Abstract
Recruitment of leukocytes is essential for eventual control of virus infections. Macrophages represent a leukocyte population involved in the first line of defense against many infections, including herpes simplex virus (HSV) infection. Through presentation of antigens to T cells and production of cytokines and chemokines, macrophages also constitute an important link between the innate and adaptive immune systems. Here, we have investigated the chemokine expression profile of macrophages after HSV infection and the virus-cell interactions involved. By reverse transcription-PCR and cDNA arrays, we found that HSV type 1 (HSV-1) and HSV-2 induced expression of the CC chemokine RANTES/CCL5 in murine macrophage cell lines and peritoneal cells. The CXC chemokine BCA-1/CXCL13 was also induced in peritoneal cells. Twenty-six other chemokines tested were not affected. Accumulation of RANTES mRNA was detectable after 5 h of infection, was sensitive to UV irradiation of the virus, and was preceded by accumulation of viral immediate-early mRNA and proteins. The viral components responsible for initiation of RANTES expression were examined with virus mutants and RAW 264.7 macrophage-like cells expressing a dominant negative mutant of the double-stranded-RNA-activated protein kinase (PKR). The PKR mutant cell line displayed reduced constitutive and HSV-inducible RANTES expression compared to the control cell line. HSV-1 mutants deficient in genes encoding the immediate-early proteins ICP4, ICP22, and ICP27 remained fully capable of inducing RANTES expression in macrophages. By contrast, the ability of an ICP0-deficient HSV-1 mutant to induce RANTES expression was compromised. Thus, HSV selectively induces expression of RANTES in macrophages through a mechanism dependent on cellular PKR and viral ICP0.
Herpes simplex virus type 1 (HSV-1) and HIV-2 are human pathogens that infect and replicate in the epithelial cells of mucosal surfaces, which is followed by establishment of latency in the ganglia of sensory neurons; they thus persist in the host as lifelong infections. Primary and recurrent HSV infections may cause a number of diseases, including gingivostomatitis, cold sores, keratitis, and encephalitis (HSV-1) as well as genital herpes, cutaneous herpes, and meningitis (HSV-2) (39). The pathology observed following HSV infections is due to the cytopathic effect (CPE) of the infection as well as to the host immune response (36).
Efficient clearance of HSV infections is dependent upon both the innate and adaptive immune response. In the first line of defense against HSV infections, macrophages and natural killer (NK) cells have been attributed particular important roles (1, 15). Antiviral mechanisms include direct effector functions as well as interactions between macrophages and other cell types. An important aspect of the latter function includes activation and regulation of T cells (15, 23, 33). In order to mount an efficient and organized host response to viral infections it is important to recruit leukocytes to the site of infection. Chemokines produced during viral infection participate actively in this process, and the localized and regulated chemokine production by virus-infected cells is an important determinant for the outcome of infection (7, 25).
Chemokines belong to a superfamily of more than 40 small cytokines which share common structural motifs. The four subfamilies of chemokines are characterized by the position of the N-terminal cysteine residues (CXC, CC, C, and CX3C) (31). The two largest families are the CC and CXC chemokines, which can be distinguished by the cell populations they attract and act on. CXC chemokines (including interleukin-8/CXCL8, IP-10/CXCL10, and MIP-2/CXCL2) preferentially attract neutrophils, whereas CC chemokines (including MIP-1α/CCL3, MIP-1β/CCL4, RANTES/CCL5, and MCP-1/CCL2) attract and activate mononuclear cells. For instance, the CC chemokine RANTES attracts monocytes, eosinophils, basophils, NK cells, mast cells, and T cells, including memory T cells (17, 20, 32).
Many chemokines are known to be induced as a primary response to viral infection, and their roles in host defense and immunopathology are now being explored in several ways, e.g., in mice deficient in specific chemokines or specific chemokine receptors (7, 38). Additionally, many viruses encode soluble chemokine-binding proteins, chemokine analogs, and decoy chemokine receptors (27), which further suggests a significant role of chemokines in elimination of viral infections.
The ability of specific chemokines to potentiate experimental vaccines against HSV-1 and -2 was recently reported (9, 14, 35). In particular, Sin et al. showed that RANTES supports recruitment of memory CD4+ T cells after infection and, moreover, is involved in the development of Th1 cells (35). The role of chemokines in HSV pathology has also been demonstrated via studies in a murine keratitis model (38).
The present study was undertaken in order to evaluate the chemokine expression profiles of macrophages after HSV-1 and HSV-2 infection and to examine the virus-cell interactions involved. Our data suggest that RANTES is the main CC chemokine induced by macrophages during a primary infection and that the double-stranded-RNA-activated protein kinase (PKR) as well as the immediate-early viral gene ICP0 are essential for the induction of RANTES expression, while three other immediate-early genes (ICP4, ICP22, and ICP27) are dispensable.
MATERIALS AND METHODS
Reagents.
The recombinant cytokines used were murine RANTES (PeproTech) and murine gamma interferon (BD Pharmingen). The antibodies used were polyclonal murine anti-RANTES (PeproTech), biotinylated polyclonal anti-RANTES (PeproTech), horseradish peroxidase (HRP)-conjugated rabbit polyclonal anti-mouse immunoglobulin (Transduction Laboratories), mouse monoclonal anti-ICP27 (Advanced Biotechnologies), rabbit polyclonal anti-VP16 (BD Clontech), mouse monoclonal anti-ICP8 (Virusys), and mouse monoclonal anti-gD (Virusys). Enzyme-linked immunosorbent assay (ELISA) plates were from Maxisorp. RNA was purified with Trizol (Life Technologies), cleared of DNA with DNase I (Stratagene), and reverse transcribed using Expand reverse transcriptase (Roche) and oligo(dT)15 (Roche). For PCR amplification, Taq2000 DNA polymerase (Stratagene) was used. DNA primers and probes were obtained from DNA Technology. G418 was obtained from Roche. The mouse cytokine expression array was from R&D systems, as were the murine cytokine-specific primers and the cDNA labeling and hybridization kit. [α-32P]dATP was supplied by Hartmann Analytics, and Sephadex G-25 spin columns (Roche) were used for removal of unincorporated radioactivity.
Mice and cell culture.
The mice used in our experiment were 7- to 8-week-old female C57BL/6 mice. The mice were from M&B (Ry, Denmark) and were kept in our animal facilities between delivery (at 4 to 6 weeks of age) and the time of the experiments. Resting peritoneal cells (PCs) were harvested from the mice by lavage of the peritoneal cavity with cold phosphate-buffered saline (PBS) supplemented with 2% lipopolysaccharide-free fetal calf serum (FCS) (HyClone) and 20 IU of heparin per ml. Inflammatory macrophages were induced by injection of 2 ml of 10% thioglycolate (TG) into the peritoneal cavities of the mice. Five days later the cells were harvested as described above. The cells were washed once in RPMI 1640 medium with 5% FCS, seeded at a density of 3.5 × 105 cells/well in 96-well tissue culture plates, and left overnight before stimulation and infection. The murine macrophage cell lines used were J774A.1 and RAW 264.7, as well as the RAW 264.7-derived cell lines RAW-pBK-CMV and RAW-PKR-M7, which are stably transfected with empty pBK vector and the PKR M7 mutant, respectively (22). The M7 mutant lacks the first double-stranded-RNA-binding domain of PKR. All cell lines were maintained in Dulbecco modified Eagle medium with 1% Glutamax I (Life Technologies), antibiotics, and 5% FCS. The growth medium for the stably transfected cell lines also contained 200 μg of G418 per ml. For experiments the cells were seeded in 2- or 10-cm2 tissue culture wells at a density of 4 × 106 or 2 × 106 cells/well, respectively, and left for 16 to 20 h before further treatment.
Viruses.
The wild-type viruses used in this study were the MS strain of HSV-2 and the KOS and 17+ strains of HSV-1. The ICP0 mutant dl1403 (37) is on a 17+ genetic background, whereas mutants lacking ICP4 (34), ICP22 (21), and ICP27 (30) are on a KOS genetic background. The viruses and mock preparations were produced essentially as described previously (8) and quantified by titration in U2OS cells, Vero cells, and/or Vero-derived cells expressing specific viral proteins. Just before use, virus was thawed and either used as infectious virus, subjected to heat inactivation at 56°C for 30 min, or inactivated by UV light for 15 min.
Isolation of RNA and reverse transcription-PCR.
Total RNA was isolated using Trizol according to the manufacturer's recommendations. The RNA to be analyzed for viral RNA was cleared of DNA contaminants by treatment with DNase I. Two micrograms of RNA was subjected to reverse transcription using oligo(dT)15 and Expand reverse transcriptase. The cDNA was amplified by PCR with the following primers: murine RANTES, 5′-ATA TGG CTC GGA CAC CAC TC-3′ (sense) and 5′-GAT GCC GAT TTT CCC AGG AC-3′ (antisense); murine MIP-2, 5′-TGG GTG GGA TGT AGC TAG TCC C-3′ (sense) and 5′-AGT TTG CCT TGA CCC TGA AGC C-3′ (antisense); murine MIP-1α, 5′-GAA GAG TCC CTC GAT GTG GCT A-3′ (sense) and 5′-CCC TTT TCT GTT CTG CTG TAC AAG-3′ (antisense); murine MIP-1β, 5′-TTC TCT CTC CTC CTG CTT GTG G-3′ (sense) and 5′-CAC ATA CTC ATT GAC CCA GGG C-3′ (antisense); murine MIP-1γ, 5′-GGG GAA ACT TGT CTG TGC CAA C-3′ (sense) and 5′-ATG GAG GGG TGG TGG GAA AAT C-3′ (antisense); murine IP-10, 5′-CGC ACC TCC ACA TAG CTT ACA G-3′ (sense) and 5′-CCT ATC CTG CCC ACC GTG TTG AG-3′ (antisense); murine MCP-1, 5′-ACA AGA GGA TCA CCA GCA GCA G-3′ (sense) and 5′-AAG GCA TCA CAG TCC GAG TCA C-3′ (antisense); ICP27-2, 5′-AGA TCG ACT ACA CGA CCG T-3′ (sense) and 5′-TGC CGT GCA CAT ATA AGG G-3′ (antisense); ICP8-2, 5′-GGT TGA AAA ACG GAA GGG GG-3′ (sense) and 5′-TGG ACA AGG TAA CCA TCG GG-3′ (antisense); gD-2, 5′-GCT ACT ATG ACA GCT TTA GCG-3′ (sense) and 5′-ATA AAT TGT GTG ATC TCC GTC C-3′ (antisense); and murine β-actin, 5′-CCA ACC GTG AAA AGA TGA CC-3′ (sense) and 5′-GCA GTA ATC TCC TTC TGC ATC C-3′ (antisense). The products spanned 330 bp (murine RANTES), 466 bp (murine MIP-2), 561 bp (murine MIP-1α), 237 bp (murine MIP-1β), 537 bp (murine MIP-1γ), 319 bp (murine MCP-1), 431 bp (murine IP-10), 174 bp (ICP27), 159 bp (ICP8), 133 bp (gD), and 616 bp (murine β-actin). As a negative control for viral transcripts, a pool of RNA (from Vero cells infected for 5, 8, and 12 h) which was subjected to PCR without prior reverse transcription was used. As a positive control, a pool of RNA (from Vero cells infected for 5, 8, and 12 h) which was reverse transcribed before PCR was used.
cDNA array analysis.
The cDNA arrays (mouse cytokine expression arrays) were rinsed in 0.2× SSPE (1× SSPE is 0.18 M NaCl, 10 mM NaH2PO4, and 1 mM EDTA [pH 7.7]) and prehybridized for 2 h at 65°C in hybridization buffer (5× SSPE, 2% sodium dodecyl sulfate [SDS], 5× Denhardt's reagent, 100 mg of salmon sperm DNA per ml). The hybridization solution was made by addition of labeled cDNA (see below) to 5 ml of hybridization buffer followed by heating for 10 min at 90°C. The prehybridization solution was discarded and exchanged with the hybridization solution. Hybridization was allowed to occur for 16 to 20 h at 65°C. Unhybridized cDNA was washed off the array by two 20-min washes with 0.5× SSPE-2% SDS and two 20-min washes with 0.1× SSPE-2% SDS. All washing steps were carried out at 65°C, with 150 ml of washing buffer used per wash. The arrays were sealed in plastic bags and subjected to autoradiography. Total RNA was labeled by using the cDNA labeling and hybridization kit and [α-32P]dATP. Briefly, 1 to 2 mg of total RNA was mixed with 4 ml of murine cytokine-specific primers and heated at 90°C for 2 min, followed by slow cooling to 42°C. The remaining components were added, mixed, and incubated for 2.5 h at 42°C. The labeled cDNA was separated from unincorporated radioactivity by centrifugation through Sephadex G-25 spin columns.
Analysis of cDNA array data.
Hybridization intensities were determined by densitometric measurement of the X-ray films after the array was aligned to the grid reference template to determine the target localization for each of the duplicate cDNAs, eight housekeeping genes, and six negative controls. The background was determined individually for each gene and subtracted from the value for the duplicate spots to yield the average hybridization intensity. For comparison between arrays, hybridization intensities were normalized to those of the housekeeping genes prior to comparison between samples from HSV- and mock-treated cells. Only those genes which reproducibly showed at least threefold upregulation or downregulation were considered to be differentially expressed.
ELISA.
For measurement of chemokine production, culture supernatants were harvested after 0 to 24 h of infection. RANTES protein levels in cell culture supernatants were determined by ELISA. Maxisorp plates were coated overnight at 4°C with 100 ml of anti-murine RANTES antibodies (0.5 mg/ml) in coating buffer (15 mM Na2CO3, 35 mM NaHCO3, 0.2% sodium azide [pH 9.6]). The wells were washed three times with PBS plus 0.05% Tween 20 and blocked with 1% bovine serum albumin in PBS supplemented with 5% sucrose and 0.05% sodium azide at room temperature for 3 h. Next, 100-μl portions of serial dilutions of recombinant murine RANTES standard or culture supernatants were added in duplicates or triplicates and incubated overnight at 4°C. The plates were washed with PBS-0.05% Tween 20 and incubated with biotinylated anti-murine RANTES antibodies (0.25 μg/ml) at room temperature for 2 h. The plates were washed and incubated with 100 μl of 1:5,000 diluted peroxidase-conjugated streptavidin at room temperature for 20 min and developed with 0.5 mg of o-phenylenediamine per ml of substrate buffer at room temperature for 15 min. The chemokine concentration was measured and calculated with an automated ELISA reader. The ELISA was able to detect RANTES concentrations of as low as 100 to 150 pg/ml.
Whole-cell extracts.
To prepare whole-cell extracts, cells were washed with ice-cold PBS and resuspended in lysis buffer (20 mM Tris-HCl [pH 8.0], 150 mM NaCl, 1.5 mM MgCl2, 1% Triton X-100, 10% glycerol, 0.5 mM EDTA, 0.5 mM phenylmethylsulfonyl fluoride [PMSF], 0.2 mM leupeptin, 0.2 mM pepstatin A). After 30 min on ice, the lysates were centrifuged at 10,000 × g for 10 min at 4°C and supernatants were isolated.
Isolation of nuclear extracts.
To isolate nuclear proteins, the cell monolayer was washed twice with ice-cold PBS, scraped off the plate, and spun down (2,000 × g for 1 min). The cells were resuspended in a hypotonic buffer (20 mM HEPES [pH 7.9], 1.5 mM MgCl2, 10 mM KCl, 0.5 mM dithiothreitol, 0.2 mM EDTA, 0.2 mM PMSF, 0.2 mM leupeptin, 0.2 mM pepstatin A, 0.1 mM Na3VO4) and left on ice for 15 min. NP-40 was added to 0.6%, and the mixture was vortexed for 15 s and centrifuged at 10,000 × g for 1 min. Extraction buffer (20 mM HEPES [pH 7.9], 20% glycerol, 1.5 mM MgCl2, 420 mM NaCl, 0.5 mM dithiothreitol, 0.2 mM EDTA, 0.2 mM PMSF, 0.2 mM leupeptin, 0.2 mM pepstatin A, 0.1 mM Na3VO4, 0.2% NP-40) was added to the nuclei and incubated for 30 min at 4°C with rocking. The samples were centrifuged at 10,000 × g for 15 min at 4°C, and the supernatants were harvested as nuclear extracts.
Western blotting.
Virus particles or whole-cell extracts were denatured in sample buffer (140 mM Tris-HCl [pH 8.5], 10% glycerol, 2% SDS, 1 mM EDTA, 0.019% Serva blue G250, 0.06% phenol red), heated to 80°C for 10 min, and subjected to SDS-polyacrylamide gel electrophoresis. The proteins were blotted onto a polyvinylidene difluoride membrane and blocked for 1 h with TBS (10 mM Tris, 140 mM NaCl) supplemented with 0.05% Tween 20 and 5% skim milk powder. Antibody was added for overnight incubation at 4oC. The membrane was washed four times for 10 min each in washing buffer (TBS with 0.05% Tween 20) and incubated for 1 h at room temperature with a polyclonal HRP-conjugated antibody against mouse immunoglobulin. The membrane was washed as described above, and the HRP-conjugated antibody was visualized using enhanced chemiluminescence. As a negative control for viral proteins, whole-cell extracts from uninfected Vero cells were used. As a positive control, a pool of extracts from Vero cells infected for 4.5, 7, and 16 h was used.
Statistical analysis.
Chemokine concentrations are presented as means ± standard errors of the means (SEMs). SEMs were calculated using the statistics analysis tools of the Sigma Plot program.
RESULTS
Induction of chemokines in a murine macrophage cell line and PCs infected with HSV-1 and -2.
In order to examine the pattern of chemokines expressed by macrophages after HSV infection, the murine macrophage-like cell line J774A.1 was infected with HSV-2 (multiplicity of infection [MOI] of 6) and RNA was harvested after various times postinfection (p.i.). The RNA was reverse transcribed and analyzed for the presence of chemokines by PCR. Among the chemokine mRNA species tested (RANTES/CCL5, MIP-1α/CCL3, MIP-1β/CCL4, MIP-1γ/CCL9, IP-10/CXCL10, MCP-1/CCL2, and MIP-2/CXCL2), only RANTES mRNA levels increased after infection, while mRNA levels for the other chemokines were unaffected by HSV-2 infection (data not shown).
To confirm this finding with primary cells, PCs were harvested from C57BL/6 mice and infected with either HSV-1 or -2. Total RNA was harvested and analyzed for the presence of chemokine RNA by use of cDNA arrays. The results are presented in Table 1. Among the 28 chemokines on the cDNA array, only RANTES and the CXC chemokine BCA-1/CXCL13 were upregulated more than threefold. This was seen in response to both HSV-1 and -2. MIP-1α production increased to a moderate extent in cells infected with HSV-2 but not in cells infected with HSV-1.
TABLE 1.
Expression of chemokines by HSV-infected PCsa
| Chemokine | Changes in expression levelb
|
|
|---|---|---|
| HSV-1 | HSV-2 | |
| 6Ckine/CCL21 | NDc | ND |
| C10/CCL6 | 0.90 | 1.38 |
| Eotaxin/CCL11 | ND | ND |
| MIP-1α/CCL3 | 1.19 | 2.18 |
| MIP-1β/CCL4 | 0.81 | 0.80 |
| MIP-1γ/CCL9 | 1.03 | 1.29 |
| MIP-2α/CXCL2 | 1.08 | 1.06 |
| MIP-3α/CCL20 | ND | ND |
| MIP-3β/CCL19 | 1.15 | 0.76 |
| JE/MCP-1/CCL2 | 0.98 | 0.90 |
| MARC/MCP-3/CCL7 | ND | ND |
| MCP-5/CCL12 | 1.01 | 1.12 |
| TCA3/CCL1 | 0.88 | 1.19 |
| MDC/CCL22 | ND | ND |
| RANTES/CCL5 | 8.07 | 9.84 |
| c-Tack/CCL27 | ND | ND |
| TECK/CCL17 | ND | ND |
| TECK/CCL25 | 0.91 | 0.54 |
| CCL28 | ND | ND |
| BLA/BCA-1/CXCL13 | 7.66 | 7.48 |
| BRAK/CXCL14 | ND | ND |
| IP-10/CXCL10 | 1.17 | 0.72 |
| GCP-2/CXCL6 | ND | ND |
| GROα/CXCL1 | 0.70 | 0.63 |
| Lungkine/CXCL15 | ND | ND |
| MIG/CXCL9 | 0.89 | 1.15 |
| SDF-1/CXCL12 | ND | ND |
| Lymphotaxin/CX3CL1 | 1.03 | 0.76 |
PCs were treated with a mock preparation or infected with HSV-1 and -2 at an MOI of 1. Twenty-four hours later, total RNA was isolated, cleared of potential DNA contaminations, and analyzed for the presence of chemokine mRNAs by using cDNA arrays.
Normalized mRNA levels in infected cells were compared to the levels in mock-treated cells. Values are ratios of levels in infected cells to those in mock-infected cells.
ND, not detectable after 30 days of exposure.
Thus, infection of macrophage-like cells and PCs rich in macrophages with HSV-1 and -2 induces expression of only a limited subset of chemokines, and among these is RANTES. Below, the expression of RANTES in HSV-infected macrophages is characterized.
Production of RANTES by macrophages after HSV-2 infection.
For initial characterization of HSV-induced RANTES production and to validate the murine macrophage cell lines RAW 264.7 and J774A.1 as models for murine PCs with respect to RANTES production during HSV infection, we compared the abilities of PCs and the cell lines to produce RANTES in response to HSV-2 infection. As seen in Fig. 1, resting and TG-elicited PCs responded to HSV-2 infection with production of RANTES after 24 h of infection with HSV-2. Thus, resting and TG-elicited murine PCs secrete RANTES in response to HSV-2 infection. Moreover, the murine macrophage cell lines RAW 264.7 and J774A.1 seem to faithfully reflect the primary cells as far as RANTES production is concerned.
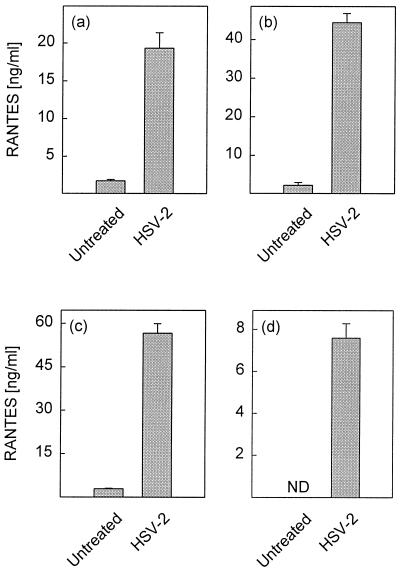
FIG. 1.
Secretion of RANTES by peritoneal macrophages and macrophage-like cells after HSV-2 infection. Cells were infected with 3 × 106 PFU of HSV-2 per ml (PCs, MOI of 1.7; cell lines, MOI of 6) and incubated for 24 h. RANTES in supernatants was measured by ELISA. (a) Resting PCs; (b) TG-elicited PCs; (c) RAW 264.7 cells; (d) J774A.1 cells. Results are shown as means from triplicate cultures ± SEMs. ND, not detectable. Essentially similar results were obtained in 3 independent experiments for panels a and b and more than 10 experiments for panels c and d.
Characterization of HSV-induced RANTES secretion.
To examine whether the induction of RANTES is dependent on a functional viral genome, the abilities of infectious and inactivated viruses to induce RANTES production were compared. Virus was inactivated by heat and UV treatment, which decreased viral titers more than 107-fold. In addition, the kinetics of RANTES secretion was examined. J774A.1 cells were seeded and infected with HSV-2 or equivalent amounts of UV- or heat-inactivated virus. Supernatants were harvested after 24 h of infection or after the indicated time points, and RANTES levels were measured by ELISA.
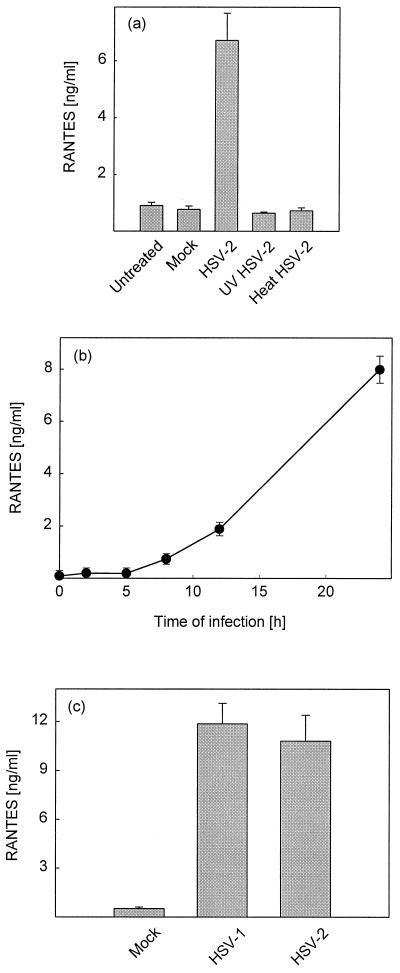
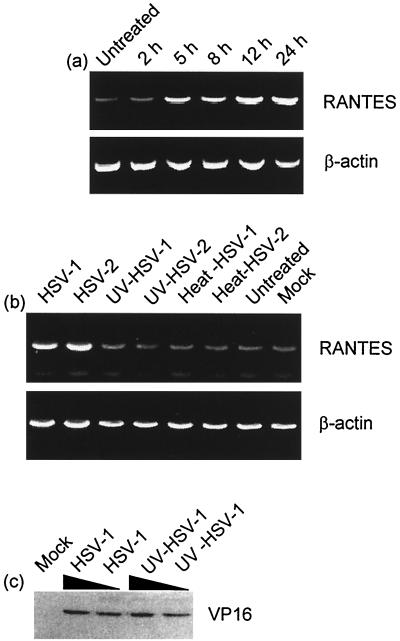
In supernatants from J774A.1 cell cultures RANTES protein was detectable from 8 h p.i., and levels subsequently increased until at least 24 h p.i (Fig. 2b). UV- or heat-inactivated HSV-2 (Fig. 2a) did not induce RANTES secretion, thus showing that a functional viral genome is essential for induction of RANTES expression. Although UV treatment prevented the virus from inducing expression of RANTES, HSV virions receiving this treatment remained able to enter the cells as assessed by accumulation of the virus tegument protein VP16 in the nuclei of J774A.1 cells (Fig. 3c)
FIG. 2.
Characterization of HSV-induced RANTES secretion. (a and b) J774A.1 cells were seeded and infected with 3 × 106 PFU of HSV-2 per ml (MOI of 6) or equivalent amounts of heat- or UV-inactivated virus. Supernatants were harvested at 24 h p.i. (a) or at the indicated time points (b) and assayed by ELISA. (c) Resting PCs were seeded and infected with 3 × 106 PFU of HSV-1 or HSV-2 per ml (MOI of 1.7). Supernatants were harvested after 24 h of infection. RANTES protein levels in the supernatants were measured by ELISA. Results are shown as means from three or four cultures ± SEMs. For all panels, essentially similar results were obtained in three independent experiments.
FIG. 3.
Characterization of HSV-induced RANTES mRNA accumulation in J774A.1 cells. (a) The cells were infected with 3 × 106 PFU of HSV-2 per ml (MOI of 6) and total RNA was harvested at the indicated time points. (b) The cells were infected with 3 × 106 PFU of HSV-2 per ml (MOI of 6) or equivalent amounts of heat- or UV-inactivated virus. Twenty-four hours later, total RNA was harvested. The RNA was reverse transcribed with oligo(dT) priming and analyzed for the presence of RANTES and β-actin by PCR. (c) J774A.1 cells were mock infected or treated with infectious or UV-inactivated HSV-1 (MOIs of 6 and 2). After 3 h of infection, nuclear extracts were prepared and analyzed for the presence of VP16 by Western blotting. For all panels, similar results were obtained in three independent experiments.
In order to compare HSV-1- and HSV-2-induced RANTES production in primary cells, PCs were harvested and infected with HSV-1 or -2. Supernatants were collected after 24 h of infection, and RANTES accumulation was measured by ELISA. In PCs both HSV-1 and HSV-2 induced strong RANTES secretion (Fig. 2c), which corroborate the above-described cDNA array data (Table 1).
Taken together, these data show that HSV-inducible RANTES secretion in macrophages is dependent on virus replication and that HSV-1 and -2 induce comparable levels of the CC chemokine.
Characterization of HSV-induced RANTES mRNA accumulation.
Given the above-described RANTES secretion profile, equivalent experiments were performed to reveal the kinetics of mRNA accumulation. Total RNA was harvested, reverse transcribed, and subsequently subjected to PCR with primers specific for murine RANTES and β-actin. Increased RANTES mRNA levels were detected from 5 h p.i., and the accumulation proceeded through the 24 h of the experiment (Fig. 3a). These results are in accordance with the elevated RANTES protein levels observed from 8 h p.i. Both HSV-1 and HSV-2 induced high levels of RANTES mRNA, which is sensitive to UV inactivation of the viruses (Fig. 3b).
Production of virus mRNA and proteins in HSV-2-infected macrophages.
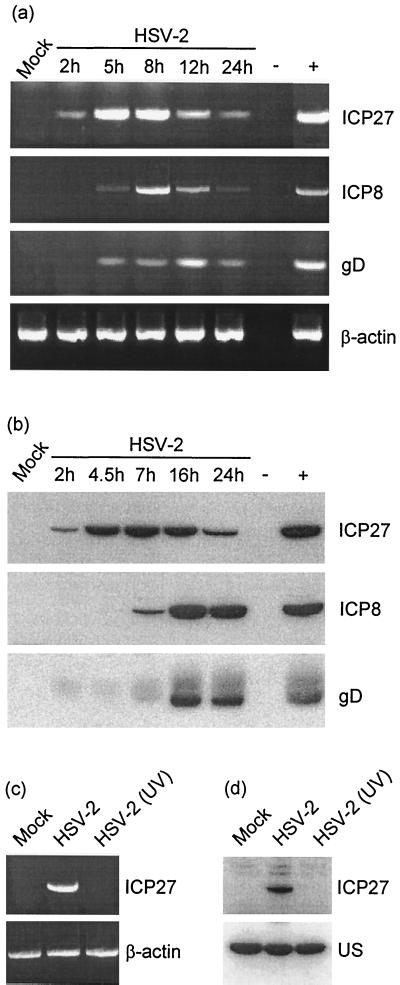
To examine the virus-cell interactions responsible for induction of RANTES in macrophages, we first examined whether a correlation between accumulation of a specific set of viral transcripts or proteins and induction of RANTES expression was noticeable. To this end, we first looked for the appearance of mRNAs transcribed from immediate-early (ICP27), early (ICP8), and late (gD) genes. As seen in Fig. 4a, no viral transcripts were observed in uninfected J774A.1 macrophages. HSV-2 infection induced ICP27 mRNA accumulation after 2 h, which was increased further after 5 and 8 h. mRNA species encoding ICP8 and gD were detected only at later time points when RANTES expression had been initiated. As to the accumulation of viral proteins in the infected macrophage-like cells, we found that all identified viral mRNA species were translated into proteins (Fig. 4b). Moreover, accumulation of immediate-early proteins preceded induction of RANTES.
FIG. 4.
Production of HSV-2 immediate-early, early, and late mRNA and proteins in J774A.1 cells. (a) The cells were infected with 3 × 106 PFU/ml of HSV-2 per ml (MOI of 6) and incubated for the indicated periods. Total RNA was isolated, and viral mRNA was detected by reverse transcription-PCR with 30 PCR cycles. (b) The cells were treated as for panel a. At the indicated time points, whole-cell extracts were prepared and analyzed for the presence of viral antigens by Western blotting. (c and d) J774A.1 cells were infected with 3 × 106 PFU of HSV-2 per ml (MOI of 6) or an equivalent amount of UV-irradiated virus. After 5 h, total RNA was isolated and whole-cell extracts were prepared and analyzed for the presence of ICP27 mRNA (c) and protein (d), respectively. US, unspecific band serving as an internal control. For all panels, essentially similar results were obtained in two independent experiments.
In Fig. 2 and 3 we showed that induction of RANTES production by HSV in macrophages is sensitive to UV irradiation of the virus. To examine further the correlation between immediate-early virus gene expression and production of RANTES, we examined the extent to which UV irradiation impaired virus immediate-early gene expression. The results (Fig. 4c and d) show that UV-irradiated HSV-2 is totally unable to bring about accumulation of immediate-early mRNA and protein.
In concert these results show that accumulation of immediate-early viral mRNA and protein precedes induction of RANTES. Moreover, since accumulation of these viral products as well as RANTES was sensitive to UV irradiation of the virus, the results raise the possibility that HSV-induced immediate-early gene products play a role in induction of RANTES in macrophages.
Involvement of PKR and viral tegument and immediate-early genes in RANTES production.
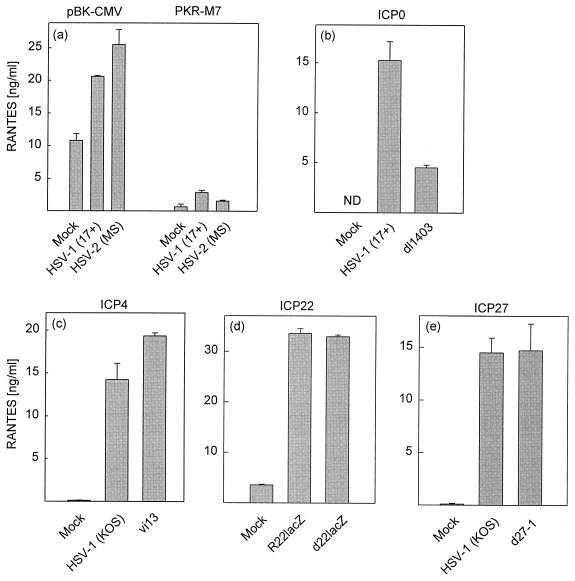
Given the above-described correlation between accumulation of immediate-early mRNA and proteins and the onset of RANTES expression, we wanted to examine this phenomenon more thoroughly. Since accumulation of viral RNA is known to be sensed in cells by the double-stranded-RNA-activated protein kinase (PKR) (40), we wanted to test the potential involvement of this kinase in RANTES expression. For this purpose we used the previously described RAW 264.7-derived PKR dominant negative cell line RAW-PKR-M7 (22). The results are presented in Fig. 5a and show that the empty vector control cell line (RAW-pBK-CMV) exhibited rather high constitutive levels of RANTES, which were further augmented after HSV-1 infection. The PKR mutant cell line displayed strongly reduced constitutive and HSV-1-inducible RANTES expression. Essentially similar results were obtained with HSV-2.
FIG. 5.
Induction of RANTES production in the absence of PKR activity and specific viral proteins. RAW-pBK-CMV and RAW-PKR-M7 cells (a) or RAW 264.7 cells (b to e) were seeded and left overnight to settle. (a) The cells were mock infected or infected with 106 PFU of HSV-1 or HSV-2 per ml (MOI of 2). (b to e) The cells were treated with mock virus preparation or infected with 106 PFU of mutant virus (ICP0 mutant [b], ICP4 mutant [c], ICP22 mutant [d], or ICP27 mutant [e]) per ml (MOI or 2), wild-type HSV-1, or the reconstituted mutants. After 24 h of infection, supernatants were harvested and RANTES levels were measured by ELISA. Results are shown as means from three or four cultures ± SEMs. ND, not detectable. Similar results were seen in four independent experiments for panels a and b and two independent experiments for panels c to e.
The role of viral immediate-early genes in RANTES expression was examined with HSV-1 mutants deficient in specific viral genes. When the ICP0 mutant was examined for RANTES-inducing activity, we found that this virus induced significantly less RANTES than the wild type (Fig. 5b). The HSV-1 ICP4 mutant was slightly more active than the wild-type virus with respect to induction of RANTES (Fig. 5c), while the mutants lacking ICP22 and ICP27 displayed wild-type ability to induce RANTES expression (Fig. 5d and e).
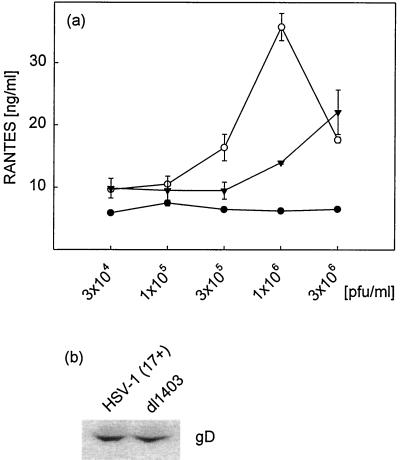
To investigate further the reduced ability of the ICP0 mutant virus to trigger RANTES production, we performed dose-response experiments. The results (Fig. 6a) showed that wild-type HSV-1 (17+) induced a dose-dependent increase in RANTES secretion by RAW 264.7 cells at concentrations of up to 106 PFU/ml (MOI of 2). At higher virus concentrations the CPE of this highly virulent strain of HSV-1 was pronounced, which correlated with reduced RANTES production. The ICP0 mutant displayed a reduced capacity to induce RANTES secretion at virus concentrations of up to 106 PFU/ml (MOI of 2). The CPE observed after infection with the wild-type virus was not induced by the ICP0 mutant, thus explaining the higher RANTES-inducing activity of the mutant at high virus concentrations. To exclude the possibility that the differences observed were due to variance in virus antigen levels, we compared the levels of gD in our virus preparations, which had been made on Vero cells and titrated on U2OS cells. As seen in Fig. 6b, the virus antigen levels were comparable in the wild-type and mutant virus preparations.
FIG. 6.
RANTES dose-response curve for wild-type (17+) and ICP0 mutant (dl1403) HSV-1 in RAW 264.7 cells. (a) The cells were seeded and treated with increasing amounts of 17+ (○), dl1403 (▾), or mock preparation (•). After 24 h of infection, supernatants were harvested and analyzed for the presence of RANTES by ELISA. Results are shown as means ± SEMs. (b) Assessment of virus antigen levels. Wild-type and ICP0 mutant virus (4 × 105 PFU) were denatured and separated by SDS-polyacrylamide gel electrophoresis. The levels of gD in the samples were assessed by Western blotting. Both results presented were seen in two independent experiments.
Together, these results show that the ability of macrophages to respond to HSV infection with production of RANTES is dependent on cellular PKR and proceeds through a mechanism involving viral ICP0.
DISCUSSION
Macrophages contribute to the innate immune response against HSV infection and constitute an important link between innate and adaptive immunity (15, 33). Part of the macrophage defense mechanisms is mediated by production of chemokines. Through recruitment and activation of leukocytes, together with their role in differentiation of T lymphocytes, chemokines have great influence on the host response and the outcome of viral infections (9, 18). Induced expression of a large number of chemokines has been observed during viral infection of various cell types. Particularly, MIP-1α, MIP-2, IP-10, MCP-1, MIG, and RANTES have been reported to be produced after viral challenges (9, 18, 19). Furthermore, it is interesting that the chemokines RANTES and MIP-1α seem to be implicated in Th1 development during HSV infections (9, 14, 35).
In this study, we report that HSV infection of macrophages specifically induces RANTES and BCA-1, suggesting a role for these chemokines in the response against the virus. RANTES is a chemoattractant for eosinophils, monocytes, basophils, NK cells, mast cells, and T lymphocytes, including T memory cells (16, 17, 20). Additionally, RANTES has been connected to Th1 differentiation and T-cell maturation (14, 28, 35). As to the role of RANTES in HSV infections, the data are sparse. However, given the ability of RANTES to support Th1 cell differentiation and attract CD4+ cells (32, 35), together with the known protective effect of a Th1-based immune response against HSV infection (9), it is tempting to speculate that RANTES plays a role in driving the immune response, eventually clearing the infection.
BCA-1 has been reported to participate in homing, recirculation, and development of B lymphocytes (3, 4), and the production of BCA-1 may have an impact on the innate as well as the adaptive immune response against HSV infections. It was recently reported that B-cell-deficient mice display an impaired ability to control HSV-1 and -2 at early stages of infection, and it was further demonstrated that the protective effect of B cells was due to production of natural antibodies (5, 12).
When we examined the mechanisms underlying RANTES expression, we found that UV-irradiated virus did not induce RANTES, which indicates dependence on the HSV genome. Subsequent experiments revealed that RANTES mRNA accumulation was preceded by immediate-early mRNA and protein but not early and late gene products. This opens up the possibility that the accumulation of immediate-early gene products plays a role in induction of RANTES in macrophages. Furthermore, experiments showed that the ICP0 mutant dl1403 induced significantly less RANTES than the wild type, whereas mutants with mutations in other immediate-early genes (ICP4, ICP22, and ICP27) did not. Virus-cell interactions in virus-induced cytokine production was recently reviewed (24), and the present findings further contribute to a molecular understanding of the immune response elicited by HSV infections.
Concerning how ICP0 might affect RANTES expression, several possibilities exist. First, ICP0 may stimulate IκB degradation. It is known that ICP0 induces protein degradation through the ubiquitin-proteasome pathway (10, 11) and that this at least in part is mediated by inhibition of SUMO-1 conjugation (26). SUMO-1 is a small ubiquitin-like protein that blocks the ubiquitin-proteasome pathway. Since a small pool of cellular IκB (the inhibitory unit of NF-κB) is SUMO-1 conjugated (6), it is possible that ICP0 acts by increasing the turnover of IκB. Second, it is possible that ICP0 mRNA forms extensive secondary structure and hence is a particular good activator of PKR. Third, ICP0 may affect RANTES expression through direct interactions with cellular transcription factors, thereby enhancing their activity, as recently shown for the cellular helix-loop-helix transcription factor BMAL1 (13).
The PKR mutant RAW-PKR-M7 secreted very low constitutive levels of RANTES compared to the empty vector cell line RAW-pBK-CMV and also produced significantly less RANTES after HSV infection. These findings suggest a role for PKR in constitutive and HSV-inducible expression of RANTES. As to the mechanism through which PKR affects RANTES expression, we have previously shown that activation of the cellular transcription factors ATF2/Jun and AP-1 by HSV in macrophages is dependent on PKR, whereas NF-κB activation occurs independently of PKR (29). Since the RANTES promoter contains functional binding sites for ATF2/Jun and AP-1 (2), it seems possible that PKR acts through these cellular pathways. The exact nature of the cellular signal transduction leading to expression of RANTES in HSV-infected macrophages is currently being investigated in our laboratory.
Collectively, the results of these studies demonstrate that RANTES and BCA-1 are specifically induced after HSV infection of macrophages and that PKR as well as products of the immediate-early gene ICP0 are implicated. The production of RANTES by macrophages may have an impact on the innate host defense through attraction of monocytes and NK cells and could also affect the adaptive immune response against HSV infection via the ability of this CC chemokine to support T-cell recruitment and Th1 cell differentiation.
Acknowledgments
This work was supported by grants from the Carlsberg Research Foundation (grant no. 990803/10-911), Fonden til lægevidenskabens fremme (grant no. 00182), and fhv. Direktør Leo Nielsen og hustru Karen Margrethe Nielsens legat for lægevidenskabelig grundforskning (grant no. LN 15/00). F.S.P. was supported by Karen Elise Jensen's Fond.
The donations of mutant viruses and cell lines by Roger D. Everett, Stephen Rice, David M. Knipe, Neal A. Deluca, and John A. Corbett are greatly appreciated. The skillful technical assistance of Birthe Søby and Elin Jacobsen has been invaluable.
REFERENCES
- 1.Biron, C. A., K. B. Nguyen, G. C. Pien, L. P. Cousens, and T. P. Salazar-Mather. 1999. Natural killer cells in antiviral defense: function and regulation by innate cytokines. Annu. Rev. Immunol. 17:189-220. [DOI] [PubMed] [Google Scholar]
- 2.Boehlk, S., S. Fessele, A. Mojaat, N. G. Miyamoto, T. Werner, E. L. Nelson, D. Schlondorff, and P. J. Nelson. 2000. ATF and Jun transcription factors, acting through an Ets/CRE promoter module, mediate lipopolysaccharide inducibility of the chemokine RANTES in monocytic Mono Mac 6 cells. Eur. J. Immunol. 30:1102-1112. [DOI] [PubMed] [Google Scholar]
- 3.Bowman, E. P., J. J. Campbell, D. Soler, Z. Dong, N. Manlongat, D. Picarella, R. R. Hardy, and E. C. Butcher. 2000. Developmental switches in chemokine response profiles during B cell differentiation and maturation. J. Exp. Med. 191:1303-1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brandes, M., D. F. Legler, B. Spoerri, P. Schaerli, and B. Moser. 2000. Activation-dependent modulation of B lymphocyte migration to chemokines. Int. Immunol. 12:1285-1292. [DOI] [PubMed] [Google Scholar]
- 5.Deshpande, S. P., U. Kumaraguru, and B. T. Rouse. 2000. Dual role of B cells in mediating innate and acquired immunity to herpes simplex virus infections. Cell. Immunol. 202:79-87. [DOI] [PubMed] [Google Scholar]
- 6.Desterro, J. M., M. S. Rodriguez, and R. T. Hay. 1998. SUMO-1 modification of IκBα inhibits NF-κB activation. Mol. Cell 2:233-239. [DOI] [PubMed] [Google Scholar]
- 7.Domachowske, J. B., C. A. Bonville, J. L. Gao, P. M. Murphy, A. J. Easton, and H. F. Rosenberg. 2000. The chemokine macrophage-inflammatory protein-1 α and its receptor CCR1 control pulmonary inflammation and antiviral host defense in paramyxovirus infection. J. Immunol. 165:2677-2682. [DOI] [PubMed] [Google Scholar]
- 8.Ellermann-Eriksen, S. 1993. Autocrine secretion of interferon-α/β and tumour necrosis factor-α synergistically activates mouse macrophages after infection with herpes simplex virus type 2. J. Gen. Virol. 74:2191-2199. [DOI] [PubMed] [Google Scholar]
- 9.Eo, S. K., S. Lee, S. Chun, and B. T. Rouse. 2001. Modulation of immunity against herpes simplex virus infection via mucosal genetic transfer of plasmid DNA encoding chemokines. J. Virol. 75:569-578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Everett, R. D., W. C. Earnshaw, J. Findlay, and P. Lomonte. 1999. Specific destruction of kinetochore protein CENP-C and disruption of cell division by herpes simplex virus immediate-early protein Vmw110. EMBO J. 18:1526-1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Everett, R. D., A. Orr, and C. M. Preston. 1998. A viral activator of gene expression functions via the ubiquitin-proteasome pathway. EMBO J. 17:7161-7169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harandi, A. M., B. Svennerholm, J. Holmgren, and K. Eriksson. 2001. Differential roles of B cells and IFN-γ-secreting CD4(+) T cells in innate and adaptive immune control of genital herpes simplex virus type 2 infection in mice. J. Gen. Virol. 82:845-853. [DOI] [PubMed] [Google Scholar]
- 13.Kawaguchi, Y., M. Tanaka, A. Yokoymama, G. Matsuda, K. Kato, H. Kagawa, K. Hirai, and B. Roizman. 2001. Herpes simplex virus 1 α regulatory protein ICP0 functionally interacts with cellular transcription factor BMAL1. Proc. Natl. Acad. Sci. USA 98:1877-1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim, J. J., L. K. Nottingham, J. I. Sin, A. Tsai, L. Morrison, J. Oh, K. Dang, Y. Hu, K. Kazahaya, M. Bennett, T. Dentchev, D. M. Wilson, A. A. Chalian, J. D. Boyer, M. G. Agadjanyan, and D. B. Weiner. 1998. CD8 positive T cells influence antigen-specific immune responses through the expression of chemokines. J. Clin. Investig. 102:1112-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kodukula, P., T. Liu, N. V. Rooijen, M. J. Jager, and R. L. Hendricks. 1999. Macrophage control of herpes simplex virus type 1 replication in the peripheral nervous system. J. Immunol. 162:2895-2905. [PubMed] [Google Scholar]
- 16.Kumar, A., Y. L. Yang, V. Flati, S. Der, S. Kadereit, A. Deb, J. Haque, L. Reis, C. Weissmann, and B. R. Williams. 1997. Deficient cytokine signaling in mouse embryo fibroblasts with a targeted deletion in the PKR gene: role of IRF-1 and NF-κB. EMBO J. 16:406-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuna, P., R. Alam, U. Ruta, and P. Gorski. 1998. RANTES induces nasal mucosal inflammation rich in eosinophils, basophils, and lymphocytes in vivo. Am. J. Respir. Crit. Care Med. 157:873-879. [DOI] [PubMed] [Google Scholar]
- 18.Liu, M. T., D. Armstrong, T. A. Hamilton, and T. E. Lane. 2001. Expression of Mig (monokine induced by interferon-γ) is important in T lymphocyte recruitment and host defense following viral infection of the central nervous system. J. Immunol. 166:1790-1795. [DOI] [PubMed] [Google Scholar]
- 19.Liu, M. T., B. P. Chen, P. Oertel, M. J. Buchmeier, D. Armstrong, T. A. Hamilton, and T. E. Lane. 2000. The T cell chemoattractant IFN-inducible protein 10 is essential in host defense against viral-induced neurologic disease. J. Immunol. 165:2327-2330. [DOI] [PubMed] [Google Scholar]
- 20.Loetscher, P., M. Seitz, I. Clark-Lewis, M. Baggiolini, and B. Moser. 1996. Activation of NK cells by CC chemokines. Chemotaxis, Calif.2+ mobilization, and enzyme release. J. Immunol. 156:322-327. [PubMed] [Google Scholar]
- 21.Long, M. C., V. Leong, P. A. Schaffer, C. A. Spencer, and S. A. Rice. 1999. ICP22 and the UL13 protein kinase are both required for herpes simplex virus-induced modification of the large subunit of RNA polymerase II. J. Virol. 73:5593-5604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maggi, L. B., Jr., M. R. Heitmeier, D. Scheuner, R. J. Kaufman, R. M. Buller, and J. A. Corbett. 2000. Potential role of PKR in double-stranded RNA-induced macrophage activation. EMBO J. 19:3630-3638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Malmgaard, L., S. R. Paludan, S. C. Mogensen, and S. Ellermann-Eriksen. 2000. Herpes simplex virus type 2 induces secretion of IL-12 by macrophages through a mechanism involving NF-κB. J. Gen. Virol. 81:3011-3020. [DOI] [PubMed] [Google Scholar]
- 24.Mogensen, T. H., and S. R. Paludan. 2001. Molecular pathways in virus-induced cytokine production. Microbiol. Mol. Biol. Rev. 65:131-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moghaddam, A., J. Koch, B. Annis, and F. Wang. 1998. Infection of human B lymphocytes with lymphocryptoviruses related to Epstein-Barr virus. J. Virol. 72:3205-3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muller, S., and A. Dejean. 1999. Viral immediate-early proteins abrogate the modification by SUMO-1 of PML and Sp100 proteins, correlating with nuclear body disruption. J. Virol. 73:5137-5143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murphy, P. M. 2001. Viral exploitation and subversion of the immune system through chemokine mimicry. Nat. Immunol. 2:116-122. [DOI] [PubMed] [Google Scholar]
- 28.Ortiz, B. D., P. J. Nelson, and A. M. Krensky. 1997. Switching gears during T-cell maturation: RANTES and late transcription. Immunol. Today 18:468-471. [DOI] [PubMed] [Google Scholar]
- 29.Paludan, S. R. 2001. Requirements for the induction of interleukin-6 by herpes simplex virus-infected leukocytes. J. Virol. 75:8008-8015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rice, S. A., and D. M. Knipe. 1990. Genetic evidence for two distinct transactivation functions of the herpes simplex virus alpha protein ICP27. J. Virol. 64:1704-1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rollins, B. J. 1997. Chemokines. Blood 90:909-928. [PubMed] [Google Scholar]
- 32.Schall, T. J., K. Bacon, K. J. Toy, and D. V. Goeddel. 1990. Selective attraction of monocytes and T lymphocytes of the memory phenotype by cytokine RANTES. Nature 347:669-671. [DOI] [PubMed] [Google Scholar]
- 33.Seid, J. M., M. Liberto, L. Bonina, K. N. Leung, and A. A. Nash. 1986. T cell-macrophage interactions in the immune response to herpes simplex virus: the significance of interferon-γ. J. Gen. Virol. 67:2799-2802. [DOI] [PubMed] [Google Scholar]
- 34.Shepard, A. A., and N. A. DeLuca. 1991. Activities of heterodimers composed of DNA-binding- and transactivation-deficient subunits of herpes simplex virus regulatory protein ICP4. J. Virol. 65:299-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sin, J. I., J. J. Kim, C. Pachuk, C. Satishchandran, and D. B. Weiner. 2000. DNA vaccines encoding interleukin-8 and RANTES enhance antigen-specific Th1-type CD4+ T-cell-mediated protective immunity against herpes simplex virus type 2 in vivo. J. Virol. 74:11173-11180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Skoldenberg, B. 1996. Herpes simplex encephalitis. Scand. J. Infect. Dis. Suppl. 100:8-13. [PubMed] [Google Scholar]
- 37.Stow, N. D., and E. C. Stow. 1986. Isolation and characterization of a herpes simplex virus type 1 mutant containing a deletion within the gene encoding the immediate early polypeptide Vmw110. J. Gen. Virol. 67:2571-2585. [DOI] [PubMed] [Google Scholar]
- 38.Tumpey, T. M., H. Cheng, D. N. Cook, O. Smithies, J. E. Oakes, and R. N. Lausch. 1998. Absence of macrophage inflammatory protein-1 α prevents the development of blinding herpes stromal keratitis. J. Virol. 72:3705-3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Whitley, R. J., and B. Roizman. 2001. Herpes simplex virus infections. Lancet 357:1513-1518. [DOI] [PubMed] [Google Scholar]
- 40.Williams, B. R. 1995. The role of the dsRNA-activated kinase, PKR, in signal transduction. Semin. Virol. 6:191-202. [Google Scholar]