Abstract
Adenovirus-mediated gene delivery via the intramuscular route efficiently promotes an immune response against the transgene product. In this study, a recombinant adenovirus vector encoding β-galactosidase (AdβGal) was used to transduce dendritic cells (DC), which are antigen-presenting cells, as well as myoblasts and endothelial cells (EC), neither of which present antigens. C57BL/6 mice received a single intramuscular injection of AdβGal-transduced DC, EC, or myoblasts and were then monitored for anti-β-galactosidase (anti-β-Gal) antibody production, induction of gamma interferon-secreting CD8+ T cells, and protection against melanoma tumor cells expressing β-Gal. While all transduced cell types were able to elicit an antibody response against the transgene product, the specific isotypes were distinct, with exclusive production of immunoglobulin G2a (IgG2a) antibodies following injection of transduced DC and EC versus equivalent IgG1 and IgG2a responses in mice inoculated with transduced myoblasts. Transduced DC induced a strong ex vivo CD8+ T-cell response at a level of 50% of the specific response obtained with the AdβGal control. In contrast, this response was 6- to 10-fold-lower in animals injected with transduced myoblasts and EC. Accordingly, only animals injected with transduced DC were protected against a β-Gal tumor challenge. Thus, in order to induce a strong and protective immune response to an adenovirus-encoded transgene product, it is necessary to transduce cells of dendritic lineage. Importantly, it will be advantageous to block the transduction of DC for adenovirus-based gene therapy strategies.
Gene transfer with adenovirus vectors is a powerful way to induce humoral and cellular immune responses to a transgene product (12, 24, 49, 53). This approach has been used to develop vaccines for infectious diseases and cancers with some success in animal models (29). Among the administration routes tested, the intramuscular (i.m.) route is one of the most potent. The majority of the adenovirus vectors currently used for vaccination are derived from human adenovirus type 5 and are replication deficient due to a deletion of the E1 genes. Their efficacy as vaccines can be explained by at least three mechanisms: (i) adenoviruses have a broad host cell range and infect a wide spectrum of cells including antigen-presenting cells (APCs) (4, 25); (ii) there is high expression of the transgene product; and (iii) adenoviral vectors induce a strong inflammatory response (18, 26, 54). Moreover, as we previously reported for humans (17), the capsid component in the adenoviral inoculum is directly implicated in neutralizing (16) and cytotoxic T-cell (35) activity. While adenoviral vectors are proposed as vehicles for clinical gene therapy protocols and vaccination, little is known regarding the relative roles of different cell types in eliciting an in vivo immune response. This information is of crucial importance for the design of vaccine strategies to optimize immune responses against the transgene product as well as for gene therapy purposes, where in vivo it may be possible to reduce or eliminate an immune response by targeting specific cell types. Many gene therapy or vaccine-mediated approaches using adenoviral vectors have targeted muscle. Several cell types are present in this tissue in varying amounts; muscle fibers or myocytes, endothelial cells (EC), and fibroblasts are present at high levels, whereas satellite cells or myoblasts, dendritic cells (DC), and macrophages are rarer. Indeed, although DC and macrophages are scattered throughout all nonlymphoid tissues, they represent less than 1 and 2% of murine skeletal muscle cells, respectively (39). The ensemble of these cells is a potential target for adenoviruses, and their transduction following adenovirus administration is dependent on their relative abundance as well as on the presence of adequate receptors at the cell surface. We and others have previously shown that in vitro, fibroblasts and macrophages are poorly transduced whereas myoblasts and EC are efficiently transduced (1, 5, 47). Murine DC have been described to be efficiently transduced with human adenovirus type 5-based vectors only at high multiplicities of infection (MOI) (20, 48). In vivo, transduction of 7 to 22% of cells has been detected on frozen muscle sections (10). Therefore, it appears that only some cell types are putative target cells following i.m. adenovirus administration.
Induction of CD8+ cytotoxic T lymphocytes (CTL) is an essential component of the immune response to various viral infections and tumors. Cultured myoblasts constitutively express only a few major histocompatibility complex class I (MHCI) molecules, no adhesion factors in the absence of tumor necrosis factor alpha (TNF-α) treatment, and no major histocompatibility complex class II (MHCII) molecules (21). In vitro, EC express MHCI and MHCII molecules as well as certain costimulatory molecules (6, 34, 40). Therefore, myoblasts and EC are not typical APCs, but it has been proposed that they may act as APCs under some conditions such as inflammation. Nevertheless, in all situations, they could be sources for antigen transfer to APCs. Recently, it has been demonstrated that professional APCs such as DC can capture exogenous antigen and stimulate an antigen-specific T-cytotoxic response (19). Studies suggest that immune responses against an adenoviral vector transgene product are induced both by direct transduction of APCs and by cross-priming from other types of transduced cells (25). However, adenovirus-mediated transduction of the DC present outside of the lymph nodes has never been demonstrated (45). Moreover, the probability that DC are infected in skeletal muscle in vivo is likely to be low, since they are only rarely present and are poorly infectible.
To define the relative contributions of various cell types to the generation of an immune response in mice, humoral and cellular responses were measured following i.m. inoculation of distinct lineages of adenovirus-transduced cells. DC were selected as a prototype of APCs, while EC and myoblasts were used as non-APC targets. Escherichia coli β-galactosidase (β-Gal) was chosen as the transgene product because of its high immunogenicity, the identification of peptides resulting in a CD8+ response in different H-2 mouse haplotypes, its ability to be easily detected in tissues, and the existence of β-Gal-expressing tumorigenic cells to assay protection in mice.
This approach allowed us to address the following questions. (i) What are the cell lineages naturally present in muscle which contribute to the generation of an adenoviral transgene-specific humoral and/or cellular immune response? (ii) Which transduced cell lineages directly present antigens in vivo? (iii) Does the immune response induced by different types of adenovirus-transduced muscle cells protect against a transgene-expressing tumor challenge? We find that all the cell lineages tested are able to induce a transgene-specific antibody response following adenoviral vector transduction, but only transduced DC induce a high specific CD8+ T-cell response and protect against a tumor challenge.
MATERIALS AND METHODS
Cell lines and cell culture.
The 293 cell line, an adenovirus-transformed human embryonic cell line which provides phenotypic complementation of the E1 genes, was used for adenovirus multiplication and titration. Cells were grown in high-glucose Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal calf serum (FCS) (GIBCO Laboratories, Grand Island, N.Y.). Myoblast cells (H-2b) were derived from primary mouse myoblasts and were the kind gift of C. Pinset and D. Montarras (Pasteur Institute, Paris, France). They were cultivated in medium containing half high-glucose DMEM and half MCDB 202 medium (both from BICEF, L'Aigle, France) containing 20% FCS (Biomedia, Boussens, France) and 2% (vol/vol) Ultroser (Biosepra, Villeneuve-la-Garenne, France) according to the manufacturer's recommendations. EC, derived from mouse heart and immortalized with simian virus 40 T antigen, were provided by D. Paulin and C. Delouis (51). These cells were grown in DMEM with 10% FCS (GIBCO). The RMAS (H-2b/d) cell lines are lymphoid cells that were cultivated in RPMI containing 10% FCS (GIBCO). B16/F10 melanoma cells (15) were a kind gift from B. Goud (Institut Curie, Paris, France). They were grown in RPMI with 10% FCS. Primary murine DC were generated from bone marrow of C57BL/6 mice as previously described (32). Briefly, total bone marrow leukocytes were plated in bacteriological petri dishes with recombinant murine granulocyte-macrophage colony-stimulating factor (20 ng/ml) at 2 × 106 cells per plate. The medium was partially changed every 2 to 3 days. On day 8 of in vitro culture, nonadherent cells were used for phenotype analysis or transduction. All media were supplemented with l-glutamine (2 mM), penicillin (50 IU/ml), streptomycin (50 μg/ml), and sodium pyruvate (1 mM). Cells were grown at 37°C in a 5% CO2 incubator except for EC and B16/F10 cells, which were grown in an 8% CO2 incubator.
Adenovirus vectors, transduction, and analysis of gene transfer.
The adenovirus β-Gal (AdβGal) vector was kindly provided by B. Klowjkowsky (National Veterinary School of Alfort, Maisons-Alfort, France). This vector is derived from human adenovirus serotype 5 and has deletions in the E1 and E3 regions. The nls lacZ gene is under the control of the cytomegalovirus promoter and is inserted in the E1 region. The virus was amplified in 293 cells, purified by two rounds of CsCl density centrifugation, and extensively dialyzed. This stock was recombinant contaminating adenovirus free. Adenovirus transduction of all cells were performed in serum-free media. Cells were exposed to the adenovirus vector at MOI ranging from 1 to 500 PFU per cell for 1 h at 37°C. Cell transduction efficiency was assayed 2 days later. Glutaraldehyde (0.05%, vol/vol)-fixed cells were analyzed for β-Gal activity by 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) histochemistry. Infected adherent cells were photographed under a stereomicroscope at a magnification of ×50. By using either photography or direct detection on a Malassez hemocytometer, visible blue nuclei as well as nonstained nuclei were counted in order to determine the percentage of transduced cells.
Vaccination with adenovirus vector or adenovirus-transduced cells and animal procedures.
Female C57BL/6 (H-2b) and DBA/2 × C57BL/6 (BDF1) (H-2b/d) mice, 7 to 12 weeks old, were purchased from the Institut Nationale de la Recherche Agronomique (INRA) animal research facility (Jouy-en-Josas, France) and Iffa Credo (L'Arbresle, France), respectively. Animals were handled in accordance with the institutional guidelines of the National Veterinary School of Alfort. Mice were anesthetized with avertine (10 μl/g of body weight). Groups of five to eight mice were injected by the i.m. route at one site in the tibialis anterior with 10 μl of inoculum. The inoculum was either AdβGal vector (109 PFU), 5 × 104 β-Gal-expressing cells transduced with the AdβGal vector, or control cells. Peripheral blood was collected at the indicated time points. Spleens were collected from animals sacrificed at days 40 to 42 postvaccination. For all analyses, serum, blood, or spleen samples were pooled from animals in the same group.
Flow cytometry analysis.
Expression of the B7.2 (CD86), B7.1 (CD80), MHCII (I-Ab), MHCI, and CD11c cell surface markers was assessed on DC and EC before and after AdβGal infection. DC were harvested and washed twice in phosphate-buffered saline (PBS). Aliquots of cells were washed in fluorescence-activated cell sorter (FACS) flow buffer (Becton Dickinson, Mountain View, Calif.) and preincubated for 15 min at room temperature with FACS flow buffer supplemented with bovine serum albumin (0.5%) and normal mouse serum (2%) to prevent binding to Fc receptors. Cells were then incubated with the primary antibodies for 30 min on ice. A second incubation with a conjugate antibody was performed when necessary. The following antibodies were used: rat IgG2a monoclonal anti-mouse B7.2 (CD86) (clone RMMP-2; catalog no. RM7004; Caltag Laboratories, Burlingame, Calif.), rat IgG2a monoclonal anti-mouse B7.1 (CD80) (clone RMMP-1; Caltag), mouse immunoglobulin M (IgM) monoclonal anti-mouse I-Ab MHCII biotin conjugate (clone 25.16.85; Caltag), mouse IgG2a monoclonal anti-mouse H-2 Kb Db MHCI (clone 5041.16.1; Cedarlane, Hornby, Ontario, Canada), hamster IgG monoclonal anti-mouse CD11c (clone N418; hybridoma obtained from American Type Culture Collection, Manassas, Va.), rat IgG2a-phycoerythrin (PE)- and -fluorescein isothiocyanate (FITC)-conjugated isotype controls (Caltag), a mouse IgG2a-FITC-conjugated isotype control (Caltag), and a mouse IgM biotin-conjugated isotype control. Cells were fixed with 1% paraformaldehyde. Samples were analyzed on a FACScan flow cytometer with Lysis II software (Becton Dickinson).
To monitor the efficiency of CD8 depletion by magnetic cell sorting (MACS), CD8 and CD4 markers were assessed on the different cell fractions. The following antibodies were used: rat anti-CD8a-PE (clone CT-CD8α; Caltag), rat anti-CD4-FITC (clone CT-CD4; Caltag), and PE- and FITC-conjugated isotype controls (Caltag).
β-Gal antigen quantitation.
Two million cells exposed to the adenovirus vector were lysed 48 h after infection (at MOI of 500 for DC, 10 for myoblasts, and 50 for EC) in 1 ml of lysis buffer. The quantity of β-Gal antigen in each lysate was measured by using a sandwich β-Gal enzyme-linked immunosorbent assay (ELISA) kit according to the manufacturer's procedure (kit no. 1539426; Roche Molecular Biochemicals, Mannheim, Germany).
Anti-β-Gal antibody assay.
Anti-β-Gal serum antibody titers were determined by ELISA. A 96-well plate (Maxisorb; Nunc) was coated overnight at 4°C with 100 μl of 0.1 M Tris-0.15 M EDTA (pH 9.6) buffer containing 500 ng of soluble β-Gal. A solution of PBS containing 0.1% (vol/vol) Tween 20 and 0.05% (wt/vol) gelatin was used as a blocking buffer. Plates were washed (with water containing 0.1% Tween 20), and dilutions of sera were incubated in the coated wells for 30 min at 37°C. Plates were again washed and then incubated with biotin-labeled anti-mouse Ig followed by streptavidin-horseradish peroxidase (Amersham Pharmacia) for 30 min at 37°C. Different anti-mouse Ig's were used: goat-anti-mouse IgG (Amersham Pharmacia), goat anti-mouse IgG1 (Caltag), and goat anti-mouse IgG2a (Caltag). Peroxidase (POD) activity was detected by using BM Blue POD as a substrate. Absorbance was measured at 405 nm. Preimmune sera were used to determine the background level. For Ig isotype titers, the first dilution tested was 1/4.
ELISPOT assay for monitoring IFN-γ release from single cells.
The gamma interferon (IFN-γ) enzyme-linked immunospot assay (ELISPOT assay) detects secreted IFN-γ molecules in the immediate vicinity of the cell from which they are secreted; each spot in the read-out represents a footprint of the original IFN-γ-producing cell.
Peripheral blood mononuclear cells (PBMC) or splenocytes were isolated from mice at different time points after i.m. injection of the adenovirus vector or AdβGal-transduced cells. The 96-well nitrocellulose plates (multiscreen HA; Millipore Corporation, Bedford, Mass.) were coated with 40 ng of capture anti-mouse IFN-γ monoclonal antibody (MAb) R4-6A2 (PharMingen, San Diego, Calif.)/well. After overnight incubation at 4°C, wells were washed repeatedly with culture medium and saturated with complete medium for 30 min at 37°C. MHCI-compatible target cells, RMAS cells pulsed with a synthetic peptide (30 μg/ml) representing the CD8 epitope of β-Gal in the H-2b and/or H-2d haplotype, were added to the ELISPOT assay. The β-Gal peptides used in this assay were the octamer ICPMYARV (I8V) and the nonamer TPHPARIGL (T9L), which are naturally presented by the H-2 Kb (38) and H-2 Ld molecules, respectively (13) (Isochem, Strasbourg, France). Target cells not pulsed with peptide were used as a negative control. Serially diluted PBMC or splenocytes (1 × 106 to 1.25 × 105 cells/wells) depleted of erythrocytes by treatment with ammonium chloride were cocultured with 105 RMAS cells in the ELISPOT wells in medium supplemented with human recombinant interleukin 2 (IL-2) (30 U/ml; Roche Molecular Biochemicals) for 24 h at 37°C under 5% CO2. As a positive control, splenocytes or PBMC were incubated with conacavalin A (5 μg/ml; Sigma, St. Louis, Mo.). After extensive washing with PBS-0.05% Tween 20, plates were incubated overnight at 4°C with 50 ng of biotinylated anti-mouse IFN-γ MAb XMG1.2 (PharMingen)/well and then with ExtrAvidin alkaline phosphatase conjugate (Sigma) for 1 h at room temperature. Spots were developed by adding an alkaline phosphatase conjugate substrate (Bio-Rad Laboratories, Hercules, Calif.). The number of spots corresponding to IFN-γ-secreting cells was determined by using a stereomicroscope or a KS ELISPOT prototype (Zeiss-Kontron, Jena, Germany). Only large spots with fuzzy borders were scored as positive. Responses were considered significant if a minimum of five spots per well were present, as long as this number was at least twice that present in the negative control wells. The β-Gal-specific T-cell response, monitored as the percentage of IFN-γ-secreting T cells in mice transplanted with AdβGal-transduced cells (cell/Ad) compared with AdβGal vector (Ad), was determined as (number of spots with peptide − number of spots without peptide for cell/Ad)/(number of spots with peptide − number of spots without peptide for Ad).
In vitro isolation of CD8+ T cells.
CD8+ T cells were isolated from 5 × 107 splenocytes by depletion with MACS CD8α (Ly-2) microbeads as described by the producer (Miltenyi Biotech, Bergisch Gladbach, Germany). The CD8+ cell fraction (attached) and the CD8− cell fraction (nonattached) were collected. Depletion of the CD8+ cell population was confirmed by flow cytometry. Both subsets were used in the ELISPOT assay.
Generation of B16-LacZ cells.
B16/F10 cells were infected twice with retroviral supernatant from the TE FLY A7 producer line (kindly provided by J.-C. Pagès) in the presence of Polybrene. The TE FLY A7 cell line produces amphotropic envelope-pseudotyped virions harboring a murine leukemia virus retroviral vector with an nls lacZ gene (9). Two days after the second infection, cells were cloned by limiting dilution. Clones were tested for β-Gal expression 10 days later. One positive clone with high cell division was amplified and named B16-LacZ.
Tumorigenicity studies.
B16-LacZ cells were harvested, washed three times in PBS, and administered to immunized mice by a subcutaneous (s.c.) flank injection (5 × 104 cells per mouse). Animals were examined three times a week for signs of a tumor. When a tumor was present, two bisecting diameters of the tumor were measured with calipers. The volume of the tumor was calculated by the formula (0.5 × L × l2) where L stands for the larger diameter and l stands for the smaller diameter. All animal were killed when the tumors reached a diameter of 15 mm. Some tumors were analyzed by histochemistry to verify that they expressed β-Gal.
Histochemistry.
Tumors were harvested, fixed for 1 h in 4% paraformaldehyde, embedded in OCT compound (Sakura Finetek Europe B.V., Zoeterwoude, The Netherlands), and then frozen in liquid nitrogen. Frozen histologic sections were analyzed for β-Gal activity by X-Gal staining. Tissues were counterstained with neutral red.
RESULTS
Efficiencies of adenoviral vector infections in distinct cell types.
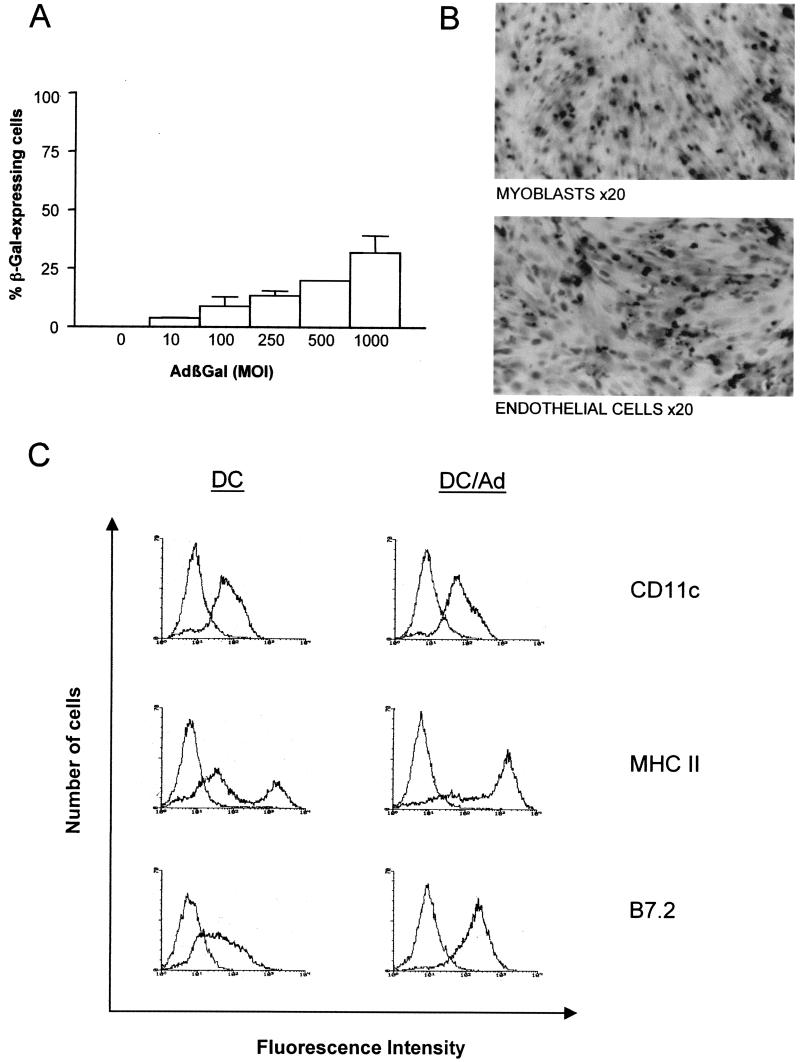
In order to analyze the infectibilities of the distinct cell types present in muscle, EC, myoblasts, and DC were infected with an AdβGal vector at various MOI. It should be noted that the DC used in this study were bone marrow-derived cells obtained by a previously established protocol (32). Cells which differentiated to immature DC in culture were used, as these cells are resident in peripheral tissues such as muscle. Infected cells were assayed for β-Gal activity at 48 h postinfection (Fig. 1A). β-Gal activity was detected in approximately 20% of DC infected at an MOI of 500. At higher MOI, transducibility was higher but was associated with a high cell mortality, probably due to adenovirus capsid toxicity (22). An MOI of 500 was chosen for further experiments because this MOI gave the best results in terms of transducibility and cell mortality. Importantly, 100% of myoblasts and EC expressed β-Gal, without any associated cell toxicity, at MOI of 10 and 50, respectively.
FIG. 1.
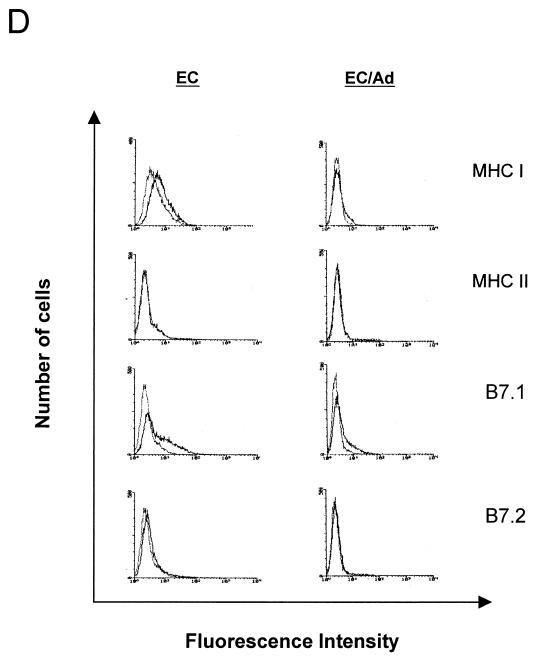
In vitro transduction of DC, myoblasts, and EC with AdβGal. (A) Transduction efficiency of AdβGal on DC at MOI of 10, 100, 250, 500, and 1,000. Transduction was assayed by β-Gal expression 48 h after vector exposure. (Error bars, standard errors of the means; n = 2). (B) Myoblasts and EC, 48 h after infection with AdβGal at 10 and 50 MOI, respectively. Cells were stained with X-Gal (original magnification, ×50). (C) Immunophenotype of primary DC before (DC) and after (DC/Ad) adenovirus exposure. Flow cytometry analyses of CD11c, MHCII (I-A), and B7.2 (CD86) cell surface molecules were performed 24 h after exposure to adenovirus vector (MOI, 500). Isotype controls are presented as negative controls. Data are representative of results obtained from two independent experiments. (D) Immunophenotype of EC 48 h after AdβGal infection (MOI, 10). Isotype controls are presented as a negative control.
The phenotype of DC was analyzed before and after infection in order to evaluate any effect of adenoviral infection on cell differentiation (Fig. 1B). This was performed by monitoring the expression of different surface markers by flow cytometry. Before infection, most of the DC population showed the phenotype of immature cells, similar to that described by Lutz and colleagues (32). All cells expressed the CD11c marker, and the majority of cells expressed moderate levels of membrane MHCII (I-Ab) and B7.2 molecules. In contrast, at 24 h postinfection, all DC showed a phenotype of more-mature cells. CD11c expression was unchanged, but MHCII and B7.2 expression was increased; 64.4% of the cells had shifted toward high MHCII expression, and 100% of cells expressed high levels of B7.2. These data demonstrate a DC maturation induced by adenovirus exposure. The MHCI, MHCII, B7.1, and B7.2 phenotype of EC was also studied before and after transduction. Neither MHCII, B7.2, nor B7.1 expression was altered in transduced cells: MHCII was absent, B7.2 was expressed, and B7.1 was expressed at low levels. MHCI was expressed at very low levels in EC prior to infection and was not detected postinfection (Fig. 1D).
In order to evaluate if the same number of β-Gal-expressing cells of different cell types produced the same quantity of β-Gal antigen, we quantified by ELISA the β-Gal protein in the three cell types transduced with the AdβGal vector. We detected 1.25, 260, and 37.5 ng in 5 × 104 β-Gal-expressing DC, myoblasts, and EC, respectively. Therefore, the β-Gal antigen is expressed in DC at levels 30 to 200 times lower than those in EC or myoblasts.
On the basis of these data, experiments designed to assess the immunogenicities of adenovirus-infected DC, myoblasts, and EC were performed following infection of these cells with the AdβGal vector at MOI of 500, 10, and 50, respectively.
Anti-β-Gal antibody response to a single inoculation of adenovirus-transduced muscle cells.
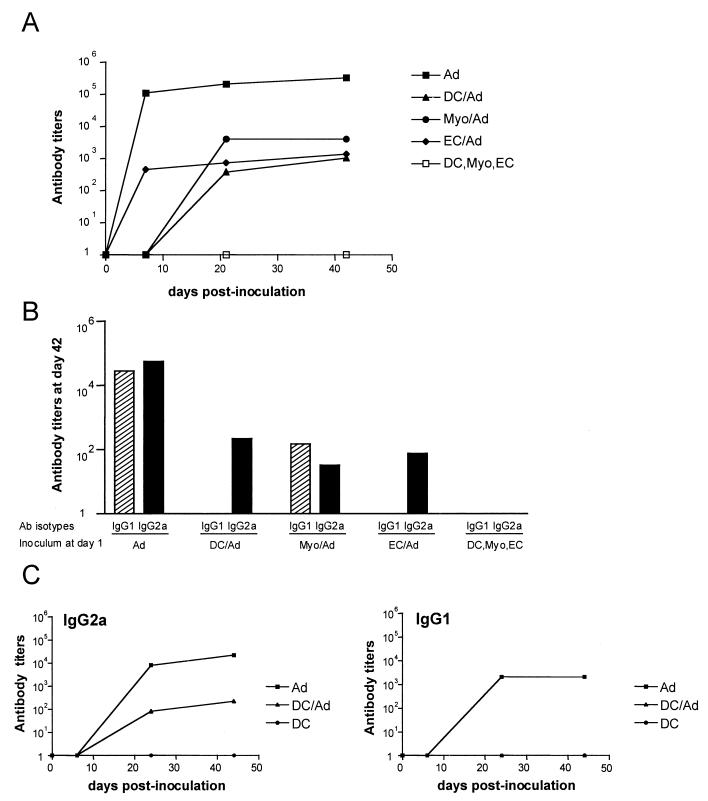
Syngeneic C57BL/6 mice (five to eight mice per group) were inoculated by the i.m. route with 5 × 104 β-Gal-expressing cells (either DC, myoblasts, or EC) transduced with the AdβGal vector. Blood samples were taken from immunized mice at days 7, 21, and 42 (the day of challenge) and were assayed for anti-β-Gal antibodies by ELISA (Fig. 2). Mice inoculated with each of the three adenovirus-transduced cell types developed relatively high and similar anti-β-Gal IgG titers at days 42 to 45, ranging from 103 to 103.5 (Fig. 2A), although differences were found in the relative levels of β-Gal antigen in the different cell types. The antibody titer at day 42 was always higher after direct i.m. injection of the AdβGal vector (104.5 to 105) than after inoculation with AdβGal-transduced cells (in eight independent experiments [data not shown]). Direct injection of AdβGal probably induces a higher production of exogenous β-Gal antigen than the graft of transduced cells and/or a more inflammatory context leading to a higher response. Kinetic analyses showed that animals injected with AdβGal-transduced EC developed an antibody response at least 7 days earlier than those injected with AdβGal-transduced DC or myoblasts. The isotypes of the antibodies produced by B cells are determined by the specific cytokine pattern produced by helper T cells (36). IgG2a and IgG1 are characteristically produced under Th1- and Th2-dominant conditions, respectively. Therefore, we investigated the production of these two IgG subclasses. Direct injection of the recombinant adenovirus vector, AdβGal, induced high levels of both IgG1 and IgG2a (Fig. 2B). In marked contrast, inoculation of AdβGal-transduced DC and EC produced only IgG2a isotype antibodies. Interestingly, injection with transduced myoblasts revealed yet another phenotype of both IgG1 and IgG2a isotypes. Moreover, we found that this isotype profile was already defined early in the antibody response and was not modified with time (Fig. 2C).
FIG. 2.
β-Gal-specific antibody titers in mice immunized with AdβGal or with AdβGal-transduced muscle cells. Mice received one i.m. injection with either 109 PFU of AdβGal (Ad) or 5 × 104 AdβGal-expressing DC (DC/Ad), myoblasts (Myo/Ad), or EC (EC/Ad). Mice injected with untransduced DC, myoblasts, or EC (DC, Myo, or EC) were used as negative controls. Mice were bled once a week, and β-Gal-specific antibody titers were measured by ELISA. (A) Generation of β-Gal-specific IgG antibodies as a function of time. (B) Levels of IgG1- and IgG2a-specific antibodies at day 42. (C) Kinetics of IgG1 and IgG2a β-Gal-specific antibodies after immunization with AdβGal-transduced DC.
Induction of β-Gal-specific CD8+ T cells in response to a single inoculation of adenovirus-transduced muscle cells.
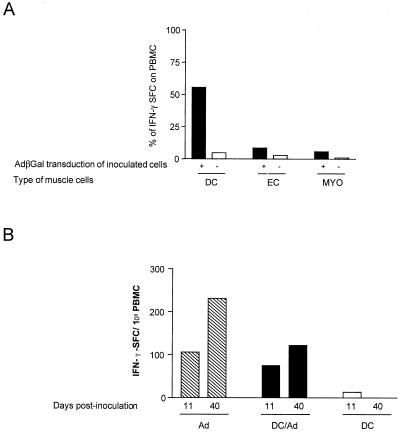
To analyze the capacity of adenovirus-infected muscle cells to induce a β-Gal-specific cellular immune response in syngeneic mice, we used an IFN-γ ELISPOT assay. This assay provides a quantitative assessment of the number of antigen-specific activated CD8+ T cells (31, 37). Freshly explanted PBMC, isolated 42 to 44 days after a single immunization, were examined for their abilities to secrete IFN-γ ex vivo in response to the H-2b-restricted β-Gal peptide (I8V) presented by RMAS cells (Fig. 3). No response, or only a weak response, was obtained with PBMC cultured with RMAS cells alone (data not shown). In contrast, significant numbers of IFN-γ-secreting cells were detected with PBMC effectors from mice injected directly with AdβGal in the presence of RMAS cells plus I8V peptide (range, 210 to 280 spot-forming cells [SFC] per 106 PBMC). PBMC from mice inoculated with AdβGal-transduced DC were able to secrete IFN-γ in response to the I8V peptide, with a frequency approximately 50% of that observed in mice immunized directly with AdβGal. In mice injected with transduced DC, CD8+ T cells recognized the β-Gal CTL epitope, ranging from 140 to 240 SFC per 106 PBMC. While PBMC collected from transduced-EC- and -myoblast-immunized mice had significant numbers of β-Gal-specific CD8+ T cells, these levels were 6- to 10-fold lower, respectively. Also, in DC-transduced mice, the β-Gal antigen-specific CD8+ T-cell response was higher at day 42 than at an early time point after injection (day 11) (Fig. 3B).Therefore, our data demonstrate that the highest specific cellular immune response was observed in mice vaccinated with transduced DC.
FIG. 3.
IFN-γ-secreting cells in mice immunized with AdβGal-transduced DC, EC, or myoblasts. The number of β-Gal peptide (I8V)-specific CD8+ T cells was determined directly ex vivo by an IFN-γ-specific ELISPOT assay. The number of IFN-γ SFC was determined after a 24-h incubation of PBMC pools with RMAS cells alone or pulsed with the I8V peptide. Data represent averages from duplicate or triplicate wells. (A) The frequency of IFN-γ SFC in mice injected with AdβGal-transduced muscle cells was compared, at 42 days postimmunization, with that in mice injected directly with AdβGal. (B) Numbers of IFN-γ SFC in mice injected with AdβGal directly (Ad), AdβGal-transduced DC (DC/Ad), and untransduced DC (DC) at days 11 and 40 postinoculation. Results are expressed as IFN-γ SFC per 106 PBMC and are calculated as the differences between the numbers of spots observed in the presence and absence of β-Gal peptide I8V.
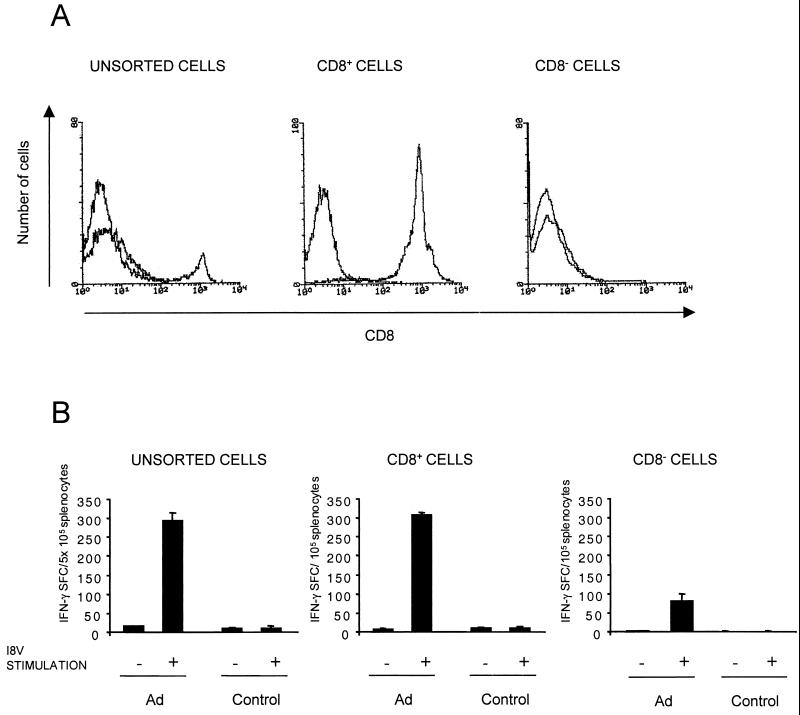
To confirm that the specific IFN-γ effector T cells were CD8+, as would be expected for a class I-restricted CTL peptide, CD8+ T cells were isolated from the splenocytes of AdβGal-injected mice. Splenocytes were used instead of PBMC so that there would be enough cells for sorting. The quality of the isolation was verified by cytometry (Fig. 4A). The two populations, CD8+ and CD8− fractions, were tested for IFN-γ secretion after stimulation with the I8V peptide. The number of IFN-γ SFC in the CD8+ fraction was 3 to 5 times higher than that in the CD8− fraction and was also approximately 5 times higher than that detected in the total splenocyte population (Fig. 4B). This is consistent with the percentage of CD8+ cells in the unsorted cells. Thus, in the case of the β-Gal antigen, an IFN-γ ELISPOT assay can be used to quantitate antigen-specific CD8+ cells from the total splenocyte population.
FIG. 4.
β-Gal-specific CD8+ T-cell effectors in AdβGal-immunized C57BL/6 mice. Splenocytes were harvested 13 days after inoculation, and CD8+ cells were depleted by MACS. Both CD8+ and CD8− populations were collected. (A) CD8+ cell surface molecules were analyzed by flow cytometry before and after cell sorting. Staining with a nonspecific isotype control antibody is shown in each histogram. (B) The number of IFN-γ SFC was determined in the presence or absence of the β-Gal peptide I8V. Splenocytes from CD8+ and CD8− fractions collected from immunized or control mice were plated at a graded density starting at 2 × 105 cells/well, whereas unsorted splenocytes were plated at a graded density starting at 1 × 106 cells/well. Data are means of duplicate samples.
Protective immunity to tumor challenge.
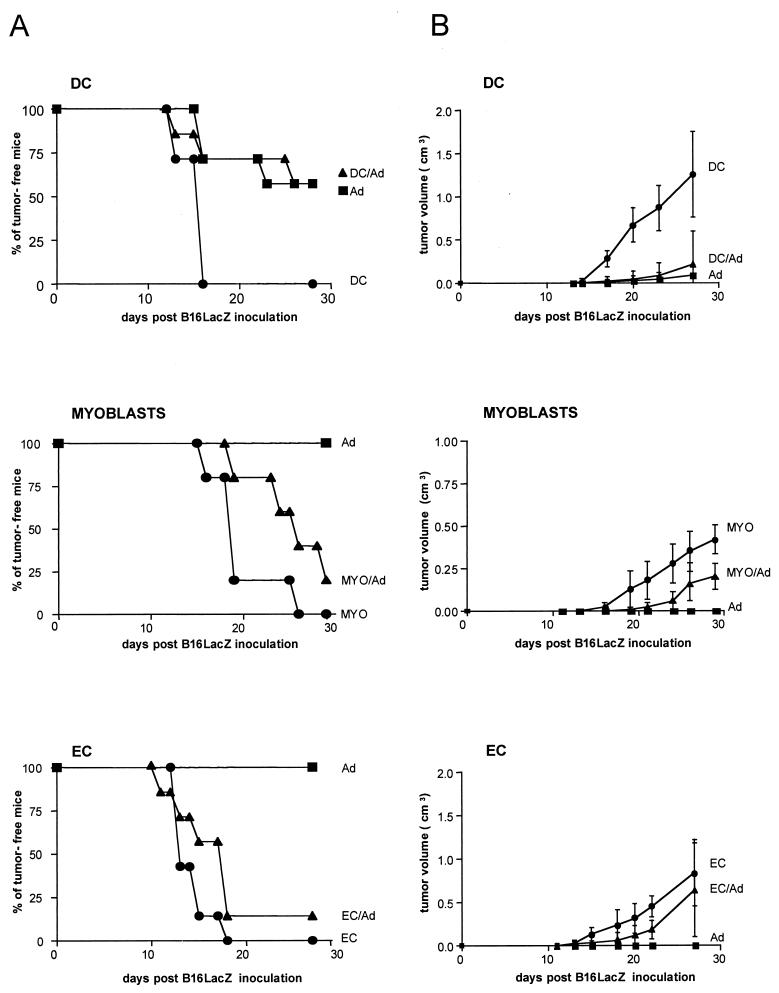
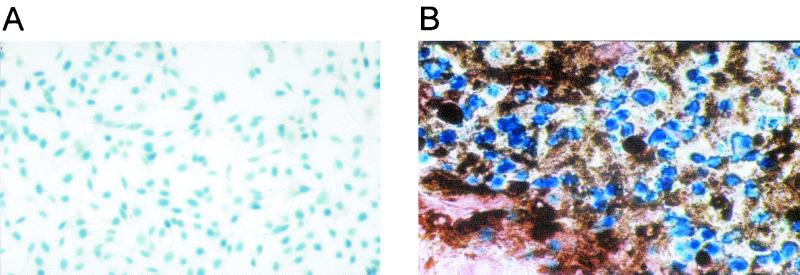
To determine the capacity of AdβGal-transduced cells to induce protective antitumor immunity, we used a tumor model based on the β-Gal murine melanoma clone B16-LacZ, expressing H-2b antigens. This clone was obtained by infecting B16/F10 cells with a retroviral vector carrying an nls lacZ gene. B16-LacZ cells did not in themselves induce higher immunogenicity than B16/F10 cells, because injection of the same number of either cell type in C57BL/6 mice resulted in the death of 100% of animals (data not shown). Forty-two days after immunization of C57BL/6 mice with AdβGal-transduced cells, 5 × 104 B16-LacZ cells were injected s.c. into the backs of the mice. Two parameters were monitored to quantify the protection: the percentage of mice not developing a tumor and, in case of tumor development, the mean volume. As shown in Fig. 5A, the development of melanoma was prevented in 57% of the mice (4 of 7) by the previous injection of AdβGal-transduced DC. The percentage of mice protected by transduced DC was similar to that observed for mice injected directly with AdβGal. In marked contrast, less than 17% of mice (1 of 7 or 1 of 6) were protected following injection with transduced EC or myoblasts. Nevertheless, in the myoblast-immunized mice, the tumor size was reduced from that for controls, whereas in EC-immunized animals, the tumor size was similar to that observed in mice inoculated with control cells. In order to verify that the tumors detected were truly due to a poor immunological tumor control and not to an escape from the immune response associated with an extinction of β-Gal expression in B16-LacZ cells, we analyzed β-Gal expression in tumors explanted from vaccinated mice and sampled at random. As shown in Fig. 6, all the B16-LacZ tumors still expressed the β-Gal protein. This observation demonstrates that the protection results are dependent on the preparations used for the immunization and are not biased by artifactual tumor cells that have escaped the immune response.
FIG. 5.
Protective immunity against tumor challenge. C57BL/6 mice (six to seven per group) were immunized i.m. with either AdβGal (squares), AdβGal-transduced cells (triangles), or untransduced cells (circles). Forty-two days postimmunization, mice were challenged s.c. with 5 × 104 B16-LacZ cells, and tumor formation was monitored every 2 days for 30 days. (A) Percent tumor-free mice. In order to facilitate the reading of the graphs, only points that determine the aspect of the curves were indicated, rather than each time point. (B) Tumor volume measured in cubic centimeters. At each time point represented, tumor sizes of all mice in the group were added up. Results are shown as mean tumor sizes ± standard errors of the means.
FIG. 6.
Histology of melanoma tumors in B16-LacZ-challenged mice. (A) B16-LacZ cells in culture after X-Gal staining (original magnification, ×50). (B) Tumor isolated from B16-LacZ-challenged mice previously vaccinated with AdβGal-transduced myoblasts (original magnification, ×360). The tissue section was stained with X-Gal and counterstained with neutral red. Black deposits correspond to melanin.
Direct or indirect priming of the immune response by transduced DC.
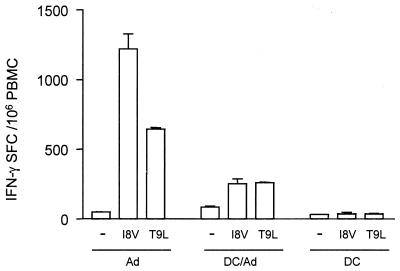
In order to determine if transduced DC were directly triggering the β-Gal-specific CD8+ T-cell response or if cells from the host mice were also participating in this process, we immunized H-2b×d mice with H-2b-transduced DC as previously described (8). This experiment was not performed with AdβGal-transduced myoblasts or EC because the CD8+ T-cell response induced was low under these conditions. PBMC from mice injected with AdβGal-transduced DC were monitored in an ex vivo IFN-γ ELISPOT assay with or without β-Gal peptide stimulation at 19 days postinoculation. Two peptides were used: the H-2b-specific I8V and the H-2d-specific T9L peptide (Fig. 7). We found that β-Gal epitopes were recognized in the context of both the H-2b- and H-2d-specific MHC molecules. This result suggests that cells from the host also participate in the presentation of the β-Gal peptides and that cross-priming may be involved in the T-cell-response induction.
FIG. 7.
IFN-γ-secreting cells in BDF1 mice. F1 (C57BL/6 × DBA2) mice were immunized as previously described with C57BL/6-transduced DC. The number of CD8+ T cells with a specificity for the I8V or T9L β-Gal peptide was directly determined ex vivo by an IFN-γ-specific ELISPOT assay 19 days after immunization. The number of IFN-γ SFC was counted after 24 h of stimulation. Results shown are mean numbers of IFN-γ-producing PBMC ± standard errors of the means from duplicate wells.
DISCUSSION
Many studies have demonstrated that in vivo transfer of foreign proteins via adenovirus vectors can result in the generation of a strong immune response. This property is clearly a drawback for gene therapy strategies but is an advantage in vaccination. Understanding the mechanisms underlying the induction of transgene-specific immune responses following adenovirus-mediated gene transfer is thus important for both vaccination and gene therapy purposes. Particularly, a major point is to determine whether the immune response is principally initiated by adenoviral transduction of APCs, which are essential for initiating naive T-lymphocyte activation, or, alternatively, whether cross-priming of these cells is sufficient. To address this issue, we compared the immune response induced by an i.m. injection of recombinant adenovirus alone with that induced by cells transduced ex vivo with the recombinant adenovirus. The natures of the humoral and cellular immune responses induced to the immunogenic β-Gal protein expressed from an adenoviral vector were studied.
An important focus of these studies was to determine whether transduced cells in the muscle induced cellular immunity. Injection of transduced myoblasts or EC was very inefficient in inducing IFN-γ-secreting CD8+ T cells compared to transduced DC. The inefficiency of myoblasts in this process was quite surprising for several reasons. First, DNA-transfected myoblasts expressing a viral antigen have been described to induce an MHCI CTL response in mice (50). In this previous study, the cells grafted were C2C12, a cell line of the H-2k haplotype. The discrepancy between this and our study may be related to differences in the number of cells injected (200-fold lower in our study), MHCI expression at the surfaces of the myoblasts, mouse haplotype, and the nature of the transgene product. Second, under certain in vitro conditions, myoblasts have been described to express the two components required for CD8+ T-cell activation, making them APCs: MHCI molecules and certain costimulatory molecules (2, 21). Nevertheless, the conditions required for expression of these molecules were likely not met in the present study, and moreover, it is possible that these components are necessary but not sufficient for induction of an immune response (23). Third, myoblasts could serve as a source of antigen that can be recaptured by resident APCs and presented on MHCI molecules. Such a mechanism of cross-priming has been suggested by different authors (8, 14). The reason why in our study the myoblasts were not a source for efficient cross-priming remains uncertain. The mechanism of cell death of the transduced myoblasts injected could be involved. Indeed, from in vitro experiments, it is still not clear whether antigen from apoptotic or necrotic dead cells can induce a CD8+ T-cell response by cross-priming (3, 46). With regard to the inefficiency of the transduced EC inducing IFN-γ-secreting CD8+ T cells, the same reasoning as for the myoblasts can be used with one major restriction: EC have been defined as semiprofessional APCs on the basis of in vitro T-cell activation studies, whereas myoblasts have not (33). Taken together, our observations suggest that in muscle, adenovirus-transduced myoblasts and EC are very poor inducers of an antigen-specific CD8+ T-cell response either by direct stimulation or by cross-priming.
Our results are consistent with earlier studies that proposed a role for DC in the induction of a CD8+ T-cell response after injection of a recombinant adenovirus in vivo (25, 45). Surprisingly, though, in our study, there was a significant difference between the CD8+ specific IFN-γ response after direct injection of recombinant adenovirus and that after inoculation of transduced DC in the muscle. If DC presenting the transgene are the only cells able to stimulate naive T cells, several hypotheses can be proposed to explain this observation. First, the possibility that injection of a high dose of free adenovirus in the muscle may be more efficient than transduction of DC directly in the lymph node cannot be excluded (45). Indeed, presentation of an antigen by MHCI molecules in a lymphoid organ even by non-APCs could directly and efficiently induce a CTL response (30). Second, transduced DC injected into muscle may not mature properly, migrate, or survive long enough to strongly prime the CD8+ T-cell response. Many studies have now demonstrated the ability of modified DC to induce a CD8+ T-cell response, and some have used human adenovirus vectors in mice (28, 43, 48, 52). However, in all these studies, the modified DC were administered through an intravenous or s.c. route. i.m. administration may be less potent, as muscle is a dense and highly organized tissue in which the rate of migration of mature DC may be slowed compared to that in a fluid or loose tissue such as the dermis. In agreement with previous studies, we found that adenovirus infection induces an upregulation of costimulatory and MHC molecules (20, 42, 44). Intriguingly, recombinant adenoviruses may block IL-12 secretion and CD83 expression, inhibiting the full maturation of DC (20, 42). Therefore, most of the transduced DC inoculated in this study might not be able to migrate properly to the lymph node and stimulate the antigen-specific CD8+ T cells. Also in agreement with this hypothesis, we and others observe that following inoculation of H-2b DC in H-2b×d mice, the T-cell response is restricted to H-2b×d (7; also this report). Taken together, our data suggest that transduction of DC is probably essential for induction of a CD8+ T-cell response after i.m. injection of recombinant adenovirus. However, following direct muscular injection of adenoviral vectors, it remains to be determined which DC cells are transduced and in which location the infection occurs, in the muscle itself or directly in the draining lymph nodes.
Many studies, especially those focusing on gene therapy strategies, have described the presence of antibodies against the transgene product, but very few have focused on B-cell induction for IgG subclass shifts following direct injection of adenovirus or grafting of distinct types of adenovirus-transduced cells (27). In this study, we observed that the graft of either DC, myoblasts, or EC transduced with adenovirus induced a strong humoral response against the transgene product, with similar IgG titers. The transgene product, the β-Gal protein, has a nuclear targeting signal and is a cell-associated antigen. In order to stimulate the B-cell response, the antigen newly synthesized after adenovirus transduction therefore needs to be released into the extracellular environment. For the three cell types, different hypotheses can be proposed to explain antigen release following death of the transduced cells. First, a small proportion of the transduced cells were already dead prior to inoculation. This explanation may hold only for transduced DC with an in vitro mortality of 3 to 5%. This mortality can be associated with various factors including high MOI, adenovirus protein toxicity, and maturation of DC leading to cell death. Second, transduced cells of all cell types can die when they traverse the needle during i.m. inoculation. This hypothesis can be eliminated, as we never observed an increase in trypan blue-stained cells immediately after a single passage through the needle (data not shown). Third, the grafted transduced cells may die in the muscle after inoculation. This hypothesis is the privileged one for myoblasts and EC but can also be suggested for DC. Rapid disappearance of transduced myoblasts was also described by Ulmer et al. (50). Two major mechanisms may induce cell death: a nonspecific defense associated with cytotoxic NK cells and a specific immunity associated with CD8+ T lymphocytes. Low MHCI expression at the cell surfaces of myoblasts and EC is compatible with sensitivity to NK cytotoxicity. That is supported by our finding that very few β-Gal-expressing cells were detected in the muscle by cytochemistry 1 day after injection of myoblasts or EC (data not shown). With regard to cell death of transduced cells by anti-β-Gal-specific cell lysis, this is unlikely in the case of transduced myoblasts and EC, as we detected only a low CD8+ T-cell response. A role for the minor histocompatibility complex is also unlikely, as the disappearance of the cells was rapid.
Importantly, we found that different types of adenovirus-transduced cells induced different IgG isotype patterns. In the case of transduced myoblasts, both IgG1 and IgG2a isotypes were detected, with equal titers. For the two other cell types, EC and DC, only IgG2a antibodies were detected. The IgG2a/IgG1 skewing is therefore significant and in favor of a predominant Th1 response. For DC, our results were unexpected, as myeloid DC have been described to favor Th2 responses in terms of antibody response in mice (41). Nevertheless, this discrepancy may be explained by the recent concept that DC function is not immutable but rather is regulated by the cells' microenvironment (41). Moreover, the DC that were injected in this study may have contained distinct cell subsets. As the isotype profile is guided mostly by cell-secreted cytokines, we postulate that adenovirus-transduced EC and DC induce the same kind of cytokine profile, whereas myoblasts induce a different one. It is known for mice that IL-12 and IFN-γ promote a Th1 response. The mechanism by which transduced DC and EC activate in vivo cytokine secretion has not yet been analyzed in detail. Nevertheless, our data comparing the IgG isotype patterns after direct adenovirus injection and graft of transduced cells suggest that several cell types participate in the generation of an antibody response.
The major focus of the present study was to obtain information regarding the mechanism of activation of the immune response against a transgene product in an adenoviral vector leading to protection as well as rejection of cells expressing the transgene. Importantly, we found that injection of transduced DC and myoblasts, but not of EC, resulted in antitumor immunity against a B16 melanoma challenge, but with different levels of efficiency. For DC the protection, measured by the percentage of tumor-free mice and tumor volumes, was similar to that obtained with free adenovirus, whereas for myoblasts the protection was limited and only affected tumor volumes. Our results with transduced DC are in agreement with those of studies performed by other immunization routes and with other antigens (28, 52). Regarding the effect of transduced myoblasts, this slowdown was unexpected, considering the low CD8+ T-cell response obtained with these cells. A role for antibodies in tumor growth control is unlikely, as the β-Gal antigen is not expressed at the cell surface of the tumor. Thus, CD4+ cells might play a role, as already described for some antitumor protection (11). Taken together, our data demonstrate that transduced DC are largely superior to their EC and myoblast counterparts in inducing a protective immune response against a transgene harbored by an adenoviral vector.
These observations have implications for the development of vaccination and gene therapy strategies. The fact that DC are essential for the initiation of a CD8+ T-cell response but are poorly transducible by human adenovirus type 5 suggests that new vaccine strategies should be investigated, such as identifying different ways of enhancing adenovirus entry into DC. A second possibility is that another inoculation route, such as direct inoculation of the adenovirus into the lymph node, may be more efficient. Thirdly, coinoculation of different factors that could recruit DC at the injection site may help the entry of the adenovirus into DC or modify their immune response. Further studies should focus on the entry route of recombinant adenoviruses in DC and the adenoviral components implicated in DC activation.
Acknowledgments
We are indebted to P. Mettens for some reagents and for help in primary DC culture. We thank K. Kacem for technical help in animal experiments; C. Pinset, D. Montarras, C. Delouis, D. Paulin, and J. Pagès for kindly providing us with cells and a retroviral vector; and B. Klonjkowski for helpful discussions. We are very grateful to N. Taylor for critical reading of the manuscript.
S.M. was supported by a fellowship from the French Ministère de la Recherche et de l'Industrie, and H.G.-S. was supported by a fellowship from the ANRS (Paris).
REFERENCES
- 1.Acsadi, G., A. Jani, J. Huard, K. Blaschuk, B. Massie, P. Holland, H. Lochmuller, and G. Karpati. 1994. Cultured human myoblasts and myotubes show markedly different transducibility by replication-defective adenovirus recombinants. Gene Ther. 1:338-340. [PubMed] [Google Scholar]
- 2.Agadjanyan, M. G., J. J. Kim, N. Trivedi, D. M. Wilson, B. Monzavi-Karbassi, L. D. Morrison, L. K. Nottingham, T. Dentchev, A. Tsai, K. Dang, A. A. Chalian, M. A. Maldonado, V. W. Williams, and D. B. Weiner. 1999. CD86 (B7-2) can function to drive MHC-restricted antigen-specific CTL responses in vivo. J. Immunol. 162:3417-3427. [PubMed] [Google Scholar]
- 3.Albert, M. L., B. Sauter, and N. Bhardwaj. 1998. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature 392:86-89. [DOI] [PubMed] [Google Scholar]
- 4.Ali, M., N. R. Lemoine, and C. J. Ring. 1994. The use of DNA viruses as vectors for gene therapy. Gene Ther. 1:367-384. [PubMed] [Google Scholar]
- 5.Ambriovic, A., M. Adam, M. Monteil, D. Paulin, and M. Eloit. 1997. Efficacy of replication-defective adenovirus-vectored vaccines: protection following intramuscular injection is linked to promoter efficiency in muscle representative cells. Virology 238:327-335. [DOI] [PubMed] [Google Scholar]
- 6.Batten, P., M. H. Yacoub, and M. L. Rose. 1996. Effect of human cytokines (IFN-γ, TNF-α, IL-1β, IL-4) on porcine endothelial cells: induction of MHC and adhesion molecules and functional significance of these changes. Immunology 87:127-133. [PMC free article] [PubMed] [Google Scholar]
- 7.Cayeux, S., G. Richter, C. Becker, A. Pezzutto, B. Dorken, and T. Blankenstein. 1999. Direct and indirect T cell priming by dendritic cell vaccines. Eur. J. Immunol. 29:225-234. [DOI] [PubMed] [Google Scholar]
- 8.Corr, M., D. J. Lee, D. A. Carson, and H. Tighe. 1996. Gene vaccination with naked plasmid DNA: mechanism of CTL priming. J. Exp. Med. 184:1555-1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cosset, F.-L., Y. Takeuchi, J.-L. Battini, R. A. Weiss, and M. K. Collins. 1995. High-titer packaging cells producing recombinant retroviruses resistant to human serum. J. Virol. 69:7430-7436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Couffinhal, T., M. Kearney, A. Sullivan, M. Silver, Y. Tsurumi, and J. M. Isner. 1997. Histochemical staining following LacZ gene transfer underestimates transfection efficiency. Hum. Gene Ther. 8:929-934. [DOI] [PubMed] [Google Scholar]
- 11.Cui, J., T. Shin, T. Kawano, H. Sato, E. Kondo, I. Toura, Y. Kanedo, M. Koseki, M. Kanno, and M. Taniguchi. 1997. Requirement for Vα14 NKT cells in IL-12-mediated rejection of tumors. Science 278:1623-1626. [DOI] [PubMed] [Google Scholar]
- 12.Dai, Y., E. M. Schwarz, D. Gu, W.-W. Zhang, N. Sarvetnick, and I. M. Verma. 1995. Cellular and humoral immune responses to adenoviral vectors containing factor IX gene: tolerization of factor IX and vector antigens allows for long-term expression. Proc. Natl. Acad. Sci. USA 92:1401-1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dick, L. R., C. Aldrich, S. C. Jameson, C. R. Moomaw, B. C. Pramanik, C. K. Doyle, G. N. DeMartino, M. J. Bevan, J. M. Forman, and C. A. Slaughter. 1994. Proteolytic processing of ovalbumin and beta-galactosidase by the proteasome to yield antigenic peptides. J. Immunol. 152:3884-3894. [PMC free article] [PubMed] [Google Scholar]
- 14.Doe, B., M. Selby, S. Barnett, J. Baenziger, and C. M. Walker. 1996. Induction of cytotoxic T lymphocytes by intramuscular immunization with plasmid DNA is facilitated by bone marrow-derived cells. Proc. Natl. Acad. Sci. USA 93:8578-8583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fidler, I. J. 1975. Biological behavior of malignant melanoma cells correlated to their survival in vivo. Cancer Res. 35:218-224. [PubMed] [Google Scholar]
- 16.Gahery-Segard, H., F. Farace, D. Godfrin, J. Gaston, R. Lengagne, T. Tursz, P. Boulanger, and J.-G. Guillet. 1998. Immune response to recombinant capsid proteins of adenovirus in humans: antifiber and anti-penton base antibodies have a synergistic effect on neutralizing activity. J. Virol. 72:2388-2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gahery-Segard, H., V. Molinier-Frenkel, C. Le Boulaire, P. Saulnier, P. Opolon, R. Lengagne, E. Gautier, A. Le Cesne, L. Zitvogel, A. Venet, C. Schatz, M. Courtney, T. Le Chevalier, T. Tursz, J.-G. Guillet, and F. Farace. 1997. Phase I trial of recombinant adenovirus gene transfer in lung cancer. Longitudinal study of the immune responses to transgene and viral products. J. Clin. Investig. 100:2218-2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ginsberg, H. S. 1996. The ups and downs of adenovirus vectors. Bull. N. Y. Acad. Med. 73:53-58. [PMC free article] [PubMed] [Google Scholar]
- 19.Heath, W., and F. Carbone. 2001. Cross-presentation, dendritic cells, tolerance and immunity. Annu. Rev. Immunol. 19:47-64. [DOI] [PubMed] [Google Scholar]
- 20.Hirschowitz, E. A., J. D. Weaver, G. E. Hidalgo, and D. E. Doherty. 2000. Murine dendritic cells infected with adenovirus vectors show signs of activation. Gene Ther. 7:1112-1120. [DOI] [PubMed] [Google Scholar]
- 21.Hohlfeld, R., and A. G. Engel. 1994. The immunobiology of muscle. Immunol. Today 15:269-274. [DOI] [PubMed] [Google Scholar]
- 22.Horowitz, M. 1996. Adenoviruses, p. 2149-2176. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Fields virology, 3rd ed. Lippincott-Raven Publishers, Philadelphia, Pa.
- 23.Iwasaki, A., C. A. Torres, P. S. Ohashi, H. L. Robinson, and B. H. Barber. 1997. The dominant role of bone marrow-derived cells in CTL induction following plasmid DNA immunization at different sites. J. Immunol. 159:11-14. [PubMed] [Google Scholar]
- 24.Jooss, K., H. C. Ertl, and J. M. Wilson. 1998. Cytotoxic T-lymphocyte target proteins and their major histocompatibility complex class I restriction in response to adenovirus vectors delivered to mouse liver. J. Virol. 72:2945-2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jooss, K., Y. Yang, K. J. Fisher, and J. M. Wilson. 1998. Transduction of dendritic cells by DNA viral vectors directs the immune response to transgene products in muscle fibers. J. Virol. 72:4212-4223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jooss, K., Y. Yang, and J. M. Wilson. 1996. Cyclophosphamide diminishes inflammation and prolongs transgene expression following delivery of adenoviral vectors to mouse liver and lung. Hum. Gene Ther. 7:1555-1566. [DOI] [PubMed] [Google Scholar]
- 27.Juillard, V., P. Villefroy, D. Godfrin, A. Pavirani, A. Venet, and J.-G. Guillet. 1995. Long-term humoral and cellular immunity induced by a single immunization with replication-defective adenovirus recombinant vector. Eur. J. Immunol. 25:3467-3473. [DOI] [PubMed] [Google Scholar]
- 28.Kaplan, J. M., Q. Yu, S. T. Piraino, S. E. Pennington, S. Shankara, L. A. Woodworth, and B. L. Roberts. 1999. Induction of antitumor immunity with dendritic cells transduced with adenovirus vector-encoding endogenous tumor-associated antigens. J. Immunol. 163:699-707. [PubMed] [Google Scholar]
- 29.Klonjkowski, B., C. Denesvre, and M. Eloit. 1999. Adenoviral vectors for vaccines, p. 163-173. In P. Seth (ed.), Adenoviruses: basic biology to gene therapy. R.G Landes Company, Austin, Tex.
- 30.Kundig, T. M., M. F. Bachmann, C. DiPaolo, J. J. Simard, M. Battegay, H. Lother, A. Gessner, K. Kuhlcke, P. S. Ohashi, H. Hengartner, and R. M. Zinkernagel. 1995. Fibroblast as efficient antigen-presenting cells in lymphoid organs. Science 268:1343-1347. [DOI] [PubMed] [Google Scholar]
- 31.Lalvani, A., R. Brookes, S. Hambleton, W. J. Britton, A. V. S. Hill, and A. J. McMichael. 1997. Rapid effector function in CD8+ memory T cells. J. Exp. Med. 186:859-865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lutz, M. B., N. Kukutsch, A. L. J. Ogilvie, S. Roßner, F. Koch, N. Romani, and G. Schuler. 1999. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J. Immunol. Methods 223:77-92. [DOI] [PubMed] [Google Scholar]
- 33.Ma, W., and J. S. Pober. 1998. Human endothelial cells effectively costimulate cytokine production by, but not differentiation of, naive CD4+ T cells. J. Immunol. 161:2158-2167. [PubMed] [Google Scholar]
- 34.Marelli-Berg, F. M., R. E. Hargreaves, P. Carmichael, A. Dorling, G. Lombardi, and R. I. Lechler. 1996. Major histocompatibility complex class II-expressing endothelial cells induce allospecific nonresponsiveness in naive T cells. J. Exp. Med. 183:1603-1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Molinier-Frenkel, V., H. Gahery-Segard, M. Mehtali, C. Le Boulaire, S. Ribault, P. Boulanger, T. Tursz, J. G. Guillet, and F. Farace. 2000. Immune response to recombinant adenovirus in humans: capsid components from viral input are targets for vector-specific cytotoxic T lymphocytes. J. Virol. 74:7678-7682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mosmann, T. R., and S. Sad. 1996. The expanding universe of T-cell subsets: Th1, Th2 and more. Immunol. Today 17:138-146. [DOI] [PubMed] [Google Scholar]
- 37.Murali-Krishna, K., J. D. Altman, M. Suresh, D. J. Sourdive, A. J. Zajac, J. D. Miller, J. Slansky, and R. Ahmed. 1998. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity 8:177-187. [DOI] [PubMed] [Google Scholar]
- 38.Oukka, M., M. Cohen-Tannoudji, Y. Tanaka, C. Babinet, and K. Kosmatopoulos. 1996. Medullary thymic epithelial cells induce tolerance to intracellular proteins. J. Immunol. 156:968-975. [PubMed] [Google Scholar]
- 39.Pimorady-Esfahani, A., M. D. Grounds, and P. G. McMenamin. 1997. Macrophages and dendritic cells in normal and regenerating murine skeletal muscle. Muscle Nerve 20:158-166. [DOI] [PubMed] [Google Scholar]
- 40.Pober, J. S. 1999. Immunobiology of human vascular endothelium. Immunol. Res. 19:225-232. [DOI] [PubMed] [Google Scholar]
- 41.Pulendran, B., E. Maraskovsky, J. Banchereau, and C. Maliszewski. 2001. Modulating the immune response with dendritic cells and their growth factors. Trends Immunol. 22:41-47. [DOI] [PubMed] [Google Scholar]
- 42.Rea, D., F. H. Schagen, R. C. Hoeben, M. Mehtali, M. J. Havenga, R. E. M. Toes, C. J. Melief, and R. Offringa. 1999. Adenoviruses activate human dendritic cells without polarization toward a T-helper type 1-inducing subset. J. Virol. 73:10245-10253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ribas, A., L. H. Butterfield, W. H. McBride, S. M. Jilani, L. A. Bui, C. M. Vollmer, R. Lau, V. B. Dissette, B. Hu, A. Y. Chen, J. A. Glaspy, and J. S. Economou. 1997. Genetic immunization for the melanoma antigen MART-1/Melan-A using recombinant adenovirus-transduced murine dendritic cells. Cancer Res. 57:2865-2869. [PubMed] [Google Scholar]
- 44.Rouard, H., A. Leon, B. Klonjkowski, J. Marquet, L. Tenneze, A. Plonquet, S. G. Agrawal, J. P. Abastado, M. Eloit, J. P. Farcet, and M. H. Delfau-Larue. 2000. Adenoviral transduction of human 'clinical grade' immature dendritic cells enhances costimulatory molecule expression and T-cell stimulatory capacity. J. Immunol. Methods 241:69-81. [DOI] [PubMed] [Google Scholar]
- 45.Sarukhan, A., S. Camugli, B. Gjata, H. von Boehmer, O. Danos, and K. Jooss. 2001. Successful interference with cellular immune responses to immunogenic proteins encoded by recombinant viral vectors. J. Virol. 75:269-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sauter, B., M. L. Albert, L. Francisco, M. Larsson, S. Somersan, and N. Bhardwaj. 2000. Consequences of cell death: exposure to necrotic tumor cells, but not primary tissue cells or apoptotic cells, induces the maturation of immunostimulatory dendritic cells. J. Exp. Med. 191:423-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Soares, M. P., A. Muniappan, E. Kaczmarek, K. Koziak, C. J. Wrighton, F. Steinhauslin, C. Ferran, H. Winkler, F. H. Bach, and J. Anrather. 1998. Adenovirus-mediated expression of a dominant negative mutant of p65/RelA inhibits proinflammatory gene expression in endothelial cells without sensitizing to apoptosis. J. Immunol. 161:4572-4582. [PubMed] [Google Scholar]
- 48.Song, W., H. L. Kong, H. Carpenter, H. Torii, R. Granstein, S. Rafii, M. A. Moore, and R. G. Crystal. 1997. Dendritic cells genetically modified with an adenovirus vector encoding the cDNA for a model antigen induce protective and therapeutic antitumor immunity. J. Exp. Med. 186:1247-1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tripathy, S. K., H. B. Black, E. Goldwasser, and J. M. Leiden. 1996. Immune responses to transgene-encoded proteins limit the stability of gene expression after injection of replication-defective adenovirus vectors. Nat. Med. 2:545-550. [DOI] [PubMed] [Google Scholar]
- 50.Ulmer, J. B., R. R. Deck, C. M. Dewitt, J. I. Donnelly, and M. A. Liu. 1996. Generation of MHC class I-restricted cytotoxic T lymphocytes by expression of a viral protein in muscle cells: antigen presentation by non-muscle cells. Immunology 89:59-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vicart, P., B. Schwartz, A. Vandewalle, M. Bens, C. Delouis, J.-J. Panthier, S. Pournin, C. Babinet, and D. Paulin. 1994. Immortalization of multiple cell types from transgenic mice using a transgene containing the vimentin promoter and a conditional oncogene. Exp. Cell Res. 214:35-45. [DOI] [PubMed] [Google Scholar]
- 52.Wan, Y., P. Emtage, Q. Zhu, R. Foley, A. Pilon, B. Roberts, and J. Gauldie. 1999. Enhanced immune response to the melanoma antigen gp100 using recombinant adenovirus-transduced dendritic cells. Cell. Immunol. 198:131-138. [DOI] [PubMed] [Google Scholar]
- 53.Yang, Y., K. U. Jooss, Q. Su, H. C. Ertl, and J. M. Wilson. 1996. Immune responses to viral antigens versus transgene product in the elimination of recombinant adenovirus-infected hepatocytes in vivo. Gene Ther. 3:137-144. [PubMed] [Google Scholar]
- 54.Zsengeller, Z., K. Otake, S.-A. Hossain, P.-Y. Berclaz, and B. C. Trapnell. 2000. Internalization of adenovirus by alveolar macrophages initiates early proinflammatory signaling during acute respiratory tract infection. J. Virol. 74:9655-9667. [DOI] [PMC free article] [PubMed] [Google Scholar]