Abstract
Both human immunodeficiency virus type 1 (HIV-1) and hepatitis C virus (HCV) lead to chronic infection in a high percentage of persons, and an expanding epidemic of HIV-1-HCV coinfection has recently been identified. These individuals provide an opportunity for simultaneous assessment of immune responses to two viral infections associated with chronic plasma viremia. In this study we analyzed the breadth and magnitude of the CD8+- and CD4+-T-lymphocyte responses in 22 individuals infected with both HIV-1 and HCV. A CD8+-T-lymphocyte response against HIV-1 was readily detected in all subjects over a broad range of viral loads. In marked contrast, HCV-specific CD8+-T-lymphocyte responses were rarely detected, despite viral loads in plasma that were on average 1,000-fold higher. The few HCV-specific responses that were observed were relatively weak and limited in breadth. CD4-proliferative responses against HIV-1 were detected in about half of the coinfected subjects tested, but no proliferative response against any HCV protein was found in these coinfected persons. These data demonstrate a major discordance in immune responses to two persistent RNA viruses. In addition, they show a consistent and profound impairment in cellular immune responses to HCV compared to HIV-1 in HIV-1-HCV-coinfected persons.
Coinfection with human immunodeficiency virus type 1 (HIV-1) and hepatitis C virus (HCV) is common and of increasing clinical relevance (8, 22). Relatively rapid progression of HCV-mediated liver disease has been described in individuals with HIV-1-related immunodeficiency (21, 47, 53, 54). There is also increasing evidence that infection with each of these viruses can alter the course of infection of the other virus (14, 22, 33), which has been hypothesized to be due to influences on immune responses. In coinfected individuals, HCV viral loads tend to be higher than in those infected with HCV alone (33). Conversely, acute HCV infection can raise HIV-1 viremia in persons with otherwise well-controlled illness, and coinfection with HCV may accelerate progression from HIV-1 infection to AIDS (22).
Cytotoxic T lymphocytes (CTL) and CD4+-T-helper cells have been shown to play an important role in both HIV-1 (27, 43, 51) and HCV (19, 37) infection. Numbers of antigen-specific CD8 cells in excess of 5% of total CD8 cells have been documented in monoinfection with HIV-1 and with HCV. Strong virus-specific CD4+ responses have also been documented in both infections in persons who spontaneously control viremia (2, 19, 37, 44, 51). However, in the majority of individuals with HIV-1 or HCV infection alone, the immune system is not capable of containing viremia.
An expanding body of evidence has provided more detailed characterization of the cellular immune responses in both HIV-1 monoinfection and HCV monoinfection. In HIV-1 infection, the vast majority of persons exhibit detectable CD8+-T-lymphocyte responses by new and more sensitive assays, such as gamma interferon (IFN-γ) production or tetramer analysis. In HCV infection, similar high levels of antigen-specific CD8+ cells can be detected in peripheral blood in some individuals, but the overall magnitude and breadth of CD8+-CTL responses is lower than observed in chronic HIV-1 infection (31, 32, 48, 58). Persons with chronic HIV-1 infection rarely demonstrate strong HIV-1-specific CD4+-proliferative responses, with the exception of individuals with long-term nonprogressive disease (27, 51). The proliferative response by CD4+ cells upon stimulation with HCV proteins is also weak in individuals with chronic HCV infection (9, 16, 19, 26), but vigorous CD4+ responses have been described in individuals with spontaneous resolution of infection (9, 16, 19, 37). In those persons who maintain long-term control of either infection, it has been suggested that specific genetic factors play a role, especially the HLA type (11, 18, 24, 29, 39, 42, 55). However, the ability of an individual's immune system to recognize these chronic viral infections simultaneously has not been determined.
In this study we have analyzed the CD8+- and CD4+-T-lymphocyte responses in 22 individuals coinfected with HIV-1 and HCV. Using a recombinant vaccinia virus-based enzyme-linked immunospot (Elispot) assay and a standard proliferation assay, we analyzed the magnitude and breadth of HIV-1- and HCV-specific responses in each individual. Intraindividual differences in the immune response against the two viruses were evaluated in subjects with different courses and stages of both HIV-1 infection and HCV infection. Our principal aim was to analyze and compare the cellular immune responses against HIV-1 and HCV in the same individual. The study was not designed to address the influence of HIV-1 on HCV-specific responses or inversely the influence of HCV on HIV-1-specific responses. However, in the course of the study a limited but nevertheless significant comparison could be made regarding the CD4+-T-cell response against the two viruses in mono- and coinfection.
MATERIALS AND METHODS
Study subjects.
Twenty-two individuals with HIV-1-HCV coinfection were studied, with the clinical characteristics outlined in Table 1. All were HCV and HIV-1 antibody positive for more than 1 year, as measured by enzyme immunoassay. Subjects on HAART had all been receiving therapy for at least 6 months.
TABLE 1.
Clinical characteristics of 22 patientsa
| Patient | Sex | Age (yr) | CD4 count | ALT level | HIV quant | HCV quant | HCV genotype | Histology | HIV Tx | HCV Tx |
|---|---|---|---|---|---|---|---|---|---|---|
| CO-1 | M | 52 | 280 | 121 | <50 | 1,334,400 | 1b | ND | HAART | None |
| CO-2 | M | 40 | 550 | 126 | <50 | 237,140 | 1a | 2, 1 | HAART | None |
| CO-3 | M | 43 | 331 | 68 | <400 | 503,820 | 1a | 2-3, 1-2 | HAART | None |
| CO-4 | F | 45 | 671 | 12 | <50 | 26,516,000 | 1a | 2-3, 2 | HAART | DC |
| CO-5 | M | 41 | 445 | 110 | <400 | 467,720 | 1a | 3, 4 | HAART | None |
| CO-6 | F | 35 | 246 | 103 | 9,420 | 118,430 | 2b | 2, 4 | HAART | None |
| CO-7 | F | 56 | 234 | 50 | <50 | Positive | 3a | ND | HAART | None |
| CO-8 | M | 46 | 1,224 | 133 | 88 | >1,000,000 | 1b | 2, 1 | HAART | None |
| CO-9 | M | 42 | 140 | 82 | <50 | 603,750 | 1b | 2, 1-2 | HAART | None |
| CO-10 | F | 31 | 278 | 198 | 251 | 23,000,000 | 1a | ND | HAART | None |
| CO-11 | M | 36 | 916 | 121 | 400 | >1,000,000 | 3a | 2, 2 | HAART | None |
| CO-12 | M | 43 | 668 | 126 | <50 | 779,250 | 1b | 2, 4 | None | NRb |
| CO-13 | M | 38 | 877 | 186 | 80 | 1,358,300 | 1a | ND | None | None |
| CO-14 | M | 43 | 815 | 76 | <50 | 793,000 | 1a | 2, 1 | None | None |
| CO-15 | F | 33 | 295 | 162 | 960 | 317,510 | ND | ND | None | None |
| CO-16 | M | 38 | 382 | 72 | 1,910 | 3,059,400 | 1a | ND | None | None |
| CO-17 | F | 47 | 318 | 28 | 319,000 | 532,100 | 1a | ND | DC | None |
| CO-18 | F | 40 | 725 | 39 | 21,300 | >1,000,000 | 3a | ND | None | None |
| CO-19 | M | 39 | 1,064 | 137 | 94,000 | Positive | ND | ND | None | None |
| CO-20 | M | 40 | 308 | 15 | 82,100 | Negative | 1a | 2, 1 | DC | ETR |
| CO-21 | F | 46 | 336 | 17 | 54 | Negative | ND | ND | HAART | ETR |
| CO-22 | M | 46 | 268 | 50 | <50 | Negative | ND | ND | HAART | Spontaneous |
ALT level, alanine aminotransferase levels in serum (units per milliliter); HIV quant, quantitative HIV PCR (viral RNA copies per milliliter); HIV Tx, treatment for HIV infection; ND not done; DC, discontinued; and ETR, end-of-treatment response. Histology results are given as grading of inflammation and staging of fibrosis (0 to 4, METAVIR score). Subjects with boldfaced patient identification were tested in the lymphoproliferative assay.
NR, no response.
Two control groups were also analyzed for their CD4+-proliferative response. Control group A included eight individuals with HIV-1 monoinfection. All were HIV-1 antibody positive for more than 1 year and were receiving highly active antiretroviral therapy (HAART) for more than 6 months with viral loads under 400 copies/ml, and CD4 cell counts ranged between 173/μl and 985/μl. Control group B was comprised of 17 individuals positive for anti-HCV but not for anti-HIV-1. Fourteen had chronic HCV infection; one had cleared HCV infection spontaneously and two had undergone successful treatment with IFN-α. All individuals gave informed written consent.
Vaccinia virus constructs.
Recombinant vaccinia viruses constructed from the HIV-1 IIIb isolate included those expressing Gag (vAbT141), reverse transcriptase (RT) (vCF21), and Env (vPE11). A recombinant vaccinia virus expressing the Nef protein was constructed from the HIV-1 SF2 isolate (generous gift of T. Yilma). Six other vaccinia constructs were used expressing overlapping segments of the complete HCV-1 sequence, a genotype 1a strain. The constructs express the following regions of HCV: 9A expresses the HCV core and the E1 region (amino acids [aa] 1 to 339), 1H expresses E2 and NS2 (aa 339 to 906), NNRD expresses E2, NS2, and NS3 (aa 364 to 1619), NS4 expresses NS4 (aa 1590 to 2050), NS5A expresses NS5A (aa 2005 to 2396), and NS5B expresses NS5B (aa 2396 to 3011). A vaccinia construct containing the beta-galactosidase gene (lac) served as a control. These constructs were the kind gift of the Chiron Corporation.
HIV-1 and HCV proteins.
HIV-1 p24 Gag and gp160 are recombinant baculovirus-derived proteins (51) and were purchased from Protein Sciences, Meriden, Conn. The following recombinant HCV proteins were kindly provided by Chiron Corporation: c22 (core, 118 aa), c33c (NS3, 265 aa), c100 (NS4, 362 aa), and NS5 (941 aa). All were expressed as COOH-terminal fusion proteins with human superoxide dismutase in yeast. Control proteins consisted of yeast extract and superoxide dismutase.
Elispot assay.
96-Well polyvinylidene plates (Millipore) were coated with 0.5 μg of recombinant human anti-IFN-γ antibody (Endogen)/ml in phosphate-buffered saline at 4°C overnight. Fresh or previously frozen peripheral blood mononuclear cells (PBMC) were added at 50,000 to 200,000 cells/well in 140 μl of R10 medium (RPMI 1640 [Sigma-Aldrich], 10% fetal calf serum [Sigma-Aldrich], and 10 mM HEPES buffer [Sigma-Aldrich]) with 2 mM glutamine and antibiotics (50 U of penicillin and 50 μg of streptomycin/ml). The vaccinia vectors were added in 10 μl of R10 at a multiplicity of infection of 3 to 5. The plate contents were incubated for 36 h at 37°C with 5% CO2. Plates were then washed, labeled with 0.25 μg of biotin-labeled anti-IFN-γ (Endogen)/ml, and developed by incubation with streptavidin-alkaline phosphatase (Bio-Rad) followed by incubation with 5-bromo-4-chloro-3-indolylphosphate-nitroblue tetrazolium (Bio-Rad) in Tris buffer (pH 9.5). The reaction was stopped by washing with tap water, and the plates were dried overnight, prior to counting by direct visualization.
Class I-peptide tetramers.
Class I peptide tetramers were prepared as previously described (37) and included tetramers specific for epitopes restricted by HLA-A2 (NS3 peptide 1073-1081, CINGVWCTV; NS4 peptide 1406-1415, KLVALGINAV; NS4 peptide 1807-1816, LLFNILGGWV; and NS5B peptide 2594-2602, ALYDVVTKL) (23, 36, 37), HLA-B7 (core peptide 41-49, GPRLGVRAT; and core peptide 111-119, DPRRRSRNL) (23, 36), and HLA-B8 (NS3 peptide 1395-1403, HSKKKLDEL; and NS3 peptide 1611-1618, LIRLKPTL) (23, 36).
Tetramer staining.
PBMC (5 × 105 to 1 × 106) were stained as described earlier (23). Flow cytometry analysis was performed with a Becton Dickinson FACSCalibur fluorescence-activated cell sorter, and analysis was performed with CellQuest software.
Proliferation assay.
Lymphocyte proliferation assays were carried out using the proteins described above at concentrations of 5 μg/ml (HIV-1 proteins) or 10 μg/ml (HCV proteins). Fresh PBMC were plated with 150 μl of R10/HAB medium (RPMI 1640 [Sigma-Aldrich], 10% human AB serum [Sigma-Aldrich], and 10 mM HEPES buffer [Sigma-Aldrich]) with 2 mM glutamine and antibiotics (50 U of penicillin and 50 μg of streptomycin/ml) and the proteins in 96 U-bottomed plates (Costar) at 100,000 cells per well for 6 days. On day 6 the plate contents were incubated for 6 h with 1 μCi of [3H]thymidine (NEN). Cells were then collected on filters, and the amount of incorporated radiolabel was measured with a beta counter. For the purposes of data interpretation, a stimulation index of 5 or greater was considered significant.
Viral load quantitation.
Plasma viral loads were measured using either the Roche Amplicor Monitor assay (detection limit of 400 HIV-1 RNA copies/ml of plasma and 100 HCV RNA copies/ml of plasma) or the Roche Ultradirect assay (detection limit of 50 HIV-1 RNA copies/ml of plasma), according to the manufacturer's specifications.
Statistical analysis.
Statistical analysis (Mann-Whitney rank sum test, Fisher's exact test, and correlation coefficient) was done using GraphPad Prism 3.0a for Macintosh.
RESULTS
Assessment of viral loads in persons with HIV-1-HCV coinfection.
All 22 coinfected individuals studied had serum antibodies to both HIV-1 and HCV, as determined by enzyme-linked immunosorbent assay. The clinical and virologic characteristics of the persons studied are provided in Table 1. Three of these individuals (CO-20, CO-21, and CO-22) had no detectable HCV RNA in plasma using a sensitive PCR assay (limit of detection 100 HCV RNA copies/ml). Two subjects (CO-20 and CO-21) had cleared HCV RNA after therapy with IFN-α; an additional subject (CO-22) had a spontaneous clearance of RNA following acute HCV infection. The remaining 19 individuals had detectable HCV RNA by PCR, and in the 15 individuals in whom quantitative testing was performed, viral loads ranged from 118,430 to 23,000,000 copies per ml of plasma. The mean HCV viral load was more than 1,000-fold higher than the HIV viral load. There was no significant correlation between HCV viral loads and HIV-1-viral loads (r = −0.44, P > 0.1) or CD4 counts (r = −0.26, P > 0.2).
HIV-1 viremia was undetectable or controlled to a level under 1,000 viral copies per ml of plasma in 16 of the 22 individuals studied. This was achieved by HAART in 12 persons, but four individuals (CO-12 to CO-15) controlled HIV-1 replication without the need for antiretroviral therapy. It is noteworthy that all four individuals spontaneously controlling HIV-1 were unable to control HCV, with HCV viral loads ranging from 317,500 to 1,358,300 copies per ml. HCV viral loads in these persons who spontaneously controlled HIV-1 viremia were not significantly different from the loads in those who were effectively treated with HAART (P > 0.8). Finally, six individuals had HIV-1 RNA levels greater than 1,000 copies per ml, one of them (CO-20) being the only person in the cohort who had been able to immunologically control HCV infection. These results suggest that the ability to maintain spontaneous virologic control of HIV-1 infection is not associated with the ability to control HCV infection.
Intraindividual comparison of HIV-1- and HCV-specific CD8+-T-cell responses in coinfected persons.
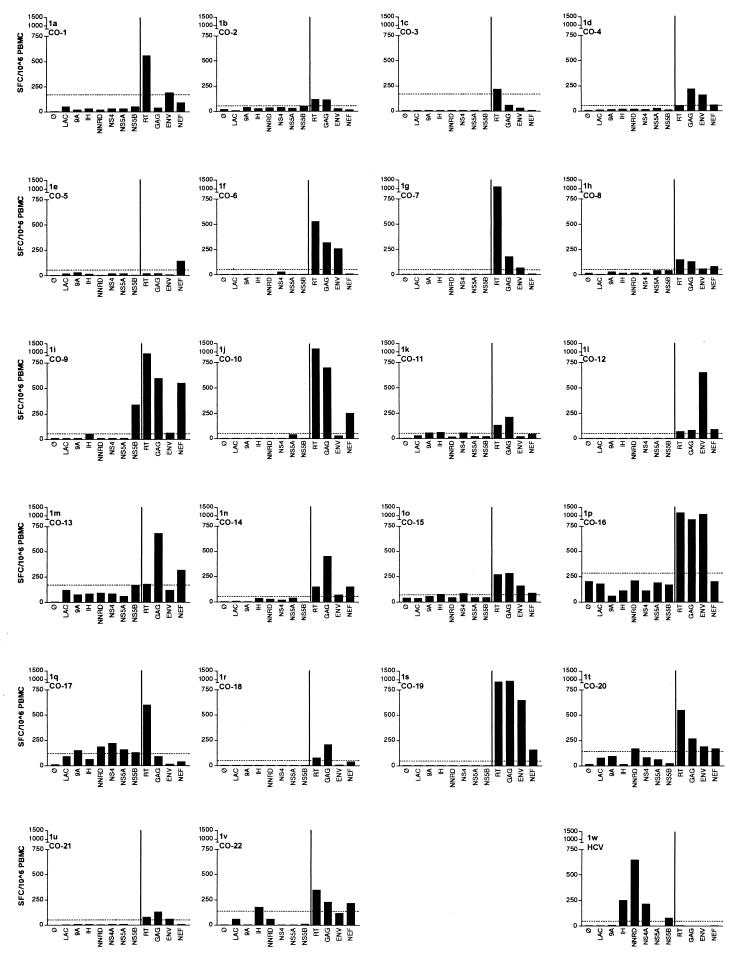
Having demonstrated marked differences in viral loads in persons with HIV-1-HCV coinfection, we next examined CD8+-T-lymphocyte responses in all 22 individuals using recombinant vaccinia viruses in an Elispot assay (Fig. 1). Background levels of activity against the control Lac vaccinia construct were usually lower than 50 spot-forming cells/106 PBMC but reached up to 180 spot-forming cells/106 PBMC in six individuals, most likely due to prior immunization against smallpox (35). We therefore established the threshold of significance separately for each individual by quadruplicate testing of the response to the control vaccinia vector expressing the Escherichia coli beta-galactosidase gene product. Responses greater than the average Lac vaccinia virus response plus 2 standard deviations were considered statistically significant. To ensure that the response measured was truly a response by CD8+ cells, we performed the Elispot assay in three randomly selected individuals after the depletion of either CD8+ or CD4+ T cells. While depletion of CD8+ T cells completely abolished the response, the result after CD4+-T-cell depletion was in a comparable range with the result using unfractionated PBMC (data not shown). Twelve of the 22 persons exhibited IFN-γ production in response to at least three of the four HIV-1 gene products tested, and all 22 were able to target at least one protein (Fig. 1). Responses were detected in persons on and off therapy in this cross-sectional analysis. RT was the most commonly recognized antigen (20 of 22), followed by Gag (17 of 22), Env (13 of 22), and Nef (11 of 22). In contrast, only one of the coinfected persons (subject CO-17) targeted more than one HCV protein above the levels of the negative controls, and HCV-specific CD8-T-cell responses were completely absent in 17 of the 22 persons. Thus, a CD8+-T-lymphocyte response to HCV was significantly less common than to HIV-1, using analogous methods of detection (P < 0.0001). This was not due to a technical problem with the assay system employed, as we had earlier examined HCV-monoinfected individuals with a significant CD8+-T-lymphocyte response in a peptide-based Elispot. All six HCV vaccinia constructs tested positive; representative results from one subject (designated “HCV”) are shown in Fig. 1w. Additionally, it was shown earlier that infection with these vaccinia constructs results not only in stimulation of IFN-γ production (37) but also in effective recognition and killing by specific CTL (58, 59). These data indicate that the CD8+-T-lymphocyte response to these two infections is highly discordant.
FIG. 1.
CTL responses against HIV-1 and HCV. Vaccinia virus Elispot responses are shown to each of the HIV-1 and HCV antigens tested (HIV-1 antigens to the right and HCV antigens to the left of the bar) as well as the negative and Lac controls. Results are given in spot-forming cells (SFC) per 100,000 PBMC. The final graph (w) represents an individual with HCV monoinfection who had cleared acute HCV infection about 2 years prior to the assay and has a strong HCV-specific CTL response.
Class I tetramer analysis does not reveal additional HCV-specific CD8+-T-cell responses undetected in the Elispot assay.
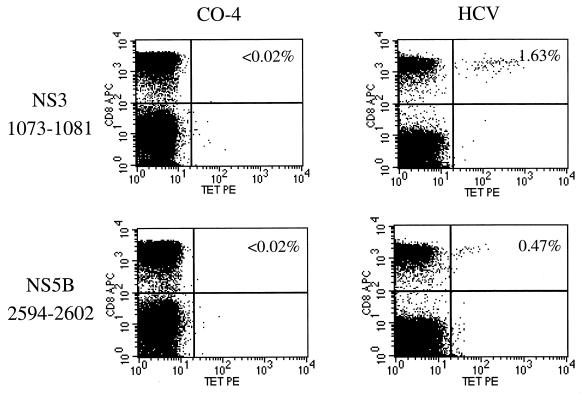
HCV-specific CD8+ T lymphocytes can be impaired in their ability to secrete IFN-γ (23). The IFN-γ Elispot assay might therefore underestimate the true HCV-specific response by CD8+ T lymphocytes, if some of these are unable to secrete IFN-γ. In contrast, class I tetramers recognize HCV-specific CD8+ T lymphocytes by directly binding to the epitope-specific T-cell receptor, thus allowing direct quantification of specific cells by flow cytometry analysis. Tetramer analysis was therefore performed in a subset of eight subjects expressing HLA alleles for which HCV tetramers were available, including HLA-A2 (three subjects and four epitopes tested), -B7 (four subjects and 2 epitopes tested) and -B8 (one subject and two epitopes tested).
None of the subjects had a detectable response to any of the epitopes tested (two representative stainings from subject CO-4 are shown in Fig. 2). This is in marked contrast to strong staining for the HLA-A2-restricted epitopes NS3 1073-1081 and NS5B 2594-2602 in a control individual infected with HCV alone who was controlling HCV viremia (designated HCV, Fig. 2). In this person responses against HCV were also detected by Elispot in the corresponding regions (Fig. 1w). These results demonstrate that the low frequency of HCV-specific CD8+ T cells in the recombinant vaccinia Elispot is unlikely to be a detection problem due to a “stunned” phenotype of HCV-specific cells (23, 37) or due to low expression of recombinant HCV vaccinia constructs.
FIG. 2.
Tetramer staining of HCV-specific CD8+ T cells. The two graphs on the left show the absence of CD8+ T cells specific for the epitopes 1073-1081 and 2594-2602 in the coinfected individual CO-4. On the right, CD8+ T cells specific for the same two peptides were readily detected in an individual who had cleared HCV monoinfection. Each person expressed the relevant HLA alleles for the tetramers tested. APC, allophycocyanin; TET PE, tetramer phycoerythrin.
Intraindividual comparison of HIV-1- and HCV-specific CD4+-T-cell responses in coinfected persons.
A considerable body of evidence indicates that the presence of strong virus-specific T-helper cell responses is required for maintenance of strong CTL function in chronic viral infections (28, 40, 61), including infection with HIV-1 (27). We therefore examined the coinfected persons for evidence of virus-specific CD4+ T cells, using a standard lymphocyte proliferation assay.
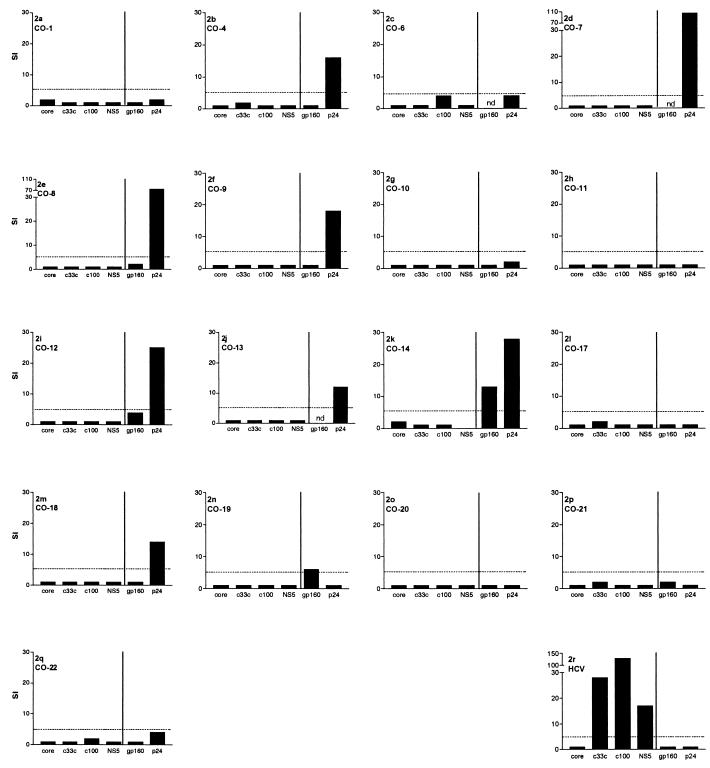
Since this assay does not deliver reliable results when applied to frozen cells (data not shown), we restricted the analysis of the CD4+-proliferative responses to the 17 individuals from whom freshly isolated cells were available. Overall, 9 of 17 subjects had a detectable HIV-1 p24-specific proliferative response to HIV-1 (Fig. 3), a higher percentage than has been typically reported in HIV monoinfection (51). HIV-1 gp160-specific T-helper cell responses were tested in 14 of the subjects and were positive in only 2. Only one individual (CO-14) responded to both HIV-1 proteins (Fig. 3k).
FIG. 3.
T-helper cell responses against HIV-1 and HCV. Stimulation indices are shown for four different HCV antigens and two HIV-1 antigens in 17 of the 22 coinfected individuals. The final graph (r) represents an individual with HCV monoinfection who had cleared acute HCV infection about 2 years prior to the assay.
Consistent with previous reports (2, 51), all three subjects tested with spontaneous control of HIV-1 replication (CO-12, CO-13, and CO-14) had detectable proliferative responses to p24 protein (Fig. 3). Surprisingly, we also found strong proliferative responses to HIV-1 p24 in four out of eight individuals who had chronic HCV-HIV coinfection but were successfully treated for HIV-1 infection with HAART (CO-4 and CO-7 to CO-9).
Lymphocyte proliferation assays were also performed with available HCV proteins in these coinfected persons and controls, using soluble proteins spanning over 60% of the expressed proteins of the virus. In contrast to the detection of HIV-1-specific responses, none of these HCV-HIV-coinfected persons had proliferative responses to HCV, despite continued exposure to HCV antigen (Fig. 3).
Comparative analysis of HIV-1- and HCV-specific CD4+-T-cell responses in coinfected and monoinfected persons.
Since the CD4+-T-cell responses to HIV-1 were strikingly high in the individuals on HAART and the responses to HCV were strikingly low in the whole cohort, we performed further analysis of the same responses in matched, monoinfected persons.
For HIV-1 we compared the results with a matched group of eight individuals on HAART who were only positive for anti-HIV-1 but not anti-HCV (control group A). Only one individual tested positive for a HIV-1 p24-proliferative response (data not shown).
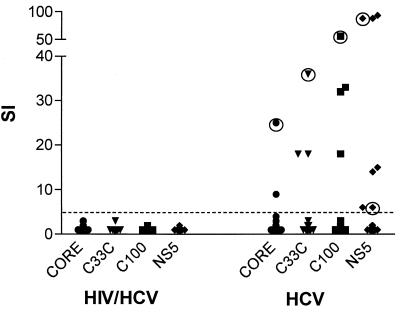
The HCV antigens were used to test HCV-monoinfected persons (control group B). In contrast to the results in the coinfected individuals, we were able to detect an HCV-specific proliferative response in 8 of 17 persons (47%) with HCV monoinfection (P < 0.002) (Fig. 4). Only three of the individuals had a response to two or more of the antigens (example in Fig. 3r), including two individuals after resolution of HCV infection. These results are similar to those reported elsewhere in the literature where HCV-specific CD4+-proliferative responses had been detected in 16 to 59% of individuals with chronic infection (9, 12, 16, 26) and in a higher percentage of persons after clearance of HCV (9, 12, 13, 19). This confirms that detectable virus-specific proliferative responses to HCV are diminished in the majority of persons with HIV-1-HCV coinfection.
FIG. 4.
Comparison of the lymphoproliferative responses to HCV antigens in 17 individuals with HIV-1-HCV coinfection and 17 with HCV monoinfection. No HCV-specific proliferative responses were detected in the coinfected individuals, but responses to all four HCV proteins were seen in the HCV-monoinfected cohort. Responses by HCV RNA-negative individuals are marked with a circle. SI, stimulation index.
DISCUSSION
In this study we have evaluated the cellular immune response in peripheral blood against HIV-1 and HCV in subjects with HIV-1-HCV coinfection. Although infection with each virus can lead to strong CTL and T-helper cell responses (19, 37, 51), we noted markedly weaker CD8+-T-lymphocyte responses to HCV than to HIV-1 in coinfected persons, regardless of disease stage. Moreover, T-helper cell responses to HCV were entirely absent in this cohort with dual infection. Notably, HAART-associated control of HIV-1 plasma viremia was associated with the frequent detection of HIV-1-specific T-helper cell responses in dually infected persons, much higher than typically reported in persons with chronic HIV-1 monoinfection (46, 49). However, none of these persons had detectable HCV-specific T-helper cell responses.
A CD8+-T-lymphocyte response against HIV-1 was readily detected in all 22 subjects, regardless of the stage or course of the HIV-1 or HCV infection and regardless of HIV-1 or HCV viral load. The CD8+-T-lymphocyte response against HIV-1 was strong and broadly directed in the majority of individuals. In contrast, HCV-specific CD8+-T-lymphocyte responses were absent in the majority of individuals, and in the few persons with detectable HCV-specific CD8+ T lymphocytes, the response was generally weak. This result was confirmed by analysis using class I tetramers, which failed to demonstrate HCV-specific CD8+ T lymphocytes in the subgroup of our cohort tested. Therefore, our findings in chronic HCV infection cannot be explained by nonfunctional HCV-specific CD8+ T lymphocytes (23, 37), which in other settings have been shown to be present by tetramer analysis but restricted in secretion of IFN-γ. Also, when comparing the HIV-1- and the HCV-specific responses, it is important that the HCV Elispot assessed CD8 T-cell responses directed against peptides representing all expressed proteins, whereas only about two-thirds of HIV-1 proteins were screened for HIV-1-specific CD8+ T lymphocytes. Since untested HIV-1 proteins, such as Tat, Rev, and Vpr, are also frequent targets for HIV-1-specific CD8+ T lymphocytes (1, 3), our results most likely underestimate the difference in the CD8+-T-lymphocyte responses against the two viruses. The observed relative lack of HCV-specific CD8+ T lymphocytes is not explained by advanced HIV-1-induced immunodeficiency, as most subjects in our cohort were controlling HIV-1, either spontaneously or with HAART. In addition, although not analyzed here, T-cell responses to other viruses, such as cytomegalovirus, Epstein-Barr virus, and herpes simplex virus, are readily detectable late in the course of HIV infection (10, 15, 30). Thus, these findings in coinfected persons are distinct and not anticipated.
The analysis of cellular immune responses was also extended to CD4+-T-cell responses. Maintenance of CD8+-lymphocyte responses in chronic viral infections is thought to be dependent upon the presence of virus-specific T-helper cell responses (27, 28, 40, 61). CD4+-T-helper cell responses against HIV-1 were, as expected, not as common as HIV-1-specific CD8+-T-lymphocyte responses, with little more than half of the individuals tested responding to one of the HIV-1-specific antigens. Interestingly, we observed robust proliferation in response to HIV-1 p24 in four out of eight coinfected individuals on HAART. In studies with HIV-1-monoinfected individuals, strong proliferative responses are usually limited to individuals with long-term nonprogressive disease (51) or those treated during acute HIV-1 infection (50). Although restoration of HIV-1 antigen-induced proliferation is typically not observed in chronically HIV-1-infected individuals after the introduction of HAART (2, 46; M. Plana, F. Garcia, T. Gallart, J. M. Miro, and J. M. Gatell, Letter, Lancet 352:1194-1195, 1998), isolated reports are beginning to appear suggesting that these responses may be produced with longer follow-up (7). When we compared these results to a matched cohort of individuals with HIV-1 monoinfection, only one out of eight individuals displayed an HIV-1 p24-proliferative response, a difference that approached but did not reach statistical significance. This finding is especially interesting in light of recent reports showing a beneficial effect of coinfection with GB virus C on survival of HIV-1 infected individuals (56, 60). GB virus C is the virus most closely related to HCV in the Flaviviridae family, but so far no disease could be associated with GB virus C. Therefore, one could speculate that both viruses have a positive effect on the HIV-1-specific immune response but that this effect is masked by the liver disease caused by HCV in HIV-1-HCV coinfection. A larger cohort of mono- and coinfected individuals needs to be studied to address this issue.
Perhaps the most striking finding in this study was the lack of detectable T-helper cell responses to HCV in persons with dual infection. HIV-1-specific proliferative responses were seen in 9 of 17 subjects tested, but not a single individual demonstrated T-helper cell responses to HCV antigens. This occurred even though, similar to CD8+-T-lymphocyte responses, a larger portion of HCV antigens than HIV-1 antigens was screened in the proliferation assay. This lack of HCV-specific T-helper cell responses is quite different from chronic HCV monoinfection, in which 16 to 59% of individuals have been reported to have detectable HCV-specific proliferative responses, even though they are usually weak and narrowly directed (9, 12, 16, 26). An even higher percentage of individuals has been shown to have HCV-specific proliferative responses after HCV has been cleared (9, 12, 13, 19), either spontaneously or under therapy. The one person in this cohort who had cleared HCV infection spontaneously had no detectable proliferative response to HCV. It will be interesting if future studies compare subjects who clear HCV infection before and after they acquire HIV-1, as differences in these two groups might reveal the mechanisms by which HIV-1 influences the CD4 response against HCV.
Because of the variations between studies regarding the HCV-specific proliferative response, we expanded our original study to include a matched cohort of persons with chronic or resolved HCV monoinfection. This confirmed a difference in the HCV-specific CD4+-T-cell response, as in the control cohort a proliferative response was detected with statistically significantly higher frequency than in the coinfected group (47 versus 0%, P < 0.002). This interesting finding does not appear to be due to HIV-1-related immune impairment: it has been shown that while other, non-HIV-1-specific proliferative responses decrease late in advanced HIV-1 disease, they improve under HAART (4, 46, 57; Plana et al., letter). The majority of the individuals in our cohort had no evidence of advanced HIV-1-induced immunocompromise, and many had been on HAART for more than 1 year, but still no HCV-specific T-helper response was detectable. This suggests that HIV-1 infection might influence the HCV-specific T-cell response by means other than simply the general failure of the immune system in more advanced stages of HIV-1 infection. Longitudinal studies are required to further evaluate the course of HCV-specific proliferative responses in coinfected individuals, especially for individuals who will be treated with HAART.
Overall, the comparison of the intraindividual cellular immune response toward HIV-1 and HCV in coinfected persons shows that an individual having a strong cellular immune response against HIV-1 generally does not have a similar response against HCV. This finding was seen in all individuals analyzed and was independent of the stage or course of either HIV-1 infection or HCV infection. One explanation for the low frequency of HCV-specific T cells in peripheral blood might be compartmentalization of these cells to the liver as the main site of infection. Indeed, there is some evidence that HCV-specific CD8+ and CD4+ T cells are enriched in the liver (20, 25, 41, 52). While this possibility certainly has its merits, previous studies were limited either by small numbers of individuals studied or by the need to expand cells in vitro before testing. Therefore, the extent to which HCV-specific cells might actually be enriched in the liver is not known. Furthermore, while compartmentalization might contribute to a lower frequency of HCV-specific cells in PBMC, it is unlikely to explain the large discordance observed. It has been shown by cloning directly from liver biopsies obtained from chronically HCV-monoinfected individuals that fewer than half of the subjects studied were found to have any CTL response against HCV (58). Furthermore, in individuals with acute HCV infection who subsequently cleared the virus, a vigorous HCV-specific CTL and T-helper cell response could be readily detected in the periphery (19, 37).
As compartmentalization alone is unlikely to explain the difference in the responses against HIV-1 and HCV, other mechanisms must contribute to the relative lack of cellular immune response against HCV compared to the response against HIV-1. It is especially surprising that the HCV-specific CD8+-T-lymphocyte response is so weak despite such high levels of viral replication, whereas HIV-1-specific CD8+ T lymphocytes are easily detected even in the absence of detectable virus. On the other hand, these high HCV loads could lead to exhaustion of antigen-specific T cells (17, 45). Another possibility is that, as HCV infects primarily hepatocytes, HCV-specific CD8+ T cells are not sufficiently generated in the first place, perhaps due to deficient priming of naive T cells (5, 6, 38) or insufficient T-help once HCV-specific naive T cells have been activated (34). These are critical issues that will need to be addressed in future studies.
In conclusion, we describe robust cellular immune responses against HIV-1 in HIV-1-HCV-coinfected individuals in different stages and with different courses of HIV-1 infection. In contrast, HCV-specific T-cell responses in the same individuals are absent or at best of low frequency and are narrowly directed despite high levels of ongoing HCV replication. These findings suggest that, while both HIV-1 and HCV are in most cases not effectively controlled by the immune system, HCV and HIV-1 might employ distinct mechanisms to evade immune control.
Acknowledgments
This work was supported by a grant to G.M.L. from the Deutsche Forschungsgemeinschaft (DFG LA 1241/1-1) and by a grant to B.D.W. from the National Institutes of Health. B.D.W. is a Doris Duke Distinguished Clinical Science Professor. P.K. was funded by the Wellcome Trust, and M.L. was funded by the European Union (5th Framework, HCVacc, grant no. QLK2-CT-1999-00356).
REFERENCES
- 1.Addo, M. M., M. Altfeld, E. S. Rosenberg, R. L. Eldridge, M. N. Philips, K. Habeeb, A. Khatri, C. Brander, G. K. Robbins, G. P. Mazzara, P. J. Goulder, and B. D. Walker. 2001. The HIV-1 regulatory proteins Tat and Rev are frequently targeted by cytotoxic T lymphocytes derived from HIV-1-infected individuals. Proc. Natl. Acad. Sci. USA 98:1781-1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altfeld, M., E. S. Rosenberg, R. Shankarappa, J. S. Mukherjee, F. M. Hecht, R. L. Eldridge, M. M. Addo, S. H. Poon, M. N. Phillips, G. K. Robbins, P. E. Sax, S. Boswell, J. O. Kahn, C. Brander, P. J. Goulder, J. A. Levy, J. I. Mullins, and B. D. Walker. 2001. Cellular immune responses and viral diversity in individuals treated during acute and early HIV-1 infection. J. Exp. Med. 193:169-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Altfeld, M. A., B. Livingston, N. Reshamwala, P. T. Nguyen, M. M. Addo, A. Shea, M. Newman, J. Fikes, J. Sidney, P. Wentworth, R. Chesnut, R. L. Eldridge, E. S. Rosenberg, G. K. Robbins, C. Brander, P. E. Sax, S. Boswell, T. Flynn, S. Buchbinder, P. J. R. Goulder, B. D. Walker, A. Sette, and S. A. Kalams. 2001. Identification of novel HLA-A2-restricted human immunodeficiency virus type 1-specific cytotoxic T-lymphocyte epitopes predicted by the HLA-A2 supertype peptide-binding motif. J. Virol. 75:1301-1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Autran, B., G. Carcelain, T. S. Li, C. Blanc, D. Mathez, R. Tubiana, C. Katlama, P. Debre, and J. Leibowitch. 1997. Positive effects of combined antiretroviral therapy on CD4+ T cell homeostasis and function in advanced HIV disease. Science 277:112-116. [DOI] [PubMed] [Google Scholar]
- 5.Bertolino, P., M. C. Trescol-Biemont, and C. Rabourdin-Combe. 1998. Hepatocytes induce functional activation of naive CD8+ T lymphocytes but fail to promote survival. Eur. J. Immunol. 28:221-236. [DOI] [PubMed] [Google Scholar]
- 6.Bertolino, P., M. C. Trescol-Biemont, J. Thomas, B. F. de St Groth, M. Pihlgren, J. Marvel, and C. Rabourdin-Combe. 1999. Death by neglect as a deletional mechanism of peripheral tolerance. Int. Immunol. 11:1225-1238. [DOI] [PubMed] [Google Scholar]
- 7.Blankson, J. N., J. E. Gallant, and R. F. Siliciano. 2001. Proliferative responses to human immunodeficiency virus type 1 (HIV-1) antigens in HIV-1-infected patients with immune reconstitution. J. Infect. Dis. 183:657-661. [DOI] [PubMed] [Google Scholar]
- 8.Bonacini, M., and M. Puoti. 2000. Hepatitis C in patients with human immunodeficiency virus infection: diagnosis, natural history, meta-analysis of sexual and vertical transmission, and therapeutic issues. Arch. Intern. Med. 160:3365-3373. [DOI] [PubMed] [Google Scholar]
- 9.Botarelli, P., M. R. Brunetto, M. A. Minutello, P. Calvo, D. Unutmaz, A. J. Weiner, Q. L. Choo, J. R. Shuster, G. Kuo, F. Bonino, et al. 1993. T-lymphocyte response to hepatitis C virus in different clinical courses of infection. Gastroenterology 104:580-587. [DOI] [PubMed] [Google Scholar]
- 10.Carmichael, A., X. Jin, P. Sissons, and L. Borysiewicz. 1993. Quantitative analysis of the human immunodeficiency virus type 1 (HIV-1)-specific cytotoxic T lymphocyte (CTL) response at different stages of HIV-1 infection: differential CTL responses to HIV-1 and Epstein-Barr virus in late disease. J. Exp. Med. 177:249-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carrington, M., G. W. Nelson, M. P. Martin, T. Kissner, D. Vlahov, J. J. Goedert, R. Kaslow, S. Buchbinder, K. Hoots, and S. J. O'Brien. 1999. HLA and HIV-1: heterozygote advantage and B*35-Cw*04 disadvantage. Science 283:1748-1752. [DOI] [PubMed] [Google Scholar]
- 12.Chang, K. M., R. Thimme, J. J. Melpolder, D. Oldach, J. Pemberton, J. Moorhead-Loudis, J. G. McHutchison, H. J. Alter, and F. V. Chisari. 2001. Differential CD4(+) and CD8(+) T-cell responsiveness in hepatitis C virus infection. Hepatology 33:267-276. [DOI] [PubMed] [Google Scholar]
- 13.Cramp, M. E., S. Rossol, S. Chokshi, P. Carucci, R. Williams, and N. V. Naoumov. 2000. Hepatitis C virus-specific T-cell reactivity during interferon and ribavirin treatment in chronic hepatitis C. Gastroenterology 118:346-355. [DOI] [PubMed] [Google Scholar]
- 14.Daar, E. S., H. Lynn, S. Donfield, E. Gomperts, S. J. O'Brien, M. W. Hilgartner, W. K. Hoots, D. Chernoff, S. Arkin, W. Y. Wong, and C. A. Winkler. 2001. Hepatitis C virus load is associated with human immunodeficiency virus type 1 disease progression in hemophiliacs. J. Infect. Dis. 183:589-595. [DOI] [PubMed] [Google Scholar]
- 15.Dalod, M., M. Dupuis, J. C. Deschemin, D. Sicard, D. Salmon, J. F. Delfraissy, A. Venet, M. Sinet, and J. G. Guillet. 1999. Broad, intense anti-human immunodeficiency virus (HIV) ex vivo CD8+ responses in HIV type 1-infected patients: comparison with anti-Epstein-Barr virus responses and changes during antiretroviral therapy. J. Virol. 73:7108-7116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferrari, C., A. Valli, L. Galati, A. Penna, P. Scaccaglia, T. Giuberti, C. Schianchi, G. Missale, M. G. Marin, and F. Fiaccadori. 1994. T-cell response to structural and nonstructural hepatitis C virus antigens in persistent and self-limited hepatitis C virus infections. Hepatology 19:286-295. [PubMed] [Google Scholar]
- 17.Gallimore, A., A. Glithero, A. Godkin, A. C. Tissot, A. Pluckthun, T. Elliott, H. Hengartner, and R. Zinkernagel. 1998. Induction and exhaustion of lymphocytic choriomeningitis virus-specific cytotoxic T lymphocytes visualized using soluble tetrameric major histocompatibility complex class I-peptide complexes. J. Exp. Med. 187:1383-1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao, X., G. W. Nelson, P. Karacki, M. P. Martin, J. Phair, R. Kaslow, J. J. Goedert, S. Buchbinder, K. Hoots, D. Vlahov, S. J. O'Brien, and M. Carrington. 2001. Effect of a single amino acid change in MHC class I molecules on the rate of progression to AIDS. N. Engl. J. Med. 344:1668-1675. [DOI] [PubMed] [Google Scholar]
- 19.Gerlach, J. T., H. M. Diepolder, M. C. Jung, N. H. Gruener, W. W. Schraut, R. Zachoval, R. Hoffmann, C. A. Schirren, T. Santantonio, and G. R. Pape. 1999. Recurrence of hepatitis C virus after loss of virus-specific CD4(+) T-cell response in acute hepatitis C. Gastroenterology 117:933-941. [DOI] [PubMed] [Google Scholar]
- 20.Grabowska, A. M., F. Lechner, P. Klenerman, P. J. Tighe, S. Ryder, J. K. Ball, B. J. Thomson, W. L. Irving, and R. A. Robins. 2001. Direct ex vivo comparison of the breadth and specificity of the T cells in the liver and peripheral blood of patients with chronic HCV infection. Eur. J. Immunol. 31:2388-2394. [DOI] [PubMed] [Google Scholar]
- 21.Graham, C. S., L. R. Baden, E. Yu, J. M. Mrus, J. Carnie, T. Heeren, and M. J. Koziel. 2001. Influence of human immunodeficiency virus infection on the course of hepatitis C virus infection: a meta-analysis. Clin. Infect. Dis. 33:562-569. [DOI] [PubMed] [Google Scholar]
- 22.Greub, G., B. Ledergerber, M. Battegay, P. Grob, L. Perrin, H. Furrer, P. Burgisser, P. Erb, K. Boggian, J. C. Piffaretti, B. Hirschel, P. Janin, P. Francioli, M. Flepp, and A. Telenti. 2000. Clinical progression, survival, and immune recovery during antiretroviral therapy in patients with HIV-1 and hepatitis C virus coinfection: the Swiss HIV Cohort Study. Lancet 356:1800-1805. [DOI] [PubMed] [Google Scholar]
- 23.Gruener, N. H., F. Lechner, M. C. Jung, H. Diepolder, T. Gerlach, G. Lauer, B. Walker, J. Sullivan, R. Phillips, G. R. Pape, and P. Klenerman. 2001. Sustained dysfunction of antiviral CD8+ T lymphocytes after infection with hepatitis C virus. J. Virol. 75:5550-5558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harcourt, G., S. Hellier, M. Bunce, J. Satsangi, J. Collier, R. Chapman, R. Phillips, and P. Klenerman. 2001. Effect of HLA class II genotype on T helper lymphocyte responses and viral control in hepatitis C virus infection. J. Viral Hepat. 8:174-179. [DOI] [PubMed] [Google Scholar]
- 25.He, X. S., B. Rehermann, F. X. Lopez-Labrador, J. Boisvert, R. Cheung, J. Mumm, H. Wedemeyer, M. Berenguer, T. L. Wright, M. M. Davis, and H. B. Greenberg. 1999. Quantitative analysis of hepatitis C virus-specific CD8(+) T cells in peripheral blood and liver using peptide-MHC tetramers. Proc. Natl. Acad. Sci. USA 96:5692-5697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoffmann, R. M., H. M. Diepolder, R. Zachoval, F. M. Zwiebel, M. C. Jung, S. Scholz, H. Nitschko, G. Riethmuller, and G. R. Pape. 1995. Mapping of immunodominant CD4+ T lymphocyte epitopes of hepatitis C virus antigens and their relevance during the course of chronic infection. Hepatology 21:632-638. [PubMed] [Google Scholar]
- 27.Kalams, S. A., S. P. Buchbinder, E. S. Rosenberg, J. M. Billingsley, D. S. Colbert, N. G. Jones, A. K. Shea, A. K. Trocha, and B. D. Walker. 1999. Association between virus-specific cytotoxic T-lymphocyte and helper responses in human immunodeficiency virus type 1 infection. J. Virol. 73:6715-6720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kalams, S. A., and B. D. Walker. 1998. The critical need for CD4 help in maintaining effective cytotoxic T lymphocyte responses. J. Exp. Med. 188:2199-2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaslow, R. A., R. Duquesnoy, M. VanRaden, L. Kingsley, M. Marrari, H. Friedman, S. Su, A. J. Saah, R. Detels, J. Phair, et al. 1990. A1, Cw7, B8, DR3 HLA antigen combination associated with rapid decline of T-helper lymphocytes in HIV-1 infection. A report from the Multicenter AIDS Cohort Study. Lancet 335:927-930. [DOI] [PubMed] [Google Scholar]
- 30.Kersten, M. J., M. R. Klein, A. M. Holwerda, F. Miedema, and M. H. van Oers. 1997. Epstein-Barr virus-specific cytotoxic T cell responses in HIV-1 infection: different kinetics in patients progressing to opportunistic infection or non-Hodgkin's lymphoma. J. Clin. Investig. 99:1525-1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koziel, M. J., D. Dudley, N. Afdhal, A. Grakoui, C. M. Rice, Q. L. Choo, M. Houghton, and B. D. Walker. 1995. HLA class I-restricted cytotoxic T lymphocytes specific for hepatitis C virus. Identification of multiple epitopes and characterization of patterns of cytokine release. J. Clin. Investig. 96:2311-2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koziel, M. J., D. Dudley, J. T. Wong, J. Dienstag, M. Houghton, R. Ralston, and B. D. Walker. 1992. Intrahepatic cytotoxic T lymphocytes specific for hepatitis C virus in persons with chronic hepatitis. J. Immunol. 149:3339-3344. (Erratum, 150:2563, 1993.) [PubMed]
- 33.Krarup, H. B., J. M. Moller, P. B. Christensen, T. Fuglsang, J. Ingerslev, T. Arnfred, and P. H. Madsen. 2000. Haemophilic patients with hepatitis C have higher viral load compared to other well-defined patient groups. J. Viral Hepat. 7:435-439. [DOI] [PubMed] [Google Scholar]
- 34.Kurts, C., F. R. Carbone, M. Barnden, E. Blanas, J. Allison, W. R. Heath, and J. F. Miller. 1997. CD4+ T cell help impairs CD8+ T cell deletion induced by cross-presentation of self-antigens and favors autoimmunity. J. Exp. Med. 186:2057-2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Larsson, M., X. Jin, B. Ramratnam, G. S. Ogg, J. Engelmayer, M. A. Demoitie, A. J. McMichael, W. I. Cox, R. M. Steinman, D. Nixon, and N. Bhardwaj. 1999. A recombinant vaccinia virus based ELISPOT assay detects high frequencies of Pol-specific CD8 T cells in HIV-1-positive individuals. AIDS 13:767-777. [DOI] [PubMed] [Google Scholar]
- 36.Lechner, F., N. H. Gruener, S. Urbani, J. Uggeri, T. Santantonio, A. R. Kammer, A. Cerny, R. Phillips, C. Ferrari, G. R. Pape, and P. Klenerman. 2000. CD8+ T lymphocyte responses are induced during acute hepatitis C virus infection but are not sustained. Eur. J. Immunol. 30:2479-2487. [DOI] [PubMed] [Google Scholar]
- 37.Lechner, F., D. K. Wong, P. R. Dunbar, R. Chapman, R. T. Chung, P. Dohrenwend, G. Robbins, R. Phillips, P. Klenerman, and B. D. Walker. 2000. Analysis of successful immune responses in persons infected with hepatitis C virus. J. Exp. Med. 191:1499-1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Limmer, A., J. Ohl, C. Kurts, H. G. Ljunggren, Y. Reiss, M. Groettrup, F. Momburg, B. Arnold, and P. A. Knolle. 2000. Efficient presentation of exogenous antigen by liver endothelial cells to CD8+ T cells results in antigen-specific T-cell tolerance. Nat. Med. 6:1348-1354. [DOI] [PubMed] [Google Scholar]
- 39.Mangia, A., R. Gentile, I. Cascavilla, M. Margaglione, M. R. Villani, F. Stella, G. Modola, V. Agostiano, C. Gaudiano, and A. Andriulli. 1999. HLA class II favors clearance of HCV infection and progression of the chronic liver damage. J. Hepatol. 30:984-989. [DOI] [PubMed] [Google Scholar]
- 40.Matloubian, M., R. J. Concepcion, and R. Ahmed. 1994. CD4+ T cells are required to sustain CD8+ cytotoxic T-cell responses during chronic viral infection. J. Virol. 68:8056-8063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Minutello, M. A., P. Pileri, D. Unutmaz, S. Censini, G. Kuo, M. Houghton, M. R. Brunetto, F. Bonino, and S. Abrignani. 1993. Compartmentalization of T lymphocytes to the site of disease: intrahepatic CD4+ T cells specific for the protein NS4 of hepatitis C virus in patients with chronic hepatitis C. J. Exp. Med. 178:17-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nelson, G. W., R. Kaslow, and D. L. Mann. 1997. Frequency of HLA allele-specific peptide motifs in HIV-1 proteins correlates with the allele's association with relative rates of disease progression after HIV-1 infection. Proc. Natl. Acad. Sci. USA 94:9802-9807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ogg, G. S., X. Jin, S. Bonhoeffer, P. R. Dunbar, M. A. Nowak, S. Monard, J. P. Segal, Y. Cao, S. L. Rowland-Jones, V. Cerundolo, A. Hurley, M. Markowitz, D. D. Ho, D. F. Nixon, and A. J. McMichael. 1998. Quantitation of HIV-1-specific cytotoxic T lymphocytes and plasma load of viral RNA. Science 279:2103-2106. [DOI] [PubMed] [Google Scholar]
- 44.Oxenius, A., D. A. Price, P. J. Easterbrook, C. A. O'Callaghan, A. D. Kelleher, J. A. Whelan, G. Sontag, A. K. Sewell, and R. E. Phillips. 2000. Early highly active antiretroviral therapy for acute HIV-1 infection preserves immune function of CD8+ and CD4+ T lymphocytes. Proc. Natl. Acad. Sci. USA 97:3382-3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oxenius, A., R. M. Zinkernagel, and H. Hengartner. 1998. Comparison of activation versus induction of unresponsiveness of virus-specific CD4+ and CD8+ T cells upon acute versus persistent viral infection. Immunity 9:449-457. [DOI] [PubMed] [Google Scholar]
- 46.Pontesilli, O., S. Kerkhof-Garde, D. W. Notermans, N. A. Foudraine, M. T. Roos, M. R. Klein, S. A. Danner, J. M. Lange, and F. Miedema. 1999. Functional T cell reconstitution and human immunodeficiency virus-1-specific cell-mediated immunity during highly active antiretroviral therapy. J. Infect. Dis. 180:76-86. [DOI] [PubMed] [Google Scholar]
- 47.Ragni, M. V., and S. H. Belle. 2001. Impact of human immunodeficiency virus infection on progression to end-stage liver disease in individuals with hemophilia and hepatitis C virus infection. J. Infect. Dis. 183:1112-1115. [DOI] [PubMed] [Google Scholar]
- 48.Rehermann, B., K. M. Chang, J. G. McHutchison, R. Kokka, M. Houghton, and F. V. Chisari. 1996. Quantitative analysis of the peripheral blood cytotoxic T lymphocyte response in patients with chronic hepatitis C virus infection. J. Clin. Investig. 98:1432-1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rinaldo, C. R., Jr., J. M. Liebmann, X. L. Huang, Z. Fan, Q. Al-Shboul, D. K. McMahon, R. D. Day, S. A. Riddler, and J. W. Mellors. 1999. Prolonged suppression of human immunodeficiency virus type 1 (HIV-1) viremia in persons with advanced disease results in enhancement of CD4 T cell reactivity to microbial antigens but not to HIV-1 antigens. J. Infect. Dis. 179:329-336. [DOI] [PubMed] [Google Scholar]
- 50.Rosenberg, E. S., M. Altfeld, S. H. Poon, M. N. Phillips, B. M. Wilkes, R. L. Eldridge, G. K. Robbins, R. T. D'Aquila, P. J. Goulder, and B. D. Walker. 2000. Immune control of HIV-1 after early treatment of acute infection. Nature 407:523-526. [DOI] [PubMed] [Google Scholar]
- 51.Rosenberg, E. S., J. M. Billingsley, A. M. Caliendo, S. L. Boswell, P. E. Sax, S. A. Kalams, and B. D. Walker. 1997. Vigorous HIV-1-specific CD4+ T cell responses associated with control of viremia. Science 278:1447-1450. [DOI] [PubMed] [Google Scholar]
- 52.Schirren, C. A., M. C. Jung, J. T. Gerlach, T. Worzfeld, G. Baretton, M. Mamin, N. Hubert Gruener, M. Houghton, and G. R. Pape. 2000. Liver-derived hepatitis C virus (HCV)-specific CD4(+) T cells recognize multiple HCV epitopes and produce interferon gamma. Hepatology 32:597-603. [DOI] [PubMed] [Google Scholar]
- 53.Soto, B., A. Sanchez-Quijano, L. Rodrigo, J. A. del Olmo, M. Garcia-Bengoechea, J. Hernandez-Quero, C. Rey, M. A. Abad, M. Rodriguez, M. Sales Gilabert, F. Gonzalez, P. Miron, A. Caruz, F. Relimpio, R. Torronteras, M. Leal, and E. Lissen. 1997. Human immunodeficiency virus infection modifies the natural history of chronic parenterally-acquired hepatitis C with an unusually rapid progression to cirrhosis. J. Hepatol. 26:1-5. [DOI] [PubMed] [Google Scholar]
- 54.Teague, W., J. Hepworth, and G. Krug. 1999. Support for patients with hepatitis C: an exploratory qualitative study of medical specialists' perceptions. Aust N. Z. J. Public Health 23:201-203. [DOI] [PubMed] [Google Scholar]
- 55.Tillmann, H. L., D. F. Chen, C. Trautwein, V. Kliem, A. Grundey, A. Berning-Haag, K. Boker, S. Kubicka, L. Pastucha, W. Stangel, and M. P. Manns. 2001. Low frequency of HLA-DRB1*11 in hepatitis C virus induced end stage liver disease. Gut 48:714-718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tillmann, H. L., H. Heiken, A. Knapik-Botor, S. Heringlake, J. Ockenga, J. C. Wilber, B. Goergen, J. Detmer, M. McMorrow, M. Stoll, R. E. Schmidt, and M. P. Manns. 2001. Infection with GB virus C and reduced mortality among HIV-infected patients. N. Engl. J. Med. 345:715-724. [DOI] [PubMed] [Google Scholar]
- 57.Wendland, T., H. Furrer, P. L. Vernazza, K. Frutig, A. Christen, L. Matter, R. Malinverni, and W. J. Pichler. 1999. HAART in HIV-infected patients: restoration of antigen-specific CD4 T-cell responses in vitro is correlated with CD4 memory T-cell reconstitution, whereas improvement in delayed type hypersensitivity is related to a decrease in viraemia. AIDS 13:1857-1862. [DOI] [PubMed] [Google Scholar]
- 58.Wong, D. K., D. D. Dudley, N. H. Afdhal, J. Dienstag, C. M. Rice, L. Wang, M. Houghton, B. D. Walker, and M. J. Koziel. 1998. Liver-derived CTL in hepatitis C virus infection: breadth and specificity of responses in a cohort of persons with chronic infection. J. Immunol. 160:1479-1488. [PubMed] [Google Scholar]
- 59.Wong, D. K., D. D. Dudley, P. B. Dohrenwend, G. M. Lauer, R. T. Chung, D. L. Thomas, and B. D. Walker. 2001. Detection of diverse hepatitis C virus (HCV)-specific cytotoxic T lymphocytes in peripheral blood of infected persons by screening for responses to all translated proteins of HCV. J. Virol. 75:1229-1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xiang, J., S. Wunschmann, D. J. Diekema, D. Klinzman, K. D. Patrick, S. L. George, and J. T. Stapleton. 2001. Effect of coinfection with GB virus C on survival among patients with HIV infection. N. Engl. J. Med. 345:707-714. [DOI] [PubMed] [Google Scholar]
- 61.Zajac, A. J., J. N. Blattman, K. Murali-Krishna, D. J. Sourdive, M. Suresh, J. D. Altman, and R. Ahmed. 1998. Viral immune evasion due to persistence of activated T cells without effector function. J. Exp. Med. 188:2205-2213. [DOI] [PMC free article] [PubMed] [Google Scholar]