Abstract
Dicistronic, selectable subgenomic replicons derived from the Con1 strain of hepatitis C virus (HCV) are capable of autonomous replication in cultured Huh7 cells (Lohmann et al., Science 285:110-113, 1999). However, adaptive mutations in the NS3, NS5A, and/or NS5B proteins are required for efficient replication of these RNAs and increase by orders of magnitude the numbers of G418-resistant colonies selected following transfection of Huh7 cells. Here, we demonstrate that a subgenomic replicon (NNeo/3-5B) derived from an infectious molecular clone of a second genotype 1b virus, HCV-N (Beard et al., Hepatology 30:316-324, 1999) is also capable of efficient replication in Huh7 cells. G418-resistant cells selected following transfection with NNeo/3-5B RNA contained abundant NS5A antigen and HCV RNA detectable by Northern analysis. Replicon RNA in one of three clonally isolated cell lines contained no mutations in the NS3-NS5B polyprotein, confirming that adaptive mutations are not required for efficient replication in these cells. However, the deletion of a unique 4-amino-acid insertion that is present within the interferon sensitivity-determining region (ISDR) of the NS5A protein in wild-type HCV-N drastically decreased the number of G418-resistant colonies obtained following transfection of Huh7 cells. This effect could be reversed by inclusion of a previously described Con1 cell culture-adaptive mutation (S2005→I), confirming that this natural insertion has a controlling role in determining the replication capacity of wild-type HCV-N RNA in Huh7 cells. Additional selectable, dicistronic RNAs encoding NS2-NS5B, E1-NS5B, or the full-length HCV polyprotein were also capable of replication and gave rise to G418-resistant cell clones following transfection of Huh7 cells. We conclude that RNA derived from this documented infectious molecular clone has a unique capacity for replication in Huh7 cells in the absence of additional cell culture-adaptive mutations.
Persistent infection with hepatitis C virus (HCV), a hepatotropic flavivirus, is the most common infectious cause of chronic liver disease in the United States and many other developed countries (1, 19). Patients with chronic hepatitis C are at risk for hepatic fibrosis, potentially culminating in life-threatening hepatic cirrhosis, as well as hepatocellular carcinoma (12, 22, 23). Although much has been learned over the past decade about the organization of the genome and the functions of the proteins it encodes (5, 17), the pace of research on hepatitis C and the development of new therapies for this disease have been slowed by the absence of a permissive cell culture system supporting efficient replication of the virus. The recent description by Lohmann et al. (16) of selectable subgenomic, dicistronic HCV RNA replicons that are capable of autonomous replication in Huh7 cells has thus transformed this field, providing an important new tool for the study of HCV replication mechanisms and allowing new approaches for the discovery and characterization of potential antiviral compounds.
The replicons described by Lohmann et al. (16) were derived from Con1, a genotype 1b strain of HCV (GenBank accession no. AJ238799). The expression of a selectable antibiotic marker, neomycin phosphotransferase (Neo), from the upstream cistron of these dicistronic RNAs under control of the natural HCV internal ribosome entry site (IRES) allowed the selection of stable cell clones containing a substantial abundance of viral RNA and proteins. The downstream cistron, encoding either the NS2-NS5B or NS3-NS5B nonstructural proteins, was placed under the translational control of an IRES derived from the picornavirus, encephalomyocarditis virus (EMCV). Since these HCV replicon RNAs lacked most of the sequence encoding the structural proteins (core and E1, E2, and p7), they were not capable of producing infectious particles, despite their robust replication in Huh7 cells.
Replicon RNAs recovered from G418-resistant cells following the transfection of subgenomic Con1 RNAs have been shown to contain a variety of mutations within the NS3, NS5A, or NS5B sequences that greatly enhance the replication of the Con1 RNA in Huh7 cells (4, 14, 15). Such cell culture-adaptive mutations appear to be required for efficient replication of Con1 replicons and increase the efficiency of selection of G418-resistant cell clones under Neo pressure by several orders of magnitude (4, 14, 15). Interestingly, many of these cell culture-adaptive mutations involve a segment of the NS5A coding region adjacent to the so-called “interferon sensitivity determining region” (ISDR), a genome segment that has been implicated in interferon resistance in human infections (4, 6, 24).
Although other research groups have confirmed the original findings of Lohmann et al. (4, 16), replication-competent replicons have been constructed thus far only from the Con1 viral sequence described in their initial report. Attempts to develop analogous dicistronic and subgenomic RNA replicons from the RNA sequences of other, known infectious cDNA sequences have not succeeded. Specifically, it has not been possible to develop such replicons from an infectious molecular clone of the Hutchinson genotype 1a strain of HCV (HCV-H) (4), despite the fact that RNA derived from this clone is capable of robust replication in vivo following its injection into the livers of chimpanzees. Moreover, only Huh7 cells have been demonstrated to support the replication of the Con1 replicons thus far. The basis for this apparently unique ability of the Con1 RNA sequence to replicate in Huh7 cells remains undefined and is made more obscure by the fact that the ability of this viral sequence to infect permissive chimpanzees in vivo has not yet been documented.
We previously constructed a full-length cDNA clone of a Japanese genotype 1b HCV virus (HCV-N) and demonstrated that RNA transcripts produced from this clone were infectious when inoculated into the liver of a chimpanzee (2). Here, we report the successful construction of replication-competent, selectable dicistronic replicons from this known infectious clone. Unlike the Con1 replicons, we show that adaptive mutations are not required for efficient replication of these HCV-N replicons in Huh7 cells or for the selection of Huh7 clones under G418 selection. We also demonstrate the replication competence of similar selectable, dicistronic RNAs incorporating the NS2-NS5B, E1-NS5B, or complete core-NS5B sequences of this virus. Our findings extend the range of replication-competent HCV replicons to a second, genotype 1b virus and show that a natural 4-amino-acid insertion within the NS5A protein of the wild-type HCV-N virus has a controlling role in determining the replication capacity of this RNA in cultured Huh7 cells.
MATERIALS AND METHODS
Plasmids.
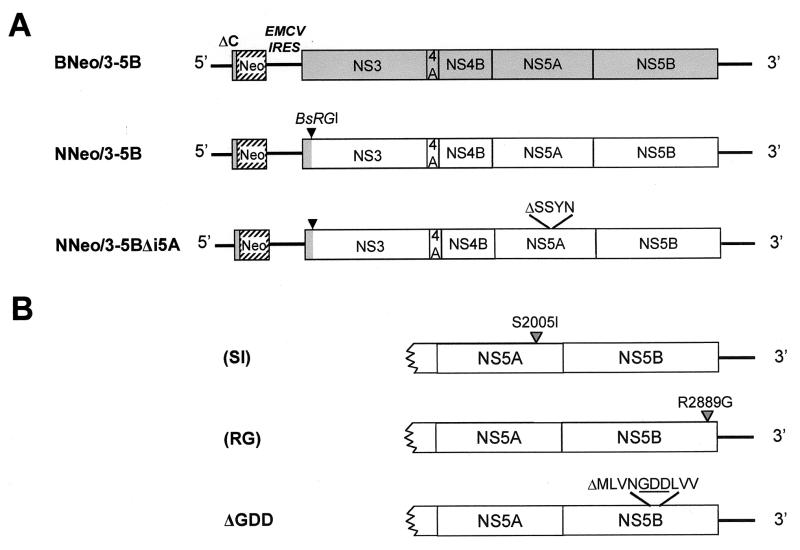
The plasmid pBNeo/3-5B (Fig. 1) contains the Con1 sequence of the I377neo/NS3-3′ replicon of Lohmann et al. (16) (GenBank accession no. AJ238799) downstream of the T7 promoter (Fig. 1). It was kindly provided by Michael Murray of the Schering-Plough Research Institute, Kenilworth, N.J. pNNeo/3-5B (Fig. 1) contains the sequence of a similar HCV replicon in which almost all of the NS3-NS5B sequence comprising the 3′ cistron is derived from an infectious molecular clone of the genotype 1b virus, HCV-N (GenBank accession no. AF139594) (2). It was constructed by replacing the large BsrGI-XbaI fragment of pBNeo/3-5B with the analogous HCV sequence derived from the plasmid pHCV-N. This fragment swap results in the NS3-NS5B sequence in pNNeo/3-5B being identical to that of HCV-N, with the exception of substitutions at 2 amino acid residues that retain the Con1 sequence: a Lys-to-Arg substitution at residue 1053 and an Ala-to-Thr substitution at residue 1099, near the N terminus (proteinase domain) of the NS3 protein. The 5′ untranslated region (′UTR) and N-terminal core protein sequences of HCV-N and the BNeo/3-5B replicon are identical.
FIG. 1.
(A) Organization of the subgenomic HCV RNA replicons employed in these studies. Open reading frames are depicted as boxes, and untranslated segments of the dicistronic RNAs are depicted as solid lines. The sequence of BNeo/3-5B (shaded box) is identical to that of I377NS3-3′/wt, described previously by Lohmann et al. (16). NNeo/3-5B contains mostly HCV-N-derived sequence (open boxes). The amino acid sequence of NS3 in NNeo/3-5B differs from that of HCV-N at only 2 amino acid residues, as described in the text, while the 5′- and 3′UTR sequences are identical. “ΔC” indicates the N-terminal segment of the HCV core protein that is expressed as a fusion with Neo in these replicons. (B) Locations of the S2205I and R2889G BNeo/3-5B-adaptive mutations that were introduced into the replicons shown in panel A.
The mutant pNNeo/3-5BΔi5A (Fig. 1) was derived from pNNeo/3-5B by an in-frame deletion removing a unique 4-amino-acid insertion that is present in the NS5A sequence of HCV-N in comparison to the consensus genotype1b sequence (2). This was accomplished by QuickChange mutagenesis (Stratagene, La Jolla, Calif.). By similar methods, additional mutations were created within the background of pNNeo/3-5B and pNNeo/3-5BΔi5A incorporating single-amino-acid substitutions within NS5A or NS5B that have previously been reported to enhance the replication capacity of the I377/NS3-3′ replicon (BNeo/3-5B) by others: the R2884G mutation described by Lohmann et al. (15), and the S1179I mutation described by Blight et al. (4). These mutations are referred to as R2889G and S2005I, respectively, for the purposes of this study, according to the location of these residues within the original full-length HCV-N polyprotein sequence. The resulting mutants were designated NNeo/3-5B(RG) and NNeo/3-5B(SI). Similar substitutions were introduced into the background of pBNeo/3-5B to generate BNeo/3-5B(RG) and BNeo/3-5B(SI). Two additional mutants, NNeo/3-5BΔGDD and BNeo/3-5BΔGDD, each possess an in-frame deletion of 10 amino acids (MLVNGDDLVV) spanning the GDD motif (underlined) within the NS5B RNA-dependent RNA polymerase of both wild-type replicons. DNA sequencing of the manipulated regions of the plasmids verified all mutations.
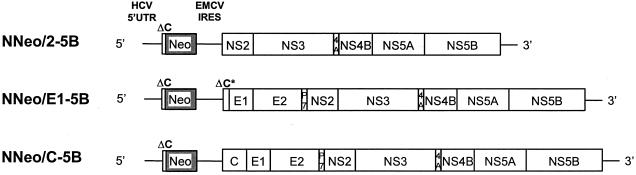
Selectable, dicistronic replicons containing part or all of the HCV-N structural protein-coding sequence within the 3′ cistron were generated as follows. The plasmid pNNeo/C-5B contains the full-length HCV-N polyprotein-coding sequence downstream of the EMCV IRES (see Fig. 8). To construct it, DNA fragments representing the EMCV IRES and HCV core protein-coding sequence were fused by overlapping PCRs. The resulting DNA was digested with RsrII and BstZ17I and then ligated with the XbaI-RsrII fragment of pBNeo/3-5B and the BstZ17I -XbaI fragment of pHCV-N. pNNeo/E1-5B contains sequence encoding the C-terminal 22 amino acids of the core protein, the downstream E1 and E2 sequences and the remainder of the HCV-N polyprotein coding sequence. To construct it, a DNA fragment containing the EMCV sequence was fused to the E1 sequence by an overlapping PCR, digested with RsrII and NotI, and then ligated to the XbaI-RsrII fragment of pBNeo/3-5B and NotI-XbaI fragment of pHCV-N. The 3′ cistron of pNNeo/2-5B contains sequence encoding the NS2-NS5B proteins of HCV-N, immediately downstream of the EMCV IRES. It was constructed in a fashion similar to pNNeo/C-5B and pNNeo/E1-5B, with fusion of the EMCV and NS2 sequences by an overlapping PCR, followed by digestion of the DNA product with RsrII and EcoRV, and ligation to the XbaI-RsrII fragment of pBNeo/3-5B and EcoRV -XbaI fragment from pHCV-N.
FIG. 8.
Organization of selectable dicistronic RNAs containing HCV-N sequence encoding NS2, the envelope proteins E1 and E2, and/or the core protein within the 3′ cistron. UTR, nontranslated region.
Cells.
Huh7 cells were cultured in Dulbecco's modified Eagle's medium (Gibco-BRL, Invitrogen Life Technologies, Carlsbad, Calif.) supplemented with 10% fetal calf serum, penicillin, and streptomycin. Transfected cells supporting the replication of HCV replicons were maintained in the presence of 1 mg of G418 (Geneticin) per ml and passaged two or three times per week at a 4:1 split ratio.
In vitro transcription and transfection of synthetic RNA.
Plasmid DNAs were linearized by XbaI and purified by passage through a column (PCR Purification Kit; Qiagen, Valencia, Calif.) prior to transcription. RNA was synthesized with T7 MEGAScript reagents (Ambion, Austin, Tex.) following the manufacturer's suggested protocol, and the reaction was stopped by digestion with RNase-free DNase. Following precipitation with lithium chloride, RNA was washed with 75% ethanol and dissolved in RNase-free water. For electroporation, Huh7 cells were washed twice with ice-cold phosphate-buffered saline (PBS) and resuspended at 107 cells/ml in PBS. RNA (1 to 10 μg) was mixed with 500 μl of the cell suspension in a cuvette with a gap width of 0. 2 cm (GenePulser II System; Bio-Rad, Hercules, Calif.). The mixture was immediately subjected to two pulses of current at 1.5 kV, 25 μF, and maximum resistance. Following 10 min of incubation at room temperature, the cells were transferred into 9 ml of growth medium and the number of viable cells assessed by staining with trypan blue. Cells were seeded into 10-cm-diameter cell culture dishes. For selection of Neo-expressing cells, the medium was replaced with fresh medium containing 1 mg of G418 per ml after 24 to 48 h in culture.
Indirect immunofluorescence.
Cells were grown on chamber slides until 70 to 80% confluent, washed three times with PBS, and fixed in methanol-acetone (1:1 [vol/vol]) for 10 min at room temperature. Dilutions of primary, murine monoclonal antibodies to residues 1 to 61 of the core protein (MAB7013; Maine Biotechnology Services, Portland) (1:25), E2 (a generous gift from Yoshiharu Matsuura and Tatsuo Miyamura of the National Institute of Health, Tokyo, Japan) (1:400), or NS5A (MAB7022P; Maine Biotechnology Services) (1:10) were prepared in PBS containing 3% bovine serum albumin and incubated with fixed cells for 2 h at room temperature. After additional washes with PBS, specific antibody binding was detected with a goat anti-mouse immunoglobulin G-fluorescein isothiocyanate-conjugated secondary antibody (Sigma-Aldrich, St. Louis, Mo.) diluted 1:70. Cells were washed with PBS, counterstained with 4′, 6′-diamidino-2-phenylindole (DAPI), and mounted in Vectashield mounting medium (Vector Laboratories, Burlingame, Calif.) prior to examination by a Zeiss AxioPlan2 fluorescence microscope.
Northern analysis.
To minimize potential variation in the intracellular abundance of HCV RNAs that might occur due to variation in the growth status of cells, RNA was extracted from freshly plated cultures after cells had reached 70 to 80% confluence. Total cellular RNAs were extracted with TRIzol reagent (Gibco-BRL) and quantified by spectrophotometry at 260 nm. RNAs were separated by denaturing agarose-formaldehyde gel electrophoresis and transferred to positively charged Hybond-N+ nylon membranes (Amersham-Pharmacia Biotec, Piscataway, N.J.) with reagents provided with the NorthernMax kit (Ambion) and the manufacturer's suggested protocol. RNAs were immobilized on the membranes by UV cross-linking (Stratagene) and stained with ethidium bromide to locate 28S rRNA on the membrane. The upper part of the membrane containing HCV replicon RNA (size greater than 28S) was hybridized with a digoxigenin-labeled, negative-sense RNA riboprobe complementary to the NS5B sequence of HCV-N, while the lower part of the membrane containing β-actin mRNA was hybridized with a digoxigenin-labeled, β-actin-specific riboprobe. For detection of the bound riboprobes, membranes were incubated with anti-digoxigenin-alkaline phosphatase conjugate, reacted with CSPD (Roche Molecular Biochemicals, Indianapolis, Ind.), and exposed to X-ray film.
RT-PCR amplification and sequencing of cDNA from replicating HCV RNAs.
Total cellular RNA was extracted from replicon-bearing cell lines as described above and used as a template for the amplification of cDNA fragments spanning the NS3-NS5B segment of the NNeo/3-5B replicon. Reverse transcription (RT) was carried out with 1 μg of RNA, 200 U of SuperScript II reverse transcriptase (Gibco-BRL), and two HCV-specific primers (N6700R, 5′-AGCCTCTTCAGCAGCTG-3′ and N9411R 5′-AGGAAATGGCCTATTGGC-3′, 1 μM), complementary to sequence in the NS4B and 3′UTR segments of the genome, in a total reaction volume of 10 μl for 60 min at 42°C. cDNAs were subsequently amplified with Pfu Turbo DNA polymerase (Stratagene) by 30 PCR cycles involving annealing at 60°C for 60 s, extension at 72°C for 120 s, and denaturation at 95°C for 30s, followed by a final extension reaction at 72°C for 2 min. Eight separate PCR primer sets were used to amplify nested segments spanning the NS3-NS5B region of the genome. The sequence of each amplified cDNA segment was determined directly with an ABI 9600 automatic DNA sequencer. The existence of mutations was confirmed by sequencing the products of at least two separate RT-PCRs.
RESULTS
Autonomous replication of subgenomic HCV replicons derived from HCV-N.
HCV-N is a genotype 1b virus (2) that shares only about 90% nucleotide identity in the NS3-NS5B region with the Con1 sequence present in the replicon RNAs described by Lohmann et al. (16) and Blight et al. (4). To determine whether subgenomic RNAs derived from a previously constructed molecular clone of this virus are capable of replication in Huh7 cells, we constructed a plasmid with a T7 transcriptional unit containing the sequence of a candidate replicon, NNeo/3-5B (Fig. 1). The organization of RNA transcripts generated from this plasmid is identical to that of the I377neo/NS3-3′ replicon of Lohmann et al. (16) (designated BNeo/3-5B in this study), with the 5′UTR of HCV and immediately downstream sequence encoding the N-terminal 12 amino acids of the core protein fused in-frame to the selectable marker, Neo, followed by the IRES of EMCV fused to the NS3-coding sequence and downstream regions of the HCV genome, including the 3′UTR (Fig. 1). The sequences of the proteins expressed by both the 5′ and 3′ cistrons of NNeo/3-5B are identical to those of HCV-N, with the exception of substitutions at 2 amino acid residues near the amino terminus of NS3, a Lys-to-Arg substitution at residue 1053 and an Ala-to-Thr substitution at residue 1099. These substitutions derive from the Con1 sequence employed in construction of this plasmid (see Materials and Methods).
In initial experiments, NNeo/3-5B transcripts were transfected into Huh7 cells, and the cells were grown in the presence of G418 to select cells with active expression of Neo from replicon RNAs undergoing amplification. BNeo/3-5B transcripts were transfected in parallel. As shown in Fig. 2, numerous G418-resistant cell colonies survived the selection process in Huh7 cultures transfected with NNeo/3-5B RNA, with the number of cell colonies isolated proportional to the quantity of RNA electroporated into the cells. However, there were no surviving G418-resistant cell colonies following transfection of NNeo/3-5BΔGDD, a mutated replicon containing an in-frame deletion spanning the GDD motif in the NS5B RNA-dependent RNA polymerase. The absence of surviving cell colonies following transfection of this RNA indicates that amplification of the NNeo/3-5B replicon is essential for G418 resistance. Despite reproducible isolation of greater than 1,000 colonies from cultures transfected with 1 μg of NNeo/3-5B RNA (Fig. 2), we were unable to isolate any colonies from cells transfected with an equivalent quantity of either BNeo/3-5B or BNeo/ΔGDD RNA (data not shown). The failure to recover G418-resistant colonies following transfection of BNeo/3-5B suggests strongly that this previously described RNA replicates significantly less efficiently than NNeo/3-5B in these Huh7 cells.
FIG. 2.
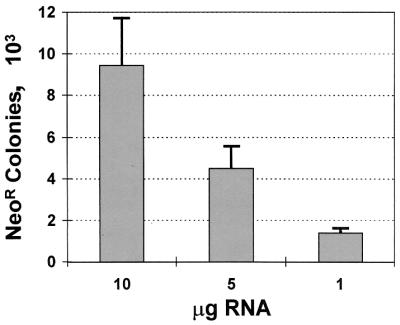
Mean number of G418-resistant cell colonies isolated per 10-cm-diameter cell culture dish 4 weeks following transfection of Huh7 cells with various amounts of the subgenomic NNeo/3-5B replicon RNA. Error bars indicate the range of values observed in duplicate experiments.
To confirm the presence of replicating subgenomic RNAs in cells selected for G418 resistance following transfection with NNeo/3-5B, three G418-resistant cell colonies were selected at random and clonally isolated. These clonal cell lines were then examined for the presence of HCV RNA by Northern analysis. As shown in Fig. 3 (lanes 4 and 5), the presence of a substantial abundance of HCV-specific RNA with a length approximating 8 kb was detected in extracts of total cellular RNA prepared from each of these stable cell lines (data shown only for clones 1 and 2). Although the abundance of the replicon RNA was significantly greater in the BNeo/3-5B(RG) cell line than in other cell lines studied in this particular experiment, we noted no consistent trends in the abundance of replicon RNA among cell lines derived with different replicon constructs. Abundant NS5A protein was also demonstrated in each of the cell lines by indirect immunofluorescence (Fig. 4). These data confirm the ability of wild-type HCV-N subgenomic replicons to undergo autonomous replication in Huh7 cells and represent an important confirmation of the results of Lohmann et al. (16) with a second, independent isolate of HCV.
FIG. 3.
(Top panel) Northern analysis of HCV-specific RNA present in G418-resistant cell lines selected following transfection of Huh7 cells with the indicated replicon RNA. Lanes 1 and 2, synthetic RNA transcribed from pNNeo/3-5B (108 and 107 genome equivalents spiked into normal cellular RNA), respectively; lane 3, normal Huh7 cells; lanes 4 and 5, two independent, clonally isolated cell lines (cell lines 1 and 2) selected following transfection with NNeo/3-5B; lanes 6 to 9, other clonally isolated cell lines. (Lower panel) Northern analysis of β-actin mRNAs in the samples loaded into the gel shown in the top panel.
FIG. 4.
Indirect immunofluorescence detection of NS5A antigen in normal Huh7 cells (A) and clonally isolated cell lines selected following transfection of Huh7 cells with NNeo/3-5B (cell line 1) (B), NNeo/3-5B(SI) (C), and NNeo/3-5B(RG) (D).
Adaptive mutations are not required for efficient replication of NNeo/3-5B RNA.
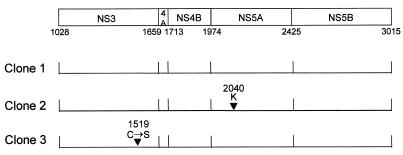
Data reported both by Lohmann et al. (15) and by Blight et al. (4) suggest that spontaneously arising, cell culture-adaptive mutations are required for efficient replication of BNeo/3-5B in Huh7 cells. Such mutations appear to be present within each replicon-bearing cell line that has been clonally isolated and characterized in detail (4, 14, 15). Cell culture-adaptive mutations have been identified within NS3, NS5A, and NS5B and have been shown to dramatically increase the efficiency of colony formation when cells are transfected and subjected to G418 selection. To determine whether such adaptive mutations are also required with NNeo/3-5B replicons derived from HCV-N, we determined the nucleotide sequences of the NS3-NS5B segment of the replicons present in the three clonal cell lines described in the preceding section. RNA extracted from these cells were reverse transcribed into cDNA and amplified by RT-PCR for direct DNA sequencing as described in Materials and Methods. Results are shown schematically in Fig. 5.
FIG. 5.
Amino acid substitutions predicted to be present in the HCV nonstructural proteins expressed by replicons present in three clonally isolated, G418-resistant cell lines selected following transfection of Huh7 cells with NNeo/3-5B RNA. There were no mutations identified within the open reading frame of the replicon RNA present in cell line 1.
Replicon RNAs in two of the three cell lines contained single-amino-acid mutations: a 3-base insertion resulting in a new Lys residue at position 2040 (NS5A) in clone 2, and a single-base change leading to a Cys-to-Ser substitution at residue 1519 (NS3 helicase domain) in clone 3. Remarkably, there were no mutations identified in the amino acid sequence of the nonstructural proteins in clone 1, despite the fact that the replicon RNA abundance in these cells was approximately equivalent to that in other G418-resistant cell lines, including clone 2, in which there was the insertion of an additional residue in NS5A (Fig. 3, compare lanes 4 and 5). These results confirm that NNeo/3-5B RNA is capable of efficient autonomous replication in the absence of adaptive mutations and suggest that the two mutations identified in Fig. 5 may have relatively little impact on the replication of this RNA.
Effect of BNeo/3-5B adaptive mutations on replication of NNeo/3-5B.
To determine whether mutations in NS5A or NS5B that have been reported previously to enhance the replication of BNeo/3-5B would further enhance the replication of NNeo/3-5B replicons, we constructed NNeo/3-5B-derived replicons with a Ser-to-Ile substitution at residue 2005, NNeo/3-5B(SI), comparable to the Con1 replicons containing the S1179→I mutation in NS5A described by Blight et al. (4), or an Arg-to-Gly substitution at residue 2889, NNeo/3-5B(RG), comparable to the replicon containing the R2884G mutation in NS5B reported by Lohmann et al. (15). Identical mutations were also introduced into BNeo/3-5B, leading to the creation of BNeo/3-5B(SI) and BNeo/3-5B(RG), respectively, and the modified NNeo/3-5B and BNeo/3-5B RNAs were transfected into Huh7 cells in parallel experiments.
The results of these experiments confirmed the cell culture-adaptive activities of these NS5A and NS5B mutations on Con1-derived replicons. As shown in Fig. 6, the introduction of S2005I into the background of BNeo/3-5B increased the efficiency of G418-resistant colony formation substantially more than the introduction of R2884G. The number of colonies generated following transfection of Huh7 cells with BNeo/3-5B(SI) RNA approximated that obtained with NNeo/3-5B RNA (Fig. 6). These results thus confirmed the importance of the S2005I substitution for replication of the BNeo/3-5B replicon, as reported previously (4). However, they also demonstrated that the wild-type NNeo/3-5B RNA is comparable to BNeo/3-5B RNAs containing adaptive mutations such as S2005I in terms of its ability to replicate in Huh7 cells and lead to the selection of G418-resistant colonies. In fact, there was no apparent difference in the abundance of HCV RNA in cell lines selected following transfection of BNeo/3-5B(SI) and NNeo/3-5B (clone 1, which contains no adaptive mutations) (Fig. 3, compare lanes 4 and 8). Interestingly, however, a cell line selected following transfection with BNeo/3-5B(RG) had a greater abundance of viral RNA (Fig. 3, lane 9) despite the substantially lower number of G418-resistant cell colonies generated with this RNA (Fig. 6). We did not determine whether this particular cell line contained additional adaptive mutations.
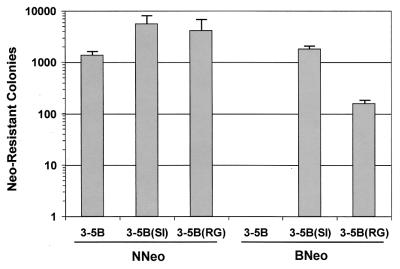
FIG. 6.
Mean number of G418-resistant cell colonies selected per 10-cm-diameter culture dish following transfection of Huh7 cells with the indicated replicon RNAs. A total of 1 μg of replicon RNA was transfected per culture dish. Error bars indicate the range of values obtained in replicate experiments.
The introduction of either of these two mutations into the background of NNeo/3-5B also resulted in an increase in the number of G418-resistant colonies, but proportionately this increase was much less that that observed with the introduction of these mutations into the BNeo/3-5B background (Fig. 6). The S2005I and R2889G mutations resulted in comparable increases in the numbers of G418-resistant colonies, although the density of colony formation made their enumeration difficult even when only 1 μg of RNA was transfected per culture dish, as in the experiments depicted in Fig. 6. However, we also compared the effects of these two mutations when introduced into the background of a similar subgenomic HCV-N replicon containing blastocidin rather than Neo as a selection marker (NBla/3-5B). In this case, where blastocidin is generally less efficient than Neo as a selectable marker, the introduction of R2889G was shown to result in an ∼5-fold higher number of G418-resistant cell colonies than the introduction of S2005I (data not shown). Importantly, the introduction of these mutations increased the number of G418-resistant colonies obtained with NNeo/3-5B replicons no more than severalfold, and far less than the 1,000-fold or greater increases seen with the comparable BNeo/3-5B replicons (Fig. 6). Neither mutation resulted in an increase in the abundance of replicon RNA in G418-resistant cell lines selected following transfection with NNeo/3-5B RNAs (Fig. 3, compare lanes 4 and 5 with lanes 6 and 7).
Enhanced replication capacity of HCV-N RNA is due to a natural 4-amino-acid insertion in NS5A.
As mentioned above, the sequence of the infectious HCV-N cDNA clone contains a unique 4-amino-acid insertion (-Ser-Ser-Tyr-Asn-) within the ISDR segment of the NS5A protein in alignments with other HCV sequences (2). This insertion comprises amino acid residues 2220 to 2223 in the HCV-N polyprotein and, although unique in the database, was present in cDNA cloned directly from the Japanese patient who served as the source of the HCV-N isolate (10). It is thus representative of the wild-type sequence of this virus. Since mutations that enhance the replication of the BNeo/3-5B replicon have been suggested to cluster near the ISDR of NS5, we questioned whether the presence of this unique insertion in the ISDR might contribute to the ability of NNeo/3-5B replicons to replicate efficiently in the absence of additional cell culture-adaptive mutations. To address this question, we deleted the 4-amino-acid insertion from NNeo/3-5B (generating NNeo/3-5BΔi5A) and assessed the ability of this NS5A deletion mutant to support the selection of G418-resistant colonies following transfection of Huh7 cells. Additional deletion mutants were generated by removal of the 4-amino-acid insertion from NNeo/3-5B(SI) and NNeo/3-5B(RG), designated NNeo/3-5B(SI)Δi5A and NNeo/3-5B(RG)Δi5A, respectively.
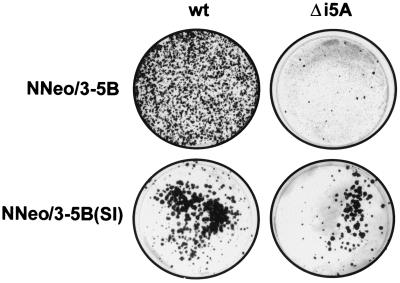
The number of G418-resistant colonies selected following transfection with NNeo/3-5BΔi5A was much lower than after transfection with NNeo/3-5B (Fig. 7, top panel). Only a small number of colonies were generated following transfection with a large amount of RNA (20 μg per culture dish), confirming the importance of this insertion to replication of this RNA in Huh7 cells. In contrast, the deletion of these 4 amino acids from the NS5A sequences of NNeo/3-5B(SI) resulted in only a modest decrease in the efficiency of colony formation, with large numbers of G418-resistant colonies selected after transfection of relatively small amounts of NNeo/3-5B(SI)Δi5A RNA (1 μg/culture dish) (Fig. 7, bottom panel). Similar results were obtained with the NNeo/3-5B(RG)Δi5A replicon, although the number of surviving G418-resistant colonies was less than that with NNeo/3-5B(SI) (data not shown). The fact that efficient G418-resistant colony-forming activity could be preserved by either of these previously described cell culture-adaptive mutations in the absence of the 4-amino-acid insertion in NS5A provides further evidence that the 4-amino-acid insertion is responsible for the inherent ability of NNeo/3-5B RNA to replicate efficiently in Huh7 cells.
FIG. 7.
G418-resistant cell colonies present 3 weeks after transfection with NNeo/3-5B and NNeo/3-5BΔi5A RNAs (20 μg of RNA per 10-cm-diameter culture dish) (top panel) or NNeo/3-5B(SI) and NNeo/3-5B(SI)Δi5A (1 μg of RNA per 10-cm-diameter culture dish) (bottom panel). Colonies were visualized by staining cells with crystal violet. wt, wild type.
Replication competence of selectable dicistronic HCV-N RNAs encoding the structural proteins of HCV.
Lohmann et al. (16) demonstrated that subgenomic Con1 replicons containing the NS2-NS5B segment of HCV also were capable of autonomous replication in Huh7 cells, although the number of G418-resistant colonies selected was somewhat less than that obtained after transfection of cells with replicon RNA containing only the NS3-NS5B segment. To determine whether the replication capacity of the HCV-N RNA would be influenced by the inclusion of NS2-coding sequence or sequences encoding the envelope and core proteins of HCV-N, we constructed a series of plasmids with transcriptional units encoding the selectable, dicistronic RNAs shown in Fig. 8. In addition to the NS3-NS5B coding sequence present in NNeo/3-5B, the 3′ cistrons of these dicistronic RNAs contain upstream wild-type HCV-N sequence encoding NS2 (NNeo/2-5B), the envelope proteins as well as NS2 (NNeo/E1-5B), or the entire polyprotein (NNeo/C-5B). RNA transcripts prepared from these plasmids were transfected into Huh7 cells, as described above, and in each case gave rise to G418-resistant colonies after several weeks of culture in G418-containing media. The number of colonies produced from each RNA diminished with the increasing length of the second cistron, with ∼160 colonies obtained with NNeo/2-5B, ∼60 colonies with NNeo/E1-5B, and only 22 colonies from NNeo/C-5B. However, stable, G418-resistant cell lines were clonally isolated from transfections with each of these RNAs, indicating that the RNA remained replication competent despite the inclusion of the additional sequence.
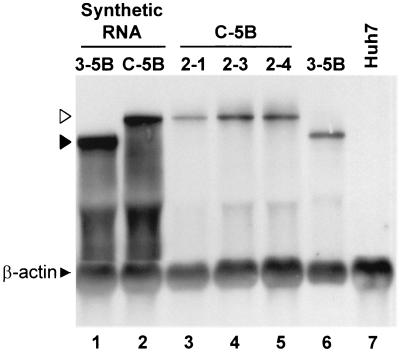
Total cellular RNA extracted from these G418-resistant cell lines was analyzed by Northern analysis for HCV RNA. Each cell line contained HCV-specific RNA of the appropriate length (data not shown), confirming the ongoing replication of HCV RNA in cell lines selected after transfection with each of the RNAs shown in Fig. 8. However, cells selected following transfection with NNeo/C-5B contained a demonstrably lower abundance of replicon RNA than cells selected following transfection with NNeo/2-5B or NNeo/E1-5B. These latter cell lines were comparable in replicon abundance to cells selected following transfection with NNeo/3-5B. Furthermore, G418-resistant cells selected with the NNeo/C-5B replicon grew slowly and failed to become completely confluent after several weeks in culture. Colonies of cells selected from one of the NNeo/C-5B cell lines were subcloned and, after passage for an additional month, demonstrated improved growth properties. Figure 9 (lanes 3 to 5) shows a Northern analysis of total cellular RNA extracted from three of these NNeo/C-5B subclones. Each subcloned cell line contained viral RNA of the appropriate length, with an abundance approximating that of replicon RNA in cell lines selected following transfection with NNeo/3-5B (Fig. 9, compare lanes 3 to 5 with lane 6).
FIG. 9.
Northern analysis of (lanes 1 to 2) synthetic T7 transcripts representing the dicistronic subgenomic NNeo/3-5B (solid arrowhead) and full-length NNeo/C-5B (open arrowhead) RNAs spiked into total cellular RNA extracted from normal Huh7 cells; (lanes 3 to 6) total cellular RNA isolated from G418-resistant cell lines, including three subcloned cell lines selected following transfection with NNeo/C-5B RNA (lanes 3 to 5) and a clonal cell line selected following transfection with NNeo/3-5B RNA (lane 6), and total cellular RNA from normal Huh7 cells (lane 7). The blot was hybridized to a β-actin probe as an internal control.
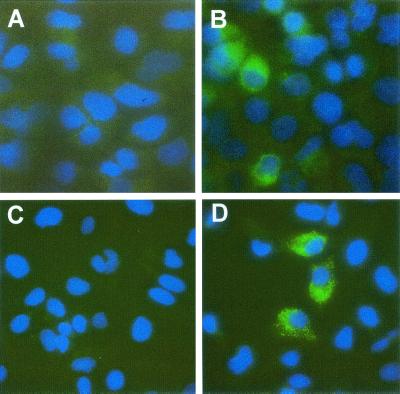
G418-resistant cell lines selected following transfection with NNeo/E1-5B or NNeo/C-5B were examined for the presence of structural protein antigens by indirect immunofluorescence. In addition to NS5A antigen (data not shown), cells selected following transfection with NNeo/E1-5B contained detectable E2 antigen (Fig. 10B), while cells selected following transfection with NNeo/C-5B RNA stained positively for core antigen (Fig. 10D). In both cases, only a proportion of the cells present in the clonally isolated cell lines contained a detectable abundance of these antigens at any single point in time. This result was different from what was observed with G418-resistant cell lines selected following transfection with NNeo/3-5B, in which almost all cells contained detectable NS5A antigen (Fig. 4). It is possible that this may reflect cell cycle dependence of the replication of these RNAs (20), because the cell lines were clonally derived and stable. Together, however, these data provide strong confirmatory evidence of the replication competence of genome-length, selectable, dicistronic HCV-N RNAs in Huh7 cells. Preliminary attempts to transfer G418 resistance to fresh cultures of Huh7 cells with filtered supernatants from these cell lines were not successful.
FIG. 10.
Indirect immunofluorescence for detection of E2 protein (A and B) or core protein (C and D) in normal Huh7 cells (A and C) or G418-resistant cells selected following transfection with NNeo/E1-5B (B) or NNeo/C-5B.
DISCUSSION
Previous studies have demonstrated that subgenomic RNA replicons derived from diverse members of the family Flaviviridae are capable of autonomous replication in permissive cell lines (3, 11). Recent reports make it evident that analogous subgenomic HCV replicons are also capable of replication in Huh7 cells (4, 16). Importantly, however, only HCV replicons derived from a single, genotype 1b strain of HCV, Con1, have been shown previously to be replication competent. Efforts to derive replication-competent, subgenomic RNAs from known infectious cDNAs of the genotype 1a, H77C virus (13, 25), have proven unsuccessful 4; our unpublished data). Here, we describe the replication competence of subgenomic RNAs that are derived from a second, completely independent HCV isolate, HCV-N. Although both the NNeo/3-5B and BNeo/3-5B replicons are derived from genotype 1b viruses, the sequences of the open reading frames in these replicons share only ∼90% nucleotide identity and ∼94% amino acid identity overall.
Prior to the development of selectable replicons, the replication competence of synthetic HCV RNA could be determined only by inoculation of the RNA into the liver of a susceptible chimpanzee (13, 25). Since attempts to infect chimpanzees with synthetic RNAs representing the Con1 sequence have not been reported, the in vivo replication competence of the Con1 RNA sequence used for construction of replicons by Lohmann et al. (16) is not known. In contrast, the inoculation of synthetic, genome-length HCV-N RNA into the liver of a susceptible chimpanzee has been shown previously to lead to both viremia and the permanent appearance of high-titer antibodies to HCV (2). Thus, the HCV-N RNA sequence present in the NNeo/3-5B replicon was derived from a molecular clone that has previously been shown to be infectious in chimpanzees. This is important, since it permits a comparison of the replication competence of this RNA in chimpanzees and Huh7 cells.
While infectious in the chimpanzee model (2), RNA derived from HCV-N has a substantially less robust replication phenotype in chimpanzees than either of the two available, infectious molecular clones of the H77C strain of HCV that have failed to produce replication-competent replicons. In contrast to the high-titer viremia (106 or greater genome equivalents/ml) that typically extends for weeks following the inoculation of chimpanzees with H77C RNA (13, 18, 25), viremia was of low level (peaking at 3.7 × 104 genome equivalents/ml) and was only intermittently detectable following intrahepatic inoculation of HCV-N RNA in a chimpanzee that was successfully infected with HCV-N RNA (2). Moreover, subsequent attempts to infect two other chimpanzees with RNA derived from this clone failed to give rise to either detectable viremia or antibody seroconversions (T. S. Wang and S. M. Lemon, unpublished data). Thus, while the number of HCV strains studied thus far is very small, there appears to be no direct relationship between the ability of an HCV RNA sequence to replicate in vivo in the chimpanzee model and its ability to replicate autonomously as subgenomic RNA in cultured Huh7 cells. Further studies will be required to understand the basis of this difference in the host ranges of these HCV RNAs.
Cell culture-adaptive mutations, particularly in the NS5A protein, are required for efficient replication of the BNeo/3-5B replicon in Huh7 cells (4, 14, 15). In contrast, adaptive mutations are not required for efficient replication of NNeo/3-5B RNA in these cells. Although mutations were found in the nonstructural proteins encoded by replicon RNAs present in two of three clonally isolated cell lines selected with G418 following transfection with NNeo/3-5B RNA, there were no mutations present in a third cell line despite comparable abundances of viral RNA and viral protein (NS5A) in these cells. Moreover, the efficiency with which NNeo/3-5B RNA led to selection of G418-resistant cell colonies was comparable to that of BNeo/3-5B(SI) and BNeo/3-5B(RG), each of which contains mutations that enhance the colony-forming capacity of the original BNeo/3-5B replicon by several orders of magnitude. The introduction of either of these mutations (one in NS5A, the other in NS5B) into NNeo/3-5B resulted in only slight enhancements of the transduction efficiency of this RNA. The increase in the number of G418-resistant colonies was minimal (on the order of 3- to 4-fold relative to NNeo/3-5B), compared with an increase of over 1,000-fold in the case of the BNeo/3-5B replicon (Fig. 6). It is interesting to note, however, that the single-amino-acid mutation identified in one of the G418-resistant cell lines (clone 2) selected following transfection with NNeo/3-5B is identical to a mutation that has been shown recently to enhance the colony-forming activity of BNeo/3-5B (M. Gale, personal communication).
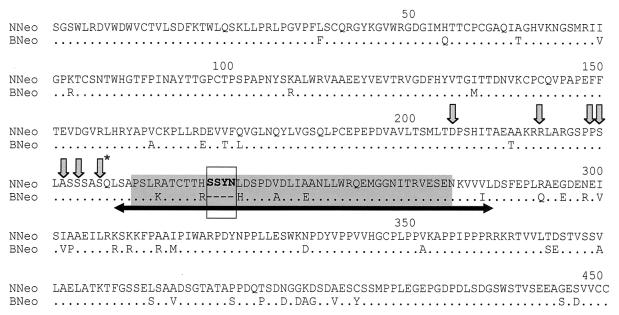
Since many of the mutations that enhance the replication of BNeo/3-5B have been localized to the NS5A sequence (4, 14), we compared the NS5A sequences of NNeo/3-5B and BNeo/3-5B. The proteins are predicted to differ at 49 of 451 (∼11%) amino acid residues (Fig. 11). Amino acid differences are scattered across the length of the protein sequence, although they are somewhat more frequent within the ISDR and C-terminal half of the protein. Interestingly, there are no differences at any of the residues at which single-amino-acid substitutions have previously been reported to enhance the replication capacity of BNeo/3-5B.
FIG. 11.
Alignment of the amino acid sequences of the NS5A proteins encoded by NNeo/3-5B and BNeo/3-5B. The ISDR is shaded, with the 4-amino-acid -Ser-Ser-Tyr-Asn- insertion in NNeo/3-5B shown in boldface type and enclosed in a box. Arrows indicate the location of single-base substitutions and insertions and the large 47-amino-acid deletion that has been shown previously to enhance the replication capacity of BNeo/3-5B (4, 14, 15). The asterisk indicates the S2005I mutation.
The most striking difference in the NS5A sequences of these replicons is the presence of the 4-amino-acid insertion within the ISDR of NNeo/3-5B. This insertion and, in fact, the entire ISDR are within a 47-amino-acid segment that was shown to have been spontaneously deleted in a cell line bearing a BNeo/3-5B replicon isolated by Blight et al. (4). This large deletion mutation significantly increased the numbers of G418-resistant cell colonies selected following transfection of BNeo/3-5B RNA (4). When the 4-amino-acid insertion was deleted from NNeo/3-5B, its capacity to generate G418-resistant colonies was substantially, although not completely, eliminated (Fig. 7, top panel). However, the ability of the RNA to efficiently generate G418-resistant colonies was preserved by introduction of the BNeo/3-5B-adaptive S2005I mutation in NS5A (Fig. 7, bottom panel) and, to a slightly lesser extent, the R2889G mutation in NS5B (data not shown). The 4-amino-acid insertion in NS5A thus accounts, at least in part, for the unique ability of the wild-type HCV-N RNA to replicate in these cells. It thus represents a natural cell culture-adaptive mutation. Although present in the synthetic HCV-N RNA that gave rise to infection in a chimpanzee, as described above (2), the persistence of this sequence polymorphism was not studied in this animal. Thus, it is not possible to comment further on its contribution to replication in vivo.
The mechanism by which diverse mutations in NS5A act to enhance the replication of subgenomic, genotype 1b RNAs remains obscure. However, the nonstructural NS5A protein appears to be involved in several distinct functions (5). These include, most likely, a role in the replicase complex, as well as a probable role in mediating resistance to interferon (6, 24). This latter effect may occur via a specific interaction of NS5A with the double-stranded RNA-activated protein kinase R (PKR) (7, 8) or through PKR-independent mechanisms that have yet to be elucidated (21). Several of the more effective cell culture-adaptive mutations identified with BNeo/3-5B replicons involve the substitution of Ser residues that are thought to be sites of phosphorylation of NS5A (Fig. 11). Although some of these mutations have been shown to result in a reduction of the phosphorylation status of the protein (4), the relevance of this observation to the replication of the viral RNA remains unknown. Two of the four residues that comprise the insertion in NS5A of HCV-N are Ser, but the impact of this insertion on the phosphorylation status of NS5A is not yet known. The diverse nature of the NS5A mutations that enhance RNA replication (including multiple substitutions at different residues, a large deletion mutation, and both single-amino-acid and larger insertions) suggests that these mutations serve to knock out a specific function of this protein, rather than specifically modify or enhance an existing function. A good possibility would seem to be the elimination of an interaction of NS5A with a cellular factor that is inhibitory to RNA replication.
It is interesting that the insertion of the 4 amino acids that exists in the NS5A sequence of HCV-N was present in cDNA cloned directed from virus present in an HCV-infected patient in Japan (10). This distinguishes it from the other mutations shown in Fig. 11, each of which was selected under G418 pressure in cultured Huh7 cells. The patient from which HCV-N was originally recovered was asymptomatic, but was reported to have had an unusually high elevation of serum alanine aminotransferase (10). Little else is known of this patient. Specifically, we do not know whether the patient had received or was receiving treatment with interferon at the time the cDNA was amplified from the blood. Thus, the selective forces that led to the evolution of the NS5A insertion in this virus in vivo are uncertain.
Selectable, dicistronic RNAs encoding the entire polyprotein of HCV-N were able to replicate in Huh7 cells to a level of abundance that was detectable by Northern analysis (Fig. 9). These results indicate that there are no major restrictions to the replication of the full-length RNAs in these cells. We did not determine whether additional adaptive mutations (within the structural proteins or elsewhere in the genome) are required for the replication of this RNA. However, the efficiency with which this RNA was able to support the selection of G418-resistant colonies was reduced from that of NNeo/3-5B. Our inability to passage G418 resistance to fresh Huh7 cells by exposure to filtered supernatant fluids from these cells may be due to a lack of appropriate receptors for the virus on Huh7 cells, because a functional receptor is not required for replication of transfected, selectable RNAs. However, additional experiments will be required to determine whether cell lines supporting the replication of the full-length NNeo/C-5B RNA are capable of producing virions that package the dicistronic RNA.
ADDENDUM IN PROOF
While this manuscript was in preparation, Guo et al. (9) reported the successful construction of replication-competent subgenomic replicons from the pHCV-N cDNA. Similar to the results reported here, subgenomic HCV-N replicons encoding the NS3-5B segment of the polyprotein appeared to have substantially greater intrinsic replication capacity than replicons derived from the Con1 sequence, although Guo et al. did not directly assess whether HCV-N-based replicons require additional cell culture-adaptive mutations for efficient replication in Huh7 cells.
Acknowledgments
This work was supported in part by a grant from the National Institute of Allergy and Infectious Diseases (U19-AI40035).
We thank Michael Murray and colleagues at the Schering Plough Research Institute for the gift of the pBNeo/3-5B plasmid, and Michael Beard, Shinji Makino, and Annette Martin for helpful discussions.
REFERENCES
- 1.Alter, M. J., H. S. Margolis, K. Krawczynski, F. N. Judson, A. Mares, W. J. Alexander, P. Y. Hu, J. K. Miller, M. A. Gerber, and R. E. Sampliner. 1992. The natural history of community-acquired hepatitis C in the United States. The Sentinel Counties Chronic non-A, non-B Hepatitis Study Team. N. Engl. J. Med. 327:1899-1905. [DOI] [PubMed] [Google Scholar]
- 2.Beard, M. R., G. Abell, M. Honda, A. Carroll, M. Gartland, B. Clarke, K. Suzuki, R. Lanford, D. V. Sangar, and S. M. Lemon. 1999. An infectious molecular clone of a Japanese genotype 1b hepatitis C virus. Hepatology 30:316-324. [DOI] [PubMed] [Google Scholar]
- 3.Behrens, S. E., C. W. Grassmann, H.-J. Thiel, G. Meyers, and N. Tautz. 1998. Characterization of an autonomous subgenomic pestivirus RNA replicon. J. Virol. 72:2364-2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blight, K. J., A. A. Kolykhalov, and C. M. Rice. 2000. Efficient initiation of HCV RNA replication in cell culture. Science 290:1972-1974. [DOI] [PubMed] [Google Scholar]
- 5.De Francesco, R., P. Neddermann, L. Tomei, C. Steinkuhler, P. Gallinari, and A. Folgori. 2000. Biochemical and immunologic properties of the nonstructural proteins of the hepatitis C virus: implications for development of antiviral agents and vaccines. Semin. Liver Dis. 20:69-83. [DOI] [PubMed] [Google Scholar]
- 6.Enomoto, N., I. Sakuma, Y. Asahina, M. Kurosaki, T. Murakami, C. Yamamoto, Y. Ogura, N. Izumi, F. Marumo, and C. Sato. 1996. Mutations in the nonstructural protein 5A gene and response to interferon in patients with chronic hepatitis C virus 1b infection. N. Engl. J. Med. 334:77-81. [DOI] [PubMed] [Google Scholar]
- 7.Gale, M., Jr., B. Kwieciszewski, M. Dossett, H. Nakao, and M. G. Katze. 1999. Antiapoptotic and oncogenic potentials of hepatitis C virus are linked to interferon resistance by viral repression of the PKR protein kinase. J. Virol. 73:6506-6516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gale, M. J., Jr., M. J. Korth, N. M. Tang, S. L. Tan, D. A. Hopkins, T. E. Dever, S. J. Polyak, D. R. Gretch, and M. G. Katze. 1997. Evidence that hepatitis C virus resistance to interferon is mediated through repression of the PKR protein kinase by the nonstructural 5A protein. Virology 230:217-227. [DOI] [PubMed] [Google Scholar]
- 9.Guo, J.-T., V. V. Bichko, and C. Seeger. 2001. Effect of alpha interferon on the hepatitis C virus replicon. J. Virol. 75:8516-8523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hayashi, N., H. Higashi, K. Kaminaka, H. Sugimoto, M. Esumi, K. Komatsu, K. Hayashi, M. Sugitani, K. Suzuki, O. Tadao, K. Mizuno, and T. Shikata. 1993. Molecular cloning and heterogeneity of the human hepatitis C virus (HCV) genome. J. Hepatol. 17:S94-S107. [DOI] [PubMed] [Google Scholar]
- 11.Khromykh, A. A., and E. G. Westaway. 1997. Subgenomic replicons of the flavivirus Kunjin: construction and applications. J. Virol. 71:1497-1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kiyosawa, K., and S. Furuta. 1994. Hepatitis C virus and hepatocellular carcinoma, p. 98-120. In H. W. Reesink (ed.), Hepatitis C virus. Karger, Basel, Switzerland. [DOI] [PubMed]
- 13.Kolykhalov, A. A., A. A. Agapov, K. J. Blight, K. Mihalik, S. M. Feinstone, and C. M. Rice. 1997. Transmission of hepatitis C by intrahepatic inoculation with transcribed RNA. Science 277:570-574. [DOI] [PubMed] [Google Scholar]
- 14.Krieger, N., V. Lohmann, and R. Bartenschlager. 2001. Enhancement of hepatitis C virus RNA replication by cell culture-adaptive mutations. J. Virol. 75:4614-4624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lohmann, V., F. Körner, A. Dobierzewska, and R. Bartenschlager. 2001. Mutations in hepatitis C virus RNAs conferring cell culture adaptation. J. Virol. 75:1437-1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lohmann, V., F. Korner, J. Koch, U. Herian, L. Theilmann, and R. Bartenschlager. 1999. Replication of subgenomic hepatitis C virus RNAs in a hepatoma cell line. Science 285:110-113. [DOI] [PubMed] [Google Scholar]
- 17.Major, M. E., and S. M. Feinstone. 1997. The molecular virology of hepatitis C. Hepatology 25:1527-1538. [DOI] [PubMed] [Google Scholar]
- 18.Major, M. E., K. Mihalik, J. Fernandez, J. Seidman, D. Kleiner, A. A. Kolykhalov, C. M. Rice, and S. M. Feinstone. 1999. Long-term follow-up of chimpanzees inoculated with the first infectious clone for hepatitis C virus. J. Virol. 73:3317-3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nishioka, K., S. Mishiro, and H. Yoshizawa. 1996. Hepatitis C virus infection in the general population of Japan: past and future. Viral Hepatitis Rev. 2:199-203. [Google Scholar]
- 20.Pietschmann, T., V. Lohmann, G. Rutter, K. Kurpanek, and R. Bartenschlager. 2001. Characterization of cell lines carrying self-replicating hepatitis C virus RNAs. J. Virol. 75:1252-1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Podevin, P., A. Sabile, R. Gajardo, N. Delhem, A. Abadie, P. Y. Lozach, L. Beretta, and C. Brechot. 2001. Expression of hepatitis C virus NS5A natural mutants in a hepatocytic cell line inhibits the antiviral effect of interferon in a PKR-independent manner. Hepatology 33:1503-1511. [DOI] [PubMed] [Google Scholar]
- 22.Poynard, T., P. Bedossa, and P. Opolon. 1997. Natural history of liver fibrosis progression in patients with chronic hepatitis C. Lancet 349:825-832. [DOI] [PubMed] [Google Scholar]
- 23.Seeff, L. B. 1997. Natural history of hepatitis C. Hepatology 26:21S-28S. [DOI] [PubMed]
- 24.Watanabe, H., N. Enomoto, K. Nagayama, N. Izumi, F. Marumo, C. Sato, and M. Watanabe. 2001. Number and position of mutations in the interferon (IFN) sensitivity-determining region of the gene for nonstructural protein 5A correlate with IFN efficacy in hepatitis C virus genotype 1b infection. J. Infect. Dis. 183:1195-1203. [DOI] [PubMed] [Google Scholar]
- 25.Yanagi, M., R. H. Purcell, S. U. Emerson, and J. Bukh. 1997. Transcripts from a single full-length cDNA clone of hepatitis C virus are infectious when directly transfected into liver of a chimpanzee. Proc. Natl. Acad. Sci. USA 97:8738-8743. [DOI] [PMC free article] [PubMed] [Google Scholar]