Abstract
The human papillomavirus type 18 (HPV-18) E7 protein promotes S-phase reentry in postmitotic, differentiated keratinocytes in squamous epithelium to facilitate vegetative viral DNA amplification. To examine the nature and fate of the differentiated cells that reenter S phase, organotypic cultures of primary human keratinocytes transduced with HPV-18 E7 were pulse-chase-pulse-labeled with 3H-thymidine (3H-TdR) and bromodeoxyuridine (BrdU). The kinetics of the appearance of doubly labeled suprabasal cells demonstrate that E7 expression did not promote prolonged S phase. Rather, there was a considerable lag before a small percentage of the cells reentered another round of S phase. Fluorescence in situ hybridization analysis, indeed, revealed a small fraction of the cells with more than 4n chromosomes in the differentiated strata. Differentiated cells positive for 3H-TdR, BrdU, or both often had enlarged nuclei or were binucleated. These results suggest that S phase is not followed by cell division, although nuclear division may occur. Interestingly, a significant fraction of differentiated cells that entered S phase subsequently accumulated p27kip1 protein with a kinetics preceding the accumulation of cyclin E. We conclude that E7-transduced, differentiated keratinocytes that enter S phase have two alternative fates: (i) a low percentage of cells undergoes endoreduplication, achieving higher than 4n ploidy, and (ii) a high percentage of cells accumulates the p27kip1, cyclin E, and p21cip1 proteins, resulting in arrest and preventing further S-phase reentry.
Human papillomaviruses (HPVs) cause benign proliferative lesions in cutaneous or mucosal epithelia. Infections by certain virus types, such as HPV type 16 (HPV-16) and HPV-18, can also lead to anogenital cancers. Despite the difference in viral pathogenesis, viral DNA amplification and progeny virion production invariably depend on squamous differentiation and occur only in benign warty growths (7). Because viral DNA replication requires the host replication machinery, HPVs induce S-phase reentry in a subset of postmitotic, differentiated keratinocytes (5), which we called unscheduled DNA synthesis. Organotypic cultures of primary human keratinocytes (PHKs) grown at the air-medium interface (abbreviated as raft cultures) resemble closely the native squamous epithelia from which the PHKs were derived (8). As in native skin, only the basal and parabasal cells maintain the ability to divide, whereas cells above are postmitotic and become progressively differentiated by histological and molecular criteria. In these cultures, expression of the HPV-18 E7 gene from its differentiation-dependent promoter located in the upstream regulatory region (URR) recapitulates the unscheduled cellular DNA synthesis observed in benign papillomas caused by the nononcogenic HPV-6 or HPV-11 (5).
The G1-to-S-phase transition during the cell cycle is normally controlled by the retinoblastoma susceptibility protein (pRb), the cyclin-dependent kinases (cdks), and cdk inhibitors. pRb binds to and represses the family of E2F transcription factors. Inactivation of pRb, leading to the activation of E2F factors, is accomplished primarily by cdk4 and cdk6 in complex with type D G1 cyclins (16). E2Fs control the transcription of a number of cellular genes necessary for S-phase entry and progression, including DNA polymerase α, ribonucleotide reductase, dihydrofolate reductase, thymidine (TdR) kinase, cyclin E, cyclin A, and others (16). The cyclin E/cdk2 kinase is critical for S-phase entry and the initiation of cellular DNA replication (17, 31), and the substrates include proteins regulating cell cycle control and proteins related to DNA replication, such as pRb (16), p27kip1 (35), CDC7 (26), E2F-5 (28), cyclin E (43), subunits of DNA polymerase α (42), and p220NPAT (25, 45). The E7 protein bypasses the requirement for type D cyclin/cdks for S-phase entry, as it binds to and inactivates the hypophosphorylated form of pRb and promotes its degradation (2-4, 21). HPV-18 E7 mutations that do not bind to pRb are incapable of inducing unscheduled DNA synthesis in epithelial raft cultures (6). Interestingly, the papillomavirus-encoded DNA helicase E1 and the primary origin recognition protein E2 are substrates of various cdks in vitro (9, 24). In particular, phosphorylation of E1 by cyclin E/cdk2 is critical for efficient initiation of HPV DNA replication (23).
The kinase activities of cyclin E/cdk2 and cyclin A/cdk2 are inhibited effectively following association with p21cip1 or p27kip1 (37). The p21cip1 mRNA is constitutively expressed in several postmitotic differentiated tissues (10, 12, 14, 15, 33, 34). We have previously shown that the p21cip1 mRNA is up-regulated independently of p53 in differentiated keratinocytes in normal skin, in HPV-caused papillomas, in normal PHK raft cultures, and in raft cultures of PHKs transduced with HPV-18 URR-E7. However, the p21cip1 protein is very unstable and undetectable in situ except in a subset of the postmitotic, differentiated cells in papillomas, in low-grade dysplasias, and in HPV-18 URR-E7-transduced raft cultures (18, 34). Furthermore, high-level p21cip1 protein colocalizes with cyclin E accumulation and vice versa, whereas cyclin E is undetectable in normal skin or raft cultures. The differentiated cells that accumulated unusually high levels of cyclin E or p21cip1 protein were not in S phase. Conversely, differentiated cells that reentered S phase did not accumulate cyclin E or p21cip1 protein (19).
We have also shown recently by in situ analysis that, unlike p21cip1, p27kip1 protein is stably expressed in many differentiated keratinocytes of the foreskin, of patient papillomas, and of raft cultures in the absence or presence of E7 and that its signal increases with differentiation in the upper strata (30). However, while all cyclin E-positive cells are positive for p27kip1, as well as p21cip1, only some of the p27kip1-positive cells are positive for cyclin E. Furthermore, none of p27kip1-positive cells was in S phase after a brief exposure of the papillomas or E7 raft cultures to BrdU in vitro. We have suggested that the normal differentiation-dependent expression of p27kip1 leads to the accumulation of cyclin E induced by E7 in an inactive complex with cdk2 when the level of the p27kip1 protein is relatively high. The stabilization and accumulation of cyclin E, in turn, prevent the degradation of the p21cip1 protein by establishing an equilibrium between the kinase-inactive complexes cyclin E/cdk2/p21cip1 and cyclin E/cdk2/p27kip1. The absence of active cyclin E/cdk2 then results in failure of S-phase reentry by the differentiated keratinocytes. Conversely, cells that have little or no p27kip1 protein can reenter S phase after E7 inactivates pRb and reactivates S-phase genes, including cyclin E. Cyclin E is subsequently down-regulated upon S-phase entry (36). Thus, neither p27kip1 nor cyclin E/p21cip1 accumulates in these cells (19, 30).
Several questions remain concerning the fate of E7-transduced postmitotic cells. In particular, do the postmitotic cells ever exit the E7-induced S phase? Do these cells divide or become polyploid? Do cells in which unscheduled DNA has taken place eventually accumulate p21cip1, p27kip1, and cyclin E, thus preventing further S-phase reentry? Conversely, can E7 overcome the accumulation of cdk inhibitors and reactivate S phase? In this study, we investigated these issues by using several sets of pulse and chase experiments. We also examined chromosome ploidy by fluorescence in situ hybridization (FISH) assay. Collectively, our results show that postmitotic cells expressing HPV-18 E7 have alternative fates. They either reenter S phase or accumulate p27kip1, cyclin E, and p21cip1 proteins and are thus prevented from doing so. Furthermore, cells in which unscheduled DNA synthesis did occur do not appear to divide. Rather, following a considerable lag, a small fraction of these cells endoreduplicate by reentering another round of S phase, whereas the majority become arrested as they accumulate p27kip1, cyclin E, and p21cip1 during differentiation.
MATERIALS AND METHODS
Recombinant retroviruses and antibodies.
Recombinant retroviruses containing vector only or HPV-18 URR-E7 have been described (5, 6). Antibodies used for immunostaining were purchased as follows: cyclin E (HE-12), Pharmingen, San Diego, Calif.; p21cip1/waf1 (OP-64), Calbiochem, La Jolla, Calif.; p27kip1, Vector, Burlingame, Calif.; bromodeoxyuridine (BrdU), Zymed, South San Francisco, Calif.; goat anti-mouse immunoglobulin G (IgG)-Texas red and BrdU-fluorescein isothiocyanate (FITC), Roche, Indianapolis, Ind.
Epithelial raft cultures.
Primary human foreskin keratinocytes were isolated and acutely infected with amphotropic, recombinant retroviruses. After a 2-day selection with G418, the bulk surviving PHKs were used to develop raft cultures that were then fixed in formalin and embedded in paraffin as previously described (32). Exposure to nucleoside analogues was performed as described previously (22), with modifications. The schemes are illustrated in each figure, and the nomenclature of each culture is defined in the text. Briefly, for experiments whose results are shown in Fig. 1A, 4A, and 5A, raft cultures were pulse-labeled with 3H-TdR (Amersham Pharmacia Biotech, Piscataway, N.J.) at 15 μCi/ml (3 μM) for 6 h at 6-h intervals, chased for different periods, and then exposed to BrdU at 50 μg/ml for the final 6 h immediately before harvest. For the last two experiments described in Fig. 1A, raft cultures were exposed to 3H-TdR at 7.5 μCi/ml on day 8 for 12 h, chased for different durations, and then exposed to BrdU at 50 μg/ml for various lengths of time before harvest. All raft cultures were harvested at the same time on day 10. Media were changed every 6 h in the experiments whose results are described in Fig. 1, 4, and 5, except for the last two experiments described in Fig. 1A, where the media were changed every 12 h.
FIG. 1.
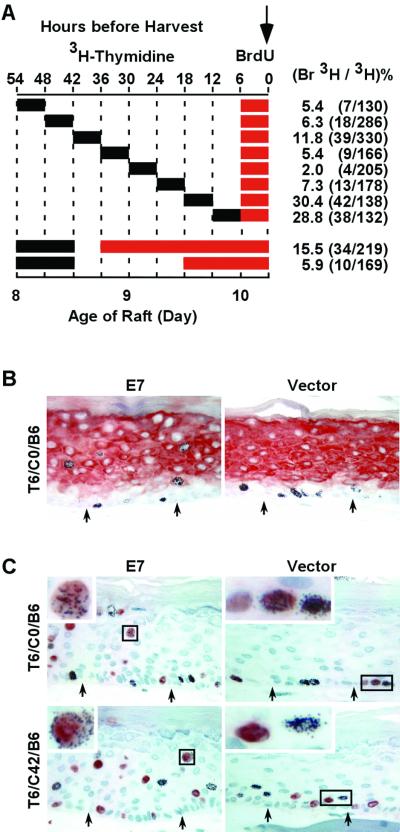
HPV-18 URR-E7 does not prolong S phase in differentiated keratinocytes of raft cultures. (A) Schematic diagram of pulse-chase-pulse experiments with two TdR analogues. Cultures were pulsed with 3H-TdR for 6 or 12 h as indicated, chased for different durations, and then exposed to BrdU for the last 6 h or longer before harvest (downward arrow). The values at the top indicate hours before harvest, whereas those below indicate the ages of raft cultures in days after they were raised to the air-medium interface. The incorporation of BrdU and 3H-TdR was detected by immunohistochemistry (in red) and autoradiography (black dots), respectively. The percentages and numbers (in parenthesis) of doubly labeled cells among all 3H-TdR-positive cells in the differentiated strata are shown on the right. Positive cells along the entire section were scored. (B) Immunostaining of keratin 1 and autoradiography of 3H-TdR in T6/C0/B6 raft cultures. (C) Example of cells positive for 3H-TdR, BrdU, or both in T6/C0/B6 and T6/C42/B6 cultures.
Immunohistochemistry and autoradiography.
Four-micrometer sections were immunostained with antibodies against BrdU (1:100), cyclin E (1:25), p27kip1 (1:20), and p21cip1 (1:20) as previously described (6); counterstained with hematoxylin; rinsed with water for 30 min; air dried; dipped into Kodak NTB-2 liquid radiographic emulsion; exposed for 7 to 10 days; and finally mounted with Aqua-Mount (Lerner Laboratories, Pittsburgh, Pa.). Images were captured with an Olympus BH2 microscope using a SPOT camera (Diagnostic Instruments) and processed with Adobe Photoshop.
FISH assay.
For the FISH assay, 5-μm (see Fig. 3A) or 10-μm (see Fig. 3C) sections of cultures that were initially labeled with BrdU were used. Hybridization with chromosome 17 (D17Z1, FITC conjugated) or chromosome X (DXZ1, cy3 conjugated) pericentromeric probes (Vysis, Downers Grove, Ill.) was performed in accordance with the manufacturer's instructions. The slides for detection of chromosome 17 were subsequently probed with an anti-BrdU antibody (1:100) and a secondary antibody conjugated with Texas red (1:100). The slides for detection of chromosome X were probed with an FITC-conjugated anti-BrdU antibody (1:100) without further signal amplification. Slides were mounted with Vectashield containing 4′,6′-diamidino-2-phenylindole (DAPI; Vector) to reveal nuclear DNA. Images were viewed or captured with a single-, double-, or triple-pass filter with a 100× oil immersion objective lens in an Olympus Provix microscope equipped with an Olympus 2000 camera.
FIG. 3.
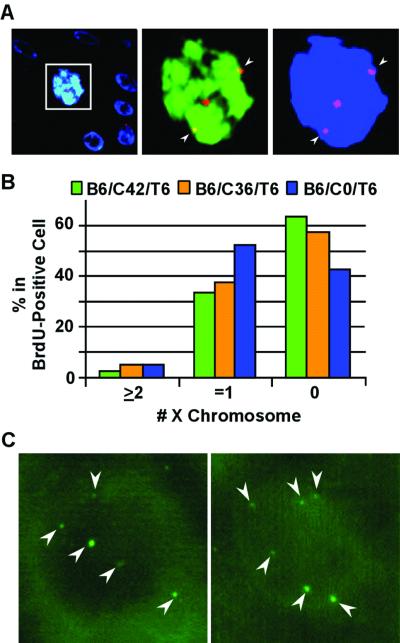
HPV-18 E7 induces endoreduplication in differentiated keratinocytes. Fluorescent images were taken from the differentiated strata in E7-transduced cultures. The labeling scheme is similar to that depicted in Fig 1A (top rows), except that the order of exposure to TdR analogues was reversed. FISH for panels A and B was conducted with cy3-conjugated, pericentromeric probes specific for the X chromosome (red), while BrdU was revealed with an FITC-conjugated anti-BrdU antibody (green) and nuclei were revealed with DAPI (in blue) in a 5-μm section. (A) B6/C42/T6 culture images were captured with a triple-pass filter (left) or with dual filters for the X chromosome and BrdU (middle) or for the X chromosome and the nucleus (right). Also note that the boxed nucleus is significantly larger than the nuclei of surrounding cells. (B) Percentages of differentiated cells containing 0, 1, or 2 or more X chromosome copies in BrdU-positive cells were scored in three E7-transduced cultures, B6/C42/T6, B6/C36/T6, and B6/C0/T6. (C) A FISH assay was conducted with fluorescein-conjugated pericentromeric probes of chromosome 17. Examples of six dots in a BrdU-negative nucleus from the B6/C36/T6 culture are shown. Weaker signal dots were not in focus because they were situated at different focal planes than the strong-signal dots. Arrowheads point to the pericentromeric signals.
Data analysis.
Some of the cells in the proliferating lower strata (basal, parabasal, and daughter cells) moved upward in the course of the pulse-chase-pulse experiments. In the E7 cultures, only 3H- or BrdU-positive cells located higher than the positive layers observed in the corresponding control raft cultures were counted in deriving the percentages of doubly positive cells. Positive cells were counted along the entire tissue section with an Olympus BH2 microscope with a 40× objective lens. The percentage was calculated as the number of differentiated cells that were positive for both 3H-TdR and BrdU, for both 3H-TdR and cyclin E, or for p27kip1 divided by the total number cells positive for 3H-TdR (usually 100 or more cells) in the differentiated strata. The X chromosome copy number in BrdU-positive cells in the differentiated strata was determined with a 100× oil immersion objective lens with a dual-pass filter, scanning along the entire length of the raft cultures.
RESULTS
To monitor the dynamics of cellular DNA synthesis or the accumulation of differentiation-dependent p27kip1 or E7-induced cyclin E in differentiated keratinocytes, we designed several pulse-chase-pulse experiments using 3H-TdR followed by BrdU or, alternatively, BrdU followed by 3H-TdR, as described for each individual experiment. By using a combination of immunohistochemistry and autoradiography, cells positive for either marker or both markers were counted. In other experiments, the synthesis of keratin 1 or the accumulation of p27kip1, cyclin E, or p21cip1, along with 3H-TdR incorporation, was counted or visualized.
Double labeling does not disturb the growth and differentiation of primary keratinocytes in raft cultures.
Raft cultures were pulsed, chased, and then pulsed with different analogues as shown in Fig. 1A. Briefly, over a period of 54 h prior to harvest on day 10, raft cultures were pulsed with 3H-TdR for 6 h, chased for different time intervals ranging from 0 to 42 h in 6-h increments, and then exposed to BrdU for the final 6 h. These cultures were designated Tx/Cy/Bz, where T, B, and C denote exposures to 3H-TdR and BrdU and the chase between them, respectively, while x, y, and z represent the durations, in hours, of the treatments. For instance, T6/C12/B6 represents a pulse with 3H-TdR for 6 h, a chase of 12 h, and a 6-h exposure to BrdU for a total of 24 h prior to harvest.
To ascertain that the exposure to two nucleoside analogues does not cause significant anomalies in cell growth and differentiation at the concentrations used, we examined the distribution of cells positive for these analogues relative to each other and to the squamous differentiation marker keratin 1. In the empty-vector-transduced cultures, keratin 1 was always restricted to the differentiated layers in the presence or absence of exposure to the TdR analogue (Fig. 1B, right side, data not shown). In cultures with no chase or with a short chase, such as T6/C0/B6 and T6/C6/B6, only basal and parabasal cells were labeled with 3H-TdR. Some positive cells were also found in the second and third strata in T6/C12/B6, T6/C18/B6, and T6/C24/B6 cultures. With a longer chase, such as T6/C30/B6, T6/C36/B6, and T6/C42/B6, some 3H-TdR-positive cells were found in the third or fourth layer from the bottom due to upward movement by some basal cells, parabasal cells, or their daughter cells upon cell division during the chase (Fig. 1C, right side, and data not shown). However, in all cases, the cells positive for BrdU were confined to the basal and parabasal strata, where cellular proliferation takes place under normal conditions (Fig. 1C, right side). These patterns of 3H-TdR- and BrdU-labeled cells relative to keratin 1 expression demonstrate that exposure to these analogues did not disturb the proliferation and differentiation programs of the keratinocytes. Similar conclusions were reached when the order of exposure to the two TdR analogues was reversed (data not shown).
E7 expression in differentiated keratinocytes does not prolong S phase.
In E7-transduced cultures treated in parallel with the vector-only cultures just described (Fig. 1A, top rows), the pattern of keratin 1 was similar to that in the control cultures (Fig. 1B, left side). However, cells positive for 3H-TdR were sporadically distributed throughout all of the strata, including those positive for keratin 1, regardless of the duration of the chase period. Similarly, cells positive for BrdU were also observed in all of the strata (Fig. 1C). These observations are consistent with the stochastic S-phase reentry induced by E7 in differentiated keratinocytes and also demonstrate that S-phase entry did not disturb keratin 1 expression, in agreement with our previous conclusion based on assays performed on adjacent tissue sections (5).
We then determined the percentages of 3H-TdR and BrdU doubly labeled cells among all of the 3H-TdR-positive cells in the differentiated strata, as described in Materials and Methods. The results are summarized in Fig. 1A (top rows), and examples are shown in Fig. 1C, left side. In these experiments, colocalization of 3H-TdR and BrdU in E7 cultures was up to 30.4% when there was no chase or a short chase between the two pulses (T6/C0/B6 and T6/C6/B6). We suggest that many, perhaps most, of the doubly labeled cells incorporated these TdR analogues while they were in the same S phase. The percentage of such cells decreased sharply with time when the chase period increased, and only 2.0% of doubly labeled cells were detected in the T6/C18/B6 culture. However, when the chase was lengthened further, there was a small increase in doubly labeled cells, reaching 11.8% in the T6/C30/B6 culture. These observations suggest that most of the E7-transduced differentiated keratinocytes are not in a continuous or prolonged S phase but that there is an approximately 24-h lag between the two S phases. It was not possible to interpret experiments with longer chases because of the migration of 3H-TdR-positive cells from the lower strata to the mid and upper epithelial strata and the anticipated loss of labeled cells in the superficial layers to cornification.
Extended exposure to BrdU following 3H-TdR incorporation does not increase doubly labeled cells in differentiated strata.
To verify that E7 expression does not trigger a continuous or prolonged S phase in differentiated keratinocytes, raft cultures were pulsed with 3H-TdR for 12 h, chased for 6 h, and then continuously labeled with BrdU for 36 h until harvesting on day 10 (designated T12/C6/B36) (Fig. 1A, second row from the bottom). If the E7-transduced cells remained in a prolonged S phase, the great majority of the 3H-TdR-positive cells should also be BrdU positive. However, only 15.5% of the 3H-TdR-positive cells were also positive for BrdU. This value decreased to 5.9% in T12/C24/B18, as indicated in Fig. 1A, bottom row. Therefore, about 9.6% of the 3H-TdR-positive cells exited the S phase during an 18-h period. These results support the conclusion that E7-transduced, differentiated cells are not maintained in a continuous or prolonged S phase.
E7 induces binucleated cells in differentiated strata.
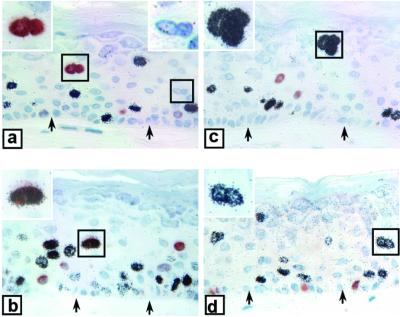
Pathognomonic features of benign papillomas and condylomas include the presence of cells with an increased nucleus-to-cytoplasm ratio, as well as binucleated cells. We believe that these phenotypes can be attributed to unscheduled DNA synthesis by differentiated cells in the absence of cytokinesis. We note that, in our raft cultures, many of the differentiated cells positive for BrdU or 3H-TdR appeared to have an enlarged nucleus or were binucleated (Fig. 2). In contrast, no such cells were observed in the control cultures (data not shown). However, because the tissues were sectioned through the nuclei at different planes, the sizes of nuclei vary so that we cannot estimate accurately the fraction of cells with an enlarged nucleus. But 4 to 11% of the cells positive for 3H-TdR or BrdU in the differentiated strata of E7-transduced cultures were binucleated. Some binucleated cells did not incorporate either nucleoside, because their S phase did not overlap with the exposure to either TdR analogue (Fig. 2a). Relatively few doubly positive, binucleated cells were observed, but one example is shown in Fig. 2b. This frequency is low probably because only a low percentage of the differentiated cells were doubly labeled (Fig. 1A) and only a small fraction of these underwent nuclear division. Occasionally, there appeared to be trinucleated cells in the differentiated strata (Fig. 2c) (with the caveat that a portion of the cells was undoubtedly cut away in the 4-μm section). In the example shown in Fig. 2c, all three nuclei were positive for 3H-TdR. The existence of enlarged nuclei or binucleated cells is consistent with the occurrence of endoreduplication.
FIG. 2.
E7 induces multinucleated cells in the suprabasal strata of raft cultures. Examples of binucleated and trinucleated cells in E7-transduced cultures. All cells were positive for 3H-TdR or BrdU, except those in panel b, which were positive for both. In panel a, one of the two binucleated cells did not incorporate any nucleoside analogue. Insets are enlarged views of selected nuclei (boxed). Arrows point to the basal stratum.
Polyploidy is detected in E7-transduced raft cultures.
As an alternative approach to investigate of whether E7 induces endoreduplication in differentiated keratinocytes or, alternatively, S phase is followed by cytokinesis, we performed FISH assays on 5- or 10-μm raft culture sections by using pericentromeric probes of chromosome 17 or X. For these experiments, we used a set of cultures that were initially pulsed for 6 h with BrdU, chased, and then exposed to 3H-TdR again for 6 h before harvest (a labeling order that is the reverse of that depicted in Fig. 1A, top rows). Since 3H-TdR incorporation is not relevant in these FISH experiments, it will not be further discussed. The reason for choosing this set of cultures was to perform doubled immunofluorescence assays on cells that had ample time to arrive at their fate, whatever it might be.
Five-micrometer sections of E7-transduced cultures harvested 6 h (B6/C0/T6), 42 h (B6/C36/T6), or 48 h (B6/C42/T6) after the BrdU pulse were hybridized with X chromosome probes and then reacted with anti-BrdU antibody (Fig. 3A). For each sample, about 200 BrdU-positive nuclei in the differentiated layers were examined for the number of FISH signals along the entire section. One signal dot was expected if the X chromosome had not yet replicated, while two or more dots were anticipated if it had replicated once or more than once without cytokinesis. However, 40 to 60% of the cells in the upper layers (above the third or fourth layer from the basal layer in B6/C36/T6 and B6/C42/T6 and above the second layer from the basal cells in B6/C0/T6) had no X chromosome signal, indicating a detection efficiency of no better than approximately 50%. This low efficiency of detection was, at least in part, attributable to the loss of a portion of the nucleus (10 μm or larger in diameter) in the 5-μm sections. Nevertheless about 40% of the cells had one signal dot and 2.1 to 4.7% had two or three dots (Fig. 3B), consistent with the presence of polyploid cells. For comparison, about 0.5% of the basal cells had two dots, indicative of actively cycling cells in which G2/M phases are short. The signals were only observed in the nuclei of the keratinocytes, and there was no signal in the mouse 3T3 feeder fibroblasts, demonstrating the specificity of the probe (data not shown).
We then probed 10-μm sections from the B6/C36/T6 culture with a pericentromeric probe of chromosome 17. In the vector-transduced cultures, no nucleus in the differentiated strata contained more than two chromosome 17 dots. In the E7-transduced cultures, the majority of the differentiated cells contained two chromosome 17 dots. Some had three dots, and a few had as many as six dots (Fig. 3C). However, we were not able to conduct statistical analysis of the data from these experiments because it was not possible to determine the number of signals through the focal planes of the thick tissue sections along their entire length without quenching the signals. Nevertheless, these observations are consistent with our interpretation that E7 induces endoreduplication in a small fraction of the differentiated cells.
Delayed accumulation of cyclin E and p21cip1 after unscheduled DNA synthesis in E7-transduced raft cultures.
The above-described experiments indicated that only a small fraction of the postmitotic differentiated cells endoreduplicate to generate more than 4n ploidy, while the majority of the cells either did not enter S phase or did so only once. What might be the reasons? We have previously shown that active unscheduled cellular DNA synthesis takes place only in differentiated cells that do not accumulate abnormally high levels of a kinase-inactive cyclin E/cdk2/p21cip1 or cyclin E/cdk2/p27kip1 complex (18, 19, 30). It is then conceivable that the majority of cells that have entered S phase subsequently accumulate high levels of the cyclin E and p21cip1 proteins, which would inhibit further rounds of S-phase reentry. To test this hypothesis, we studied the kinetics of cyclin E accumulation in cells that had at least one E7-induced S phase, as determined by the incorporation of 3H-TdR, in the raft cultures described in Fig. 1A (also shown in Fig. 4A).
FIG. 4.
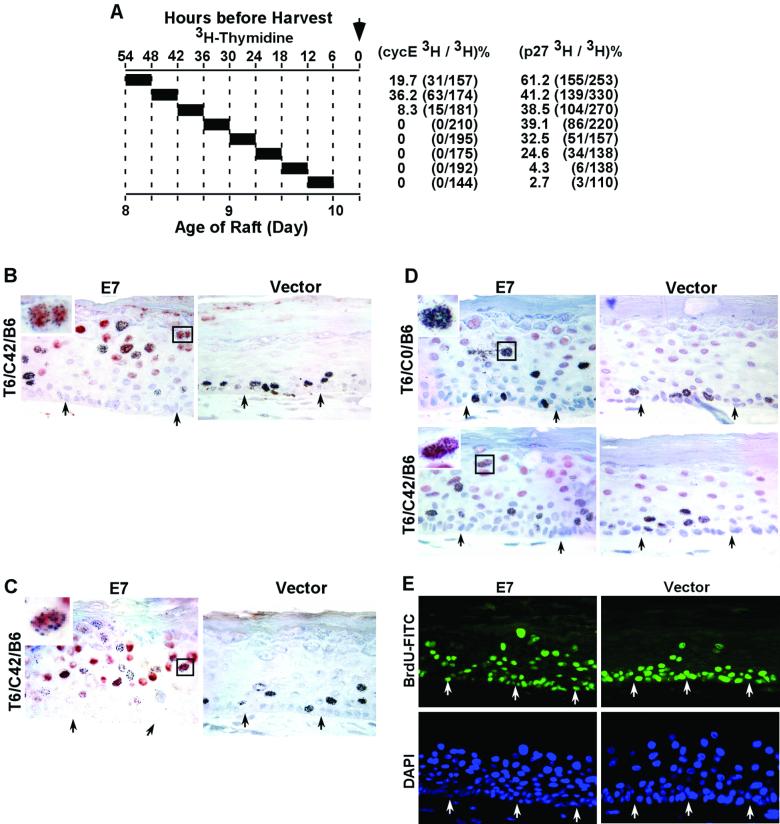
Delayed accumulation of abundant p27kip1, cyclin E, and p21cip1 proteins in HPV-18 URR-E7-transduced, differentiated keratinocytes that have reentered S phase prevents further rounds of S phase. The cultures were those described in Fig. 1A (top), except that BrdU was not assayed in these experiments. Sections were immunostained for the protein of interest (in red) and then autoradiographed to reveal 3H-TdR incorporation. (A) 3H-TdR labeling scheme and the percentage of cells positive for both 3H-TdR and cyclin E or p27kip1 among 3H-TdR-positive cells in the differentiated strata. (B) Cyclin E immunostaining in control and E7-transduced raft cultures. Immunostaining of p21cip1 (C) and p27kip1 (D) in control and E7-transduced cultures. The insets in panels B and D are enlarged images of selected (boxed) binucleated, doubly positive cells. The inset in panel C is an enlarged view of the selected (boxed), doubly positive cell with an enlarged nucleus. (E) Images of control and E7-transduced raft cultures that had been exposed to BrdU continuously for 96 h. BrdU incorporation was revealed by FITC-conjugated anti-BrdU (green), and nuclei were stained by DAPI (blue). Arrows in panels B through E point to the basal stratum.
Cyclin E was detected only in the E7-transduced cultures and not in the control culture. In the T6/C0/B6, T6/C6/B6, T6/C12/B6, T6/C18/B6, and T6/C24/B6 cultures, cyclin E was found only in differentiated cells that were negative for 3H-TdR (data not shown), in agreement with our previous report (19). Colocalization of 3H-TdR and cyclin E began in the T6/C30/B6 culture and reached a maximum of 36.2% in the T6/C36/B6 culture (Fig. 4A and B). These data are also summarized in Fig. 5A. As expected from the colocalization of cyclin E and p21cip1 (19), 3H-TdR and p21cip1 colocalization was also observed in T6/C30/B6, T6/C36/B6, and T6/C42/B6 cultures but not in cultures with shorter chases (Fig. 4C; data not shown). These observations demonstrate that abnormally high levels of cyclin E and p21cip1 indeed accumulate in cells that have previously experienced unscheduled DNA synthesis, but detectable accumulation occurred only after a time lag of at least 30 to 36 h.
FIG. 5.
Alternative cell fates of HPV-18 URR-E7-transduced keratinocytes during squamous differentiation. (A) Kinetic profiles of percentages of differentiated keratinocytes doubly positive for 3H-TdR and BrdU (open triangles), for 3H-TdR and cyclin E (filled squares), or for 3H-TdR and p27kip1 (open circles) in E7-transduced cultures. The data were taken from Fig. 1A, and 4A. (B) Proposed model of alternative paths induced by HPV-18 E7 in differentiated keratinocytes. Lightly shaded ovals represent nuclei capable of entering S phase, whereas filled ovals represent nuclei accumulating abundant p27kip1 or cyclin E/p21cip1 as well. The size of the nucleus reflects its DNA content. Nuclear division without cytokinesis generates binucleated cells. Attrition of cells to the lower path leads to low and decreasing percentages of cells capable of entering into one or more rounds of S phase.
Accumulation of p27kip1 precedes that of cyclin E following E7-induced unscheduled DNA synthesis.
We then investigated whether p27kip1 accumulates in 3H-TdR-positive cells in the differentiated strata and whether this accumulation precedes that of cyclin E and p21cip1, as we previously proposed (30). Tissue sections from cultures described in Fig. 4A were used. In agreement with our previous observation, in normal control raft cultures, p27kip1 was detected in many postmitotic, differentiated cells but not in 3H-TdR-positive cells in the basal and parabasal strata (Fig. 4D, right side). The results obtained with E7 cultures are summarized in Fig. 5A. Six or 12 h after the termination of 3H-TdR exposure, fewer than 5% of 3H-TdR-positive, differentiated keratinocytes were also positive for p27kip1. Colocalization was dramatically increased with longer chases, 24.6% in T6/C12/B6 cells and up to 61.2% in T6/C42/B6 cells (Fig. 4A and D, left side). Thus, accumulation of p27kip1 preceded that of cyclin E (Fig. 4A and B and 5A) and p21cip1 (Fig. 4C and data not shown) in cells that had previously experienced unscheduled DNA synthesis. These results are consistent with our hypothesis that stabilization and accumulation of the cyclin E and p21cip1 proteins are secondary to inhibition of S-phase entry by the stable, endogenous p27kip1 protein (30).
Prolonged BrdU exposure does not significantly increase the fractions of S-phase cells in the differentiated strata.
Our experiments described so far did not directly address the fate of cells that accumulated high concentrations of p27kip1, p21cip1, and cyclin E. Could they reenter S phase, or are they arrested permanently? We used a different strategy to probe this issue. We exposed cultures to BrdU continuously for the last 96 h before harvest on day 10 and stained them with an FITC-conjugated anti-BrdU antibody. Our reasoning was the following. The majority of the cells in the differentiated strata are usually positive for p27kip1 or additionally for cyclin E/p21cip1 in E7-expressing cultures (18, 19, 30) (Fig. 4B). Had E7 been able to overcome the inhibitory effect of one or both cdk inhibitor proteins within the 96-h period and reenter S phase as reported in cycling cells (13, 20), we would have expected a substantial increase in the percentage of BrdU-positive cells in the differentiated strata. However, the pattern of BrdU incorporation remained heterogeneous in suprabasal nuclei in different regions along the entire length of the tissue section, ranging from a few percent to 30% (Fig. 4E), not significantly different from the results seen with a shorter duration of BrdU exposure (Fig. 1 and 2). In the control culture, a few spinous cells were also positive for BrdU due to upward movement of differentiated cells. In contrast, in the control and E7-transduced cultures, the majority of the basal and parabasal cells were positive for BrdU, as expected of cycling basal and parabasal cells (Fig. 4E). These observations are consistent with the interpretation that, within the time frame of this experiment, high levels of p27kip, p21cip1, and cyclin E in differentiated cells effectively prevent S-phase entry. This conclusion agrees with our observation that the level of p27kip1 protein does not decrease in E7-transduced raft cultures relative to that in control cultures (30), as one might have expected had E7 overcome the inhibitory effect of p27kip1 by activating cyclin E/cdk2, which would have then phosphorylated p27kip1, leading to its degradation (35).
DISCUSSION
Expression of E7 in differentiated keratinocytes promotes S-phase entry in only a subset of cells, while a separate subset accumulates high levels of the p27kip1, cyclin E, and p21cip1 proteins. Similarly, most of the differentiated cells containing high copy numbers of HPV DNA in papillomas or condylomas usually do not have abundant cyclin E or p21cip1, suggesting that the high levels of cyclin E/p21cip1 inhibit the amplification of both host and viral DNAs (18, 19, 30). In this study, we investigated the fate of E7-transduced PHKs during squamous differentiation by following the kinetics of S-phase reentry by using sequential exposure to 3H-TdR and BrdU, as well as the kinetics of cyclin E and p27kip1 accumulation in 3H-TdR-positive differentiated cells (Fig. 5A). Our results indicate that E7 does not increase the duration of or prevent exit from S phase. In fact, two consecutive E7-induced S phases were separated by about 18 to 24 h (Fig. 1A). Furthermore, after exiting the first S phase induced by E7, the postmitotic, differentiated cells had one of two alternative fates: the majority stayed tetraploid because of arrest by the abundant p27kip1, cyclin E, and p21cip1 proteins that subsequently accumulated, whereas the minority endoreduplicated by reentering another round of S phase.
Several pieces of evidence led to the above conclusions. First, pulse-chase-pulse experiments demonstrated that a small fraction of the differentiated cells that were positive for 3H-TdR later became positive for BrdU, indicating more than one round of S-phase reentry (Fig. 1A). Second, chromosome ploidy studies showed that, in the differentiated strata, a low percentage of individual nuclei had up to six copies of chromosome 17 and two or more copies of X chromosomes, consistent with two or more rounds of S-phase reentry without cytokinesis (Fig. 3). Third, compared to those of control cultures, nuclei in some of the differentiated cells were enlarged. An enlarged nucleus is expected from a DNA content higher than 2n. Moreover, among the differentiated cells positive for either nucleoside analogue, 4 to 11% were multinucleated, indicating polyploidy and nuclear division without cytokinesis (Fig. 2). In vivo, both binucleated cells and an increase in the nucleus-to-cytoplasm ratio are pathognomonic changes for HPV-induced benign papillomas and condylomas (27). Lastly, a high percentage of 3H-TdR positive cells in the differentiated strata eventually accumulated high levels of p27kip1, followed by cyclin E and p21cip1 (Fig. 4 and 5A).
The normal G2/M transition is regulated by cyclin A/cdc2 and cyclin B/cdc2. Polyploidy naturally occurs in maize endosperm, Drosophila salivary glands, and mammalian trophoblasts and megakaryocytes and is accompanied by a reduction in cyclin B/cdc2 activity (11). However, the mechanism leading to endoreduplication is not well understood. Recent studies showed that pRb-deficient proliferating cells undergo endoreduplication when there is an elevated level of p21cip1 or p27kip1 (1, 29, 40). Similarly, E7 abrogation of the mitotic-spindle checkpoint leading to polyploidy has been attributed to the elimination of the pRb function and up-regulation of the p21cip1 protein (41). The conditions under which E7 induces endoreduplication in differentiated keratinocytes have both of the above features, as these cells are phenotypically pRb deficient while constitutively expressing p21cip1. Moreover, as the cells differentiate, p27kip1 is stably made and may also play an important role in promoting this outcome.
We noted that the percentage of polyploid cells, as detected by FISH, is low compared with that of cells doubly positive for 3H-TdR and BrdU. There are several possible explanations for the inefficient detection of target chromosomes. First, portions of nuclei are invariably sectioned off in the 5- or 10-μm sections used, especially when the nuclei are enlarged due to a higher DNA content. A second reason is that the FISH assay measures DNA content per DAPI-stained nucleus. However, up to 11% of differentiated cells positive for either nucleoside analogue are multinucleated. This population of polyploid cells was counted as diploid in the FISH assay. It is also possible that the particular chromosomes did not replicate to completion by the time of harvest. As the centromere is the last region of a chromosome to be duplicated, incomplete replication would then result in signals that underestimate the true DNA content when pericentromeric probes are used. Similar low detection efficiencies of polyploidy have been reported in patient specimens and in raft cultures transduced with HPV-18 E7 or HPV-18 genome DNA using probes for other chromosomes (38, 39). The highly keratinized raft cultures prevented efficient recovery of individual cells after enzymatic digestion and consequently we were unable to quantify accurately the percentages of cells with 4n or higher ploidy by fluorescence-activated cell sorter analysis.
Most keratinocytes stably express the p27kip1 protein as they differentiate, regardless of the presence or the absence of E7. In p27kip1-positive cells in E7-transduced raft cultures and in patient papillomas, unscheduled DNA synthesis does not occur (30). The observation that postmitotic cells that had reentered S phase subsequently accumulated p27kip1 and cyclin E (Fig. 5A) suggests that the amount of E7 protein in individual cells is not the only determinant of cell fate. Rather, what matters is the amount of p27kip1, which increases during differentiation. Furthermore, the observation that p27kip1 accumulation precedes that of cyclin E supports our previous hypothesis that the normal differentiation-dependent p27kip1 accumulation leads, in the presence of E7, to the accumulation of kinase-inactive cyclin E/cdk2, which, in turn, stabilizes the otherwise unstable p21cip1 protein. Taken together, these observations strongly suggest that E7-induced unscheduled DNA synthesis is arrested by high levels of the p27kip1 and p21cip1 proteins (30).
In HPV-induced papillomas, condylomas, and low-grade dysplasia, some koilocytes, which may contain high numbers of HPV DNA copies, were also found to be positive for cyclin E (38) or p21cip1 (44). However, our experiments with raft cultures or patient specimens clearly demonstrate that cells with active DNA replication at the time of tissue fixation did not simultaneously have detectable levels of cyclin E, p21cip1, or p27kip1 protein (19, 30). Our kinetic studies have now provided a probable interpretation of these seemingly contradictory observations. We suggest that, in patient specimens, viral DNA amplification preceded the accumulation of cyclin E or p21cip1 protein.
On the basis of this study and our previous observations in vivo and in vitro (18, 19, 30, 34), we propose that postmitotic, differentiated keratinocytes that express HPV-18 E7 face alternative fates (Fig. 5B): to enter S phase and become tetraploid with one or two nuclei or to accumulate high levels of p27kip1 and, with time, cyclin E/p21cip1 as well. The latter population of cells is arrested for a minimum of several days, if not for the remaining lifetime of the cells. Cells that have successfully entered S phase follow, after a long lag, either of the same two alternative paths again (Fig. 5B). As the fractions of cells that accumulate p27kip1, or additionally cyclin E and p21cip1, are greater than that of cells in S phase at any time, this attrition process then significantly limits the percentage of cells capable of supporting cellular and viral DNA replication. We propose that these virus-host interactions not only serve as a natural host defense but are also beneficial to virus persistence. They allow sustained but low-level virus shedding with minimal pathological consequences in typical benign and productive infections.
Acknowledgments
This research was supported by USPHS grant CA36200.
We thank the nurses of the newborn nursery at Cooper Green Hospital for collecting neonatal foreskins and Ge Jin for embedding and sectioning raft cultures.
REFERENCES
- 1.Bates, S., K. M. Ryan, A. C. Phillips, and K. H. Vousden. 1998. Cell cycle arrest and DNA endoreduplication following p21Waf1/Cip1 expression. Oncogene 17:1691-1703. [DOI] [PubMed] [Google Scholar]
- 2.Berezutskaya, E., and S. Bagchi. 1997. The human papillomavirus E7 oncoprotein functionally interacts with the S4 subunit of the 26S proteasome. J. Biol. Chem. 272:30135-30140. [DOI] [PubMed] [Google Scholar]
- 3.Berezutskaya, E., B. Yu, A. Morozov, P. Raychaudhuri, and S. Bagchi. 1997. Differential regulation of the pocket domains of the retinoblastoma family proteins by the HPV16 E7 oncoprotein. Cell Growth Differ. 8:1277-1286. [PubMed] [Google Scholar]
- 4.Boyer, S. N., D. E. Wazer, and V. Band. 1996. E7 protein of human papilloma virus-16 induces degradation of retinoblastoma protein through the ubiquitin-proteasome pathway. Cancer Res. 56:4620-4624. [PubMed] [Google Scholar]
- 5.Cheng, S., D.-C. Schmidt-Grimminger, T. Murant, T. R. Broker, and L. T. Chow. 1995. Differentiation-dependent up-regulation of the human papillomavirus E7 gene reactivates cellular DNA replication in suprabasal differentiated keratinocytes. Genes Dev. 9:2335-2349. [DOI] [PubMed] [Google Scholar]
- 6.Chien, W.-M., J. N. Parker, D. C. Schmidt-Grimminger, T. R. Broker, and L. T. Chow. 2000. Casein kinase II phosphorylation of the human papillomavirus-18 E7 protein is critical for promoting S-phase entry. Cell Growth Differ. 11:425-435. [PubMed] [Google Scholar]
- 7.Chow, L. T., and T. R. Broker. 1997. Small DNA tumor viruses, p. 267-301. In N. Nathanson (ed.), Viral pathogenesis. Lippincott-Raven Publishers, Philadelphia, Pa.
- 8.Chow, L. T., and T. R. Broker. 1997. In vitro experimental systems for HPV: epithelial raft cultures for investigations of viral reproduction and pathogenesis and for genetic analyses of viral proteins and regulatory sequences. Clin. Dermatol. 15:217-227. [DOI] [PubMed] [Google Scholar]
- 9.Cueille, N., R. Nougarede, F. Mechali, M. Philippe, and C. Bonne-Andrea. 1998. Functional interaction between the bovine papillomavirus virus type 1 replicative helicase E1 and cyclin E-Cdk2. J. Virol. 72:7255-7262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dyson, N. 1998. The regulation of E2F by pRB-family proteins. Genes Dev. 12:2245-2262. [DOI] [PubMed] [Google Scholar]
- 11.Edgar, B. A., and T. L. Orr-Weaver. 2001. Endoreplication cell cycles: more for less. Cell 105:297-306. [DOI] [PubMed] [Google Scholar]
- 12.El-Deiry, W. S. 1998. p21/p53, cellular growth control and genomic integrity. Curr. Top. Microbiol. Immunol. 227:121-137. [DOI] [PubMed] [Google Scholar]
- 13.Funk, J. O., S. Waga, J. B. Harry, E. Espling, B. Stillman, and D. A. Galloway. 1997. Inhibition of CDK activity and PCNA-dependent DNA replication by p21 is blocked by interaction with the HPV-16 E7 oncoprotein. Genes Dev. 11:2090-2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Halevy, O., B. G. Novitch, D. B. Spicer, S. X. Skapek, J. Rhee, G. J. Hannon, D. Beach, and A. B. Lassar. 1995. Correlation of terminal cell cycle arrest of skeletal muscle with induction of p21 by MyoD. Science 267:1018-1021. [DOI] [PubMed] [Google Scholar]
- 15.Hamamoto, R., K. Yamada, M. Kamihira, and S. Iijima. 1998. Differentiation and proliferation of primary rat hepatocytes cultured as spheroids. J. Biochem. (Tokyo) 124:972-979. [DOI] [PubMed] [Google Scholar]
- 16.Herwig, S., and M. Strauss. 1997. The retinoblastoma protein: a master regulator of cell cycle, differentiation and apoptosis. Eur. J. Biochem. 246:581-601. [DOI] [PubMed] [Google Scholar]
- 17.Jackson, P. K., S. Chevalier, M. Philippe, and M. W. Kirschner. 1995. Early events in DNA replication require cyclin E and are blocked by p21CIP1. J. Cell Biol. 130:755-769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jian, Y., D.-C. Schmidt-Grimminger, W. M. Chien, X. Wu, T. R. Broker, and L. T. Chow. 1998. Post-transcriptional induction of p21cip1 protein by human papillomavirus E7 inhibits unscheduled DNA synthesis reactivated in differentiated keratinocytes. Oncogene 17:2027-2038. [DOI] [PubMed] [Google Scholar]
- 19.Jian, Y., B. A. Van Tine, W.-M. Chien, G. M. Shaw, T. R. Broker, and L. T. Chow. 1999. Concordant induction of cyclin E and p21cip1 in differentiated keratinocytes by the human papillomavirus E7 protein inhibits cellular and viral DNA synthesis. Cell Growth Differ. 10:101-111. [PubMed] [Google Scholar]
- 20.Jones, D. L., R. M. Alani, and K. Münger. 1997. The human papillomavirus E7 oncoprotein can uncouple cellular differentiation and proliferation in human keratinocytes by abrogating p21Cip1-mediated inhibition of cdk2. Genes Dev. 11:2101-2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones, D. L., D. A. Thompson, and K. Münger. 1997. Destabilization of the RB tumor suppressor protein and stabilization of p53 contribute to HPV type 16 E7-induced apoptosis. Virology 239:97-107. [DOI] [PubMed] [Google Scholar]
- 22.Lehrer, M. S., T. T. Sun, and R. M. Lavker. 1998. Strategies of epithelial repair: modulation of stem cell and transit amplifying cell proliferation. J. Cell Sci. 111:2867-2875. [DOI] [PubMed] [Google Scholar]
- 23.Lin, B.-Y., T. Ma, J.-S. Liu, S.-R. Kuo, L. Jin, T. R. Broker, J. W. Harper, and L. T. Chow. 2000. HeLa cells are phenotypically limiting in cyclin E/CDK2 for efficient human papillomavirus DNA replication. J. Biol. Chem. 275:6167-6174. [DOI] [PubMed] [Google Scholar]
- 24.Ma, T., N. Zou, B.-Y. Lin, L. T. Chow, and J. W. Harper. 1999. Interaction between cyclin-dependent kinases and human papillomavirus replication-initiation protein E1 is required for efficient viral replication. Proc. Natl. Acad. Sci. USA 96:382-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ma, T., B. A. Van Tine, W. Yue, M. Garrett, D. Nelson, P. D. Adams, J. Wang, L. T. Chow, and J. W. Harper. 2000. Cell cycle-regulated phosphorylation of p220NPAT by cyclin E/Cdk2 in Cajal bodies promotes histone gene transcription. Genes Dev. 14:2298-2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Masai, H., E. Matsui, Z. You, Y. Ishimi, K. Tamai, and K. Arai. 2000. Human Cdc7-related kinase complex. In vitro phosphorylation of MCM by concerted actions of Cdks and Cdc7 and that of a critical threonine residue of Cdc7 by Cdks. J. Biol. Chem. 275:29042-29052. [DOI] [PubMed] [Google Scholar]
- 27.Meisels, A., and R. Fortin. 1976. Condylomatous lesions of the cervix and vagina. I. Cytologic patterns. Acta Cytol. 20:505-509. [PubMed] [Google Scholar]
- 28.Morris, L., K. E. Allen, and N. B. La Thangue. 2000. Regulation of E2F transcription by cyclin E-Cdk2 kinase mediated through p300/CBP co-activators. Nat. Cell Biol. 2:232-239. [DOI] [PubMed] [Google Scholar]
- 29.Niculescu, A. B., III, X. Chen, M. Smeets, L. Hengst, C. Prives, and S. I. Reed. 1998. Effects of p21(Cip1/Waf1) at both the G1/S and the G2/M cell cycle transitions: pRb is a critical determinant in blocking DNA replication and in preventing endoreduplication. Mol. Cell. Biol. 18:629-643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Noya, F., W.-M. Chien, T. R. Broker, and L. T. Chow. 2001. p21cip1 degradation in differentiated keratinocytes is abrogated by costabilization with cyclin E induced by human papillomavirus E7. J. Virol. 75:6121-6134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ohtsubo, M., A. M. Theodoras, J. Schumacher, J. M. Roberts, and M. Pagano. 1995. Human cyclin E, a nuclear protein essential for the G1-to-S phase transition. Mol. Cell. Biol. 15:2612-2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parker, J. N., W. Zhao, K. J. Askins, T. R. Broker, and L. T. Chow. 1997. Mutational analyses of differentiation-dependent human papillomavirus type 18 enhancer elements in epithelial raft cultures of neonatal foreskin keratinocytes. Cell Growth Differ. 8:751-762. [PubMed] [Google Scholar]
- 33.Reichert, M., and D. Eick. 1999. Analysis of cell cycle arrest in adipocyte differentiation. Oncogene 18:459-466. [DOI] [PubMed] [Google Scholar]
- 34.Schmidt-Grimminger, D.-C., X. Wu, Y. Jian, T. R. Broker, and L. T. Chow. 1998. Post-transcriptional induction of p21cip1 protein in condylomata and dysplasias is inversely related to human papillomavirus activities. Am. J. Pathol. 152:1015-1024. [PMC free article] [PubMed] [Google Scholar]
- 35.Sheaff, R. J., M. Groudine, M. Gordon, J. M. Roberts, and B. E. Clurman. 1997. Cyclin E-CDK2 is a regulator of p27Kip1. Genes Dev. 11:1464-1478. [DOI] [PubMed] [Google Scholar]
- 36.Sherr, C. J. 1996. Cancer cell cycles. Science 274:1672-1677. [DOI] [PubMed] [Google Scholar]
- 37.Sherr, C. J., and J. M. Roberts. 1999. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 13:1501-1512. [DOI] [PubMed] [Google Scholar]
- 38.Southern, S. A., and C. S. Herrington. 1998. Differential cell cycle regulation by low- and high-risk human papillomaviruses in low-grade squamous intraepithelial lesions of the cervix. Cancer Res. 58:2941-2945. [PubMed] [Google Scholar]
- 39.Southern, S. A., F. Noya, C. Meyers, T. R. Broker, L. T. Chow, and C. S. Herrington. 2001. Tetrasomy is induced by human papillomavirus type 18 E7 gene expression in keratinocyte raft cultures. Cancer Res. 61:4858-4863. [PubMed] [Google Scholar]
- 40.Stewart, Z. A., S. D. Leach, and J. A. Pietenpol. 1999. p21(Waf1/Cip1) inhibition of cyclin E/Cdk2 activity prevents endoreduplication after mitotic spindle disruption. Mol. Cell. Biol. 19:205-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thomas, J. T., and L. A. Laimins. 1998. Human papillomavirus oncoproteins E6 and E7 independently abrogate the mitotic spindle checkpoint. J. Virol. 72:1131-1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Voitenleitner, C., C. Rehfuess, M. Hilmes, L. O'Rear, P. C. Liao, D. A. Gage, R. Ott, H. P. Nasheuer, and E. Fanning. 1999. Cell cycle-dependent regulation of human DNA polymerase alpha-primase activity by phosphorylation. Mol. Cell. Biol. 19:646-656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Won, K. A., and S. I. Reed. 1996. Activation of cyclin E/CDK2 is coupled to site-specific autophosphorylation and ubiquitin-dependent degradation of cyclin E. EMBO J. 15:4182-4193. [PMC free article] [PubMed] [Google Scholar]
- 44.Zehbe, I., A. Ratsch, M. Alunni-Fabbroni, A. Burzlaff, E. Bakos, M. Dürst, E. Wilander, and M. Tommasino. 1999. Overriding of cyclin-dependent kinase inhibitors by high and low risk human papillomavirus types: evidence for an in vivo role in cervical lesions. Oncogene 18:2201-2211. [DOI] [PubMed] [Google Scholar]
- 45.Zhao, J., B. K. Kennedy, B. D. Lawrence, D. A. Barbie, A. G. Matera, and E. Harlow. 2000. NPAT links cyclin E-Cdk2 to the regulation of replication-dependent histone gene transcription. Genes Dev. 14:2283-2297. [PMC free article] [PubMed] [Google Scholar]