Abstract
Following the introduction of highly active antiretroviral therapy (HAART), the incidence of Kaposi's sarcoma (KS) has significantly declined in human immunodeficiency virus type 1 (HIV-1)-positive (HIV-1+) individuals and clinical remission is often observed. We hypothesize that these effects are partly due to anti-KS-associated herpesvirus (KSHV) immune restoration. Here, 15-mer overlapping peptides from proteins K12 and K8.1 were used to identify novel KSHV-specific cytotoxic T-lymphocyte epitopes. Three immunogenic peptides, two lytic and one latent, were subsequently used to monitor the anti-KSHV CD8+ T-cell responses in a cohort of 19 HIV-1+ KSHV+/− KS+/− individuals during 52 weeks of HAART. KSHV and HIV-1 loads, KSHV antibody titers, and both CD4+ and CD8+ T-lymphocyte counts were enumerated. Prior to HAART, the total number of spot-forming cells (SFC) for all three peptides correlated with both CD4+ and CD8+ T-lymphocyte counts (P ≤ 0.05) in the KSHV-positive KS-positive cohort (n = 11). Following 52 weeks of HAART, significant decreases in HIV-1 and KSHV loads were associated with significant increases in CD4+ T-lymphocyte counts and number of SFC for the three KSHV-specific peptides. Although these increases were modest in comparison to the number of SFC observed with the HIV-1 gag peptide SLYNTVATL, they represented a fourfold increase from the baseline, continuing an upward trend to week 52.
Kaposi's sarcoma (KS)-associated herpesvirus (KSHV, or human herpesvirus 8) is the etiological agent of KS (23). In the early 1980s, a KS epidemic was described among young homosexual men in the United States (3) and became one of the first AIDS-defining conditions. AIDS-KS was found to be more common in human immunodeficiency virus type 1 (HIV-1)-infected homosexual men than in other HIV-1 risk groups (7). However, since the introduction of highly active antiretroviral therapy (HAART) in developed countries, the incidence of AIDS-KS has rapidly declined (8, 15), with many case reports documenting the resolution of KS lesions following therapy (4, 14, 16, 17, 25).
Cellular immune responses are a critical part of host defense against viral infections, with CD8+ T lymphocytes assuming a pivotal role in the control of infection. Virus-specific cytotoxic T lymphocytes (CTL) have been shown to be important in controlling viremia in primary HIV-1 and herpesvirus infections (2, 22, 24). However, only two groups to date have reported CTL responses to both lytic and latent antigens of KSHV, with no dominant response to a specific KSHV antigen being defined (20, 27, 28). KSHV-specific CD8+ T lymphocytes are likely to be an important component in the control of KSHV infection, and one report has suggested that CTL responses decrease 2 to 3 years after primary infection in HIV-1-seronegative individuals (27).
We hypothesize that, like Epstein-Barr virus (EBV), KSHV establishes a persistent infection which is normally controlled by the immune system, with the number of KSHV-infected cells being under immunological control. As a result of HIV-1 infection, immunosuppression leads to an increase in the number of KSHV-infected cells, resulting in the development of KSHV-related tumors and the expression of lytic viral genes. The human gammaherpesviruses establish latent infections in lymphoid cells, where only a limited number of viral genes, the so-called latent genes, are expressed. In EBV infection, virus-specific CTL activity directed at peptides from both lytic and latent proteins is an important factor in the pathogenesis of EBV-associated diseases (22). In this study, we have used overlapping peptides from the unique lytic (K8.1) and latent (K12) proteins of KSHV to identify novel KSHV-specific CTL target epitopes which exclude cross-reactivity with EBV-specific CTL. We have used these peptides to longitudinally monitor anti-KSHV-specific CTL responses in a cohort of HIV-1-positive (HIV-1+) KSHV+/− KS+/− individuals receiving HAART for 52 weeks to determine the level of immune reconstitution toward KSHV.
(This work was presented in part at the 3rd International Workshop on Kaposi's Sarcoma-Associated Herpesvirus and Related Agents, University of Massachusetts, Amherst, 2000 [abstr. 104], and the HIV Pathogenesis Symposia, Keystone, Colo., 2001 [abstr. 447].)
MATERIALS AND METHODS
Patients and study design.
Two cohorts of HIV-1+ patients were recruited from the Chelsea and Westminster Hospital following informed consent and local ethical approval. Cohort A comprised 10 randomly selected HAART-treated HIV-1+ KSHV-positive (KSHV+) KS+/− patients recruited for the identification of KSHV-specific CTL epitopes against K12 and K8.1 proteins by use of overlapping peptides. These patients had mixed HLA types and were bled at a single time point only. As a result of HAART, the cohort demonstrated a mean CD4+ T-lymphocyte count of 547 cells/μl (standard error [SE], ±140) and a mean log HIV-1 load of 2.19 (±0.3) and a mean log KSHV load of 677 (±448) copies/106 peripheral blood mononuclear cells (PBMC). Six HIV-1- and KSHV-seronegative individuals were used as controls. Cohort B comprised 19 HIV-1+ patients who were monitored longitudinally over time pre- and post-HAART. These 19 individuals were placed in three study cohorts prior to the initiation of antiretroviral therapy and consisted of 11 KSHV+ KS-positive (KS+), 4 KSHV+ KS-negative (KS−), and 4 KSHV-negative (KSHV−) KS− individuals (Table 1). Serial blood samples were collected in lithium-heparin-potassium-EDTA tubes at 0, 2, 4, 8, 12, 18, 24, 36, and 52 weeks of HAART. Serologic testing of KSHV-specific antibodies, quantification of KSHV and HIV-1 loads, and T-lymphocyte phenotyping and enumeration were performed at these time points. In addition, PBMC were collected, cryopreserved, and batch tested in a gamma interferon (IFN-γ) enzyme-linked immunospot (ELIspot) assay. All patients were HLA typed to determine peptide HLA class I restriction as previously described (20).
TABLE 1.
Baseline characteristics for HIV-1 patients receiving 52 weeks of HAART
| Group (n) | Mean ± SE
|
||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cells/μl at indicated wk:
|
Log plasma HIV-1 copies/ml at wk:
|
Log KSHV copies/106 PBMC at wk:
|
KSHV antibody titer at wk:
|
KSHV peptides (P) (SFC/106 PBMC) at indicated wk:
|
|||||||||||||||
| CD4+
|
CD8+
|
K12 P2
|
K8.1 P14
|
K8.1 P22
|
Total for P2, P14,and P22
|
||||||||||||||
| 0 | 52 | 0 | 52 | 0 | 52 | 0 | 52 | 0 | 52 | 0 | 52 | 0 | 52 | 0 | 52 | 0 | 52 | ||
| IKSHV− KS− (4)a | 285 ± 52 | 510 ± 130 | 937 ± 111 | 828 ± 98 | 5.18 ± 0.37 | 1.7 ± 0 | 1.0 ± 0 | 1.0 ± 0 | 2,200 ± 1,438 | 6,400 ± 1,848 | 0 ± 0 | 10 ± 10 | 14 ± 8 | 5 ± 5 | 39 ± 32 | 35 ± 35 | 53 ± 28 | 50 ± 18 | |
| IKSHV+ KS+ (11) | 134 ± 36 | 293 ± 41 | 771 ± 115 | 1,211 ± 190 | 5.19 ± 0.15 | 2.46 ± 0.45 | 3.52 ± 0.35 | 2.46 ± 0.23 | 76,055 ± 49,735 | 121,600 ± 66,223 | 5 ± 2 | 19 ± 10 | 14 ± 7 | 51 ± 20 | 10 ± 4 | 40 ± 16 | 29 ± 12 | 110 ± 30 | |
| IKSHV+ KS− (4) | 223 ± 72 | 276 ± 145 | 923 ± 260 | 674 ± 203 | 4.39 ± 0.17 | 1.7 ± 0 | 3.5 ± 0.4 | 1.67 ± 0.67 | 35,466 ± 33,474 | 115,200 ± 126,714 | 5 ± 5 | 30 ± 30 | 10 ± 8 | 3 ± 3 | 7 ± 3 | 0 ± 0 | 22 ± 15 | 33 ± 33 | |
The KSHV− KS− cohort demonstrated KSHV antibody titers throughout HAART.
Viral serology.
Antibodies against latent nuclear antigen 1 of KSHV were detected by an indirect immunofluorescence assay as previously described (5, 29). To determine anti-KSHV antibody titers, twofold serial dilutions of patient serum were made starting with a dilution of 1:200.
Quantification of KSHV load.
For KSHV load quantification, DNA was extracted from whole blood by using a QIAamp blood kit (Qiagen, Sussex, United Kingdom) according to the manufacturer's instructions. The purified DNA was amplified by PCR with a real-time PCR TaqMan assay (Perkin-Elmer Biosystems 7700 sequence detector) as described previously (12). PCR primers were used to quantify the human albumin gene copy number (1), and sequences from the open reading frame 73 gene were used as PCR primers to quantify KSHV (12). A plasmid containing sequences for both KSHV and the human albumin gene was constructed as a positive control with serial dilutions generating a standard curve. PCR product formation, leading to the activation of AmpliTaq Gold, releases a fluorescent reporter. The number of PCR cycles required for each sample to reach a threshold limit of fluorescence was compared to the standard curve. KSHV load is presented as copies per 106 PBMC, with a cutoff for detection of <100 copies/106 PBMC.
Quantification of HIV-1 load.
Plasma HIV-1 load was determined with an HIV-1 RNA 3.0 assay (Bayer, Berkshire, United Kingdom). The lower limit of detection was <50 HIV RNA copies/ml.
Peptides.
Peptides with a length of 15 amino acids (aa) and with a 5-aa overlap were synthesized (Advanced Biotechnology Center, Charing Cross Hospital, Imperial College School of Medicine, London, United Kingdom) for both the latent (K12) (kaposin A) and the lytic (K8.1) (gp35/37) proteins of KSHV. Each peptide was initially solubilized in 100% dimethyl sulfoxide, and sterile phosphate-buffered saline was added to give a final peptide stock concentration of 1 mM. For K12, there were six 15-mer peptides, designated P1 to P6; for K8.1, there were 23, P1 to P23. Following optimization experiments, the KSHV peptides were used in the ELIspot assay at a concentration of 25 μM. Additionally, the HLA-A*0201-restricted HIV-1 peptide SLYNTVATL (SL9, HIV-1 p17, aa 77 to 85) was used to determine the frequency of gag-specific IFN-γ-secreting cells in four HLA-A2 HIV-1+ KSHV+ KS+ patients pre- and post-HAART (6). The HIV-1 antigen was used in the IFN-γ ELIspot assay at a concentration of 10 μg/ml.
T-lymphocyte phenotyping and enumeration.
Monoclonal antibodies to CD3, CD4, and CD8 (Beckman Coulter, High Wycombe, United Kingdom) were used to determine absolute T-lymphocyte counts. A EPICSXL-MCL apparatus (Beckman Coulter) was used for flow cytometric analyses of whole blood.
IFN-γ ELIspot assay.
Detection of single-cell IFN-γ release by the ELIspot assay was performed as previously described (13). Cryopreserved PBMC were rapidly thawed, viability was assessed by 0.4% trypan blue dye exclusion, and the PBMC batch was tested in the ELIspot assay to avoid interassay variation. Briefly, 2 × 105 cells/well were cultured in 10% AB plasma-RPMI medium (Sigma, Dorset, United Kingdom) in a 96-well polyvinylidene difluoride-backed plate (Millipore, Bedford, Mass.) coated with an anti-IFN-γ monoclonal antibody (Mabtech, Nacka, Sweden). Cells were stimulated with appropriate peptides and phytohemagglutinin (5 μg/ml; Sigma), which acted as a positive control, in 10% AB plasma-RPMI medium. The negative control comprised cells cultured with medium alone to allow evaluation of background IFN-γ release. Plates were incubated for 36 h at 37°C with 5% CO2. Spot-forming cells (SFC) were then detected according to the antibody manufacturer's instructions (Mabtech) and counted under a stereomicroscope. For each peptide, background values were subtracted and the number of spots was expressed as SFC per 106 PBMC. To determine major histocompatibility complex class I peptide restriction, monoclonal antibody W6/32 (Dako, Glostrup, Denmark) was added to patient PBMC at 1 μg/ml for 30 min at 4°C. The cells were washed twice in 10% AB plasma-RPMI medium and plated by use of the ELIspot assay.
CTL assay (chromium release assay).
Confirmation of CTL activity was determined by using a chromium release assay as previously described (20). Briefly, patient PBMC were cultured in 10% AB plasma-RPMI medium at 2 × 106 cells/ml and stimulated with 50 μM relevant peptide, and 50 U of interleukin 2 (Roche Diagnostics Ltd., Sussex, United Kingdom)/ml was added on days 0, 3, and 7 of culturing. Effector cells were assessed for specific killing of 51Cr-labeled EBV-transformed autologous B cells (target cells) (pulsed with each peptide) at effector/target ratios of 20:1, 40:1, and 80:1 on day 10.
Statistical methods.
Statistical analysis was performed by using Statview 4.5 software (Abacus, Berkeley, Calif.). Comparisons between two independent groups were tested by using the Mann-Whitney U test with a P value of <0.05 as the cutoff for significance. Regression analysis was performed to determine correlations between KSHV load and the other biological parameters measured.
RESULTS
Identification of CTL epitopes for lytic and latent genes of KSHV. (i) Computer predictions of binding.
To identify potential HLA class I-restricted epitopes within KSHV K12 and K8.1, the amino acid sequences of both proteins were analyzed by a computer program designed to predict HLA-binding peptides (http://bimas.dcrt.nih.gov/molbio/hla__bind) based on an estimation of the half-time disassociation of the HLA-peptide complex (21). For K12, peptide 2 (P2) was predicted to confer the strongest binding to the HLA-A2 molecule, with a score of 1,008. Low binding scores were predicted for binding of the remaining peptide sequences to the HLA-A2 molecule and to all remaining HLA types. P2 and peptide 21 and peptides 19 and 20 of K8.1 demonstrated the strongest binding to HLA-A2 and HLA-A24, respectively (scores of 592, 459, 420, and 300, respectively). No other peptides from K8.1 demonstrated high HLA binding scores for any of the other HLA molecules assessed.
(ii) K12 ELIspot assay and class I blocking.
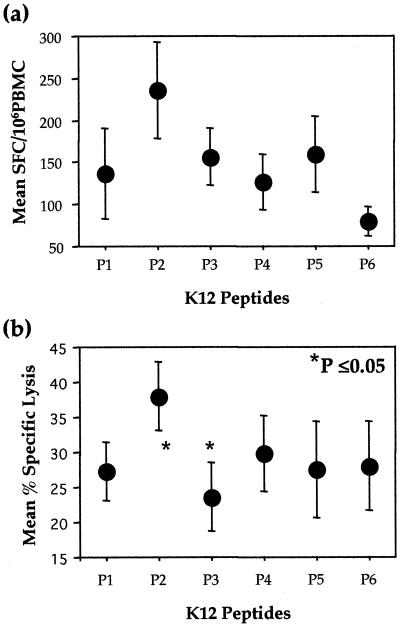
KSHV-specific SFC were observed for all six overlapping peptides of K12 (Fig. 1a). The strongest responses were seen for K12 P2, with a mean of 236 SFC (±58)/106 PBMC. Mean responses to the remaining five peptides ranged from 80 to 160 SFC. Peptide responses in the seronegative controls were significantly lower, with means of <15 SFC for the six peptides. To further define the KSHV-specific CTL epitope, seven 9-mer overlapping peptides from P2, designated P2.1 to P2.7, were synthesized. Mean responses to P2.1 to P2.7 were 19 (±8), 33 (±14), 39 (±12), 24 (±11), 25 (±11), 41 (±11), and 74 (±20) SFC, respectively (n = 10). These studies suggest that P2.7 (LLNGWRWRL) at positions 17 to 25 of K12 is the optimal epitope.
FIG. 1.
Determination of KSHV K12 peptide-specific CTL in KSHV+ KS+ individuals by using an ELIspot assay (n = 10) (a) and a chromium release assay (n = 5) (b). Means and SEs are shown.
HLA class I restriction was confirmed by using the anti-HLA class I monoclonal antibody W6/32 such that the mean number of SFC in four HIV-1+ KSHV+ KS+ individuals was reduced to the level observed in seronegative controls upon pretreatment of the PBMC with the antibody (data not shown).
(iii) K12 chromium release assay.
Confirmation of cell killing of targets presenting peptides 1 to 6 was determined by using a standard chromium release assay. KSHV-specific CTL responses were greatest to K12 P2, with a mean specific cytotoxicity of 38% (±5%) at a 40:1 effector/target ratio (Fig. 1b). The mean percent specific cytotoxicities for the other five peptides ranged from 24 to 30%. For K12 P2, a correlation was observed between the number of SFC in the ELIspot assay and the KSHV-specific cytotoxicity in the chromium release assay (r = 0.83, P = 0.08).
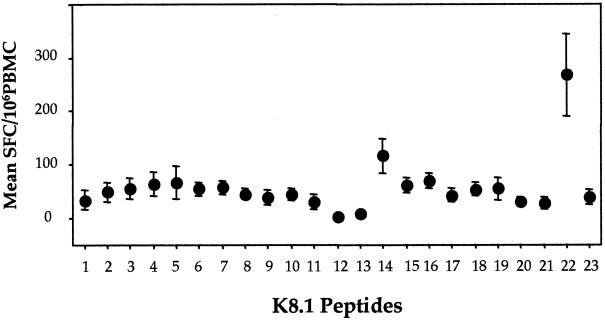
(iv) K8.1 ELIspot assay.
Differential responses to the 23 peptides of K8.1 were observed (Fig. 2). The numbers of SFC were the highest for peptides 22 (P22) and 14 (P14), with mean numbers of 268 (±76) and 116 (±33) SFC/106 PBMC, respectively. The response to K8.1 P22 was significantly greater than that to any of the other overlapping peptides (P < 0.02), for which, with the exception of P14, the mean number of SFC was <70.
FIG. 2.
Determination of K8.1 peptide-specific IFN-γ-producing PBMC in KSHV+ KS+individuals (n = 10). Means and SEs are shown.
Longitudinal analysis of KSHV-specific CTL epitopes in patients receiving HAART.
As a result of these studies, P2 (VHVPDVLLNGWRWRL) of K12 and P14 (ELTDALISAFSGSYS) and P22 (LILYLCVPRCRRKKP) of K8.1 were used to longitudinally monitor the reconstitution of KSHV-specific CTL in a cohort of 19 patients receiving HAART for 52 weeks.
(i) Treatment and clinical response.
Of the 11 HIV-1+ KSHV+ KS+ patients at day 0, 7 received HAART alone, 1 received radiotherapy in addition to HAART, and 3 received chemotherapy in addition to HAART. The HIV-1+ KSHV+ KS− and HIV-1+ KSHV− KS− patients received HAART alone. Four of the 11 individuals resolved their KS lesions, 4 showed a marked improvement in their KS lesions, 2 demonstrated no change, and 1 showed worse symptoms by week 52. Three of the four patients receiving additional therapy with HAART resolved their KS lesions; the fourth was the single patient who showed an increase in KS lesions at week 52. The four patients with KS resolution at week 52 demonstrated decreases to undetectable levels of both HIV-1 and KSHV loads by week 52. The four patients showing an improvement in KS demonstrated undetectable HIV-1 loads and reductions in KSHV loads; however, as expected, with the presence of KS lesions at week 52, they did not show undetectable levels. Similarly, the two individuals with KS present at week 52 demonstrated undetectable HIV-1 loads but positive KSHV loads, although these were reduced from day 0. The single patient with deteriorating KS status by week 52 demonstrated positive HIV-1 and KSHV loads throughout the study.
(ii) Baseline characteristics.
The baseline characteristics of the three cohorts of 19 HIV-1+ KSHV+/− KS+/− patients were generally well matched prior to the initiation of therapy (Table 1). Significant differences between the cohort parameters were observed, including, as expected, a significantly lower KSHV load in the KSHV− KS− cohort than in both the KSHV+ KS+ and the KSHV+ KS− cohorts (P = 0.03 and 0.004, respectively). In addition, the KSHV+ KS+ cohort demonstrated a significantly lower CD4+ T-lymphocyte count (P = 0.04) than the KSHV− KS− cohort and a significantly higher HIV-1 load (P = 0.05) than the KSHV+ KS− cohort at baseline.
(iii) Correlation of KSHV peptide-specific SFC with biological markers prior to HAART.
At baseline, the number of KSHV peptide-specific SFC for K8.1 P22 significantly correlated with both CD4+ and CD8+ T-lymphocyte counts (r = 0.7 and 0.68 and P = 0.02 and 0.02, respectively), such that higher numbers of IFN-γ-secreting cells were associated with higher numbers of both T-lymphocyte subsets. A similar trend was observed for K12 P2, with higher numbers of SFC being associated with higher numbers of both CD4+ and CD8+ T-lymphocytes at baseline (r = 0.59 and 0.56 and P = 0.06 and 0.07, respectively). The total number of SFC for all three peptides combined (P2, P14, and P22) also positively correlated with the numbers of CD4+ and CD8+ T-lymphocytes at baseline (r = 0.65 and 0.62 and P = 0.03 and 0.04, respectively). There were no other correlations between the number of KSHV peptide-specific SFC and any other biological parameter measured.
(iv) Immune reconstitution with HAART. (a) Effects of HAART on T-lymphocyte count and viral load following 52 weeks of therapy.
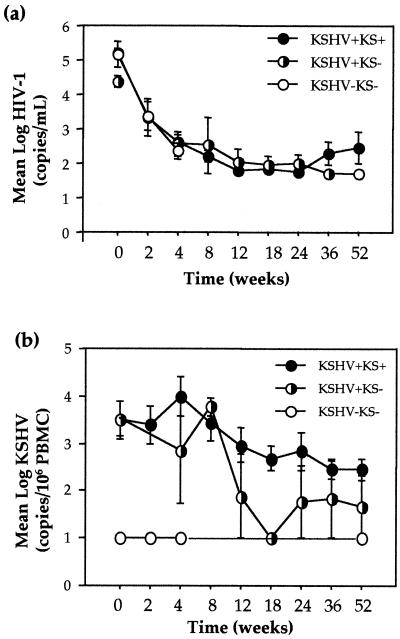
Characteristically, mean log plasma HIV-1 loads significantly decreased in all three cohorts following 52 weeks of HAART to 288 copies/ml in the KSHV+ KS+ cohort (P = 0.01) and to below detectable levels (<50 copies/ml) in the KSHV+ KS− (P = 0.05) and KSHV− KS− (P = 0.03) cohorts (Fig. 3a). Mean CD4+ T-lymphocyte counts increased by 159, 53, and 225 cells/μl and mean CD8+ T-lymphocyte counts increased by 152 cells/μl and decreased by 249 and 109 cells/μl in the KSHV+ KS+, KSHV+ KS−, and KSHV− KS− cohorts, respectively. Additionally,we noted a significant mean log reduction of 1.1 in KSHV load in the KSHV+ KS+ cohort to 290 copies/106 PBMC (P = 0.04), a mean log reduction of 1.83 to 47 copies/106 PBMC in the KSHV+ KS− cohort (P = 0.08), and no change in the KSHV− KS− cohort, in which the load remained undetectable at <100 copies/106 PBMC (Fig. 3b). The mean indirect immunofluorescence assay KSHV antigen titers increased in all three cohorts by 45,546, 79,733, and 4,200 to 121,600, 115,200, and 6,400 at week 52 for the KSHV+ KS+, KSHV+ KS−, and KSHV− KS− cohorts, respectively, suggesting increased B-cell functionality.
FIG. 3.
Mean viral loads in the three cohorts of HIV-1-infected patients receiving HAART. (a) Mean and SE plasma HIV-1 load. (b) Mean and SE KSHV load.
(b) Reconstitution of KSHV-specific IFN-γ-producing T lymphocytes in patients receiving HAART.
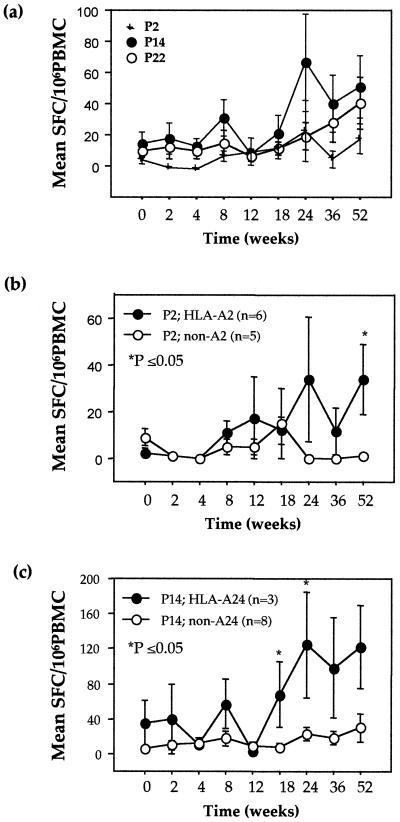
Following 52 weeks of HAART, we noted 3.8-, 1.5-, and 1.4-fold increases from baseline in the total numbers of SFC for the three KSHV-specific peptides in the 11 KSHV+ KS+, 4 KSHV+ KS−, and 4 KSHV− KS− patients, respectively. In the KSHV+ KS+ cohort, these data represented a significant increase from baseline at weeks 24 (P = 0.04) and 52 (P = 0.02) from 29 to 109 (week 24) and 110 (week 52) SFC/106 PBMC for P2, P14, and P22 combined. Individual peptide responses in this cohort equated to 3.8-, 3.6-, and 4-fold increases for K12 P2 and K8.1 P14 and P22, respectively, at week 52. Mean peak responses were 24 ± 19 SFC at week 24 for K12 P2, a significant increase from baseline (P = 0.02); 66 ± 31 SFC at week 24 for K8.1 P14; and 40 ± 16 SFC at week 52 for K8.1 P22 (Fig. 4a). At week 52, the numbers of SFC for the two peptides of the K8.1 lytic protein demonstrated further increases, while responses to P2 of the K12 latent protein were transient, with the expression of between 5 and 24 SFC throughout therapy. Positive correlations between K8.1 P22 SFC and both CD8+ T-lymphocyte number (r = 0.79, P = 0.01) and log KSHV load (r = 0.64, P = 0.09) at week 52 suggested an association between IFN-γ release and effector cell number and antigenic stimulation. Such an association was not observed for K12 P2 or K8.1 P14.
FIG. 4.
Numbers of IFN-γ-secreting SFC in 11 HIV-1+ KSHV+ KS+ individuals for KSHV K12 and K8.1 P2, P14, and P22 (a) and for P2 and P14 when grouped according to HLA types HLA-A2 (b) and HLA-A24 (c), respectively. Means and SEs are shown.
(c) HLA restriction.
A comparison of the KSHV peptide-specific responses between individual HLA types was performed to determine peptide restriction in the 11 KSHV+ KS+ patients. In support of the peptide-binding predictions for K12 P2, there was a significant increase in the mean number of SFC in HLA-A2 individuals (n = 6) compared to non-HLA-A2 individuals (n = 5) at week 52 of HAART (P = 0.02) (Fig. 4b). The HLA-A2 individuals demonstrated mean increases from baseline to week 52 of 31 (P = 0.02), 49, and 14 SFC while the non-HLA-A2 individuals demonstrated a decrease of 8 SFC and increases of 21 and 52 SFC for the KSHV-specific peptides K12 P2 and K8.1 P14 and P22, respectively. This difference in responses between HLA-A2 and non-HLA-A2 individuals, with an increase in SFC in HLA-A2 individuals, was significant for P2 (P = 0.02) alone, suggesting HLA-A2 restriction for this peptide. Similarly, there was a suggestion that K8.1 P14 was restricted through HLA-A24 (Fig. 4c). When the KSHV+ KS+ cohort was again divided by HLA type, the HLA-A24 individuals (n = 3) demonstrated significantly higher numbers of P14-specific SFC at weeks 18 (P = 0.04) and 24 (P = 0.03) of HAART than the non-HLA-A24 individuals (n = 8), a trend that remained to week 52 (P = 0.08). Increases in SFC from baseline in the HLA-A24 cohort peaked at week 24 with 125 ± 60 SFC and remained at 123 ± 48 SFC at week 52. In contrast, the number of SFC in the non-HLA-A24 cohort remained at <30 throughout HAART. No HLA restriction could be determined for K8.1 P22 despite strong computer predictions for the binding of closely associated K8.1 P20 and P21 to HLA-A24 and HLA-A2, respectively. Smaller overlapping peptides of between 8 and 10 aa may be required to determine HLA restriction for P22.
(d) Immune reconstitution toward HIV-1 in patients receiving HAART.
To confirm that the low level of SFC observed for the KSHV peptides was a genuine level of expression, an HIV-1 peptide was used as an internal control. The frequency of gag-specific IFN-γ-secreting cells was determined by using the HLA-A2-restricted peptide SLYNTVATL. At baseline, the four HIV-1+ KSHV+ KS+ patients demonstrated a mean of 115 ± 43 SFC/106 PBMC; this level decreased to 57 ± 57 and 43 ± 23 SFC at weeks 24 and 36, respectively. This loss of SFC was accompanied by a decrease in mean HIV-1 load to below detectable levels (<50 copies/ml) at week 24. At week 36, the cohort demonstrated a viral rebound with an increase in mean HIV-1 load to 347 ± 7 copies/ml, which resulted in a mean increase in the level of gag-specific IFN-γ-producing cells to 163 ± 134 SFC/106 PBMC at week 52.
DISCUSSION
This study provides a novel description of virus-specific CTL epitopes for KSHV. We identified three dominant epitopes, one from the latent gene K12 (recently confirmed by Micheletti et al. in a preliminary manner [F. Micheletti, P. Monini, C. Fortini, P. Rimessi, M. Bazzaro, M. Andreoni, B. Ensoli, and R. Gavioli, Abstr. 4th Int. Conf. HHV-6, -7 and -8, abstr. 015, 2001]) and two immunogenic peptides from the lytic gene K8.1 that both induced CD8+ IFN-γ release and direct cell lysis. The CD8+ CTL epitopes were major histocompatibility complex class I restricted, with a propensity for HLA-A2 and HLA-24 restriction, as predicted by an analysis of HLA-peptide binding. However, CD8+ T-lymphocyte responses were observed in non-HLA-A2 and non-HLA-A24 individuals, and K8.1 P22 could not be associated with HLA restriction, suggesting that there may be more promiscuous HLA restriction with these peptides. Additionally, we characterized CD8+ CTL responses to these immunogenic KSHV peptides in a cohort of HIV-1+ KSHV+ KS+ individuals receiving 52 weeks of HAART and showed an increase in their numbers with antiretroviral therapy. Such immune reconstitution toward KSHV was not previously described. The numbers of KSHV-specific CTL detected in this study were relatively low, in support of the results of other studies (20, 28) which detected KSHV-specific CTL and which also demonstrated low frequencies. However, similar frequencies of CD8+ T-cell responses have been reported for HIV-1, EBV, and cytomegalovirus antigens (9). Despite the low level of immune reconstitution toward KSHV, there was an approximate fourfold increase in CD8+ T-lymphocyte responses to all three immunogenic peptides at week 52 of HAART.
Following initial experiments to confirm cell lysis in a chromium release assay, we chose the ELIspot assay over the more conventional chromium release assay because it was easier to use and resulted in similar data, as shown by regression analysis. In addition, the frequencies of IFN-γ-releasing cells were shown to be higher than precursor CTL frequencies obtained by limiting dilution assays (18, 27); this aspect is an advantage in the presence of low CD8+ effector frequencies.
Following HAART, there was an upward trend in the number of SFC for the K8.1 lytic antigens P14 and P22 at week 52, and the CD8+ T-lymphocyte response may have increased further with prolonged antiretroviral therapy. The increased number of IFN-γ-releasing cells and the wide variation in individual responses, as shown by wide SE bars for cohort A, may be a reflection of the amount of time each individual has been receiving HAART. We observed a greater reconstitution of the immune system toward the lytic peptides of KSHV than toward the latent peptide (K12), a finding that was previously reported for whole KSHV proteins (28). Interestingly, it is the lytic cycle proteins of EBV that are targets for CD8+ CTL during both primary and chronic infections (26). This CD8+ response to the lytic antigens, in addition to the correlation between KSHV load and K8.1 P22 SFC numbers at week 52, suggests that there may be ongoing KSHV replication within the HIV-1+ KSHV+ KS+ cohort, similar to the antigenic stimulation required for CD8+ responses in HIV-1 infection (19, 30). In other words, ongoing KSHV replication may be a requirement to induce CD8+ responses, as with HIV-1, an idea which is supported by our data obtained with the HIV-1 peptide SLYNTVATL.
Following 52 weeks of HAART, decreases in KSHV load were greater in patients without KS than in those with KS, suggesting a positive role for early therapeutic intervention. The higher level of viral replication in the KSHV+ KS+ cohort was associated with a higher number of SFC to the lytic peptides of KSHV compared to the results obtained for the KSHV+ KS− cohort. However, the increases in the CD8+ cell-mediated responses to the latent peptide of KSHV were the same for both cohorts. This result suggests that virus is replicating faster in patients with KS than in those without KS and also that it may be important to induce immune reconstitution specific for latent proteins, which could prevent progression to KS in KSHV+ KS− patients.
In addition to KSHV replication, the data suggest that CD4+ T-lymphocyte help may also be required for anti-KSHV CD8+ T-cell responses. The correlation observed between KSHV peptide responses and numbers of T lymphocytes pre- and post-HAART suggests that high numbers of effector cells are required in conjunction with high numbers of CD4+ T-helper cells, a concept that has been well documented for HIV-1 (10, 11). The increased response to the immunogenic peptides in cohort A may therefore be a reflection of the higher number of CD4+ T lymphocytes in these individuals than in cohort B individuals.
In addition, we showed an increase in anti-KSHV antibody titer over 52 weeks of HAART, indicating a possible restoration of humoral immune responses. These responses may influence KSHV pathogenesis and KS lesion regression.
In this study, we have identified class I-restricted CTL against KSHV, allowing us to evaluate the effectiveness of HAART in the reconstitution of KSHV-specific CTL. Following confirmation of this restriction with smaller overlapping peptides, we will develop a KSHV tetramer with which we can measure the frequency of KSHV-specific CTL ex vivo. Our data suggest that with the reduction of HIV-1 load with effective antiretroviral therapy, the immune system is able to deal with other host infections. The numbers of KSHV-specific CTL are relatively low in comparison to the numbers of HIV-1-specific CTL, but we have detected these responses in chronically HIV-1-infected individuals who have a CD4+ count of <200 cells/μl but who are still able to demonstrate increasing responses over time on HAART. However, the identification of KSHV-specific CTL epitopes is a major advance in the understanding of KSHV-immune interactions and may lead to successful prophylactic and therapeutic vaccines.
Acknowledgments
John Wilkinson and Alethea Cope contributed equally to this work.
We thank all the patients who were involved in this study.
This work was funded by MRC grant 35 G9825083. Frances Gotch and Chris Boshoff are supported by funding from the UK MRC, CRC, Wellcome Trust, LRF, NIH, and EU.
REFERENCES
- 1.Bieche, I., M. Olivi, M. Champeme, D. Vidaud, R. Lidereau, and M. Vidaud. 1998. Novel approach to quantitative polymerase chain reaction using real-time detection: application to the detection of gene amplification in breast cancer. Int. J. Cancer 78:661-666. [DOI] [PubMed] [Google Scholar]
- 2.Borrow, P., H. Lewick, B. Hahn, G. Shaw, and M. Oldstone. 1994. Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J. Virol. 68:6103-6110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. 1981. Kaposi's sarcoma and pneumocystis pneumonia among homosexual men. Morb. Mortal. Wkly. Rep. 30:305-308. [PubMed] [Google Scholar]
- 4.De Milito, A., M. Catucci, G. Venturi, L. Romano, L. Incandela, P. Valensin, and M. Zazzi. 1999. Antiretroviral therapy with protease inhibitors in HIV-1 and human herpesvirus 8-coinfected patients. J. Med. Virol. 57:140-144. [DOI] [PubMed] [Google Scholar]
- 5.Gao, S., L. Kingsley, M. Li, W. Zheng, C. Parravinci, J. Ziegler, R. Newton, C. Rinaldo, A. Saah, J. Phair, R. Detels, Y. Chang, and P. Moore. 1996. KSHV antibodies among Americans, Italians and Ugandans with and without Kaposi's sarcoma. Nat. Med. 2:925-928. [DOI] [PubMed] [Google Scholar]
- 6.Goulder, P., A. Sewell, D. Lalloo, D. Price, J. Whelan, J. Evans, G. Taylor, G. Luzzi, P. Giangrande, R. Phillips, and A. McMichael. 1997. Patterns of immunodominance in HIV-1-specific cytotoxic T lymphocyte responses in two HLA-identical siblings with HLA-A*0201 are influenced by epitope mutation. J. Exp. Med. 185:1423-1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haverkos, H., and D. Drotman. 1985. Prevalence of Kaposi's sarcoma among patients with AIDS. N. Engl. J. Med. 312:1518.. [PubMed] [Google Scholar]
- 8.International Collaboration on HIV-1 and Cancer. 2000. Highly active antiretroviral therapy and the incidence of cancer in HIV-infected adults. J. Natl. Cancer Inst. 92:1823-1830. [DOI] [PubMed] [Google Scholar]
- 9.Jin, X., M.-A. Demoitie, S. M. Donahoe, G. S. Ogg, S. Bonhoeffer, W. M. Kakimoto, G. Gillespie, P. A. Moss, W. Dyer, M. G. Kurilla, S. R. Riddell, J. Downie, J. S. Sullivan, A. J. McMichael, C. Workman, and D. F. Nixon. 2000. High frequency of cytomegalovirus-specific cytotoxic T-effector cells in HLA-A*0201-positive subjects during multiple viral coinfections. J. Infect. Dis. 181:165-175. [DOI] [PubMed] [Google Scholar]
- 10.Kalams, S., S. Buchbinder, E. Rosenberg, J. Billingsley, D. Colbert, N. Jones, A. Shea, A. Trocha, and B. Walker. 1999. Association between virus-specific cytotoxic T-lymphocyte and helper responses in human immunodeficiency virus type 1 infection. J. Virol. 73:6715-6720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kalams, S., and B. Walker. 1998. The critical need for CD4 help in maintaining effective cytotoxic T lymphocyte responses. J. Exp. Med. 188:2199-2204. [DOI] [PMC free article] [PubMed]
- 12.Lallemand, F., N. Desire, W. Rozenbaum, J. Nicholas, and V. Marechal. 2000. Quantitative analysis of human herpesvirus 8 viral load using a real-time PCR assay. J. Clin. Microbiol. 38:1404-1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lalvani, A., R. Brookes, S. Hambleton, W. Britton, A. Hill, and A. McMichael. 1997. Rapid effector function in CD8+ memory T cells. J. Exp. Med. 186:859-865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lebbe, C., L. Blum, C. Pellet, G. Blanchard, O. Verola, P. Morel, O. Danne, and F. Calvo. 1998. Clinical and biological impact of antiretroviral therapy with protease inhibitors on HIV-related Kaposi's sarcoma. AIDS 12:F45-F49. [DOI] [PubMed]
- 15.Ledergerber, B., A. Telenti, and M. Egger. 1999. Risk of HIV related Kaposi's sarcoma and non-Hodgkin's lymphoma with potent antiretroviral therapy: prospective cohort study. Swiss HIV Cohort Study. Br. Med. J. 319:23-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martinelli, C., M. Zazzi, S. Ambu, D. Bartolozzi, P. Corsi, and F. Leoncini. 1998. Complete regression of AIDS-related Kaposi's sarcoma-associated herpesvirus-8 during therapy with indinavir. AIDS 12:1717-1719. [PubMed] [Google Scholar]
- 17.Niehues, T., G. Horneff, M. Megahed, H. Schroten, and V. Wahn. 1999. Complete regression of AIDS-related Kaposi's sarcoma in a child treated with highly active antiretroviral therapy. AIDS 13:1148-1149. [DOI] [PubMed] [Google Scholar]
- 18.Ogg, G., and A. McMichael. 1999. Quantitation of antigen-specific CD8+ T-cell responses. Immunol. Lett. 66:77-80. [DOI] [PubMed] [Google Scholar]
- 19.Ogg, G. S., X. Jin, S. Bonhoeffer, P. R. Dunbar, M. A. Nowak, S. Monard, J. P. Segal, Y. Cao, S. L. Rowland-Jones, V. Cerundolo, A. Hurley, M. Markowitz, D. D. Ho, D. F. Nixon, and A. J. McMichael. 1998. Quantitation of HIV-1-specific cytotoxic T lymphocytes and plasma load of viral RNA. Science 279:2103-2106. [DOI] [PubMed] [Google Scholar]
- 20.Osman, M., T. Kubo, J. Gill, F. Neipel, M. Becker, G. Smith, R. Weiss, B. Gazzard, C. Boshoff, and F. Gotch. 1999. Identification of human herpesvirus 8-specific cytotoxic T-cell responses. J. Virol. 73:6136-6140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parker, K., M. Bednarek, and J. Coligan. 1994. Scheme for ranking potential HLA-A2 binding peptides based on independent binding of individual peptide side-chains. J. Immunol. 152:163-175. [PubMed] [Google Scholar]
- 22.Rickinson, A., and D. Moss. 1997. Human cytotoxic T lymphocyte responses to Epstein-Barr virus infection. Annu. Rev. Immunol. 15:405-431. [DOI] [PubMed] [Google Scholar]
- 23.Sarid, R., S. Olsen, and P. Moore. 1999. Kaposi's sarcoma-associated herpesvirus: epidemiology, virology and molecular biology. Adv. Virus Res. 52:139-232. [DOI] [PubMed] [Google Scholar]
- 24.Schmitz, J. E., M. J. Kuroda, S. Santra, V. G. Sasseville, M. A. Simon, M. A. Lifton, P. Racz, K. Tenner-Racz, M. Dalesandro, B. J. Scallon, J. Ghrayeb, M. A. Forman, D. C. Montefiori, E. P. Rieber, N. L. Letvin, and K. A. Reimann. 1999. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science 283:857-860. [DOI] [PubMed] [Google Scholar]
- 25.Shaw, A., and K. McLean. 1999. Kaposi's sarcoma regression following treatment with a triple antiretroviral regimen containing nevirapine. Int. J. STD AIDS 10:417-418. [PubMed] [Google Scholar]
- 26.Steven, N., N. Annels, A. Kumar, A. Leese, M. Kurilla, and A. Rickinson. 1997. Immediate early and early lytic cycle proteins are frequent targets of the Epstein-Barr virus-induced cytotoxic T cell response. J. Exp. Med. 185:1605-1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang, Q., F. Jenkins, L. Jacobson, L. Kingsley, R. Day, Z. Zhang, Y. Meng, P. Pellet, K. Kousoulas, A. Baghian, and C. Rinaldo. 2001. Primary human herpesvirus 8 infection generates a broadly specific CD8+ T-cell response to viral lytic cycle proteins. Blood 97:2366-2373. [DOI] [PubMed] [Google Scholar]
- 28.Wang, Q., F. Jenkins, L. Jacobson, Y. Meng, P. Pellet, L. Kingsley, K. Kousoulas, A. Baghian, and C. Rinaldo. 2000. CD8+ cytotoxic T lymphocyte responses to lytic proteins of human herpes virus 8 in HIV-1-infected and uninfected individuals. J. Infect. Dis. 182:928-932. [DOI] [PubMed] [Google Scholar]
- 29.Whitby, D., M. Luppi, P. Barozzi, C. Boshoff, R. Weiss, and G. Torelli. 2001. HHV8 seroprevalence in blood donors and lymphoma patients from different regions of Italy. J. Natl. Cancer Inst. 90:395-397. [DOI] [PubMed] [Google Scholar]
- 30.Wilkinson, J., J. Zaunders, A. Carr, and D. Cooper. 1999. CD8+ anti-HIV-1 suppressor activity (CASA) in response to antiretroviral therapy: loss of CASA is associated with loss of viremia. J. Infect. Dis. 180:68-75. [DOI] [PubMed] [Google Scholar]