Abstract
We investigated the primary cellular immune responses to human immunodeficiency virus type 1 (HIV-1) Env and Gag proteins elicited by recombinant vesicular stomatitis viruses (rVSVs). The primary response to Env peaked 5 to 7 days after intraperitoneal vaccination, at which time 40% of CD8+ cells were Env tetramer positive and activated (CD62LLo). These freshly isolated cells actively lysed target cells pulsed with the p18-I10 peptide and secreted gamma interferon and tumor necrosis factor alpha after stimulation with the Env p18-I10 peptide. The primary response to Env elicited by rVSVs was sixfold higher than that elicited by recombinant vaccinia viruses (rVVs) at 5 days postvaccination. An intranasal route of vaccination with VSV-Env also elicited a strong primary response to Env. The primary immune response to Gag elicited by rVSV peaked 7 days after vaccination, at which time 3% of CD8+ cells were Gag tetramer positive and CD62LLo and functional by intracellular cytokine staining. This response was eightfold higher than that elicited by rVV expressing Gag. VSV-GagEnv, which expresses both Gag and Env from a single recombinant, also induced strong cytotoxic T-lymphocyte (CTL) responses to both Env and Gag. Our quantitative analyses illustrate the potency of the VSV vector system in CTL induction.
Cytotoxic T lymphocytes (CTL) are believed to play an important role in the control of human immunodeficiency virus type 1 (HIV-1) infection. HIV-specific CTL appear within the first few weeks of primary HIV-1 infection (23). The absence of an early CTL response is associated with prolonged clinical symptoms and plasma viremia (6, 23). Plasma viral RNA load is correlated with progression to AIDS (33) but is inversely correlated with the percentage of HIV-specific CTL (34). Later in infection, the frequency of HIV-specific memory CTL is associated with a lower median level of plasma viral RNA and better clinical performance (32).
Vesicular stomatitis virus (VSV) is a nonsegmented, negative-strand RNA virus that encodes five structural proteins. The development of a system for recovering VSV from plasmid DNA has allowed the manipulation of the viral genome and the expression of foreign genes in recombinant VSV (24, 42). Recombinant VSVs (rVSVs) expressing foreign viral glycoproteins elicit strong, protective humoral immune responses to the corresponding viruses (36, 37, 40), but there has been little quantitative analysis of the cellular immune responses to foreign viral proteins expressed from VSV.
CTL play a major role in clearing or controlling viral infections (reviewed in reference 13). Upon encountering antigen in the context of major histocompatibility complex (MHC) class I molecules, naïve CD8+ T cells with the appropriate T-cell receptor undergo clonal expansion and differentiate into effector cells capable of lysing infected target cells. CTL can also reduce viral replication in the absence of cytolysis by the release of cytokines, such as gamma interferon (IFN-γ) and tumor necrosis factor alpha (TNF-α) (14, 39).
Although the immune response to VSV in its natural livestock host has not been well characterized, VSV has been used extensively in the study of the cellular immune response to cytopathic viruses in the mouse model (18). In contrast to non-cytopathic viruses like lymphocytic choriomeningitis virus, CD8+ cells are not required to clear VSV infection (19). However, a strong cellular immune response to VSV N and G proteins is elicited 6 to 10 days after infection (35, 50, 51), with up to 17% of the CD8+ splenocytes of C57Bl/6 mice recognizing a single immunodominant peptide from the N protein (26, 27, 48). These strong primary responses result in a substantial long-term memory response to VSV (27).
To examine the primary immune response to foreign viral proteins expressed in rVSVs, we used recombinants expressing HIV-1 Gag (VSV-Gag), HIV-1 Env (IIIb) (VSV-Env), and both Gag and Env (VSV-GagEnv) (12, 16). The HIV-1 gag gene encodes HIV's internal structural proteins (matrix, capsid, and nucleocapsid). The env gene encodes the viral envelope glycoprotein that is used by HIV for attachment and entry. We used recombinant vaccinia viruses (rVVs) expressing Gag and Env for comparison of the magnitude of the CTL responses (10, 20). Vaccinia is a cytopathic, enveloped double-stranded DNA virus with a relatively large genome (∼180 kbp) containing ∼185 open reading frames, and it is commonly used as a vaccine vector.
The method of MHC class I tetramer staining allows quantitative measurement of antigen-specific CTL during the primary response to viral infection (1, 8, 11, 31). Corresponding quantitative functional data can be gathered with intracellular cytokine staining after stimulating cells with specific peptide antigens (4). The gag and env genes used in this study contain defined immunodominant CTL epitopes restricted to H-2d MHC alleles, allowing the application of MHC class I tetramer staining to quantitate the primary response in BALB/c mice. An H-2Kd-restricted immunodominant epitope has been identified in the p24 (capsid) region of Gag (28), and the IIIb strain of Env contains the well-characterized p18-I10 immunodominant epitope, which is unusual in that it is presented by multiple class I alleles (45, 46).
MATERIALS AND METHODS
Virus preparation and analysis.
rVSVs expressing HIV-1 Env and Gag separately or together have been described previously (12, 15, 16), as has the corresponding recovered wild-type VSV (VSV-rwt) (24). Stocks of VSV and recombinants were grown on BHK-21 cells. Viral titers were determined by plaque assay on BHK cells. Stocks of virus were stored at −70°C.
rVVs expressing HIV-1 IIIb Env (vPE16) (10) and HXB2 Gag (vVK1) (20) under the early/late p7.5 promoter were obtained from the National Institutes of Health AIDS Research and Reference Reagent Program (Rockville, Md.). Stocks of the rVVs were grown on ∼107 HeLa cells in 15-cm-diameter tissue culture dishes in Dulbecco's modified Eagle medium containing 10% fetal bovine serum (DMEM-10). Two days after infection, the infected cells were scraped from the culture dishes, pelleted by centrifugation at 500 × g for 10 min, and resuspended in 2 ml of phosphate-buffered saline. Vaccinia virions were harvested from infected cells by sonication and 3 to 5 cycles of freezing and thawing. Viral titers were determined by plaque assay on BHK cells. Stocks of recombinant vaccinia were stored at −70°C.
Metabolic labeling of proteins was used to confirm the production of HIV-1 Gag and Env proteins in rVSV-infected cells. Briefly, ∼5 × 105 BHK cells in 35-mm-diameter tissue culture dishes were infected with rVSVs at a multiplicity of infection (MOI) of 10 to 20. Four hours later the culture medium was replaced with methionine-free DMEM containing 100 μCi of [35S]methionine (Easy tag EXPRESS protein labeling mix; NEN Life Sciences, Boston, Mass.), and the cells were incubated for 1 h. Cells were lysed on ice for 15 min in 500 μl of lysis buffer (1% Nonidet P-40, 0.4% deoxycholate, 66 mM EDTA, and 10 mM Tris-Cl, pH 7.4), and the nuclei were removed by centrifugation for 2 min at room temperature in an Eppendorf centrifuge. Protein extracts (10 μl of each sample) were fractionated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE, 10% acrylamide), and proteins were visualized on a PhosphorImager (Molecular Dynamics, Sunnyvale, Calif.).
Animals, inoculation, and isolation of splenocytes.
All animal experiments were approved by the Institutional Animal Care and Use Committee of Yale University. Five- to 7-week-old female BALB/c mice obtained from Charles Rivers Laboratories were accommodated to the animal facility for at least 1 week before inoculation with rVSV or rVV expressing HIV-1 Gag and Env. Animals were maintained in microisolator cages in a BL-2-equipped animal facility and given were food and water ad libitum. Viral stocks were diluted in an appropriate volume of serum-free DMEM before inoculating mice. For intraperitoneal (i.p.) vaccinations, mice were inoculated with 5 × 106 PFU of each virus in volumes of 100 to 300 μl. For intranasal (i.n.) vaccinations, mice were lightly anesthetized with vaporized methoxyflurane (Mallinckrodt Veterinary, Mundelein, Ill.), and 106 PFU in a 20-μl volume was administered into the nostrils with a Pipetteman. At various times during the primary response, animals were euthanatized with CO2 and their spleens were isolated. Bulk splenocytes were isolated by forcing the spleen through a metal strainer with ∼1-mm2 holes. Erythrocytes were lysed in buffer (pH 7.4) containing 0.15 M NH4Cl, 10 mM KHCO3, and 0.1 mM EDTA.
MHC class I tetramer preparation.
The Gag immunodominant peptide (N-AMQMLKETI-C) (28) was obtained from Research Genetics (Huntsville, Ala.). An MHC class I Kd tetramer was synthesized with this peptide as previously described (1, 7). Briefly, β2m and a H-2Kd heavy chain that was modified to contain a BirA recognition site (for biotinylation) at its carboxy terminus were purified from Escherichia coli. The purified heavy chain and β2m were dissolved in 8 M urea and diluted into refolding buffer containing Gag peptide (60 μg/ml) to generate soluble MHC class I complexes. The complexes were then purified by gel filtration over a Superdex 200HR column (Pharmacia Biotech AB, Piscataway, N.H.). The purified complexes were biotinylated in vitro for 12 h at 20°C in the presence of 15 μg of BirA (AVIDITY, Boulder, Colo.), 80 μM biotin, 10 mM ATP, 10 mM magnesium acetate, 20 mM bicine, and 10 mM Tris-HCl (pH 8.3). The complexes were purified a second time by gel filtration to remove excess biotin and were tetramerized by the addition of phycoerythrin (PE)-conjugated streptavidin (Molecular Probes, Eugene, Oreg.) at a molar ratio of 4 to 1. The tetrameric complexes were purified again by gel filtration over a Superdex 200HR column and stored at 4°C.
The p18-I10 peptide (N-RGPGRAFVTI-C) (46) used in these experiments was obtained from Research Genetics. A PE-conjugated MHC class I Dd tetramer was synthesized with this peptide by the National Institute of Allergy and Infectious Disease MHC Tetramer Core Facility (Atlanta, Ga.).
Tetramer staining and flow cytometric analysis.
At various time points after vaccination, bulk splenocytes were isolated as described above. Splenocytes were resuspended in flow cytometry sample buffer (SB; phosphate-buffered saline containing 0.5% bovine serum albumin, 0.02% NaN3), and ∼5 × 106 cells were added to wells of a V-bottom 96-well plate for staining. Cells were first blocked with unconjugated streptavidin (Molecular Probes) and Fc-block (Pharmingen, San Diego, Calif.) at 4°C for 15 min and were pelleted at 500 × g for 5 min. Subsequently the cells were stained with PE-conjugated tetramers, a fluorescein isothiocyanate (FITC)-conjugated anti-CD62L antibody (Pharmingen), and an allophycocyanin (APC)-conjugated anti-CD8 antibody (Pharmingen) at predetermined optimal dilutions for 30 min on ice. The cells were then washed three times in SB and were transferred to sample tubes for flow cytometric analysis. Just before analysis, 1 μl of propidium iodide staining solution (50 μg/ml; Pharmingen) was added to each sample to identify dead cells. Data were gathered with a FACSCalibur flow cytometer (Becton-Dickinson, San Jose, Calif.) and were analyzed by using FlowJo analysis software (Tree Star, Inc., San Carlos, Calif.). In the analysis, lymphocytes were selected from forward and side scatter characteristics, propidium iodide-stained cells were gated out, and CD8+ cells were selected. The data for CD8+ cells are plotted as tetramer (PE) versus CD62L (FITC).
Chromium release assay.
Seven days after immunization with rVSV, bulk splenocytes were isolated as described above and were used directly ex vivo in a standard chromium release assay. On the day of the assay, 106 MHC-matched (H-2d) P815 target cells (American Type Culture Collection, Manassas, Va.) were incubated with 200 μCi of [51Cr]sodium chromate (NEN) and 10−6 M p18-I10 peptide or Gag immunodominant peptide at 37°C for 2 h. P815 cells were also incubated without peptide to serve as media-pulsed controls for nonspecific lysis. Excess chromium and peptide were removed by 3 washes in serum-free DMEM, and the cells were resuspended at 105 cells/ml in DMEM-5. For the assay, 100 μl (104) of cells were added to the wells of a 96-well V-bottom plate. Bulk splenocytes containing effector cells were diluted to 107 cells/ml in DMEM-5. For an effector-to-target ratio of 100:1 based on bulk splenocyte counts, 100 μl of effectors was added to target cells in the 96-well plate. Serial twofold dilutions were used to dilute effectors for the 50:1 and 25:1 bulk effector-to-target ratios. To determine maximal and spontaneous release, 100 μl of 1% NP-40 or DMEM-5, respectively, was added to target cells. The cells were incubated at 37°C and 5% CO2 for 5 h. Subsequently, 100 μl of each sample was collected and the chromium release was determined by being counted in a gamma counter. The percentage of specific lysis was determined from the following equation: {[CPM (experimental) − CPM (spontaneous)]/[CPM (maximal) − CPM (spontaneous)]} × 100.
Intracellular cytokine staining.
Intracellular cytokine staining using the Golgi Plug Kit (Pharmingen) was performed essentially as recommended by the manufacturer. Briefly, bulk splenocytes were harvested as described above, and 5 × 106 cells in 1 ml of DMEM-10 were added to 2 wells of a 24-well plate. One well served as an unstimulated control. Cells in the other well were stimulated with 10−5 M peptide (p18-I10 or Gag immunodominant peptide). The cells were incubated at 37°C with 5% CO2 for 2 h, 1 μl of brefeldin A was added to each well, and the cells were incubated for an additional 3 h.
The cells were harvested by centrifuging the 24-well plates at 500 × g for 5 min at 4°C, aspirating the medium, and resuspending the cells in SB. The cells from each well were transferred to wells of a 96-well V-bottom plate and were blocked with Fc-block for 15 min at 4°C and were pelleted at 500 × g at 4°C. The cells were then stained with an APC-conjugated anti-CD8 antibody (Pharmingen) for 30 min at 4°C and were washed three times with SB. The cells were then fixed in 100 μl of cytofix/cytoperm solution (Pharmingen) for 10 to 20 min on ice and were washed twice in 1× Perm/Wash solution (Pharmingen). Intracellular cytokines were then stained with FITC-conjugated rat anti-mouse IFN-γ or anti-mouse TNF-α antibodies or an FITC-conjugated rat isotype control immunoglobulin (Pharmingen) to identify background staining. The cells were analyzed by flow cytometry and are plotted as cytokine (FITC) intensity versus CD8 (APC) intensity. The percent of CD8+ cells producing cytokine is reported in the upper right quadrant.
RESULTS
Recombinant VSV expression of HIV-1 Gag and Env proteins.
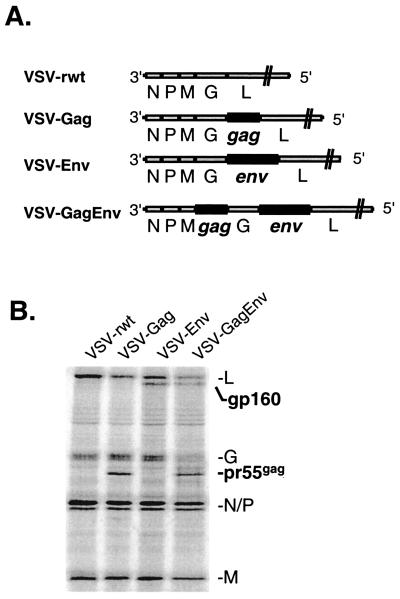
We used three recombinant VSV expressing HIV-1 Gag and Env to determine the CTL response to foreign viral proteins expressed in VSV (Fig. 1A). The gene for the uncleaved precursor form of HIV-1 Gag (pr55gag) is contained within the genome of VSV-Gag (12, 29). VSV-Env carries the env gene from the IIIb strain of HIV-1 (16). VSV-GagEnv expresses both the Gag and Env genes from within a single recombinant VSV.
FIG. 1.
Diagram of VSV/HIV recombinants and protein expression in infected cells. (A) The genomes of VSV-rwt and the three VSV/HIV recombinants are diagrammed in a 3′-to-5′ orientation on the negative-stranded viral RNA. Additional genes are expressed by duplication of the conserved VSV transcription start and stop signals (42). VSV-Gag and VSV-Env express the gag gene and the IIIb env gene, respectively, between the G and L genes of VSV. VSV-GagEnv contains both the gag and env genes in a single recombinant. (B) An SDS-polyacrylamide gel showing the expression of VSV and HIV proteins in rVSV-infected cells. BHK cells were infected with the recombinants shown at an MOI of 10 to 20. Four hours later, the medium was replaced with methionine-free medium containing [35S]methionine. The cells were lysed 1 h later, and the extracts were fractionated by SDS-PAGE. The samples shown here were refractionated after normalization for VSV N protein content.
To confirm the expression of HIV-1 proteins from the recombinants used in this study, we infected BHK cells with the rVSVs, metabolically labeled proteins with [35S]methionine, and analyzed the products by SDS-PAGE. Since VSV causes a rapid shutoff of host cell protein synthesis, only proteins expressed in VSV are seen by 4 h after infection. As can be seen in Fig. 1B, both VSV-Gag and VSV-GagEnv express a 55-kDa protein corresponding to pr55gag. Similarly, VSV-Env and VSV-GagEnv express a 160-kDa protein corresponding to HIV-1 Env gp160.
Primary response to HIV-1 Env measured with MHC class I tetramers.
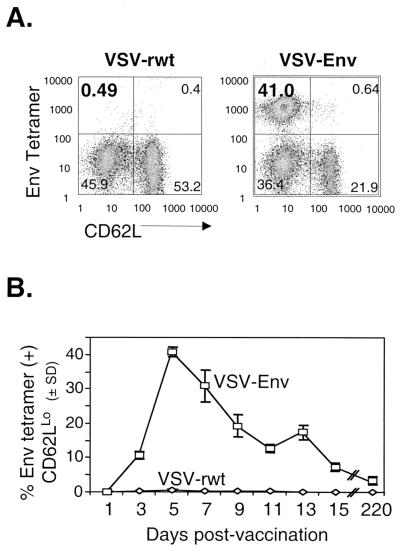
The HIV-1 Env protein of the IIIb strain contains the immunodominant p18 peptide that has been well characterized in other systems. This peptide binds multiple class I alleles but shows the strongest binding to the H-2Kd allele (45, 46). To identify a subset of Env-specific CTL in BALB/c mice, we used a PE-conjugated MHC class I tetramer bound to the optimal length core sequence of the p18 peptide (p18-I10) (46). At various points after vaccination, splenocytes were isolated from vaccinated mice and stained directly ex vivo with Env tetramer, APC-conjugated anti-CD8 antibody, and FITC-conjugated anti-CD62L antibody. After flow cytometric analysis, dead cells were gated out and only CD8+ cells were included in the analysis. Therefore, the data shown include only CD8+ cells and are plotted as CD62L staining versus Env tetramer staining. Downregulation of CD62L, a lymph node homing receptor, is associated with recent activation of T cells (2, 3, 17, 30). The upper left quadrant of each graph shows the percentage of CD8+, activated (CD62LLo), tetramer-positive cells.
Mice were vaccinated i.p. with 5 × 106 PFU of VSV-Env or VSV-rwt. Five days after vaccination with VSV-Env, 41% of CD8+ splenocytes were Env tetramer positive and CD62LLo (Fig. 2A). In all mice vaccinated with VSV-rwt, a background level of staining of ≤0.5% was detected. Since the peak of the primary response to wild-type VSV proteins occurs 6 to 10 days after vaccination (51), we determined if the response to Env expressed in rVSV followed a similar time course. Splenocytes were analyzed in 2-day increments between 1 and 15 days after vaccination (Fig. 2B). Three days after vaccination, ∼12% of CD8+ splenocytes were Env tetramer positive. The primary response to Env peaked 5 to 7 days after vaccination. After the peak response, tetramer-positive CD8+ cells gradually decreased from ∼19% on day 9 to approximately 7% by day 15. At day 220 postvaccination, ∼4% of CD8+ splenocytes were Env tetramer positive and CD62LLo.
FIG. 2.
MHC class I tetramer quantitation of the primary response to Env after VSV-Env vaccination. (A) Five days after vaccination with 5 × 106 PFU of VSV-Env (n = 3) or VSV-rwt (n = 2), bulk splenocytes were isolated from vaccinated BALB/c mice and were stained with the Env tetramer and anti-CD8 and anti-CD62L antibodies. Flow cytometry data were collected, and only live CD8+ lymphocytes were included in the analysis. The upper left quadrant shows the percent of CD8+ cells that were Env tetramer-positive and CD62LLo. (B) Mice were vaccinated as above, and the primary response was examined at 2-day intervals between 3 and 15 days after vaccination. Bulk splenocytes were stained as above. The data are plotted as the mean percentage of Env-tetramer-positive and CD62LLo, CD8+ cells versus days after vaccination. Error bars indicate the standard deviation (SD).
Functional assays for the primary response to Env.
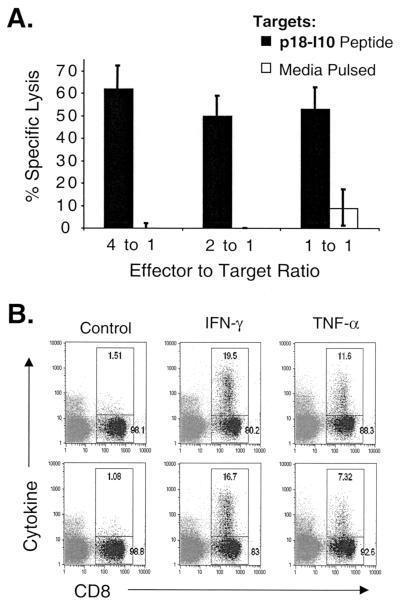
To determine if splenocytes from VSV-Env-vaccinated mice were functional in target cell lysis, we used splenocytes directly ex vivo in a standard chromium release assay against target cells pulsed with the p18-I10 peptide or media alone. The effector-to-target ratios based on bulk splenocyte counts were normalized to the actual number of p18-peptide-reactive CTL based on the mean percentage of CD8+, Env tetramer-positive cells. One characteristic example of the chromium release data is shown in Fig. 3A. Approximately 62% specific lysis was present at an effector-to-target ratio of 4 to 1. Approximately 50% specific lysis was present at the lowest effector-to-target ratio of 1 to 1.
FIG. 3.
Functional assays for CTL specific for the Env p18-I10 epitope. (A) Seven days after vaccination with 5 × 106 PFU of VSV-Env (n = 2), bulk splenocytes were used in a standard chromium release assay against p18-I10 peptide-pulsed or media-pulsed control P815 target cells. The effector-to-target ratios have been normalized for the percentage of Env-tetramer-positive CD8+ cells. One representative assay is shown. Error bars indicate the standard deviation for the assay. (B) Seven days after vaccination with 5 × 106 VSV-Env (n = 2), bulk splenocytes were stimulated with the p18-I10 peptide for 5 h in the presence of brefeldin A and were stained with anti-CD8 antibody and either isotype control, anti-IFN-γ, or anti-TNF-α antibodies and were analyzed by flow cytometry. The gate and corresponding number in the upper right indicate the percentage of cytokine- or isotype control-positive CD8+ cells. The results for both mice are shown.
We also used intracellular cytokine staining for IFN-γ and TNF-α to identify CTL that were functionally reactive to the p18-I10 peptide. Splenocytes were isolated from VSV-Env-vaccinated mice 7 days after vaccination with VSV-Env. The cells were incubated with 10−5 M p18-I10 peptide or no peptide (control) for 5 h in the presence of brefeldin A. To identify CD8+ cells producing cytokine, they were stained with an APC-conjugated anti-CD8 antibody and FITC-conjugated antibodies to either IFN-γ, TNF-α, or an isotype control immunoglobulin. The results for both mice are shown in Fig. 3B. Flow cytometric analysis revealed that 16 to 19% of CD8+ cells stained positively for IFN-γ, and approximately 7 to 11% of CD8+ splenocytes stained positively for TNF-α after stimulation with 10−5 M p18-I10 peptide. Each of the mice used in this experiment had 24% Env-tetramer-positive CD8+ cells (data not shown). Background IFN-γ and TNF-α staining in splenocytes from VSV-rwt-vaccinated mice and splenocytes from VSV-Env-vaccinated mice that were not stimulated with peptide were at low levels, similar to the background staining with the isotype control immunoglobulin (data not shown).
Primary response to HIV-1 Gag.
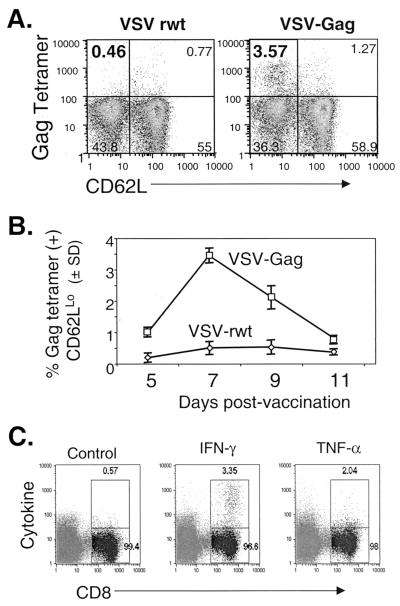
An H-2Kb-restricted immunodominant CTL epitope has been identified in the HXB2 gag gene (28). To measure the CTL response to Gag in BALB/c mice vaccinated with rVSV expressing Gag, we constructed a PE-conjugated MHC class I tetramer containing this immunodominant peptide. We stained splenocytes with the tetramer directly ex vivo at various time points after vaccination with VSV-Gag. Analysis with the anti-CD8 and anti-CD62L antibodies was as described above. Background staining of splenocytes from VSV-rwt-vaccinated mice was always ≤0.6%.
Mice were vaccinated i.p. with 5 × 106 PFU of VSV-Gag or VSV-rwt. Seven days after vaccination, ∼3.5% of CD8+ cells were specific for the Gag immunodominant peptide and were CD62LLo (Fig. 4A). We also determined the timing of the primary response to Gag by staining splenocytes with the Gag tetramer 5, 7, 9, and 11 days after vaccination. Like the response to VSV proteins, the primary response to Gag expressed in VSV peaked 7 to 9 days after vaccination and was only slightly above background (VSV-rwt) at 5 and 11 days after vaccination (Fig. 4B).
FIG. 4.
MHC class I tetramer and intracellular cytokine quantitation of the primary response to Gag after VSV-Gag vaccination. (A) Five days after vaccination with 5 × 106 PFU of VSV-Gag (n = 4) or VSV-rwt (n = 3), bulk splenocytes were isolated from vaccinated BALB/c mice and were stained with the Gag tetramer and anti-CD8 and anti-CD62L antibodies. Flow cytometry data was collected, and only CD8+ lymphocytes were included in the analysis. The upper left quadrant shows the percentage of CD8+ cells that were Gag tetramer positive and CD62LLo. (B) Mice were vaccinated as above, and the primary response was examined at 2-day intervals between 5 and 11 days after vaccination. Bulk splenocytes were stained as above. The data are plotted as the mean percentage of tetramer-positive, CD62LLo, CD8+ cells versus days after vaccination. Error bars indicate the standard deviation (SD). (C) Seven days after vaccination with 5 × 106 PFU of VSV-Gag (n = 2), bulk splenocytes were stimulated with the Gag immunodominant peptide for 5 h in the presence of brefeldin A, stained with anti-CD8 antibody and either isotype control, anti IFN-γ, or anti-TNF-α antibodies, and analyzed by flow cytometry. The gate and corresponding number in the upper right indicates the percentage of cytokine- or isotype control-positive CD8 cells. The results for one representative mouse are shown.
To determine if functionally lytic CTL were elicited by VSV-Gag vaccination, we attempted to use a standard chromium release assay with bulk splenocyte effectors directly ex vivo and target cells pulsed with Gag peptide. Splenocytes from VSV-Gag-vaccinated mice displayed a low level of lytic activity above the level seen for VSV-rwt-vaccinated mice. However, the specific lysis was below 10% (data not shown). To obtain more quantitative functional data, we used intracellular cytokine staining at the peak of the primary response to Gag 7 days after vaccination. In agreement with data obtained from Gag tetramer staining, ∼3% of CD8+ cells produced IFN-γ (Fig. 4C).
Vaccination with a recombinant VSV expressing both Gag and Env elicits CTL specific for both proteins.
VSV-GagEnv expresses both HIV-1 Gag and Env from within a single recombinant virus but grows in vitro to titers approximately three- to fourfold lower than those of VSV-rwt (12). To examine the CTL response generated by this rVSV expressing two foreign proteins simultaneously, we vaccinated BALB/c mice i.p. with 5 × 106 VSV-GagEnv or VSV-rwt and analyzed the response by MHC class I tetramer staining.
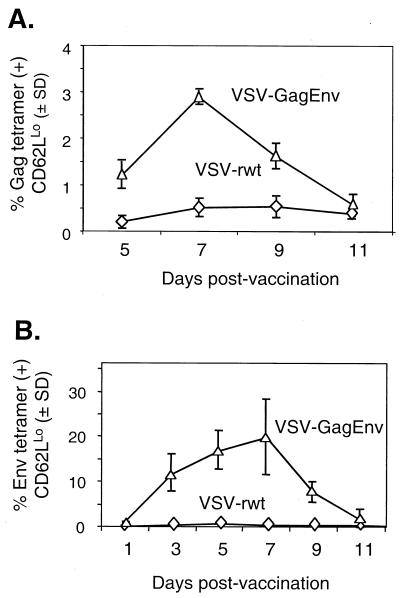
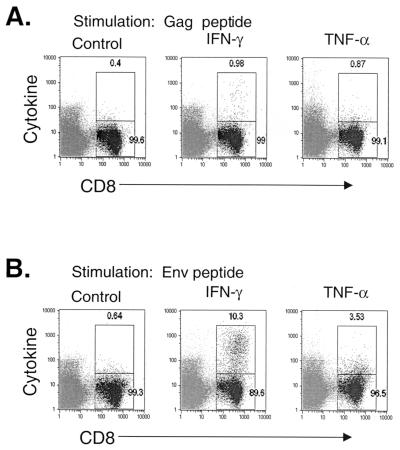
The primary response to HIV-1 Gag after vaccination with VSV-GagEnv showed a time course similar to that after vaccination with VSV-Gag (Fig. 5A). The response peaked 7 days after vaccination, at which time approximately 2.9% of CD8+ cells were Gag tetramer positive and CD62LLo. Intracellular cytokine staining revealed that ∼0.5% (above background) of CD8+ cells produced IFN-γ after stimulation with the Gag immunodominant peptide (Fig. 6A).
FIG. 5.
Quantitation and time course of the primary response to Gag and Env after VSV-GagEnv vaccination. Bulk splenocytes were collected from BALB/c mice at the indicated points after vaccination with 5 × 106 PFU of VSV-GagEnv (n = 4/time point) or VSV-rwt (n = 3/time point). Splenocytes were stained with the Gag (A) and Env (B) tetramers in addition to anti-CD8 and anti-CD62L antibodies. The data are plotted as the mean percentage of tetramer-positive, CD62LLo, CD8+ cells versus days after vaccination. Error bars indicate the standard deviation (SD).
FIG. 6.
Intracellular cytokine staining of Gag- and Env-specific CTL after VSV-GagEnv vaccination. Seven days after vaccination with 5 × 106 PFU of VSV-Env (n = 2), bulk splenocytes were stimulated with the Gag immunodominant peptide (A) or the p18-I10 peptide (B) for 5 h in the presence of brefeldin A. Cells were then stained with anti-CD8 antibody and either isotype control, anti-IFN-γ, or anti-TNF-α antibodies and were analyzed by flow cytometry. The gate and corresponding number in the upper right indicates the percentage of cytokine- or isotype control-positive CD8 cells. The results for one representative mouse are shown.
Similar to the response to Env after vaccination with VSV-Env, the primary response to HIV-1 Env peaked 5 to 7 days after vaccination with VSV-GagEnv. At 5 and 7 days after vaccination, ∼17 and ∼20% of CD8+ cells were Env tetramer positive and CD62LLo, respectively. These values were approximately half those observed for VSV-Env-vaccinated mice. The response to Env after VSV-GagEnv vaccination reached significant levels (∼12%) as early as 3 days after vaccination and returned to baseline by day 11 postvaccination. Intracellular cytokine staining 7 days after vaccination revealed that ∼10% of cells produced IFN-γ after stimulation with the p18-I10 peptide (Fig. 6B).
Comparison of VSV and vaccinia vectors for CTL induction.
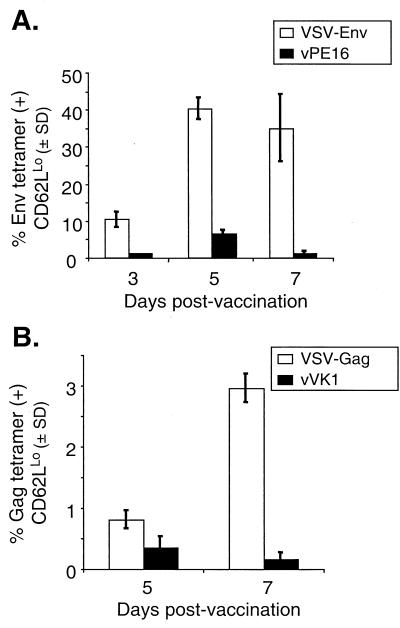
To determine the relative effect of the vector in which HIV-1 Gag and Env were expressed on the level of the primary response, we compared the primary response elicited by rVSVs with those elicited by another vaccine vector, rVV. We vaccinated BALB/c mice with either 5 × 106 PFU of VSV-Env or 5 × 106 PFU of vPE16, a vaccinia recombinant expressing the HIV-1 IIIb Env protein, and analyzed the primary response with the Env tetramer. Figure 7A shows the percentage of Env-tetramer-positive, CD62LLo cells after subtracting background tetramer staining (staining of splenocytes from mice vaccinated with an analogous Gag-expressing vaccinia recombinant vVK1). Background staining of splenocytes from VSV-rwt-vaccinated mice has also been subtracted from the data from VSV-Env-vaccinated mice. The primary response to recombinant vaccinia peaked 5 days after vaccination, at which time ∼6.3% were activated, Env-tetramer-positive cells. On day 3 after vaccination, only ∼1% of splenocytes from vPE16-vaccinated mice were activated and specific for the p18-I10 peptide. At its peak, the response to Env in VSV-Env-vaccinated mice was approximately sixfold higher (∼40% Env tetramer positive, CD62LLo) than that in vPE16-vaccinated mice.
FIG. 7.
Comparison of the primary response to Gag and Env elicited by rVSVs or rVVs. Mice were vaccinated i.p. with either 5 × 106 PFU of VSV-Env (n = 3) or 5 × 106 PFU of vPE16 (n = 2), an rVV expressing HIV-1 Env (A), or with 5 × 106 PFU of VSV-Gag (n = 3) or 5 × 106 PFU of vVK1 (n = 2), an rVV expressing HIV-1 Gag-pol (B). Bulk splenocytes were isolated at the indicated number of days after vaccination and were stained with Env (A) or Gag (B) tetramers in addition to anti-CD8 and anti-CD62L antibodies. Background tetramer staining (<0.6%) has been subtracted from each value. Error bars indicate the standard deviation (SD).
To conduct a similar comparison of the response to Gag, we vaccinated mice i.p. with 5 × 106 PFU of VSV-Gag or 5 × 106 PFU of vVK1, a recombinant vaccinia expressing Gag, and stained splenocytes with the Gag tetramer. Figure 7B shows the responses to Gag after subtracting mean background tetramer staining from each time point. The primary response to Gag peaked 5 days after vaccination in vVK1-vaccinated mice, whereas it peaked 7 days after vaccination in VSV-Gag-vaccinated mice. Comparing only the peak responses for each virus revealed that the response to Gag elicited by VSV-Gag was approximately eightfold higher than that elicited by vVK1.
Comparison of mucosal and i.p. immunization.
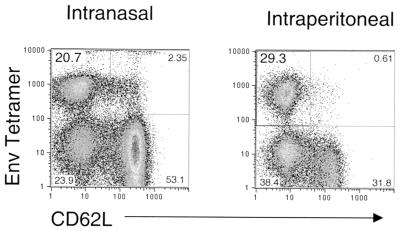
To determine if a mucosal route of vaccination with VSV vectors would generate CTL specific for the Env immunodominant p18-I10 epitope, we vaccinated BALB/c mice i.n. with 106 PFU of VSV-Env in a 25-μl volume. Seven days after vaccination, we isolated splenocytes and stained them with the Env tetramer as described above. Figure 8 shows a comparison of the data obtained from two mice vaccinated i.n. and i.p. with VSV-Env. After i.n. vaccination with VSV-Env, ∼20% of CD8+ cells were tetramer positive and CD62LLo, whereas ∼29% were tetramer positive and CD62LLo after i.p. vaccination with VSV-Env. Comparisons of data from four mice showed that i.p. vaccination was 1.8× more effective than i.n. vaccination for CTL induction.
FIG. 8.
Comparison of i.n. and i.p. vaccination with VSV-Env. Mice were vaccinated i.n. with 106 PFU of VSV-Env in volumes of 10 to 20 μl (n = 4) or i.p. with 5 × 106 PFU of VSV-Env (n = 3). Seven days after vaccination, bulk splenocytes were isolated and stained with Env tetramers. The flow cytometry data include only CD8+ cells. Two representative plots are shown. The means for all mice are reported in the text.
DISCUSSION
We describe here a quantitative characterization of the primary CTL response to foreign viral proteins expressed in rVSV. Although it has been long known that VSV elicits a strong CTL response to its own proteins (35, 50, 51), the primary CTL response to foreign viral proteins expressed in rVSVs had not been quantitated. We have shown that immunization of mice with rVSVs expressing HIV-1 Gag and Env elicits a potent CTL response to immunodominant epitopes in Gag and Env. We were unable make a direct comparison with the response to VSV's own proteins, since no immunodominant peptides have yet been defined in H-2d mice. However, to put the data reported here in perspective, Masopust et al. (26, 27) recently reported that ∼11 to 17% of CD8+ splenocytes are specific for the VSV N52-59 immunodominant epitope in C57Bl/6 mice 6 to 8 days after vaccination.
The primary response to Env after inoculation with rVSVs was particularly robust (Fig. 2). At the peak of the primary response 5 days after vaccination, ∼40% of CD8+ splenocytes were recognized by the Env tetramer and displayed markers of recent activation. These responses fell rapidly but maintained a detectable level of ∼3.9% for at least 220 days. Splenocytes isolated at the peak of the primary response were functional in the killing of peptide-pulsed target cells at low effector-to-target ratios (Fig. 3A). Intracellular cytokine staining in two mice with 24% Env-tetramer-positive cells revealed that 15 to 19% of CD8+ cells secreted IFN-γ in response to stimulation with the p18-I10 peptide (Fig. 3B). Therefore, the percentage of p18-I10-specific cells identified by functional intracellular cytokine staining approximated that identified by tetramer staining, a correlation also observed by others (31). The slight discrepancy between tetramer and cytokine staining may have been caused by suboptimal peptide stimulation or lysis in culture. It is not surprising, however, that the percentage of TNF-α-positive cells (7 to 10%) is less than that identified by tetramer staining, since TNF-α staining often underestimates the percentage of antigen-specific cells after stimulation at moderate peptide concentrations (4).
The remarkable response to Env led us to question whether or not a mucosal route of immunization would elicit a response similar to that of i.p. immunization. A strong response (20% tetramer positive) was observed 7 days after vaccination with 106 PFU of VSV-Env (Fig. 7). This response was approximately half that yielded by i.p. vaccination.
The response to Gag generated by VSV-Gag was somewhat less than that to Env, but it was still substantial. At its peak approximately 3% of CD8+ cells were tetramer positive (Fig. 4), and these cells produced IFN-γ when stimulated with peptide. Based on the results of Lefrançois et al. (26, 27), we would expect to find even higher frequencies of recently activated CTL to Gag and Env in mucosal sites, such as the lamina propria. This will be examined in future studies, as will the recall response elicited by different boosting vectors expressing Gag and Env.
We compared the abilities of rVSVs and rVVs to elicit primary CTL responses to Gag and Env. At all time points, the responses generated by rVSVs were higher than those generated by rVVs (Fig. 7). The response to Env peaked at the same time postvaccination for both viruses, but the response elicited by VSV-Env was about sixfold higher than that elicited by vPE16. The response to Gag elicited by vVK1 peaked earlier than that to VSV-Gag. However, VSV-Gag elicited an eightfold higher response than did vVK1 when only the peak responses are compared. The differences in the responses might be explained by differences in the viruses themselves. VSV has a smaller genome and fewer encoded proteins to compete for the immune response than does vaccinia virus. Vaccinia virus also carries several immunosuppressive genes, such as cytokine receptor homologues, which may reduce its ability to elicit CTL responses (47).
VSV appears to be incredibly versatile in its ability to accommodate the addition of genes to its basic 11-kb genome. In early studies of rVSVs, it was found that additional genes were accommodated by a physical lengthening of the virion (41). VSV-GagEnv expands the genome of the virus approximately 40%, from 11.1 to 15.4 kb, and expresses two additional genes with only a three- to fourfold reduction in viral titer (12). We have shown here that this recombinant also has the ability to generate CTL responses to both proteins. The CTL response to Gag elicited by VSV-GagEnv was approximately 80% of that elicited by VSV-Gag. The response to Env elicited by VSV-GagEnv was approximately 50% that elicited by VSV-Env. The lower responses to both proteins in VSV-GagEnv might have been caused by competition for class I sites between other epitopes in Gag and Env or by the slower growth of VSV-GagEnv (12).
Other laboratories have used an rVSV expressing ovalbumin (VSV-Ova) to define the requirements for mucosal CTL responses and memory in adoptive-transfer experiments (21, 22, 25, 49). They have also shown that 6 days after VSV-Ova infection, ∼7% of CD8+ splenocytes were Ova specific by tetramer staining (49). The latter study and the results presented here demonstrate that primary response to foreign proteins expressed in VSV are readily detectable by MHC class I tetramer staining. For this reason, we believe that rVSVs, particularly VSV-Env, may have practical application in basic immunological studies of foreign proteins. For example, the antibody KP15 binds the Dd MHC-p18-I10 complex and blocks induction of CTL recognizing this complex (9). VSV-Env might be useful in testing the effects of KP15 without the need for in vitro restimulation.
In a recent study it was shown that recombinant VSVs expressing simian immunodeficiency virus (SIV) Gag and HIV-1 Env are effective in protecting monkeys from challenge with a highly pathogenic SIV/HIV hybrid (SHIV) (38). Although quantitative tetramer studies were not possible in that study, IFN-γ elispot analysis indicated that a significant amount of CTL activity to SIV-Gag was elicited by rVSVs in a primary response to VSV-Gag vectors (38). Here we have compared the relative efficiencies of rVV and rVSV to elicit primary CTL responses in a mouse model and found that rVSV elicited a much stronger response. Comparison of the primary responses to VSV and vaccinia constructs in rhesus macaques is not possible from published data, but typically the primary CTL responses to modified vaccinia Ankara Gag are very low or are undetectable by MHC class I tetramer analysis of an immunodominant Gag epitope (5, 43, 44). The ability of rVSV to induce a high-level CTL response against foreign viral proteins probably explains the effectiveness of VSV vectors as an AIDS vaccine in monkeys.
Acknowledgments
We thank members of the Rose and Pamer laboratories for helpful comments and suggestions during the preparation of the manuscript and JoAnn Falato for providing valuable administrative assistance. We also gratefully acknowledge Leo Lefrançois, James Huleatt, and Roman Tuma for advice about chromium release assays. We are grateful to the National Institute of Allergy and Infectious Disease MHC Tetramer Core Facility for providing the Env tetramer. We also thank Gouzel Tokmoulina and Roberto Mercado for helpful advice and assistance with flow cytometry, and the Howard Hughes Medical Institute at Yale University for use of the flow cytometer.
K.H. was supported by a fellowship from the Bayer Corporation. This study was supported by National Institutes of Health grants AI40357 and AI24345 to J.K.R. and AI42135 to E.P.
REFERENCES
- 1.Altman, J. D., P. A. Moss, P. J. Goulder, D. H. Barouch, M. G. McHeyzer-Williams, J. I. Bell, A. J. McMichael, and M. M. Davis. 1996. Phenotypic analysis of antigen-specific T lymphocytes. Science 274:94-96. [DOI] [PubMed] [Google Scholar]
- 2.Andersson, E. C., J. P. Christensen, O. Marker, and A. R. Thomsen. 1994. Changes in cell adhesion molecule expression on T cells associated with systemic virus infection. J. Immunol. 152:1237-1245. [PubMed] [Google Scholar]
- 3.Andersson, E. C., J. P. Christensen, A. Scheynius, O. Marker, and A. R. Thomsen. 1995. Lymphocytic choriomeningitis virus infection is associated with long-standing perturbation of LFA-1 expression on CD8+ T cells. Scand. J. Immunol. 42:110-118. [DOI] [PubMed] [Google Scholar]
- 4.Badovinac, V. P., and J. T. Harty. 2000. Intracellular staining for TNF and IFN-gamma detects different frequencies of antigen-specific CD8+ T cells. J. Immunol. Methods 238:107-117. [DOI] [PubMed] [Google Scholar]
- 5.Barouch, D. H., A. Craiu, S. Santra, M. A. Egan, J. E. Schmitz, M. J. Kuroda, T. M. Fu, J. H. Nam, L. S. Wyatt, M. A. Lifton, G. R. Krivulka, C. E. Nickerson, C. I. Lord, B. Moss, M. G. Lewis, V. M. Hirsch, J. W. Shiver, and N. L. Letvin. 2001. Elicitation of high-frequency cytotoxic T-lymphocyte responses against both dominant and subdominant simian-human immunodeficiency virus epitopes by DNA vaccination of rhesus monkeys. J. Virol. 75:2462-2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borrow, P., H. Lewicki, B. H. Hahn, G. M. Shaw, and M. B. A. Oldstone. 1994. Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J. Virol. 68:6103-6110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Busch, D. H., I. M. Pilip, S. Vijh, and E. G. Pamer. 1998. Coordinate regulation of complex T cell populations responding to bacterial infection. Immunity 8:353-362. [DOI] [PubMed] [Google Scholar]
- 8.Callan, M. F., L. Tan, N. Annels, G. S. Ogg, J. D. Wilson, C. A. O'Callaghan, N. Steven, A. J. McMichael, and A. B. Rickinson. 1998. Direct visualization of antigen-specific CD8+ T cells during the primary immune response to Epstein-Barr virus In vivo. J. Exp. Med. 187:1395-1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chung, D. H., I. M. Belyakov, M. A. Derby, J. Wang, L. F. Boyd, J. A. Berzofsky, and D. H. Margulies. 2001. Competitive inhibition in vivo and skewing of the T cell repertoire of antigen-specific CTL priming by an anti-peptide-MHC monoclonal antibody. J. Immunol. 167:699-707. [DOI] [PubMed] [Google Scholar]
- 10.Earl, P. L., S. Koenig, and B. Moss. 1991. Biological and immunological properties of human immunodeficiency virus type 1 envelope glycoprotein: analysis of proteins with truncations and deletions expressed by recombinant vaccinia viruses. J. Virol. 65:31-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gallimore, A., A. Glithero, A. Godkin, A. C. Tissot, A. Pluckthun, T. Elliott, H. Hengartner, and R. Zinkernagel. 1998. Induction and exhaustion of lymphocytic choriomeningitis virus-specific cytotoxic T lymphocytes visualized using soluble tetrameric major histocompatibility complex class I-peptide complexes. J. Exp. Med. 187:1383-1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haglund, K., J. Forman, H. G. Krausslich, and J. K. Rose. 2000. Expression of human immunodeficiency virus type 1 Gag protein precursor and envelope proteins from a vesicular stomatitis virus recombinant: high-level production of virus-like particles containing HIV envelope. Virology 268:112-121. [DOI] [PubMed] [Google Scholar]
- 13.Harty, J. T., A. R. Tvinnereim, and D. W. White. 2000. CD8+ T cell effector mechanisms in resistance to infection. Annu. Rev. Immunol. 18:275-308. [DOI] [PubMed] [Google Scholar]
- 14.Herbein, G., and W. A. O'Brien. 2000. Tumor necrosis factor (TNF)-alpha and TNF receptors in viral pathogenesis. Proc. Soc. Exp. Biol. Med. 223:241-257. [DOI] [PubMed] [Google Scholar]
- 15.Johnson, J. E., W. Rodgers, and J. K. Rose. 1998. A plasma membrane localization signal in the HIV-1 envelope cytoplasmic domain prevents localization at sites of vesicular stomatitis virus budding and incorporation into VSV virions. Virology 251:244-252. [DOI] [PubMed] [Google Scholar]
- 16.Johnson, J. E., M. J. Schnell, L. Buonocore, and J. K. Rose. 1997. Specific targeting to CD4+ cells of recombinant vesicular stomatitis viruses encoding human immunodeficiency virus envelope proteins. J. Virol. 71:5065-5068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jung, T. M., W. M. Gallatin, I. L. Weissman, and M. O. Dailey. 1988. Down-regulation of homing receptors after T cell activation. J. Immunol. 141:4110-4117. [PubMed] [Google Scholar]
- 18.Kagi, D., and H. Hengartner. 1996. Different roles for cytotoxic T cells in the control of infections with cytopathic versus noncytopathic viruses. Curr. Opin. Immunol. 8:472-477. [DOI] [PubMed] [Google Scholar]
- 19.Kagi, D., P. Seiler, J. Pavlovic, B. Ledermann, K. Burki, R. M. Zinkernagel, and H. Hengartner. 1995. The roles of perforin- and Fas-dependent cytotoxicity in protection against cytopathic and noncytopathic viruses. Eur. J. Immunol. 25:3256-3262. [DOI] [PubMed] [Google Scholar]
- 20.Karacostas, V., K. Nagashima, M. A. Gonda, and B. Moss. 1989. Human immunodeficiency virus-like particles produced by a vaccinia virus expression vector. Proc. Natl. Acad. Sci. USA 86:8964-8967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim, S. K., D. S. Reed, S. Olson, M. J. Schnell, J. K. Rose, P. A. Morton, and L. Lefrancois. 1998. Generation of mucosal cytotoxic T cells against soluble protein by tissue-specific environmental and costimulatory signals. Proc. Natl. Acad. Sci. USA 95:10814-10819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim, S. K., K. S. Schluns, and L. Lefrancois. 1999. Induction and visualization of mucosal memory CD8 T cells following systemic virus infection. J. Immunol. 163:4125-4132. [PubMed] [Google Scholar]
- 23.Koup, R. A., J. T. Safrit, Y. Cao, C. A. Andrews, G. McLeod, W. Borkowsky, C. Farthing, and D. D. Ho. 1994. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J. Virol. 68:4650-4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lawson, N. D., E. A. Stillman, M. A. Whitt, and J. K. Rose. 1995. Recombinant vesicular stomatitis viruses from DNA. Proc. Natl. Acad. Sci. USA 92:4477-4481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lefrancois, L., C. M. Parker, S. Olson, W. Muller, N. Wagner, M. P. Schon, and L. Puddington. 1999. The role of beta7 integrins in CD8 T cell trafficking during an antiviral immune response. J. Exp. Med. 189:1631-1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Masopust, D., J. Jiang, H. Shen, and L. Lefrancois. 2001. Direct analysis of the dynamics of the intestinal mucosa CD8 T cell response to systemic virus infection. J. Immunol. 166:2348-2356. [DOI] [PubMed] [Google Scholar]
- 27.Masopust, D., V. Vezys, A. L. Marzo, and L. Lefrancois. 2001. Preferential localization of effector memory cells in nonlymphoid tissue. Science 291:2413-2417. [DOI] [PubMed] [Google Scholar]
- 28.Mata, M., P. J. Travers, Q. Liu, F. R. Frankel, and Y. Paterson. 1998. The MHC class I-restricted immune response to HIV-gag in BALB/c mice selects a single epitope that does not have a predictable MHC-binding motif and binds to Kd through interactions between a glutamine at P3 and pocket D. J. Immunol. 161:2985-2993. [PubMed] [Google Scholar]
- 29.Mergener, K., M. Facke, R. Welker, V. Brinkmann, H. R. Gelderblom, and H. Krausslich. 1992. Analysis of HIV particle formation using transient expression of subviral constucts in mammalian cells. Virology 186:25-39. [DOI] [PubMed] [Google Scholar]
- 30.Mueller, C., H. K. Gershenfeld, C. G. Lobe, C. Y. Okada, R. C. Bleackley, and I. L. Weissman. 1988. A high proportion of T lymphocytes that infiltrate H-2-incompatible heart allografts in vivo express genes encoding cytotoxic cell-specific serine proteases, but do not express the MEL-14-defined lymph node homing receptor. J. Exp. Med. 167:1124-1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murali-Krishna, K., J. D. Altman, M. Suresh, D. J. Sourdive, A. J. Zajac, J. D. Miller, J. Slansky, and R. Ahmed. 1998. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity 8:177-187. [DOI] [PubMed] [Google Scholar]
- 32.Musey, L., J. Hughes, T. Schacker, T. Shea, L. Corey, and M. J. McElrath. 1997. Cytotoxic-T-cell responses, viral load, and disease progression in early human immunodeficiency virus type 1 infection. N. Engl. J. Med. 337:1267-1274. [DOI] [PubMed] [Google Scholar]
- 33.O'Brien, T. R., W. A. Blattner, D. Waters, E. Eyster, M. W. Hilgartner, A. R. Cohen, N. Luban, A. Hatzakis, L. M. Aledort, P. S. Rosenberg, W. J. Miley, B. L. Kroner, and J. J. Goedert. 1996. Serum HIV-1 RNA levels and time to development of AIDS in the Multicenter Hemophilia Cohort Study. JAMA 276:105-110. [PubMed] [Google Scholar]
- 34.Ogg, G. S., X. Jin, S. Bonhoeffer, P. R. Dunbar, M. A. Nowak, S. Monard, J. P. Segal, Y. Cao, S. L. Rowland-Jones, V. Cerundolo, A. Hurley, M. Markowitz, D. D. Ho, D. F. Nixon, and A. J. McMichael. 1998. Quantitation of HIV-1-specific cytotoxic T lymphocytes and plasma load of viral RNA. Science 279:2103-2106. [DOI] [PubMed] [Google Scholar]
- 35.Puddington, L., M. J. Bevan, J. K. Rose, and L. Lefrancois. 1986. N protein is the predominant antigen recognized by vesicular stomatitis virus-specific cytotoxic T cells. J. Virol. 60:708-717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roberts, A., L. Buonocore, R. Price, J. Forman, and J. K. Rose. 1999. Attenuated vesicular stomatitis viruses as vaccine vectors. J. Virol. 73:3723-3732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roberts, A., E. Kretzschmar, A. S. Perkins, J. Forman, R. Price, L. Buonocore, Y. Kawaoka, and J. K. Rose. 1998. Vaccination with a recombinant vesicular stomatitis virus expressing an influenza virus hemagglutinin provides complete protection from influenza virus challenge. J. Virol. 72:4704-4711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rose, N. F., P. A. Marx, A. Luckay, D. F. Nixon, W. J. Moretto, S. M. Donahoe, D. Montefiori, A. Roberts, L. Buonocore, and J. K. Rose. 2001. An effective AIDS vaccine based on live attenuated vesicular stomatitis virus recombinants. Cell 106:539-549. [DOI] [PubMed] [Google Scholar]
- 39.Ruby, J., and I. Ramshaw. 1991. The antiviral activity of immune CD8+ T cells is dependent on interferon-gamma. Lymphokine Cytokine Res. 10:353-358. [PubMed] [Google Scholar]
- 40.Schlereth, B., J. K. Rose, L. Buonocore, V. ter Meulen, and S. Niewiesk. 2000. Successful vaccine-induced seroconversion by single-dose immunization in the presence of measles virus-specific maternal antibodies. J. Virol. 74:4652-4657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schnell, M. J., L. Buonocore, E. Kretzschmar, E. Johnson, and J. K. Rose. 1996. Foreign glycoproteins expressed from recombinant vesicular stomatitis viruses are incorporated efficiently into virus particles. Proc. Natl. Acad. Sci. USA 93:11359-11365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schnell, M. J., L. Buonocore, M. A. Whitt, and J. K. Rose. 1996. The minimal conserved transcription stop-start signal promotes stable expression of a foreign gene in vesicular stomatitis virus. J. Virol. 70:2318-2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Seth, A., I. Ourmanov, M. J. Kuroda, J. E. Schmitz, M. W. Carroll, L. S. Wyatt, B. Moss, M. A. Forman, V. M. Hirsch, and N. L. Letvin. 1998. Recombinant modified vaccinia virus Ankara-simian immunodeficiency virus gag pol elicits cytotoxic T lymphocytes in rhesus monkeys detected by a major histocompatibility complex class I/peptide tetramer. Proc. Natl. Acad. Sci. USA 95:10112-10116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Seth, A., I. Ourmanov, J. E. Schmitz, M. J. Kuroda, M. A. Lifton, C. E. Nickerson, L. Wyatt, M. Carroll, B. Moss, D. Venzon, N. L. Letvin, and V. M. Hirsch. 2000. Immunization with a modified vaccinia virus expressing simian immunodeficiency virus (SIV) Gag-Pol primes for an anamnestic Gag-specific cytotoxic T-lymphocyte response and is associated with reduction of viremia after SIV challenge. J. Virol. 74:2502-2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shirai, M., S. Kozlowski, D. H. Margulies, and J. A. Berzofsky. 1997. Degenerate MHC restriction reveals the contribution of class I MHC molecules in determining the fine specificity of CTL recognition of an immunodominant determinant of HIV-1 gp160 V3 loop. J. Immunol. 158:3181-3188. [PubMed] [Google Scholar]
- 46.Shirai, M., C. D. Pendleton, and J. A. Berzofsky. 1992. Broad recognition of cytotoxic T cell epitopes from the HIV-1 envelope protein with multiple class I histocompatibility molecules. J. Immunol. 148:1657-1667. [PubMed] [Google Scholar]
- 47.Smith, G. L. 1999. Vaccinia virus immune evasion. Immunol. Lett. 65:55-62. [DOI] [PubMed] [Google Scholar]
- 48.Van Bleek, G. M., and S. G. Nathenson. 1990. Isolation of an endogenously processed immunodominant viral peptide from the class I H-2Kb molecule. Nature 348:213-216. [DOI] [PubMed] [Google Scholar]
- 49.Vezys, V., S. Olson, and L. Lefrancois. 2000. Expression of intestine-specific antigen reveals novel pathways of CD8 T cell tolerance induction. Immunity 12:505-514. [DOI] [PubMed] [Google Scholar]
- 50.Yewdell, J. W., J. R. Bennink, M. Mackett, L. Lefrancois, D. S. Lyles, and B. Moss. 1986. Recognition of cloned vesicular stomatitis virus internal and external gene products by cytotoxic T lymphocytes. J. Exp. Med. 163:1529-1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zinkernagel, R. M., B. Adler, and J. J. Holland. 1978. Cell-mediated immunity to vesicular stomatitis virus infections in mice. Exp. Cell Biol. 46:53-70. [DOI] [PubMed] [Google Scholar]