Abstract
Using bacterial artificial chromosome (BAC) technology, we have constructed and characterized a human cytomegalovirus recombinant virus with a mutation in the exon specific for the major immediate-early region 2 (IE2) gene product. The resulting IE2 86-kDa protein (IE2 86) has an internal deletion of amino acids 136 to 290 and is fused at the carboxy terminus to enhanced green fluorescent protein (EGFP). The deletion also removes the promoter and initiator methionine for the p40 form of IE2 and initiator methionine for the p60 form of the protein, and therefore, these late gene products are not produced. The mutant virus IE2 86ΔSX-EGFP is viable but exhibits altered growth characteristics in tissue culture compared with a full-length wild-type (wt) IE2 86-EGFP virus or a revertant virus. When cells are infected with the mutant virus at a low multiplicity of infection (MOI), there is a marked delay in the production of infectious virus. This is associated with slower cell-to-cell spread of the virus. By immunofluorescence and Western blot analyses, we show that the early steps in the replication of the mutant virus are comparable to those for the wt. Although there is significantly less IE2 protein in the cells infected with the mutant, there is only a modest lag in the initial accumulation of IE1 72 and viral early proteins, and viral DNA replication proceeds normally. The mutation also has only a small effect on the synthesis of the viral major capsid protein. The most notable molecular defect in the mutant virus infection is that the steady-state levels of the pp65 (UL83) and pp28 (UL99) matrix proteins are greatly reduced. In the case of UL83, but not UL99, there is also a corresponding decrease in the amount of mRNA present in cells infected with the mutant virus.
Human cytomegalovirus (HCMV) is a common pathogen that is the leading viral cause of birth defects (49). HCMV infection also results in significant morbidity and mortality in immunosuppressed individuals and may be one of the factors contributing to atherosclerosis and restenosis following coronary angioplasty (74).
The survival of HCMV and its ability to establish both acute and latent infections depend on a complex set of interactions between the virus and the host cell machinery that optimize the environment for viral replication (3, 16). During the productive infection, there are three major phases of gene expression. The immediate-early (IE) genes are transcribed after viral entry and rely mainly on host factors for their expression, although some input virion proteins contribute to their activation. Early genes are synthesized prior to viral DNA replication, and their expression requires one or more viral IE gene products. Included in this early class are viral proteins required to “activate” the cell to a metabolic state most conducive for viral DNA synthesis as well as proteins involved in the replication process itself (for a review, see reference 16). Concurrent with these effects on cellular metabolism, viral DNA synthesis begins at ∼18 h postinfection (p.i.). Finally, late genes are transcribed in abundance after viral DNA replication, and virus is released at ∼96 h p.i.
A primary site of IE transcription includes two genetic units, IE1 and IE2 (for a review, see references 18 and 46). The predominant IE RNA (IE1) is 1.9 kb and consists of four exons; a single open reading frame (ORF) (UL123) initiates in exon 2 and specifies a 72-kDa nuclear phosphoprotein, designated IE1 72. The major IE2 gene product, IE2 86 (ORF UL122), is an 86-kDa phosphoprotein that shares 85 amino acids (aa) at its amino terminus with the IE1 72 protein. Other IE2 transcripts include a low-abundance splice variant detected in human monocytes (34) and a late unspliced RNA that encodes a 40-kDa protein that represents the C-terminal half of IE2 86 (30, 54, 64). It should be noted that the Towne IE2 86 protein has 579 aa while the AD169 protein has 580 aa (an extra Ser is found in the set of Ser at aa 258 to 264). Since the Towne numbering has most commonly been used in publications, it will serve as the reference in this paper, although strain AD169 is the parent virus in the studies presented.
Using transient expression assays, members of our group and others have demonstrated that multiple HCMV early promoters and heterologous viral promoters can be activated by the IE1 and IE2 gene products (for a review, see references 18 and 46). IE2 86 appears to play a major role in activating HCMV early promoters and in repressing the major IE promoter (its own promoter), while IE1 72 stimulates the IE promoter and may augment the activating effect of IE2 86. The late 40-kDa IE2 protein can also repress the IE promoter and activate some promoters in the presence of IE1 72 (30). The observation that transient expression of IE2 86 alone is able to block cell division by arresting the cells either at G1/S or shortly after the initiation of cell DNA synthesis has also lent support to the hypothesis that IE2 86 may have a role in the observed dysregulation of the cell cycle (9, 48, 70, 71). There is also evidence that IE2 86 in association with IE1 72 may be able to interfere with apoptosis (40, 75).
Any consideration of the mechanism by which IE2 86 functions must take into account the fact that it can engage in multiple protein-protein and protein-DNA interactions. The IE2 86 protein interacts with itself and an HCMV early protein (UL84), as well as with specific cellular transcription factors and components of the basal transcription complex, the products of the tumor suppressor genes Rb and p53, the ubiquitin-homologous proteins SUMO-1 and hSMT3b, and the histone acetyltransferase P/CAF (8, 10, 14, 15, 19, 21, 22, 26, 27, 32, 36, 41, 42, 58, 60-63, 68, 73). IE2 86 also represses expression of its own promoter by binding to the cis-repression signal (CRS) DNA element located between the TATAA box and transcription start site (13, 14, 37-39, 43, 50). In addition, other DNA binding sites for IE2 86 have been identified just upstream of the TATAA box on HCMV promoters for several early genes (4, 11, 12, 28, 59, 60).
To delineate the functional domains of IE2 86, members of our group and others have constructed a large number of single-site and deletion mutants of this protein and have tested them with respect to autoregulation, DNA binding, promoter activation, and protein-protein interaction. The general consensus is that the carboxy-terminal half of IE2 86 (aa 313 to 579) is responsible for autoregulation and DNA binding to its own promoter and to HCMV early promoters (1, 4, 5, 14, 19, 25, 30-32, 37, 43, 50-52, 59-61, 65, 72). The region of IE2 86 involved in protein-protein interactions is more extensive and maps within aa 86 to 542 (10, 14, 17, 21, 32, 60, 61). The studies directed at mapping the activation domains of IE2 86 have primarily involved transient expression assays and have yielded results that are variable and depend on the target promoter used and, to a lesser extent, on the host cell (for a review, see reference 18).
Posttranslational modifications of IE2 86 may also be very important for its function. There is a cluster of serines from aa 258 to 275 that lie within a consensus casein kinase II site, and this region of IE2 86 shows the highest level of phosphorylation in vitro when incubated with cell extracts. Phosphorylation of IE2 86 has been found to have an adverse effect on the ability of the protein to bind to DNA (23, 24, 67). Harel and Alwine have also presented data suggesting that mutation of several potential phosphorylation sites in IE2 86 affects its transactivating ability in transient transfections. Interestingly, IE2 86 mutants with substitutions of alanine at aa T233/S234 or S144 functioned in transient assays as stronger activators of transcription than did wild-type IE2 (24). In addition to phosphorylation, IE2 86 can be modified by the addition of SUMO (2, 26), and recent evidence indicates that the SUMOylation of aa 175 and 180 is required for activation of the HCMV UL112-113 promoter (26).
Although the above-mentioned studies have provided useful information, it is recognized that they did not necessarily present an accurate picture of the events that occur during a normal infection. Because the transcriptional regulatory functions of IE2 86 were primarily studied in transient expression assays and the majority of the protein-protein and protein-DNA interactions were observed either in cells where the proteins were overexpressed or in in vitro binding assays, the biological relevance of the results is uncertain. Most agree that the best approach for understanding the function of viral regulatory proteins is to test mutants of these genes in the context of the viral genome.
In the past, the generation of viruses with mutations in essential genes required that they be isolated and propagated in cells expressing the wild-type proteins. This has provided a tremendous challenge in the case of IE2 86 as several groups have attempted to establish cell lines capable of complementing ie2 mutants without success. The single factor that has probably been most important in overcoming this obstacle has been the development of an approach for constructing herpesvirus mutants that is based on the cloning and mutagenesis of the viral genomes as bacterial artificial chromosomes (BACs) in Escherichia coli (6). The tremendous advantage of this method is that any mutation is easily introduced into the cloned viral genome, and the mutagenized genome is physically characterized while still a BAC. The mutated genome is then transfected into permissive cells with an HCMV pp71 (UL82) expression plasmid, generating mutant viruses that are free of the wild-type virus. Using this approach, Marchini et al. have recently constructed an HCMV recombinant virus with a deletion of the majority of the IE2 86 gene and shown that replication of this mutant virus is blocked at the early phase of the cycle (44).
In this paper, we have used the BAC technology to isolate and analyze the functional properties of a viral mutant that has an internal deletion within the IE2 86 gene corresponding to aa 136 to 290. For ease of analysis, we also fused the carboxy terminus to enhanced green fluorescent protein (EGFP). Our choice of this deletion was based in part on the studies described above regarding the effect of the posttranslational modifications occurring in this region on transactivation and protein-protein interactions. In addition, members of our group and others had found that deletion of the entire region reduced but did not eliminate the transactivating function of IE2 86 in transient expression assays (26, 61). Based on these prior observations, it seemed likely that the recombinant virus would still be viable and that we would be able to dissect the pleiotropic functions of the protein in the context of the entire infection. Our studies show that the mutant virus is viable, but there is a significant delay in the production of infectious virus. One effect of the deletion is that the level of IE2 protein is significantly lower in the cells infected with the mutant virus. Despite the reduced amount of IE2 protein, the early phase of the infection and the kinetics of viral DNA replication are comparable to that of the wild-type virus. At late times, however, the steady-state levels of the pp65 (UL83) and pp28 (UL99) matrix proteins are greatly reduced. This is accompanied by a decrease in the levels of the mRNA encoding UL83, but not UL99.
MATERIALS AND METHODS
Cells and virus.
Human foreskin fibroblasts (HFF) were maintained in minimum essential medium with Earle's salts containing 10% fetal bovine serum (FBS) except where noted otherwise. Methods for cell culture and viral infection have been described elsewhere (66).
Molecular cloning.
The plasmid pHCMV EcoRI J (66) containing the entire major immediate-early region of HCMV strain AD169 was used as the starting material for the construction of all of the mutations in IE2 86.
To make the plasmid pJΔSX, which is missing aa 136 to 290 of the IE2 86 protein, separate aliquots of pHCMV EcoRI J were digested with either SmaI or XhoI. The ends of the XhoI digest were filled in using Klenow enzyme. Both digests were then subjected to digestion with XbaI, and fragments of 7.6 and 6.25 kbp were gel purified. These fragments were subsequently ligated together to form the plasmid pJΔSX. The mutation was verified by DNA sequencing to ensure that the deletion was present and that the correct IE2 86 reading frame was maintained. A 2-kbp BglII-StuI fragment containing the 3′ half of exon 4 and the majority of exon 5 down to the StuI site was taken from the plasmid pHCMV EcoRI J and subcloned into the vector pSG5 (Stratagene) to generate the plasmid pSG5-J (BglII-StuI). A 1.5-kbp BglII-StuI fragment from pJΔSX was also subcloned into pSG5 to yield the plasmid pSG5-JΔSX (BglII-StuI). These plasmids were used in the preparation of the JΔSX-EGFP revertant virus.
The wt IE2 86 and IE2 86ΔSX-EGFP fusion plasmids were constructed as follows. A 668-bp AvrII-StuI fragment, containing the 3′ end of the IE2 86 ORF, was isolated from pHCMV EcoRI J and subcloned into the vector pFastBacI (Gibco-BRL). A SwaI site was introduced, replacing the stop codon at the 3′ end of exon 5 using the QuickChange site-directed mutagenesis kit (Stratagene) and two oligonucleotides (sense, 5′-CAAGTCTCAGTATTTAAATAACTGGAAAGAG-3′; antisense, 5′-CTCTTTCCAGTTATTTAAATACTGAGACTTG-3′). The resulting plasmid was designated pFB-J(AvrII-StuI)/SwaI. To isolate EGFP, the plasmid pEGFP-N3 (Clontech) was digested with NotI, treated with Klenow enzyme, and then digested with SmaI to obtain a 742-bp fragment that contained the EGFP ORF. This fragment was cloned into the SwaI site of pFB-J (AvrII-StuI)/SwaI, resulting in the plasmid pFB-J(AvrII-StuI)-EGFP. The correct orientation of the insert was confirmed by restriction analysis. The AvrII-StuI fragment containing the EGFP fused to the 3′ end of exon 5 was then subcloned back into pHCMV EcoRI J and pJΔSX to generate the plasmids pHCMV EcoRI J-EGFP and pJΔSX-EGFP, respectively.
The recombination shuttle plasmid used for the BAC mutagenesis, pSTKS11, was a kind gift from M. Messerle, Max von Pettenkofer Institute, Munich, Germany. The EcoRI inserts from the plasmids pHCMV EcoRI J, pHCMV EcoRI J-EGFP, and pJΔSX-EGFP were subcloned into this vector prior to the BAC mutagenesis.
BAC mutagenesis.
The E. coli strain CBTS containing the HCMV AD169 genome as a BAC (pHB5) was also a gift from M. Messerle. The CBTS strain is recA negative at 30°C and recA positive at temperatures higher than 37°C. A chloramphenicol resistance marker is located within the BAC sequences residing in the HCMV genome. The pSTKS11 shuttle plasmid encodes a kanamycin resistance marker, the negative selection marker SacB, and a temperature-sensitive origin of replication. It replicates poorly at temperatures above 37°C and in the presence of sucrose. Mutagenesis of the HCMV BAC plasmid was performed as previously described (6).
The protocol for generating mutants in the viral BAC genome relies on a two-step replacement strategy. Briefly, the IE2 86 mutations within the pSTKS11 shuttle vector were electroporated into the CBTS bacteria, and transformants were selected at 30°C on Luria-Bertani (LB) agar plates containing chloramphenicol (30 μg/ml) and kanamycin (30 μg/ml). Recombination between the homologous regions in the IE1-IE2 sequence yields a cointegrate. Clones that had formed cointegrates were selected for by incubating them at 43°C on LB agar plates containing both of the above antibiotics. Confirmed cointegrate clones were streaked onto LB agar plates containing chloramphenicol only and grown at 30°C. At this temperature recA is active and the cointegrates can resolve. Clones that had resolved cointegrates were selected for on LB agar plates containing chloramphenicol and 5% sucrose at 30°C. Kanamycin-sensitive clones were confirmed to be pure mutants by either Southern blot and/or by cycle sequencing of the BAC DNA using the Thermo Sequenase Radiolabeled Terminator Cycle Sequencing Kit (United States Biochemical). pHB5, p86-EGFP, and p86ΔSX-EGFP BAC DNAs were propagated in the recA-negative bacterial strain DH10B (Gibco-BRL) and purified from these cells using Nucleobond Nucleic Acid purification columns (Clontech).
Reconstitution of the IE2 86 mutant BAC viruses.
HFF cells (3.2 × 106 cells) were electroporated with 6.25 μg of BAC DNA and 3.75 μg of the pp71 expression plasmid pcDNApp71tag (kindly provided by B. Plachter, University of Mainz, Mainz, Germany). Electroporations were carried out using a BTX ECM-600 electroporator (Genetronics, Inc.) as previously described (47). Following electroporation, the cells were seeded into either 12-well dishes containing sterile coverslips for immunofluorescence assays (IFA) or 6-cm tissue culture dishes for monitoring of plaque development. IE2 86ΔSX-EGFP mutant plaques were checked to see that they had not reverted to the wt by cycle sequencing of genomic DNA from infected cells harvested when viral cytopathic effect was at its maximum. Stocks of wt AD169 virus, prepared from pHB5 BAC, as well as the wt IE2 86-EGFP and IE2 86ΔSX-EGFP mutant viruses, were prepared and titered as previously described (66).
Preparation of IE2 86 mutant revertant viruses.
The plasmids pSG5-J (BglII-StuI) and pSG5-JΔSX (BglII-StuI) were digested with BglII and StuI, which liberated 2- and 1.5-kbp inserts containing wt IE2 86-EGFP and IE2 86ΔSX-EGFP sequences, respectively. Twenty micrograms of DNA from each of these digests was coelectroporated into HFF cells along with 6.25 μg of p86ΔSX-EGFP BAC DNA and 3.75 μg of pCDNApp71tag. Plaques resulting from this electroporation were picked and used to infect fresh monolayers of cells. Viral stocks were prepared from this infection and titered for use in subsequent analyses. Genomic DNA was also harvested from the infected cells and sequenced to confirm that the IE2 86ΔSX mutation had indeed reverted to the wt only when the wt IE2 86-EGFP was cotransfected with the mutant BAC DNA.
Time course infection with wt IE2 86-EGFP, IE2 86ΔSX-EGFP, and IE2 86ΔSX-EGFP revertant viruses.
HFF cells were seeded onto coverslips for IFA, into flasks for protein and DNA analyses, or into 12-well dishes for growth cycle analysis the day before infection. Cells were mock infected or infected at an MOI of 0.5 with each of the above viruses in media containing 10% FBS. Harvests were taken at 8, 24, 48, 72, 96, and 120 h p.i. for viral protein analysis, and at 24, 48, 72, 96, 120 h p.i. and 7 days p.i. for viral DNA analysis. Cells were fixed for IFA at 24, 72, and 120 h p.i. and 8 days p.i. Supernatants were taken for viral titers 3, 5, 7, 9, 11 and 14 days p.i. Infections for viral growth cycle analysis were done in triplicate.
Southern blot and viral DNA slot blot analyses.
Total cell genomic DNA was prepared from wt IE2 86-EGFP-, IE2 86ΔSX-EGFP-, IE2 86ΔSX-EGFP revertant-, or mock-infected cells at 24, 48, 72, 96, 120, and 168 h p.i., using the QIAamp DNA Blood Mini Kit (Qiagen). DNAs were quantified with a UV spectrophotometer and slotted onto a nylon filter using the Minifold II Slot Blotter System. The filter was hybridized to a 32P-labeled, 4.5-kbp ApaLI-NcoI DNA fragment isolated from pHCMV EcoRI B and subjected to autoradiography. The autoradiograph was scanned, and the number of pixels in each slot was determined using the Histogram function of the program Adobe Photoshop 5.5.
For the Southern blot, pHB5, wt IE2 86-EGFP, and IE2 86ΔSX-EGFP BAC DNAs were digested with BamHI and subjected to Southern blot analysis using standard methods. Blots were hybridized to a 32P-labeled, 4.5-kbp PmlI-ApaLI fragment isolated from pHCMV EcoRI fragment J and subjected to autoradiography.
Western blotting.
HFF cells were infected with IE2 86-EGFP, IE2 86ΔSX-EGFP, or IE2 86ΔSX-EGFP revertant viruses at an MOI of 0.5 in media containing 10% FBS. Cells were harvested at various time points. Cell pellets were lysed in reducing sample buffer (RSB) (50 mM Tris [pH 6.8], 0.2% sodium dodecyl sulfate, 10% glycerol, 5% 2-mercaptoethanol, 25 mM sodium fluoride, 1 mM sodium orthovanadate, 5 mM β-glycerophosphate, 1 mM phenylmethylsulfonyl fluoride, 50 μM leupeptin, and 100 μM pepstatin A) without bromophenol blue, sonicated, and boiled for 5 min. Protein content was determined by comparing the A280 of samples to known standards as previously described (45). Either 80 μg (8 and 24 h p.i.) or 40 μg (48, 72, 96, and 120 h p.i.) of protein in RSB with bromophenol blue was resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) on Bio-Rad 7.5% polyacrylamide, Tris-HCl Ready Gels, or 10% polyacrylamide gels for pp28. Proteins were transferred to nitrocellulose as previously described (55). Primary antibodies were diluted in Blotto (5% nonfat milk and 0.5% Tween 20 in Tris-buffered saline [pH 7.4]) as follows: CH16.0 (1:10,000; Goodwin Institute), anti-IE1 72 monoclonal antibody (MAb) p63-27 (1:5,000; William Britt), anti-EGFP polyclonal rabbit antibody (1:500; Clontech), rabbit anti-UL112-113 proteins (1:1,000) (61), anti-UL44 (1:5,000; Goodwin Institute), anti-major capsid protein (MCP) MAb 28-4 (1:10; William Britt), anti-pp65 MAb (1:7,500; Goodwin Institute), and anti-pp28 MAb 41-18 (1:10; William Britt). Horseradish peroxidase (HRP)-conjugated secondary antibodies against mouse and rabbit were purchased from Calbiochem and diluted 1:1,000 to 1:10,000. SuperSignal chemiluminescent substrate was purchased from Pierce and used per the manufacturer's instructions.
Immunofluorescence.
HFF cells were seeded on glass coverslips and infected with virus at an MOI of 0.5 in media containing 10% FBS. At various time points, cells were fixed in 2% paraformaldehyde in phosphate-buffered saline (PBS) and processed as previously described (55). Briefly, cells were permeabilized in 0.2% Triton X-100 in PBS for 5 min at room temperature (RT). After three washes in PBS, cells were blocked with 10% normal goat serum in PBS for 30 min at RT. Primary antibodies described above were diluted in PBS containing 5% normal goat serum as follows: IE1 72 (1:500), UL44 (1:1,000), MCP and pp65-specific MAb 28-19 (no dilution; from William Britt). Coverslips were incubated with primary antibody for 30 min at RT. After being washed, coverslips were incubated with goat anti-mouse immunoglobulin G tetramethyl rhodamine isocyanate (TRITC)-conjugated antibody (1:200 dilution in PBS with 5% normal goat serum; Jackson Immunologicals) and Hoechst for 30 min at RT. Washed coverslips were mounted onto slides with SlowFade antiphotobleaching reagent (Molecular Probes). Images were captured and processed in Adobe Photoshop.
Northern blot analysis.
Messenger RNA was isolated from IE2 86-EGFP-, IE2 86ΔSX-EGFP-, or IE2 86ΔSX-EGFP revertant virus-infected cells at 24, 48, and 96 h p.i. using the FastTrack 2.0 kit (Invitrogen). Approximately 1.3 × 107 cells per sample were infected at an MOI of 0.5 in media containing 10% FBS. The Northern blots were performed using the NorthernMax kit (Ambion) and approximately 1 μg of mRNA was loaded per lane. The 32P-labeled probes were synthesized by random priming (StripEZ DNA kit; Ambion) of the following DNA fragments: a 1.1-kbp SstII-SstII fragment from pp65, a 0.75-kbp XhoI-StuI fragment from exon 5 of IE2 86, a 0.5-kbp HindIII-BglII fragment of pp71, a 0.7-kbp fragment encoding pp28, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) decatemplate (Ambion). All reactions were performed according to the manufacturer's recommendations. After hybridization, filters were subjected to autoradiography.
RESULTS
Construction of recombinant HCMV and revertant viruses.
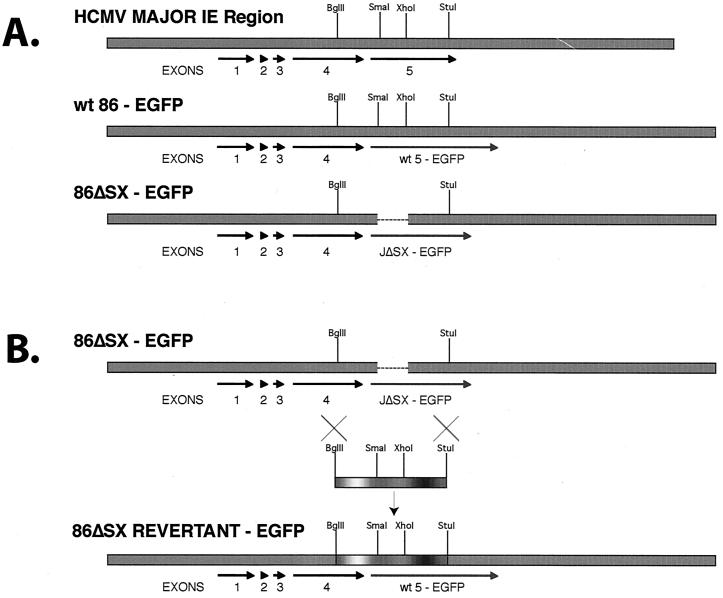
To generate the in-frame deletion (aa 136 to 290) within the genomic IE2 86 gene, we first deleted a 0.9-kbp SmaI-XhoI fragment from the HCMV (strain AD169) 10-kbp EcoRI fragment J (66) (Fig. 1A). The deletion also removes the promoter for the p40 form of IE2 86 as well as the initiator methionine for the p60 form of the protein. Both of these forms usually appear at late times p.i. (30, 53). We also fused the carboxy termini of both the mutant and wild-type genes to EGFP to facilitate the analysis. Both constructs were subcloned into the BAC shuttle vector pSTKS11, and the resulting vectors were then used to generate recombinant wt and mutant BAC plasmids, IE2 86-EGFP and IE2 86ΔSX-EGFP, respectively, as outlined in Materials and Methods. The BAC DNAs were checked for the integrity of the viral sequences, and the mutations were confirmed by Southern blotting and DNA sequencing.
FIG. 1.
Preparation of IE2 86ΔSX-EGFP and IE2 86ΔSX-EGFP revertant viruses. (A) A 0.9-kbp SmaI-XhoI fragment (corresponding to aa 136 to 290 of IE2 86) from exon 5 was removed, and the remaining fragment was cloned in-frame with EGFP to make IE2 86ΔSX-EGFP. The entire EcoRI J fragment containing this mutation was put into the BAC containing HCMV AD169 via recombination in E. coli. (B) Mutant BAC DNA was harvested and transfected with a wt BglII-StuI fragment into HFF to recover revertant virus.
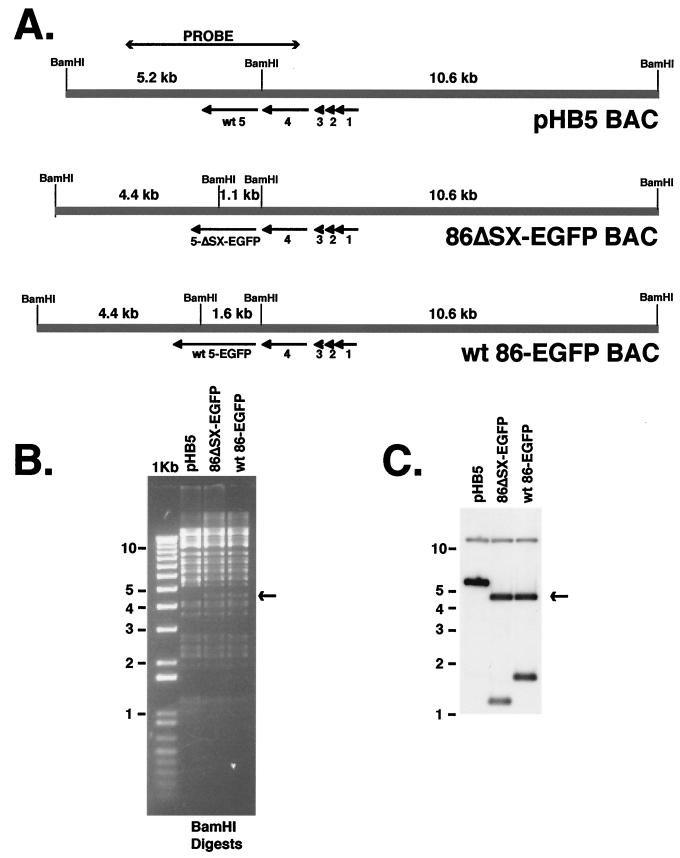
Figure 2A shows the BamHI restriction digest pattern of the BAC DNAs containing wt IE2 86-EGFP and IE2 86ΔSX-EGFP sequences. For comparison, we also show the original HCMV BAC plasmid pHB5 (6) with wt IE2 86 sequences. As the probe for the Southern blot, we chose a 4.5-kbp PmlI-ApaLI restriction fragment that spans the BamHI site located between exons 4 and 5. The ethidium bromide-stained agarose gel and Southern autoradiograph are shown in panels B and C, respectively. In the parental BAC, pHB5, the probe hybridizes to two fragments, of 5.2 and 10.6 kbp. In both the wt IE2 86-EGFP and IE2 86ΔSX-EGFP BACs, an additional BamHI site is introduced within the EGFP sequences. Because of this, the downstream BamHI fragment is cleaved into two smaller fragments, of 4.4 and 1.6 kbp in the case of the wt IE2 86-EGFP BAC and 4.4 and 1.1 kbp for IE2 86ΔSX-EGFP BAC. The 500-bp reduction in the size of the internal BamHI fragment of IE2 86ΔSX-EGFP BAC is due to the ΔSX deletion. The presence of the 4.4-kbp BamHI fragment can be easily visualized in the wt IE2 86-EGFP BAC and IE2 86ΔSX-EGFP BAC lanes of the ethidium bromide-stained gel (Fig. 2B) as well as in the autoradiograph.
FIG. 2.
Southern blot analysis of wt (pHB5), IE2 86-EGFP, and IE2 86ΔSX-EGFP BAC DNA. (A) A 4.5-kbp PmlI-ApaLI fragment encompassing exon 5 of IE2 86 of HCMV EcoRI J was used as a probe for Southern blots. (B) BAC DNAs were digested with BamHI, and fragments were separated by agarose gel electrophoresis. The arrow designates the position of a specific fragment resulting from an additional BamHI site introduced by the EGFP fusion. (C) Southern blot of BamHI digests of BAC DNAs.
To reconstitute the recombinant HCMV, BAC DNA containing wt IE2 86-EGFP or IE2 86ΔSX-EGFP sequences was electroporated into HFF cells. EGFP-expressing plaques were evident in the wt IE2 86-EGFP-transfected cells by day 6 postelectroporation. These plaques spread quickly until the entire monolayer was infected. High-titer viral stocks (>2 × 107 PFU/ml) were prepared from this transfection. In contrast, cells transfected with the IE2 86ΔSX-EGFP BAC did not demonstrate plaques until day 9 postelectroporation, and these plaques were much smaller and more isolated than the wt plaques. When the cells were maintained in 10% FBS, the IE2 86ΔSX-EGFP virus was unable to spread sufficiently to infect all of the cells in the culture even after several passages of the cells. Under these conditions, the cells appeared to be dividing at a faster rate than the infection could spread, thus establishing a persistent infection. Peak viral titers resulting from the electroporation were only between 105 and 106 PFU/ml. To ensure that the virus resulting from this transfection was the IE2 86ΔSX-EGFP mutant and had not spontaneously reverted back to the wt, DNA from transfected cells was sequenced and the mutation was confirmed.
To confirm that the IE2 86ΔSX-EGFP phenotype was due only to the deletion within IE2 86, we constructed an IE2 86ΔSX-EGFP revertant virus. A 2-kbp BglII-StuI fragment was isolated from wt HCMV EcoRI J and cotransfected into HFF cells along with the IE2 86ΔSX-EGFP mutant BAC (Fig. 1B). EGFP-expressing plaques that exhibited wt growth characteristics were picked and plaque purified to homogeneity. Virus stocks prepared from these plaques were checked by DNA sequencing to ensure that they had reverted to the wt.
Cell cultures infected with a low MOI of the IE2 86ΔSX-EGFP mutant virus show delayed kinetics in generating infectious virus.
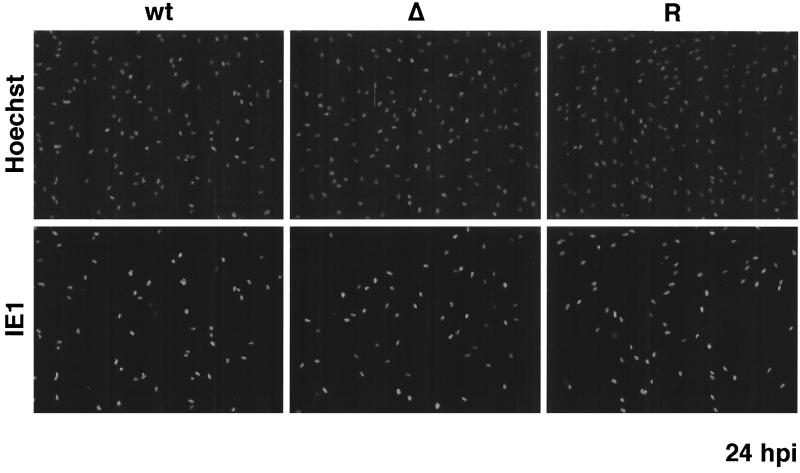
To investigate the kinetics of viral replication and spread, HFF cells were infected in triplicate with wt IE2 86-EGFP, IE2 86ΔSX-EGFP, or IE2 86ΔSX-EGFP revertant viruses at an MOI of 0.5. Supernatants were obtained at 3, 5, 7, 9, 11, and 14 days p.i., and the viral titer of each supernatant was determined by standard plaque assay on HFF cells. As a control for the first round of infection, cells seeded onto coverslips and infected at the same MOI were subjected to immunofluorescent staining with IE1-specific antibody at 24 h p.i. As shown in Fig. 3, the percentage of IE1-positive cells within each of the three infected cultures was similar at this time point. The kinetics of virus accumulation and peak viral titers for the wt IE2 86-EGFP infection were identical to those for the IE2 86ΔSX-EGFP revertant virus (Fig. 4). In contrast, the IE2 86ΔSX-EGFP mutant virus showed delayed growth kinetics. Peak titers for the wt and revertant viruses were reached by day 9. In contrast, the titer of the mutant virus remained 1 to 2 logs below that of the wt and revertant viruses until day 11. There was a small increase in the titer of the mutant virus between days 11 and 14, resulting in a peak titer that was slightly higher than the peak reached by the wt and revertant viruses at day 9. These results were consistent with the continued growth of the cells and slower spread of the virus in the cultures infected with the mutant.
FIG. 3.
IE1 72 expression in cultures infected with wt IE2 86-EGFP (wt), IE2 86ΔSX-EGFP (Δ), or IE2 86ΔSX-EGFP revertant (R) viruses. HFF cells were seeded on coverslips and infected with virus at an MOI of 0.5 in media containing 10% FBS. At 24 h p.i., cells were fixed in 2% paraformaldehyde. After permeabilization, cells were stained with IE1 72-specific MAb p63-27 followed by anti-mouse IgG TRITC-conjugated secondary antibody. Hoechst stain was used to visualize the total number of nuclei in the field. Magnification, ×87.
FIG. 4.
Single-step growth curves of IE2 86-EGFP (wt), IE2 86ΔSX-EGFP (Δ), and IE2 86ΔSX-EGFP revertant (R) viruses. HFF cells were infected with recombinant viruses at an MOI of 0.5 in media containing 10% FBS. Supernatants were collected at various times p.i. Titers of virus were measured by standard plaque assays on HFF cells. The results shown here and in Fig. 5, 6, 8, 9, 10, 11, 12, and 13 are from the same experiment shown in Fig. 3.
Accumulation of IE1 72 is only slightly delayed in cells infected with the IE2ΔSX-EGFP mutant virus.
To determine which stages of the viral infection were affected by the mutation in IE2 86, cell cultures were infected at a MOI of 0.5. At various times p.i., the cells were harvested and the infected cell proteins were analyzed by Western blot with antibodies directed against selected IE, early, and late HCMV proteins. At the same time points, coverslips of infected cells were fixed and analyzed by IFA.
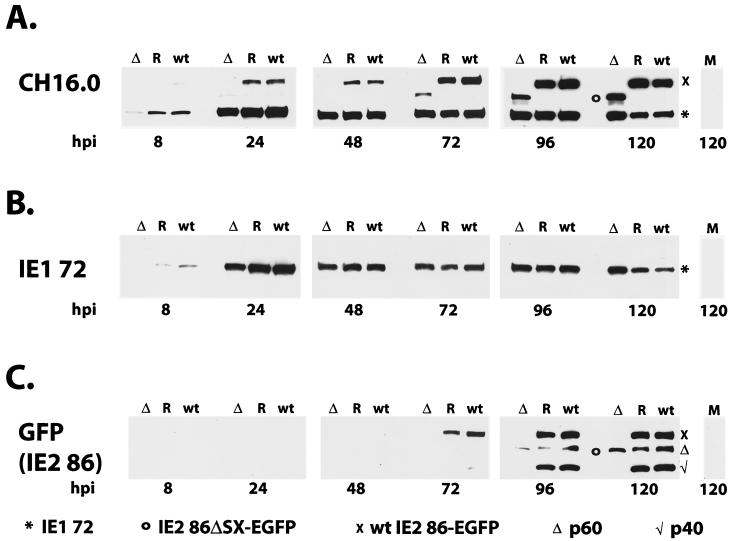
The Western blot in Fig. 5 shows the synthesis and steady-state accumulation of the major wt and mutant IE gene products encoded by the IE1/IE2 region of the viral genome. For this experiment, we first used a highly sensitive antibody (CH16.0) that is directed against the amino-terminal domain common to the IE1 72 and IE2 86-EGFP proteins (Fig. 5A). Levels of IE1 72 were comparable for the mutant, wt, and revertant viruses throughout most of the time course. Although at 8 h p.i. we observed a small lag in IE1 72 production for the IE2 86ΔSX-EGFP mutant, by 24 h p.i. the levels of this protein were similar in all three viral infections. This pattern continued until late in the infection (120 h p.i.), at which time there appeared to be slightly more IE1 72 accumulating in the cultures infected with the IE2 86ΔSX-EGFP mutant. The identity of the IE1 72 was confirmed with an antibody that is specific for a region of the protein encoded by exon 4 (Fig. 5B).
FIG. 5.
Western blots of IE proteins. HFF cells were infected with IE2 86-EGFP (wt), IE2 86ΔSX-EGFP (Δ), or IE2 86ΔSX-EGFP revertant (R) viruses at an MOI of 0.5 in media containing 10% FBS. Cells were harvested at the time points indicated. Either 80 μg (8 and 24 h p.i.) or 40 μg (48, 72, 96, and 120 h p.i.) of protein was resolved by SDS-PAGE and transferred to nitrocellulose. Filters were probed with an exon 2-specific MAb, CH16.0 (A), an exon 4-specific MAb, p63-27 (IE1 72) (B), or a rabbit antibody specific for EGFP (IE2 86) (C). Reactive proteins were visualized by chemiluminescence. Symbols denote the positions of specific proteins, as indicated.
We determined the distribution of the IE1 72 protein within individual cells in the cultures as the infection progressed by IFA. As shown in Fig. 6, examination of the cells with an antibody specific for the IE1 72 protein supported the data from the Western blot analysis. At 72 h p.i., there were similar numbers of IE1 72-positive cells in the wt-, revertant-, and mutant virus-infected cultures (Fig. 6A). As expected for an infection at an MOI of 0.5, not all of the cells appeared to be infected at this time point. By 120 h p.i., a second round of infection had already begun, and more cells were positive for IE1 72 (Fig. 6B). However, at this time point, there were clear differences between the cultures infected with mutant virus and those infected with either wt or revertant viruses. Cultures infected with the ΔSX mutant virus contained more cells than those infected with the wt control. As a result, although the total number of IE1 72-positive cells was similar in the wt-, revertant-, and mutant virus-infected cultures, the percentage of positive cells differed. In both the wt and revertant cultures, almost 100% of the cells were IE1 72 positive, while in the cultures infected with the mutant virus only 50 to 60% of cells were positive at 120 h p.i.
FIG. 6.
IE1 72 expression in cultures infected with wt IE2 86-EGFP (wt), IE2 86ΔSX-EGFP (Δ), or IE2 86ΔSX-EGFP revertant (R) viruses. HFF cells were seeded on coverslips and infected with virus at an MOI of 0.5 in media containing 10% FBS. At the time points indicated, cells were fixed in 2% paraformaldehyde. After permeabilization, cells were stained with IE1 72-specific MAb p63-27 followed by antimouse IgG TRITC-conjugated secondary antibody. Hoechst stain was used to visualize the total number of nuclei in the field. Magnification, ×80.
There is significantly less IE2 protein in cells infected with the mutant virus.
In contrast to the relatively similar levels of IE1 72 for all three infections, there was a significant difference in the accumulation of the wt IE2 86-EGFP and mutant IE2 86ΔSX-EGFP proteins. In both the wt and revertant virus infections, the fusion protein could easily be detected at 24 h p.i. with the CH16.0 antibody, and with long exposures of the Western blot, it was apparent at 8 h p.i. (Fig. 5A and data not shown). However, the mutant IE2 86ΔSX-EGFP protein could not be detected until 48 to 72 h p.i., and its level remained below that of the wt protein throughout the time course. With an antibody that was directed specifically against the EGFP carboxy-terminal domain of the IE2 86 fusion proteins, the pattern of accumulation of the wt and mutant IE2 86 proteins was similar to that observed with the CH16.0 antibody, although at early times the levels of the protein were below the limit of detection with this less-sensitive antibody (Fig. 5C). The anti-EGFP antibody also revealed the p40 and p60 forms of the IE2 proteins that initiate within exon 5 and are synthesized later in the infection. As expected, these forms of the protein were not observed in cells infected with IE2 86ΔSX-EGFP, since the deletion removes the promoter for p40 as well as the initiator methionine for p60. The above-described results suggested that there was a defect in either the synthesis or the stability of the IE2 86ΔSX-EGFP RNA or protein. In order to determine the nature of the decrease, we also analyzed the IE2 86-EGFP transcripts produced by these viruses by Northern blotting using a probe which recognized full-length IE2 86, p60, and p40 mRNAs (Fig. 7). We observed that the mutant virus produced levels of the IE2 86ΔSX-EGFP deletion mRNA that were similar to those of full-length IE2 86-EGFP transcripts in the wt and revertant samples. This observation suggested that the lower level of IE2 86ΔSX-EGFP observed in cells infected with the mutant virus was due to decreased protein stability. We also detected a small amount of a shorter transcript that was consistent with being a truncated form of the IE2 p60 mRNA in cells infected with the mutant virus; however, we were unable to obtain evidence for expression of a truncated protein.
FIG. 7.
Northern blot analysis of IE2-related transcripts expressed in HFF cells infected with IE2 86-EGFP (wt), IE2 86ΔSX-EGFP (Δ), or IE2 86ΔSX-EGFP revertant (R) viruses at an MOI of 0.5 in media containing 10% FBS. Cells were collected at the time points indicated. One microgram of mRNA per sample was resolved by agarose gel electrophoresis and transferred to nylon filters. Blots were hybridized to an IE2 86-specific probe spanning the 3′ end of exon 5 (A) and a GAPDH-specific probe as a loading control (B). Symbols denote the position of specific mRNAs: arrowhead, full-length wt IE2 86 mRNA; ∗, ΔSX deletion IE2 86 mRNA;], p40/p60 mRNA; open circle, ΔSX truncated p60 mRNA.
Synthesis of viral early proteins is only modestly affected in cells infected with IE2 86ΔSX-EGFP mutant virus during the first round of infection.
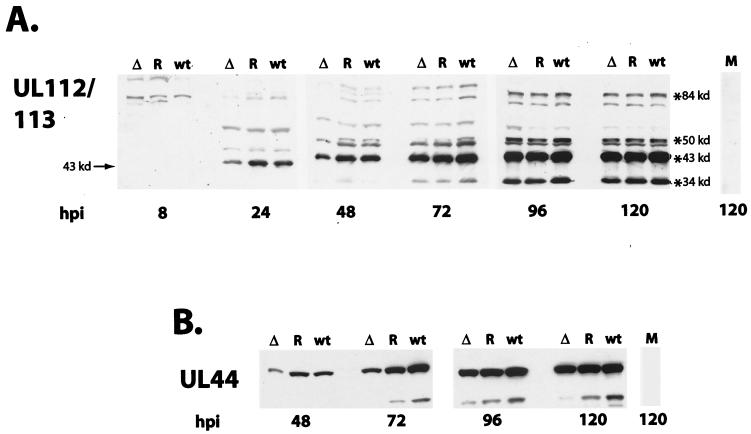
To assess early gene expression in the cells infected with the IE2 86ΔSX-EGFP mutant virus, we probed Western blots with two antibodies. One was directed against the four proteins (with masses of 84, 50, 43, and 34 kDa) encoded by UL112/113, and the other was specific for the protein encoded by UL44 (52 kDa). As shown in Fig. 8, the synthesis and accumulation of these representative early proteins in cells infected with the mutant virus showed a small lag relative to that observed in either the wt or revertant virus infections. This pattern was similar to what was observed for IE1 72. The major early protein detected by the UL112/113 antisera has a mass of 43 kDa, and this gene product is one of the earliest to appear after the synthesis of the IE proteins. Although in the IE2 86ΔSX-EGFP infection there was a slightly lower level of the p43 phosphoprotein at 24 h p.i. (and of the other three proteins at 48 h p.i.), the levels of all four phosphoproteins were comparable by 72 h p.i. (Fig. 8A). The major 52-kDa UL44 protein is considered to be a delayed early gene product. It was detected in the cultures infected with all three viruses at 48 h p.i., with the level in the cells infected with the mutant being approximately twofold lower at 48 and 72 h p.i. (Fig. 8B).
FIG. 8.
Western blots of early viral proteins. HFF cells were infected with IE2 86-EGFP (wt), IE2 86ΔSX-EGFP (Δ), or IE2 86ΔSX-EGFP revertant (R) viruses at an MOI of 0.5 in media containing 10% FBS. Cells were harvested at the time points indicated. Either 80 μg (8 and 24 h p.i.) or 40 μg (48, 72, 96, and 120 h p.i.) of protein was resolved by SDS-PAGE and transferred to nitrocellulose. Filters were probed with a rabbit antibody against the UL112-113 family of proteins (A) and a UL44-specific monoclonal antibody (B). Reactive proteins were visualized by chemiluminescence. The positions of the specific proteins are indicated.
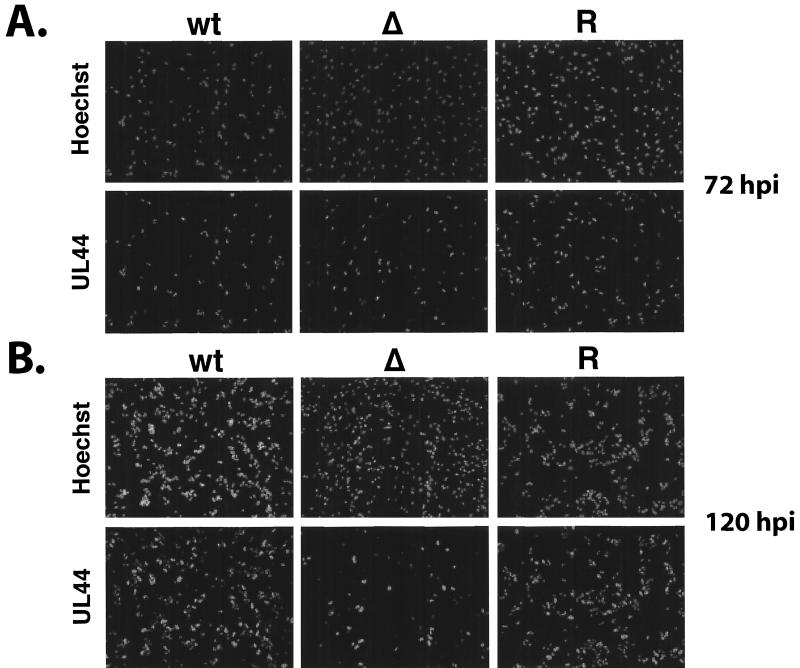
We also looked at the level and distribution of UL44 by IFA at 72 and 120 h p.i. (Fig. 9). At 72 h p.i. there were similar numbers of UL44-positive cells in the cultures infected with the wt, revertant, and mutant virus; however, at 120 h p.i., two important differences were noted. First, as described above, there were more cells in the cultures infected with IE2 86ΔSX-EGFP. Second, in the wt and revertant cultures, there was a significant increase in the total number of UL44-positive cells, while the total number of positive cells in the cultures infected with the mutant virus was approximately the same at both 72 and 120 h p.i. Since the number of IE1 72 cells in the cultures infected with the mutant virus had increased during this interval, these results indicated that there was a lag in the second round of infection. These observations were also consistent with a delay in the spread of the virus. If this were the case, then the cultures infected with the mutant virus would be at a much earlier point in the secondary infection than the cultures infected with the wt and revertant viruses.
FIG. 9.
UL44 expression in cultures infected with wt IE2 86-EGFP (wt), IE2 86ΔSX-EGFP (Δ), or IE2 86ΔSX-EGFP revertant (R) viruses. HFF cells were seeded on coverslips and infected with virus at an MOI of 0.5 in media containing 10% FBS. At the time points indicated, cells were fixed in 2% paraformaldehyde. After permeabilization, cells were stained with UL44-specific monoclonal antibody followed by antimouse IgG TRITC-conjugated secondary antibody. Hoechst stain was used to visualize the total number of nuclei in the field. Magnification, ×81.
Viral DNA replication is not affected by the deletion in IE2 86.
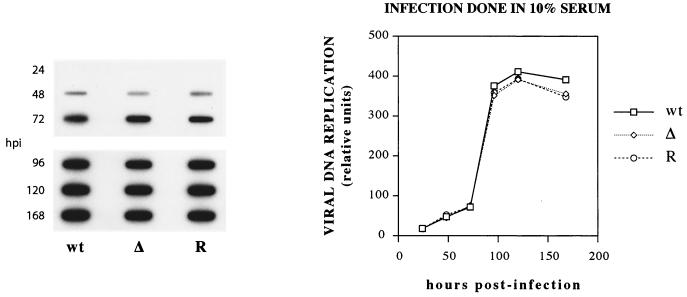
The above-described results indicated that the accumulation of IE1 72 and the viral early proteins was only modestly affected in cells initially infected with IE2 86ΔSX-EGFP mutant virus. However, the deletion within the IE2 86 protein resulted in lower steady-state levels of IE2-specific proteins in the cells infected with the mutant. To determine whether viral DNA replication was affected, cells infected with wt, mutant, and revertant virus were harvested at various time points p.i. and total cellular DNA was subjected to slot blot hybridization analysis. The blot was probed for viral sequences, and individual slots on the autoradiograph were quantified using the Histogram function of Adobe Photoshop. Figure 10 shows that the kinetics of DNA replication and levels of viral DNA for the mutant were very similar to those for the wt and revertant. These results suggested that the delay in virus production observed in cells infected with IE2 86ΔSX-EGFP was most likely due to a defect that occurs after the viral DNA has been replicated.
FIG. 10.
Kinetics of viral DNA replication in HFF cells infected with IE2 86-EGFP (wt), IE2 86ΔSX-EGFP (Δ), and IE2 86ΔSX-EGFP revertant (R) viruses. HFF cells were infected with recombinant viruses at an MOI of 0.5 in media containing 10% FBS. Virus-infected cells were collected at various times postinfection. Viral DNA was measured by slot blot and quantified using Adobe Photoshop 5.5.
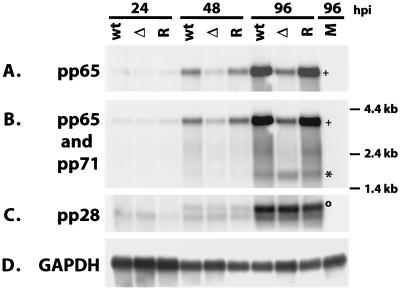
Cells infected with the mutant virus show a significant defect in the accumulation of the tegument proteins encoded by UL83 (pp65) and UL99 (pp28).
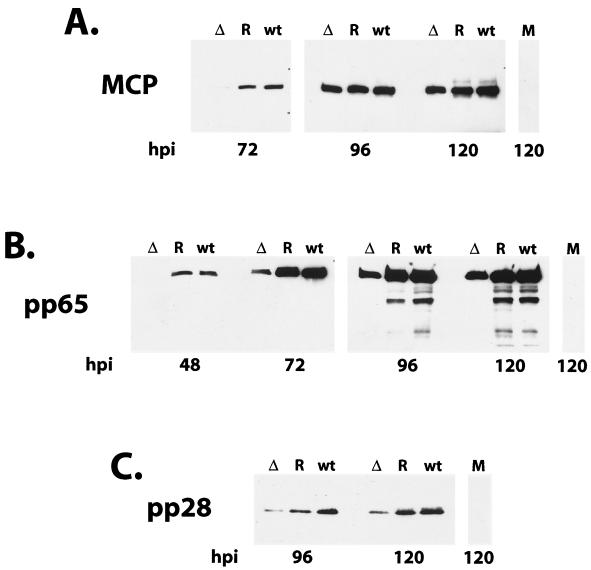
Since the above-described results indicated that some event late in the infection was affected by the mutation within IE2 86, we proceeded to assess synthesis of the structural components of the virus, specifically the MCP UL86 and the tegument proteins pp65 (UL83) and pp28 (UL99). By Western blot analysis, we detected a slight lag in the accumulation of MCP in cells infected with the IE2 86ΔSX-EGFP mutant at 72 h p.i., but by 24 h later, the levels were comparable to those observed in the cells infected with the wt and revertant viruses (Fig. 11A). In contrast, there was a dramatic decrease in the levels of the tegument protein pp65 in the cells infected with the IE2 86ΔSX-EGFP mutant (Fig. 11B). At 48 h p.i., the protein could be readily detected in the cells infected with the wt and revertant viruses but not in the cells infected with the mutant. Unlike the short delay observed for the accumulation of some of the other viral proteins, the disparity in the pp65 levels in the cells infected with the mutant versus the wt and revertant viruses was maintained over several time points. In addition, we did not detect the 50- or 52-kDa forms of pp65 in the IE2 86ΔSX-EGFP infection (7). A similar pattern was observed for the accumulation of the true late protein pp28 (Fig. 11C).
FIG. 11.
Western blots of late viral antigens. HFF cells were infected with IE2 86-EGFP (wt), IE2 86ΔSX-EGFP (Δ), or IE2 86ΔSX-EGFP revertant (R) viruses at an MOI of 0.5 in media containing 10% FBS. Cells were harvested at the time points indicated. Forty micrograms of protein was resolved by SDS-PAGE and transferred to nitrocellulose. Filters were probed with a MAb specific for the MCP (A), pp65 (B), and pp28 (C). Reactive proteins were visualized by chemiluminescence.
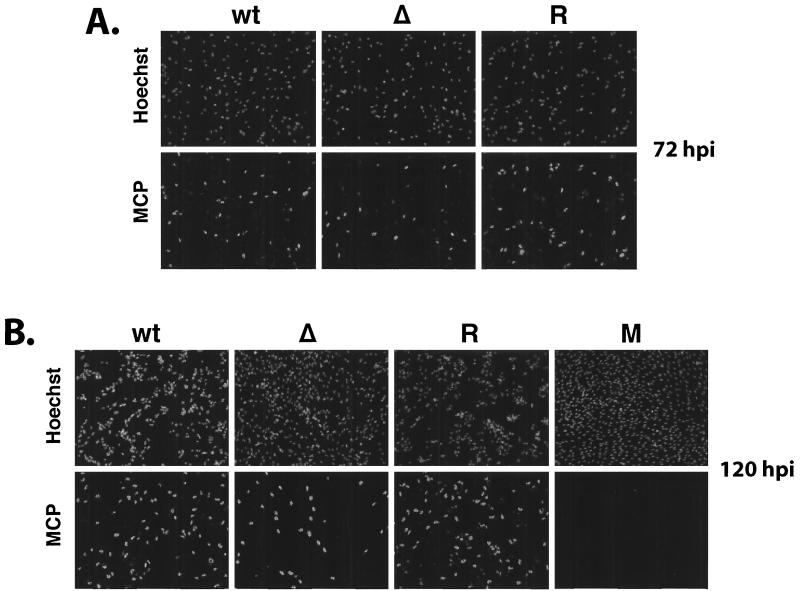
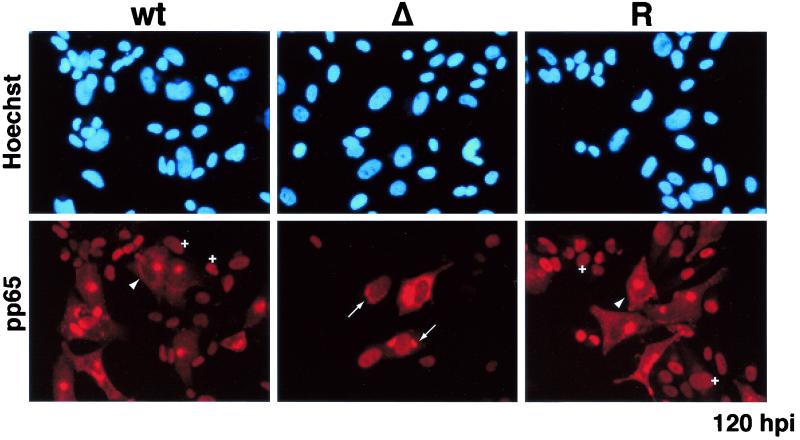
By IFA, the distribution of the MCP was comparable to that observed for IE1 72 and UL44 (Fig. 12). The percentage of MCP-positive cells was similar for all three viruses at 72 h p.i. However, at 120 h p.i., the number of MCP-positive cells had increased in the cultures infected with wt and revertant virus but not in those infected with the mutant virus. Taken together, these data supported the notion that the IE2 86ΔSX-EGFP virus was defective in some aspect of virion production and spread. This was most evident when we determined the distribution of pp65 in infected cells at 120 h p.i. (Fig. 13). In the cultures infected with the wt and revertant virus, pp65 was detected in almost all of the cells and two different patterns of localization were observed. In cells infected with the primary inoculum and thus at the late stages of infection at 120 h p.i., pp65 accumulated in defined juxtanuclear structures (56). We also detected pp65 in the nuclei of cells that did not contain replication centers (data not shown) and therefore represented the newly infected cells of the second round of infection (20, 57, 69). In contrast, a much smaller number of cells in the cultures infected with the mutant virus were positive for pp65, and these cells had a nuclear distribution of the protein that was characteristic of earlier stages in the infection. Moreover, when pp65 was present in the cytoplasm it was more often distributed around the nucleus rather than in defined structures or foci.
FIG. 12.
MCP expression in cultures infected with wt IE2 86-EGFP (wt), IE2 86ΔSX-EGFP (Δ), or IE2 86ΔSX-EGFP revertant (R) viruses. HFF cells were seeded on coverslips and infected with virus at an MOI of 0.5 in media containing 10% FBS. At the time points indicated, cells were fixed in 2% paraformaldehyde. After permeabilization, cells were stained with MCP-specific MAb followed by antimouse IgG TRITC-conjugated secondary antibody. Hoechst stain was used to visualize the total number of nuclei in the field. A field of mock-infected cells (M) was shown for comparison. Magnification, ×71.
FIG. 13.
Distribution of pp65 in cells infected with IE2 86-EGFP (wt), IE2 86ΔSX-EGFP (Δ), or IE2 86ΔSX-EGFP revertant (R) viruses. HFF cells were seeded on coverslips and infected with virus at an MOI of 0.5 in media containing 10% FBS. Cells were fixed in 2% paraformaldehyde at 120 h p.i. After permeabilization, cells were stained with pp65-specific monoclonal antibody followed by antimouse IgG TRITC-conjugated secondary antibody. Hoechst stain was used to visualize the total number of nuclei in the field. Arrowheads indicate cells in the late phases of infection in which pp65 is localized to a defined juxtanuclear compartment. Crosses (+) indicate newly infected cells with pp65 targeted to the nucleus. Arrows indicate cells in which pp65 is localized to both the nucleus and the cytoplasm. Magnification, ×332.
The levels of pp65 mRNA are reduced in cells infected with the IE2 86ΔSX mutant virus.
In order to determine if the reduced level of pp65 expression in cells infected with IE2 86ΔSX-EGFP virus was at the level of transcription, we analyzed the mRNA by a Northern blot (Fig. 14). HFF cells were infected with IE2 86-EGFP, IE2 86ΔSX-EGFP, or IE2 86ΔSX-EGFP revertant viruses at an MOI of 0.5 in media containing 10% FBS or mock infected. Equal quantities of mRNA were analyzed. We observed a decrease in the levels of pp65 mRNA in cells infected with IE2 86ΔSX-EGFP compared to controls as early as 24 h p.i. (Fig. 14A and B). This effect was specific for the pp65 mRNA, since a probe which detected both the pp65 and pp71 mRNAs failed to demonstrate a decrease in the pp71 transcript in the ΔSX samples (Fig. 14B). Similarly, we did not observe a decrease in the levels of the 1.6-kb pp28 mRNA in cells infected with the ΔSX mutant (Fig. 14C). These data suggested that the presence of aa 136 to 290 in IE2 86 was important for accumulation of pp65 transcripts.
FIG. 14.
Northern blot analysis of cells infected with IE2 86-EGFP (wt), IE2 86ΔSX-EGFP (Δ), or IE2 86ΔSX-EGFP revertant (R) viruses at an MOI of 0.5 in media containing 10% FBS. Cells were collected at the time points indicated. One microgram of mRNA per sample was resolved by agarose gel electrophoresis and transferred to nylon filters. The blot shown in Fig. 7 was stripped and hybridized to a pp65-specific probe within the pp65 coding region (A), a probe reactive with pp65 and pp71 coterminal transcripts (B), a pp28-specific probe (C), and a GAPDH-specific probe (D). Symbols denote the positions of specific mRNAs: ∗, pp71; o, pp28; +, pp65.
DISCUSSION
The IE2 86 protein is a key regulator of the HCMV infectious cycle. Much of what is known about the functions of IE2 86 comes from studies utilizing transient expression assays in which the domains necessary for the transactivating properties of IE2 86 were elucidated by mutational analysis. Based on these and other studies, the importance of IE2 86 in the progression of HCMV infection has been suggested; however, the precise effects of these mutations in IE2 86 in the context of viral infection have been difficult to ascertain. One major drawback has been the inability to generate a system in which IE2 86 is efficiently provided in trans so that viruses with mutations in the IE2 86 ORF can be propagated. Previous attempts to introduce mutations into this protein in the absence of a complementation system have demonstrated that IE2 86 is essential, since these mutant viruses were not viable and progeny virus was not produced. This obstacle has now been eliminated with the advent of BAC technology, which allows the introduction of specific mutations into the IE2 86 coding region followed by amplification of infectious viral DNA in bacteria, bypassing the need for complementation of the defect. Using this technology, Marchini and colleagues have elegantly shown that IE2 86 is indeed essential for HCMV replication (44). A BAC recombinant virus lacking the majority of the IE2 86 ORF failed to produce mRNA for any of the viral early genes examined or to produce plaques when transfected in permissive cells. In the present report we describe the construction and characterization of the first viable HCMV IE2 86 mutant with a deletion corresponding to aa 136 to 290, IE2 86ΔSX-EGFP.
Early work from our laboratory showed that aa 136 to 290 of IE2 86 were inhibitory for the binding of this protein to cellular factors that modulate transcription (61). Deletion of these amino acids resulted in enhanced binding to TATA-binding protein and Rb, but there was a modest decrease in the ability of this construct to activate transcription of the UL112-113 promoter in transient assays (61). More recent reports have mapped several phosphorylation and SUMOylation sites to this region of the protein (24, 26). Harel and Alwine reported that in cotransfection assays the phosphorylation of T233/S234 was important for down-regulating the transcriptional activity of IE2 86 (24). In contrast, mutation of SUMOylation sites at aa 175 and 180 resulted in reduced transcription in transient assays, suggesting that this modification had a positive effect on IE2 86-mediated transcription (26). Taken together, these studies suggested that sites within the domain encompassing aa 136 to 290 were important for regulating the activity of IE2 86. Surprisingly, our results indicated that in the context of the infection, this domain was dispensable for viral replication in culture, although growth of the IE2 86ΔSX-EGFP virus was restricted.
Primary analyses of IE2 86ΔSX-EGFP growth kinetics revealed that this viral mutant had a defect in the production of extracellular virus. The titers of virus produced by the mutant were consistently 1 to 2 logs lower than those for the control viruses until later time points, when peak titers of the mutant virus were produced (Fig. 4). In contrast, the levels of viral DNA in cells infected with each of the three viruses tested were comparable, suggesting that the defect observed in the production of IE2 86ΔSX-EGFP progeny virions resided at a point following viral DNA replication (Fig. 10).
Consistent with the growth analyses data for the IE2 86ΔSX-EGFP virus, the expression of IE and early genes necessary for viral DNA replication was only slightly affected in cells infected with the mutant virus, with the exception of IE2 86-EGFP levels. As measured by Western blot, the delays observed in the expression of IE1 72, UL112-113 proteins, and UL44 in IE2 86ΔSX-EGFP virus-infected cells relative to the controls were quickly overcome, with levels becoming comparable within the next time interval (Fig. 5 and 8). Together with the results from the viral DNA analyses, these data suggest that the deletion of aa 136 to 290 of IE2 86-EGFP has little effect on the early stages of viral infection. These observations were unexpected given the large deletion made in the coding region of the IE2 86 protein and the greatly reduced amount of IE2 86ΔSX-EGFP produced by the mutant virus. In addition, we did not detect the corresponding p40 and p60 forms of IE2-EGFP in cells infected with the mutant virus (Fig. 5C). It is tempting to speculate that it is the lack of p40 and p60 expression that results in the phenotype of IE2 86ΔSX-EGFP, as these isoforms of IE2 are normally expressed at the later stages of infection with the wt virus (30, 53).
Examination of virion structural proteins revealed a more significant lag in expression of specific viral proteins in cells infected with the IE2 86ΔSX-EGFP mutant virus. While the accumulation of MCP was slightly delayed in IE2 86ΔSX-infected cells relative to the controls, there was a pronounced reduction in the levels of pp65 and pp28 in these cells over several time intervals (Fig. 11). We also did not observe the 50- or 52-kDa forms of pp65 in lysates from cells infected with the mutant virus (Fig. 11B). We do not know the significance of this result except that these forms of pp65 are normally detected later in infection (7). Given the reduction in the pp65 and pp28 levels, it seemed likely that the decrease in extracellular virion production by the mutant virus could be attributed to a defect in virion assembly and/or release from the infected cells. Several observations support this hypothesis. First, careful investigation of pp65 and pp28 localization within infected cells by IFA showed that the distribution of both of these proteins was altered in cells infected with IE2 86ΔSX-EGFP. At 120 h p.i., there were significantly fewer pp65-positive cells in cultures infected with the mutant virus (Fig. 13). In addition, pp65 was predominantly localized to the nuclei of cells in the first round of virus infection based on cell morphology and IE2 86ΔSX-EGFP intensity (data not shown). This is in contrast to the wt and revertant virus-infected cell cultures that contained cells late in infection in which pp65 was detected in the juxtanuclear assembly compartment as well as newly infected cells with input pp65 targeted to the nucleus (56). The IE2 86ΔSX-EGFP infected cultures did contain a small percentage of cells that displayed a pp65 distribution which was more consistent with a normal infection. Some cells appeared to be at an intermediate stage, with pp65 localized to both the nucleus and the cytoplasm. These observations support the notion that there is a defect in shifting from early to late stages of infection in cells infected with the IE2 86ΔSX-EGFP mutant virus. As with pp65, the distribution of pp28 in cells infected with the mutant virus was aberrant in that the localization to a well-defined juxtanuclear structure was not detected (data not shown) (56).
Other evidence indicates that cells infected with IE2 86ΔSX-EGFP were defective in viral spread in that cultures contained a relatively small number of newly infected, pp65-positive cells at 120 h p.i. This effect was also apparent in the reduced number of UL44-positive cells in cultures infected with the mutant virus compared to the control cultures at 120 h p.i. (Fig. 9 and 13). Finally, the observed increase in cell density in cultures infected with the IE2 86ΔSX-EGFP mutant virus was consistent with a delay in cell lysis and virion release. The cells infected in the first round were still present in the culture, and the uninfected cells continued to divide. This phenotype was somewhat reminiscent of a persistent infection, but at this time we have not measured how long the viral infection can be maintained in culture. Infection of cells with the IE2 86ΔSX-EGFP mutant was also similar to infection with a clinical isolate in which pp65 mRNA is not abundantly expressed and extracellular virus production is reduced relative to results with laboratory-adapted strains (29, 35). It should be noted, however, that factors other than the decrease in pp65 expression might contribute to the phenotype of IE2 86ΔSX-EGFP, since there are numerous viral gene products that have not been evaluated.
Results from the Northern blot analysis confirmed that the level of pp65 mRNA was also reduced in cells infected with the IE2 86ΔSX-EGFP mutant virus (Fig. 14A and B). In contrast, the mRNA encoding pp71 was not affected, suggesting that the decrease in pp65 mRNA was specific and not an effect of reduced transcription from that locus. In addition, the Northern blots showed that the levels of the transcript for IE2 86ΔSX-EGFP were similar to those of the wt, full-length IE2 86-EGFP (Fig. 7A). These data suggest that the IE2 86ΔSX-EGFP protein is inherently unstable, although we have not measured the half-life of this protein at this time (Fig. 5A and C). We did not detect a truncated form of p60 in cells infected with the mutant virus by a Western blot even though an mRNA consistent with the truncated p60 IE2 86ΔSX-EGFP was produced, albeit at a much reduced level (Fig. 7A). We have not determined whether the effect on pp65 gene transcription was the result of reduced IE2 86ΔSX-EGFP expression or the lack of detectable p40 and/or p60 in the infected cells. In any case, these data may prompt a reexamination of the expression of IE2 isoforms by clinical strains and a possible correlation to the production of extracellular virus.
We did not observe a decrease in pp28 mRNA transcripts in cells infected with the ΔSX mutant (Fig. 14), even though we did detect a decrease in pp28 by Western blotting (Fig. 11) and immunofluorescence (data not shown). Previous work by Kerry et al. described posttranscriptional regulation of pp28 at the level of translation of its mRNA (33). It is possible that the decrease in pp28 in cells infected with IE2 86ΔSX-EGFP was due to a defect in translational control of pp28 mRNA (33). Additional experiments are under way to address these issues.
In summary, we have generated a viable HCMV recombinant virus lacking aa 136 to 290 of IE2 86. Our data suggest that this region of IE2 86 is dispensable for viral replication but may be important for the function of IE2 86 in the later stages of infection. Specifically, our results suggest a role for IE2 86 in the accumulation of pp65 and the production of extracellular virus.
Acknowledgments
We thank Martin Messerle for providing the E. coli strain CBTS containing pHB5; Bodo Plachter for providing the expression plasmid for pp71; and William Britt for the plasmids containing pp65 and pp28 and monoclonal antibodies to IE1 72, pp65, pp28, and MCP. We thank Randall Johnson for his help with microscopy. We also acknowledge the members of the laboratory for critical reading of the manuscript.
This work was supported by NIH grants CA34729 and CA73490. J.Y.Y. was supported by NIH training grant T32 GM07240.
REFERENCES
- 1.Ahn, J.-H., C. J. Chiou, and G. S. Hayward. 1998. Evaluation and mapping of the DNA binding and oligomerization domains of the IE2 regulatory protein of human cytomegalovirus using yeast one and two hybrid interaction assays. Gene 210:25-36. [DOI] [PubMed] [Google Scholar]
- 2.Ahn, J.-H., Y. Xu, W.-J. Jang, M. J. Matunis, and G. S. Hayward. 2001. Evaluation of interactions of human cytomegalovirus immediate-early IE2 regulatory protein with small ubiquitin-like modifiers and their conjugation enzyme Ubc9. J. Virol. 75:3859-3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Albrecht, T., M. P. Fons, I. Boldogh, S. AbuBakar, C. Z. Deng, and D. Millinoff. 1991. Metabolic and cellular effects of human cytomegalovirus infection. Transplant. Proc. 23:48-55. [PubMed] [Google Scholar]
- 4.Arlt, H., D. Lang, S. Gebert, and T. Stamminger. 1994. Identification of binding sites for the 86-kilodalton IE2 protein of human cytomegalovirus within an IE2-responsive viral early promoter. J. Virol. 68:4117-4125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baracchini, E., E. Glezer, K. Fish, R. M. Stenberg, J. A. Nelson, and P. Ghazal. 1992. An isoform variant of the human cytomegalovirus immediate-early auto repressor functions as a transcriptional activator. Virology 188:518-529. [DOI] [PubMed] [Google Scholar]
- 6.Borst, E. M., G. Hahn, U. H. Koszinowski, and M. Messerle. 1999. Cloning of the human cytomegalovirus (HCMV) genome as an infectious bacterial artificial chromosome in Escherichia coli: a new approach for construction of HCMV mutants. J. Virol. 73:8320-8329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Britt, W. J., and L. Vugler. 1987. Structural and immunological characterization of the intracellular forms of an abundant 68,000 Mr human cytomegalovirus protein. J. Gen. Virol. 68:1897-1907. [DOI] [PubMed] [Google Scholar]
- 8.Bryant, L. A., P. Mixon, M. Davidson, A. J. Bannister, T. Kouzarides, and J. H. Sinclair. 2000. The human cytomegalovirus 86-kilodalton major immediate-early protein interacts physically and functionally with histone acetyltransferase P/CAF. J. Virol. 74:7230-7237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Castillo, J. P., A. Yurochko, and T. F. Kowalik. 2000. Role of human cytomegalovirus immediate-early proteins in cell growth control. J. Virol. 74:8028-8037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caswell, R., C. Hagemeier, C.-J. Chiou, G. Hayward, T. Kouzarides, and J. Sinclair. 1993. The human cytomegalovirus 86K immediate-early (IE) 2 protein requires the basic region of the TATA-box binding protein (TBP) for binding, and interacts with TBP and transcription factor TFIIB via regions of IE2 required for transcriptional regulation. J. Gen. Virol. 74:2691-2698. [DOI] [PubMed] [Google Scholar]
- 11.Chang, C.-P., C. L. Malone, and M. F. Stinski. 1989. A human cytomegalovirus early gene has three inducible promoters that are regulated differentially at various times after infection. J. Virol. 63:281-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang, C.-P., D. H. Vesole, J. Nelson, M. B. A. Oldstone, and M. F. Stinski. 1989. Identification and expression of a human cytomegalovirus early glycoprotein. J. Virol. 63:3330-3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cherrington, J. M., E. L. Khoury, and E. S. Mocarski. 1991. Human cytomegalovirus IE2 negatively regulates α gene expression via a short target sequence near the transcription start site. J. Virol. 65:887-896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chiou, C.-J., J. Zong, I. Waheed, and G. S. Hayward. 1993. Identification and mapping of dimerization and DNA-binding domains in the C terminus of the IE2 regulatory protein of human cytomegalovirus. J. Virol. 67:6201-6214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choi, K. S., S.-J. Kim, and S. Kim. 1995. The retinoblastoma gene product negatively regulates transcriptional activation mediated by the human cytomegalovirus IE2 protein. Virology 208:450-456. [DOI] [PubMed] [Google Scholar]
- 16.Fortunato, E. A., A. K. McElroy, V. Sanchez, and D. H. Spector. 2000. Exploitation of cellular signaling and regulatory pathways by human cytomegalovirus. Trends Microbiol. 8:111-119. [DOI] [PubMed] [Google Scholar]
- 17.Fortunato, E. A., M. H. Sommer, K. Yoder, and D. H. Spector. 1997. Identification of domains within the human cytomegalovirus major immediate-early 86-kilodalton protein and the retinoblastoma protein required for physical and functional interaction with each other. J. Virol. 71:8176-8185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fortunato, E. A., and D. H. Spector. 1999. Regulation of human cytomegalovirus gene expression. Adv. Virus Res. 54:61-128. [DOI] [PubMed] [Google Scholar]
- 19.Furnari, B. A., E. Poma, T. F. Kowalik, S.-M. Huong, and E.-S. Huang. 1993. Human cytomegalovirus immediate-early gene 2 interacts with itself and with several novel cellular proteins. J. Virol. 67:4981-4991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gallina, A., E. Percivalle, L. Simoncini, M. Revello, G. Gerna, and G. Milanesi. 1996. Human cytomegalovirus pp65 lower matrix phosphoprotein harbours two transplantable nuclear localization signals. J. Gen. Virol. 77:1151-1157. [DOI] [PubMed] [Google Scholar]
- 21.Hagemeier, C., R. Caswell, G. Hayhurst, J. Sinclair, and T. Kouzarides. 1994. Functional interaction between the HCMV IE2 transactivator and the retinoblastoma protein. EMBO J. 13:2897-2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hagemeier, C., S. Walker, R. Caswell, T. Kouzarides, and J. Sinclair. 1992. The human cytomegalovirus 80-kilodalton but not the 72-kilodalton immediate-early protein transactivates heterologous promoters in a TATA box-dependent mechanism and interacts directly with TFIID. J. Virol. 66:4452-4456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hardy, S., D. A. Engel, and T. Shenk. 1989. An adenovirus early region 4 gene product is required for induction of the infection-specific form of cellular E2F activity. Genes Dev. 3:1062-1074. [DOI] [PubMed] [Google Scholar]
- 24.Harel, N. Y., and J. C. Alwine. 1998. Phosphorylation of the human cytomegalovirus 86-kilodalton immediate-early protein IE2. J. Virol. 72:5481-5492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hermiston, T. W., C. L. Malone, and M. F. Stinski. 1990. Human cytomegalovirus immediate-early two-protein region involved in negative regulation of the major immediate-early promoter. J. Virol. 64:3532-3536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hofmann, H., S. Floss, and T. Stamminger. 2000. Covalent modification of the transactivator protein IE2-p86 of human cytomegalovirus by conjugation to the ubiquitin-homologous proteins SUMO-1 and hSMT3b. J. Virol. 74:2510-2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang, C., Y. Wang, D. Tsao, S. Tung, Y. Lin, and C. Wu. 2000. Antagonism between members of the CNC-bZIP family and the immediate-early protein IE2 of human cytomegalovirus. J. Biol. Chem. 275:12313-12320. [DOI] [PubMed] [Google Scholar]
- 28.Huang, L., and M. F. Stinski. 1995. Binding of cellular repressor protein or the IE2 protein to a cis-acting negative regulatory element upstream of a human cytomegalovirus early promoter. J. Virol. 69:7612-7621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jahn, G., B. C. Scholl, B. Traupe, and B. Fleckenstein. 1987. The two major structural phosphoproteins (pp65 and pp150) of human cytomegalovirus and their antigenic properties. J. Gen. Virol. 68:1327-1337. [DOI] [PubMed] [Google Scholar]
- 30.Jenkins, D. E., C. L. Martens, and E. S. Mocarski. 1994. Human cytomegalovirus late protein encoded by ie2: a transactivator as well as a repressor of gene expression. J. Gen. Virol. 75:2337-2348. [DOI] [PubMed] [Google Scholar]
- 31.Jupp, R., S. Hoffmann, A. Depto, R. M. Stenberg, P. Ghazal, and J. A. Nelson. 1993. Direct interaction of the human cytomegalovirus IE86 protein with the cis repression signal does not preclude TBP from binding to the TATA box. J. Virol. 67:5595-5604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jupp, R., S. Hoffmann, R. M. Stenberg, J. A. Nelson, and P. Ghazal. 1993. Human cytomegalovirus IE86 protein interacts with promoter-bound TATA-binding protein via a specific region distinct from the autorepression domain. J. Virol. 67:7539-7546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kerry, J. A., M. Priddy, C. Kohler, T. L. Staley, D. Weber, T. R. Jones, and R. M. Stenberg. 1997. Translational regulation of the human cytomegalovirus pp28 (UL99) late gene. J. Virol. 71:981-987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kerry, J. A., A. Sehgal, S. W. Barlow, V. J. Cavanaugh, K. Fish, J. A. Nelson, and R. M. Stenberg. 1995. Isolation and characterization of a low-abundance splice variant from the human cytomegalovirus major immediate-early gene region. J. Virol. 69:3868-3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klages, S., B. Ruger, and G. Jahn. 1989. Multiplicity dependent expression of the predominant phosphoprotein pp65 of human cytomegalovirus. Virus Res. 12:159-168. [DOI] [PubMed] [Google Scholar]
- 36.Lang, D., S. Gebert, H. Arlt, and T. Stamminger. 1995. Functional interaction between the human cytomegalovirus 86-kilodalton IE2 protein and the cellular transcription factor CREB. J. Virol. 69:6030-6037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lang, D., and T. Stamminger. 1993. The 86-kilodalton IE-2 protein of human cytomegalovirus is a sequence-specific DNA-binding protein that interacts directly with the negative autoregulatory response element located near the cap site of the IE-1/2 enhancer-promoter. J. Virol. 67:323-331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lang, D., and T. Stamminger. 1994. Minor groove contacts are essential for an interaction of the human cytomegalovirus IE2 protein with its DNA target. Nucleic Acids Res. 22:3331-3338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu, B., T. W. Hermiston, and M. F. Stinski. 1991. A cis-acting element in the major immediate-early (IE) promoter of human cytomegalovirus is required for negative regulation by IE2. J. Virol. 65:897-903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lukac, D. M., and J. C. Alwine. 1999. Effects of human cytomegalovirus major immediate-early proteins in controlling the cell cycle and inhibiting apoptosis: studies with ts13 cells. J. Virol. 73:2825-2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lukac, D. M., N. Y. Harel, N. Tanese, and J. C. Alwine. 1997. TAF-like functions of human cytomegalovirus immediate-early proteins. J. Virol. 71:7227-7239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lukac, D. M., J. R. Manuppello, and J. C. Alwine. 1994. Transcriptional activation by the human cytomegalovirus immediate-early proteins: requirements for simple promoter structures and interactions with multiple components of the transcription complex. J. Virol. 68:5184-5193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Macias, M. P., and M. F. Stinski. 1993. An in vitro system for human cytomegalovirus immediate early protein (IE-2)-mediated site-dependent repression of transcription and direct binding of IE2 to the major immediate-early promoter. Proc. Natl. Acad. Sci. USA 90:707-711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marchini, A., H. Liu, and H. Zhu. 2001. Human cytomegalovirus with IE-2 (UL122) deleted fails to express early lytic genes. J. Virol. 75:1870-1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McElroy, A. K., R. S. Dwarakanath, and D. H. Spector. 2000. Dysregulation of cyclin E gene expression in human cytomegalovirus-infected cells requires viral early gene expression and is associated with changes in the Rb-related protein p130. J. Virol. 74:4192-4206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mocarski, E. S., and C. T. Courcelle. 2001. Cytomegaloviruses and their replication, p. 2629-2673. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed., vol. 2. Lippincott Williams & Wilkins, Philadelphia, Pa. [Google Scholar]
- 47.Morello, C. S., L. D. Cranmer, and D. H. Spector. 1999. In vivo replication, latency, and immunogenicity of murine cytomegalovirus mutants with deletions in the M83 and M84 genes, the putative homologs of human cytomegalovirus pp65 (UL83). J. Virol. 73:7678-7693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Murphy, E. A., D. N. Streblow, J. A. Nelson, and M. F. Stinski. 2000. The human cytomegalovirus IE86 protein can block cell cycle progression after inducing transition into the S phase of permissive cells. J. Virol. 74:7108-7118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pass, R. F. 2001. Cytomegalovirus, p. 2675-2705. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed., vol. 2. Lippincott Williams & Wilkins, Philadelphia, Pa. [Google Scholar]
- 50.Pizzorno, M. C., and G. S. Hayward. 1990. The IE2 gene products of human cytomegalovirus specifically down-regulate expression from the major immediate-early promoter through a target sequence located near the cap site. J. Virol. 64:6154-6165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pizzorno, M. C., M.-A. Mullen, Y.-C. Chang, and G. S. Hayward. 1991. The functionally active IE2 immediate-early regulatory protein of human cytomegalovirus is an 80-kilodalton polypeptide that contains two distinct activator domains and a duplicated nuclear localization signal. J. Virol. 65:3839-3852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pizzorno, M. C., P. O'Hare, L. Sha, R. L. LaFemina, and G. S. Hayward. 1988. trans-activation and autoregulation of gene expression by the immediate-early region 2 gene products of human cytomegalovirus. J. Virol. 62:1167-1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Plachter, B., W. J. Britt, T. Vornhagen, T. Stamminger, and G. Jahn. 1993. Analysis of proteins encoded by IE regions 1 and 2 of human cytomegalovirus using monoclonal antibodies generated against recombinant antigens. Virology 193:642-652. [DOI] [PubMed] [Google Scholar]
- 54.Puchtler, E., and T. Stamminger. 1991. An inducible promoter mediates abundant expression from the immediate-early 2 gene region of human cytomegalovirus at late times after infection. J. Virol. 65:6301-6303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sanchez, V., P. C. Angeletti, J. A. Engler, and W. J. Britt. 1998. Localization of human cytomegalovirus structural proteins to the nuclear matrix of infected human fibroblasts. J. Virol. 72:3321-3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sanchez, V., K. Greis, E. Sztul, and W. J. Britt. 2000. Accumulation of virion tegument and envelope proteins in a stable cytoplasmic compartment during human cytomegalovirus replication: characterization of a potential site of virus assembly. J. Virol. 74:975-986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schmolke, S., P. Drescher, G. Jahn, and B. Plachter. 1995. Nuclear targeting of the tegument protein pp65 (UL83) of human cytomegalovirus: an unusual bipartite nuclear localization signal functions with other portions of the protein to mediate its efficient nuclear transport. J. Virol. 69:1071-1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schwartz, R., B. Helmich, and D. H. Spector. 1996. CREB and CREB-binding proteins play an important role in the IE2 86-kilodalton protein-mediated transactivation of the human cytomegalovirus 2.2-kilobase RNA promoter. J. Virol. 70:6955-6966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schwartz, R., M. H. Sommer, A. Scully, and D. H. Spector. 1994. Site-specific binding of the human cytomegalovirus IE2 86-kilodalton protein to an early gene promoter. J. Virol. 68:5613-5622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Scully, A. L., M. H. Sommer, R. Schwartz, and D. H. Spector. 1995. The human cytomegalovirus IE2 86-kDa protein interacts with an early gene promoter via site-specific DNA binding and protein-protein associations. J. Virol. 69:6533-6540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sommer, M. H., A. L. Scully, and D. H. Spector. 1994. Trans-activation by the human cytomegalovirus IE2 86-kDa protein requires a domain that binds to both TBP and RB. J. Virol. 68:6223-6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Spector, D. J., and M. J. Tevethia. 1994. Protein-protein interactions between human cytomegalovirus IE2-580aa and pUL84 in lytically infected cells. J. Virol. 68:7549-7553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Speir, E., R. Modali, E.-S. Huang, M. B. Leon, F. Sahwl, T. Finkel, and S. E. Epstein. 1994. Potential role of human cytomegalovirus and p53 interaction in coronary restenosis. Science 265:391-394. [DOI] [PubMed] [Google Scholar]
- 64.Stenberg, R. M., A. S. Depto, J. Fortney, and J. A. Nelson. 1989. Regulated expression of early and late RNAs and proteins from the human cytomegalovirus immediate-early gene region. J. Virol. 63:2699-2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stenberg, R. M., J. Fortney, S. W. Barlow, B. P. Magrane, J. A. Nelson, and P. Ghazal. 1990. Promoter-specific trans activation and repression by human cytomegalovirus immediate-early proteins involves common and unique protein domains. J. Virol. 64:1556-1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tamashiro, J. C., L. J. Hock, and D. H. Spector. 1982. Construction of a cloned library of the EcoRI fragments from the human cytomegalovirus genome (strain AD169). J. Virol. 42:547-557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Waheed, I., C. Chiou, J. Ahn, and G. Hayward. 1998. Binding of the human cytomegalovirus 80-kDa immediate-early protein (IE2) to minor groove A/T-rich sequences bounded by CG dinucleotides is regulated by protein oligomerization and phosphorylation. Virology 252:235-257. [DOI] [PubMed] [Google Scholar]
- 68.Wara-Aswapati, N., Z. Yang, W. Waterman, Y. Koyama, S. Tetradis, B. Choy, A. Webb, and P. Auron. 1999. Cytomegalovirus IE2 protein stimulates interleukin 1β gene transcription via tethering to Spi-1/PU.1. Mol. Cell. Biol. 19:6803-6814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Weiner, D., W. Gibson, and K. L. Fields. 1985. Anti-complement immunofluorescence establishes nuclear localization of human cytomegalovirus matrix protein. Virology 147:19-28. [DOI] [PubMed] [Google Scholar]
- 70.Wiebusch, L., and C. Hagemeier. 1999. Human cytomegalovirus 86-kilodalton IE2 protein blocks cell cycle progression in G1. J. Virol. 73:9274-9283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wiebusch, L., and C. Hagemeier. 2001. The human cytomegalovirus immediate early 2 protein dissociates cellular DNA synthesis from cyclin dependent kinase activation. EMBO J. 20:1086-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wu, J., R. Jupp, R. M. Stenberg, J. A. Nelson, and P. Ghazal. 1993. Site-specific inhibition of RNA polymerase II preinitiation complex assembly by human cytomegalovirus IE86 protein. J. Virol. 67:7547-7555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yoo, Y. D., C.-J. Chiou, K. S. Choi, Y. Yi, S. Michelson, S. Kim, G. S. Hayward, and S.-J. Kim. 1996. The IE2 regulatory protein of human cytomegalovirus induces expression of the human transforming growth factor β1 gene through an Egr-1 binding site. J. Virol. 70:7062-7070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhou, Y. F., M. B. Leon, M. A. Waclawiw, J. J. Popma, Z. X. Yu, T. Finkel, and S. E. Epstein. 1996. Association between prior cytomegalovirus infection and risk of restenosis after coronary atherectomy. N. Engl. J. Med. 335:624-630. [DOI] [PubMed] [Google Scholar]
- 75.Zhu, H., Y. Shen, and T. Shenk. 1995. Human cytomegalovirus IE1 and IE2 proteins block apoptosis. J. Virol. 69:7960-7970. [DOI] [PMC free article] [PubMed] [Google Scholar]