 1997. Simian immunodeficiency virus variants with differential T-cell and macrophage tropism use CCR5 and an unidentified cofactor expressed in CEMX174 cells for efficient entry. J. Virol. 71:6509-6516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kolchinsky, P., E. Kiprilov, P. Bartley, R. Rubinstein, and J. Sodroski. 2001. Loss of a single N-linked glycan allows CD4-independent human immunodeficiency virus type 1 infection by altering the position of the gp120 V1/V2 variable loops. J. Virol. 75:3435-3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kolchinsky, P., E. Kiprilov, and J. Sodroski. 2001. Increased neutralization sensitivity of CD4-independent human immunodeficiency virus variants. J. Virol. 75:2041-2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kolchinsky, P., T. Mirzabekov, M. Farzan, E. Kiprilov, M. Cayabyab, L. J. Mooney, H. Choe, and J. Sodroski. 1999. Adaptation of a CCR5-using, primary human immunodeficiency type 1 isolate for CD4-independent replication. J. Virol. 73:8120-8126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kwong, P. D., R. Wyatt, J. Robinson, R. W. Sweet, J. Sodroski, and W. A. Hendrickson. 1998. Structure of an HIV gp120 envelope glycoprotein in complex with the CD4 receptor and a neutralizing human antibody. Nature 393:648-659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.LaBranche, C. C., T. L. Hoffman, J. Romano, B. S. Haggarty, T. G. Edwards, T. J. Matthews, R. W. Doms, and J. A. Hoxie. 1999. Determinants of CD4 independence for a human immunodeficiency virus type 1 variant map outside regions required for coreceptor specificity. J. Virol. 73:10310-10319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lapham, C. K., J. Ouyang, B. Chandrasekhar, N. Y. Nguyen, D. S. Dimitrov, and H. Golding. 1996. Evidence for cell-surface association between Fusin and the CD4-gp120 complex in human cell lines. Science 274:602-605. [DOI] [PubMed] [Google Scholar]
- 36.Lee, B., M. Sharron, C. Blanpain, B. J. Doranz, J. Vakili, P. Setoh, E. Berg, G. Liu, H. R. Guy, S. R. Durell, M. Parmentier, C. N. Chang, K. Price, M. Tsang, and R. W. Doms. 1999. Epitope mapping of CCR5 reveals multiple conformational states and distinct but overlapping structures involved in chemokine and coreceptor function. J. Biol. Chem. 274:9617-9626. [DOI] [PubMed] [Google Scholar]
- 37.Lu, M., S. C. Blacklow, and P. S. Kim. 1995. A trimeric structural domain of the HIV-1 transmembrane glycoprotein. Nat. Struct. Biol. 2:1075-1082. [DOI] [PubMed] [Google Scholar]
- 38.Luciw, P. A., K. E. Shaw, R. E. Unger, V. Planelles, M. W. Stout, J. E. Lackner, E. Pratt-Lowe, N. J. Leung, B. Banapour, and M. L. Marthas. 1992. Genetic and biological comparisons of pathogenic and nonpathogenic molecular clones of simian immunodeficiency virus (SIVmac). AIDS Res. Hum. Retrovir. 8:395-402. [DOI] [PubMed] [Google Scholar]
- 39.Mankowski, J. L., M. T. Flaherty, J. P. Spelman, D. A. Hauer, P. J. Didier, A. M. Amedee, M. Murphey-Corb, L. M. Kirstein, A. Munoz, J. E. Clements, and M. C. Zink. 1997. Pathogenesis of simian immunodeficiency virus encephalitis: viral determinants of neurovirulence. J. Virol. 71:6055-6060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marcon, L., H. Choe, K. A. Martin, M. Farzan, P. D. Ponath, L. Wu, W. Newman, N. Gerard, C. Gerard, and J. Sodroski. 1997. Utilization of C-C chemokine receptor 5 by the envelope glycoproteins of a pathogenic simian immunodeficiency virus, SIVmac239. J. Virol. 71:2522-2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martin, K. A., R. Wyatt, M. Farzan, H. Choe, L. Marcon, E. Desjardins, J. Robinson, J. Sodroski, C. Gerard, and N. P. Gerard. 1997. CD4-independent binding of SIV gp120 to rhesus CCR5. Science 278:1470-1473. [DOI] [PubMed] [Google Scholar]
- 42.Means, R. E., T. Matthews, J. A. Hoxie, M. H. Malim, T. Kodama, and R. C. Desrosiers. 2001. Ability of the V3 loop of simian immunodeficiency virus to serve as a target for antibody-mediated neutralization: correlation of neutralization sensitivity, growth in macrophages, and decreased dependence on CD4. J. Virol. 75:3903-3915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mori, K., D. J. Ringler, T. Kodama, and R. C. Desrosiers. 1992. Complex determinants of macrophage tropism in env of simian immunodeficiency virus. J. Virol. 66:2067-2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mori, K., M. Rosenzweig, and R. C. Desrosiers. 2000. Mechanisms for adaptation of simian immunodeficiency virus to replication in alveolar macrophages. J. Virol. 74:10852-10859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.O'Doherty, U., W. J. Swiggard, and M. H. Malim. 2000. Human immunodeficiency virus type 1 spinoculation enhances infection through virus binding. J. Virol. 74:10074-10080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pöhlmann, S., B. Lee, S. Meister, M. Krumbiegel, G. Leslie, R. W. Doms, and F. Kirchhoff. 2000. Simian immunodeficiency virus utilizes human and sooty mangabey but not rhesus macaque STRL33 for efficient entry. J. Virol. 74:5075-5082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reeves, J. D., and T. F. Schultz. 1997. The CD4-independent tropism of human immunodeficiency virus type 2 involves several regions of the envelope protein and correlates with a reduced activation threshold for envelope-mediated fusion. J. Virol. 71:1453-1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reitter, J. N., R. E. Means, and R. C. Desrosiers. 1998. A role for carbohydrates in immune evasion in AIDS. Nat. Med. 4:679-684. [DOI] [PubMed] [Google Scholar]
- 49.Rizzuto, C. D., R. Wyatt, N. Hernandez-Ramos, Y. Sun, P. D. Kwong, W. A. Hendrickson, and J. Sodroski. 1998. A conserved HIV gp120 glycoprotein structure involved in chemokine receptor binding. Science 280:1949-1953. [DOI] [PubMed] [Google Scholar]
- 50.Rucker, J., B. J. Doranz, A. E. Edinger, D. Long, J. F. Berson, and R. W. Doms. 1997. Use of a cell-cell fusion assay to study the role of chemokine receptors in human immunodeficiency virus type 1 (HIV-1) entry. Methods Enzymol. 288:118-133. [DOI] [PubMed] [Google Scholar]
- 51.Schenten, D., L. Marcon, G. B. Karlsson, C. Parolin, T. Kodama, N. Gerard, and J. Sodroski. 1999. Effects of soluble CD4 on simian immunodeficiency virus infection of CD4-positive and CD4-negative cells. J. Virol. 73:5373-5380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sharma, D. P., M. C. Zink, M. Anderson, R. J. Adams, J. E. Clements, S. V. Joag, and O. Narayan. 1992. Derivation of neutrotropic simian immunodeficiency virus from exclusively lymphocyte-tropic parental virus: pathogenesis of infection in macaques. J. Virol. 66:3550-3556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sharron, M., S. Pohlmann, K. Price, E. Lolis, M. Tsang, F. Kirchhoff, R. W. Doms, and B. Lee. 2000. Expression and coreceptor activity of STRL33/Bonzo on primary peripheral blood lymphocytes. Blood 96:41-49. [PubMed] [Google Scholar]
- 54.Trkola, A., T. Dragic, J. Arthos, J. M. Binley, W. C. Olson, G. P. Allaway, C. Cheng-Mayer, J. Robinson, P. J. Maddon, and J. P. Moore. 1996. CD4-dependent, antibody-sensitive interactions between HIV-1 and its co-receptor CCR-5. Nature 384:184-187. [DOI] [PubMed] [Google Scholar]
- 55.Watry, D., T. E. Lane, M. Streb, and H. S. Fox. 1995. Transfer of neuropathogenic simian immunodeficiency virus with naturally infected microglia. Am. J. Pathol. 146:914-923. [PMC free article] [PubMed] [Google Scholar]
- 56.Weissenhorn, W., A. Dessen, S. C. Harrison, J. J. Skehel, and D. C. Wiley. 1997. Atomic structure of the ectodomain from HIV-1 gp41. Nature 387:426-430. [DOI] [PubMed] [Google Scholar]
- 57.Wu, L., N. P. Gerard, R. Wyatt, H. Choe, C. Parolin, N. Ruffing, A. Borsetti, A. A. Cardoso, E. Desjardin, W. Newman, C. Gerard, and J. Sodroski. 1996. CD4-induced interaction of primary HIV-1 gp120 glycoproteins with the chemokine receptor CCR-5. Nature 384:179-183. [DOI] [PubMed] [Google Scholar]
- 58.Wyatt, R., P. D. Kwong, E. Desjardins, R. W. Sweet, J. Robinson, W. A. Hendrickson, and J. G. Sodroski. 1998. The antigenic structure of the HIV gp120 envelope glycoprotein. Nature 393:705-711. [DOI] [PubMed] [Google Scholar]
- 59.Wyatt, R., and J. Sodroski. 1998. The HIV-1 envelope glycoproteins: fusogens, antigens, and immunogens. Science 280:1884-1888. [DOI] [PubMed] [Google Scholar]
- 60.Yang, X., L. Florin, M. Farzan, P. Kolchinsky, P. D. Kwong, J. Sodroski, and R. Wyatt. 2000. Modifications that stabilize human immunodeficiency virus envelope glycoprotein trimers in solution. J. Virol. 74:4746-4754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhuge, W., F. Jia, I. Adany, O. Narayan, and E. B. Stephens. 1997. Plasmas from lymphocyte- and macrophage-tropic SIVmac-infected macaques have antibodies with a broader spectrum of virus neutralization activity in macrophage versus lymphocyte cultures. Virology 227:24-33. [DOI] [PubMed] [Google Scholar]