Abstract
An E1-deletion-containing adenoviral recombinant based on the chimpanzee serotype 68 (AdC68) was developed to express the rabies virus glycoprotein. Mice immunized with this construct (AdC68rab.gp) developed antibodies to rabies virus and remained resistant to challenge with an otherwise lethal dose of rabies virus. In naïve mice immunized intranasally, the rabies virus-specific antibody responses elicited by AdC68rab.gp were comparable with regard to both titers and isotype profiles to those induced by an adenoviral recombinant based on human serotype 5 (Adhu5) expressing the same transgene product. In contrast, subcutaneous immunization with the AdC68rab.gp vaccine resulted in markedly lower antibody responses to the rabies virus glycoprotein than the corresponding Adhu5 vaccine. Antibodies from AdC68rab.gp-immunized mice were strongly biased towards the immunoglobulin G2a isotype. The antibody response to the rabies virus glycoprotein presented by Adhu5rab.gp was severely compromised in animals preexposed to the homologous adenovirus. In contrast, the rabies virus-specific antibody response to the AdC68rab.gp vaccine was at most marginally affected by preexisting immunity to common human adenovirus serotypes, such as 2, 4, 5, 7, and 12. This novel vaccine carrier thus offers a distinct advantage over adenoviral vaccines based on common human serotypes.
E1-deletion-containing replication-defective adenoviral recombinants based on human serotype 5 (Adhu5) have been tested widely as carriers for gene therapy (2, 21). Gene therapy trials demonstrated high-level expression of the transgene product in a variety of different cell types. Nevertheless, expression was transient in vivo due to clearance of adenovirus-infected cells by CD8+ T cells directed against antigens of the adenovirus as well as against the transgene product (4, 26). Vaccine studies based on the rabies virus glycoprotein (22), the circumsporozoite protein of Plasmodium falciparum (17), the E6 and E7 oncoproteins of human papillomavirus type 16 (HPV-16) (9), and others (9; J. Fitzgerald, G.-P. Gao, A. Reyes-Sandoval, G. N. Pavlakis, Z. Q. Xiang, A. P. Wlazlo, W. Giles-Davis, J. Wilson, and H. C. J. Ertl, submitted for publication) demonstrated that E1-deletion-containing adenoviral recombinants induce, even if given at moderate doses, superb B-cell and CD8+-T-cell responses in experimental animals. The immune responses to the transgene products far surpass those achieved with other types of subunit vaccines, such as vaccinia virus recombinants or DNA vaccines (9, 22, 23; J. Shiver, AIDS Vaccines 2001, abstr. LB5, 2001). The high immunogenicity of adenoviral recombinants relates in part to the noncytopathic nature of such viruses, which permits sustained antigen expression (22). In addition, adenoviruses that enter cells primarily, although not exclusively, through interaction with the coxsackie-adenovirus receptor (CAR) (3) efficiently transduce dendritic cells (27), which are the main cell population able to present antigen to a naïve immune system.
Nevertheless, although E1-deletion-containing human adenoviral recombinants have yielded highly promising results as vaccines in rodents, canines, and nonhuman primates (9, 18, 19, 22; Fitzgerald et al., submitted; Shiver, AIDS Vaccines 2001), preexisting immunity in humans, who frequently encounter these ubiquitous viruses and generally seroconvert within their first years of life, is expected to interfere with the efficacy of such vaccines. We showed previously that the efficacy of Adhu5 recombinant vaccines was impaired in mice which had had prior exposure to the same serotype of adenovirus. The response could be rescued either by increasing the dose of the vaccine, which augments the cost and the risk of side effects, or by using a DNA vaccine expressing the same transgene product for priming (22, 23). However, prime booster regimens increase the cost of a vaccine, and their use is subject to logistic problems, especially in less developed countries. Furthermore, although both prime booster vaccinations and increases in the vaccine dose restored the antibody response to the transgene product in preimmune rodents, humans are expected to encounter the common serotypes of human adenoviruses more frequently. The resulting immunological memory may not be as readily overcome as the more moderate response in rodents to a single immunization with a virus that fails to replicate in this species. We therefore developed an adenoviral recombinant vaccine based on a chimpanzee serotype, i.e., serotype 68 (1) using the well-defined rabies virus glycoprotein as our model antigen. This serotype of adenovirus does not circulate in humans and lacks neutralizing B-cell epitopes cross-reacting with those of common human serotypes (7).
MATERIALS AND METHODS
Mice.
Female 6- to 8-week-old C3H/He mice were purchased from Jackson Laboratory, Bar Harbor, Maine. Outbred ICR mice were purchased from Charles River (Wilmington, Mass.). Mice were kept in the Animal Facility of the Wistar Institute.
Cell lines.
Mammalian cells, i.e., baby hamster kidney 21 (BHK-21) cells, E1-transfected 293 cells, thymidine kinase-negative (TK−) 143B human osteosarcoma cells (Wistar Institute), and L929 mouse fibroblast cells, were propagated in Dulbecco's modified Eagle's medium supplemented with glutamine, sodium pyruvate, nonessential amino acids, HEPES buffer, antibiotic, and 10% fetal bovine serum.
Rabies viruses.
Rabies virus of the Evelyn Rokitniki-Abelseth (ERA) and challenge virus standard 11 (CVS-11) strains were propagated on BHK-21 cells. ERA was purified over a sucrose gradient, inactivated by treatment with β-propionolactone, and adjusted to a protein concentration of 0.1 mg/ml. CVS-11 was titrated on BHK-21 cells and by intracerebral injection into adult ICR mice (24).
Adenoviruses.
Adenoviruses of the human serotypes 2, 4, 5, 7, and 12 and the chimpanzee serotype 68 were propagated and titrated on human 293 cells. The recombinant Adhu5 constructs expressing the glycoprotein of rabies virus strain ERA or the L1 protein of HPV-16 have been described previously (11, 22). An expression system using an E1-deletion-containing adenoviral recombinant based on the chimpanzee serotype 68 (AdC68) was developed as described previously (7). Briefly, the full-length coding sequence for the glycoprotein of rabies virus was ligated into the NotI site of the pC68-CMV-AP shuttle vector, replacing the alkaline phosphatase (AP) sequence. The resulting vector, pC60CMVrab.gp, was digested with BglII and PacI. A 4.6-kb BglII/PacI fragment containing C68 map units 0 to 1, the rab.gp minigene cassette, and C68 m.u.9-11 was isolated from this digestion and subcloned into the BglII and PacI sites of the pCMVAPMU32 vector, resulting in pC68-CMVrab.gpMU36. This construct (8 μg) was cotransfected with 2 μg of SspI-digested AdC68 genome in which E1 had been replaced with the gene for green fluorescent protein (AdC68CMVGFP) into 293 cells. White plaques were isolated and characterized as described previously (7). Viruses were propagated on 293 cells transfected with the E1 from Adhu5 (8). Viruses were harvested by freeze-thawing of the cells. For some experiments, virus was purified by CsCl gradient purification. For other experiments, cleared supernatant of the infected cells subjected to three rounds of freeze-thawing was used. Viruses were titrated on 293 cells to determine the number of PFU.
Expression of the transgene product by the adenoviral recombinants. (i) Immunoprecipitation.
TK− cells (106 per sample) were infected, at 5 PFU/cell, with the AdC68rab.gp or Adhu5rab.gp construct or control constructs expressing unrelated viral antigens. After 48 h, cells were washed twice with sterile phosphate-buffered saline (PBS) and then incubated for 90 min in serum-free medium prior to the addition of 20 μl of 35S-labeled cysteine and methionine (Promix; NEN, Boston, Mass.). After 4 h of incubation, cells were washed with PBS and then treated for 20 min with 1 ml of radioimmunoprecipitation assay buffer containing protease inhibitors. Cells and cell debris were removed from the wells, vortexed briefly, and centrifuged for 2 min at 12,000 rpm in an Eppendorf microcentrifuge. The supernatant was incubated for 90 min at 4°C with of ascitic fluid (15 μl/ml) containing the 509-6 monoclonal antibody to the rabies virus glycoprotein. Protein G-Sepharose was added at 75 μl per sample and incubated at 4°C with mild agitation for 30 min. The samples were pelleted by centrifugation and washed four times with radioimmunoprecipitation assay buffer. The pellets were resuspended in 80 μl of loading buffer and boiled for 4 min. Samples (20 μl) were then separated over a sodium dodecyl sulfate-12% polyacrylamide gel along with a molecular weight standard. Gels were dried onto filter papers, which were exposed for 48 h to Kodak scientific imaging film (X-Omat Blue XB-1).
(ii) Immunofluorescence.
L929 and TK− cells (106 per sample) were infected for 48 h with 5 PFU of Adhu5rab.gp or AdC68rab.gp per cell. Control cells were left uninfected. Cells were treated for 45 min on ice with 100 μl of a 1:1,000 dilution of the 509-6 antibody in PBS or with PBS only. Cells were then washed and treated for 45 min with 100 μl of a 1:100 dilution of a fluorescein isothiocyanate-labeled anti-mouse immunoglobulin (Ig). Cells were washed and analyzed by flow cytometry.
Immunization and challenge of mice.
Mice were vaccinated with various doses of the adenoviruses or the adenoviral recombinants given in 100 μl of saline subcutaneously or in 50 μl intranasally. Mice were challenged with rabies virus strain CVS-11 given at 10 mean lethal doses (LD50) intracerebrally. After challenge, mice were checked every 24 to 48 h for at least 21 days. They were euthanized once they developed complete hind leg paralysis, which is indicative of terminal rabies virus encephalitis.
Serological assays. (i) ELISA.
Enzyme-linked immunosorbent assays (ELISAs) were performed basically as described before (22). Mice were bled by retro-orbital puncture a various intervals after immunization. Sera were prepared and tested for antibodies to rabies virus on plates coated with 0.1 μg of inactivated rabies virus per well. Sera were tested for antibodies to adenovirus on plates coated with 5 × 109 virus particles of purified E1-deletion-containing adenovirus recombinants to green fluorescent protein of human serotype 5 or chimpanzee serotype 68 per well. Plates were coated overnight and then blocked for 24 h with PBS containing 3% bovine serum albumin. After washing, sera diluted in PBS-3% bovine serum albumin were added for 60 min. After washing, a 1:100 dilution of AP-conjugated goat anti-mouse Ig (Cappel) was added for 1 h at room temperature. After washing, substrate was added for 20 to 30 min at room temperature. Optical density was read at 405 nm.
(ii) Isotype profile of antibodies.
Isotypes of antibodies to rabies virus were determined by an ELISA on plates coated with inactivated ERA virus with a Calbiochem (La Jolla, Calif.) hybridoma subisotyping kit with some minor modifications as previously described (22). Sera were tested at a 1:400 dilution.
(iii) Virus neutralization assays.
Sera were tested for neutralizing antibodies to rabies virus strain CVS-11, which is antigenically closely related to the ERA strain, as described previously (25). A World Health Organization reference serum was used for comparison. Titers are expressed in international units.
RESULTS
Expression of the rabies virus glycoprotein by the adenoviral recombinants.
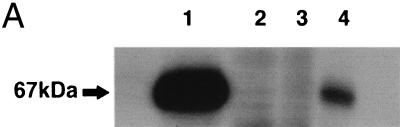
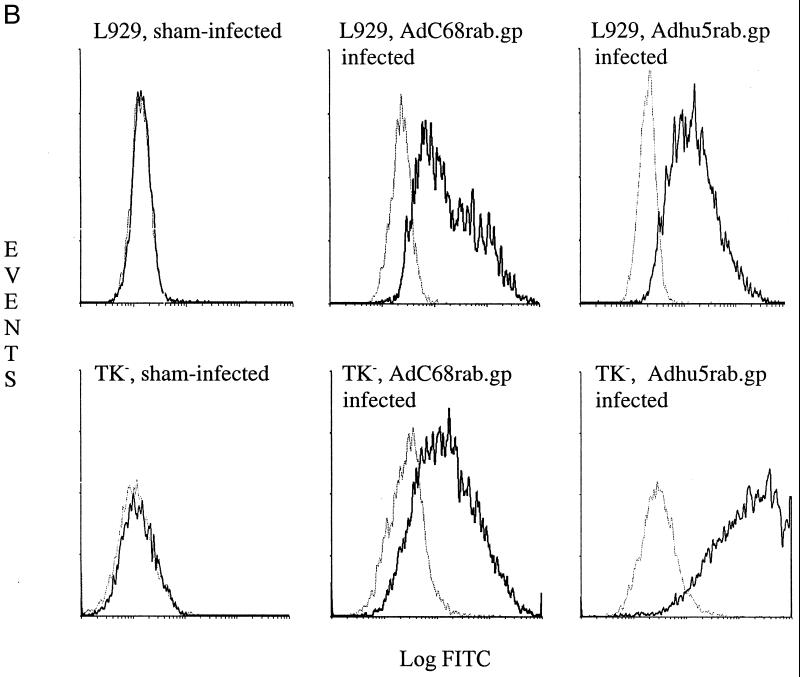
The AdC68rab.gp recombinant was generated in 293 cells transfected with E1 of human adenovirus serotype 5 (7). Viral clones were initially screened by indirect immunofluorescence with monoclonal antibody 509-6, which recognizes a conformation-dependent epitope of the rabies virus glycoprotein. After selection of a stable adenoviral subclone, expression of full-length rabies virus glycoprotein by AdC68rab.gp in infected TK− cells was confirmed by immunoprecipitation. As shown in Fig. 1, both AdC68rab.gp and Adhu5rab.gp expressed a protein of the expected size that was precipitated by the 509-6 antibody. Expression of correctly folded rabies virus glycoprotein in AdC68rab.gp-infected cells was further demonstrated by indirect immunofluorescence followed by flow-cytometric analysis again using the 509-6 antibody. In transduced TK− cells, the Adhu5rab.gp construct appeared to result in higher levels of rabies virus glycoprotein, as did transduction with the AdC68rab.gp construct. However, in other cell lines (data shown only for L929 cells), such as mouse L929 fibroblasts, equal levels of transgene expression were achieved with both vectors.
FIG. 1.
Expression of the rabies virus glycoprotein in cells transduced with the Adhu5 or AdC68 recombinants. (A) Immunoprecipitation. Radiolabeled lysates from cells infected for 48 h with 5-PFU/cell doses of the Adhu5rab.gp recombinant (lane 1), an Adhu5 construct expressing HIV-1 Gag (lane 2), an AdC68 construct expressing HIV-1 Gag (lane 3), or the AdC68rab.gp recombinant (lane 4) were immunoprecipitated with a monoclonal antibody to the rabies virus glycoprotein and separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. (B) Immunofluorescence. Uninfected, AdC68rab.gp-infected, and Adhu5rab.gp-infected L929 and TK− cells were treated with PBS (gray line) or the 509-6 antibody (black line). Cells were then treated with a fluorescein isothiocyanate (FITC)-labeled antibody to mouse Ig and analyzed by flow cytometry. The graph shows events over green fluorescent intensity.
Induction of a B-cell response by the adenoviral recombinants to the rabies virus glycoprotein.
Vaccine-induced protection to rabies virus correlates with virus-neutralizing antibodies (VNAs) (25). Our studies thus focused on stimulation of this arm of the immune system. In all experiments, mice were immunized either subcutaneously or intranasally with AdC68rab.gp or the previously described Adrab.gp construct (22). This recombinant is referred to here as Adhu5rab.gp.
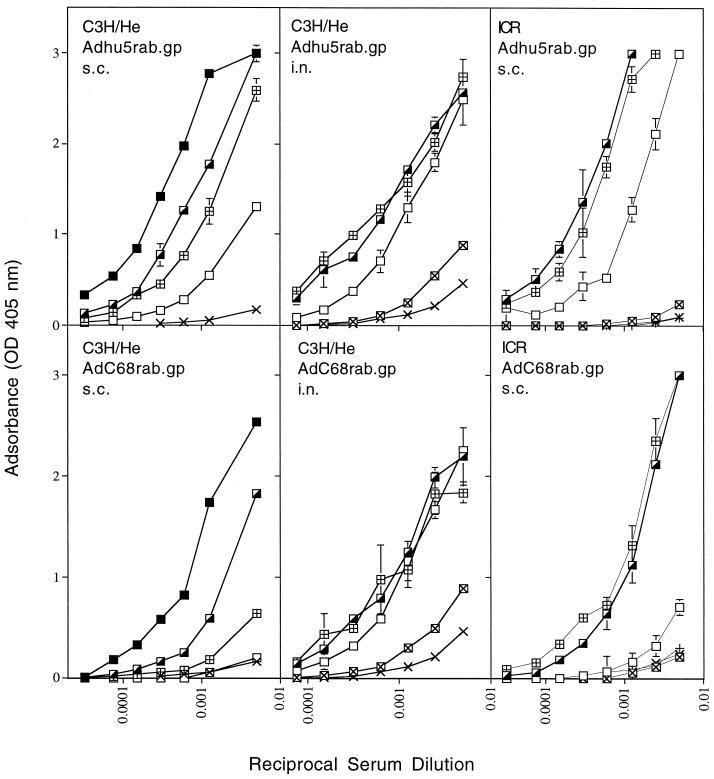
The rabies virus-specific antibody response was tested in inbred and outbred strains of mice immunized with serial dilutions of either of the recombinants. Sera were harvested 14 days after immunization and tested for antibodies to the rabies virus glycoprotein by an ELISA (Fig. 2) and a virus neutralization assay (Table 1). Adenoviral recombinants encoding an unrelated viral transgene used as controls (8) failed to induce an antibody response to rabies virus detectable by either assay.
FIG. 2.
Antibody response to rabies virus. Groups of five C3H/He or ICR mice were immunized with 5 × 107 (▪; tested in subcutaneously [s.c.] immunized C3H mice only), 5 × 106 (┌), 5 × 105 (⊞), 5 × 104 (□), or 5 × 103 (⊠; tested in intranasally [i.n.] immunized C3H and ICR mice only) PFU of Adhu5rab.gp or AdC68rab.gp virus or no virus (×). Mice were bled 2 weeks later, and sera were tested for antibodies to rabies virus by an ELISA. Data are means of duplicate values.
TABLE 1.
Induction of VNAs and protective immunity to rabies virus challenge
| Host mouse strain and immunization routea | Vaccine | (% survival) with VNA titer vaccine dose (PFU)b |
|||||
|---|---|---|---|---|---|---|---|
| 5 × 107 | 5 × 106 | 5 × 105 | 5 × 104 | 5 × 103 | 0 | ||
| C3H (s.c.) | Adhu5rab.gp | 972 (100) | 324 (100) | 108 (100) | 12 (100) | NT | <0.5 (0) |
| AdC68rab.gp | 240 (100) | 36 (100) | 12 (80) | 8 (80) | NT | <0.5 (0) | |
| ICR (s.c.) | Adhu5rab.gp | NT | 162 (100) | 162 (100) | 18 (100) | 6 (0) | <0.5 (0) |
| AdC68rab.gp | NT | 54 (NT) | 54 (100) | 6 (20) | 0.3 (0) | <0.5 (0) | |
| C3H (i.n.) | Adhu5rab.gp | NT | 162 (100) | 162 (100) | 18 (50) | 6 (0) | <0.5 (0) |
| AdC68rab.gp | NT | 54 (100) | 162 (100) | 18 (100) | 6 (0) | <0.5 (0) | |
Groups of five C3H/He or ICR mice were immunized with various doses of Adhu5rab.gp or AdC68rab.gp subcutaneously (s.c.) or intranasally (i.n.). They were bled 2 weeks later, and sera were tested for VNAs to rabies virus.
VNA titers are in international units and are means of samples tested in duplicate. Sera from mice immunized with either recombinant expressing an unrelated viral antigen were tested in parallel. None of these sera contained detectable antibody titers to rabies virus (data not shown). Mice were challenged 3 weeks after vaccination with 10 LD50 of CVS-11 virus, and survival was recorded. All of the mice injected with the corresponding highest dose of adenoviral recombinants expressing an unrelated transgene product died upon infection (not shown). NT, not tested.
When administered subcutaneously, the AdC68rab.gp virus induced a less potent antibody response to the transgene product than did the Adhu5rab.gp construct. The difference in magnitude of the antibody response, which was observed at all time points tested, depended on the mouse strain and was less pronounced in outbred ICR than in inbred C3H/He mice. In contrast, when administered intranasally, the vaccines induced comparable titers of antibodies as determined by ELISAs (Fig. 2) and by virus neutralization assays (Table 1).
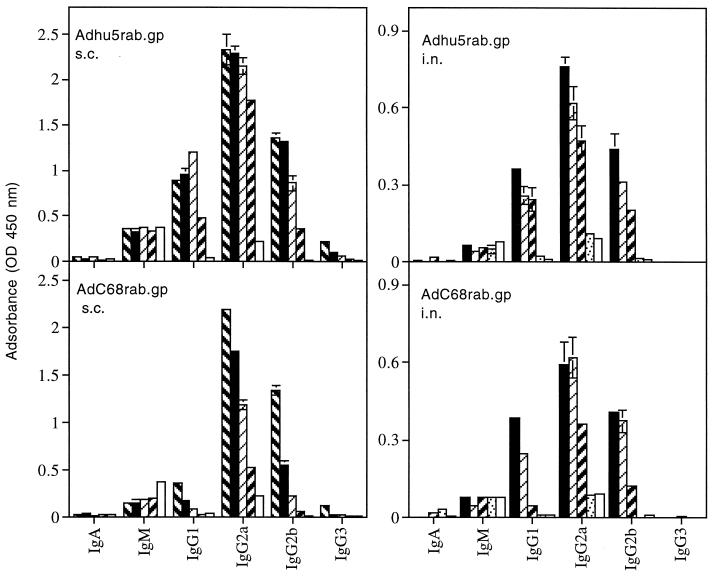
After subcutaneous immunization, antibodies induced by the Adhu5rab.gp and AdC68rab.gp vaccines differed in their isotype profile. This difference was not observed upon intranasal immunization (Fig. 3). Both recombinants, delivered by either route of inoculation, elicited IgG2a antibodies to the antigen of rabies virus, suggesting induction of a Th1-type immune response. Both recombinants upon intranasal immunization and Adhu5rab.gp upon subcutaneous administration also induced a pronounced IgG1 response indicative of Th2 help, which was lacking in the response to the AdC68rab.gp construct given subcutaneously.
FIG. 3.
Sera from C3H/He mice immunized with 5 × 107 (), 5 × 106 (▪), 5 × 105 (▨), 5 × 104 ( ), or 5 × 103 (░⃞) PFU of Adhu5rab.gp or AdC68rab.gp were tested for isotypes of antibodies to the rabies virus antigen. Normal mouse serum (□) was tested for comparison. Data were obtained with a 1:400 dilution of the sera and are means of duplicate values plus standard deviations.
The pronounced Th1-linked antibody response to the transgene product of AdC68rab.gp given subcutaneously may relate to the “adjuvant effect” of this particular serotype of adenovirus. Splenocytes from naive C3H/He mice cultured in vitro with an E1-deletion-containing AdC68 construct rapidly secreted interferon (IFN) which could not be neutralized by an antibody to gamma interferon (IFN-γ) and thus represented IFN-α/β (Table 2). This was not observed upon culture of splenocytes with a corresponding Adhu5 construct.
TABLE 2.
Adjuvant effect of the adenoviral recombinantsa
| Source of cytokine | Amt of IFN (U) |
|
|---|---|---|
| Without XMG1 | With XMG1 | |
| Splenocytes + Adhu5rab.gp | 2 | 2 |
| Splenocytes + AdC68rab.gp | 12 | 12 |
| Splenocytes without virus | ≤0.5 | |
| Recombinant mouse IFN-γ | 1024 | 8 |
Splenocytes from naïve C3H/He mice were cultured with 10 PFU of the adenoviral recombinants per cell. Cell-free supernatants were tested for IFN without and with addition of the XMG1 antibody to IFN-γ. A recombinant IFN-γ was tested in parallel.
Induction of protection against challenge with a virulent strain of rabies virus.
Both adenoviral recombinants induced protection against infection of mice challenge with rabies virus (Table 1). C3H/He mice immunized subcutaneously with 5 × 106 PFU of either of the adenoviral recombinants remained disease-free when challenged 3 weeks later with 10 LD50 of rabies virus of the CVS strain. This strain is antigenically closely related to the ERA strain but is more virulent in rodents. At 5 × 105 PFU, Adhu5rab.gp still provided complete protection while a small percentage of AdC68rab.gp-immunized mice succumbed to infection. Further reduction of the vaccine dose resulted in loss of efficacy of the AdC68rab.gp vaccine: at 5 × 104 PFU 20% of subcutaneously immunized C3H/He and 80% of subcutaneously vaccinated ICR mice succumbed to the challenge with rabies virus. With nasal immunization, both vaccines provided complete protection if given at 5 × 105 PFU. At 5 × 104 PFU, 50% of mice vaccinated with Adhu5rab.gp developed progressive disease while those immunized with this dose of AdC68rab.gp were protected. All of the mice immunized with control adenoviral recombinants of either serotype or with only 5 × 103 PFU of either of the adenoviral recombinants to the rabies virus glycoprotein developed fatal rabies encephalitis.
Effect of preexisting immunity to different serotypes of human adenoviruses on the antibody response to rabies virus glycoprotein.
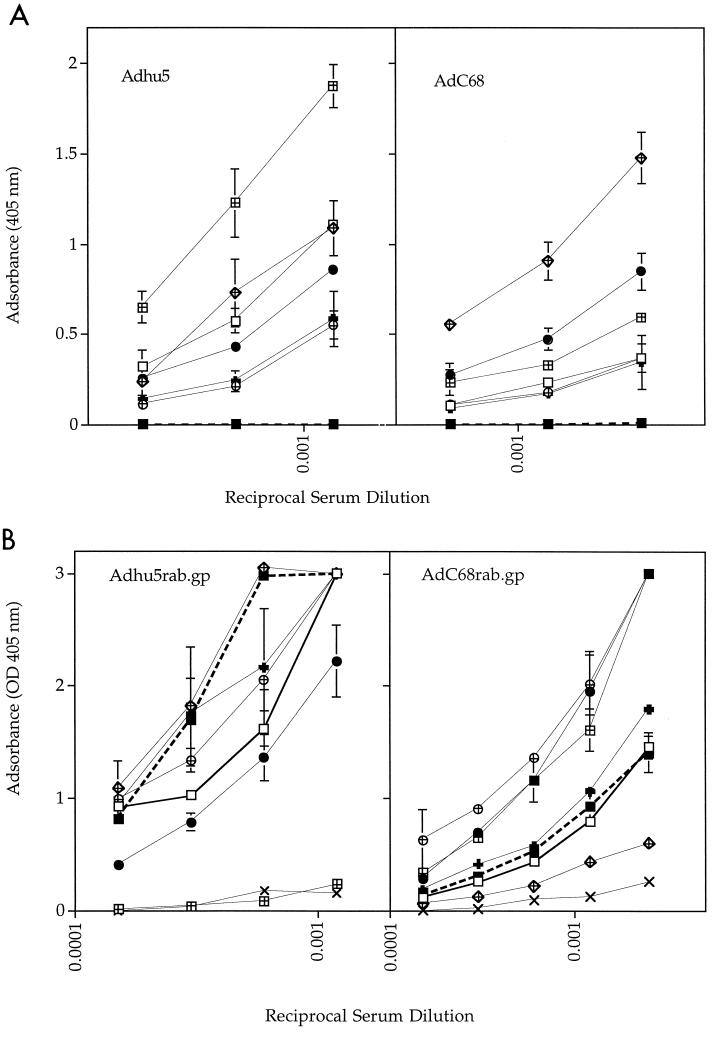
We reported previously that mice preimmune to Adhu5 develop a reduced antibody response to vaccination with the Adhu5rab.gp recombinant compared to naïve mice (23). Human adults are immune not only to Adhu5 but also to other serotypes, including 2, 4, 7, and 12 (12). To test if preexposure to any of these common serotypes of human adenoviruses would inhibit the antibody response to the AdC68rab.gp vaccine, groups of C3H/He mice were immunized with 4 × 108 PFU of replication-competent adenoviruses of human serotype 2, 4, 5, 7, or 12 or with the chimpanzee serotype 68 (the latter contained an E1 deletion). Two weeks later, mice were bled and sera were tested by an ELISA (Fig. 4A) for antibodies to adenoviral antigens. Sera from mice immunized with the different serotypes showed extensive cross-reactivity. The highest antibody titers were achieved in mice inoculated with the homologous construct. Sera from mice immunized with Adhu4, whose known sequences show ∼90% homology with those of AdC68, had higher titers to AdC68 than did sera from mice injected with any of the other human serotypes. After sera had been harvested, mice were vaccinated subcutaneously with either Adhu5rab.gp or AdC68rab.gp. Adhu5rab.gp was used at a dose of 2 × 105 PFU per mouse; AdC68rab.gp, which induces only a marginal antibody response in C3H/He mice when given subcutaneously at such a low dose, was injected at 2 × 107 PFU per mouse. Sera were harvested 2 weeks later and tested for antibodies to the rabies virus glycoprotein by an ELISA (Fig. 4B) or a virus neutralization assay (Table 3). The rabies virus-specific response to Adhu5rab.gp in naïve mice was slightly superior to that elicited by AdC68rab.gp. The response to Adhu5rab.gp was completely inhibited in mice preimmune to Adhu5. Some reduction was also seen for rabies virus glycoprotein-specific ELISA titers in mice preimmune to human adenovirus serotypes 2, 4, 7, and 12. VNA titers showed only a twofold reduction in Adhu2-, Adhu7-, and Adhu12-preimmune mice, which was not considered biologically significant. Adhu4-preimmune mice showed a more pronounced reduction in VNA titers to rabies virus.
FIG. 4.
Effect of preexisting immunity on the antibody response to the transgene product presented by Adhu5 or AdC68. (A) Cross-reactivity by different serotypes of human adenovirus. Groups of C3H/He mice were immunized subcutaneously with 4 × 108 PFU of Adhu2 (□), Adhu4 (•), Adhu5 (⊞), Adhu7 (⊕), Adhu12 (✚), AdC68 (), or nothing (□). Mice were bled 2 weeks later, and titers of serum antibody to Adhu5 and AdC68 were determined by an ELISA. (B) Inhibition of the transgene specific antibody response by preexposure to common human serotypes of adenovirus. Three weeks after immunization with adenoviruses of different serotypes, the groups of mice used for panel A were injected with 2 × 105 PFU of Adhu5rab.gp or 2 × 107 PFU of AdC68. Sera were harvested 2 weeks later and tested for antibodies to rabies virus by an ELISA using normal mouse serum (×) for comparison. Symbols are as in panel A. The bold solid and dashed lines indicate mice immunized with the homologous serotype used for preexposure and mice that had not been preexposed, respectively.
TABLE 3.
Effect of preexisting immunity to Adhu5 on vaccine efficacya
| Vaccine (dose [PFU]) | VNA titer (IU) (% survival upon challenge) with preexposure |
|
|---|---|---|
| None | Adhu5 | |
| Adhu5rab.gp (2 × 107) | 972 (100) | 486 (100) |
| Adhu5rab.gp (2 × 106) | 486 (100) | 54 (100) |
| Adhu5rab.gp (2 × 105) | 486 (100) | 18 (40) |
| AdC68rab.gp (2 × 107) | 729 (100) | 729 (100) |
| AdC68rab.gp (2 × 106) | 486 (100) | 486 (100) |
| AdC68rab.gp (2 × 105) | 54 (100) | 54 (100) |
| None | <0.5 (0) | |
Groups of five C3H/He mice were injected with 4 × 108 PFU of an E1-deletion-containing Adhu5 virus expressing the L1 protein of HPV-16. Mice were injected 3 weeks later with various doses of Adhu5rab.gp or AdC68rab.gp. Sera were harvested 2 weeks later and tested for VNAs to rabies virus. Mice were then challenged with 10 LD50 of CVS-11 virus, and survival was recorded.
The rabies virus-specific antibody response to the Adhu5rab.gp vaccine was not affected in mice that had been preexposed to AdC68. The response to AdC68rab.gp was strongly inhibited in mice that were preimmune to the homologous virus. Mice that had previously encountered Adhu2 showed a slight reduction of the antibody response to the rabies virus antigen presented by the AdC68 vaccine, as seen by ELISA and virus neutralization assays. Mice inoculated with any of the other serotypes of human adenoviruses developed ELISA titers of antibody to rabies virus upon AdC68rab.gp immunization that were either equal to or higher than those in mice that were naïve prior to vaccination. In particular, mice preimmune to Adhu5 developed higher antibody titers upon vaccination with the AdC68rab.gp construct; this may reflect the presence of cross-reactive T helper cells that promoted the B-cell response to the transgene product.
To further determine if at equal vaccine doses the AdC68rab.gp vaccine induced antibody titers superior to those induced by Adhu5rab.gp in mice preimmune to Adhu5, we conducted a vaccine titration experiment. Groups of C3H/He mice were immunized subcutaneously with 4 × 108 PFU of an E1-deletion-containing Adhu5 recombinant encoding the L1 antigen of HPV-16. Mice were vaccinated 2 weeks later with either Adhu5rab.gp or AdC68rab.gp given subcutaneously at various doses. Mice were bled 2 weeks later, and titers of serum antibody to rabies virus were determined by an ELISA (not shown) and a virus neutralization assay (Table 3). Neither assay showed a significant reduction in the antibody response to the AdC68rab.gp construct in Adhu5-immune mice. The extent of the reduction of titers of antibody to rabies virus presented by the Adhu5 construct in mice preimmune to the homologous virus depended on the vaccine dose. The antibody response to lower doses of vaccine was more affected than the response to higher vaccine doses. At the highest vaccine dose, VNA titers were halved in Adhu5-preimmune mice; at the two lower vaccine doses, titers were reduced 9- to 27-fold. At all doses tested, AdC68rab.gp induced higher VNA titers to rabies virus in Adhu5-preimmune mice than did an equal dose of Adhu5rab.gp. The detrimental effect of preexisting immunity to Adhu5 on the efficacy of the Adhu5 vaccine was demonstrated further in a protection experiment (Table 3). Naïve mice immunized with 2 × 105 PFU of Adhu5rab.gp or AdC68rab.gp were completely protected against challenge with CVS-11 virus. The majority (60%) of Adhu5-preimmune mice immunized with this dose of the Adhu5rab.gp vaccine succumbed to a rabies virus infection, while those vaccinated with the same dose of AdC68rab.gp remained protected. Increasing the dose of Adhu5rab.gp to 2 × 106 PFU per mouse restored the efficacy of the vaccine.
DISCUSSION
E1-deletion-containing Adhu5 recombinants have been used extensively as gene therapy vehicles. Although they efficiently transduce a variety of mammalian cells, their high immunogenicity, which results in potent T- and B-cell-mediated immune responses to both the adenoviral antigens and the transgene product, has severely limited their usefulness for long-term replacement of missing or faulty genes. Replication-competent and replication-defective adenoviral recombinants based on common human serotypes have been tested in experimental animals as vaccine carriers for a variety of antigens derived from viruses, parasites, or tumor cells. In each case, E1-deletion-containing adenoviral recombinants elicited potent cell-mediated and humoral immune responses to the transgene product that even at low doses of vaccine provided protection to challenge with the pathogen or the tumor cells (6, 9, 15, 22). Adhu5 is a ubiquitous common-cold virus that infects most humans within their first year of life. Recurrent asymptomatic reinfections result in persistent titers of antibodies to Adhu5, and approximately one-third of human adults have high levels of circulating neutralizing antibodies to adenovirus (7). Such antibodies have been shown in experimental animals to reduce the efficacy of adenoviral recombinant vaccines based on the homologous viral serotype (23; Shiver, AIDS Vaccines 2001). The underlying mechanism presumably involves antibody-mediated neutralization of the vaccine, which reduces the number of recombinant virus particles able to infect cells and to synthesize the transgene product. Additional pathways, such as lysis of adenovirus-transduced antigen-presenting cells by effector-memory CD8+ T cells, may also dampen the induction of transgene-specific immune responses; this remains to be tested.
Alternative adenoviral recombinants based on nonhuman serotypes were developed for gene therapy to circumvent interference by circulating neutralizing antibodies in human patients (10, 14). We developed such a recombinant as a vehicle for vaccination. The recombinant is based on an adenovirus that was initially isolated from mesenteric lymph nodes of a chimpanzee. Like Adhu5, AdC68 enters cells upon binding of fiber to the CAR (5). Alternative but less efficient pathways circumventing the CAR may involve interactions such as those between the viral penton and cell integrins (20). It remains to be investigated if AdC68 uses the same alternative mechanisms of transduction as Adhu5 and to what degree potential differences affect the tropism and the vaccine efficacy of either viral recombinant.
Mice immunized subcutaneously with the AdC68rab.gp vaccine developed markedly lower antibody titers than did those vaccinated with the Adhu5rab.gp construct, a difference that was not observed upon mucosal immunization. This is most likely linked to differences in the interactions of the two viral constructs with the innate immune system; the AdC68 construct triggered rapid release of IFN-α/β in splenocyte cultures. A similar induction of this cytokine in vivo would bias the immune response towards the Th1 pathway, as is indeed suggested by the strongly biased IgG2a response upon subcutaneous immunization with the AdC68rab.gp vaccine. The strongly biased Th1 response to the AdC68rab.gp vaccine that was not observed upon immunization with the Adhu5rab.gp vaccine may have favored induction of cell-mediated rather than humoral immune responses and may thus have contributed to the less potent B-cell response to our novel vaccine carrier given systemically. This was suggested in another system in which the Gag antigen of human immunodeficiency virus type 1 (HIV-1) was delivered by E1-deletion-containing Adhu5 and AdC68 recombinants. In that system, the AdC68 vaccine given systemically induced markedly higher CD8+-T-cell responses to Gag than the corresponding Adhu5 vaccine (Fitzgerald et al., submitted).
Upon mucosal vaccination, both adenoviral vaccines induced a more balanced Th1/Th2 response, as determined by IgG2a/IgG1 ratios, and accordingly induced antibody responses to the transgene product that were comparable in magnitude.
Most importantly, the antibody response to the transgene product expressed by the AdC68 recombinant was not affected by preexisting immunity to common human adenovirus serotypes. In contrast, after preimmunization with replication-competent viruses, the immune response to the Adhu5rab.gp vaccine was abolished in Adhu5-preimmune mice and reduced in mice preimmune to other human adenoviruses, such as serotypes 2 and 4. The response to the AdC68 recombinant was, as expected, inhibited in mice preimmune to the homologous virus. This is not of clinical concern, as AdC68 does not circulate in the human population (7) and common human serotypes do not share neutralizing epitopes with AdC68.
Preexposure to replication-defective Adhu5 also reduced the antibody response to the rabies virus glycoprotein encoded by Adhu5 recombinants, although the impact was not as severe as in mice previously infected with E1-containing virus. Such virus replicates in its natural host but fails to replicate in mice. Nevertheless, even in mice, replication-competent adenovirus is expected to synthesize a higher antigenic load of structural viral proteins than E1-deletion-containing adenoviral recombinants. Sera from mice preimmune to replication-defective Adhu5 developed reduced but readily detectable antibodies to rabies virus upon immunization with the Adhu5rab.gp vaccine. Increasing the dose of the Adhu5rab.gp construct could in part circumvent the impact of preexisting immunity. Vaccine-induced protection against rabies virus requires VNAs, which were not induced as efficiently in preimmune mice by the Adhu5 vaccine, especially when it was used at lower doses. In Adhu5-preimmune mice, the VNA response to AdC68rab.gp was superior to that to the Adhu5 vaccine at all doses, more than compensating for the slightly lower potency of this vaccine upon subcutaneous immunization.
AdC68 recombinants thus provide an attractive alternative as a vaccine carrier for use in humans. As shown here, they are efficacious even when applied at low doses of 2 × 105 PFU through noninvasive routes of administration, such as the upper airways. Mucosal immunization by intranasal application has the added advantage of favoring induction of responses of the common mucosal immune system (13, 16, 23), which is distinct from, albeit interconnected with, the central immune system targeted by injected vaccines. Most pathogens, including influenza A and B viruses, hepatitis viruses, rotaviruses, human immunodeficiency virus, and cancer-associated types of human papillomaviruses, invade their hosts through mucosal surfaces of the airways, the intestine, or the genital tract. They might thus best be combated by vaccines that upon application to mucosal membranes induce robust mucosal immune responses, including IgA antibodies, which are poorly induced upon systemic immunization (23). As shown here, preexisting immunity to common serotypes of human adenoviruses found in the majority of the human population impairs the efficacy of recombinants based on such human serotypes. This can in part be overcome by increasing the dose of the vaccine or more elegantly and cost-efficiently by using an adenoviral recombinant based on a mammalian serotype that does not circulate in the human population, such as AdC68. E1-deletion-containing AdC68 recombinants have another advantage over currently used replication-defective Adhu5 vaccines. The Adhu5 vaccines are propagated in cell lines that transcomplement the E1 protein derived from the same virus serotype. Homologous recombination between the cell-derived E1 gene and the E1-deletion-containing Adhu5 genome is inevitable and contaminates the vaccine preparations with replication-competent virus. These pose an added risk to patients, especially those who are immunocompromised, such as the very young and the elderly. In the replication-defective AdC68 constructs, the E1 of human serotype 5 transcomplements the E1-deletion-containing adenoviral genome. The flanking sequences of the human serotype 5 E1 are nonhomologous with those of the AdC68 serotype, thus disallowing homologous recombination. Vaccine preparations are therefore less likely to carry replication-competent virus.
Acknowledgments
The technical support of the Vector and Immunology Cores of the Institute for Human Gene Therapy is greatly appreciated.
This work was supported by NIH (NIDDK P30, NHLBI P01, NIAID R01), CF Foundation, and Genovo. J. M. Wilson owns equity in Targeted Genetics (formerly Genovo).
REFERENCES
- 1.Basnight, M., N. G. Rogers, C. J. Gibbs, and D. C. Gajdusek. 1971. Characterization of four new adenovirus serotypes isolated from chimpanzee tissue explants. Am. J. Epidemiol. 94:166-171. [DOI] [PubMed] [Google Scholar]
- 2.Batshaw, M. L., J. M. Wilson, S. Raper, M. Yudkoff, and M. B. Robinson. 1999. Recombinant adenovirus gene transfer in adults with partial ornithine transcarbamylase deficiency (OTCD). Hum. Gene Ther. 10:2419-2437. [DOI] [PubMed] [Google Scholar]
- 3.Bergelson, J. M., J. A. Cunningham, G. Droguett, E. A. Kurt-Jones, A. Krithivas, J. S. Hong, M. S. Horwitz, R. L. Crowell, and R. W. Finberg. 1997. Isolation of a common receptor for coxsackie B viruses and adenoviruses 2 and 5. Science 275:1320-1323. [DOI] [PubMed] [Google Scholar]
- 4.Chirmule, N., K. Propert, S. Magosin, Y. Qian, R. Qian, and J. M. Wilson. 1999. Immune responses to adenovirus and adeno-associated virus in humans. Gene Ther. 6:1574-1583. [DOI] [PubMed] [Google Scholar]
- 5.Cohen, C. J., Z.-Q. Xiang, G.-P. Gao, H. C. J. Ertl, J. M. Wilson, and C. V. Bergelson. 2002. Chimpanzee adenovirus 68 adapted as gene delivery vector interacts with the coxsackievirus and adenovirus receptor. J. Gen. Virol., 83:151-155. [DOI] [PubMed]
- 6.Elahi, S. M., S. H. Shen, B. G. Talbot, B. Massie, S. Harpin, and Y. Elazhary. 1999. Induction of humoral and cellular immune responses against the nucleocapsid of bovine viral diarrhea virus by an adenovirus vector with an inducible promoter. Virology 261:1-7. [DOI] [PubMed] [Google Scholar]
- 7.Farina, S., G.-P. Gao, Z. Q. Xiang, J. J. Rux, R. M. Burnett, M. Alvira, H. C. J. Ertl, and J. M. Wilson. 2001. Development of a replication-defective vector based on a chimpanzee adenovirus. J. Virol. 75:11603-11613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Graham, F. L., J. Smiley, W. C. Russell, and R. Nairn. 1977. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J. Gen. Virol. 36:59-74. [DOI] [PubMed] [Google Scholar]
- 9.He, Z., A. P. Wlazlo, D. W. Kowalczyk, J. Cheng, Z. Q. Xiang, W. Giles-Davis, and H. C. J. Ertl. 2000. Viral recombinant vaccines to the E6 and E7 antigens of HPV-16. Virology 270:146-161. [DOI] [PubMed] [Google Scholar]
- 10.Hofmann, C., P. Loser, G. Cichon, W. Arnold, G. W. Both, and M. Strauss. 1999. Ovine adenovirus vectors overcome preexisting humoral immunity against human adenoviruses in vivo. J. Virol. 73:6930-6936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kowalczyk, D., A. P. Wlazlo, S. Shane, and H. C. J. Ertl. 2001. Vaccine regimen for prevention of sexually transmitted human papillomavirus type 16. Vaccine 19:3583-3590. [DOI] [PubMed] [Google Scholar]
- 12.Ludwig, S. L., J. F. Brundage, P. W. Kelley, R. Nang, C. Towle, D. P. Schnurr, L. Crawford-Miksza, and J. C. Gaydos. 1998. Prevalence of antibodies to adenovirus serotypes 4 and 7 among unimmunized US Army trainees: results of a retrospective nationwide seroprevalence survey. J. Infect. Dis. 178:1776-1778. [DOI] [PubMed] [Google Scholar]
- 13.McGhee, J. R., J. Xu-Amano, C. J. Miller, R. J. Jackson, K. Fujihashi, H. F. Staats, and H. Kiyono. 1994. The common mucosal immune system: from basic principles to enteric vaccines with relevance for the female reproductive tract. Reprod. Fertil. Dev. 6:369-379. [DOI] [PubMed] [Google Scholar]
- 14.Moffatt, S., J. Hays, H. HogenEsch, and S. K. Mittal. 2000. Circumvention of vector-specific neutralizing antibody response by alternating use of human and non-human adenoviruses: implications in gene therapy. Virology 272:159-167. [DOI] [PubMed] [Google Scholar]
- 15.Natuk, R. J., A. R. Davis, P. K. Chanda, M. D. Lubeck, M. Chengalvala, S. C. Murthy, M. S. Wade, S. K. Dheer, B. M. Bhat, and K. K. Murthy. 1994. Adenovirus vectored vaccines. Dev. Biol. Stand. 82:71-77. [PubMed] [Google Scholar]
- 16.Neutra, M. R., E. Pringault, and J. P. Kraehenbuhl. 1996. Antigen sampling across epithelial barriers and induction of mucosal immune responses. Annu. Rev. Immunol. 14:275-300. [DOI] [PubMed] [Google Scholar]
- 17.Rodrigues, E. G., F. Zavala, D. Eichinger, J. M. Wilson, and M. Tsuji. 1997. Single immunizing dose of recombinant adenovirus efficiently induces CD8+ T cell-mediated protective immunity against malaria. J. Immunol. 158:1268-1274. [PubMed] [Google Scholar]
- 18.Sullivan, N. J., A. Sanchez, P. E. Rollin, Z. Y. Yang, and G. J. Nabel. 2000. Development of a preventive vaccine for Ebola virus infection in primates. Nature 408:605-609. [DOI] [PubMed] [Google Scholar]
- 19.Tims, T., D. J. Briggs, R. Davis, S. M. Moore, Z. Q. Xiang, H. C. J. Ertl, and Z. F. Fu. 2000. Dogs vaccinated with recombinant adenovirus glycoprotein develop high titers of neutralizing antibodies. Vaccine 18:2804-2807. [DOI] [PubMed] [Google Scholar]
- 20.Wickham, T. J., P. Mathias, D. A. Cheresh, and G. R. Nemerow. 1993. Integrins αvβ3 and αvβ5 promote adenovirus internalization but not virus attachment. Cell 73:309-319. [DOI] [PubMed] [Google Scholar]
- 21.Wilson, J. M. 1996. Adenoviruses as gene-delivery vehicles. N. Engl. J. Med. 334:1185-1187. [DOI] [PubMed] [Google Scholar]
- 22.Xiang, Z. Q., Y. Yang, J. M. Wilson, and H. C. J. Ertl. 1996. A replication-defective human adenovirus recombinant serves as a highly efficacious vaccine carrier. Virology 219:220-227. [DOI] [PubMed] [Google Scholar]
- 23.Xiang, Z. Q., S. Pasquini, and H. C. J. Ertl. 1999. Induction of genital immunity by DNA priming and intranasal booster immunization with a replication-defective adenoviral recombinant. J. Immunol. 162:6716-6723. [PubMed] [Google Scholar]
- 24.Xiang, Z. Q., and H. C. J. Ertl. 1994. A simple method to test the ability of individual viral proteins to induce immune responses. J. Virol. Methods 47:103-116. [DOI] [PubMed] [Google Scholar]
- 25.Xiang, Z. Q., B. B. Knowles, J. W. McCarrick, and H. C. Ertl. 1995. Immune effector mechanism required for protection to rabies virus. Virology 214:398-404. [DOI] [PubMed] [Google Scholar]
- 26.Yang, Y., K. U. Jooss, Q. Su, H. C. J. Ertl, and J. M. Wilson. 1996. Immune responses to viral antigens versus transgene product in the elimination of recombinant adenovirus-infected hepatocytes in vivo. Gene Ther. 3:137-144. [PubMed] [Google Scholar]
- 27.Zhong, L., A. Granelli-Piperno, M. Pope, R. Ignatius, M. G. Lewis, S. S. Frankel, and R. M. Steinman. 2000. Presentation of SIVgag to monkey T cells using dendritic cells transfected with a recombinant adenovirus. Eur. J. Immunol. 30:3281-3290. [DOI] [PubMed] [Google Scholar]