Abstract
The application of adenoviral vectors in cancer gene therapy is hampered by low receptor expression on tumor cells and high receptor expression on normal epithelial cells. Targeting adenoviral vectors toward tumor cells may improve cancer gene therapy procedures by providing augmented tumor transduction and decreased toxicity to normal tissues. Targeting requires both the complete abolition of native tropism and the addition of a new specific binding ligand onto the viral capsid. Here we accomplished this by using doubly ablated adenoviral vectors, lacking coxsackievirus-adenovirus receptor and αv integrin binding capacities, together with bispecific single-chain antibodies targeted toward human epidermal growth factor receptor (EGFR) or the epithelial cell adhesion molecule. These vectors efficiently and selectively targeted both alternative receptors on the surface of human cancer cells. Targeted doubly ablated adenoviral vectors were also very efficient and specific with primary human tumor specimens. With primary glioma cell cultures, EGFR targeting augmented the median gene transfer efficiency of doubly ablated adenoviral vectors 123-fold. Moreover, EGFR-targeted doubly ablated vectors were selective for human brain tumors versus the surrounding normal brain tissue. They transduced organotypic glioma and meningioma spheroids with efficiencies similar to those of native adenoviral vectors, while exhibiting greater-than-10-fold-reduced background levels on normal brain explants from the same patients. As a result, EGFR-targeted doubly ablated adenoviral vectors had a 5- to 38-fold-improved tumor-to-normal brain targeting index compared to native vectors. Hence, single-chain targeted doubly ablated adenoviral vectors are promising tools for cancer gene therapy. They should provide an improved therapeutic index with efficient tumor transduction and effective protection of normal tissue.
Recombinant adenoviral vectors (AdV) appear to be promising for therapeutic interventions in humans, including gene therapy for cancer and cardiovascular diseases. In this regard, the principle attribute of AdV is their superior in vivo gene transfer efficiency on many different human tissues. However, this broad tropism at the same time represents an important limitation for their use in therapeutic applications where specific gene transfer is required. In addition, several potential target cells for gene therapy are poorly transduced by AdV due to scarcity of an appropriate cell surface receptor (29, 43, 45). Notably, many primary tumors express low levels of the coxsackievirus-adenovirus receptor (CAR), resulting in low levels of gene delivery into these cancer cells (5, 12, 24, 27, 28). Targeting AdV toward alternative surface receptors on specific cell types may overcome these limitations. This requires abolition of native viral tropism and introduction of a novel binding affinity.
Two general strategies are currently being considered to target AdV in order to enhance vector infectivity and specificity. In the first approach, AdV are genetically modified to alter the binding specificity of the viral capsid, thus creating a stable single-reagent genetic medicine (22). Presently, the major limitation for further development of this type of vector is incomplete knowledge of the restrictions to successful ligand incorporation in adenovirus capsid proteins. In the second approach, AdV are complexed with bispecific molecules that on one side bind to the viral capsid and on the other side redirect the virus to a novel receptor (7-9, 14, 15, 17, 28, 38, 43). Alternatively, a new ligand is chemically coupled onto the viral capsid (35). The biggest advantage of this two-component strategy is its versatility. The continuous identification of high-affinity peptide ligands and antibodies vastly increases the number of potential targets for this type of vector. However, a major disadvantage of AdV targeted with bispecific molecules is that inhibition of native receptor binding relies on neutralization by the targeting molecules. Therefore, until now the one-component approach was considered to offer the best advantages for manufacture of gene therapeutics, while the two-component strategy was used mainly as a powerful means for validating the utility of alternative receptors as potential targets for AdV-mediated gene delivery.
Recently, specific mutations which eliminate the interaction with CAR were identified in the adenovirus fiber knob (20, 21, 34). Such mutated AdV show reduced transduction of CAR-expressing cells in vitro but retain significant CAR-independent infectivity in vivo (11, 40). Because residual transduction was found to be integrin dependent and mediated through the adenovirus penton base protein (34, 40), AdV with both mutations in the fiber knob eliminating the interaction with CAR and a deletion of their αv integrin-binding penton base RGD motif were constructed. These doubly ablated AdV exhibited dramatically reduced tissue transduction after intravenous administration in mice (11).
Here we show that by combining doubly ablated AdV with bispecific targeting molecules, an important drawback of the two-component strategy for AdV targeting has been overcome. In this new system, abolition of native tropism no longer depends on neutralization by the targeting molecule but is inherent to the doubly ablated AdV. The bispecific targeting molecules that we used efficiently redirected the doubly ablated AdV toward alternative receptors on human cancer cells and primary brain tumors, allowing CAR- and integrin-independent gene delivery. This resulted in an improved targeting index of tumor to normal tissue transduction. Hence, targeted AdV such as those described here are likely to improve the therapeutic potential of cancer gene therapy.
MATERIALS AND METHODS
Cell lines, primary tumor cells, and organotypic spheroids.
Rat2 fibroblasts and the human cancer cell lines OVCAR-3 (ovary carcinoma), HT29 (colon carcinoma), 11B (head and neck squamous carcinoma), and U373MG (anaplastic astrocytoma) were purchased from the American Type Culture Collection (Manassas, Va.). LNCaP (prostate carcinoma) was a kind gift of L. Blok (Erasmus University Rotterdam, The Netherlands), MKN-28 (gastric carcinoma) was kindly provided by M. Tsujii (Osaka University School of Medicine, Osaka, Japan), and U118MG (glioblastoma multiforme [GBM]) was a kind gift of J. T. Douglas (Gene Therapy Center, University of Alabama, Birmingham). LNCaP cells were grown in RPMI 1640 with 10% fetal calf serum (FCS) and antibiotics; all other lines were maintained in F12-supplemented Dulbecco modified Eagle medium (DMEM) with 10% FCS and antibiotics. All culture media and supplements were purchased from Gibco BRL Life Technologies, Paisley, United Kingdom.
Fresh material was collected during surgery of patients with brain tumors after informed consent and processed within 3 h after dissection. Pathological confirmation of the diagnosis was made on the tumor material that was processed for cell and spheroid culture. Primary glioma cell cultures were obtained after mechanical dissociation from confirmed GBM, according to the technique described by Darling (6). The primary cells were cultured in DMEM supplemented with 10% FCS and antibiotics. Glial origin of the cultured cells was confirmed by morphology and by staining of early passages with anti-GFAP monoclonal antibody (MAb) clone 6F2 (Dako, Glostrup, Denmark). Gene transfer experiments were done before passage 5. Organotypic multicellular spheroids (OMS) were prepared from three supratentorial GBM and one infratentorial meningioma by the technique originally described by Bjerkvig et al. (2). Briefly, the tumor tissue was cut with 21-gauge needles into 0.3- to 0.5-mm pieces, which were incubated individually in 48-well plates coated with agarose (2% in phosphate-buffered saline [PBS]) in DMEM with 10% FCS, antibiotics, and 2 mM l-glutamine. After cells were checked for viability by morphology, spheroids of similar diameter (400 to 500 μm) were used for gene transfer experiments. Additionally, from each individual patient explants were made from small pieces of damaged brain removed from the corticotomy tract or from the surroundings of the tumor that was resected during surgery and were cultured according to the same procedure. Gene therapy experiments on tumor spheroids and normal brain explants were performed within the first 2 weeks after tumor removal.
Construction of recombinant AdV.
AdL is a replication-defective AdV derived from human adenovirus serotype 5 (Ad5), with deleted E3 sequences (1.9-kb XbaI fragment deleted with XbaI site filled) and a cytomegalovirus promoter-driven firefly luciferase gene expression cassette in place of the E1 region. All other AdV are derivatives of AdL with specific mutations in the fiber and penton base genes that eliminate binding to CAR and αv integrins, respectively. AdL.F(RAEK-HA) contains four point mutations in the AB loop of the fiber knob (R412S, A415G, E416G, and K417G) that abolish binding to CAR, and AdL.F(TAYT-HA) comprises a deletion of amino acids T489AYT492 in the FG loop of the fiber knob that abolishes binding to CAR (34). In addition, AdL.F(RAEK-HA) and AdL.F(TAYT-HA) carry an insertion of the amino acid sequence SRGFKSYPYDVPDYAG in the HI loop of the fiber knob between amino acids G443 and D444, where the underlined amino acids represent a hemagglutinin (HA) peptide motif that enables propagation of AdV with CAR binding abolished on 293-HA cells (10). AdL.PB(HA)F(TAYT-HA) comprises the mutations described for AdL.F(TAYT-HA) and, additionally, has αv integrin binding eliminated through replacement of amino acids H337AIRGDTF344 in the penton base (41) with amino acids SRGYPYDVPDYAGTS, where the underlined sequence again represents an HA peptide motif.
Purified virus stocks were prepared by two successive bandings on CsCl gradients. Virus was stored at −80°C in 10 mM Tris (pH 7.8)-150 mM NaCl-10 mM MgCl2-40% glycerol until use. Viral particle (VP) titers were determined by measurement of optical density at 260 nm, and focus-forming unit titers were determined on 293-HA cells by staining with anti-DBP-fluorescein isothiocyanate antibody (Strategic Biosolutions, Newark, Del.) at 24 h postinfection. Ratios of VP to focus-forming units were 11 for AdL, 18 for AdL.F(RAEK-HA), 142 for AdL.F(TAYT-HA), and 256 for AdL.PB(HA)F(TAYT-HA).
Production of bispecific single-chain targeting antibodies.
Bispecific scFv expression constructs comprising the adenovirus infection-neutralizing S11 antibody (38) were made as previously described (14), by inserting scFv fusion genes into pSTCF (1). This creates in-frame fusions with an amino-terminal murine immunoglobulin κ-chain leader sequence and carboxy-terminal Myc-His6 tag. Anti-epidermal growth factor receptor (anti-EGFR)-targeting bispecific scFv 425-S11 has been described before (14); anti-epithelial cell adhesion molecule (anti-EpCAM) bispecific scFv C28-S11 was constructed according to the same procedure by using anti-EpCAM scFv C28, a derivative of UBS-54 (19) (kindly provided by T. Logtenberg, Crucell Holland B.V., Leiden, The Netherlands). CHO cells were stably transfected with these constructs and cultured in a CL 350 cultivation system (INTEGRA Biosciences AG, Wallisellen, Switzerland) in CHO-S-SFM II medium (Life Technologies). Undiluted culture supernatant of the same batch was used for all targeting experiments. The relative bispecific scFv concentration was determined by enzyme-linked immunosorbent assay using recombinant fiber knob protein (23) as the antigen and anti-Myc MAb 9E10 (4) followed by rabbit anti-mouse immunoglobulin G-horseradish peroxidase conjugate and o-phenylenediamine substrate (Dako) for detection. The concentration of 425-S11 was approximately 15 times higher than that of C28-S11.
AdV-mediated transduction.
Cell lines and primary cell cultures were seeded at 104 cells/well in 96-well plates or at 105 cells/well in 24-well plates and cultured overnight before AdV transduction. For targeting, AdV were incubated at 5 × 109 VP/ml in 50% (vol/vol) bispecific scFv CHO supernatant and 50% (vol/vol) F12-supplemented DMEM with 2% FCS for 30 min at room temperature. Control transduction experiments with 425-S11 on CAR-deficient U118MG cells showed that the amount of bispecific scFv used was at least 10-fold higher than that needed to obtain maximal enhancement of gene delivery. After incubation, AdV were diluted to their final concentration in F12-supplemented DMEM with 2% FCS, except for transduction of LNCaP cells, which was done in RPMI 1640 with 10% FCS, and used to transduce cells at the indicated multiplicity of infection (MOI). In all experiments, the same number of VP per cell was used for the different AdV and their complexes with bispecific scFv. After incubation at 37°C for 1 h in most experiments, or for periods ranging from 2 min to 24 h for the time-dependent transduction experiment, the virus was removed from the cells and replaced by the standard culture medium. In one experiment, transduced cells were treated with trypsin (BioWhittaker, Verviers, Belgium) in PBS for 5 min at room temperature immediately following exposure to virus before addition of standard culture medium. Analysis of transduction efficiency by luciferase activity measurement was performed at 24 h after infection.
To transduce a mixed population of OVCAR-3 and U373MG cells, these cells were seeded in a 1:2 proportion at low density in 96-well plates and allowed to form mixed monolayers of OVCAR-3 islands within a U373MG network. Transduction was performed as described above for 1 h at 108 VP per well. Gene transfer was detected by luciferase immunocytochemistry at 48 h after infection.
Brain tumor spheroids and normal brain explants were cultured in separate wells of a 96-well plate coated with agarose in 150 μl of DMEM with 10% FCS. Fifty microliters of medium with 108 VP of AdV or AdV-bispecific scFv complexes produced as described above was added per well, and transduction was allowed to proceed at 37°C overnight. Analysis of transduction efficiency by luciferase activity measurement was performed after 20 to 22 h.
Analysis of luciferase expression.
Luciferase activity was measured using the luciferase chemiluminescence assay system (Promega, Madison, Wis.). For cells, culture medium was replaced with reporter lysis buffer and the whole culture plate was subjected to a single freeze-thaw cycle. Spheroids and brain explants were removed from their culture well, rinsed in PBS, and subjected to three cycles of freeze-thawing and vigorous vortexing in reporter lysis buffer. Chemiluminescence was measured with a Lumat LB 9507 luminometer (EG&G Berthold, Bad Wildbad, Germany) during the 10 s immediately after addition of the cell extract to the luciferase assay reagent. Relative light unit values were normalized per number of cells seeded per well, or per spheroid or explant, after subtraction of the background. In the experiment where cells were treated with trypsin following transduction, data were corrected for loss of viable cells by measuring WST-1 (Roche Diagnostics, Mannheim, Germany) conversion during 1 h prior to cell harvest.
Immunocytochemistry for luciferase expression was done after fixation of cells in tissue culture plates by using 50:50 (vol/vol) methanol-acetone with 2.5 μg of antiluciferase MAb LUC-Y (J. Grill et al., submitted for publication) per ml, followed by rabbit anti-mouse immunoglobulin G-alkaline phosphatase conjugate (Dako) and nitroblue tetrazolium-5-bromo-4-chloro-3-indolylphosphate substrate (Dako). Stained cells were photographed at a magnification of ×100 or ×200.
RESULTS
Targeting AdV with native tropism abolished by using bispecific single-chain antibodies.
Previously, we have successfully redirected AdV with native tropism toward EpCAM or EGFR by using bispecific antibodies that bind on one side to the adenovirus fiber knob and on the other side to EpCAM or EGFR, respectively (14, 15). To investigate whether bispecific scFv bind to AdV with fiber knob mutations eliminating CAR binding, we incubated three different AdV expressing the firefly luciferase marker gene with the EGFR-targeting bispecific scFv 425-S11 (14). These AdV were AdL with native Ad5 tropism and the Ad5-derived vectors with CAR binding abolished, i.e., AdL.F(RAEK-HA) and AdL.F(TAYT-HA) (34), carrying mutations in the fiber knob AB loop and FG loop, respectively. The three AdV and their complexes with 425-S11 were used to infect CAR- and EGFR-positive OVCAR-3 cancer cells, and gene transfer was measured by luciferase activity assay. To discriminate between CAR-dependent and CAR-independent transduction, the cells were preincubated with excess recombinant Ad5 fiber knob protein (23). As can be seen in Fig. 1A, both mutations eliminating CAR binding reduced gene transfer to a level similar to that obtained with native AdL after competition with recombinant Ad5 fiber knob. Targeting with 425-S11 protein augmented gene delivery by AdL and AdL.F(TAYT-HA) but not that by AdL.F(RAEK-HA). The 425-S11-mediated gene transfer was not inhibited by recombinant Ad5 fiber knob, confirming that it was CAR independent. Thus, the 425-S11 bispecific scFv mediated CAR-independent gene transfer of AdV with native fibers and with FG loop-mutated fibers. The AdV with the FG loop mutation could also be used in conjunction with chemical antibody conjugates based on the anti-fiber knob antibody 1D6.14 (8, 15) (data not shown). Apparently, the AB loop mutation eliminated the binding epitope for S11, whereas the FG loop mutation abolished CAR binding but did not affect the epitope for S11 or 1D6.14 antibodies. Therefore, all further experiments to explore targeting of vectors lacking CAR binding with bispecific scFv were done with FG loop mutant AdV only.
FIG. 1.
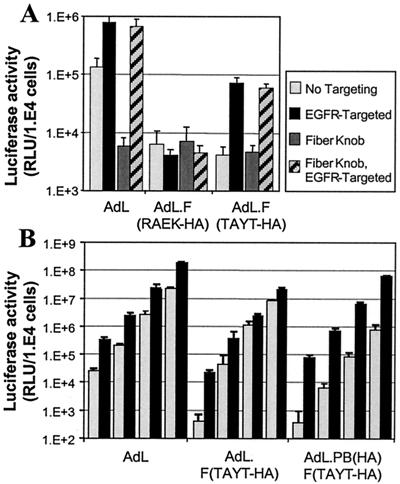
EGFR targeting of AdV with CAR and integrin binding abolished. OVCAR-3 cells were transduced for 1 h with AdL, AdL.F(RAEK-HA), AdL.F(TAYT-HA), or AdL.PB(HA)F(TAYT-HA) at an MOI of 100 VP/cell (A) or of 10, 100, 1,000, or 10,000 VP/cell shown from left to right (B), with or without EGFR targeting using 425-S11 bispecific scFv. (A) Where indicated, OVCAR-3 cells were preincubated with fiber knob protein before transduction with or without EGFR targeting. Twenty-four hours after infection, cells were analyzed for luciferase activity. The data are the means and standard deviations from three independent experiments performed in duplicate (A) or from a representative experiment performed in triplicate (B). RLU, relative light units.
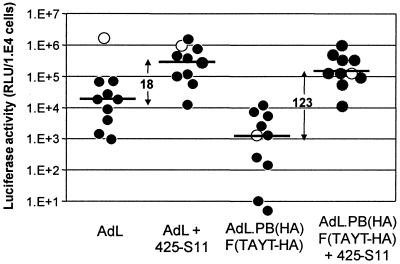
Since AdV with CAR binding abolished retain significant penton base-mediated transduction (11, 34, 40), the use of vectors with the penton base RGD motif also deleted is preferable. To investigate whether targeting with bispecific scFv is also possible in the absence of penton base-integrin interactions, we compared 425-S11-mediated gene delivery by native AdL, AdL.F(TAYT-HA) (with CAR binding eliminated), and doubly ablated AdL.PB(HA)F(TAYT-HA) (with CAR binding plus penton base RGD eliminated) at various MOIs. Figure 1B shows that, in the tested range of 10 to 10,000 VP per cell, transduction by native AdV and by EGFR-targeted native AdV was linearly related to the MOI. Transduction by AdV with CAR binding eliminated was strongly reduced at low MOI, but this reduction was mostly lost at MOIs of 100 VP/cell and higher. Doubly ablated AdV, however, exhibited a constant, approximately 1.5-log-unit-reduced transduction compared to native AdV over the entire MOI range. EGFR targeting by 425-S11 bispecific scFv worked quite efficiently in combination with doubly ablated AdV. Even at the high MOI of 10,000 particles per cell, EGFR-targeted doubly ablated AdV retained a high targeting index (i.e., targeted transduction with bispecific scFv over control transduction without bispecific scFv) of 81, while the targeting index of AdV with CAR binding eliminated was only 3 at this MOI. Thus, especially at higher AdV concentrations, it is preferable to combine bispecific scFv with doubly ablated AdV to achieve selective gene delivery.
Efficiency and kinetics of targeted gene transfer with mutated AdV on human cancer cell lines.
To investigate the versatility of our targeting approach, we decided to compare CAR- and integrin-independent transduction on a panel of human cancer cell lines from different tissue origins, using bispecific scFv targeted toward EGFR or EpCAM. These two cell surface molecules are completely unrelated in aspects relevant for AdV transduction. EGFR is rapidly internalized via endocytosis (39), and EGFR ligation activates phosphatidylinositol 3-OH kinase (PI3K)-dependent signaling (18), a process involved in adenovirus entry (26). In contrast, the pan-carcinoma antigen EpCAM is very slowly internalized (15, 44) and has no known interaction with signaling pathways.
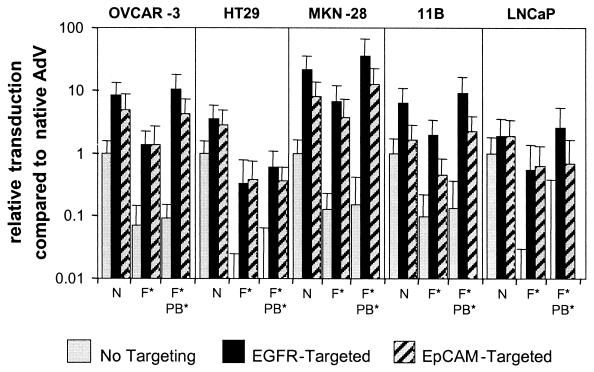
Five human cancer cell lines that expressed all three potential primary binding molecules for AdV and targeted AdV, i.e., CAR, EGFR, and EpCAM, as confirmed by fluorescence-activated cell sorter analysis (not shown), were selected. The cell lines, which represented carcinomas from ovary, colon, stomach, squamous head and neck, and prostate, were transduced with AdL, AdL.F(TAYT-HA), or AdL.PB(HA)F(TAYT-HA) with or without anti-EGFR 425-S11 or anti-EpCAM C28-S11. Figure 2 shows that both EGFR and EpCAM served as targets for AdV-mediated transduction on all five cell lines. In general, EGFR-mediated gene delivery was more efficient than EpCAM-mediated gene transfer. Importantly, neither of the two alternative entry pathways required CAR or integrin binding by fiber knob and penton base, respectively. Moreover, most cell lines were more efficiently transduced by targeted doubly ablated AdV than by targeted AdV with CAR binding abolished. EGFR targeting was four- to sevenfold more efficient in the absence of integrin interaction on all lines except HT29, and EpCAM targeting was three- to fivefold more efficient in the absence of integrin interaction on the OVCAR-3, MKN-28, and 11B lines. In addition, with the exception of colon carcinoma line HT29, all lines were transduced by targeted native AdV and targeted doubly ablated AdV with similar efficiencies. Thus, replacing native AdV with doubly ablated AdV did not compromise the efficacy of targeted gene delivery. Most importantly, the use of CAR- or doubly ablated vectors increased the gene transfer specificity on all cancer cell lines, as can be deduced from their augmented targeting indices compared to native AdV. For EGFR targeting, the average targeting index increased from 8 (range, 2 to 21) for AdL to 36 (range, 19 to 54) for AdL.F(TAYT-HA) to 146 (range, 59 to 252) for AdL.PB(HA)F(TAYT-HA). For EpCAM targeting, the values were 4 (range, 2 to 8), 31 (range, 5 to 63), and 49 (range, 17 to 81), respectively. Hence, the most specific, target molecule-dependent transduction was obtained using the doubly ablated AdV.
FIG. 2.
Targeting toward EGFR and EpCAM on a panel of human cancer cell lines. Human carcinoma cell lines (OVCAR-3, ovary; HT29, colon; MKN-28, stomach; 11B, head and neck; and LNCaP, prostate) with confirmed expression of CAR, EGFR, and EpCAM were transduced for 1 h with AdL (N), AdL.F(TAYT-HA) (F*), or AdL.PB(HA)F(TAYT-HA) (F*PB*) at an MOI of 100 or 1,000 VP/cell, with or without EGFR-specific scFv 425-S11 or EpCAM-specific scFv C28-S11. Luciferase activity was measured after 24 h. The cell lines exhibited considerable diversity in sensitivity to AdV transduction. Therefore, all data were normalized on the basis of native AdV transduction, which was set at 1. The data shown are the means and standard deviations from two independent experiments performed in triplicate. Relative targeted transduction was compared between AdL.F(TAYT-HA) and AdL.PB(HA)F(TAYT-HA) by using Student's t test. Significant augmentation by penton base RGD deletion was observed for EGFR targeting on OVCAR-3 (6.9-fold; P < 0.02), MKN-28 (4.4-fold; P < 0.02), 11B (4.8-fold; P < 0.02), and LNCaP (5.4-fold; P < 0.02) and for EpCAM targeting on OVCAR-3 (3.7-fold; P < 0.03), MKN-28 (3.0-fold; P < 0.006), and 11B (4.8-fold; P < 0.0003).
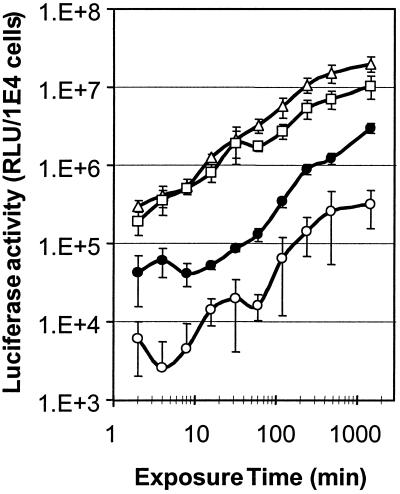
To gain more insight in the targeted transduction process, OVCAR-3 cells were exposed to AdL, AdL.PB(HA)F(TAYT-HA), EGFR-targeted AdL.PB(HA)F(TAYT-HA), or EpCAM-targeted AdL.PB(HA)F(TAYT-HA) for different periods, ranging from 2 min to 24 h (Fig. 3). Surprisingly, the four vectors exhibited quite similar transduction kinetics, with a rather constant rate of gene delivery over the entire period. This showed that the targeting indices determined using a 1-h transduction protocol are relevant for shorter and longer exposures as well. The rate of gene delivery differed by only a factor 2 between EGFR-mediated and EpCAM-mediated gene transfer. In addition, when bound but not yet internalized virus was removed from the cell surface by treatment with trypsin, the rate of EpCAM-mediated gene transfer was reduced by only approximately 40%, while native and EGFR-mediated transduction was not significantly affected (data not shown). Thus, despite a very large difference in inherent internalization rate between EGFR and EpCAM, AdV uptake via these molecules was only two- to fourfold different. Furthermore, it could be deduced that nonspecific uptake of untargeted AdL.PB(HA)F(TAYT-HA) requires more than 100-fold-longer exposure of the cells to reach the same level of transduction as EGFR- or EpCAM-targeted gene delivery (Fig. 3).
FIG. 3.
Time-dependent transduction by EGFR-targeted and EpCAM-targeted AdV. OVCAR-3 cells were exposed to AdL (closed circles), AdL.PB(HA)F(TAYT-HA) (open circles), EGFR-targeted AdL.PB(HA)F(TAYT-HA) (triangles), or EpCAM-targeted AdL.PB(HA)F(TAYT-HA) (squares) at 100 VP/cell for periods ranging from 2 min to 24 h. Luciferase activity was measured at 24 h after infection. Each data point represents the mean and standard deviation from two experiments performed in triplicate. During the span of the experiment, a transduction saturation point was not reached. Therefore, transduction rates could be deduced from the slopes of the graphs (relative light units [RLU] per 104 cells per minute) by regression analysis. Transduction rates were 2.1 × 103 for AdL (r = 0.99), 2.3 × 102 for AdL.PB(HA)F(TAYT-HA) (r = 0.90), 1.4 × 104 for EGFR-targeted AdL.PB(HA)F(TAYT-HA) (r = 0.90), and 7.0 × 103 for EpCAM-targeted AdL.PB(HA)F(TAYT-HA) (r = 0.92).
Specificity of targeted doubly ablated AdV for tumor cells expressing the target molecule.
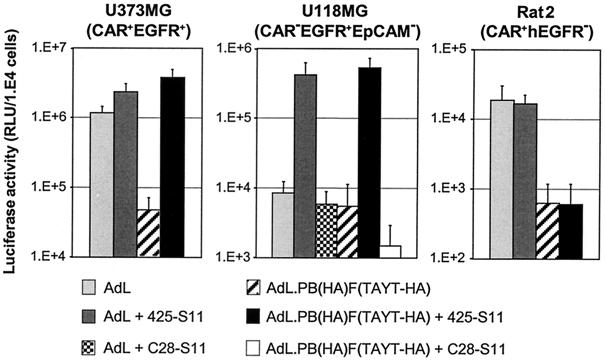
We confirmed the specificity of EGFR-targeted doubly ablated AdV for cells expressing human EGFR by using three cell lines with different CAR and EGFR expression profiles. The human glioblastoma cell lines U373MG and U118MG are CAR-EGFR double positive and CAR deficient, EGFR positive, respectively. As a surrogate for EGFR-deficient cells, we used the rodent cell line Rat2. Rat2 cells express rat CAR and rat EGFR, but because the 425-S11 bispecific scFv is specific for human EGFR, they should not be transduced by EGFR-targeted doubly ablated vectors. As can be seen in Fig. 4, 425-S11 scFv targeted the doubly ablated AdV toward U118MG cells but not toward Rat2 cells. This showed that the targeted gene transfer was dependent on human EGFR and independent of CAR. To further confirm the specificity for EGFR, the 425-S11 antibody was replaced by C28-S11. For glioma cells, which are deficient in EpCAM, C28-S11 is an irrelevant bispecific scFv targeting moiety. As expected, C28-S11 targeted doubly ablated AdV did not transduce U118MG cells. It can furthermore be seen in Fig. 4 that native AdL and EGFR-targeted AdL transduced Rat2 cells equally efficiently. Thus, while the degree of 425-S11 saturation on the viral particles was optimal for targeting, it was insufficient to neutralize CAR-mediated uptake. This finding underscored the need to use AdV with native tropism abolished in conjunction with bispecific targeting molecules.
FIG. 4.
Specificity of EGFR-targeted doubly ablated AdV for human EGFR-expressing cells. Human U373MG and U118MG glioblastoma cell lines and rat fibroblast cell line Rat2 were transduced with AdL or AdL.PB(HA)F(TAYT-HA), with or without human EGFR-specific scFv 425-S11 or human EpCAM-specific scFv C28-S11, for 1 h at an MOI of 100 VP/cell. Luciferase activity was measured at 24 h after infection. The data shown are the means and standard deviations from two (U373MG) or four (U118MG and Rat2) independent experiments performed in triplicate. RLU, relative light units.
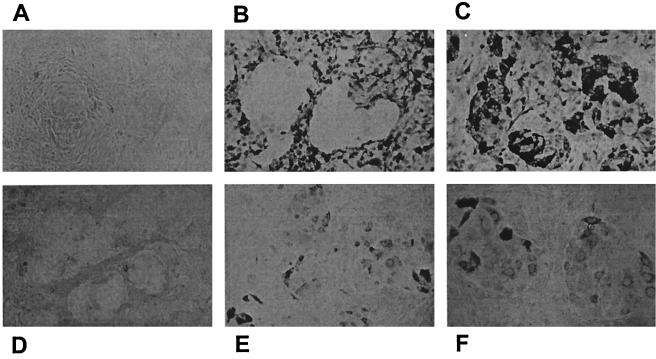
Next, we tested the targeted AdV in the context of a mixed cell population containing cells positive and negative for the target molecule. To this end, EpCAM-positive OVCAR-3 cells and EpCAM-negative U373MG cells were seeded at low density and allowed to form morphologically distinguishable monolayer structures. The cells were subjected to infection with AdL or AdL.PB(HA)F(TAYT-HA) at a high MOI with or without EpCAM-specific C28-S11 and stained for luciferase expression 2 days later. The staining method has a narrow detection window that allows assessment of approximately fivefold differences in transduction efficiency. Figure 5 shows that native AdL transduced U373MG cells more efficiently than OVCAR-3 cells (Fig. 5B), while AdL.PB(HA)F(TAYT-HA) did not exhibit detectable infectivity on either cell type (Fig. 5D). EpCAM targeting augmented transduction of OVCAR-3 cells by AdL but did not prevent transduction of U373MG cells through native AdL tropism (Fig. 5C). In contrast, EpCAM-targeted AdL.PB(HA)F(TAYT-HA) transduced only EpCAM-expressing OVCAR-3 cells (Fig. 5E and F). Most OVCAR-3 cells stained positive after transduction with EpCAM-targeted doubly ablated AdV, but their staining was less intense than that of OVCAR-3 cells transduced with EpCAM-targeted native AdV. This indicated a less-than-fivefold difference in transduction efficiency between the two vectors in this experiment. In conclusion, the targeted doubly ablated vector was specific for the subpopulation of cells expressing the target molecule.
FIG. 5.
Specificity of EpCAM-targeted doubly ablated AdV for EpCAM-positive cells within a mixed cell population. EpCAM-positive OVCAR-3 and EpCAM-negative U373MG cells were allowed to form a mixed cell layer, in which OVCAR-3 cells grow into tight colonies while U373MG cells form a network surrounding these colonies. The cells were not transduced (A) or were transduced with AdL (B and C) or AdL.PB(HA)F(TAYT-HA) (D to F), with (C, E, and F) or without (B and D) human EpCAM-specific scFv C28-S11, for 1 h at 108 VP/well. After 2 days, cells were stained with an antiluciferase MAb and photographed at a magnification of ×100 (A to E) or ×200 (F).
EGFR-targeted gene transfer into primary human glioblastoma cells.
We decided to further explore the utility of EGFR-targeted doubly ablated AdV for gene therapy of human malignant gliomas. Such vectors would be particularly useful in this context, because primary malignant gliomas are often low in CAR expression and high in EGFR expression, while CAR expression is high and EGFR is absent in the normal surrounding brain (12). Tumor specimens were obtained from 10 patients undergoing surgery for GBM, and single-cell cultures were established. After pathological confirmation of the diagnosis with the processed material, early passages of the cell cultures were used for further experiments. Fluorescence-activated cell sorter analysis for CAR showed that most samples expressed only very low or undetectable levels, while one specimen was high in CAR (data not shown). All samples were subjected to infection with AdL or AdL.PB(HA)F(TAYT-HA) with or without EGFR targeting by 425-S11 antibody. Figure 6 shows that the native vector AdL transduced cells from different patients with high variability, with the most efficient gene delivery into the high-CAR specimen. As expected, gene delivery by the doubly ablated vector was reduced on all samples. EGFR targeting augmented gene transfer into the entire panel of primary gliomas except the high-CAR sample. The median efficiency of EGFR-targeted gene transfer was similar for native and doubly ablated vectors. Only transduction of the high-CAR sample was clearly lower with the doubly ablated AdV than with native AdV. This showed that on this material most of the transduction by the targeted native AdV was CAR mediated. The doubly ablated AdV gave a better EGFR targeting index than the native AdV (123 versus 18). Hence, the highly specific and effective transduction by EGFR-targeted doubly ablated AdV observed with human cancer cell lines as described above was confirmed with primary glioblastoma specimens.
FIG. 6.
Transduction of primary human glioblastoma cells with EGFR-targeted AdV. Early passages of cell cultures established from 10 different GBM tumors were transduced with AdL or AdL.PB(HA)F(TAYT-HA), with or without 425-S11 antibody, for 1 h at an MOI of 100 VP/cell. Luciferase activity was measured at 24 h after infection. Each data point represents the average from triplicate infections; the lines give the median of each set of 10 data points. Closed symbols are for specimens with low or undetectable CAR expression; open symbols show the results from an exceptional high-CAR glioblastoma. RLU, relative light units.
Evaluation of the efficiency and selectivity of EGFR-targeted gene transfer into primary human brain tumors.
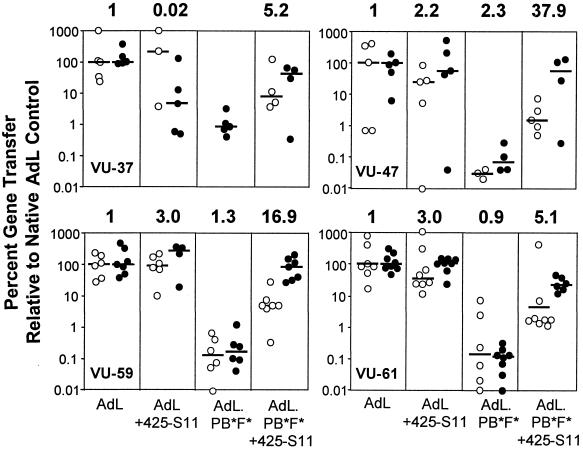
Finally, we determined the selectivity of EGFR-targeted AdV for human brain tumors relative to the normal surrounding brain tissue. To this end, we employed OMS established from the brain tumors of four patients. These tumors were GBM (patients VU-37, VU-47, and VU-61) and meningioma (patient VU-59). In addition, we cultured explants from small samples of damaged brain, taken from the corticotomy tract or from the immediate surroundings of the tumors of all four patients. Immunohistochemistry analysis showed that many cells in the surrounding brain were CAR positive and most cells were EGFR negative, while brain tumor cells were heterogeneous for CAR and EGFR expression (not shown). OMS and brain explants were individually cultured and transduced with AdL or AdL.PB(HA)F(TAYT-HA), with or without 425-S11. In general, AdL transduced OMS and brain explants of similar size with similar efficiency. To correct for differences in sample quality, gene transfer efficiencies were expressed relative to native AdV transduction for each individual patient. As can be seen in Fig. 7, the transduction efficiency of native AdL varied considerably between individual OMS and brain explants from the same donor, generally within a range of 2 orders of magnitude. Transduction by AdL.PB(HA)F(TAYT-HA) into both tumor and normal brain was strongly reduced for all four patients, with median efficiencies below 1% of those for the native control. EGFR targeting slightly enhanced the tumor-to-normal brain targeting index of native AdV in three of the four cases (up to threefold). EGFR targeting of doubly ablated AdV, however, resulted in a clear and reproducible augmentation of the targeting index (mean, 16.3-fold; range, 5.1- to 37.9-fold). Figure 7 furthermore shows that EGFR targeting of doubly ablated AdV enhanced transduction not only of tumor spheroids but also, to a lesser extent, of normal brain explants. To investigate the nature of the latter transduction, we exposed VU-59 normal brain explants to 425-S11 or C28-S11 targeted AdL.PB(HA)F(TAYT-HA). EGFR targeting with 425-S11 augmented gene delivery into individual explants on average 96-fold (range, 0.3- to 595-fold), while irrelevant EpCAM targeting with C28-S11 did not significantly enhance the transduction efficiency (mean, 2.7-fold; range, 0.03- to 8.4-fold). Hence, augmented gene transfer was 425-S11 specific, suggesting that the normal brain explants comprised some EGFR-positive cells. Indeed, some EGFR-positive microglial cells were detected by immunohistochemistry on normal brain explants of patient VU-37, and pathological evaluation of VU-61 normal brain explants revealed infiltrated tumor cells (not shown). In conclusion, EGFR-targeted doubly ablated AdV more efficiently transduced primary brain tumors, relative to the surrounding normal brain, than did either native AdL or EGFR-targeted native AdL.
FIG. 7.
Transduction of primary human brain tumor spheroids and normal brain explants. Glioma (patients VU-37, VU-47, and VU-61) or meningioma (patient VU-59) OMS and normal brain explants from the same patients were cultured individually and exposed overnight to AdL or AdL.PB(HA)F(TAYT-HA), with or without 425-S11 antibody, at 108 particles per spheroid. After 20 to 22 h, spheroids and explants were lysed and luciferase activity was measured. Each data point represents a single tumor spheroid (closed symbols) or brain explant (open symbols). The lines indicate the median values for each experimental group. All values are given as the percentage of the median transduction by the control vector AdL for the same patient. The relative tumor-to-normal brain targeting index is given above each panel, with the index of the control AdL set to 1.
DISCUSSION
A prime objective in the development of AdV for gene therapy is cell type-specific targeting through vector tropism modification. Targeting may augment gene delivery to tissues of interest that do not express sufficient native AdV receptor and may diminish immunogenicity, toxicity, and in vivo sequestration of the vector. Toward this goal, the native AdV tropism needs to be abolished completely, and a new binding ligand with specificity for the target tissue must be added to the AdV. The present study provides such targeted AdV, where the native interaction with primary and secondary AdV receptors is eliminated through genetic means and new binding affinities are introduced through bispecific adapter molecules. These vectors obviate an important drawback of previous two-component vector systems where abolition of native tropism depended on neutralization by the adapter molecule. Standardized production of the latter type of targeted vector with completely blocked native tropism is not trivial. In addition, the stability of such targeting complexes might be insufficient in vivo to retain full neutralization. The best results after systemic vascular delivery of two-component targeted vectors reported so far were obtained using AdV with bispecific antibodies that block the CAR binding domain in the fiber knob. Although this approach considerably reduced non-target organ transduction (13, 32), more than 90% of gene transfer still occurred in normal tissues (32). Thus, these studies clearly indicated that more stringent methods for abolition of native tropism were warranted.
By combining specific adenovirus capsid mutations that eliminate CAR and αv integrin interaction (11, 34, 41) with bispecific scFv fusion proteins (14), we constructed targeted AdV that provide stringent target molecule specificity. Neither exposure of cells deficient in the target molecule nor use of AdV targeted with irrelevant bispecific scFv led to considerable transduction. The gene transfer efficiencies obtained with vectors with specificity for EGFR or EpCAM on human cancer cell lines were at or well above native AdV infectivity. This information could not be gathered before, because native AdV targeted with bispecific scFv always retained a native transduction component that precluded measurement of the true efficiency of targeted gene transfer. On most cell lines, EGFR-mediated transduction was more efficient than EpCAM-mediated gene transfer. This difference may be a reflection of differences in receptor densities on the cell surface or, alternatively, may be caused by the lower concentration of the batch of EpCAM-specific targeting molecules. The kinetics of the targeted gene delivery suggested that the internalization rate of the target molecule was a less important factor determining transduction efficiency. This implies that many cell surface molecules may serve as efficient targets for AdV-mediated gene transfer, provided that high-affinity binding to the viral capsid is established. Our bispecific scFv expression cassette serves as a versatile adapter for this purpose, since it allows simple exchange of scFv with different specificities.
Our study also provided more insight into the targeted AdV transduction process. Native AdV cell entry is promoted by αv integrin interaction and requires PI3K activation (26, 42). Recently, however, it was observed that inhibition of the penton base-integrin interaction with neutralizing antibodies enhanced targeted AdV transduction (9). This suggested that while αv integrin interaction augments native adenovirus infection, it might not be involved in targeted AdV uptake. Integrin-independent targeted AdV transduction has so far been reported only with targeting moieties comprising active mitogenic ligands for tyrosine kinase growth factor receptors, including basic fibroblast growth factor, insulin-like growth factor 1, EGF, and tumor necrosis factor alpha (9, 25). Targeting toward the bombesin/gastrin-releasing peptide receptor, on the other hand, appeared to still depend on penton base-activated endocytosis (17). Inhibitors of tyrosine kinase receptor- and PI3K-mediated signal transduction had contradictory effects on the targeted AdV transduction in different studies (16, 25), leaving the role of signal transduction in the targeted AdV uptake pathway unresolved. All of these previous studies used neutralizing peptides, antibodies, or Ad protein or pharmacologic signal transduction inhibitors to interfere with penton base-initiated signaling. Their conflicting results may have been caused by incomplete inhibition on the one hand or pleiotropic effects of the inhibitors on the other hand. We now for the first time targeted AdV toward alternative cell surface receptors by using vectors that inherently lack αv integrin interaction due to absence of the penton base RGD motif. This allowed a more direct study of integrin-independent AdV entry, without any further addition of inhibitory molecules. EGFR- or EpCAM-targeted doubly ablated AdV had an efficiency similar to that of targeted native AdV and were more efficient than targeted AdV with CAR binding abolished on most cancer cell lines. The bispecific scFv that targets toward EGFR comprises the anti-EGFR scFv 425, which is an EGFR antagonist and thus should not trigger the EGFR-mediated signaling cascade (33, 37). Moreover, EpCAM, which is not involved in PI3K activation at all, served as an efficient target molecule for AdV transduction as well. These findings suggested, therefore, that penton base-triggered PI3K activation is not required for efficient targeted AdV entry and does not need to be complemented through tyrosine kinase activation. In fact, the interaction of targeted AdV with integrins in many cases even antagonized gene delivery. Deletion of the penton base RGD motif strongly enhanced the targeted gene transfer efficiency (three- to sevenfold) on most tested cancer cell lines. This suggested that disengaging targeted AdV transduction completely from known native entry pathways yields the most efficient targeting strategy. This was at least true for EGFR- and EpCAM-mediated transduction but may apply to other target molecules on cancer cells as well. The lack of a need for triggering cell signaling pathways could be an advantage in the context of cancer gene therapy, where cell activation is preferably avoided. In addition, it may be beneficial for in vivo gene therapy in general, because triggering of signaling events may potentiate early immune responses to the vector (3). Thus, our findings support further exploration of AdV with penton base RGD deleted for targeted gene delivery.
The improved specificity, as indicated by the targeting index, of EGFR-targeted doubly ablated AdV on cancer cell lines was confirmed with a panel of low-passage primary glioma cell cultures. EGFR-mediated gene delivery into primary cancer cells was 2 orders of magnitude more efficient than untargeted transduction. To investigate the performance of EGFR-targeted doubly ablated AdV in a clinically relevant microenvironment for brain tumors, we employed OMS. These structures retain the complex tissue architecture present in the tumor, including (heterogeneous) tumor cells, connective tissue, immune cells, and capillaries, with intact cell-cell and cell-matrix contacts (30, 36). They thus represent the most clinically relevant in vitro model for human tumors. Native AdV transduced tumor OMS and brain explants with similar efficiencies, demonstrating the need for targeting strategies in gene therapy for brain tumors. EGFR-targeted doubly ablated AdV exhibited a clear preference for transduction of EGFR-expressing brain tumor OMS compared to EGFR-deficient normal brain explants. On average, the tumor-to-normal brain transduction ratio was improved 16-fold (range, 5- to 38-fold) compared to native AdV. In fact, this ratio probably underestimates the true vector specificity, because in the normal brain samples of at least two of the four patients, infiltrated tumor cells or activated EGFR-positive microglial cells were detected, and this was not ruled out in the other two cases. These observations were not unexpected, since the normal brain explants were established from tissue adjacent to the tumor and proliferating reactive microglial and astroglial cells expressing EGFR can be found in brain lesions and local inflammations (31). Thus, targeted transduction of these EGFR-positive cells may account for most of the gene delivery measured in normal brain explants.
Taken together, our results support further consideration of doubly ablated AdV targeted with bispecific scFv as vectors for gene therapy. To this end, their efficacy and specificity will need to be confirmed under in vivo conditions. Depending on the route of administration, these conditions may be quite different from those in tissue culture. After intratumor injection, a high concentration of cells will be exposed to a high vector concentration for extended periods, while vascular vector administration may result in a very short virus-cell interaction at low concentration. In this respect, it is very promising that we observed a rather constant targeting index over a range of AdV concentrations (MOI of 10 to 10,000) and exposure times (2 min to 24 h). Moreover, selective transduction was also seen in spheroids, where the tumor and normal tissue architecture and composition were retained. Hence, the present study has encouraging implications for the development of human clinical gene therapy approaches.
Acknowledgments
This work was supported by Spinoza Award 1997 from The Netherlands Organization for Scientific Research to Herbert Pinedo and by a research grant from the Pasman Foundation. Victor van Beusechem is supported by a research fellowship of the Royal Netherlands Academy of Arts and Sciences (KNAW), and Jacques Grill was supported by the Federation Nationale des Centres de Lutte Contre le Cancer (FNLCC).
REFERENCES
- 1.Arafat, W., J. Gomez-Navarro, J. Xiang, G. P. Siegal, R. D. Alvarez, and D. T. Curiel. 2000. Antineoplastic effect of anti-erbB-2 intrabody is not correlated with scFv affinity for its target. Cancer Gene Ther. 7:1250-1256. [DOI] [PubMed] [Google Scholar]
- 2.Bjerkvig, R., A. Tonnesen, O. D. Laerum, and E. O. Backlund. 1990. Multicellular tumor spheroids from human gliomas maintained in organ culture. J. Neurosurg. 72:463-475. [DOI] [PubMed] [Google Scholar]
- 3.Bruder, J. T., and I. Kovesdi. 1997. Adenovirus infection stimulates the Raf/MAPK signaling pathway and induces interleukin-8 expression. J. Virol. 71:398-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chan, S., H. Gabra, F. Hill, G. Evan, and K. Sikora. 1987. A novel tumour marker related to the c-myc oncogene product. Mol. Cell. Probes 1:73-82. [DOI] [PubMed] [Google Scholar]
- 5.Cripe, T. P., E. J. Dunphy, A. D. Holub, A. Saini, N. H. Vasi, Y. Y. Mahller, M. H. Collins, J. D. Snyder, V. Krasnykh, D. T. Curiel, T. J. Wickham, J. DeGregori, J. M. Bergelson, and M. A. Currier. 2001. Fiber knob modifications overcome low, heterogeneous expression of the coxsackievirus-adenovirus receptor that limits adenovirus gene transfer and oncolysis for human rhabdomyosarcoma cells. Cancer Res. 61:2953-2960. [PubMed] [Google Scholar]
- 6.Darling, J. L. 1990. The in vitro biology of human brain tumors, p. 1-25. In D. G. T. Thomas (ed.), Neuro-oncology: primary malignant brain tumors. Johns Hopkins University Press, Baltimore, Md.
- 7.Dmitriev, I., E. Kashentseva, B. E. Rogers, V. Krasnykh, and D. T. Curiel. 2000. Ectodomain of coxsackievirus and adenovirus receptor genetically fused to epidermal growth factor mediates adenovirus targeting to epidermal growth factor receptor-positive cells. J. Virol. 74:6875-6884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Douglas, J. T., B. E. Rogers, M. E. Rosenfeld, S. I. Michael, M. Feng, and D. T. Curiel. 1996. Targeted gene delivery by tropism-modified adenoviral vectors. Nat. Biotechnol. 14:1574-1578. [DOI] [PubMed] [Google Scholar]
- 9.Doukas, J., D. K. Hoganson, M. Ong, W. Ying, D. L. Lacey, A. Baird, G. F. Pierce, and B. A. Sosnowski. 1999. Retargeted delivery of adenoviral vectors through fibroblast growth factor receptors involves unique cellular pathways. FASEB J. 13:1459-1466. [DOI] [PubMed] [Google Scholar]
- 10.Einfeld, D. A., D. E. Brough, P. W. Roelvink, I. Kovesdi, and T. J. Wickham. 1999. Construction of a pseudoreceptor that mediates transduction by adenoviruses expressing a ligand in fiber or penton base. J. Virol. 73:9130-9136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Einfeld, D. A., R. Schroeder, P. W. Roelvink, A. Lizonova, C. R. King, I. Kovesdi, and T. J. Wickham. 2001. Reducing the native tropism of adenovirus vectors requires removal of both CAR and integrin interactions. J. Virol. 75:11284-11291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grill, J., V. W. Van Beusechem, P. Van Der Valk, C. M. Dirven, A. Leonhart, D. S. Pherai, H. J. Haisma, H. M. Pinedo, D. T. Curiel, and W. R. Gerritsen. 2001. Combined targeting of adenoviruses to integrins and epidermal growth factor receptors increases gene transfer into primary glioma cells and spheroids. Clin. Cancer Res. 7:641-650. [PubMed] [Google Scholar]
- 13.Gu, D. L., A. M. Gonzalez, M. A. Printz, J. Doukas, W. Ying, M. D'Andrea, D. K. Hoganson, D. T. Curiel, J. T. Douglas, B. A. Sosnowski, A. Baird, S. L. Aukerman, and G. F. Pierce. 1999. Fibroblast growth factor 2 retargeted adenovirus has redirected cellular tropism: evidence for reduced toxicity and enhanced antitumor activity in mice. Cancer Res. 59:2608-2614. [PubMed] [Google Scholar]
- 14.Haisma, H. J., J. Grill, D. T. Curiel, S. Hoogeland, V. W. van Beusechem, H. M. Pinedo, and W. R. Gerritsen. 2000. Targeting of adenoviral vectors through a bispecific single-chain antibody. Cancer Gene Ther. 7:901-904. [DOI] [PubMed] [Google Scholar]
- 15.Haisma, H. J., H. M. Pinedo, A. Rijswijk, I. van der Meulen-Muileman, B. A. Sosnowski, W. Ying, V. W. van Beusechem, B. W. Tillman, W. R. Gerritsen, and D. T. Curiel. 1999. Tumor-specific gene transfer via an adenoviral vector targeted to the pan-carcinoma antigen EpCAM. Gene Ther. 6:1469-1474. [DOI] [PubMed] [Google Scholar]
- 16.Hoganson, D. K., B. A. Sosnowski, G. F. Pierce, and J. Doukas. 2001. Uptake of adenoviral vectors via fibroblast growth factor receptors involves intracellular pathways that differ from the targeting ligand. Mol. Ther. 3:105-112. [DOI] [PubMed] [Google Scholar]
- 17.Hong, S. S., A. Galaup, R. Peytavi, N. Chazal, and P. Boulanger. 1999. Enhancement of adenovirus-mediated gene delivery by use of an oligopeptide with dual binding specificity. Hum. Gene Ther. 10:2577-2586. [DOI] [PubMed] [Google Scholar]
- 18.Hu, P., B. Margolis, E. Y. Skolnik, R. Lammers, A. Ullrich, and J. Schlessinger. 1992. Interaction of phosphatidylinositol 3-kinase-associated p85 with epidermal growth factor and platelet-derived growth factor receptors. Mol. Cell. Biol. 12:981-990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huls, G. A., I. A. Heijnen, M. E. Cuomo, J. C. Koningsberger, L. Wiegman, E. Boel, A. R. van der Vuurst de Vries, S. A. Loyson, W. Helfrich, G. P. van Berge Henegouwen, M. van Meijer, J. de Kruif, and T. Logtenberg. 1999. A recombinant, fully human monoclonal antibody with antitumor activity constructed from phage-displayed antibody fragments. Nat. Biotechnol. 17:276-281. [DOI] [PubMed] [Google Scholar]
- 20.Jakubczak, J. L., M. L. Rollence, D. A. Stewart, J. D. Jafari, D. J. Von Seggern, G. R. Nemerow, S. C. Stevenson, and P. L. Hallenbeck. 2001. Adenovirus type 5 viral particles pseudotyped with mutagenized fiber proteins show diminished infectivity of coxsackie B-adenovirus receptor-bearing cells. J. Virol. 75:2972-2981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kirby, I., E. Davison, A. J. Beavil, C. P. Soh, T. J. Wickham, P. W. Roelvink, I. Kovesdi, B. J. Sutton, and G. Santis. 1999. Mutations in the DG loop of adenovirus type 5 fiber knob protein abolish high-affinity binding to its cellular receptor CAR. J. Virol. 73:9508-9514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krasnykh, V. N., J. T. Douglas, and V. W. van Beusechem. 2000. Genetic targeting of adenoviral vectors. Mol. Ther. 1:391-405. [DOI] [PubMed] [Google Scholar]
- 23.Krasnykh, V. N., G. V. Mikheeva, J. T. Douglas, and D. T. Curiel. 1996. Generation of recombinant adenovirus vectors with modified fibers for altering viral tropism. J. Virol. 70:6839-6846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li, D., L. Duan, P. Freimuth, and B. W. O'Malley, Jr. 1999. Variability of adenovirus receptor density influences gene transfer efficiency and therapeutic response in head and neck cancer. Clin. Cancer Res. 5:4175-4181. [PubMed] [Google Scholar]
- 25.Li, E., S. L. Brown, D. J. Von Seggern, G. B. Brown, and G. R. Nemerow. 2000. Signaling antibodies complexed with adenovirus circumvent CAR and integrin interactions and improve gene delivery. Gene Ther. 7:1593-1599. [DOI] [PubMed] [Google Scholar]
- 26.Li, E., D. Stupack, R. Klemke, D. A. Cheresh, and G. R. Nemerow. 1998. Adenovirus endocytosis via alpha(v) integrins requires phosphoinositide-3-OH kinase. J. Virol. 72:2055-2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li, Y., R. C. Pong, J. M. Bergelson, M. C. Hall, A. I. Sagalowsky, C. P. Tseng, Z. Wang, and J. T. Hsieh. 1999. Loss of adenoviral receptor expression in human bladder cancer cells: a potential impact on the efficacy of gene therapy. Cancer Res. 59:325-330. [PubMed] [Google Scholar]
- 28.Miller, C. R., D. J. Buchsbaum, P. N. Reynolds, J. T. Douglas, G. Y. Gillespie, M. S. Mayo, D. Raben, and D. T. Curiel. 1998. Differential susceptibility of primary and established human glioma cells to adenovirus infection: targeting via the epidermal growth factor receptor achieves fiber receptor-independent gene transfer. Cancer Res. 58:5738-5748. [PubMed] [Google Scholar]
- 29.Nalbantoglu, J., G. Pari, G. Karpati, and P. C. Holland. 1999. Expression of the primary coxsackie and adenovirus receptor is downregulated during skeletal muscle maturation and limits the efficacy of adenovirus-mediated gene delivery to muscle cells. Hum. Gene Ther. 10:1009-1019. [DOI] [PubMed] [Google Scholar]
- 30.Paulus, W., C. Huettner, and J. C. Tonn. 1994. Collagens, integrins and the mesenchymal drift in glioblastomas: a comparison of biopsy specimens, spheroid and early monolayer cultures. Int. J. Cancer 58:841-846. [DOI] [PubMed] [Google Scholar]
- 31.Planas, A. M., C. Justicia, M. A. Soriano, and I. Ferrer. 1998. Epidermal growth factor receptor in proliferating reactive glia following transient focal ischemia in the rat brain. Glia 23:120-129. [DOI] [PubMed] [Google Scholar]
- 32.Reynolds, P. N., K. R. Zinn, V. D. Gavrilyuk, I. V. Balyasnikova, B. E. Rogers, D. J. Buchsbaum, M. H. Wang, D. J. Miletich, W. E. Grizzle, J. T. Douglas, S. M. Danilov, and D. T. Curiel. 2000. A targetable, injectable adenoviral vector for selective gene delivery to pulmonary endothelium in vivo. Mol. Ther. 2:562-578. [DOI] [PubMed] [Google Scholar]
- 33.Rodeck, U., M. Herlyn, D. Herlyn, C. Molthoff, B. Atkinson, M. Varello, Z. Steplewski, and H. Koprowski. 1987. Tumor growth modulation by a monoclonal antibody to the epidermal growth factor receptor: immunologically mediated and effector cell-independent effects. Cancer Res. 47:3692-3696. [PubMed] [Google Scholar]
- 34.Roelvink, P. W., G. Mi Lee, D. A. Einfeld, I. Kovesdi, and T. J. Wickham. 1999. Identification of a conserved receptor-binding site on the fiber proteins of CAR-recognizing adenoviridae. Science 286:1568-1571. [DOI] [PubMed] [Google Scholar]
- 35.Romanczuk, H., C. E. Galer, J. Zabner, G. Barsomian, S. C. Wadsworth, and C. R. O'Riordan. 1999. Modification of an adenoviral vector with biologically selected peptides: a novel strategy for gene delivery to cells of choice. Hum. Gene Ther. 10:2615-2626. [DOI] [PubMed] [Google Scholar]
- 36.Sutherland, R. M. 1988. Cell and environment interactions in tumor microregions: the multicell spheroid model. Science 240:177-184. [DOI] [PubMed] [Google Scholar]
- 37.Takahashi, H., D. Herlyn, B. Atkinson, J. Powe, U. Rodeck, A. Alavi, D. A. Bruce, and H. Koprowski. 1987. Radioimmunodetection of human glioma xenografts by monoclonal antibody to epidermal growth factor receptor. Cancer Res. 47:3847-3850. [PubMed] [Google Scholar]
- 38.Watkins, S. J., V. V. Mesyanzhinov, L. P. Kurochkina, and R. E. Hawkins. 1997. The 'adenobody' approach to viral targeting: specific and enhanced adenoviral gene delivery. Gene Ther. 4:1004-1012. [DOI] [PubMed] [Google Scholar]
- 39.West, M. A., M. S. Bretscher, and C. Watts. 1989. Distinct endocytotic pathways in epidermal growth factor-stimulated human carcinoma A431 cells. J. Cell Biol. 109:2731-2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wickham, T. J. 2000. Targeting adenovirus. Gene Ther. 7:110-114. [DOI] [PubMed] [Google Scholar]
- 41.Wickham, T. J., M. E. Carrion, and I. Kovesdi. 1995. Targeting of adenovirus penton base to new receptors through replacement of its RGD motif with other receptor-specific peptide motifs. Gene Ther. 2:750-756. [PubMed] [Google Scholar]
- 42.Wickham, T. J., P. Mathias, D. A. Cheresh, and G. R. Nemerow. 1993. Integrins alpha v beta 3 and alpha v beta 5 promote adenovirus internalization but not virus attachment. Cell 73:309-319. [DOI] [PubMed] [Google Scholar]
- 43.Wickham, T. J., D. M. Segal, P. W. Roelvink, M. E. Carrion, A. Lizonova, G. M. Lee, and I. Kovesdi. 1996. Targeted adenovirus gene transfer to endothelial and smooth muscle cells by using bispecific antibodies. J. Virol. 70:6831-6838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Woo, D. V., D. Li, J. A. Mattis, and Z. Steplewski. 1989. Selective chromosomal damage and cytotoxicity of 125I-labeled monoclonal antibody 17-1a in human cancer cells. Cancer Res. 49:2952-2958. [PubMed] [Google Scholar]
- 45.Zabner, J., P. Freimuth, A. Puga, A. Fabrega, and M. J. Welsh. 1997. Lack of high affinity fiber receptor activity explains the resistance of ciliated airway epithelia to adenovirus infection. J. Clin. Investig. 100:1144-1149. [DOI] [PMC free article] [PubMed] [Google Scholar]