Abstract
The role of CD28-dependent costimulatory interactions in the development and maintenance of antiviral immune responses was investigated in a mouse model of gammaherpesvirus infection. CD28−/− mice could clear a productive infection with murine gammaherpesvirus 68 (MHV-68), although early lung viral titers were significantly increased. Both CD28−/− and CD28+/+ mice maintained effective long-term control of MHV-68. Gamma interferon responses appeared to develop more slowly in CD28−/− mice, while cytotoxic T-cell activity was similar to that in wild-type mice. Splenomegaly developed normally in CD28−/− mice, whereas virus-specific antibody responses were significantly reduced and aberrant class switching was observed. This work demonstrates that costimulatory interactions involving CD28 are not an absolute requirement for the control of infection with MHV-68.
Murine gammaherpesvirus 68 (MHV-68) is a naturally occurring rodent pathogen (5) which is closely related to Epstein-Barr virus, the Kaposi's sarcoma-associated human herpesvirus 8, and herpesvirus saimiri (11, 35). Intranasal administration of MHV-68 results in acute, productive infection of lung alveolar epithelial cells and a latent infection in several cell types, including B lymphocytes, dendritic cells, macrophages, and epithelia (12, 13, 33, 36). Infectious virus is cleared from the lungs 10 to 13 days after infection by a T-cell-mediated process (7, 12). The antibody response develops several weeks after infection (32). Control of latent virus, once established, can be maintained by either T- or B-cell-mediated pathways ( 31, 33). Mechanisms which control latent virus do not develop efficiently in the absence of CD4 T cells, leading to viral reactivation in the lungs (7, 26). CD4 T cells can provide help for CD8 T cells or B cells and can also function independently in the long-term control of MHV-68 (7, 33). It seems likely that cytokines or costimulatory molecules expressed by CD4 T cells are necessary for the generation and/or maintenance of CD8 T-cell- and B-cell-mediated control of latent MHV-68. This contention is supported by the fact that CD40L−/− (6) also showed reactivation of MHV-68 in the lungs. Furthermore, agonistic antibodies to CD40 could replace CD4 T-cell function in preventing viral reactivation (27). CD40L is present on the surface of activated CD4 T cells and interacts with CD40, which is expressed by several cell types, including B cells, dendritic cells, and macrophages (4, 15, 20, 22). CD40 ligation on an antigen-presenting cell upregulates surface expression of B7.1 and B7.2, which interact with CD28 on T cells (2, 8). The interaction of CD28 with B7.1 or B7.2 leads to upregulation of additional molecules and thus initiates “cross talk” between CD8 T cells and antigen-presenting cells, resulting in further activation of both cell types. In view of the important role of CD40 in B-cell and memory T-cell responses to MHV-68 (6, 27), it was of great interest to determine whether CD28 also played a critical role in either acute or long-term control of the virus. Therefore, in this study, viral clearance and cellular and humoral immune responses were compared in CD28+/+ and CD28−/− mice.
CD28−/− (28) or wild-type CD28+/+ C57BL/6 mice were obtained from the Jackson Laboratory (Bar Harbor, Maine) and were housed under specific-pathogen-free conditions in the La Jolla Institute for Allergy and Immunology (LIAI) animal resource center, which is an Association for Assessment and Accreditation of Laboratory Animal Care-accredited facility. All experiments were performed in accordance with a protocol approved by the Animal Care and Use Committee of LIAI, in compliance with the National Institutes of Health U.S. Public Health Service guidelines for the care and use of animals. The genotypes of CD28+/+ or CD28−/− mice were verified on sacrifice of the animals by flow cytometry analysis of splenocytes dually stained with antibodies to CD28 and CD4 or CD8. Age-matched female 6- to 20-week-old CD28+/+ and CD28−/− mice were used in all experiments.
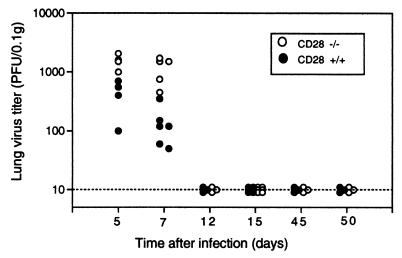
CD28−/− mice were able to clear infectious MHV-68 with normal kinetics (Fig. 1). Both CD28−/− and CD28+/+ mice had cleared infectious virus from their lungs by day 12 after an intranasal challenge with 2 × 105 PFU of MHV-68. However, at days 5 and 7 after infection, the lung virus titers of the CD28−/− mice were significantly higher than those of wild-type mice (P < 0.01, day 5; P < 0.02, day 7 [Mann-Whitney rank sum test]), suggesting a defect in the early immune response to the virus. The lungs of both CD28−/− and CD28+/+ mice remained clear of replicating virus when tested on days 45 and 50 after infection (Fig. 1). These data suggest that CD28-B7 interactions are not required for clearance of a primary challenge with MHV-68 or for long-term control of the virus. However, early immune responses appear to be more effective in CD28+/+ mice.
FIG. 1.
Lung virus titers in CD28−/− and CD28+/+ mice. CD28−/− or CD28+/+ mice were infected intranasally with 2 × 105 PFU of MHV-68 (clone G2.4). At various times after infection, lungs were harvested and virus titers were determined in lung homogenates by plaque assay as described previously (27). Data are expressed as log10 PFU/0.1 g of lung tissue for individual mice. The detection limit of this assay is 10 PFU/0.1 g of lung tissue.
In contrast to CD28−/− mice, CD40−/− mice were unable to maintain effective long-term control of MHV-68, in agreement with a previous study using CD40L−/− mice (6). Six- to 8-week-old female CD40−/− C57BL/6 mice were kindly provided by S. Schoenberger and were infected intranasally with 2 × 105 PFU of MHV-68. CD40−/− mice showed significant lung viral titers at day 50 after infection (n = 3 mice; lung viral titers of 64, 856, and 120 PFU/0.1 g of lung tissue), and there were some deaths in this group between days 70 and 90 after infection (two of four mice died; lung viral titers in the surviving mice at day 90 were 184 and 88 PFU/0.1 g of lung tissue). No deaths or viral reactivation occurred on either day 45 or 90 postinfection in groups of wild-type C57BL/6 mice infected with MHV-68 at the same time as the CD40−/− mice (n = 3 mice at each time point).
Latent virus was assessed in CD28+/+ and CD28−/− mice by an infectious center assay as described previously (7) at day 15 after infection, which is when the peak number of latently infected cells is observed in this viral model. The frequency of infectious centers in splenocytes from CD28−/− mice (123 ± 54/107 splenocytes, mean ± standard error, n = 6) was not significantly different from that in CD28+/+ mice (215 ± 77/107 splenocytes, mean ± standard error, n = 6). Means of duplicate determinations in two independent experiments with three animals per group in each experiment were compared (total of six mice in each group from two combined experiments). No replicating virus was detected in the spleens of either CD28−/− or CD28+/+ mice (the limit of detection was 8 PFU/107 splenocytes).
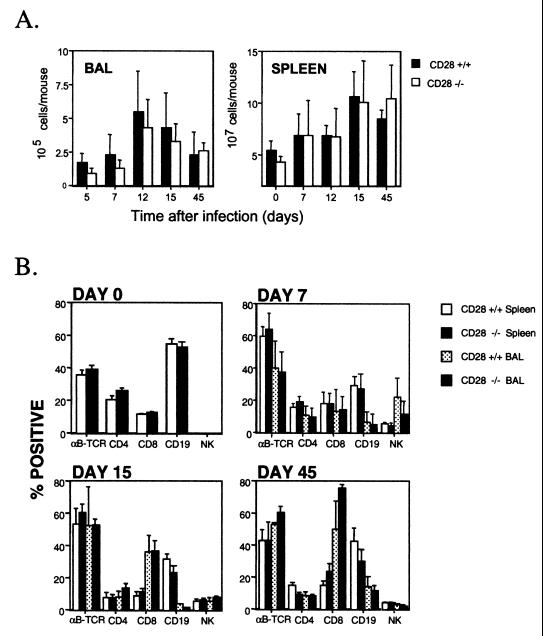
The numbers and types of cells infiltrating the lungs were assessed at various intervals after infection. Although there appeared to be fewer cells in the bronchoalveolar lavage (BAL) of CD28−/− than in CD28+/+ mice, at early times after infection, the difference was not statistically significant (Fig. 2A). Maximal cell numbers were recovered from the lungs of both groups of mice at day 12 after infection. Very few cells were recovered from the lungs of uninfected mice. The proportion of various lymphocyte subsets in the BAL was determined by flow cytometry analysis. The percentages of αβ T-cell receptor+ cells, NK cells, B cells, and CD4 and CD8 T cells were similar in CD28+/+ and CD28−/− mice (Fig. 2B).
FIG. 2.
Cell numbers and lymphocyte subsets in the BAL or spleen of CD28+/+ and CD28−/− mice. (A) Cell numbers in the BAL or spleen were determined at intervals after intranasal infection of CD28+/+ and CD28−/− mice with MHV-68. Single-cell suspensions were prepared from the spleens of individual mice, as described previously (1), and viable cell counts were determined by trypan blue exclusion. Data are means + standard deviations of cell counts for three to eight mice at each time point. (B) BAL or spleen cells were stained with phycoerythrin- or fluorescein isothiocyanate-conjugated monoclonal antibodies, as previously described (25). The resulting populations were analyzed by flow cytometry. The detection limit was less than 1% based on staining with isotype-matched control antibodies. The means + standard deviations of data from two separate experiments at each time point are shown, with the exception of day 0, which was a single experiment. Groups of three to five mice were used at each time point. TCR, T-cell receptor.
MHV-68 infection of wild-type mice is characterized by splenomegaly, which is dependent on both T and B cells and requires CD40-CD40L interactions (6). However, splenomegaly developed normally in CD28−/− mice, indicating that costimulatory interactions involving CD28 were not essential for this response. Thus, the number of cells in the spleens of both wild-type and CD28−/− mice had increased considerably by day 15 after infection (Fig. 2B) and was similar in both groups of mice at all time points examined. There was no significant difference in the percentages of splenic CD4 or CD8 T cells, B cells, or NK cells in the two groups of mice at any of the time points examined (Fig 2B).
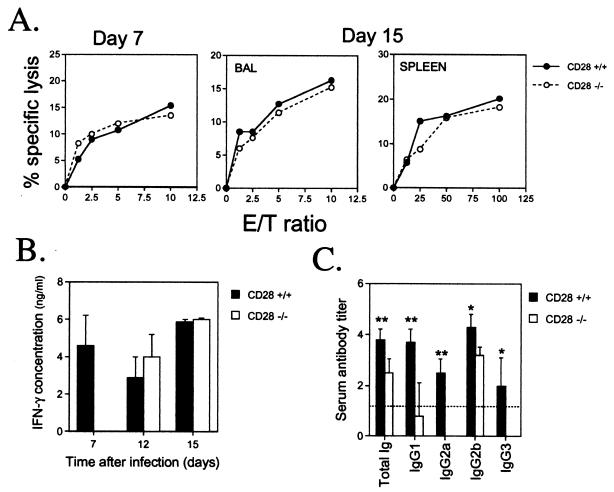
Cytotoxic T-lymphocyte (CTL) activity was assayed in the lungs or spleens of MHV-68-infected CD28−/− and wild-type mice. Previous studies have shown that the majority of the CTL activity is mediated by CD8 T cells in this model (7). The CTL activity of BAL cells was similar in CD28−/− and wild-type mice on days 7 and 15 after infection (Fig. 3A). Similarly, splenic CTL activity was comparable in CD28−/− and CD28+/+ mice at day 15 after infection, while no significant activity was detected in either group at day 7 postinfection. In contrast, production of gamma interferon (IFN-γ) by splenocytes from virus-infected mice following restimulation with virus-infected splenic antigen-presenting cells in vitro was significantly attenuated in CD28−/− mice (P < 0.01, Mann-Whitney rank sum test) at day 7 after infection (Fig. 3B). However, by day 12 after infection, splenic recall IFN-γ production in CD28−/− had reached a level comparable to that in wild-type mice. A similar result was obtained at day 15 after infection (Fig. 3B).
FIG. 3.
Cell-mediated and humoral immune responses to MHV-68 in CD28+/+ and CD28−/− mice. (A) Cytotoxic T-cell responses. Single-cell suspensions were prepared from BAL or spleens of individual mice at days 7 and 15 after intranasal infection with MHV-68 CTL activity was determined in a 6-h 51Cr release assay using MHV-68-infected MEF-1 cells as targets, as described previously (30). Mean percent specific lysis is shown for three separate experiments at day 7 and two at day 15. Groups of two or three mice were used in each experiment. No CTL activity was detected in the spleens of either CD28+/+ or CD28−/− mice at day 7 after infection. (B) Splenic recall IFN-γ responses. Spleens were harvested 15 or 30 days after infection with MHV-68. Splenocytes were restimulated with MHV-68-infected antigen-presenting cells, and supernatants were collected 24 to 96 h later for analysis of IFN-γ levels by sandwich ELISA, as previously described (25). Peak values (which usually occurred after 72 h in culture) are reported. Data are expressed as mean IFN-γ concentrations (nanograms/milliliter) + standard deviations for two separate experiments at day 7 and one each at days 12 and 15. Splenocyte cultures from three individual CD28+/+ or CD28−/− mice were tested in each experiment. (C) MHV-68-specific antibody responses. Serum was collected from CD28+/+ and CD28−/− mice 45 to 50 days after infection with MHV-68. Virus-specific antibody responses were determined by ELISA, as described previously (27). The titer of a serum sample was taken as the log10 of the highest dilution that gave an absorbance reading of <0.1. Data are expressed as mean serum antibody titers + standard deviations for two separate experiments. Sera from three individual mice were tested in each experiment. Asterisks denote that there was a statistically significant difference in antibody titers in CD28−/− and CD28+/+ mice. ∗, P < 0.05; ∗∗, P < 0.01 (Mann-Whitney rank sum test). E/T, effector-to-target.
In addition to defects in cell-mediated immunity, substantial changes in humoral immune responses were noted in CD28−/− mice. Virus-specific serum antibody titers were determined by enzyme-linked immunosorbent assay (ELISA) 45 to 50 days after infection with MHV-68. There was a significant reduction in the total level of immunoglobulin in CD28−/− mice compared to that in wild-type mice (P < 0.005 [Fig. 3C]). However, there was a more dramatic reduction for some of the immunoglobulin G (IgG) subclasses than for others. For example, levels of IgG1 and IgG2a were reduced to levels close to or below the limit of detection in this assay (P < 0.005 for either subclass). The same applied to IgG3 (P < 0.05), which was undetectable in CD28−/− mice. IgG2b levels were also significantly reduced in CD28−/− mice (P < 0.05) but not to the same extent as the other subclasses (Fig. 3C). IgA was undetectable (data not shown) in both CD28+/+ and CD28−/− mice. In addition to virus-neutralizing activity, which could be mediated by any of the immunoglobulin subclasses, antibodies specific for viral proteins expressed on the surface of infected cells might contribute to the control of viral replication by facilitating the destruction of virus-infected cells by complement fixation, antibody-dependent cellular cytotoxicity, or phagocytosis. With the exception of IgG1, all IgG subclasses fix complement efficiently. IgG2a and -2b show the strongest binding to Fc receptors, which is required for antibody-dependent cellular cytotoxicity or phagocytosis. Thus, the IgG2b response to MHV-68 in CD28−/− mice could play a role in the long-term control of MHV-68 replication. These data show that CD28−/− mice can mount significant humoral responses to MHV-68 and that class switching still occurs, although the response is significantly attenuated compared to that in wild-type mice.
Although CD28−/− mice showed defects in both cell-mediated and humoral immunity, they were still able to clear infectious MHV-68 from their lungs and maintained effective long-term control of the virus. Thus, the residual immune responses were sufficient to control the virus. In contrast, studies with CD40−/− (see above) or CD40L−/− (6) mice revealed an essential role for CD40-CD40L interactions in the long-term control of MHV-68. Thus, although CD40 ligation is known to upregulate B7 molecules, which activate T cells via interactions with CD28 (2, 8), it appears that CD40 can function via a CD28-independent pathway in the long-term control of MHV-68.
Previous studies on the role of costimulatory molecules in the immune response to viral infection have shown that the requirements for CD28 costimulation differ in different viral infection models. Shahinian et al. (28) showed that CD28−/− mice made normal CD8 CTL and delayed hypersensitivity responses to lymphocytic choriomeningitis virus (LCMV). Studies with CD28−/− mice, B7−/− mice, and CTLA4-Ig transgenic mice have shown that B7 costimulation plays a critical role in antibody class switching and CTL generation to vesicular stomatitis virus (VSV) (21, 28, 29, 37). Similarly, CTL responses to influenza were shown to be dependent on B7-CD28 interactions (10, 18, 19). In the present study, we saw no significant defect in CTL responses to MHV-68 in CD28−/− mice. This contrasts with the influenza and VSV models but is consistent with the findings in the LCMV model. However, our observation of reduced antibody production and class switching in CD28−/− mice is in agreement with findings in both LCMV and VSV models.
It is unclear why immune responses to different viruses appear to be differentially dependent on costimulatory interactions. It has been postulated that the ability of some viruses to infect and activate antigen-presenting cells can bypass the requirements for CD4 T-cell help (3, 24). Other studies have shown that the binding affinity of a peptide epitope for major histocompatibility complex class I is a key factor in determining the helper requirement for CTL priming (14). Thus, the type of costimulation required would depend on the nature of the viral epitopes recognized. However, the CD28-independent CTL response induced by LCMV (which is also CD4 independent) does not correlate with any particular viral epitope (17). Kundig et al. (17) suggested that prolonged viral replication in lymphoid tissue correlated with the CD28 independence of the CTL response. MHV-68 does not show extensive viral replication in the lymphoid tissue of intranasally infected mice. However, viral latency is established in various lymphoid tissue cell types, which could potentially induce cellular events resulting in the development of CD28-independent responses.
Alternatively, costimulatory molecules other than CD28 may be upregulated either on T cells or antigen-presenting cells during infection with MHV-68, leading to CD28-independent immune responses. For example, 41BB-41BBL interactions can deliver a costimulatory signal to a T cell in the absence of CD28 (9, 10, 23, 34). Furthermore, other CD28 family members, such as inducible costimulatory molecule (ICOS), which share some of the functions of CD28, have been characterized recently (16). However, these CD28-independent pathways of T-cell activation cannot fully replace CD28 function. Thus, MHV-68-specific antibody responses and class switching and early IFN-γ responses were significantly impaired in CD28−/− mice. The increase in early lung virus was also consistent with slower development of the immune response. However, this is unlikely to be dependent on early IFN-γ production by CD4 T cells, as early lung viral titers are not increased in either CD4 T-cell- or IFN-γ-deficient mice, although viral clearance is slightly delayed in both models (7, 12, 26). There was also a trend toward there being lower numbers of T cells in the lungs of CD28−/− mice at days 5 and 7 after infection, which might also indicate slower development of the immune response.
In summary, our data show that costimulation via CD28 plays an important role in the development of optimal immune responses to MHV-68 but is not an absolute requirement for either long- or short-term control of the virus.
Acknowledgments
This work was supported in part by grant AI-44247-01A1 to S.R.S from the National Institutes of Health.
Footnotes
This is manuscript no. 432 from the La Jolla Institute for Allergy and Immunology.
REFERENCES
- 1.Allan, W., Z. Tabi, A. Cleary, and P. C. Doherty. 1990. Cellular events in the lymph node and lung of mice with influenza. Consequences of depleting CD4+ T cells. J. Immunol. 144:3980-3986. [PubMed] [Google Scholar]
- 2.Allison, J. P. 1994. CD28-B7 interactions in T-cell activation. Curr. Opin. Immunol. 6:414-419. [DOI] [PubMed] [Google Scholar]
- 3.Bachmann, M. F., R. M. Zinkernagel, and A. Oxenius. 1998. Immune responses in the absence of costimulation: viruses know the trick. J. Immunol. 161:5791-5794. [PubMed] [Google Scholar]
- 4.Banchereau, J., F. Bazan, D. Blanchard, F. Briere, J. P. Galizzi, C. van Kooten, Y. J. Liu, F. Rousset, and S. Saeland. 1994. The CD40 antigen and its ligand. Annu. Rev. Immunol. 12:881-922. [DOI] [PubMed] [Google Scholar]
- 5.Blaskovic, D., M. Stancekova, J. Svobodova, and J. Mistrikova. 1980. Isolation of five strains of herpesviruses from two species of free living small rodents. Acta Virol. 24:468.. [PubMed] [Google Scholar]
- 6.Brooks, J. W., A. M. Hamilton-Easton, J. P. Christensen, R. D. Cardin, C. L. Hardy, and P. C. Doherty. 1999. Requirement for CD40 ligand, CD4+ T cells, and B cells in an infectious mononucleosis-like syndrome. J. Virol. 73:9650-9654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cardin, R. D., J. W. Brooks, S. R. Sarawar, and P. C. Doherty. 1996. Progressive loss of CD8+ T cell-mediated control of a γ-herpesvirus in the absence of CD4+ T cells. J. Exp. Med. 184:863-871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cella, M., D. Scheidegger, K. Palmer Lehmann, P. Lane, A. Lanzavecchia, and G. Alber. 1996. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacity: T-T help via APC activation. J. Exp. Med. 184:747-752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DeBenedette, M. A., A. Shahinian, T. W. Mak, and T. H. Watts. 1997. Costimulation of CD28− T lymphocytes by 4-1BB ligand. J. Immunol. 158:551-559. [PubMed] [Google Scholar]
- 10.DeBenedette, M. A., T. Wen, M. F. Bachmann, P. S. Ohashi, B. H. Barber, K. L. Stocking, J. J. Peschon, and T. H. Watts. 1999. Analysis of 4-1BB ligand-deficient mice and of mice lacking both 4-1BBL and CD28 reveals a role for 4-1BBL in skin allograft rejection and in the cytotoxic T cell response to influenza virus. J. Immunol. 163:4833-4841. [PubMed] [Google Scholar]
- 11.Efstathiou, S., Y. M. Ho, and A. C. Minson. 1990. Cloning and molecular characterization of the murine herpesvirus 68 genome. J. Gen. Virol. 71:1355-1364. [DOI] [PubMed] [Google Scholar]
- 12.Ehtisham, S., N. P. Sunil Chandra, and A. A. Nash. 1993. Pathogenesis of murine gammaherpesvirus infection in mice deficient in CD4 and CD8 T cells. J. Virol. 67:5247-5252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flano, E., S. M. Husain, J. T. Sample, D. L. Woodland, and M. A. Blackman. 2000. Latent murine gammaherpesvirus infection is established in activated B cells, dendritic cells, and macrophages. J. Immunol. 165:1075-1081. [DOI] [PubMed] [Google Scholar]
- 14.Franco, A., D. A. Tilly, I. Gramaglia, M. Croft, L. Cipolla, M. Meldal, and H. M. Grey. 2000. Epitope affinity for MHC class I determines helper requirement for CTL priming. Nat. Immunol. 1:145-150. [DOI] [PubMed] [Google Scholar]
- 15.Grewal, I. S., P. Borrow, E. G. Pamer, M. B. Oldstone, and R. A. Flavell. 1997. The CD40-CD154 system in anti-infective host defense. Curr. Opin. Immunol. 9:491-497. [DOI] [PubMed] [Google Scholar]
- 16.Hutloff, A., A. M. Dittrich, K. C. Beier, B. Eljaschewitsch, R. Kraft, I. Anagnostopoulos, and R. A. Kroczek. 1999. ICOS is an inducible T-cell co-stimulator structurally and functionally related to CD28. Nature 397:263-266. [DOI] [PubMed] [Google Scholar]
- 17.Kundig, T. M., A. Shahinian, K. Kawai, H. W. Mittrucker, E. Sebzda, M. F. Bachmann, T. W. Mak, and P. S. Ohashi. 1996. Duration of TCR stimulation determines costimulatory requirement of T cells. Immunity 5:41-52. [DOI] [PubMed] [Google Scholar]
- 18.Liu, Y., R. H. Wenger, M. Zhao, and P. J. Nielsen. 1997. Distinct costimulatory molecules are required for the induction of effector and memory cytotoxic T lymphocytes. J. Exp. Med. 185:251-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lumsden, J. M., J. M. Roberts, N. L. Harris, R. J. Peach, and F. Ronchese. 2000. Differential requirement for CD80 and CD80/CD86-dependent costimulation in the lung immune response to an influenza virus infection. J. Immunol. 164:79-85. [DOI] [PubMed] [Google Scholar]
- 20.Mackey, M. F., R. J. Barth, Jr., and R. J. Noelle. 1998. The role of CD40/CD154 interactions in the priming, differentiation, and effector function of helper and cytotoxic T cells. J. Leukoc. Biol. 63:418-428. [DOI] [PubMed] [Google Scholar]
- 21.McAdam, A. J., E. A. Farkash, B. E. Gewurz, and A. H. Sharpe. 2000. B7 costimulation is critical for antibody class switching and CD8+ cytotoxic T-lymphocyte generation in the host response to vesicular stomatitis virus. J. Virol. 74:203-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Noelle, R. J., J. A. Ledbetter, and A. Aruffo. 1992. CD40 and its ligand, an essential ligand-receptor pair for thymus-dependent B-cell activation. Immunol. Today 13:431-433. [DOI] [PubMed] [Google Scholar]
- 23.Pollok, K. E., Y. J. Kim, Z. Zhou, J. Hurtado, K. K. Kim, R. T. Pickard, and B. S. Kwon. 1993. Inducible T cell antigen 4-1BB. Analysis of expression and function. J. Immunol. 150:771-781. [PubMed] [Google Scholar]
- 24.Ridge, J. P., F. Di Rosa, and P. Matzinger. 1998. A conditioned dendritic cell can be a temporal bridge between a CD4+ T-helper and a T-killer cell. Nature 393:474-478. [DOI] [PubMed] [Google Scholar]
- 25.Sarawar, S. R., and P. C. Doherty. 1994. Concurrent production of interleukin-2, interleukin-10, and gamma interferon in the regional lymph nodes of mice with influenza pneumonia. J. Virol. 68:3112-3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sarawar, S. R., R. D. Cardin, J. W. Brooks, M. Mehrpooya, A. M. Hamilton-Easton, X. Y. Mo, and P. C. Doherty. 1997. Gamma interferon is not essential for recovery from acute infection with murine gammaherpesvirus 68. J. Virol. 71:3916-3921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sarawar, S. R., B. J. Lee, S. K. Reiter, and S. P. Schoenberger. 2001. Stimulation via CD40 can substitute for CD4 T cell function in preventing reactivation of a latent herpesvirus. Proc. Natl. Acad. Sci. USA 98:6325-6329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shahinian, A., K. Pfeffer, K. P. Lee, T. M. Kundig, K. Kishihara, A. Wakeham, K. Kawai, P. S. Ohashi, C. B. Thompson, and T. W. Mak. 1993. Differential T cell costimulatory requirements in CD28-deficient mice. Science 261:609-612. [DOI] [PubMed] [Google Scholar]
- 29.Sigal, L. J., H. Reiser, and K. L. Rock. 1998. The role of B7-1 and B7-2 costimulation for the generation of CTL responses in vivo. J. Immunol. 161:2740-2745. [PubMed] [Google Scholar]
- 30.Stevenson, P. G., G. T. Belz, J. D. Altman, and P. C. Doherty. 1998. Virus-specific CD8(+) T cell numbers are maintained during gamma-herpesvirus reactivation in CD4-deficient mice. Proc. Natl. Acad. Sci. USA 95:15565-15570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stevenson, P. G., G. T. Belz, J. D. Altman, and P. C. Doherty. 1999. Changing patterns of dominance in the CD8+ T cell response during acute and persistent murine gamma-herpesvirus infection. Eur J. Immunol. 29:1059-1067. [DOI] [PubMed] [Google Scholar]
- 32.Stevenson, P. G., and P. C. Doherty. 1998. Kinetic analysis of the specific host response to a murine gammaherpesvirus. J. Virol. 72:943-949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stewart, J. P., E. J. Usherwood, A. Ross, H. Dyson, and T. Nash. 1998. Lung epithelial cells are a major site of murine gammaherpesvirus persistence. J. Exp. Med. 187:1941-1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tan, J. T., J. K. Whitmire, R. Ahmed, T. C. Pearson, and C. P. Larsen. 1999. 4-1BB ligand, a member of the TNF family, is important for the generation of antiviral CD8 T cell responses. J. Immunol. 163:4859-4868. [PubMed] [Google Scholar]
- 35.Virgin, H. W., P. Latreille, P. Wamsley, K. Hallsworth, K. E. Weck, A. J. Dal Canto, and S. H. Speck. 1997. Complete sequence and genomic analysis of murine gammaherpesvirus 68. J. Virol. 71:5894-5904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weck, K. E., S. S. Kim, H. W. Virgin IV, and S. H. Speck. 1999. Macrophages are the major reservoir of latent murine gammaherpesvirus 68 in peritoneal cells. J. Virol. 73:3273-3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zimmermann, C., P. Seiler, P. Lane, and R. M. Zinkernagel. 1997. Antiviral immune responses in CTLA4 transgenic mice. J. Virol. 71:1802-1807. [DOI] [PMC free article] [PubMed] [Google Scholar]