Abstract
Human cytomegalovirus (CMV) glycoproteins H, L, and O (gH, gL, and gO, respectively) form a heterotrimeric disulfide-bonded complex that participates in the fusion of the viral envelope with the host cell membrane. During virus maturation, this complex undergoes a series of intracellular assembly and processing events which are not entirely defined (M. T. Huber and T. Compton, J. Virol. 73:3886-3892, 1999). Here, we demonstrate that gO does not undergo the same posttranslational processing in transfected cells as it does in infected cells. We further determined that gO is modified by O-linked glycosylation and that this terminally processed form is highly enriched in virions. However, during studies of gO processing, novel gO complexes were discovered in CMV virions. The newly identified gO complexes, including gO-gL heterodimers, were not readily detected in CMV-infected cells. Further characterization of the trafficking of gO through the secretory pathway of infected cells localized gH, gL, and gO primarily to the Golgi apparatus and trans-Golgi network, supporting the conclusion that the novel virion-associated gO complexes arise in a post-Golgi compartment of infected cells.
Human cytomegalovirus (CMV) causes serious disease in the immunocompromised human host. Because CMV infects multiple cells and tissues, CMV disease is manifested in many ways, including birth defects, retinitis, and pneumonitis (16). The broad cellular tropism of this virus stems from the wide array of glycoproteins studding its envelope and allowing entry into nearly all human cell types (2). A member of the Herpesviridae family, CMV is a large, enveloped virus with a double-stranded linear DNA genome. The CMV genome contains over 200 open reading frames and is enclosed within an icosahedral nucleocapsid (36).
As many as 50 of these open reading frames are predicted to encode glycoproteins, and many have been identified as virion structural proteins (7). At least six of these glycoproteins are known to be involved in cell entry of CMV in tissue culture systems (5, 10, 17, 23, 27, 28, 44). A viral glycoprotein complex composed of glycoproteins H, L, and O (gH, gL, and gO, respectively) is necessary for the final stage of virus entry—pH-independent fusion of the viral envelope with the host cell plasma membrane (9, 23, 29). gH and gL form heterodimers which are conserved throughout the Herpesviridae (22, 41). gO, however, was only recently characterized as a member of this complex in CMV and is unique to the betaherpesvirus subfamily (19). The disulfide-bonded tripartite gH-gL-gO complex undergoes multiple stages of assembly and posttranslational processing prior to being displayed on the surface of infected cells (20).
The current work describes posttranslational processing and trafficking of multiple forms of gO, which can be found in various stages of assembly, depending on the context of expression. In cells transfected with a gO expression plasmid, gO does not acquire the same complex sugar modifications as are found in infected cells. This finding is consistent with the results of previous studies of CMV gH showing that it also does not acquire Golgi-associated modifications in the absence of gL (22, 41). Consistent with its incomplete processing, the electrophoretic mobility of gO seen in transfected cells also differs from that observed in CMV-infected cells. A comparison of cell-associated forms of gO with those found in virions reveals novel forms of gO in CMV virions, including gO-gL heterodimers. Trafficking of gO through the cell was examined by analysis of carbohydrate modifications as well as indirect immunofluorescence studies. Our data show that gO is found in multiple compartments of the secretory pathway in CMV-infected cells but accumulates in the Golgi apparatus and trans-Golgi network (TGN), along with gH and gL. Therefore, the virion-specific forms of gO likely arise in a post-Golgi region of infected cells or after viral egress.
MATERIALS AND METHODS
Cells and viruses.
Human foreskin fibroblasts and immortalized fibroblasts were cultured as previously described (8). HCMV strain AD169 was propagated and titers were determined as previously described (8). AD169 virions were purified by pelleting extracellular virions through a 20% sorbitol cushion. Virions were then resuspended in Tris-buffered saline-100 μg of bacitracin/ml and applied to discontinuous sorbitol gradients. As previously demonstrated (9), infectious virions accumulated at the 50 to 60% sorbitol interface after centrifugation at 21,000 rpm in a Beckman SW41 rotor for 60 min at 18°C. Virions were aspirated from the interface with a pipette and concentrated by centrifugation at 19,000 rpm in a Sorvall ss34 rotor. Infectious virions purified by this method were assessed for purity by electron microscopy as described previously (9).
293-T cells were grown in Dulbecco modified Eagle medium supplemented with 1% Pen/Strep Fungizone (PSF), 0.3% glutamine, and 10% fetal bovine serum (Harlan Biosciences).
Antibodies.
UL74 antiserum (recognizing gO) was previously described (19). Antipeptide rabbit polyclonal antibody 6824 (recognizing gH) was also previously described (18). Anti-gL rabbit polyclonal antiserum 26388 was donated by A. Minson (18), and monoclonal antibodies 27-78 (glycoprotein B) and AP86 (gH) were gifts from W. Britt (6, 46). Rabbit antibody to calreticulin was purchased from Stressgen, mouse antibody to β-COP was obtained from Sigma, and mouse antibody to TGN38 was obtained from BD Biosciences. gH monoclonal antibody 1G6 (34) was generously donated by L. Rasmussen.
Plasmid transfections.
Plasmid DNA was purified by polyethylene glycol precipitation (37). Cells were transfected by using genePorter lipid transfection reagent (Gene Transfer Systems) according to the manufacturer's instructions. The gO expression plasmid, pCaggs.gO, was generated by PCR amplification of the full-length gO coding region by using primers 5′GGAATTCACCATGGGGAGAAAAGAGATG3′ and 5′AAACCGCTCGAGTTACTGCAACCACCA3′. The product was cut with EcoRI (5′) and XhoI (3′) and was inserted into the multiple cloning site of vector pCaggs (donated by Y. Kawaoka, University of Wisconsin).
Immunoblotting.
Sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) and immunoblotting were performed essentially as previously described (18, 19). Briefly, nitrocellulose membranes were washed in Tris-buffered saline containing 0.05% SDS, 0.5% Tween 20, and 20 mg of powdered skim milk/ml (wash buffer). Horseradish peroxidase-conjugated secondary antibodies were purchased from Pierce, and activity was detected by using Renaissance enhanced chemiluminescence reagent (New England Nuclear). Immunoblots probed with UL74 antiserum were hybridized and washed in the presence of wash buffer and additional cell lysate (100 mM dish-confluent 293-T, cos-7, or immortalized fibroblast cells lysed in 1 ml of 1% SDS).
Carbohydrate analysis.
Endoglycosidase H (endo H), peptide N-glycosidase F (PNGase), and neuraminidase were purchased from New England Biolabs and used according to the manufacturer's instructions. Jacalin-conjugated agarose beads (Sigma) were equilibrated in radioimmunoprecipitation assay (RIPA) medium-0.5% bovine serum albumin (BSA; Sigma) prior to use. For the pulldown experiments, virions were solubilized in RIPA medium-0.5% BSA-protease inhibitor cocktail, precleared with streptavidin-agarose beads (Vector Laboratories), and incubated overnight at 4°C with streptavidin or jacalin beads. Beads were washed twice in RIPA medium-BSA and then twice in RIPA medium. Proteins were eluted by boiling in SDS-PAGE sample buffer (containing a final concentration of 2% SDS) prior to SDS-PAGE and immunoblotting.
Immunofluorescence.
Human fibroblasts grown on glass coverslips were infected with CMV strain AD169 at a multiplicity of infection of 0.5. Six days postinfection, they were fixed in 3% paraformaldehyde-phosphate-buffered saline (PBS) and permeabilized in 1% Triton X-100-PBS. A blocking step was performed with 20% normal goat serum diluted in PBS-0.2% gelatin. Primary antibodies were diluted in PBS-0.2% gelatin-2% normal goat serum, and secondary antibodies (rhodamine-conjugated goat anti-rabbit [Pierce] and fluorescein isothiocyanate-conjugated goat anti-mouse [Molecular Probes]) were diluted in the same solution with the addition of Hoechst stain. After antibody incubations, cells were washed with PBS-0.2% gelatin-0.2% Tween 20. Coverslips were inverted onto a small drop of Gelvatol fixative on glass slides. Images were viewed and captured on a Zeiss Axiophot fluorescence microscope equipped with a digital camera.
RESULTS
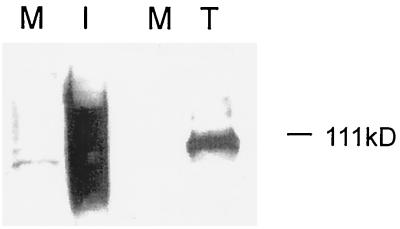
It was recently demonstrated that gO, although a soluble protein in transfected cells, showed membrane association in CMV-infected cells (42). During studies of transfected cells expressing gO, it was evident that the electrophoretic mobility of gO differed from that in CMV-infected cells (Fig. 1). The form of gO observed in transfected cells resembled a previously characterized precursor form of gO, pgO (20), and studies showed that gO was endo H sensitive in transfected cells (42). Pulse-chase studies of transiently expressed gO also showed complete endo H sensitivity of gO as late as 4 h postchase (data not shown). In pulse-chase studies of infected cells, however, gO gained partial endo H resistance by 4 h postchase (20), indicating that it had trafficked beyond the medial Golgi apparatus (25, 30, 31).
FIG. 1.
Electrophoretic mobilities of different forms of gO. CMV-infected human fibroblasts and transfected cells expressing gO were lysed and analyzed by SDS-PAGE followed by immunoblotting with gO antibody. M, mock-infected cells; I, infected human fibroblasts; T, transfected 293-T cells expressing gO.
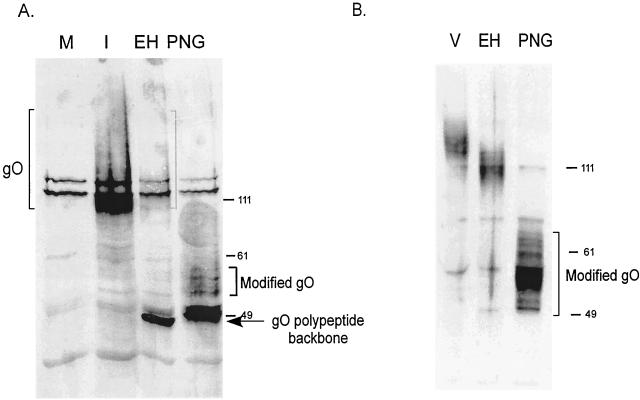
For a steady-state comparison of gO in infected cells, CMV-infected cells were harvested 7 days postinfection, lysed, and digested with endo H. As shown in Fig. 2A, the majority of gO was deglycosylated to the peptide backbone size of approximately 48 kDa. The endo H-resistant population was diffusely migrating, and after complete N deglycosylation with PNGase, this population of gO migrated above the peptide backbone size. This form of gO is thought to bear a non-N-glycans unidentified posttranslational modification acquired late during processing. This modification caused an increase in the size of the endo H-resistant form of gO (120 kDa) compared to that of the endo H-sensitive pgO (100 kDa) (20). As shown in Fig. 1, transfected cells produced only pgO, indicating incomplete processing of gO when expressed without other viral proteins.
FIG. 2.
Endo H digestion of gO. (A) Mock-infected cells (M) or CMV-infected cells were harvested 7 days postinfection and then treated with no enzyme (I), endo H (EH), or PNGase (PNG). Proteins were analyzed by SDS-PAGE and immunoblotting with gO antibody. The diffusely migrating, endo H-resistant form of gO is indicated by brackets. (B) Solubilized CMV virions were mock digested (V), treated with endo H (EH), or digested with PNGase (PNG). Proteins were analyzed as described for panel A. Numbers beside panels show kilodaltons.
To compare the steady-state pool of gO present in infected cells to the final form found in virions, virions purified from media of infected cells were similarly analyzed. Figure 2B shows that gO found in CMV virions was largely endo H resistant, with a moderate shift in electrophoretic mobility compared to the large shift seen in infected cells. In addition, the majority of virion gO had acquired the unknown modification, as indicated by its migration above the 49-kDa marker after PNGase treatment. These experiments verified that the unidentified gO modification correlated with endo H resistance and thus was acquired after exit from the endoplasmic reticulum (ER). They also raised questions, however, regarding the additional stages of glycoprotein processing which occurred between the intracellular accumulation of gO (Fig. 2A) and the egress of virions.
The unknown modification in gO was the first and most obvious difference noted between pgO and gO. The modification was most striking in virion-associated gO, so we examined virions in an effort to identify the modification. gO contains a region rich in serine, threonine, and proline residues that may serve as a linkage site for O-glycans (7, 19). Studies with a single O-glycanase failed to detect O-glycans on gO (20). However, due to the specificity of the enzyme and the diversity of O-glycans, enzymatic characterization of O glycosylation is unreliable when negative results are obtained. Thus, given the diffuse nature of the mobility shift in gO, we hypothesized that the most likely Golgi-associated modification was O glycosylation.
As an experimental alternative to O-glycanase digestion, jackfruit lectin (jacalin), which binds specifically to the galactose core of O-glycans (1, 39), was tested for its ability to interact with gO. Solubilized, reduced, PNGase-treated virions were incubated with jacalin-conjugated agarose beads. PNGase treatment ensured that lectin binding was not due to the presence of galactose-decorated N-glycans (12). Proteins were eluted from the beads and analyzed by immunoblotting with antibodies to gO. As shown in Fig. 3, the diffusely migrating N-deglycosylated gO bound to the lectin beads but not to control agarose beads. Analysis of proteins bound under nonreducing conditions and in the absence of PNGase treatment revealed that the population of gO that bound to the lectin beads was found only in high-molecular-weight gH-gL-gO complexes. This analysis revealed another finding shown in Fig. 3B: several uncharacterized forms of gO were found in virions in addition to the O-glycosylated tripartite complex. The additional forms did not bind jacalin, suggesting that O glycosylation of gO occurred only when it was complexed with gH and gL. Another interpretation is that only gO containing O-glycans was incorporated into tripartite complexes, while other forms of gO were excluded from gH-gL-gO complexes.
FIG. 3.
Analysis of O-linked carbohydrate on gO and gO complexes. Solubilized virions were incubated overnight with jacalin-conjugated agarose beads. After washing was done, bound proteins were eluted from the beads and analyzed by SDS-PAGE and immunoblotting with gO antibody. V, total solubilized virion proteins; A, proteins eluted from control streptavidin-agarose beads; J, proteins eluted from jacalin-agarose beads. (A) Prior to incubation with beads, virion proteins were boiled, reduced, and treated with PNGase to remove N-glycans. (B) Unreduced, fully glycosylated virion proteins were incubated with jacalin beads. Arrows indicate gH-gL-gO complexes, and arrowheads indicate previously unidentified forms of gO. Numbers beside panels show kilodaltons.
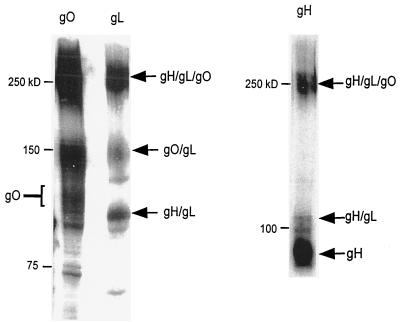
As noted above, the analysis of virion-associated gO revealed the presence of two previously uncharacterized forms of the protein (Fig. 3B). The 150-kDa gO species and the 50-kDa species were not detected in immunoblots of virions under reducing conditions (Fig. 2B), suggesting that they were comprised of disulfide-bonded components. Further analysis showed that the 150-kDa complex reacted with antibody to gL (Fig. 4) but not with monoclonal or polyclonal antibody to gH (data not shown). We concluded that the 150-kDa complex was most likely composed of 125-kDa gO disulfide bonded to 34-kDa gL. This experiment also verified the presence of monomeric gH and gO as well as gH-gL heterodimers in purified CMV virions (26). The identity of the 50-kDa species has not yet been determined, but it may represent a proteolytic cleavage product of gO or of gO complexes.
FIG. 4.
gO-gL heterodimers identified by immunoblotting. Virion proteins were solubilized and subjected to nonreducing SDS-PAGE followed by immunoblotting. Strips of the same membrane were blotted with antibodies to gO and gL. gH complexes were characterized by blotting with monoclonal antibody AP86.
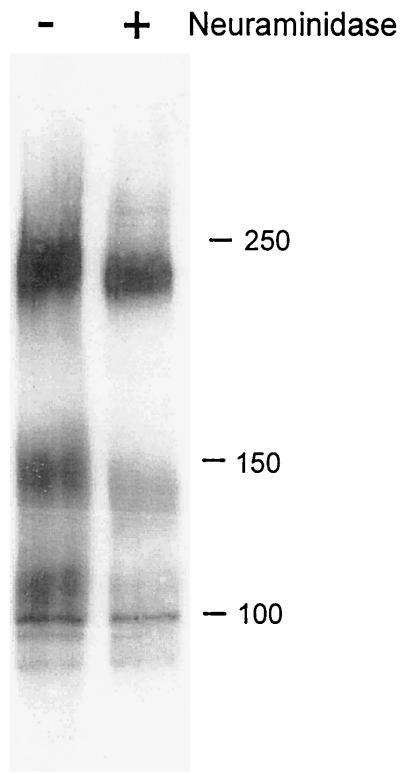
Because the novel gO complexes were not detected in analyses of infected cells (19, 20), we hypothesized that their trafficking and/or posttranslational modification may differ from that of previously characterized complexes. To address this notion, the gO complexes in virions were analyzed to determine the extent of processing of their N-glycans. All of the complexes were largely endo H resistant (data not shown) and contained sialic acid, as indicated by their mobility shift after neuraminidase treatment (Fig. 5). Earlier studies of neuraminidase-treated gO from infected cells showed no mobility shift (20). Collectively, these data indicate that a large part of the steady-state pool of gO accumulated in the infected cell has not acquired terminal carbohydrate modifications but that virion-associated gO has undergone mannose trimming and sialic acid addition.
FIG. 5.
Glycosidase treatment of multimeric complexes. CMV virions were either incubated with neuraminidase (+) or mock digested (−) and then subjected to nonreducing SDS-PAGE and immunoblotting with gO antibody. Numbers beside panels show kilodaltons.
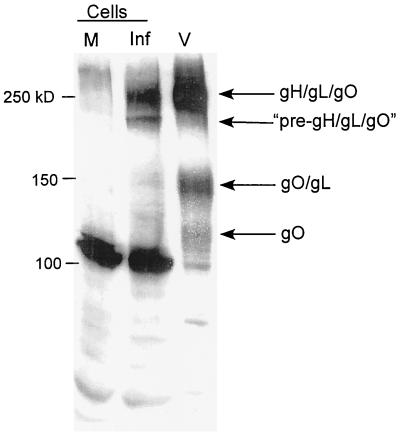
Only monomeric gO and the tripartite complex had been observed previously in CMV-infected cells (20). To directly examine differences between cell-associated and virion-associated gO complexes, infected cells and virions were solubilized under nonreducing conditions and analyzed by immunoblotting. The 240-kDa tripartite gH-gL-gO complex in both the immature endo H-sensitive precursor form and the mature endo H-resistant form (20) was abundant in infected-cell lysates (Fig. 6). Little monomeric gO was found, however, and almost no gO-gL heterodimers could be detected in infected cells. In contrast, CMV virions contained monomeric gO and gO-gL heterodimers, in addition to mature gH-gL-gO complexes. Because the novel virion-associated complexes were not abundant in infected cells, we postulated that they arose late in the secretory pathway, after the point of gH-gL-gO accumulation. These data, combined with the pulse-chase data from infected cells (20), suggest that the novel complexes arise by rearrangement or proteolytic cleavage of previously observed forms of gH-gL-gO.
FIG. 6.
Comparison of gO complexes in infected cells and virions. Mock-infected cells (M) and CMV-infected cells (Inf) were directly compared to CMV virions (V) by immunoblotting of solubilized, unreduced samples with gO antibody. Cells and purified virions were solubilized in RIPA medium prior to the addition of SDS-PAGE sample buffer.
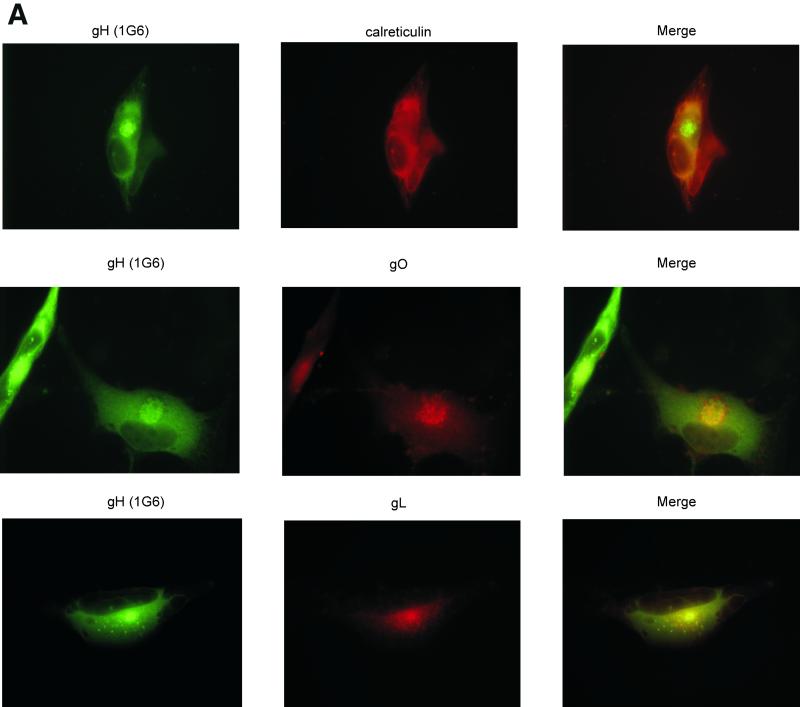
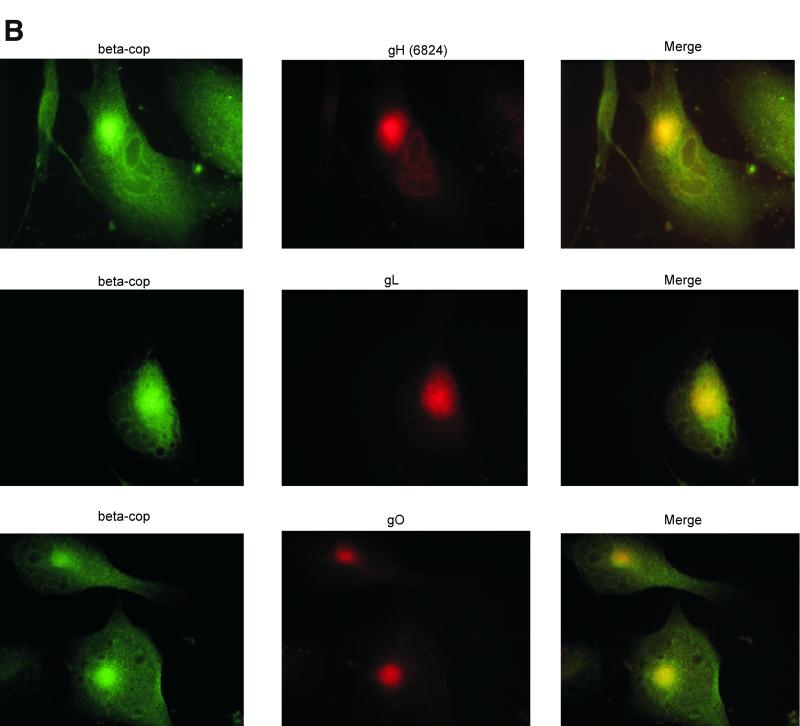
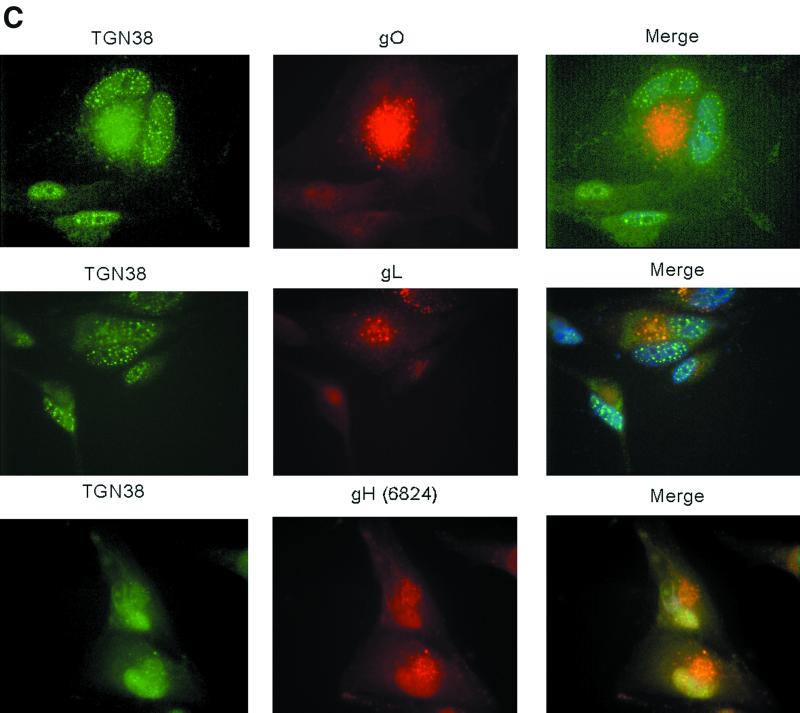
Thus, to better localize the site of intracellular accumulation of gO, gH, and gL, we conducted a series of indirect immunofluorescence studies. Other reports localized intracellular gH to a juxtanuclear compartment (11, 35, 38, 45), and our studies yielded similar results (Fig. 7A). The gO and gL antibodies colocalized with gH monoclonal antibody 1G6 in juxtanuclear regions of infected cells. Little overlap was observed when these antibodies were used in conjunction with an antibody to calreticulin, an ER marker (Fig. 7A and data not shown), indicating that the glycoprotein complexes were largely found in post-ER regions. Additional colocalization studies with an antibody to β-COP, a Golgi apparatus marker (32), showed significant overlap between the Golgi apparatus and gH, gL, and gO (Fig. 7B). Experiments with a marker for vesicles of the TGN showed that gH, gL, and gO were found in punctate compartments that colocalized with the TGN (Fig. 7C) (21, 33). Only a subset of TGN38 vesicles colocalized with gL and gO, but gH showed complete colocalization in the juxtanuclear area and in what appeared to be the nuclear envelope. All three proteins were also detected on the surface of nonpermeabilized cells (data not shown), in accordance with previous studies (11, 20, 26). These immunofluorescence observations provided additional evidence for the accumulation of gO, gH, and gL in a Golgi apparatus-derived compartment of CMV-infected cells. The three proteins also trafficked in smaller amounts beyond the TGN to appear on the cell surface.
FIG. 7.
Immunofluorescence colocalization of gH, gL, and gO. Human fibroblasts were infected with CMV at a multiplicity of infection of approximately 0.5 and harvested 6 days postinfection. Cells were fixed and stained for indirect immunofluorescence; each horizontal series represents a single field. Virus-specific rabbit antibodies (middle columns) were detected with rhodamine-conjugated goat anti-rabbit immunoglobulin G (IgG). Monoclonal antibody 1G6 (anti-gH) (A), an β-COP monoclonal antibody (B), and a TGN38 monoclonal antibody (C) were detected with fluorescein isothiocyanate-conjugated goat anti-mouse IgG (left columns). Nuclei were stained with Hoechst dye (blue). The right columns represent the merged images.
DISCUSSION
For many years it has been known that herpesvirus gH homologs do not traffic correctly when expressed alone but that coexpression with gL allows proper trafficking and processing of gH (11, 34, 41). Thus, it was not surprising to find that gO, the third component of the tripartite gH-gL-gO complex, does not undergo normal posttranslational processing when expressed alone. Experiments are under way to analyze the trafficking and posttranslational modification of gO after in vitro reconstitution of tripartite gH-gL-gO complexes (24). The high level of endo H sensitivity of gO in CMV-infected cells, as well as the presence of multiple forms of gO in the cells, correlated with earlier data regarding the kinetics of processing of gH (4). Bogner et al. (4) identified several forms of gH in CMV-infected cells and virions, one of which was endo H sensitive and localized to the nuclear envelope. Our data did not support selective retention of gO in the nuclear envelope. However, immunofluorescence studies with one antibody, 6824, localized gH to the nuclear membrane of infected cells. The implications of gH trafficking to the nucleus were not clear.
What did become clear during the course of these experiments was the extensive processing of glycoproteins that occurs between their intracellular accumulation and their presence in free virus. Differences in gH processing between infected cells and virions were observed previously (4), with endo H resistance of some intracellular gH and of all virion-associated gH. The same pattern of differential endo H sensitivity was observed for gO, suggesting that intracellular glycoproteins are distributed throughout the secretory pathway, with some having undergone medial Golgi modifications. More unexpected, however, was the appearance of entirely new virion-associated gO complexes that were not previously detected in infected cells. The maturation of gH-gL-gO in Golgi and/or post-Golgi compartments, then, not only involves carbohydrate trimming and sialic acid addition but also may include disulfide bond rearrangement and proteolysis.
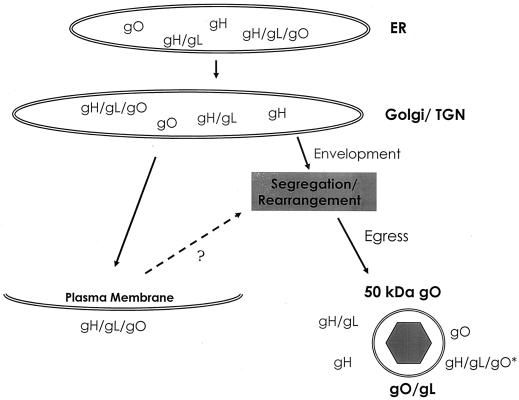
Our model of assembly must account for data showing trafficking of only the tripartite gH-gL-gO complex to the surface of infected cells (20). Earlier experiments detected no smaller forms of gO or gH at the surface of infected cells, ruling out the possibility that gO-gL heterodimers and other virion-associated complexes simply arise in the TGN or in exocytotic vesicles en route to the cell surface. In fact, our model must now explain the presence of gH, gH-gL, gO-gL, gO, and 50-kDa gO—none of which reaches the cell surface—in the viral envelope (Fig. 8). The colocalization of gH, gL, and gO in the Golgi apparatus and TGN vesicles leads to the conclusion that virion-specific forms of gO arise after passage through the TGN. If they are formed prior to or within the TGN, they should be abundant in lysates of infected cells.
FIG. 8.
Model of gH-gL-gO complex assembly and trafficking in CMV-infected cells. The appearance of various gH, gL, and gO complexes is illustrated temporally from top to bottom. Proteins denoted with a slash (e.g., gH/gL) are held together by disulfide linkages. The glycoproteins are cotranslationally inserted into the ER. Subsequently, glycosylated proteins travel to the Golgi apparatus and then to the TGN. gH, gL, and gO accumulate in Golgi apparatus-derived vesicles, although some gH-gL-gO appears on the surface of infected cells. Cell surface tripartite complexes may or may not recycle via endocytosis (?). Multiple TGN-derived complexes likely incorporate into virions during envelopment. Rearrangement of gH-gL-gO complexes results in the emergence of previously undetected forms of gO, including gO-gL heterodimers. Subsequent events in viral egress are not well characterized. Bold letters indicate gO complexes that arise between the Golgi/TGN and the point of virion collection. gH-gL-gO∗, tripartite complex in which gO contains O-glycans.
A kinetic model of gH-gL-gO complex trafficking asserts that exit from the Golgi apparatus must occur more slowly than entrance into it, thus allowing for the accumulation of proteins observed by immunofluorescence and by carbohydrate analysis (Fig. 8). In this model, endo H-resistant gH-gL-gO complexes leave the TGN and traffic to the plasma membrane. Once there, the fate of the complexes is unknown. Like glycoprotein B, the gH-gL-gO complex could undergo endocytosis and recycle back into the TGN via endosomes (43). As the gH-gL-gO complex promotes cell-cell fusion (29), it could also reside indefinitely on the cell surface. The complex could undergo endocytosis and lysosomal degradation. Currently, no data are available regarding the ultimate fate of gH-gL-gO displayed on the cell surface. Finally, if tripartite gH-gL-gO is the source of gO-gL heterodimers and 50-kDa gO, then it must consist of a mixture of forms with and without O-glycans. The absence of O-glycans on the novel gO complexes also suggests potential selection or segregation of the variously modified forms of gO, with rearrangement of non-O-glycosylated complexes occurring at some stage of virion assembly.
Data are accumulating to support a model of herpesvirus envelopment at vesicles of the TGN (13-15, 38, 40). Thus, glycoproteins and lipids accumulating in the TGN constitute the viral envelope, and viral egress occurs by subsequent fusion of vesicles with the plasma membrane. We have observed the accumulation of three viral glycoproteins in the Golgi/TGN, in agreement with this model (38). The site of envelopment, however, is not necessarily the site at which the novel gO complexes arise. They could arise at any point beyond the TGN—even during or after viral egress, with the exception of vesicles carrying tripartite complexes to the plasma membrane. Envelope glycoprotein gp120 of human immunodeficiency virus is known to undergo proteolytic processing in a post-TGN division of either the constitutive or the regulated secretory pathway (3, 47). A similar mechanism may underlie the appearance of the 50-kDa gO complex, which most likely is a proteolytic fragment of 125-kDa gO and appears in a post-Golgi compartment. Figure 8 summarizes the forms of gH-gL-gO found at various stages of trafficking and assembly.
This work has verified the presence of four different disulfide-linked forms of gH, gL, and gO complexes in gradient-purified CMV virions (Fig. 8). In addition, we have demonstrated that monomeric gH and gO are incorporated into the viral envelope. This new assembly model necessitates a reevaluation of envelopment. Because multiple forms of gH, gL, and gO are incorporated into virions, the quality control mechanisms of virion assembly may be inefficient. Perhaps the large number of viral glycoproteins produced by infected cells overwhelms the processing and quality control mechanisms in the secretory pathway, resulting in error-prone assembly of glycoproteins and nonspecific incorporation into the viral envelope.
An alternative interpretation of the data considers that each form of the gH-gL-gO complex plays a specific functional role in the viral life cycle. Thus, trafficking of only tripartite complexes to the plasma membrane may occur because only tripartite complexes are necessary to mediate cell-cell fusion. As a corollary, perhaps selective O glycosylation of tripartite complexes facilitates targeting to the cell surface. Enrichment of gO-gL heterodimers in virions similarly implies that they function specifically in the context of the viral envelope. Finally, the data further support the notion that the complement of glycoproteins mediating viral entry may not be identical to those necessary for cell-cell fusion.
Acknowledgments
This research was funded by U.S. Public Health Service grant RO1 A144203. Support for R.N.T. was provided by Molecular Biosciences training grant T32 GM 07215.
R.N.T. thanks Mary Huber for invaluable technical and intellectual contributions to this work. We also thank Rebecca Montgomery for helpful discussions.
REFERENCES
- 1.Ahmed, H., and B. P. Chatterjee. 1989. Further characterization and immunochemical studies on the carbohydrate specificity of jackfruit (Artocarpus integrifolia) lectin. J. Biol. Chem. 264:9365-9372. [PubMed]
- 2.Alford, C. A., and W. J. Britt. 1993. Cytomegalovirus, p. 227-255. In B. Roizman, R. J. Whitley, and C. Lopez (ed.), Human herpesviruses. Raven Press, New York, N.Y.
- 3.Bendjennat, M., B. Bahbouhi, and E. Bahraoui. 2001. Purification and characterization of a Ca2+-independent endoprotease activity from peripheral blood lymphocytes: involvement in HIV-1 gp160 maturation. Biochemistry 40:4800-4810. [DOI] [PubMed] [Google Scholar]
- 4.Bogner, E., M. Reschke, B. Reis, E. Reis, W. Britt, and K. Radsak. 1992. Recognition of compartmentalized intracellular analogs of glycoprotein H of human cytomegalovirus. Arch. Virol. 126:67-80. [DOI] [PubMed] [Google Scholar]
- 5.Boyle, K. A., and T. Compton. 1998. Receptor-binding properties of a soluble form of human cytomegalovirus glycoprotein B. J. Virol. 72:1826-1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Britt, W. J., L. Vulger, E. J. Butfiloski, and E. B. Stephens. 1990. Cell surface expression of human cytomegalovirus (HCMV) gp55-116 (gB): use of HCMV-recombinant vaccinia virus-infected cells in analysis of the human neutralizing antibody response. J. Virol. 64:1079-1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chee, M. S., A. T. Bankier, S. Beck, R. Bohni, C. M. Brown, R. Cerny, T. Horsnell, C. A. Hutchison, I. I. I., T. Kouzarides, J. A. Martignetti, E. Preddie, S. C. Satchwell, P. Tomlinson, K. M. Weston, and B. G. Barrell. 1990. Analysis of the protein-coding content of the sequence of human cytomegalovirus strain AD169. Curr. Top. Microbiol. Immunol. 154:125-169. [DOI] [PubMed] [Google Scholar]
- 8.Compton, T. 1993. An immortalized human fibroblast cell line is permissive for human cytomegalovirus infection. J. Virol. 67:3644-3648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Compton, T., R. R. Nepomuceno, and D. M. Nowlin. 1992. Human cytomegalovirus penetrates host cells by pH-independent fusion at the cell surface. Virology 191:387-395. [DOI] [PubMed] [Google Scholar]
- 10.Cranage, M. P., T. Kouzarides, A. T. Bankier, S. Satchwell, K. Weston, P. Tomlinson, B. Barrell, H. Hart, S. E. Bell, A. C. Minson, et al. 1986. Identification of the human cytomegalovirus glycoprotein B gene and induction of neutralizing antibodies via its expression in recombinant vaccinia virus. EMBO J. 5:3057-3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cranage, M. P., G. L. Smith, S. E. Bell, H. Hart, C. Brown, A. T. Bankier, P. Tomlinson, B. G. Barrell, and T. C. Minson. 1988. Identification and expression of a human cytomegalovirus glycoprotein with homology to the Epstein-Barr virus BXLF2 product, varicella-zoster virus gpIII, and herpes simplex virus type 1 glycoprotein H. J. Virol. 62:1416-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Do, S. I., and K. Y. Lee. 1998. Jacalin interacts with Asn-linked glycopeptides containing multi-antennary oligosaccharide structure with terminal alpha-linked galactose. FEBS Lett. 421:169-173. [DOI] [PubMed] [Google Scholar]
- 13.Eggers, M., E. Bogner, B. Agricola, H. F. Kern, and K. Radsak. 1992. Inhibition of human cytomegalovirus maturation by brefeldin A. J. Gen. Virol. 73:2679-2692. [DOI] [PubMed] [Google Scholar]
- 14.Granzow, H., B. Klupp, W. Fuchs, J. Veits, N. Osterrieder, and T. Mettenleiter. 2001. Egress of alphaherperviruses: comparative ultrastructural study. J. Virol. 75:3675-3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harley, C., A. Dasgupta, and D. Wilson. 2001. Characterization of herpes simplex virus-containing organelles by subcellular fractionation: role for organelle acidification in assembly of infectious particles. J. Virol. 75:1236-1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hirsch, M. 1998. Cytomegalovirus and human herpesvirus types 6, 7, and 8. In A. Fauci et al. (ed.), Harrison's principles of internal medicine, 14th ed. McGraw-Hill Book Co., New York, N.Y.
- 17.Hobom, U., W. Brune, M. Messerle, G. Hahn, and U. H. Koszinowski. 2000. Fast screening procedures for random transposon libraries of cloned herpesvirus genomes: mutational analysis of human cytomegalovirus envelope glycoprotein genes. J. Virol. 74:7720-7729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huber, M. T., and T. Compton. 1997. Characterization of a novel third member of the human cytomegalovirus glycoprotein H-glycoprotein L complex. J. Virol. 71:5391-5398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huber, M. T., and T. Compton. 1998. The human cytomegalvirus UL74 gene encodes the third component of the glycoprotein H-glycoprotein L-containing envelope complex. J. Virol. 72:8191-8197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huber, M. T., and T. Compton. 1999. Intracellular formation and processing of the heterotrimeric gH-gL-gO (gCIII) glycoprotein envelope complex of human cytomegalovirus. J. Virol. 73:3886-3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kain, R., K. Angata, D. Kerjaschki, and M. Fukuda. 1998. Molecular cloning and expression of a novel human trans-Golgi network glycoprotein, TGN51, that contains multiple tyrosine-containing motifs. J. Biol. Chem. 273:981-988. [DOI] [PubMed] [Google Scholar]
- 22.Kaye, J. F., U. A. Gompels, and A. C. Minson. 1992. Glycoprotein H of human cytomegalovirus (HCMV) forms a stable complex with the HCMV UL115 gene product. J. Gen. Virol. 73:2693-2698. [DOI] [PubMed] [Google Scholar]
- 23.Keay, S., and B. Baldwin. 1991. Anti-idiotype antibodies that mimic gp86 of human cytomegalovirus inhibit viral fusion but not attachment. J. Virol. 65:5124-5128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kinzler, E., R. Theiler, and T. Compton. Expression and reconstitution of the gH/gL/gO complex of human cytomegalovirus. J. Clin. Virol., in press. [DOI] [PubMed]
- 25.Kornfeld, R., and S. Kornfeld. 1985. Assembly of asparagine-linked oligosaccharides. Ann. Rev. Biochem. 54:631-664. [DOI] [PubMed] [Google Scholar]
- 26.Li, L., J. A. Nelson, and W. J. Britt. 1997. Glycoprotein H-related complexes of human cytomegalovirus: identification of a third protein in the gCIII complex. J. Virol. 71:3090-3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mach, M., B. Kropff, P. dal Monte, and W. Britt. 2000. Complex formation by human cytomegalovirus glycoproteins M (gpUL100) and N (gpUL73). J. Virol. 74:11881-11892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marshall, G. S., G. P. Rabalais, G. G. Stout, and S. L. Waldeyer. 1992. Antibodies to recombinant-derived glycoprotein B after natural human cytomegalovirus infection correlate with neutralizing activity. J. Infect. Dis. 165:381-384. [DOI] [PubMed] [Google Scholar]
- 29.Milne, R. S., D. A. Paterson, and J. C. Booth. 1998. Human cytomegalovirus glycoprotein H/glycoprotein L complex modulates fusion-from-without. J. Gen. Virol. 79:855-865. [DOI] [PubMed] [Google Scholar]
- 30.Mottola, G., N. Jourdan, G. Castaldo, N. Malagolini, A. Lahm, F. Serafini-Cessi, G. Migliaccio, and S. Bonatti. 2000. A new determinant of endoplasmic reticulum localization is contained in the juxtamembrane region of the ectodomain of hepatitis C virus glycoprotein E1. J. Biol. Chem. 275:24070-24079. [DOI] [PubMed] [Google Scholar]
- 31.Naim, H. Y., G. Joberty, M. Alfalah, and R. Jacob. 1999. Temporal association of the N- and O-linked glycosylation events and their implication in the polarized sorting of intestinal brush border sucrase-isomaltase, aminopeptidase N, and dipeptidyl peptidase IV. J. Biol. Chem. 274:17961-17967. [DOI] [PubMed] [Google Scholar]
- 32.Pepperkok, R., J. Scheel, H. Horstmann, H. P. Hauri, G. Griffiths, and T. E. Kreis. 1993. Beta-COP is essential for biosynthetic membrane transport from the endoplasmic reticulum to the Golgi complex in vivo. Cell 74:71-82. [DOI] [PubMed] [Google Scholar]
- 33.Pouli, A. E., H. J. Kennedy, J. G. Schofield, and G. A. Rutter. 1998. Insulin targeting to the regulated secretory pathway after fusion with green fluorescent protein and firefly luciferase. Biochem. J. 331:669-675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rasmussen, L., M. Nelson, M. Neff, and T. J. Merigan. 1988. Characterization of two different human cytomegalovirus glycoproteins which are targets for virus neutralizing antibody. Virology 163:308-318. [DOI] [PubMed] [Google Scholar]
- 35.Rasmussen, L., R. Nelson, D. Kelsall, and T. Merigan. 1984. Murine monoclonal antibody to a single protein neutralizes the infectivity of human cytomegalovirus. Proc. Natl. Acad. Sci. USA 81:876-880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roizman, B., R. J. Whitley, and C. Lopez (ed.). 1993. Human herpesviruses. Raven Press, New York, N.Y.
- 37.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 38.Sanchez, V., K. Greis, E. Sztul, and W. Britt. 2000. Accumulation of virion tegument and envelope proteins in a stable cytoplasmic compartment during human cytomegalovirus replication: characterization of a potential site of virus assembly. J. Virol. 74:975-986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sastry, M. V., P. Banarjee, S. R. Patanjali, M. J. Swamy, G. V. Swarnalatha, and A. Surolia. 1986. Analysis of saccharide binding to Artocarpus integrifolia lectin reveals specific recognition of T-antigen (beta-d-Gal(1—3)d-GalNAc). J. Biol. Chem. 261:11726-11733. [PubMed] [Google Scholar]
- 40.Severi, B., M. P. Landini, and E. Govoni. 1988. Human cytomegalovirus morphogenesis: an ultrastructural study of the late cytoplasmic phases. Arch. Virol. 98:51-64. [DOI] [PubMed] [Google Scholar]
- 41.Spaete, R. R., K. Perot, P. I. Scott, J. A. Nelson, M. F. Stinski, and C. Pachl. 1993. Coexpression of truncated human cytomegalovirus gH with the UL115 gene product or the truncated human fibroblast growth factor receptor results in the transport of gH to the cell surface. Virology 193:853-861. [DOI] [PubMed] [Google Scholar]
- 42.Theiler, R. N., and T. Compton. 2001. Characterization of the signal peptide processing and membrane association of human cytomegalovirus glycoprotein O. J. Biol. Chem. 276:39226-39231. [DOI] [PubMed] [Google Scholar]
- 43.Tugizov, S., E. Maidji, J. Xiao, and L. Pereira. 1999. An acidic cluster in the cytosolic domain of human cytomegalovirus glycoprotein B is a signal for endocytosis from the plasma membrane. J. Virol. 73:8677-8688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tugizov, S., D. Navarro, P. Paz, Y. L. Wang, I. Qadri, and L. Pereira. 1994. Function of human cytomegalovirus glycoprotein B: syncytium formation in cells constitutively expressing gB is blocked by virus-neutralizing antibodies. Virology 201:263-276. [DOI] [PubMed] [Google Scholar]
- 45.Urban, M., W. Britt, and M. Mach. 1992. The dominant linear neutralizing antibody-binding site of glycoprotein gp86 of human cytomegalovirus is strain specific. J. Virol. 66:1303-1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Urban, M., W. Britt, and M. Mach. 1992. The dominant linear neutralizing antibody-binding site of glycoprotein gp86 of human cytomegalovirus is strain specific. J. Virol. 66:1303-1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vollenweider, F., S. Benjannet, E. Decroly, D. Savaria, C. Lazure, G. Thomas, M. Chretien, and N. G. Seidah. 1996. Comparative cellular processing of the human immunodeficiency virus (HIV-1) envelope glycoprotein gp160 by the mammalian subtilisin/kexin-like convertases. Biochem. J. 314:521-532. [DOI] [PMC free article] [PubMed] [Google Scholar]