Abstract
Tumor relapse and cytomegalovirus (CMV) infection are major concerns in the therapy of hematopoietic malignancies by bone marrow transplantation (BMT). Little attention so far has been given to a possible pathogenetic interplay between CMV and lymphomas. CMV inhibits stem cell engraftment and hematopoietic reconstitution. Thus, by causing maintenance of bone marrow aplasia and immunodeficiency, CMV could promote tumor relapse. Alternatively, CMV could aid tumor remission. One might think of cytopathogenic infection of tumor cells, induction of apoptosis or inhibitory cytokines, interference with tumor cell extravasation or tumor vascularization, or bystander stimulation of an antitumoral immune response. To approach these questions, the established model of experimental BMT and murine CMV infection was extended by the introduction of liver-infiltrating, highly tumorigenic variant clone E12E of BALB/c-derived B-cell lymphoma A20. We document a remarkable retardation of lymphoma progression. First-guess explanations were ruled out: (i) lymphoma cells were not infected; (ii) lymphoma cells located next to infected hepatocytes did not express executioner caspase 3 but were viable and proliferated; (iii) an inhibitory effect of virus on the formation of tumor nodules in the liver became apparent by day 7 after BMT, long before the reconstitution of immune cells; and (iv) recombinant tumor necrosis factor alpha (TNF-α) did not substitute for virus; accordingly anti-TNF-α did not prevent the inhibition. Notably, while the antitumoral effect required replicative virus, prevention of cytopathogenic infection of the liver by antiviral CD8 T cells did not abolish lymphoma control. These findings are paradigmatic for a novel virus-associated antitumoral mechanism distinct from oncolysis.
Graft failure, graft-versus-host disease, infections, and in particular tumor relapse from a minimal residual leukemia/lymphoma (MRL) are major complications in the therapy of hematopoietic malignancies by hematoablative treatment followed by bone marrow transplantation (BMT) (for reviews, see references 34, 48, and 56). Cytomegalovirus (CMV) infection is the most frequent and most severe viral disease in BMT patients (for reviews, see references 33 and 52). Surprisingly, a pathogenetic interplay between CMV infection and residual leukemia has so far not been investigated, even though the multiple consequences of an infection that targets the hematopoietic system are likely to have a bearing on the control of hematopoietic malignancies.
There are good arguments to predict that CMV infection may promote tumor relapse. Human CMV (hCMV) and murine CMV (mCMV) are both known to contribute to bone marrow graft failure by infecting the bone marrow stroma. Several studies have shown that CMVs infect bone marrow stromal cells and thereby inhibit hematopoiesis in long-term bone marrow cell cultures (2, 6, 30, 58; for an editorial discussion, see reference 7). Moreover, hCMV infection in BMT recipients was found to be associated with impaired hematopoietic reconstitution (9). In the model of experimental syngeneic BMT with BALB/c mice as donors and recipients, mCMV was shown to cause graft failure and to inhibit endogenous reconstitution, resulting in bone marrow aplasia (38, 42, 63). Specifically, in vivo mCMV infection of bone marrow stroma reduced the expression of essential hemopoietins, including stem cell factor, interleukin-6 (IL-6), and granulocyte-monocyte colony-stimulating factor, although the infection was not cytolytic and did not disrupt the stromal architecture (38). By using male donors and female recipients for the BMT, a study involving Y chromosome tdy gene-specific PCR directly documented the inhibition of donor hematopoietic stem and progenitor cell engraftment in the bone marrow stroma of infected recipients (63). Impaired hematopoietic reconstitution, and thus a prolonged period of immunodeficiency and absence of tumor-specific immune functions, should favor tumor relapse. In addition, infection may facilitate tumor relapse by the induction of growth-promoting cytokines.
However, as CMV is a potentially cytopathogenic virus with a very broad cell type tropism, tumor cells of hematopoietic origin could be a direct target of the infection. Specifically, hematopoietic progenitor cells of the myeloid differentiation lineage are a source of latent hCMV and mature progeny thereof, namely, macrophages (15, 16, 19, 40, 60) and dendritic cells (1, 53), can be productively infected by hCMV and mCMV in the respective hosts (for an overview, see reference 41). Apparently, oncolytic infection cannot be a general mechanism of tumor control but trivially applies only to malignant cell types that are permissive for the infection. Given that the infection takes a productive and cytopathogenic course in the infected tumor cell, one expects a contribution to tumor remission. It must be considered, however, that the impact of virus on the fate of a cell is not restricted to productive infection. Attachment of virus to the cell membrane, specifically the binding of glycoprotein gB (57), as well as virion proteins delivered to cells through the entry process, can trigger a signaling cascade that alters the expression of a multitude of cellular genes and of interferon-responsive genes in particular (57, 67). Depending on cell type, viral regulatory proteins can be involved in cell cycle progression or in cell cycle arrest. Likewise, they can have an antiapoptotic or an apoptosis-inducing function (reviewed in reference 41). All in all, depending on cell type and many other parameters, interaction of CMV with tumor cells could contribute to tumor relapse or to tumor remission.
There are a number of additional arguments for a possible role for CMV in tumor remission by indirect mechanisms, which may be less dependent on tumor cell type and might thus be of more general applicability. For instance, viral “apoptins” (43) secreted by infected cells or set free by the lysis of infected cells could function as death ligands, triggering apoptosis in uninfected leukemic cells. Furthermore, CMV infection can induce inhibitory cytokines such as cellular IL-10 (51), and hCMV even encodes an IL-10 homolog (26). Induction of tumor necrosis factor alpha (TNF-α) in the course of an inflammatory response could lead to tumor remission by a direct cytotoxic effect on the tumor cells or by tumor necrosis associated with microvascular injury and impaired angiogenesis, resulting in a failure of tumor vascularization (reviewed in reference 20). Furthermore, viral or virally induced chemokines might interfere with migration and extravasation of leukemic cells. Finally, an antiviral immune response may exert an antileukemic bystander effect. Clearly, this list of immediate ideas is not comprehensive, and more mechanisms may enter the discussion as the project moves on.
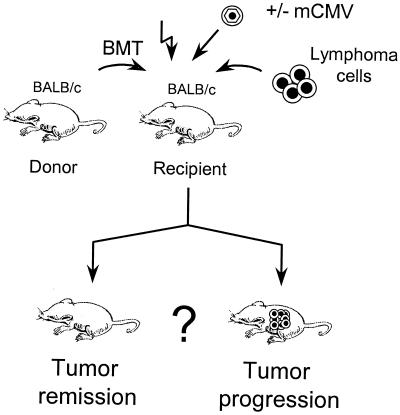
Our first aim was to decide between the fundamental alternatives of tumor relapse and tumor remission. We have here established an animal model that gives us an in vivo setting for studying the interplay between CMV infection and a lymphoma under the specific conditions of BMT. This new model is based on the previously established and well-characterized model of mCMV infection and experimental syngeneic BMT using BALB/c mice (haplotype H-2d) as donors and recipients (17, 46, 47, 62, 63). As a new parameter, liver-infiltrating, highly tumorigenic variant clone E12E (this report) of BALB/c-derived B-cell lymphoma A20 (23) is here introduced. The results document an impressive inhibition of tumor cell invasion of the liver by a very early event during in vivo mCMV infection.
MATERIALS AND METHODS
In vivo treatments.
Animal experiments were approved by the Ethics Commission, permission no.177-07/001-3, according to German federal law.
(i) BMT.
Hematoablative conditioning of BMT recipients was performed 24 h prior to BMT (referred to as day −1) by total-body gamma irradiation with a single dose of 6 or 7 Gy delivered by a 137Cs gamma ray source. A dose of 7 Gy was chosen in most experiments with the intention to minimize the influence of innate immunity defense mechanisms and to prevent an adaptive immune response. Syngeneic BMT was performed on day 0 as described previously (17, 46) with 8-week-old, female BALB/c (H-2d haplotype) mice as donors and recipients. The numbers of femoral and tibial bone marrow (BM) cells (with or without lymphoma cells) indicated in the figure legends were injected into the tail veins of the recipients in a volume of 0.5 ml in RPMI 1640 medium with no supplements (catalog no. 31870-025; Life Technologies Gibco BRL).
(ii) Tumor.
E12E lymphoma cells (see below), usually 106 cells, were injected intravenously at 24 h after the gamma irradiation either alone or along with the BM cells. The two cell populations were mixed just before the intravenous injection.
(iii) Infection.
Intraplantar (left hind footpad) infection of the recipients was performed immediately after BMT and/or lymphoma injection with 105 PFU of cell culture-propagated, sucrose gradient-purified mCMV, strain Smith (ATCC VR-194/1981) (28). In control groups, mice were likewise inoculated with UV light (254 nm)-inactivated mCMV at the doses, referred to as PFUUV, indicated in Fig. 9.
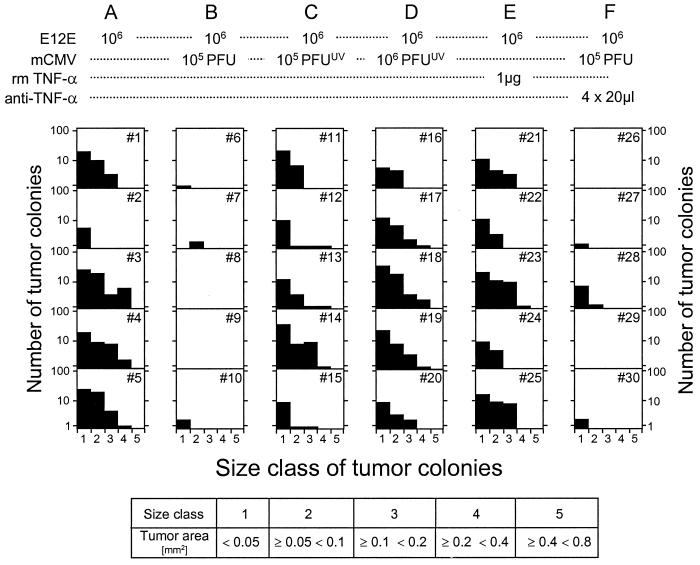
FIG. 9.
Approaches to modulate lymphoma colonization in the liver. Recipients (n = 5 mice per group, numbered individually) received the combinations of treatments specified for groups A to F. Mice of all groups were immunocompromised by gamma irradiation with a dose of 7 Gy on day −1. On day 11 after intravenous application of E12E lymphoma cells, number and sizes of E12E lymphoma colonies in IHC-stained liver sections were determined as defined for Fig. 5.
(iv) Antiviral cytoimmunotherapy.
A cytolytic T-lymphocyte line (CTLL) specific for mCMV immediate-early (IE) protein IE1 (pp89)-derived peptide 168YPHFMPTNL176 (49, 50) was generated as described recently (18). The cytolytic T lymphocytes (CTL; 106 cells per recipient, suspended in 0.5 ml of RPMI 1640) were administered intravenously ca. 24 h after the infection.
(v) Cytokine treatment.
Recombinant murine TNF-α (rmTNF-α; catalog no. 410-MT-050; R&D Systems) was administered intravenously at 24 h after injection of the lymphoma cells into uninfected, gamma-irradiated (7 Gy) recipients in a single sublethal dose of 1 μg dissolved in 0.5 ml of phosphate-buffered saline (PBS; catalog no. BE17-512F; BioWhittaker Europe). The 50% lethal dose of rmTNF-α was ca. 2 μg in this particular experimental setting.
(vi) In vivo neutralization of TNF-α.
Rabbit antiserum with the capacity to neutralize 2 μg of mTNF-α per ml was generously provided by S. Jonjic (University of Rijeka, Rijeka, Croatia). Intravenous doses of 20 μl of antiserum, capable of neutralizing 40 ng of mTNF-α, were given every other day (on days 0, 2, 4, and 6, four doses altogether). The first dose was given 4 h after infection.
(vii) Monitoring of disease.
The health status of the mice was controlled daily. Onset of illness was defined by lethargy and ruffling; later stages were defined by wasting syndrome/cachexia. For death attributed to tumor, the cause of death was verified upon autopsy, and only verified cases were included in the inverted Kaplan-Meyer plots. Death from manifestations of viral disease, such as interstitial pneumonia, hepatitis, adrenalitis, and bone marrow aplasia (38, 46, 47), was confirmed by histopathological analysis. It was known from previous work that illness and death by virus typically occur between 2 and 4 weeks after BMT.
Lymphoma cells. (i) Parental A20 cells.
The A20 cell line is a BALB/c (H-2d haplotype, female genotype) B-cell lymphoma line derived from a spontaneous reticulum cell neoplasm (23). A20 cells have a diploid karyotype with a reported modal number of 37 chromosomes (range, 33 to 38) (23). Known surface markers include immunoglobulin (Ig), major histocompatibility complex class I and class II, CD45R (B220), CD86 (B7-2) (44), and CD95 (Fas). A20 cells were purchased from the American Type Culture Collection (ATTC TIB-208; 1999). Passages were counted beginning with the first reseeding (passage 1) after the start culture (passage 0). A20 cells were propagated in 2-ml cultures (24-well flat-bottom culture plates; catalog no. 662102; Greiner bio-one, Frickenhausen, Germany) in RPMI 1640 culture medium supplemented with 7.5% (vol/vol) fetal calf serum, 5 × 10−5 M 2-mercaptoethanol, 2 mM l-glutamine, 10 mM HEPES buffer (pH 7.2), 0.1 mg of streptomycin sulfate per ml, and 100 U of penicillin per ml. Cell seeding was performed at a density of 4 × 105 cells per 2-ml culture, and passages were made every third day. Karyotype analysis performed with A20 cells in passage 13 revealed a diploid karyotype with a modal number of 40 chromosomes (range, 36 to 41).
(ii) Insertion of a reporter gene into A20 cells.
A20 cells in passage 21 were transfected with vector pcDNA3/lacZ by electroporation. Vector pcDNA3/lacZ was derived from vector pcDNA3 (catalog no. V790-20; Invitrogen, Leek, The Netherlands). In the first step, the hCMV major IE promoter-enhancer was excised by digestion with NruI (position 209) and XhoI (position 972). A 4.5-kbp fragment encompassing lacZ, the Rous sarcoma virus promoter, and the simian virus 40 poly(A) tail was excised from plasmid lacZ/gpt16 (kindly provided by Martin Messerle, Genzentrum, Munich, Germany) with HindIII and XhoI. Sticky ends were filled up by Klenow polymerase. Finally, this 4.5-kbp fragment was ligated into the pcDNA3 backbone containing the neomycin phosphotransferase gene. On the day before transfection, 5 × 106 A20 cells in a volume of 10 ml of culture medium were seeded in 10-cm-diameter petri dishes. For transfection, cells were transferred into a 50-ml tube, sedimented at 150 × g for 10 min, washed with PBS, and resuspended in 500 μl of RPMI 1640 (with no supplements) containing 20 μg of linearized pcDNA3/lacZ. Electroporation was performed at 400 V and 960 μF (Gene Pulser system model 2; Bio-Rad, Munich, Germany). Transfectants were propagated in 2-ml cultures. Starting at day 2 after electroporation, selection was performed with G418 sulfate (Geneticin catalog no. 345810; Calbiochem-Novabiochem, La Jolla, Calif.) at a concentration of 1 mg per ml. Expression of β-galactosidase and silencing of lacZ gene expression were tested by using established protocols of X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) staining of cells in suspension or of frozen tissue sections. Transfected cell line A20-lacZ consisted of 85% expressor cells (i.e., blue cells) and 15% nonexpressor cells.
(iii) Selection of A20-derived liver-adapted variant clone E12E.
After 24 passages of cell line A20-lacZ, cells were cloned by using limiting-dilution cultures set up with a λ of 0.3 cells per microwell (960 cultures in 96-well microplates), that is, with a clone probability, F(1)/[1 − F(0)], of 0.86 based on the Poisson distribution function (31), where F(0) = e−λ and F(1) = λ × F(0). Out of 112 growing tumor cell clones, 25 clones stably expressed the reporter gene in cell culture in all cells. Most clones showed variegated expression. Usually, the expression rate within a clone that showed no or variegated reporter gene expression increased with continued passaging. Compared to the parental A20 cell line, transfectants VIIIE10 (100% high expressor, dark blue), IVG6 (100% high expressor), XG11 (100% low expressor, light blue), and IID11 (50% high expressor), all used in passage 50 after the cloning, were attenuated to various degrees in terms of lethality in recipients irradiated with 6 Gy of gamma radiation. The degree of attenuation did not correlate with reporter gene expression in cell culture. In contrast to reported data on in situ lacZ gene expression in liver-infiltrating T-cell lymphoma transfectants (27), the reporter gene was found to be rapidly silenced in tumor colonies in vivo. It should be noted that nonexpressor clones as well as ex tumore-reisolated lymphoma cells still contained the reporter gene, as revealed by lacZ-specific PCR (not shown). The highest percentage of β-galactosidase-expressing cells was ca. 20% in a single mouse out of five mice carrying tumors in the liver on day 48 after intravenous transfer of 106 IID11 cells. Lymphoma cells were isolated from another portion of this IID11 lymphoma-bearing liver by excision of a macroscopically visible tumor mass. A single-cell suspension was prepared by passing the tumor tissue through a stainless steel mesh. Clumps were removed by filtration through a nylon mesh. After nine cell culture passages in the presence of G418, tumor cells were recloned by limiting dilution (see above). Out of 58 growing tumor cell colonies, 3 were selected for further analyses: clones H11H, E12E, and E2B, characterized by 80, 80, and 0% β-galactosidase expression, respectively. Based on the highest tumorigenicity in the liver, subclone E12E (frozen in passage 8 after the ex tumore recloning of clone IID11) was selected for the experiments reported herein. Karyotype analysis of clone E12E performed in passage 22 revealed a tetraploid karyotype with a broad range in chromosome numbers, from 73 to 81. All reported experiments were performed with the passage 8 stock after a further 18 to 26 passages of expansion, depending on the number of cells required.
Documentation of lymphoma progression. (i) Liver macroscopy.
Photographs of tumor-bearing livers were taken with a Minolta Dynax 500si camera using 35-mm Elitechrome 100 film (iso 100/21°; catalog no. 3788239; Kodak). Diapositives were digitalized by scanning (LS-100 35-mm film desktop scanner; Nikon, Tokyo, Japan) and arranged into a figure with Adobe Photoshop, version 6.0.
(ii) Immunohistochemical (IHC) detection of tumor colonies in liver sections.
Paraffin sections (2 μm) of liver tissue were prepared and dewaxed with xylene by standard histological protocols. Endogenous peroxidase was inactivated by an incubation for 30 min at 20°C in 0.5% (vol/vol) hydrogen peroxide in a 1:1 mixture of methanol and PBS. Tumor cells were labeled for 1 h at 20°C with a 1:1,000 dilution (in PBS) of monoclonal antibody (MAb) anti-mouse CD45R/B220 (rat IgG2a; κ; clone RA3-6B2; BD Pharmingen; catalog no. 553084 [previous Pharmingen product 01121D]) (21). Staining of the sections was performed with the avidin-biotin complex (ABC) method. In detail, the sections were incubated for 30 min at ca 20°C with a 1:100 dilution (in PBS) of a biotinylated polyclonal goat antibody (Ab) directed against rat Ig (BD Pharmingen; catalog no. 554014 [previously 12112D]), followed by detection with ABC-peroxidase (1:100 dilution in PBS for 30 min at 20°C) (peroxidase ABC kit standard PK-4000; Vector Laboratories, Burlingame, Calif.) using 3,3′-diaminobenzidine tetrahydrochloride (DAB; catalog no. D5637; Sigma) as the substrate. The staining was enhanced by ammonium nickel sulfate hexahydrate, yielding a black precipitate. The sections were lightly counterstained for 5 s with hematoxylin. It is important to note that detection of the CD45R/B220 antigen on B cells (as shown for follicles in the spleen) requires a partial digestion with trypsin followed by antigen unmasking (see below). Based on this staining difference in conjunction with the significant difference in cell size, distinction between B lymphoma cells and normal B cells was no technical problem.
Low-magnification stereomicroscopy (stereo zoom microscope model SZX12; Olympus) at a magnification of ×3.5 was used to show section overviews. Digital images were taken with a ColorView12 digital camera using analySIS software, version 3.0 (Software Imaging Systems GmbH, Münster, Germany). The documented pictures represent mosaics of 15 individual images arranged with Adobe Photoshop, version 6.0. High-magnification microscopy (Axiophot research microscope equipped with plan-Neofluar oil immersion optics; Carl Zeiss Jena GmbH, Jena, Germany) was used for the documentation of details. Diapositives (Fujichrome 64T-RTP 135) of microphotographs were digitalized and electronically processed as described above.
(iii) Histomorphometric determination of tumor size.
The area occupied by a tumor within the liver sections and the sizes of individual tumor foci (two-dimensional size in the tissue sections) were measured by using the area morphometry utility of the analySIS, version 3.0, software for image analysis (Software Imaging Systems GmbH). To do this, tumor foci were encircled by a virtual line and the enclosed area was calculated by the software routine. Since the lymphoma is space demanding, leading to increasing hepatomegaly as the tumor progresses, the percentage of tumor area was calculated for a constant hepatic tissue area of 100 mm2 according to the formula T/(H + T) × 100%, where T = tumor area and H = 100 mm2 (hepatic tissue area). Likewise, the number of tumor foci was determined for an H of 100 mm2. Based on their sizes, tumor foci were categorized into 10 classes, with area doubling from class to class, starting with an area of 0.05 mm2, which represents ca. 400 tumor cells.
Quantitation of liver infection.
IHC staining for the in situ detection of infected cells was performed essentially as described previously (17, 46). In brief, infected liver cells were visualized by staining of the intranuclear viral IE1 protein (pp89) with MAb CROMA 101 (kindly provided by S. Jonjic, University of Rijeka) using the alkaline phosphatase-anti-alkaline phosphatase (APAAP) method with new fuchsin as the substrate, yielding a brilliant red color. Sections were analyzed microscopically, and infected cells were counted for a hepatic tissue area of 5 to 100 mm2 depending on the degree of infection. Data are given for an area of 100 mm2.
Immunohistological analysis of virus-lymphoma interrelation. (i) Two-color IHC staining of cell surface CD45R and intranuclear viral IE1.
In the first step, cell membrane CD45R/B220 was stained black precisely as described above for the single-color IHC staining. After being dried, sections were treated for 15 min at 37°C with trypsin (0.125% [wt/vol]). The IE1 protein was labeled for 1 h at ca. 20°C with MAb CROMA 101 followed by incubation for 30 min at ca. 20°C with a 1:20 dilution (in PBS) of a polyclonal goat Ab directed against mouse Ig (catalog no. M5899; Sigma). Staining was performed with the murine APAAP complex (mouse IgG1 MAb clone AP1B9 directed against alkaline phosphatase with bound calf intestinal alkaline phosphatase; catalog no. A7827; Sigma) and new fuchsin as the substrate, yielding a brilliant red color.
(ii) In situ detection of proliferating cells.
Antigen unmasking (heat-induced epitope retrieval) was performed by boiling the sections for 5 min in 10 mM trisodium-citrate-dihydrate (dissolved in water; pH 6.0). After the sections were slowly cooled down to ca. 20°C, proliferating cell nuclear antigen (PCNA) was labeled for 1 h at ca. 20°C with a 1:25 dilution (in PBS) of MAb clone PC10 (mouse IgG2a; κ; catalog no. 555566 [previously 32551A]; BD Pharmingen) (12), followed by an incubation for 30 min at ca. 20°C with a 1:200 dilution of a biotinylated polyclonal goat Ab directed against mouse IgG Fab fragments (catalog. no. B0529; Sigma). Brown staining was performed with ABC-peroxidase with DAB as the substrate and no enhancement by nickel.
(iii) In situ detection of apoptosis.
After antigen unmasking (see above), active caspase 3 was labeled for 1 h at ca. 20°C with a 1:50 dilution (in PBS) of specific, affinity-purified rabbit IgG (catalog. no. AF835; R&D Systems) (61), followed by incubation for 30 min at ca. 20°C with a 1:500 dilution (in PBS) of a biotinylated polyclonal goat Ab directed against rabbit IgG (catalog. no. B8895; Sigma). Brown staining was achieved with the avidin-biotin-peroxidase complex with DAB as the substrate and no enhancement by nickel.
RESULTS
Experimental model for studying virus-tumor interplay.
It was the starting idea of this research project that two of the major problems of BMT, namely, CMV infection and residual leukemia, are unlikely to have no influence on each other. With this rationale in mind we established an experimental tumor model that was based on the well-established model of mCMV infection after syngeneic BMT performed with BALB/c mice as BM cell donors and recipients. Depending on the precise conditions of BMT, different outcomes can be predicted and range from a tight immune control of infection to lethal, multiple-organ CMV disease (17, 46, 47, 62, 63). On these firm grounds we have here added a lymphoma as a further parameter to the model (Fig. 1). In the clinical situation, a lymphoma arises as a relapse tumor from a minimal residual tumor that has survived the cytotoxic therapy in the BMT recipients (for reviews, see references 48 and 56). We therefore had to choose for the model a lymphoma syngeneic to the recipient. A second criterion for the choice was that the lymphoma should not be a cellular target of infection, because this would be a trivial mechanism of tumor control. The BALB/c-derived B-cell lymphoma A20 (23) fulfilled both conditions. However, it soon became evident that the parental A20 line, ATCC TIB-208, is not optimal for the model, because A20 cells are highly aggressive and neuroinvasive in immunodeficient recipients, leading to death within 2 to 3 weeks. On the other hand, A20 cells proved to be too immunogenic, and thus too indolent, in recipients immunoreconstituted by BMT (data not shown).
FIG. 1.
Model for studying the interplay between virus and B-cell lymphoma. A syngeneic BMT is carried out with female BALB/c mice as BM cell donors and recipients. Hematoablative conditioning of the recipients is achieved by gamma irradiation with a single dose of 6 or 7 Gy. Recipients receive B-lymphoma cells of A20 (ATCC TIB-208)-derived liver-adapted clone E12E. One group of recipients is infected with mCMV, and another group is left uninfected for control. Tumor pathogenicity is tested by monitoring survival and symptoms of illness, and tumorigenicity is determined by histomorphometrical quantitation of lymphoma growth in the liver.
The model was refined by selecting a lacZ-transfected clonal variant of line A20, referred to as clone E12E. Based on the rationale that lymphoma cells isolated from a tumor metastasis in the liver might have a particular propensity to colonize the liver, E12E was derived by ex tumore recloning of a lacZ-transfected A20-derived clone. Compared with the parental A20 line, clone E12E is less pathogenic in the immunodeficient recipient, metastasizes more efficiently to the liver, and is highly tumorigenic in the immunoreconstituted recipient.
Retardation and prevention of E12E B-lymphoma pathogenicity by mCMV infection.
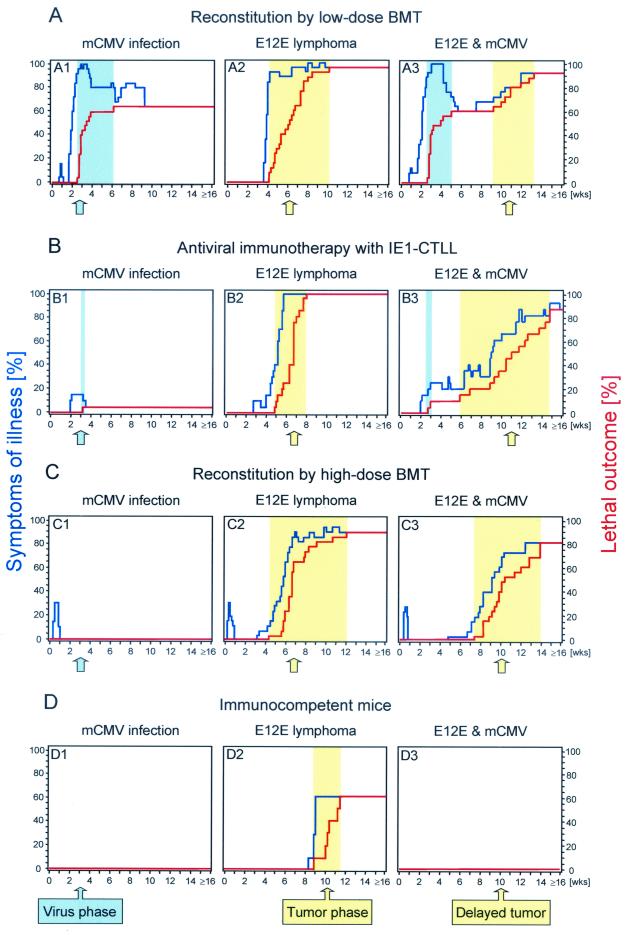
In a first protocol (Fig. 2A), recipients received hematoablative treatment by gamma irradiation with a dose of 7 Gy and were thereafter reconstituted with a low dose of BM cells, namely, with 106 cells. In accordance with all our previous experience with the viral disease model (17, 62, 63), this is an experimental condition in which the hematopoietic reconstitution of protective, antiviral CD8 T cells is not strong enough to prevent CMV disease in mCMV-infected recipients. In the specific experiment shown in Fig. 2 A1, all infected recipients developed symptoms of CMV disease, typically around the third week, and ca. 60% died of multiple organ failure, including viral hepatitis. Likewise, these conditions for BMT also failed to control the liver-infiltrating E12E lymphoma (Fig. 2, A2). Symptoms of disease set in abruptly around the fourth week, and almost all recipients died within the following 6 weeks. Thus, in this setting, lethality by the virus precedes lethality by the tumor. What happens if both diseases merge? The experiment gave two notable answers (Fig. 2, A3). First, the course of viral disease was untouched by the presence of the tumor. Second, after an interim of 1 month, with no further mortality between weeks 5 and 9, recipients started to die from lymphoma, that is with a delay of 5 weeks compared to the group of uninfected tumor-bearing recipients. It should be emphasized that the cause of death was routinely verified upon autopsy. This second wave of dying was thus definitely due to the tumor and not to a recurrent viral infection. In essence, the infection interfered with tumor progression, but it did not eliminate the tumor in this particular experimental protocol.
FIG. 2.
Attenuation of E12E lymphoma pathogenicity by mCMV in different experimental settings. Recipients, except those in panel D, were conditioned for BMT on day −1 by hematoablative gamma irradiation with a dose of 7 Gy. (A) Recipients of low-dose BMT, defined by reconstitution with 106 BM cells (n = 25 mice per group). (B) Recipients of low-dose BMT and preemptive (day 1) antiviral immunotherapy with 106 cells of a CD8 T-cell line (IE1-CTLL) specific for the IE1 peptide of mCMV (n = 20 mice per group). (C) Recipients of a high-dose BMT, defined by reconstitution with 107 BM cells (n = 25 mice per group). (D) Immunocompetent mice (n = 10 mice per group). (A1 to D1) Intraplantar infection of the recipients with 105 PFU of mCMV on day 0. (A2 to D2) Intravenous application of 106 E12E lymphoma cells on day 0. (A3 to D3) Infection and lymphoma combined. Symptoms of illness (blue line) and cases of death (red line) were monitored daily.
As acute mCMV disease involves the liver (18, 47, 55), destruction of liver parenchyma could be a reason for inhibition of a liver-infiltrating lymphoma, and late tumor progression might correlate with liver regeneration in the survivors of CMV disease. Preemptive cytoimmunotherapy with antiviral CTLL can substitute for an inefficient reconstitution of CD8 T cells after low-dose BMT and can prevent mCMV disease in general and hepatitis in particular (18, 62). Assuming that mCMV operates by destroying the “homing bed” of the tumor, one would have predicted that adoptive transfer of CTL specific for an antigen presented by infected cells, for instance, mCMV IE1 protein (pp89)-derived peptide 168YPHFMPTNL176 (18, 49, 50), would prevent both effects of mCMV, lethality from infection and the inhibition of lymphoma progression (Fig. 2B). The result of the experiment was a great surprise: IE1 CTL indeed largely prevented morbidity and lethality from mCMV (Fig. 2, B1), and they did not control the lymphoma, for which they are not specific (Fig. 2, B2). Yet, contrary to the prediction, the inhibition of lymphoma progression remained fully operative (Fig. 2, B3).
Manifestations of mCMV disease can alternatively be prevented by BMT performed with a high dose of BM cells, for instance with 107 cells. It has previously been demonstrated that this protection is based on an efficient reconstitution of antiviral CD8 T cells (17, 46, 47, 62). In fact, the antiviral protection by high-dose BMT was here reproduced (Fig. 2, C1). Unlike the transfer of antiviral CTL, the hematopoietic reconstitution of CD8 T cells could include effector cells directed against the lymphoma. Yet, as shown in Fig. 2, C2, high-dose BMT had minimal effect on the lymphoma, even though there was some delay in lethality and there were a few more long-term survivors. Most importantly, the inhibition of lymphoma progression by mCMV was operative also in this experimental setting (Fig. 2, C3).
Finally, we asked about the course of disease in immunocompetent mice (Fig. 2D). It is known that mCMV, like hCMV, does not cause clinical manifestations of CMV disease in the immunocompetent host. The virus infects cells at the site of inoculation, which is the planta in this particular model, disseminates via macrophages (16, 54, 64), and replicates preferentially in salivary gland epithelial cells. Accordingly, none of the recipients showed signs of illness (Fig. 2, D1). The E12E lymphoma was pathogenic also in the presence of full immunocompetence: the onset of dying was delayed by ca. 1 month compared that from the BMT model, but ca. 60% of the recipients subsequently succumbed to the lymphoma (Fig. 2, D2). Notably, in this setting, mCMV infection was curative (Fig. 2, D3). In conclusion, the antileukemic effect of mCMV is still operative under conditions of CMV disease control.
Quantitative and kinetic histological analysis of E12E lymphoma progression.
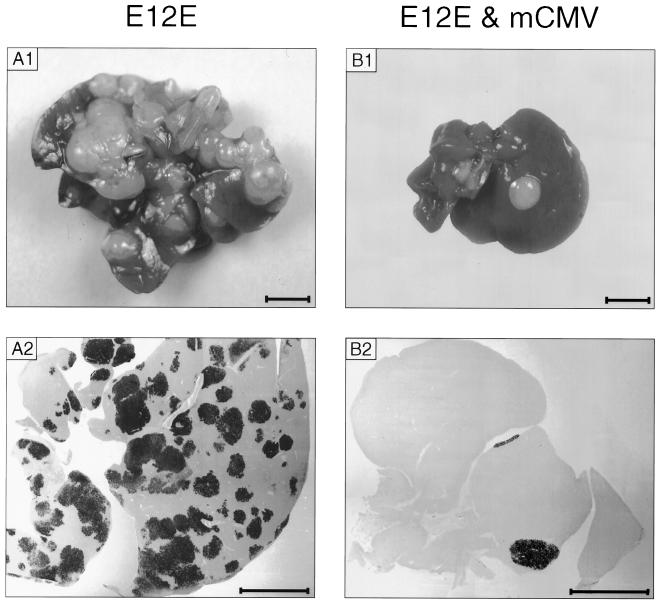
So far, the experiments were focused on pathogenicity and lethality caused by the E12E lymphoma in the absence or the presence of mCMV infection. We now take a closer look at tumorigenicity, that is, the colonization of the liver and the progression of the lymphoma within the liver. The analysis was made under the conditions of low-dose BMT corresponding to those for Fig. 2A. Macroscopic pictures of the livers were taken on day 28, which is the time point of onset of dying for the uninfected group (Fig. 3; compare with Fig. 2, A2). In the absence of infection, space demand of the lymphoma causes hepatomegaly, and the whole organ is covered with huge lymphoma colonies, macroscopically visible as hyaloid protuberances (Fig. 3, A1 shows a representative case). A liver section with black IHC staining of the lymphoma by means of cell membrane expression of CD45R/B220 (21) shows that the liver is interpenetrated by the lymphoma (Fig. 3, A2). In striking contrast, only a few lymphoma colonies had developed in livers of infected mice (Fig. 3, B1 and B2 show a selected case with two colonies visible: mouse 39 from Fig. 5).
FIG. 3.
Lymphoma remission by mCMV infection. (A1 and B1) Liver macroscopy. Photographs were taken on day 28 after inoculation of the E12E lymphoma in the absence (A1) or presence (B1) of mCMV infection under the experimental conditions of low-dose BMT (as defined for Fig. 2A). Lymphoma colonies are evident as hyaloid protuberances. (A2 and B2) Corresponding liver sections shown at low magnification. Tumor colonies interpenetrating the liver tissue were visualized by black IHC staining of cell membrane CD45R/B220. Counterstaining was with hematoxylin. Bars, 5 mm.
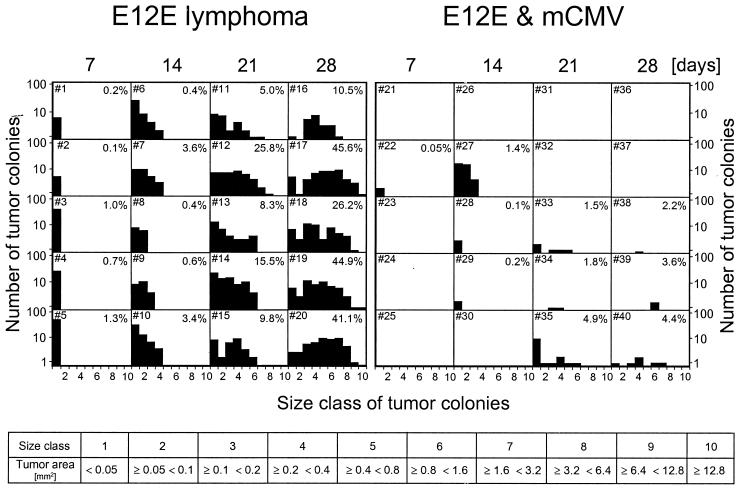
FIG. 5.
Quantitative analysis of E12E lymphoma progression in the liver. At the indicated time points after low-dose BMT (experimental setting as for Fig. 2A; same experiment as those shown in Fig. 3 and 4), number and sizes of E12E lymphoma colonies in IHC-stained liver sections were determined by image analysis using area morphometry software. Data refer to 100 mm2 of hepatic tissue to correct for the different space demand of the lymphoma. Recipients were individually numbered (upper left corners), and the percentages of total liver tissue area occupied by lymphomas are indicated (upper right corners). Ten size classes for the lymphoma colonies were defined (table).
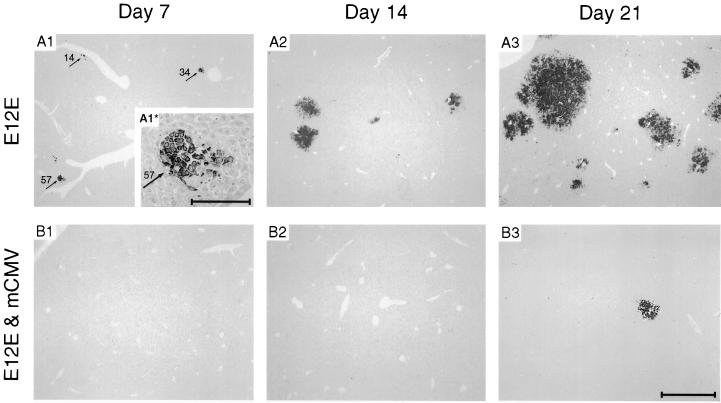
Two alternatives were considered: first, lymphoma cells could initially colonize the livers in both groups with the same efficacy. Upon virus replication in the liver, which peaks around day 14, colonies could die down in the infected liver while growing progressively in the uninfected liver. Alternatively, the infection could interfere with liver colonization at an early stage, for instance, at the stage of extravasation and transendothelial migration of the tumor cells. The question was answered by the kinetics of lymphoma development, studied by immunohistology (Fig. 4). In the uninfected group, young colonies consisting of 10 to 100 lymphoma cells (referring to a two-dimensional section) were frequently detected on day 7 in proximity to blood vessels (Fig. 4, A1 shows three perivascular lymphoma cell colonies, consisting of 14, 34, and 57 cells) and rapidly expanded with time (Fig. 4, A2 and A3). By contrast, colonies were rarely detected on day 7 in the infected group (Fig. 4, B1), and on day 14 there was no indication of remnants of necrotic or apoptotic tumor colonies (Fig. 4, B2). Obviously, some tumor cells must nevertheless have made their way into the liver, as colonies were detected occasionally at later time points (Fig. 3 and 4, B3), and because, as discussed above, the tumor relapsed weeks later (Fig. 2, A3).
FIG. 4.
Kinetics of E12E lymphoma progression in the liver. Earlier time points of the experiment of Fig. 3 were studied at higher microscopic resolution. (A1 to A3) Lymphoma progression in absence of infection. (B1 to B3) Reduced liver colonization and rare lymphoma colonies in the presence of mCMV infection. Representative sections were analyzed on days 7, 14, and 21. Tumor colonies were visualized by IHC staining of the CD45R/B220 antigen. Arrows (A1), young perivascular lymphoma colonies. Cell numbers (in two-dimensional section) are indicated. One colony is resolved to greater detail (inset A1* [bar, 50 μm]). Conterstaining was with hematoxylin. Bar (B3), 1 mm (applies to all panels except inset A1*).
While histology gives an authentic visual impression, histomorphometric quantitation of tumor size in five recipients for each time point was chosen for documenting reproducibility and variance (Fig. 5). For a representative hepatic tissue area of 100 mm2, the number and sizes of E12E lymphoma colonies were determined on days 7, 14, 21, and 28 for uninfected and infected recipients. At a glance, it was seen that E12E colonized the livers of all uninfected recipients and grew progressively with time. Specifically, many small colonies (<0.05 mm2) were present on day 7 in each of the five mice tested at that time point (mice 1 through 5). On day 28 (mice 16 through 20), lymphoma tissue occupied a large portion (ranging from 10.5 to 45.6%) of the liver section area. By contrast, lymphoma colonies were detected only sporadically in the group of infected recipients, apparently representing cases in which a few of the tumor cells had overcome the inhibitory effect of virus. The liver section area occupied by the lymphoma was <5% at any time point. Note that lymphoma-free livers (always referring to 100 mm2) were found for every time point. Specifically, on day 7, just two colonies were detected in only one (mouse 22) out of five recipients tested.
In conclusion, mCMV infection strongly inhibits the development of E12E lymphoma in the liver. The difference in liver colonization apparent on day 7 points to an interference at a very early stage.
Infection of liver tissue does not inhibit tumor growth within the liver.
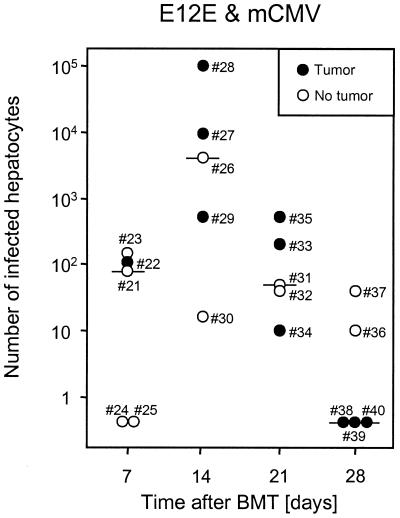
The early effect of the virus on colonization of the liver by the lymphoma cells did not formally exclude a subsequently operative additional effect of cytopathogenic infection of liver tissue on the progression of the lymphoma. If this applies, we would expect that absence of tumor colonies correlates with a high rate of tissue infection. Likewise, a low rate of tissue infection should favor the escape of the tumor. Out of altogether 20 infected recipients included in the experiment shown in Fig. 5, the lymphoma grew in 10 and failed to grow in 10, an ideal constellation for a correlation analysis (Fig. 6). The number of infected hepatocytes reached a peak level on day 14, with a high variance ranging from ca. 20 cells (mouse 30, tumor-free liver) to ca. 105 cells (mouse 28, tumor-bearing liver) per 100 mm2 of hepatic tissue. All in all, it is evident that there is no correlation between rate of liver cell infection and tumor growth in the liver parenchyma (P > 0.1; rank sum test [4]).
FIG. 6.
Lack of correlation between degree of liver infection and presence of lymphoma. Corresponding to the quantitation of E12E lymphoma in infected recipients (Fig. 5), a determination of the degree of liver infection was performed by IHC staining of intranuclear viral IE1 protein followed by counting infected hepatocytes. Data refer to 100 mm2 of hepatic liver tissue to correct for the different space demand of the lymphoma. Numbers signify individual mice, as in Fig. 5. Horizontal bars, median values.
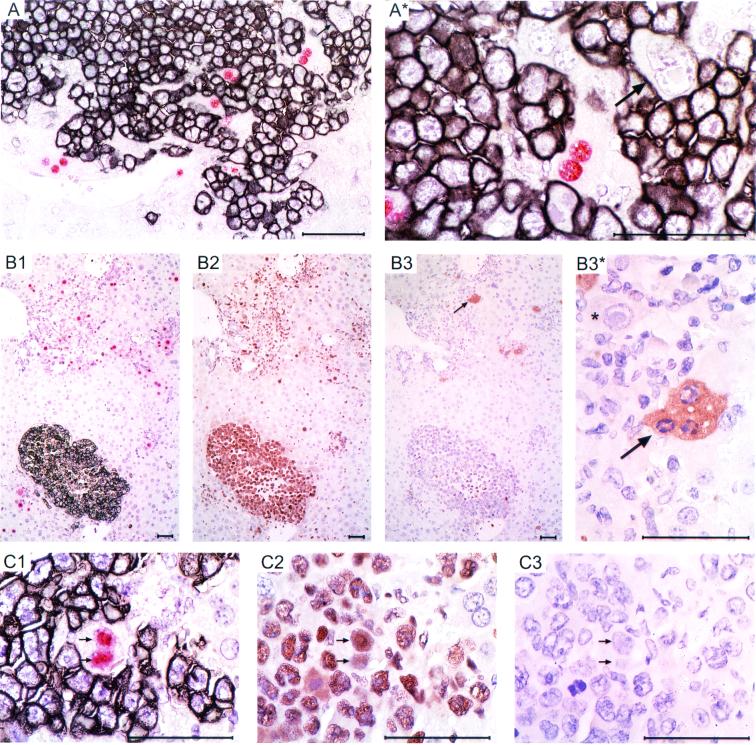
Direct evidence against inhibition of tumor growth by infected hepatocytes was provided by in situ two-color IHC analysis with red staining of intranuclear IE1 protein in infected hepatocytes and black staining of cell surface CD45R/B220 expressed by the E12E lymphoma cells (Fig. 7A [overview] and A* [details]; see also Fig. 5, mouse 35, day 21). Note that tumor cell nuclei did not stain red, that is, tumor cells did not express IE1 and were thus not infected in situ. Infected hepatocytes were found amid a tumor mass. Viable tumor cells were located in intimate proximity to the infected hepatocytes, and even dividing tumor cells were observed. Accordingly, as shown in serial sections, almost all tumor cells in such a colony (Fig. 7, B1, black staining) expressed polymerase processivity factor PCNA (12, 37) (Fig. 7, B2, brown staining), while only a few cells in the center of the tumor mass expressed the active form of effector caspase 3 (14, 61) (Fig. 7, B3). Clusters of cells undergoing apoptosis, as defined by cytoplasmic caspase 3 (brown staining) and the typical condensation and margination of chromatin (35), were seen only within the foci of infection (Fig. 7, B3*). Note, however, that the infected hepatocytes were usually not apoptotic. This is documented in Fig. 7, B3* and by a second set of serial sections (Fig. 7, C1 to C3). Specifically, Fig. 7, C1 highlights an infected, binucleated hepatocyte in which both nuclei contain the IE1 protein within an inclusion body, the site of nucleocapsid assembly and viral DNA packaging during the L phase of the viral replication cycle. The same nuclei also contain PCNA (Fig. 7, C2), which is consistent with the known stimulation of PCNA expression and cell cycle regulation by CMV genes (8, 39, 59). Accordingly, this infected hepatocyte did not express cytoplasmic caspase 3 (Fig. 7, C3), which is in agreement with an antiapoptotic function of mCMV E gene M45 (5).
FIG. 7.
Immunohistological analysis of virus-lymphoma interrelation in the liver. All IHC stainings were made on liver tissue sections derived on day 21 from infected and lymphoma-bearing mouse 35 of the experiment shown in Fig. 5 and 6. (A [overview] and A* [detail of A]) Two-color IHC staining of intranuclear IE1 protein in infected hepatocytes (red) and membrane CD45R/B220 expressed by the E12E lymphoma cells (black). Arrow, dividing tumor cell in the stage of cytokinesis. (B1 to B3 and B3* [detail of B3]) Serial sections (in 2-μm distances) stained red and black for IE1 and CD45R/B220, respectively (B1), brown for PCNA (B2), and again brown for apoptosis marker active caspase 3 (B3 and B3*). Arrow, group of apoptotic cells within the focus of infection. Note the typical clustering of chromatin in the nuclei. ∗ (B3*), infected but caspase 3-negative hepatocyte bearing the characteristic intranuclear inclusion body. (C1 to C3) Serial sections shown at high magnification after staining for IE1 and CD45R/B220 (C1), PCNA (C2), and active caspase 3 (C3). Twin arrows, infected hepatocyte containing two nuclei with both carrying an intranuclear inclusion body containing viral DNA (visible by in situ hybridization; not shown here [see reference 47]), IE1 protein, and PCNA. Note that this infected hepatocyte did not express cytoplasmic caspase 3. Throughout, sections were counterstained with hematoxylin. Bars, 50 μm.
Lack of susceptibility of the E12E lymphoma to infection and to TNF-α in cell culture.
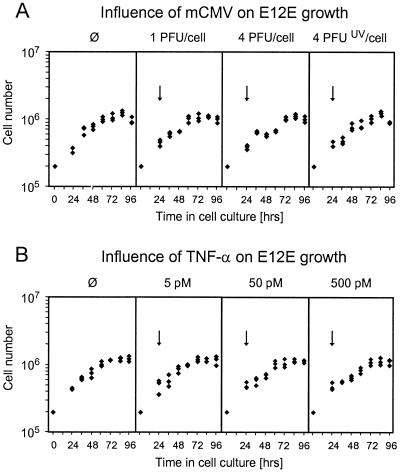
The in situ findings have shown that E12E cells do not express an immunohistologically detectable amount of the IE1 protein, which is bona fide evidence against infection of E12E. Furthermore, the lymphoma cells remained viable and even proliferated within infected liver tissue. However, an influence of infection on the doubling time of the tumor cells, that is, on the speed of lymphoma progression, was not formally excluded. It must be emphasized that virus can affect cells even in the absence of viral gene expression. Increasing evidence suggests a profound effect of virion proteins, such as gB, on signaling pathways and on cellular gene expression upon their binding to cell surface receptors or upon uptake (for an overview, see reference 41). We therefore tested the effect of different doses of infectious virus as well as of a high dose of UV light-inactivated virions on the growth of E12E in cell culture. It should be recalled that 1 PFU of mCMV corresponds to ca. 500 viral genomes contained within ca. 150 monocapsid and 100 multicapsid virions (28). Thus, a dose of 4 PFU/cell corresponds to 1,000 viral particles per cell. Apparently, there was no direct or indirect cytopathogenic effect that would have inhibited the proliferation of E12E cells (Fig. 8A). In accordance with this result, the IE1 protein was not detected in nuclei of E12E cells by immunofluorescence (not shown).
FIG. 8.
E12E lymphoma growth characteristics in cell culture. E12E cells were seeded in 2-ml cultures at a cell density of 2 × 105 cells per culture. The first counting was performed after 24 h, followed by counting in 12-h intervals (aliquots taken from the cultures) until 96 h. ø, growth curves in the absence of infection. The doubling time in the exponential phase was found to be ca. 30 h in two independent measurements. Diamonds, cell numbers determined for three individual cultures. (A) Influence of mCMV on lymphoma growth. Infectious or UV-inactivated mCMV was added after 24 h of cultivation (arrows) at the indicated doses, referring to the number of cells present in the cultures at 24 h. (B) Influence of TNF-α on lymphoma growth. rmTNF-α was added to the cultures at 24 h (arrows) in the indicated final concentrations.
Many types of tumor cells are susceptible to direct cytotoxic effects of TNF-α (20). Since TNF-α might be produced in response to mCMV by macrophages or dendritic cells located at the local site of intraplantar infection, TNF-α was a candidate for mediating the antitumoral effect of mCMV. Sensitive tumor cell types die at picomolar concentrations of TNF-α. In contrast, up to 500 pM rmTNF-α had no significant influence on viability and growth of E12E lymphoma cells (Fig. 8B).
In vivo approaches to an understanding of the early antitumoral events.
All the in vivo data pointed to a mechanism that operates before the lymphoma cells have settled down in the liver, that is, before day 7. We used this fact for establishing a more rapid assay not involving long-term reconstitution by BMT. Since death from mCMV disease after hematoablative treatment occurs during the third week, usually not starting before day 12, there was no problem in counting lymphoma colonies in the liver between days 10 and 12 (day 11 in the specific experiment of Fig. 9).
Under these modified conditions, colonization of the liver by the E12E lymphoma was reproduced (Fig. 9A). The inhibitory effect of mCMV was also reproduced in that only three out of five infected recipients showed a few tumor colonies in the liver (Fig. 9B). This finding gave the important new information that BM cells, in all preceding experiments replenished by BMT, are not needed as mediators of the antitumoral effect of mCMV. We then asked whether virion proteins could substitute for replicative virus. The dose of UV light-inactivated virus that corresponded to the virus dose used for infection, namely, 105 PFUUV, did not inhibit the lymphoma (Fig. 9C), and even a 10-fold-higher dose didn't give the slightest hint of inhibition (Fig. 9D). In accordance with these findings, 105 PFU of hCMV strain Towne did not inhibit the lymphoma (data not shown). It should be recalled that hCMV does not replicate in mouse cells, but it can penetrate and express IE genes, at least in certain murine cell lines (29).
As shown above, the E12E lymphoma is insensitive to direct cytotoxic or cytostatic effects of TNF-α. However, in vivo, TNF-α has frequently been implicated in the control of tumors due to its systemic effect on the endothelium: it causes microvascular injury (20). If we assume that tissue macrophages and dendritic cells present in the skin and connective tissue at the plantar site of infection are still functional after a 7-Gy gamma irradiation, a role for TNF-α had still to be considered. However, production of TNF-α, if any, was found to be minimal under these experimental conditions. Specifically, the concentration of TNF-α in the serum never exceeded the assay detection level of 10 pg per ml (J. Podlech, unpublished data). Furthermore, the supraphysiological dose of 1 μg of rmTNF-α administered intravenously to uninfected recipients on the day after inoculation of the lymphoma cells had no effect on liver colonization and lymphoma progression (Fig. 9E). Accordingly, repetitive administrations of antibodies capable of neutralizing 40 ng of murine TNF-α per dose did not abolish the protective effect of mCMV in the infected recipients (Fig. 9F).
DISCUSSION
CMV infection and leukemia/lymphoma relapse represent two major problems in the therapy of hematopoietic malignancies by BMT. It is therefore surprising that an influence of hCMV on tumor relapse or remission has not so far been a topic of broad clinical research. Certainly, numerous follow-up studies of BMT recipients have monitored tumor relapse, and numerous studies have monitored primary or recurrent hCMV infection. However, to the best of our knowledge, these two events were not tested for a positive or negative correlation. Is there any interplay at all, and, if so, does CMV infection enhance the risk of tumor relapse or does it contribute to tumor remission? We are aware of a single set of studies by Fujiwara et al. (10, 11) on the relationship between hCMV antigenemia and the clinical course of adult T-cell leukemia/lymphoma (ATLL). Notably, infection occurring in early stages of the clinical course suppressed ATLL activity and contributed to a somewhat longer survival of the patients. The authors concluded that hCMV infection may be related to prolonged, temporal remission of ATLL.
It is almost trivial that viruses can cause tumor remission in cases in which the tumor cell type is a target of cytopathogenic infection. There is great current interest in oncolytic effects of replicating normal or genetically engineered viruses and their potential use in cancer therapy (for a recent commentary, see reference 3; for reviews, see references 24 and 66). Some selected examples of oncolytic viruses or virus variants include Newcastle disease virus, vesicular stomatitis virus, parvoviruses, reoviruses, replication-conditional herpes simplex virus type 1, and adenoviruses. In particular replication-selective adenovirus d/1520, also known as Onyx-015, has entered clinical phase I to III trials (22, 24). Recently, live-attenuated measles virus vaccine strain MV-Edmonston was shown to induce regression of established human B-cell lymphoma xenografts in severe combined immunodeficient (SCID) mice after intratumoral injection, and intravenous infection resulted in a slowing of tumor progression. The tumoricidal effect required replicative virus and was attributed to the fusogenic activity of the virus (13).
CMVs are cytopathogenic viruses that have a very broad cell type tropism in vivo. They infect various types of epithelial cells, including glandular epithelial cells, pneumocytes, enterocytes, hepatocytes, and brain ventricle ependymal cells, as well as a number of other cell types such as endothelial cells, myocytes, brown fat adipocytes, connective tissue fibrocytes, bone marrow stromal cells, macrophages, and dendritic cells (1, 16, 19, 45, 47, 60). Clearly, if we had selected a tumor of any of these permissive cell types for the model, intratumoral injection of mCMV would likely have caused tumor remission by cytocidal infection. However, it was not the intention of this project to add CMVs to the growing list of oncolytic viruses.
The novelty in our work is the paradigmatic finding that a viral infection can strongly inhibit lymphoma progression by a mechanism that is clearly distinct from oncolysis. Absence of intranuclear IE1 protein in E12E B-lymphoma cells residing in an infected liver and in those exposed to virus in cell culture showed that E12E cells are not permissive for productive mCMV infection. Cytopathogenic effects of virus in the absence of viral gene expression, for instance, by virion protein binding to receptors on the tumor cells or by uptake of virion proteins, were also ruled out. Specifically, exposure to high doses of virus in cell culture had no influence on the growth curve, a finding that excludes any cytotoxic or cytostatic effects. The in situ findings for the tumor-infiltrated and mCMV-infected liver were in accordance with the in vitro data: the presence of tumors and the extent of tissue infection were not inversely correlated. In fact, tumor cells located next to infected hepatocytes were viable and proliferated, as we concluded from the expression of polymerase processivity factor PCNA and from the presence of cells undergoing mitosis. Tumor cells in apoptosis, as defined by cytoplasmic expression of executioner caspase 3 in conjunction with the characteristic morphological changes in chromatin organization, were sometimes observed in the starving centers of the tumor colonies but not at the colony borders, which were exposed to infected liver parenchyma. These findings not only rule out antitumoral effects of direct virus replication or of shed virion proteins, they also argue against a role for any cytostatic, cytotoxic, or apoptosis-triggering virokines or virus-induced cytokines/chemokines that might be released from infected liver cells or from liver-infiltrating or liver-resident leukocytes, such as natural killer cells and macrophages/Kupffer cells, which participate in an inflammatory antiviral response in the liver (55).
That infection of the liver is not the reason for the inhibition of tumor growth, either by physical destruction of the homing bed of the lymphoma or by any of the direct or indirect factors discussed above, explains the at first glance surprising finding that prevention of mCMV organ disease by efficient endogenous reconstitution of protective antiviral CD8 T cells or by preemptive antiviral cytoimmunotherapy with IE1-CTLL did not abolish the antitumoral effect of the infection. This is an important observation as it allows a separation of the beneficial antitumoral effect from the pathogenic consequences of viral infection. In the lymphoma-inoculated immunocompetent host, mCMV infection was indeed curative, while in the BMT experiments reported herein the benefit from infection was temporary and most recipients eventually died of delayed tumor progression. However, this is not a limitation in general but is rather a matter of lymphoma cell load. The experiments were performed with a very high dose of lymphoma cells, and the histological study of the kinetics of liver colonization in the infected BMT recipients showed sporadic cases of tumor colonies in the liver. Apparently, a low number of tumor cells had escaped from the antitumoral mechanism of the virus. Experiments in progress indicate that the frequency of escaping tumor cells declines with decreasing initial tumor cell numbers and that mCMV infection can under those conditions be curative in BMT recipients too. We also plan to use reporter gene lacZ to answer the question of whether the clinical remission of the E12E lymphoma in the survivors is associated with tumor cell clearance or with tumor cell dormancy.
Is there any role for an antitumoral immune response in this BMT setting? In contrast to that of the parental A20 cells, the pathogenicity of liver-infiltrating variant E12E was not significantly modulated by hematopoietic reconstitution. Apparently, E12E is not very immunogenic. The adaptation to the liver, for which this clone was actually selected, may contribute to the failure in immune control, because the liver is known to be a tolerogenic organ site (reviewed in reference 25). The fact that immune cells failed in preventing late lymphoma progression in the infected recipients suggests that infection did not prevent the proposed tolerance induction, at least not enduringly. An immunohistological analysis of lymphoma-bearing livers indeed revealed a high number of liver-infiltrating “observer” T cells, which were apparently unable to prevent lymphoma progression (J. Podlech, unpublished data). A functional analysis of these liver T cells is planned.
While we currently do not exclude a role for the immune response at late stages, we are quite convinced that the early antitumoral effect of mCMV is not immune cell mediated. The strongest argument in favor of this opinion is the finding that the antitumoral effect became evident as early as on day 7 after infection and was operative in recipients that did not receive BM cells for reconstitution after the hematoablative treatment. The immunodeficiency caused by the hematoablative treatment was so substantial that the infection was no longer controlled, and all recipients died of multiple-organ CMV disease between days 12 and 18. Admittedly, after gamma irradiation with a dose of 7 Gy some radioresistant, residual innate immunity might still exist and might be triggered by the infection to perform an antitumoral first-line defense response. Yet, if this applies, one would expect a markedly enhanced antitumoral effect after BMT, as BMT immediately replenishes BM macrophages, granulocytes, and natural killer cells in addition to the stem and progenitor cells that reconstitute long-term hematopoiesis. Yet, BMT was found not to improve the antitumoral effect of mCMV infection.
What finally is the mechanism? Frankly, we can only speculate at the moment. The key to the phenomenon is the kinetics of liver colonization by the lymphoma cells, in particular the situation found on day 7 (recall Fig. 4). In absence of infection, E12E had established small perivascular colonies, indicating that the lymphoma cells had successfully transmigrated the endothelium, whereas these seeding colonies were absent in the infected recipients. It has long been known that most tumor cells in the circulation are rapidly killed by mechanical trauma, actually within 24 h, and that extravasation by diapedesis is the critical rate regulator for metastasis (65; for an overview, see reference 36). We therefore propose that mCMV infection might interfere with the communication between the lymphoma cells and the capillary endothelial cells. Alternatively, or in addition, one might also still consider an effect on the regulation of angiogenesis at a very early stage in tumor vascularization (32). There is extensive literature on phenotypic and functional changes induced in endothelial cells by CMV infection. However, the situation here is more complicated, as the antitumoral effect precedes the infection of liver endothelium. It is open to question whether a viral protein exerts the effect directly or whether a cellular mediator cytokine/chemokine is involved. Notably, replicative virus is needed for this direct or indirect viral function.
Conclusion.
We have here entered an unexplored field by describing an antitumoral function of CMV, paradigmatically for mCMV. Likewise, this function is also paradigmatic for an antitumoral effect of virus infection that is clearly distinct from viral oncolysis or immune system-mediated lysis of infected tumor cells. While the definition of the precise cellular and molecular mechanisms awaits further studies, the experiments have already ruled out a number of first-guess mechanisms. The viral effect was mapped to the time period around the highly critical events of tumor cell extravasation and induction of angiogenesis. The quantitative immunohistological determination of early liver colonization by the lymphoma can serve as a precise and reasonably fast assay for testing an antitumoral effect of cytokines/chemokines or of mCMV mutants. This assay also provides the basis for the identification of the responsible viral gene(s) by a large-scale screening of viral gene expression libraries.
Acknowledgments
K.C.E. and J.P. contributed equally to the project
We thank Christoph Huber (speaker of the SFB 432) for having encouraged us to take up this interdisciplinary project and all the members of the SFB 432 for critical discussion and advice. We greatly appreciated the cooperation of our animal facility staff, of Kurt Reifenberg in particular. Karyotype analysis was performed with the help of Jürgen Olert (Institute for Pathology, Department of Pediatric Pathology, Mainz, Germany). Natascha K. A. Grzimek gave us her advice regarding the generation of transfected lymphoma cell sublines. Rafaela Holtappels and Doris Thomas supplied us with the IE1-CTLL. Stipan Jonjic (University of Rijeka) provided neutralizing antiserum against TNF-α, and Martin Messerle (Genzentrum, Munich, Germany) kindly gave us plasmid lacZ/gpt 16.
This work was supported by the Deutsche Forschungsgemeinschaft, Sonderforschungsbereich (Collaborative Research Center) 432, “Mechanisms of tumor defense and their therapeutic implications,” individual project A10, “Influence of cytomegalovirus infection on the risk of leukemia relapse after bone marrow transplantation.” Publication costs were defrayed in part by the “Fonds der chemischen Industrie (FCI),” Frankfurt, Germany.
REFERENCES
- 1.Andrews, D. M., C. D. Andoniou, F. Granucci, P. Ricciardi-Castagnoli, and M. A. Degli-Esposti. 2001. Infection of dendritic cells by murine cytomegalovirus induces functional paralysis. Nat. Immunol. 2:1077-1084. [DOI] [PubMed] [Google Scholar]
- 2.Apperley, J. F., C. Dowding, J. Hibbin, J. Buiter, E. Matutes, P. J. Sissons, M. Gordon, and J. M. Goldman. 1989. The effect of cytomegalovirus on hematopoiesis: in vitro evidence for selective infection of marrow stromal cells. Exp. Hematol. 17:38-45. [PubMed] [Google Scholar]
- 3.Bradbury, J. 2001. Oncolytic viral anti-cancer therapy: a magic bullet? Lancet 357:614.. [DOI] [PubMed] [Google Scholar]
- 4.Bradley, J. V. 1968. Distribution-free statistical tests. Prentice Hall, Englewood Cliffs, N.J.
- 5.Brune, W., C. Menard, J. Heesemann, and U. H. Koszinowski. 2001. A ribonucleotide reductase homolog of cytomegalovirus and endothelial cell tropism. Science 291:303-305. [DOI] [PubMed] [Google Scholar]
- 6.Busch, F. W., W. Mutter, U. H. Koszinowski, and M. J. Reddehase. 1991. Rescue of myeloid lineage-committed preprogenitor cells from cytomegalovirus-infected bone marrow stroma. J. Virol. 65:981-994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Childs, B., and D. Emanuel. 1993. Cytomegalovirus infection and compromise. Exp. Hematol. 21:198-200. [PubMed] [Google Scholar]
- 8.Dittmer, D., and E. S. Mocarski. 1997. Human cytomegalovirus infection inhibits G1/S transition. J. Virol. 71:1629-1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fries, B. C., D. Khaira, M. S. Pepe, and B. Torok-Storb. 1993. Declining lymphocyte counts following cytomegalovirus (CMV) infection are associated with fatal CMV disease in bone marrow transplant patients. Exp. Hematol. 21:1387-1392. [PubMed] [Google Scholar]
- 10.Fujiwara, H., Y. Eizuru, T. Matsumoto, T. Kukita, R. Imaizumi, H. Kawada, H. Ohtsubo, K. Matsushita, N. Arima, and C. Tei. 2001. The significance of cytomegalovirus infection over the clinical course of adult T-cell leukemia/lymphoma. Microbiol. Immunol. 45:97-100. [DOI] [PubMed] [Google Scholar]
- 11.Fujiwara, H., T. Matsumoto, Y. Eizuru, K. Matsushita, H. Ohtsubo, T. Kukita, R. Imaizumi, M. Matsumoto, S. Hidaka, N. Arima, and C. Tei. 2000. Cytomegalovirus infection is not necessarily a poor prognostic factor in adult T-cell leukemia/lymphoma. J. Med. Virol. 62:140-143. [PubMed] [Google Scholar]
- 12.Garcia, R. L., M. D. Coltrera, and A. M. Gown. 1989. Analysis of proliferative grade using anti-PCNA/cyclin monoclonal antibodies in fixed, embedded tissues. Comparison with flow cytometric analysis. Am. J. Pathol. 134:733-739. [PMC free article] [PubMed] [Google Scholar]
- 13.Grote, D., S. J. Russell, T. I. Cornu, R. Cattaneo, R. Vile, G. A. Poland, and A. K. Fielding. 2001. Live attenuated measles virus induces regression of human lymphoma xenografts in immunodeficient mice. Blood 97:3746-3754. [DOI] [PubMed] [Google Scholar]
- 14.Grütter, M. G. 2000. Caspases: key players in programmed cell death. Curr. Opin. Struct. Biol. 10:649-655. [DOI] [PubMed] [Google Scholar]
- 15.Hanson, L. K., J. S. Slater, Z. Karabekian, G. Ciocco-Schmitt, and A. E. Campbell. 2001. Products of US22 genes M140 and M141 confer efficient replication of murine cytomegalovirus in macrophages and spleen. J. Virol. 75:6292-6302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hanson, L. K., J. S. Slater, Z. Karabekian, H. W. Virgin IV, C. A. Biron, M. C. Ruzek, N. van Rooijen, R. P. Ciavarra, R. M. Stenberg, and A. E. Campbell. 1999. Replication of murine cytomegalovirus in differentiated macrophages as a determinant of viral pathogenesis. J. Virol. 73:5970-5980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holtappels, R., J. Podlech, G. Geginat, H.-P. Steffens, D. Thomas, and M. J. Reddehase. 1998. Control of murine cytomegalovirus in the lungs: relative but not absolute immunodominance of the immediate-early 1 nonapeptide during the antiviral cytolytic T-lymphocyte response in pulmonary infiltrates. J. Virol. 72:7201-7212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holtappels, R., D. Thomas, J. Podlech, and M. J. Reddehase. 2002. Two antigenic peptides from genes m123 and m164 of murine cytomegalovirus quantitatively dominate CD8 T-cell memory in the H-2d haplotype. J. Virol. 76:151-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ibanez, C. E., R. Schrier, P. Ghazal, C. Wiley, and J. A. Nelson. 1991. Human cytomegalovirus productively infects primary differentiated macrophages. J. Virol. 65:6581-6588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jäättelä, M. 1991. Biology of disease. Biologic activities and mechanisms of action of tumor necrosis factor-α/cachectin. Lab. Investig. 64:724-742. [PubMed] [Google Scholar]
- 21.Johnson, P., A. Maiti, and D. H. W. Ng. 1997. CD45: a family of leukocyte-specific cell surface glycoproteins, p. 62.1-62.16. In L. A. Herzenberg, D. M. Weir, L. A. Herzenberg, and C. Blackwell (ed.), Weir's handbook of experimental immunology, vol. 2. Blackwell Science, Cambridge, Mass.L. A. [Google Scholar]
- 22.Khuri, F. R., J. Nemunaitis, I. Ganly, J. Arseneau, I. F. Tannock, L. Romel, M. Gore, J. Ironside, R. H. MacDougall, C. Heise, B. Randlev, A. M. Gillenwater, P. Bruso, S. B. Kaye, W. K. Hong, and D. H. Kirn. 2000. A controlled trial of intratumoral ONYX-015, a selectively-replicating adenovirus, in combination with cisplatin and 5-fluorouracil in patients with recurrent head and neck cancer. Nat. Med. 6:862-863. [DOI] [PubMed] [Google Scholar]
- 23.Kim, K. J., C. Kanellopoulos-Langevin, R. M. Merwin, D. H. Sachs, and R. Asofsky. 1979. Establishment and characterization of BALB/c lymphoma lines with B cell properties. J. Immunol. 122:549-554. [PubMed] [Google Scholar]
- 24.Kirn, D. H., R. L. Martuza, and J. Zwiebel. 2001. Replication-selective virotherapy for cancer: biological principles, risk management and future directions. Nat. Med. 7:781-787. [DOI] [PubMed] [Google Scholar]
- 25.Knolle, P. A., and G. Gerken. 2000. Local control of the immune response in the liver. Immunol. Rev. 174:21-34. [DOI] [PubMed] [Google Scholar]
- 26.Kotenko, S. V., S. Saccani, L. S. Izotova, O. V. Mirochnitchenko, and S. Pestka. 2000. Human cytomegalovirus harbors its own unique IL-10 homolog (cmvIL-10). Proc. Natl. Acad. Sci. USA 97:1695-1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kruger, A., V. Schirrmacher, and R. Khokha. 1999. The bacterial lacZ gene: an important tool for metastasis research and evaluation of new cancer therapies. Cancer Metastasis Rev. 17:285-294. [DOI] [PubMed] [Google Scholar]
- 28.Kurz, S., H.-P. Steffens, A. Mayer, J. R. Harris, and M. J. Reddehase. 1997. Latency versus persistence or intermittent recurrences: evidence for a latent state of murine cytomegalovirus in the lungs. J. Virol. 71:2980-2987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lafemina, R. L., and G. S. Hayward. 1988. Differences in cell-type-specific blocks to immediate early gene expression and DNA replication of human, simian, and murine cytomegalovirus. J. Gen. Virol. 69:355-374. [DOI] [PubMed] [Google Scholar]
- 30.Lagneaux, L., A. Delforge, R. Snoeck, E. Bosmans, D. Schols, E. DeClercq, P. Stryckmans, and D. Bron. 1996. Imbalance in production of cytokines by bone marrow stromal cells following cytomegalovirus infection. J. Infect. Dis. 174:913-919. [DOI] [PubMed] [Google Scholar]
- 31.Lefkovits, I., and H. Waldmann. 1979. Limiting dilution analysis of cells in the immune system, p. 89-90. Cambridge University Press, Cambridge, England.
- 32.Li, C.-Y., S. Shan, Q. Huang, R. D. Braun, J. Lanzen, K. Hu, P. Lin, and M. W. Dewhirst. 2000. Initial stages of tumor cell-induced angiogenesis: evaluation via skin window chambers in rodent models. J. Natl. Cancer Inst. 92:143-147. [DOI] [PubMed] [Google Scholar]
- 33.Ljungman, P., and H. Einsele. 1994. Cytomegalovirus infection. Curr. Opin. Hematol. 1:418-422. [PubMed] [Google Scholar]
- 34.Madrigal, J. A., I. Scott, R. Arguello, R. Szydlo, A. M. Little, and J. M. Goldman. 1997. Factors influencing the outcome of bone marrow transplants using unrelated donors. Immunol. Rev. 157:153-166. [DOI] [PubMed] [Google Scholar]
- 35.Majno, G., and I. Joris. 1996. Cells, tissues, and disease, p. 175-227. Blackwell Science, Cambridge, Mass.
- 36.Majno, G., and I. Joris. 1996. Cells, tissues, and disease, p. 781-816. Blackwell Science, Cambridge, Mass.
- 37.Mathews, M. B., R. M. Bernstein, B. R. Franza, Jr., and J. I. Garrels. 1984. Identity of the proliferating cell nuclear antigen and cyclin. Nature 309:374-376. [DOI] [PubMed] [Google Scholar]
- 38.Mayer, A., J. Podlech, S. Kurz, H.-P. Steffens, S. Maiberger, K. Thalmeier, P. Angele, L. Dreher, and M. J. Reddehase. 1997. Bone marrow failure by cytomegalovirus is associated with an in vivo deficiency in the expression of stromal hemopoietin genes. J. Virol. 71:4589-4598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McElroy, A. K., R. S. Dwarakanath, and D. H. Spector. 2000. Dysregulation of cyclin E gene expression in human cytomegalovirus-infected cells requires viral early gene expression and is associated with changes in the Rb-related protein p130. J. Virol. 74:4192-4206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Minton, E. S., C. Tysoe, J. H. Sinclair, and J. G. Sissons. 1994. Human cytomegalovirus infection of the monocyte/macrophage lineage in bone marrow. J. Virol. 68:4017-4021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mocarski, E. S., and C. T. Courcelle. 2001. Cytomegaloviruses and their replication, p. 2629-2673. In D. M. Knipe and P. M. Howley (ed.). Fields virology. Lippincott Williams & Williams, Philadelphia, Pa.
- 42.Mutter, W., M. J. Reddehase, F. W. Busch, H. J. Bühring, and U. H. Koszinowski. 1988. Failure in generating hematopoietic stem cells is the primary cause of death from cytomegalovirus disease in the immunocompromised host. J. Exp. Med. 167:1645-1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pietersen, A., and H. M. Noteborn. 2000. Apoptin. Adv. Exp. Med. Biol. 465:153-161. [PubMed] [Google Scholar]
- 44.Pizzoferrato, E., N. R. Chu, T. S. Hawley, F. H. L. Lieu, B. H. Barber, R. G. Hawley, T. H. Watts, and N. L. Berinstein. 1997. Enhanced immunogenicity of B cell lymphoma genetically engineered to express both B7-1 and interleukin-12. Hum. Gene Ther. 8:2217-2228. [DOI] [PubMed] [Google Scholar]
- 45.Plachter, B., C. Sinzger, and G. Jahn. 1996. Cell types involved in replication and distribution of human cytomegalovirus. Adv. Virus Res. 46:195-261. [DOI] [PubMed] [Google Scholar]
- 46.Podlech, J., R. Holtappels, M.-F. Pahl-Seibert, H.-P. Steffens, and M. J. Reddehase. 2000. Murine model of interstitial cytomegalovirus pneumonia in syngeneic bone marrow transplantation: persistence of protective pulmonary CD8-T-cell infiltrates after clearance of acute infection. J. Virol. 74:7496-7507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Podlech, J., R. Holtappels, N. Wirtz, H.-P. Steffens, and M. J. Reddehase. 1998. Reconstitution of CD8 T cells is essential for the prevention of multiple-organ cytomegalovirus histopathology after bone marrow transplantation. J. Gen. Virol. 79:2099-2104. [DOI] [PubMed] [Google Scholar]
- 48.Radich, J. P. 2000. Clinical applicability of the evaluation of minimal residual disease in acute leukemia. Curr. Opin. Oncol. 12:36-40. [DOI] [PubMed] [Google Scholar]
- 49.Reddehase, M. J. 2000. The immunogenicity of human and murine cytomegaloviruses. Curr. Opin. Immunol. 12:390-396. [DOI] [PubMed] [Google Scholar]
- 50.Reddehase, M. J., J. B. Rothbard, and U. H. Koszinowski. 1989. A pentapeptide as minimal antigenic determinant for MHC class I-restricted T lymphocytes. Nature (London) 337:651-653. [DOI] [PubMed] [Google Scholar]
- 51.Redpath, S., A. Angulo, N. R. Gascoigne, and P. Ghazal. 1999. Murine cytomegalovirus infection down-regulates MHC class II expression on macrophages by induction of IL-10. J. Immunol. 162:6701-6707. [PubMed] [Google Scholar]
- 52.Riddell, S. R. 1995. Pathogenesis of cytomegalovirus pneumonia in immunocompromised hosts. Semin. Respir. Infect. 10:199-208. [PubMed] [Google Scholar]
- 53.Riegler, D. E., H. Hebart, H. Einsele, P. Brossart, G. Jahn, and C. Sinzger. 2000. Monocyte-derived dendritic cells are permissive to the complete replicative cycle of human cytomegalovirus. J. Gen. Virol. 81:393-399. [DOI] [PubMed] [Google Scholar]
- 54.Saederup, N., Y. C. Lin, D. J. Dairaghi, T. J. Schall, and E. S. Mocarski. 1999. Cytomegalovirus-encoded beta chemokine promotes monocyte-associated viremia in the host. Proc. Natl. Acad. Sci. USA 96:10881-10886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Salazar-Mather, T. P., J. S. Orange, and C. A. Biron. 1998. Early murine cytomegalovirus (MCMV) infection induces liver natural killer (NK) cell inflammation and protection through macrophage inflammatory protein 1 alpha (MIP-1alpha)-dependent pathways. J. Exp. Med. 187:1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sievers, E. L., and J. P. Radich. 2000. Detection of minimal residual disease in acute leukemia. Curr. Opin. Hematol. 7:212-216. [DOI] [PubMed] [Google Scholar]
- 57.Simmen, K. A., J. Singh, B. G. Luukkonen, M. Lopper, A. Bittner, N. E. Miller, M. R. Jackson, T. Compton, and K. Früh. 2001. Global modulation of cellular transcription by human cytomegalovirus is initiated by viral glycoprotein B. Proc. Natl. Acad. Sci. USA 98:7140-7145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Simmons, P., K. Kaushansky, and B. Torok-Storb. 1990. Mechanisms of cytomegalovirus-mediated myelosuppression: perturbation of stromal cell function versus direct infection of myeloid cells. Proc. Natl. Acad. Sci. USA 87:1386-1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sinclair, J., J. Baillie, L. Bryant, and R. Caswell. 2000. Human cytomegalovirus mediates cell cycle progression through G(1) into early S phase in terminally differentiated cells. J. Gen. Virol. 81:1553-1565. [DOI] [PubMed] [Google Scholar]
- 60.Sinzger, C., B. Plachter, A. Grefte, T. H. The, and G. Jahn. 1996. Tissue macrophages are infected by human cytomegalovirus in vivo. J. Infect. Dis. 173:240-245. [DOI] [PubMed] [Google Scholar]
- 61.Stadelmann, C., and H. Lassmann. 2000. Detection of apoptosis in tissue sections. Cell Tissue Res. 301:19-31. [DOI] [PubMed] [Google Scholar]
- 62.Steffens, H.-P., S. Kurz, R. Holtappels, and M. J. Reddehase. 1998. Preemptive CD8 T-cell immunotherapy of acute cytomegalovirus infection prevents lethal disease, limits the burden of latent viral genomes, and reduces the risk of virus recurrence. J. Virol. 72:1797-1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Steffens, H.-P., J. Podlech, S. Kurz, P. Angele, D. Dreis, and M. J. Reddehase. 1998. Cytomegalovirus inhibits the engraftment of donor bone marrow cells by downregulation of hematopoietin gene expression in recipient stroma. J. Virol. 72:5006-5015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stoddard, C. A., R. D. Cardin, J. M. Boname, W. C. Manning, G. B. Abenes, and E. S. Mocarski. 1994. Peripheral blood mononuclear phagocytes mediate dissemination of murine cytomegalovirus. J. Virol. 68:6243-6253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Weiss, L., F. W. Orr, and K. V. Honn. 1989. Interactions between cancer cells and the microvasculature: a rate-regulator for metastasis. Clin. Exp. Metastasis 7:127-167. [DOI] [PubMed] [Google Scholar]
- 66.Wodarz, D. 2001. Viruses as antitumor weapons: defining conditions for tumor remission. Cancer Res. 61:3501-3507. [PubMed] [Google Scholar]
- 67.Zhu, H., J. P. Cong, G. Mamtora, T. Gingeras, and T. Shenk. 1998. Cellular gene expression altered by human cytomegalovirus: global monitoring with oligonucleotide arrays. Proc. Natl. Acad. Sci. USA 95:14470-14475. [DOI] [PMC free article] [PubMed] [Google Scholar]