Abstract
Human T-cell leukemia virus type 1 (HTLV-1) and HTLV-2 are retroviruses with similar biological properties. Whereas HTLV-1 is the causative agent of an aggressive T-cell leukemia, HTLV-2 has been associated with only a few cases of lymphoproliferative disorders. Tax1 and Tax2 are the transcriptional activators of HTLV-1 and HTLV-2, respectively. Here we show that Tax2 transformed a Rat-1 fibroblast cell line to form colonies in soft agar, but the size and number of the colonies were lower than those of Tax1. Use of a chimeric Tax protein showed that the C-terminal amino acids 300 to 353 were responsible for the high transforming activity of Tax1. Activation of cellular genes by Tax1 through transcription factor NF-κB is reportedly essential for the transformation of Rat-1 cells. Tax2 also activated the transcription through NF-κB in Rat-1 cells, and such activity was equivalent to that induced by Tax1. Thus, the high transforming activity of Tax1 is mediated by mechanisms other than NF-κB activation. Our results showed that Tax2 has a lower transforming activity than Tax1 and suggest that the high transforming activity of Tax1 is involved in the leukemogenic property of HTLV-1.
Human T-cell leukemia virus type 1 (HTLV-1) infection is associated with adult T-cell leukemia (ATL), which is an aggressive malignancy of CD4+ T cells (23, 37, 58). HTLV-1 transforms primary human CD4+ T cells in the presence or absence of interleukin-2 (IL-2) in vitro (32, 60). The viral tax gene is thought to play critical roles in the transformation of T cells, and thereby in leukemogenesis, because of its transforming activity in vitro. For instance, Tax in rodent fibroblast cell lines induces colony formation in soft agar, and the cells form tumors in nude mice (54). Transgenic mice carrying the tax gene develop various types of malignancies such as fibrosarcoma and large granular cell leukemia (21, 34). In addition, Tax immortalizes primary human CD4+ T cells in the presence of IL-2 (4, 20) and converts the cell growth of a mouse T-cell line from being IL-2 dependent to being IL-2 independent (25).
Tax was originally identified as a trans activator of its own promoter in the long terminal repeat (13, 17, 33, 45, 50, 64). Thereafter, Tax has been shown to have multiple activities in T cells. For example, Tax activates the transcription of numerous cellular genes, such as genes encoding cytokines (IL-2 and IL-8), the cytokine receptors (α-chain of IL-2 receptor), proto-oncogenes (c-fos, c-jun, fra-1, and c-rel), chemokines (IL-8 and SDF-1), the antiapoptotic gene bcl-xl, and the cell cycle regulator cyclin D2 (2, 5-7, 11, 15, 16, 24, 30, 44, 56, 57). In addition, Tax represses the transcription of certain cellular genes, such as that for DNA polymerase β (26). Moreover, Tax inactivates several tumor suppressor gene products, such as p53, p16INK, and hDLG (3, 36, 52).
Accumulating evidence indicates that activation of cellular genes through the transcription factor nuclear factor κB (NF-κB) by Tax is a critical factor for the transforming activity. For instance, a mutant Tax lacking activity toward NF-κB and a recombinant HTLV-1 carrying the mutant Tax lacking activity toward NF-κB are unable to transform primary human T cells (1, 38). Moreover, IκB, a specific NF-κB inhibitor, abolished the transformation of a rat fibroblast cell line by Tax (61). On the other hand, two reports showed that Tax activation of cellular genes through NF-κB is dispensable for the transformation of primary human T cells and a fibroblast cell line, and Tax activation of cellular genes through CREB is associated with the transformation (39, 49). Although the reason for the difference is unclear, it is likely that Tax has multiple transforming activities, all of which are required for maximum activity.
In resting T cells, NF-κB is inactivated by interacting with the NF-κB-inhibitory proteins, such as IκBα and IκBβ, in the cytoplasm. Tax activation of NF-κB involves phosphorylation and degradation of IκBα and IκBβ (9, 18, 59, 63). IκBs are phosphorylated by a multisubunit IκB kinase (IKK) complex, which is composed of two catalytic components, IKKα and IKKβ, and a noncatalytic component, IKKγ/NEMO. Tax interacts with IKKγ/NEMO to activate the catalytic activity of the IKK complex (10, 22, 27, 62, 63).
HTLV-2 was isolated from a cell line established from a variant form of hairy cell leukemia (8, 51). HTLV-2 has nucleotide sequence similarity to HTLV-1 and similar biological properties (47, 51). For instance, HTLV-2 transforms primary human T cells with efficiency similar to that of HTLV-1 in vitro. HTLV-2, however, is rarely associated with leukemias similar to ATL and has been associated with only a few cases of lymphoproliferative diseases (51). Thus, HTLV-2 appears not to induce malignant growth of infected cells in vivo and hence is a useful tool to understand the pathogenesis of ATL.
HTLV-2 also encodes a trans-activator protein, Tax2 (29, 48), which exhibits more than 70% amino acid identity with Tax1. Ross et al. (41) showed that mutation of the tax2 gene in an infectious HTLV-2 molecular clone eliminates the transforming activity of Tax2 in primary human T cells. However, whether Tax2 by itself has the transforming activity and relative potency of Tax2 has not yet been elucidated. Here we show that Tax2 transformed a Rat-1 fibroblast cell line to form colonies in soft agar, but such activity was lower than that of Tax1. This observation is discussed in the context of the different pathogenic capabilities of HTLV-1 and HTLV-2.
MATERIALS AND METHODS
Plasmids.
The tax2A cDNA was isolated from a genomic tax2A gene in an expression plasmid pC-Xc by PCR (35). The primers used to amplify the tax2A gene were ttgaattcagatctCCATGGCCCATTTCCCAGGATTCGGA and tggatccTTTTAGGCCGATGACTCGT. The tax2B cDNA was derived from plasmid pCAGGS-Tax2B (29). Lowercase letters in the sequence are the restriction enzyme sites for EcoRI and BamHI to facilitate the subcloning of the fragments into the expression plasmid and do not exist in the pC-Xc plasmid sequence. Chimeric genes tax211 and tax122 were constructed by using a unique MluI restriction enzyme site located in the same amino acid position of the tax1 and tax2 genes. The tax221 gene was constructed by PCR using chimeric primers corresponding to amino acids 297 to 306 of the tax1 and tax2 genes. The nucleotide sequences of the chimera primers were ATACAATACTCCTCCTTTCATAGTTTACAT and ATGTAAACTATGAAAGGAGGAGTATTGTAT. The nucleotide sequence of tax221 was determined by DNA sequencing. The tax1, tax2A, and tax2B genes and their chimeric genes were cloned into pHβPr-1-neo, which has a β-actin promoter for protein expression in mammalian cells and a neomycin resistance gene as a selection marker (31). κB-Luc is a luciferase expression plasmid regulated by the κB element of the IL-2 receptor α-chain gene and the minimal HTLV-1 promoter. pRL-TK is an expression plasmid of Renilla luciferase and is used to normalize the transfection efficiency.
Transient transfection and luciferase assay.
Rat-1 cells were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal calf serum (FCS). For luciferase assay, Rat-1 cells were seeded at 105 cells per 35-mm-diameter plate in DMEM-10% FCS and cultured overnight. They were then cotransfected with the Tax expression plasmid together with κB-Luc by the lipofection (FuGENE 6) method according to the instructions provided by the manufacturer (Roche Molecular Systems, Inc., Branchburg, N.J.). Cell lysate was prepared from transfected cells, and the activity of luciferase as well as Renilla luciferase in the lysate was determined by a luminometer. The activity of luciferase was normalized to that of Renilla luciferase. The assay was carried out four times to confirm reproducibility.
Assay of colony formation in soft agar (CFSA).
A Tax expression plasmid was transfected into Rat-1 cells by the calcium phosphate precipitation technique described previously (19), and the cells were cultured for more than 2 weeks in a selection medium containing 600 μg of G418 per ml. Pools of resistant cells (5 × 103 cells) were seeded in 0.33% agarose-containing DMEM-10% FCS overlaid on 0.5% agarose in a 60-mm-diameter plate. After 3 weeks of culture, the size and number of colonies were examined under a light microscope, and colony formation efficiency was calculated as the proportion of colonies measuring more than 60 μm in diameter relative to colonies less than 60 μm in diameter.
Western blotting.
Cell lysates (25 μg) prepared from Rat-1 cells were size separated by electrophoresis under reducing conditions in 10% polyacrylamide gel with sodium dodecyl sulfate, and the proteins in the gel were electronically transferred onto a polyvinylidene difluoride membrane. The membrane was incubated with Block Ace (Dainippon Seiyaku, Suita, Osaka, Japan) for 1 h at room temperature to inhibit nonspecific binding and was further incubated with either anti-Tax mouse monoclonal antibody (Taxy-8) (55) or rabbit anti-Tax2B polyclonal serum (29). After being washed with TBS-T (10 mM Tris-HCl [pH 8.0], 150 mM NaCl, and 0.05% Tween 20), the membranes were further incubated with either anti-mouse or anti-rabbit immunoglobulins conjugated with horseradish peroxidase (Bio-Rad Technologies, Richmond, Calif.). Proteins recognized by the antibodies in the membrane were visualized using the ECL Western blotting detection system (Amersham Pharmacia Biotech, Buckinghamshire, United Kingdom). Yuetsu Tanaka (Ryukyu University) kindly provided the Taxy-8 antibody.
EMSA.
To prepare nuclear extracts, Rat-1 cells (107) were washed with phosphate-buffered saline containing 1 mM Na3VO4 and 5 mM NaF and then treated with 0.2% NP-40 in lysis buffer containing 20 mM HEPES (pH 7.9), 20 mM NaF, 1 mM Na3VO4, 1 mM EDTA, 1 mM dithiothreitol, 0.5 mM phenylmethylsulfonyl fluoride, 1 μg of leupeptin per ml, and 1 μg of aprotinin per ml. The cell lysates were centrifuged, and the pellets were further treated with high-salt lysis buffer supplemented with 420 mM NaCl and 20% glycerol at 4°C for 30 min followed by centrifugation. The resultant supernatant was used as nuclear extract in electrophoretic mobility shift assay (EMSA). For EMSA, nuclear extract (50 μg) was preincubated with 1 μg of poly(dI:dC) in 20 μl of a binding buffer containing 13 mM HEPES (pH 7.9), 65 mM NaCl, 0.15 mM EDTA, 8% glycerol, and 1 mM dithiothreitol for 15 min on ice. Approximately 1 ng of labeled oligonucleotide was added to the reaction mixture and further incubated for 15 min at room temperature. The complexes formed were separated from the unbound probe by electrophoresis in a 5% polyacrylamide gel containing 0.5× Tris-borate-EDTA. After separation, the gel was dried, and radioactivity in the dried gel was analyzed with a BAS5000 instrument (Fuji Film, Kanagawa, Japan). A double-stranded synthetic oligonucleotide corresponding to the κB element (agctTTGGGAAATTCCTCGGGTGGTAC) from the interferon beta gene was labeled with [γ-32P]ATP by using polynucleotide kinase and was used as the NF-κB site probe. Lowercase letters in the sequence are the restriction enzyme sites for BamHI to facilitate the subcloning of the fragments into the plasmid and do not exist in the original genomic sequence. A tetradecanoyl phorbol acetate-responsive element oligonucleotide (gatcGTGACTCAGCGCG) was used as an unrelated competitor in EMSA.
RESULTS
Tax2 transforms a rat fibroblast cell line.
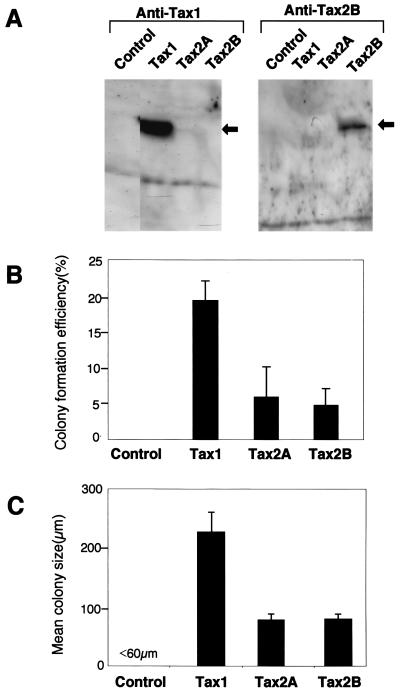
There are two major subtypes of HTLV-2, HTLV-2a and HTLV-2b, and they have Tax2A and Tax2B proteins, respectively (12, 29). Tax1 and Tax2A or Tax2B exhibit more than 70% amino acid identity. However, they show several amino acid differences throughout the protein. In addition, the C termini of both Tax2A and Tax2B are different from that of Tax1 (Fig. 1); Tax2A has a 22-amino-acid truncation relative to Tax1, and Tax2B has 25 totally different amino acids in the C terminus. cDNA encoding Tax2A, Tax2B, or Tax1 was introduced into a mammalian expression vector (pHβPr-1-neo) carrying a neomycin resistance gene (31). To examine the transforming activity (i.e., CFSA), these expression vectors were transfected into Rat-1 cells and were selected with neomycin (G418). The expression of Tax1, Tax2A, and Tax2B in Rat-1 transfectants was then examined by Western blotting analysis (Fig. 2A). Anti-Tax1 monoclonal antibody detected Tax1 but not Tax2A or Tax2B in Rat-1 transfectants. On the other hand, anti-Tax2B polyclonal antibody detected Tax2B but not Tax2A or Tax1. Anti-Tax2B occasionally detected Tax2A as well as Tax1 in Rat-1 cells, in which case Tax2B, Tax2A, and Tax1 were almost equivalently detected (data not shown). Although the reason for the inconsistent detection of Tax1 and Tax2A by anti-Tax2B is unclear, the detection may be influenced by subtle differences in the assay conditions. To measure the transforming activity, these cell lines were seeded in soft agar. Tax1 transfectants formed large and multiple colonies in soft agar (Fig. 2B and C) relative to control cells, as described previously (31, 54). Rat-1 cells expressing either Tax2A or Tax2B also formed colonies in soft agar, but their size and number were reproducibly lower than those of Tax1. Reproducible results were obtained in seven independent experiments. These results indicated that Tax2 (either Tax2A or Tax2B) transforms Rat-1 cells but that the activity is less than that of Tax1.
FIG. 1.
Structures of Tax1, Tax2A, and Tax2B proteins. The amino acid lengths of the respective proteins and the amino acids that are different in Tax2A and Tax2B are indicated.
FIG. 2.
Transforming activities of Tax1 and Tax2. (A) Cell lysates were prepared from Rat-1 cells transfected with the indicated plasmids, and the amount of Tax in each lysate was measured by Western blot analysis using an anti-Tax1 antibody (left) or an anti-Tax2B antibody (right). Arrows indicate the Tax proteins recognized by the antibodies. (B and C) Rat-1 cells transfected with the indicated plasmids were seeded in soft agar. Three weeks after the inoculation, the number (B) and the size (C) of colonies were determined by light microscopy. CFSA assay was carried out in duplicate, and the data presented are averages and standard deviations from seven experiments.
The C-terminal region of Tax1 is responsible for the high transforming activity.
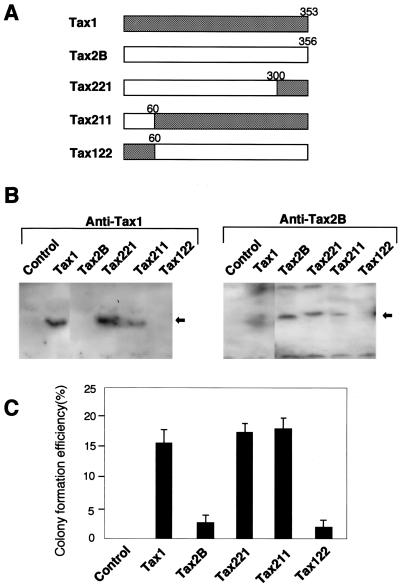
To determine the domain of Tax1 responsible for the high transforming activity, we constructed three chimeric genes between tax1 and tax2B, and stable Rat-1 cell lines expressing respective chimeric proteins were established (Fig. 3). Chimera Tax211 and Tax221 transformed Rat-1 cells as efficiently as Tax1. On the other hand, the transforming activity of Tax122 in Rat-1 cells was equivalent to that of the control plasmid. Since Tax122 was detected by neither anti-Tax1 nor anti-Tax2B (Fig. 3B), the failure of Tax122 to transform Rat-1 cells may be due to low expression of the protein. The chimeric proteins, except for Tax122, were detected by anti-Tax1 and/or anti-Tax2B in Rat-1 cells. The Tax221 and Tax211 chimeras were detected by both anti-Tax1 and anti-Tax2B, and they were expressed equivalently to Tax1 and Tax2B, indicating that the different transforming activities of Tax1, Tax221, and Tax211 with Tax2B were not due to different expression levels of the proteins in Rat-1 cells. Considered together, these results indicated that C-terminal amino acids 300 to 353 of Tax1 are responsible for the elevated transforming activity in Rat-1 cells.
FIG. 3.
Tax1 domain responsible for high transforming activity. (A) Structures of the chimeric genes and the position of the boundary between Tax1 and Tax2B. (B) Cell lysates were prepared from Rat-1 cells transfected with the indicated plasmids, and the amount of Tax in each lysate was measured by Western blot analysis using an anti-Tax1 antibody (left) or an anti-Tax2B antibody (right). Arrows indicate the Tax proteins recognized by the antibodies. (C) Rat-1 cells transfected with the indicated plasmids were seeded in soft agar. Three week after the culture, the number of the colonies was counted by light microscopy. CFSA assay was carried out in duplicate, and the data presented are averages and standard deviations from seven experiments.
Tax2 activates transcription through NF-κB equivalently to Tax1.
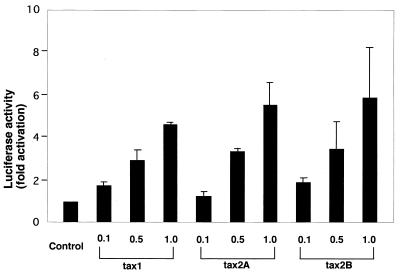
Tax1 and Tax2 activate the transcription of several cellular genes through the κB element and cyclic AMP-responsive element-like sequence (CRE) (33, 64), and the activity of Tax1 toward the κB element is essential for the transformation of Rat-1 cells by Tax, while that toward CRE is dispensable (31, 61). To measure the transcriptional activities of Tax1 and Tax2 toward the κB element, the luciferase expression plasmid regulated by the κB element was transiently transfected into Rat-1 cells together with the Tax expression plasmid, and the luciferase activity in Rat-1 transfectants was measured (Fig. 4). Tax1 and Tax2B activated the expression of the luciferase gene through the κB element in a dose-dependent manner, and the activity of Tax2B was equivalent to that of Tax1 at any dose of the plasmid. The chimeric Tax proteins also did not show any significant difference in the activities toward NF-κB (data not shown). These results suggested that the activity of Tax other than NF-κB in Rat-1 cells is involved in the variable transforming activity.
FIG. 4.
trans activation of the κB element by Tax2. Rat-1 cells were cotransfected with the indicated Tax expression plasmid (0.1 to 1.0 μg) together with the luciferase plasmid regulated by the κB element by the Lipofectin method. Cell lysates were prepared from transfected cells, and luciferase activity in the lysate was determined with a luminometer. The fold activation represents luciferase activity of cells transfected with the Tax plasmid relative to that with the control plasmid. Error bars indicate standard deviations.
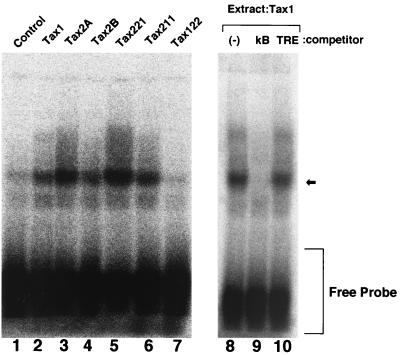
Tax activates the transcription through NF-κB by stimulating DNA binding activity (7, 33, 43). To establish that NF-κB is not responsible for the variable transforming activity between Tax1 and Tax2B, we further examined the DNA binding activity of NF-κB in the stable Rat-1 cell lines characterized as described above. EMSA using the NF-κB binding sequence as a probe showed that Rat-1 cells expressing Tax1 possessed mainly one mobility-shifted complex specific to the κB element, since the complex was competed by excess cold homologous oligonucleotide carrying the NF-κB binding sequence but not by an unrelated one (Fig. 5). The binding was higher in Rat-1 cells expressing Tax1 than in control cells, indicating that Tax1 constitutively activates DNA binding activity of NF-κB in Rat-1 cells, as reported previously (31, 61). Rat-1 cells expressing Tax2A or Tax2B also showed higher NF-κB DNA binding activity than control cells, and the activity was equivalent to that of Tax1. Similarly, Rat-1 cell lines expressing Tax221 and Tax211 but not Tax122 showed high NF-κB activity relative to control cells, and the binding activity did not correlate with the transforming activity. Considering this together with the transient assay, we concluded that Tax2 activates the NF-κB-dependent transcription in a manner equivalent to Tax1 in Rat-1 cells and that the NF-κB-dependent transcriptional activation is not responsible for the different transforming activities of Tax1 and Tax2.
FIG. 5.
Tax2 stimulates NF-κB DNA binding activity in Rat-1 cells. Nuclear extracts prepared from Rat-1 cells transfected with the indicated plasmids were incubated with the NF-κB site probe in the absence of competitor (lanes 1 to 8) or in the presence of the homologous competitor (lane 9) or the unrelated tetradecanoyl phorbol acetate-responsive element (TRE) competitor (lane 10). The binding activity to the NF-κB site probe was measured by EMSA as described in Materials and Methods. The arrow indicates the specific main complex with the κB element.
DISCUSSION
Employing a molecular HTLV-2 clone, recent studies have demonstrated that Tax2 is essential for the transformation of primary human T cells by HTLV-2 (41). However, those studies could not determine whether Tax2 directly transforms T cells or whether it transforms human cells indirectly by controlling other viral proteins. Here we showed that both Tax2A and Tax2B transformed a fibroblast cell line, Rat-1, to form colonies in soft agar (Fig. 2). Thus, Tax2 by itself can induce transformation similarly to Tax1 and is likely to play a role in the transformation of primary human T cells by HTLV-2. Our results, however, showed that the transforming activity of Tax2 in Rat-1 cells was less than that of Tax1. Unlike HTLV-1, HTLV-2 is thought to rarely cause leukemia, including disorders similar to ATL (51). Thus, our results suggest that the low activity of Tax2 may explain the poor leukemogenic property of HTLV-2.
HTLV-2 can transform primary human T cells as efficiently as HTLV-1 in vitro. How can this be achieved when the transforming activity of Tax2 is lower than that of Tax1? It is possible that the HTLV-2 promoter is stronger than that of HTLV-1, resulting in higher expression of Tax2 relative to Tax1 in HTLV-transformed T-cell lines. If this is the case, HTLV-2 may experience a stronger immune attack by cytotoxic T cells against Tax in vivo, since Tax is the main target of cytotoxic T cells. This could account for the poor leukemogenic property of HTLV-2. Alternatively, the high transforming activity of Tax1 detected in Rat-1 cells may be dispensable for the in vitro transformation of human T cells by HTLV-1.
In our study, both transient luciferase assay and EMSA showed that Tax2A as well as Tax2B activates NF-κB-dependent transcription in Rat-1 cells. Since NF-κB activation by Tax1 is essential for the transformation of Rat-1 cells (31, 61), NF-κB is likely to be also involved in the transformation of Rat-1 cells by Tax2. Interestingly, Tax2 activated NF-κB to a level equivalent to that by Tax1, irrespective of the weak transforming activity. This is in agreement with the results of previous studies, which showed that Tax1 and Tax2 equally activate NF-κB-dependent transcription in several cell lines (40, 46). Taken together, these results indicate that activity of Tax1 distinct from that involving NF-κB plays a role in the transformation of Rat-1 cells and that this effect may explain the relatively high transformation capacity of Tax1.
The chimera study showed that amino acids 300 to 353 of Tax1 were the region responsible for the high transforming activity. This region of Tax1 differs most from Tax2A and Tax2B (12). In particular, the C-terminal 22 amino acids of Tax1 are totally different from those of Tax2A and Tax2B (Fig. 6). Tax2A exhibits a 22-amino-acid truncation relative to Tax1. While the length of the C terminus of Tax2B is equivalent to that of Tax1, the amino acid sequence is totally different. Interestingly, this C-terminal Tax1 fragment contains the binding motif for the PDZ domain protein, TXI/V, and the motif is absent in both Tax2A and Tax2B (12, 42). Tax1 interacts with the tumor suppressor gene product hDLG with the PDZ domain via this motif and inhibits the growth-inhibitory function (42, 53). Thus, the interaction of Tax1 with PDZ domain proteins such as hDLG may be responsible for the high transforming activity. Previous reports showed that several transforming proteins of malignant, but not benign, viruses, such as the E6 protein of human papillomavirus, have a PDZ binding sequence, and the viral protein-binding motif interaction is strongly associated with the high transforming activity in a rat fibroblast cell line, 3Y1 (28). In addition to binding of PDZ domain proteins, the C-terminal end of Tax1 has been mapped to contain the micronucleus DNA damage function (46). Thus, we are further examining the role of a C-terminal region of Tax1 in the elevated activity relative to Tax2.
FIG. 6.
Comparison of C-terminal peptides of Tax1 with those of Tax2B and Tax2A. The amino acids of Tax2 that are identical to those of Tax1 are indicated by dashes.
Feuer et al. showed that HTLV-1-transformed T-cell lines are more tumorigenic than HTLV-2-transformed ones in SCID mice (14). CFSA of many oncogenic proteins correlates well with tumorigenicity in vivo, such as for tumor formation in nude mice. Thus, the high CFSA of Tax1 relative to Tax2 may explain the more strict in vivo immortalization of HTLV-1-infected cells than HTLV-2-infected ones, which consequently results in the occasional malignant outgrowth of HTLV-1-infected cells. Thus, we consider that present and future studies designed to examine the difference between Tax1 and Tax2 should advance our understanding of malignant progression of HTLV-1-infected T cells in vivo.
Acknowledgments
We thank Yuetsu Tanaka, Kayoko Matsumoto, Jun-Ichi Fujisawa, and Kunitada Shimotohno for anti-Tax1 antibody (Taxy-8), κB-Luc, and tax1 and tax2 plasmids, respectively.
This work was supported in part by grants from the Ministry of Education, Science, Sports and Culture of Japan. W.W.H. is supported by the Japanese Foundation for AIDS Prevention.
REFERENCES
- 1.Akagi, T., H. Ono, H. Nyunoya, and K. Shimotohno. 1997. Characterization of peripheral blood T-lymphocytes transduced with HTLV-I Tax mutants with different trans-activating phenotypes. Oncogene 14:2071-2078. [DOI] [PubMed] [Google Scholar]
- 2.Akagi, T., H. Ono, and K. Shimotohno. 1996. Expression of cell-cycle regulatory genes in HTLV-I infected T-cell lines: possible involvement of Tax1 in the altered expression of cyclin D2, p18Ink4 and p21Waf1/Cip1/Sdi1. Oncogene 12:1645-1652. [PubMed] [Google Scholar]
- 3.Akagi, T., H. Ono, N. Tsuchida, and K. Shimotohno. 1997. Aberrant expression and function of p53 in T-cells immortalized by HTLV-I Tax1. FEBS Lett. 406:263-266. [DOI] [PubMed] [Google Scholar]
- 4.Akagi, T., and K. Shimotohno. 1993. Proliferative response of Tax1-transduced primary human T cells to anti-CD3 antibody stimulation by an interleukin-2-independent pathway. J. Virol. 67:1211-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arai, M., T. Ohashi, N. Yamamoto, M. Kannagi, and M. Fujii. 1998. Human T-cell leukemia virus type 1 Tax protein induces the expression of a chemokine, SDF-1/PBSF. Virology 241:298-303. [DOI] [PubMed] [Google Scholar]
- 6.Arima, N., J. A. Molitor, M. R. Smith, J. H. Kim, Y. Daitoku, and W. C. Greene. 1991. Human T-cell leukemia virus type I Tax induces expression of the Rel-related family of kappa B enhancer-binding proteins: evidence for a pretranslational component of regulation. J. Virol. 65:6892-6899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ballard, D. W., E. Bohnlein, J. W. Lowenthal, Y. Wano, B. R. Franza, and W. C. Greene. 1988. HTLV-I tax induces cellular proteins that activate the kappa B element in the IL-2 receptor alpha gene. Science 241:1652-1655. [DOI] [PubMed] [Google Scholar]
- 8.Chen, I. S., J. McLaughlin, J. C. Gasson, S. C. Clark, and D. W. Golde. 1983. Molecular characterization of genome of a novel human T-cell leukaemia virus. Nature 305:502-505. [DOI] [PubMed] [Google Scholar]
- 9.Chu, Z. L., J. A. DiDonato, J. Hawiger, and D. W. Ballard. 1998. The tax oncoprotein of human T-cell leukemia virus type 1 associates with and persistently activates IkappaB kinases containing IKKalpha and IKKbeta. J. Biol. Chem. 273:15891-15894. [DOI] [PubMed] [Google Scholar]
- 10.Chu, Z. L., Y. A. Shin, J. M. Yang, J. A. DiDonato, and D. W. Ballard. 1999. IKKgamma mediates the interaction of cellular IkappaB kinases with the tax transforming protein of human T cell leukemia virus type 1. J. Biol. Chem. 274:15297-15300. [DOI] [PubMed] [Google Scholar]
- 11.Cross, S. L., M. B. Feinberg, J. B. Wolf, N. J. Holbrook, F. Wong-Staal, and W. J. Leonard. 1987. Regulation of the human interleukin-2 receptor alpha chain promoter: activation of a nonfunctional promoter by the transactivator gene of HTLV-I. Cell 49:47-56. [DOI] [PubMed] [Google Scholar]
- 12.Eiraku, N., P. Novoa, M. da Costa Ferreira, C. Monken, R. Ishak, O. da Costa Ferreira, S. W. Zhu, R. Lorenco, M. Ishak, V. Azvedo, J. Guerreiro, M. P. de Oliveira, P. Loureiro, N. Hammerschlak, S. Ijichi, and W. M. Hall. 1996. Identification and characterization of a new and distinct molecular subtype of human T-cell lymphotropic virus type 2. J. Virol. 70:1481-1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Felber, B. K., H. Paskaris, C. Kleinman-Ewing, F. Wong-Staal, and G. N. Pavlakis. 1985. The pX protein of HTLV-I is a transcriptional activator of its long terminal repeats. Science 229:675-679. [DOI] [PubMed] [Google Scholar]
- 14.Feuer, G., S. A. Stewart, S. M. Baird, F. Lee, R. Feuer, and I. S. Y. Chen. 1995. Potential role of natural killer cells in controlling tumorigenesis by human T-cell leukemia viruses. J. Virol. 69:1328-1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fujii, M., C. P. Sassone, and I. M. Verma. 1988. c-fos promoter trans-activation by the tax1 protein of human T-cell leukemia virus type I. Proc. Natl. Acad. Sci. USA 85:8526-8530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fujii, M., T. Niki, T. Mori, T. Matsuda, M. Matsui, N. Nomura, and M. Seiki. 1991. HTLV-1 Tax induces expression of various immediate early serum responsive genes. Oncogene 6:1023-1029. [PubMed] [Google Scholar]
- 17.Fujisawa, J., M. Seiki, and M Yoshida. 1985. Functional activation of the human T-cell leukemia virus type I by trans-acting factor. Proc. Natl. Acad. Sci. USA 82:2277-2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Geleziunas, R., S. Ferrell, X. Lin, Y. Mu, E. J. Cunningham, M. Grant, M. A. Connelly, J. E. Hambor, K. B. Marcu, and W. C. Greene. 1998. Human T-cell leukemia virus type 1 Tax induction of NF-κB involves activation of the IκB kinase alpha (IKKalpha) and IKKbeta cellular kinases. Mol. Cell. Biol. 18:5157-5165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gorman, C. M., L. F. Moffat, and B. H. Howard. 1982. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol. Cell. Biol. 2:1044-1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grassmann, R., S. Berchtold, I. Radant, M. Alt, B. Fleckenstein, J. G. Sodroski, W. A. Haseltine, and U. Ramstedt. 1992. Role of human T-cell leukemia virus type 1 X region proteins in immortalization of primary human lymphocytes in culture. J. Virol. 66:4570-4575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grossman, W. J., J. T. Kimata, F. H. Wong, M. Zutter, T. J. Ley, and L. Ratner. 1995. Development of leukemia in mice transgenic for the tax gene of human T-cell leukemia virus type I. Proc. Natl. Acad. Sci. USA 92:1057-1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harhaj, E. W., and S. C. Sun. 1999. IKKgamma serves as a docking subunit of the IkappaB kinase (IKK) and mediates interaction of IKK with the human T-cell leukemia virus Tax protein. J. Biol. Chem. 274:22911-22914. [DOI] [PubMed] [Google Scholar]
- 23.Hinuma, Y., K. Nagata, M. Hanaoka, M. Nakai, T. Matsumoto, K. I. Kinoshita, S. Shirakawa, and I. Miyoshi. 1981. Adult T-cell leukemia: antigen in an ATL cell line and detection of antibodies to the antigen in human sera. Proc. Natl. Acad. Sci. USA 78:6476-6480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Inoue, J., M. Seiki, T. Taniguchi, S. Tsuru, and M. Yoshida. 1986. Induction of interleukin 2 receptor gene expression by p40x encoded by human T-cell leukemia virus type I. EMBO J. 5:2883-2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iwanaga, Y., T. Tsukahara, T. Ohashi, Y. Tanaka, M. Arai, M. Nakamura, K. Ohtani, Y. Koya, M. Kannagi, N. Yamamoto, and M. Fujii. 1999. Human T-cell leukemia virus type 1 Tax protein abrogates interleukin 2 dependence in a mouse T-cell line. J. Virol. 73:1271-1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jeang, K. T., S. G. Widen, O. t. Semmes, and S. H. Wilson. 1990. HTLV-I trans-activator protein, tax, is a trans-repressor of the human beta-polymerase gene. Science 247:1082-1084. [DOI] [PubMed] [Google Scholar]
- 27.Jin, D. Y., V. Giordano, K. V. Kibler, H. Nakano, and K. T. Jeang. 1999. Role of adapter function in oncoprotein-mediated activation of NF-kappaB. Human T-cell leukemia virus type I Tax interacts directly with IkappaB kinase gamma. J. Biol. Chem. 274:17402-17405. [DOI] [PubMed] [Google Scholar]
- 28.Kiyono, T., A. Hiraiwa, M. Fujita, Y. Hayashi, T. Akiyama, and M. Ishibashi. 1997. Binding of high-risk human papillomavirus E6 oncoproteins to the human homologue of the Drosophila discs large tumor suppressor protein. Proc. Natl. Acad. Sci. USA 94:11612-11616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lewis, M. J., P. Novoa, R. Ishak, M. Ishak, M. Salemi, A. M. Vandamme, M. H. Kaplan, and W. W. Hall. 2000. Isolation, cloning, and complete nucleotide sequence of a phenotypically distinct Brazilian isolate of human T-lymphotropic virus type II (HTLV-II). Virology 271:142-154. [DOI] [PubMed] [Google Scholar]
- 30.Maruyama, M., H. Shibuya, H. Harada, M. Hatakeyama, M. Seiki, T. Fujita, J. Inoue, M. Yoshida, and T. Taniguchi. 1987. Evidence for aberrant activation of the interleukin-2 autocrine loop by HTLV-I-encoded p40x and T3/Ti complex triggering. Cell 48:343-350. [DOI] [PubMed] [Google Scholar]
- 31.Matsumoto, K., H. Shibata, J. I. Fujisawa, H. Inoue, A. Hakura, T. Tsukahara, and M. Fujii. 1997. Human T-cell leukemia virus type 1 Tax protein transforms rat fibroblasts via two distinct pathways. J. Virol. 71:4445-4451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miyoshi, I., I. Kubonishi, S. Yoshimoto, T. Akagi, Y. Ohtsuki, Y. Shiraishi, K. Nagata, and Y. Hinuma. 1981. Type C virus particles in a cord T-cell line derived by co-cultivating normal human cord leukocytes and human leukaemic T cells. Nature 294:770-771. [DOI] [PubMed] [Google Scholar]
- 33.Mori, N., N. Yamamoto, and M. Fujii. 1999. Activation of Nuclear Factor kappa B transcription factor by human T-cell leukemia virus type I. Acta Med. Biol. 47:85-96. [Google Scholar]
- 34.Nerenberg, M., S. H. Hinrichs, R. K. Reynolds, G. Khoury, and G. Jay. 1987. The tat gene of human T-lymphotropic virus type 1 induces mesenchymal tumors in transgenic mice. Science 237:1324-1329. [DOI] [PubMed] [Google Scholar]
- 35.Ohta, M., H. Nyunoyama, H. Tanaka, T. Okamoto, T. Akagi, and K. Shimotohno. 1988. Identification of a cis-regulatory element involved in accumulation of human T-cell leukemia virus type II genomic mRNA. J. Virol. 62:4445-4451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pise-Masison, C. A., K. S. Choi, M. Radonovich, J. Dittmer, S. J. Kim, and J. N. Brady. 1998. Inhibition of p53 transactivation function by the human T-cell lymphotropic virus type 1 Tax protein. J. Virol. 72:1165-1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Poiesz, B. J., F. W. Ruscetti, A. F. Gazdar, P. A. Bunn, J. D. Minna, and R. C. Gallo. 1980. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc. Natl. Acad. Sci. USA 77:7415-7419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Robek, M. D., and L. Ratner. 1999. Immortalization of CD4+ and CD8+ T lymphocytes by human T-cell leukemia virus type 1 Tax mutants expressed in a functional molecular clone. J. Virol. 73:4856-4865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rosin, O., C. Koch, I. Schmitt, O. J. Semmes, K. T. Jeang, and R. Grassmann. 1998. A human T-cell leukemia virus Tax variant incapable of activating NF-kappaB retains its immortalizing potential for primary T-lymphocytes. J. Biol. Chem. 273:6698-6703. [DOI] [PubMed] [Google Scholar]
- 40.Ross, T. M., A. C. Minella, Z. Y. Fang, S. M. Pettiford, and P. L. Green. 1997. Mutational analysis of human T-cell leukemia virus type 2 Tax. J. Virol. 71:8912-8917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ross, T. M., M. Narayan, Z. Y. Fang, A. C. Minella, and P. L. Green. 2000. Human T-cell leukemia virus type 2 Tax mutants that selectively abrogate NF-κB or CREB/ATF activation fail to transform primary human T cells. J. Virol. 74:2655-2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rousset, R., S. Fabre, C. Desbois, F. Bantignies, and P. Jalinot. 1998. The C-terminus of the HTLV-1 Tax oncoprotein mediates interaction with the PDZ domain of cellular proteins. Oncogene 16:643-654. [DOI] [PubMed] [Google Scholar]
- 43.Ruben, S., H. Poteat, T. H. Tan, K. Kawakami, R. Roeder, W. Haseltine, and C. A. Rosen. 1988. Cellular transcription factors and regulation of IL-2 receptor gene expression by HTLV-I tax gene product. Science 241:89-92. [DOI] [PubMed] [Google Scholar]
- 44.Santiago, F., E. Clark, S. Chong, C. Molina, F. Mozafari, R. Mahieux, M. Fujii, N. Azimi, and F. Kashanchi. 1999. Transcriptional up-regulation of the cyclin D2 gene and acquisition of new cyclin-dependent kinase partners in human T-cell leukemia virus type 1-infected cells. J. Virol. 73:9917-9927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Seiki, M., S. Hattori, Y. Hirayama, and M. Yoshida. 1986. Direct evidence that p40x of human T-cell leukemia virus type I is a trans-acting transcriptional activator. EMBO J. 5:561-565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Semmes, O. J., F. Majone, C. Cantemir, L. Turchetto, B. Hjelle, and K. T. Jeang. 1996. HTLV-I and HTLV-II Tax: differences in induction of micronuclei in cells and transcriptional activation of viral LTRs. Virology 217:373-379. [DOI] [PubMed] [Google Scholar]
- 47.Shimotohno, K., Y. Takahashi, N. Shimizu, T. Gojobori, D. W. Golde, I. S. Chen, M. Miwa, and T. Sugimura. 1985. Complete nucleotide sequence of an infectious clone of human T-cell leukemia virus type II: an open reading frame for the protease gene. Proc. Natl. Acad. Sci. USA 82:3101-3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Slamon, D., K. Shimotohno, M. J. Cline, D. W. Golde, and I. S. Y. Chen. 1984. Identification of the putative transforming protein of the human T-cell leukemia virus HTLV-I and HTLV-II. Science 226:61-65. [DOI] [PubMed] [Google Scholar]
- 49.Smith, M. R., and W. C. Greene. 1991. Type I human T cell leukemia virus tax protein transforms rat fibroblasts through the cyclic adenosine monophosphate response element binding protein/activating transcription factor pathway. J. Clin. Investig. 88:1038-1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sodroski, J. C., C. A. Rosen, and W. A. Haselteine. 1984. trans-acting transcriptional activation of the long terminal repeat of human T lymphotropic viruses in infected cells. Science 225:381-385. [DOI] [PubMed] [Google Scholar]
- 51.Sugamura, K., and Y. Hinuma. 1993. Human retroviruses: HTLV-I and HTLV-II, vol. 2, p. 399-435. In J. A. Levy (ed.), The Retroviridae. Plenum Press, New York, N.Y. [Google Scholar]
- 52.Suzuki, T., T. Narita, M. Uchida-Toita, and M. Yoshida. 1999. Down-regulation of the INK4 family of cyclin-dependent kinase inhibitors by tax protein of HTLV-1 through two distinct mechanisms. Virology 259:384-391. [DOI] [PubMed] [Google Scholar]
- 53.Suzuki, T., Y. Ohsugi, M. Uchida-Toita, T. Akiyama, and M. Yoshida. 1999. Tax oncoprotein of HTLV-1 binds to the human homologue of Drosophila discs large tumor suppressor protein, hDLG, and perturbs its function in cell growth control. Oncogene 18:5967-5972. [DOI] [PubMed] [Google Scholar]
- 54.Tanaka, A., C. Takahashi, S. Yamaoka, T. Nosaka, M. Maki, and M. Hatanaka. 1990. Oncogenic transformation by the tax gene of human T-cell leukemia virus type I in vitro. Proc. Natl. Acad. Sci. USA 87:1071-1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tanaka, Y., A. Yoshida, H. Tozawa, H. Shida, H. Nyunoya, and K. Shimotohno. 1991. Production of a recombinant human T-cell leukemia virus type-I trans-activator (tax1) antigen and its utilization for generation of monoclonal antibodies against various epitopes on the tax1 antigen. Int. J. Cancer 48:623-630. [DOI] [PubMed] [Google Scholar]
- 56.Tsuchiya, H., M. Fujii, T. Niki, M. Tokuhara, M. Matsui, and M. Seiki. 1993. Human T-cell leukemia virus type 1 Tax activates transcription of the human fra-1 gene through multiple cis elements responsive to transmembrane signals. J. Virol. 67:7001-7007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tsukahara, T., M. Kannagi, T. Ohashi, H. Kato, M. Arai, G. Nunez, Y. Iwanaga, N. Yamamoto, K. Ohtani, M. Nakamura, and M. Fujii. 1999. Induction of Bcl-x(L) expression by human T-cell leukemia virus type 1 Tax through NF-κB in apoptosis-resistant T-cell transfectants with Tax. J. Virol. 73:7981-7987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Uchiyama, T., J. Yodoi, K. Sagawa, K. Takatsuki, and H. Uchino. 1977. Adult T-cell leukemia: clinical and hematologic features of 16 cases. Blood 50:481-492. [PubMed] [Google Scholar]
- 59.Uhlik, M., L. Good, G. Xiao, E. W. Harhaj, E. Zandi, M. Karin, and S. C. Sun. 1998. NF-kappaB-inducing kinase and IkappaB kinase participate in human T-cell leukemia virus I Tax-mediated NF-kappaB activation. J. Biol. Chem. 273:21132-21136. [DOI] [PubMed] [Google Scholar]
- 60.Yamamoto, N., M. Okada, Y. Koyanagi, M. Kannagi, and Y. Hinuma. 1982. Transformation of human leukocytes by cocultivation with an adult T cell leukemia virus producer cell line. Science 217:737-739. [DOI] [PubMed] [Google Scholar]
- 61.Yamaoka, S., J. Inoue, M. Sakurai, T. Sugiyama, M. Hazama, T. Yamada, and M. Hatanaka. 1996. Constitutive activation of NF-kB is essential for transformation of rat fibroblasts by the human T-cell leukemia virus type I Tax protein. EMBO J. 15:873-887. [PMC free article] [PubMed] [Google Scholar]
- 62.Yamaoka, S., G. Courtois, C. Bessia, S. T. Whiteside, R. Weil, F. Agou, H. E. Kirk, R. J. Kay, and A. Israel. 1998. Complementation cloning of NEMO, a component of the IkappaB kinase complex essential for NF-kappaB activation. Cell 93:1231-1240. [DOI] [PubMed] [Google Scholar]
- 63.Yin, M. J., L. B. Christerson, Y. Yamamoto, Y. T. Kwak, S. Xu, F. Mercurio, M. Barbosa, M. H. Cobb, and R. B. Gaynor. 1998. HTLV-I Tax protein binds to MEKK1 to stimulate IkappaB kinase activity and NF-kappaB activation. Cell 93:875-884. [DOI] [PubMed] [Google Scholar]
- 64.Yoshida, M. 2001. Multiple viral strategies of HTLV-1 for dysregulation of cell growth control. Annu. Rev. Immunol. 19:475-496. [DOI] [PubMed] [Google Scholar]