Abstract
Objective:
To evaluate pre- and postoperative clinical parameters associated with improvement of diabetes up to 4 years after laparoscopic Roux-en-Y gastric bypass (LRYGBP) in patients with type 2 diabetes mellitus (T2DM).
Summary Background Data:
The surgical treatment of morbid obesity leads to dramatic improvement in the comorbidity status of most patients with T2DM. However, little is known concerning what preoperative clinical factors are associated with postoperative long-term improvement in diabetes in the morbidly obese patient with diabetes.
Methods:
We evaluated pre- and postoperative data, including demographics, duration of diabetes, metabolic parameters, and clinical outcomes, in all patients with impaired fasting glucose (IFG) and type T2DM undergoing LRYGBP from July 1997 to May 2002.
Results:
During this 5-year period, 1160 patients underwent LRYGBP and 240 (21%) had IFG or T2DM. Follow up was possible in 191 of 240 patients (80%). There were 144 females (75%) with a mean preoperative age of 48 years (range, 26–67 years). After surgery, weight and body mass index decreased from 308 lbs and 50.1 kg/m2 to 211 lbs and 34 kg/m2 for a mean weight loss of 97 lbs and mean excess weight loss of 60%. Fasting plasma glucose and glycosylated hemoglobin concentrations returned to normal levels (83%) or markedly improved (17%) in all patients. A significant reduction in use of oral antidiabetic agents (80%) and insulin (79%) followed surgical treatment. Patients with the shortest duration (<5 years), the mildest form of T2DM (diet controlled), and the greatest weight loss after surgery were most likely to achieve complete resolution of T2DM.
Conclusion:
LRYGBP resulted in significant weight loss (60% percent of excess body weight loss) and resolution (83%) of T2DM. Patients with the shortest duration and mildest form of T2DM had a higher rate of T2DM resolution after surgery, suggesting that early surgical intervention is warranted to increase the likelihood of rendering patients euglycemic.
In patients with type 2 diabetes (T2DM), laparoscopic Roux-en-Y gastric bypass resulted in 60% percent of excess body weight loss and 83% resolution of diabetes. Patients with T2DM < 5 years and mild disease had a much higher rate of resolution after surgery (95% and 97%, respectively) than patients with T2DM >10 years or severe disease (54% and 62%, respectively).
Since the introduction of Laparoscopic Roux-en-Y gastric bypass (LRYGBP) in the early 1990s, several investigators have demonstrated that LRYGBP is as effective as open roux-en-Y gastric bypass (RYGBP) in achieving significant long-term weight loss (60–80% percent of excess body weight loss [%EWL]) in morbidly obese patients while significantly reducing perioperative morbidity and recovery time.1–5 In 2000, our group reported (n = 275 patients) a mean excess weight loss of 77% (mean body mass index [BMI] reduced from 48 to 27 kg/m2) at 30 months and a major morbidity and mortality rate of 3.3% and 0.4%, respectively.2 Quality of life was significantly improved and all obesity comorbidities, with the exception of depression, were significantly improved or resolved. Although that study did not focus specifically on type 2 diabetes mellitus (T2DM), we did find that 82% of T2DM patients (n = 18) achieved clinical resolution (withdrawal of all antidiabetic medication) whereas the remaining 18% had significant improvement. Other investigators have also demonstrated that not only Roux-en-Y gastric bypass but other bariatric operations may result in significant clinical improvement in T2DM after weight loss.2,5–13 However, little is known concerning the effect of weight loss surgery on the degree of glycemic control that is achieved and its impact on antidiabetic medication requirement. Furthermore, factors that may be associated with postoperative resolution versus improvement in diabetes have not been fully elucidated.
The goal of this study then was to evaluate the effect of LRYGBP on morbidly obese patients with T2DM and their related comorbidites and diabetes-specific complications. Our hypothesis was that LRYGBP would result in sustained weight loss that would correspond to significant improvement in glycemic control, leading to clinical resolution or improvement in T2DM and related comorbidites and complications. We specifically evaluated postoperative outcomes, including surgical complications and weight loss as well as changes in fasting plasma glucose (FPG), glycosylated hemoglobin (HbA1C), diabetic medication requirement, and changes in comorbidity and diabetes complications.
PATIENTS AND METHODS
This study was performed with approval of the University of Pittsburgh Institutional Review Board (approval # 0304091x) and meets all HIPAA requirements. The study group consisted of all patients with T2DM who had undergone LRYGBP from July 1997 to May 2002 at the University of Pittsburgh Bariatric Surgery Center and had at least 6 months of follow-up. Patients were selected for LRYGBP if they met criteria for bariatric surgery proposed by the National Institutes of Health Consensus Development Panel report of 1991.14 Patients between the ages of 14 and 75 years were accepted if they had a BMI ≥35 kg/m2 with comorbidities, such as diabetes, obstructive sleep apnea, or arthritis or a BMI ≥40 kg/m2 with or without comorbidities. Contraindications to surgery included a history of unresolved alcohol or substance abuse, unstable psychiatric illness, an inability or unwillingness to cooperate in long-term follow-up, a lack of understanding of the operation and its consequences, an unrealistic expectation of outcome, and adverse surgical risk.
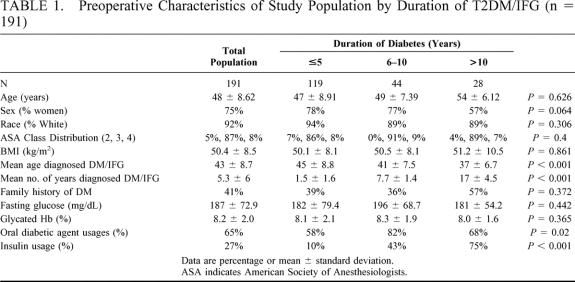
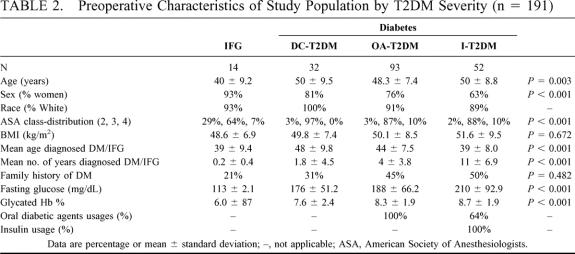
Diagnosis and classification of T2DM was based on FPG concentrations according to criteria established by the American Diabetes Association.15 The categories of FPG include (1) normal fasting glucose defined as FPG <110 mg/dL, (2) impaired fasting glucose (IFG) defined as FPG ≥ 110 mg/dL and < 126 mg/dL, and (3) clinical diabetes defined as FPG ≥ 126 mg/dL. IFG refers to a metabolic stage intermediate between normal glucose homeostasis and diabetes, now referred to as prediabetes. Patients with a positive history of T2DM and using diabetic medication before surgery were also classified as having T2DM despite normal values for FPG or HbA1C while on medication. Patients were stratified into groups based on duration of T2DM (≤5 years, 6–10 years, ≥10 years) and severity. Severity was classified from least severe to most severe according to the treatment required to maintain the patient in fair glycemic control (HbA1c ≤ 9%). Severity classifications included patients with (1) IFG, (2) diet-controlled T2DM (DC-T2DM), (3) oral agent users with T2DM (OA-T2DM), and (4) insulin users with T2DM (I-T2DM).
Preoperative Evaluation and Preparation
An extensive preoperative evaluation, including history and physical examination, nutritional and psychiatric evaluation, and indicated specialty consultations, was performed on all patients. All patients were screened for diabetes using FPG, and a careful history of diabetes, risk factors for diabetes, and comorbidities associated with diabetes and obesity were recorded. Preoperative studies included complete blood count, urinalysis, serum chemistries, nutritional indices, pregnancy test (in women younger than age 50), electrocardiogram, chest roentgenogram, and abdominal sonogram. If gallstones were detected sonographically, then laparoscopic cholecystectomy was performed concomitantly at the discretion of the surgeon.
Patient preparation for surgery consisted of a detailed explanation in written and oral form of LRYGBP, its benefits, alternatives, and risks including short- and long-term complications, side effects, nutritional sequelae, and the possibility of conversion to the open procedure. Written informed consent was obtained from all patients. Preoperative bowel cleansing and perioperative antibiotics were administered. Prophylaxis against venous thrombosis and pulmonary embolus consisted of perioperative pneumatic compression devices and low-dose subcutaneous heparin (5000 units, every 8 hours) or low molecular weight heparin 30–40 mg every 12 hours.
Surgical Technique
Our initial operative techniques for LRYGBP have been described in detail previously.2 The operations were performed initially by one attending surgeon (P.R.S.) with the addition of a second attending surgeon (S.I.) after approximately 100 patients. Four additional fellowship-trained attending surgeons (G.H., G.E., S.M., R.R.) joined the University of Pittsburgh Bariatric Surgery Center from 2001–2002. Our technique has undergone 3 major modifications since 1997. Patients #1-#150 received a 15-mL gastric pouch, a 12- to 14-mm diameter gastrojejunal anastomosis using a 21 mm circular stapler (advanced through the esophagus), and a retrocolic, retrogastric Roux-limb. For patients #151-#850, we changed to a 12- to 14-mm diameter gastrojejunal anastomosis with a linear stapler. For patients #851-#1160 we changed to an ante-colic, ante-gastric Roux-limb (Fig. 1). For all 3 techniques, Roux-limb length varied according to preoperative BMI (75 cm for BMI < 50 and 150 cm for BMI ≥ 50). Relative advantages of each technique have been described in other reports.16–18
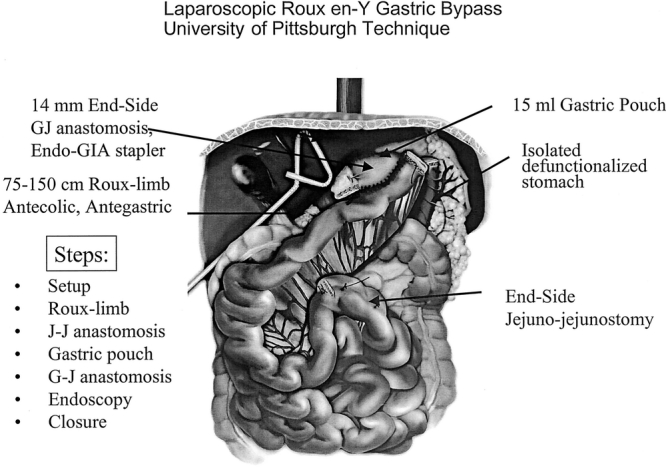
FIGURE 1. Laparoscopic Roux en-Y gastric bypass: University of Pittsburgh technique.
Postoperative Management
All patients were extubated and transferred to the surgical ward after surgery unless they required ventilatory support or close observation in the intensive care unit. Postoperative pulmonary care included incentive spirometry and deep-breathing exercises. Patient-controlled analgesia using intravenous morphine was started in the recovery room. Patients were encouraged to ambulate on the same operative day. A water-soluble contrast study was performed on the first postoperative day. A clear liquid diet was started after confirmation of an intact anastomosis without evidence of leak or obstruction. Patients were discharged from the hospital when oral fluid was tolerated. The diet was advanced to solid food by the fourth postoperative week.
Outcome Measures
Patient data were collected prospectively and verified retrospectively then entered into the University of Pittsburgh Bariatric Surgery Clinical database—a customized computer database (Access, Microsoft, Inc). Data sources included office charts, follow-up notes, hospital charts, and patient interview. Parameters included patient demographics, BMI, American Society of Anesthesia operative risk classification, comorbidity, length of hospital stay, complications, weight loss, and change in comorbidity. Patient follow-up was scheduled for every 3 months with laboratory evaluation every 6 months until weight loss stabilization occurred then at least once per year.
Weight Loss
Follow-up weights were obtained from the University of Pittsburgh Bariatric Surgery Clinic scale with a capacity of 400 kg. On occasion (< 30%), official weights were obtained from physician office scales or telephone interview. Weight loss was expressed in terms of %EWL or change in BMI. Ideal body weight was determined according to the Metropolitan Life Insurance Company1983 height/weight tables; for a given height, the middle weight for a medium frame person was chosen as the ideal body weight.
Diabetes Control
Biochemical Markers
FPG concentration (8 hours minimum fast) and HbA1c concentration were obtained on all patients postoperatively at 6 months, 12 months, and annually in the UPMC Bariatric Surgery follow-up clinic and used as biochemical markers of diabetes control. FPG was specifically used as a marker for diabetes diagnosis, resolution, and improvement after surgery. Plasma HbA1c was used to measure glycemic control. HbA1c is a measure of the degree to which hemoglobin is glycosylated in erythrocytes and is expressed as a percentage of total hemoglobin concentration.19 It reflects the exposure of erythrocytes to glucose in an irreversible and time- and concentration-dependent manner. HbA1c levels provide an indication of the average blood glucose concentration during the preceding 2–3 months, incorporating both pre- and postprandial glycemia. Because blood glucose concentrations vary widely during a 24-hour period and from day to day in diabetes, the measurement of HbA1c is the most accepted indicator of long-term glycemic control. However, the HbA1c level does not provide a measure of the magnitude or frequency of short-term fluctuations of blood glucose. Normal values (5–6%) indicate very good glycemic control, 7–8% indicate good glycemic control, 8–9% indicate fair glycemic control, and > 9 indicates poor glycemic control.
Change in Diabetes Medication Usage
Because the usage and daily dose of antidiabetic medication varies directly with the severity of T2DM, we used medication usage as an indicator of disease severity and disease status (improvement or exacerbation). Diabetic medication usage (requirement), dose, number of agents, and rate of medication withdrawal were therefore used to assess clinical improvement and resolution (remission) of T2DM. All patients had a careful assessment of dose and frequency of all diabetes medications including all oral antidiabetic agents and insulin (including its varieties) both pre- and postoperatively. Patient's primary physicians were responsible for preoperative selection of agent, dose and frequency as well as responsible for reducing, discontinuing or adding diabetic medications postoperatively when clinically appropriate.
Change in Diabetes Status
Diabetes was considered resolved in patients who had a normal FBG (≤110 mg/dL), a normal HgA1c, and who required no diabetic medications after surgery. Patients were considered improved if there was significant improvement in FBG (by >25 mg/dL) or if there was a significant reduction of HgA1c (by >1%), or if there was a significant reduction in diabetes medication or dose (by discontinuing one agent or 1/2 reduction in dose). Patients were considered unchanged if no significant improvement was obtained and aggravated if there was any worsening of the biochemical markers, or addition of a diabetic agent, or increase in medication dose. How quickly (in days) patients achieved resolution of diabetes in relation to the surgery date was also used an indicator of response to surgery. This was determined by querying patients as to when their medications were discontinued.
Change in Diabetes-Related Comorbidity
Major obesity and diabetes comorbidities, including hypertension, hypercholesterolemia, hypertriglyceridemia, and obstructive sleep apnea, were assessed for changes postoperatively. Hypertension was considered resolved if the patient was normotensive without medication, or improved if the patient was normotensive with medication, or a decrease in antihypertensive agent usage or dosage. Hypercholesterolemia and hypertriglyceridemia were considered resolved if serum levels fell to normal range without medication and improved if there was a reduction in medication usage or dosage. Sleep apnea was considered resolved if the patient obtained a normal sleep study evaluation or improved if she had a significant reduction in continuous positive airway pressure (CPAP) settings or symptoms.
Changes in Diabetes-Specific Complications
After surgery, patients completed a questionnaire that assessed improvement in chronic diabetic-related complications, including neuropathy and erectile dysfunction. They were asked if they noticed improvement, much improvement, no improvement or worsening of that specific condition. Other known complications of diabetes, including retinopathy, foot ulcer, atherosclerotic heart disease, peripheral vascular disease, or nephropathy, were not subject to evaluation in this study because they were considered not amendable to self evaluation or because their incidence in this population was too infrequent for evaluation.
Statistical Analysis
All parametric data were analyzed using Student's t test (paired and unpaired), or analysis of variance when appropriate. All nonparametric data were analyzed using Mann–Whitney tests or analysis of variance based on ranks. The z test and χ2 test were used to identify significant differences between proportions and categorical variables. A P value <0.05 was considered statistically significant. The statistical software Sigma Stat 2.0 for Windows (SPSS, Chicago, IL) was used for all analysis.
RESULTS
From 7/97 to 5/2002, 1160 patients underwent attempted LRYGBP at the University of Pittsburgh, and of these 240 patients (21%) met diagnostic criteria for T2DM or IFG. Complete follow-up information was achieved in 192 of 240 patients (80%), and the mean duration of follow-up was 19.7 months (range, 6–54 months). One patient who had diabetes since childhood and was considered insulin dependent was excluded from these analyses, which decreased the total population for analysis down to 191. The 49 patients for whom follow-up was not available were excluded from this study. These included 5 patients with incomplete data, 9 patients who declined to participate, 34 patients who could not be contacted despite multiple attempts, and 1 patient (0.5%) who died suddenly 28 days after surgery from an unknown cause (no autopsy). Preoperative demographics, operative risk, and diabetes-related data for the total population and subgroups according to duration and severity of diabetes are listed in Tables 1 and 2. In this study 54% of patients were superobese (BMI ≥ 50) and 15% had a BMI ≥ 60. A total of 1430 comorbidities were identified in the 191 diabetic patients (7.5 per patient), and 30 (16%) were newly diagnosed with T2DM during the preoperative assessment. The most common comorbidities included hypertension (70%), degenerative joint disease (57%), sleep apnea (57%), hypercholesterolemia (56%), hypertriglyceridemia (45%), depression (42%), gastroesophageal reflux disease (39%), asthma (18%), urinary stress incontinence (17%), fatty liver disease by history (10%), and cholelithiasis (3%). Diabetic complications diagnosed by clinical criteria and their prevalence in the study group included neuropathy (25%), erectile dysfunction (22%), coronary artery disease (12%), retinopathy (10%) nephropathy (7%), and diabetic foot ulcer (5%).
TABLE 1. Preoperative Characteristics of Study Population by Duration of T2DM/IFG (n = 191)
TABLE 2. Preoperative Characteristics of Study Population by T2DM Severity (n = 191)
Operative Outcomes
Primary operations performed included LRYGBP- Roux limb (75 cm) in 57 patients (30%), LRYGBP-Roux limb (150 cm) in 116 patients (61%), and LRYGBP- Roux limb (200–250 cm) in 18 patients (9%). Two patients had laparoscopic conversions of a failed VBG to RYGBP (1%) and 3 patients had intraoperative conversions from laparoscopic to open RYGBP (1.6%) because of severe adhesions. The median length of hospital stay was 3.3 days for all patients, 2.5 days for IFG patients, 3.0 for DC-T2DM patients, 3.0 days for OA-T2DM patients, and 3.9 days for I-T2DM patients (P > 0.05). When patients were stratified by duration of T2DM (<5 years, 6–10 years, >10 years) the median hospital stay for all groups was 3.0 days.
Complications
There were 16 early major complications (8.4%), most commonly pneumonia, and gastrojejunal leaks and 29 early minor complications (15%), most commonly contained gastrojejunal leaks (with out peritonitis or abscess) and wound infections. There were 10 late major complications (5.2%), most commonly small bowel obstruction and deep venous thrombosis. Late minor complications occurred in 19 patients (9.9%), most commonly prolonged emesis and marginal ulcer. The overall major complication rate was (13.6%) and minor complication rate was (24.9%). One mortality (0.5%) occurred suddenly on postoperative day 28 with no known cause (no autopsy) but pulmonary embolus or cardiac dysrhythmia was suspected.
Weight Loss
The mean total EWL for the population was 60.1% with a mean follow-up of 20 months. There were no differences in excess weight loss according to duration of T2DM: 61.2% for < 5 years, 58% for 6–10 years, and 59.1% for > 10 years (P = 0.568). However, there were differences in excess weight loss according to T2DM severity. Patients with IFG achieved a mean EWL of 73% compared with 65%, 57%, and 59% for patients with DC-T2DM, OA-T2DM, andI-T2DM, respectively (P = 0.01). Significant weight loss according to change in BMI (15–18 BMI points) is noted after LRYGBP and is largely sustained out to 4 years (P < 0.001; Fig. 2). Most patients, however, remain with a BMI above 30 kg/m2 (threshold for obesity). All subgroups di-vided according to duration of T2DM and severity achieved significant weight loss (P < 0.001; Fig. 3). Weight loss did not correlate with duration of disease, but patients with the least severe disease (IFG) had the best weight loss (BMI = 30.0) compared with patients with the most severe disease (IT2DM, BMI = 35.3; P < 0.03).
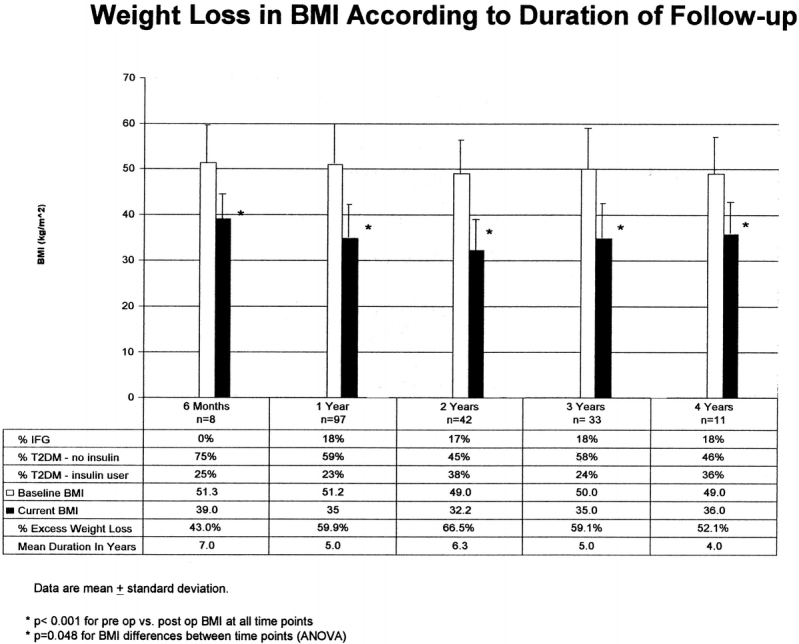
FIGURE 2. Weight loss in BMI according to duration of follow-up.
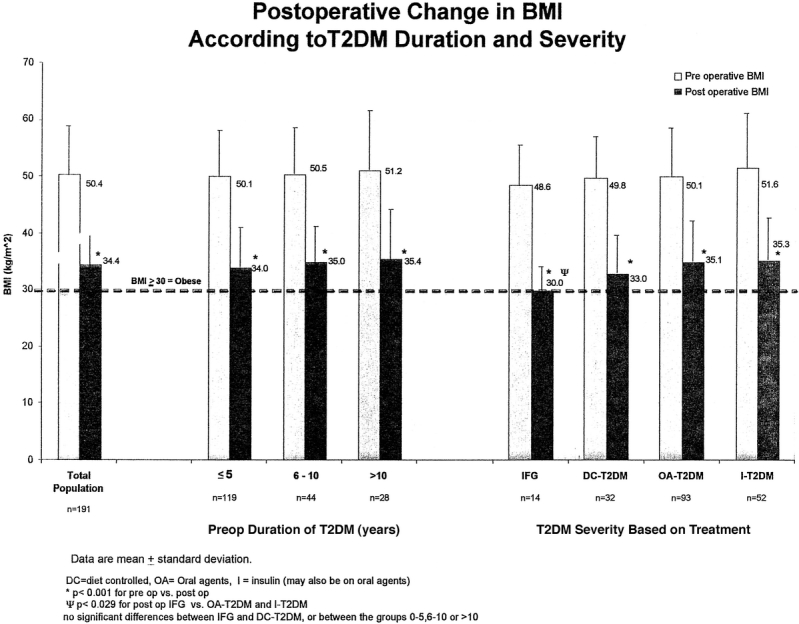
FIGURE 3. Postoperative change in BMI according to T2DM duration and severity.
Impact on Diabetes
Biochemical Markers
Surgically induced weight loss had a major impact on diabetes. Biochemical markers of diabetes severity (FPG) and long-term control (HbA1c) were markedly improved or returned to normal levels in all patients (Figs. 4 and 5). For the total population, mean FPG decreased post surgery from 187 mg/dL to 100 mg/dL and mean HbA1c decreased from 8.2% to 5.5% (P < 0.001). Patients with less severe disease (IFG) had significantly better post surgery FPG and HbA1c compared with insulin-requiring patients with more severe disease (86 mg/dL and 5.0% vs. 113 mg/dl and 6.0%, respectively, P < 0.001). Preoperative duration of T2DM did not result in significant differences in postoperative FPG or HbA1c among the groups (P = 0.418 and P = 0.148, respectively).
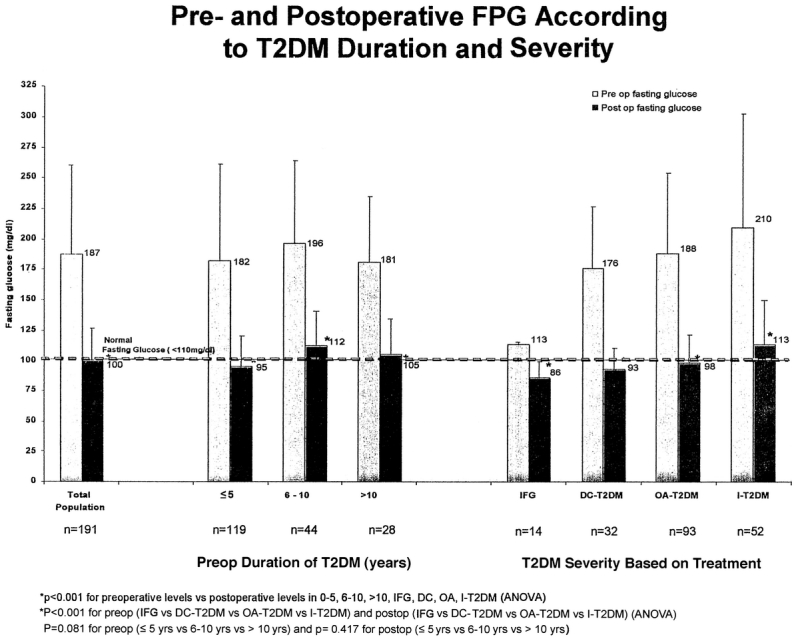
FIGURE 4. Pre- and postoperative FPG according to T2DM duration and severity.
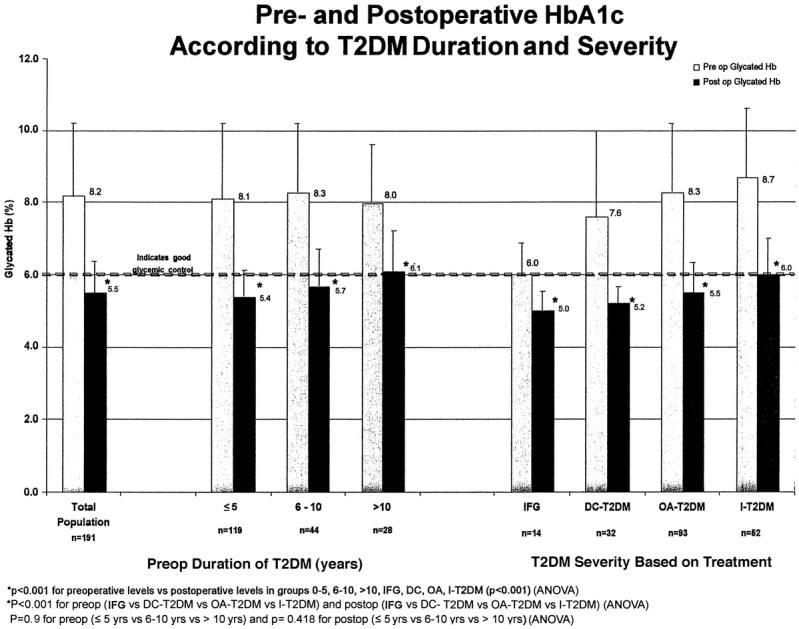
FIGURE 5. Pre- and postoperative HbA1c according to T2DM severity and duration.
Diabetes Medication Usage
A significant reduction in percent usage of oral antidiabetic agents and insulin (Fig. 6A and B) followed surgical treatment of all patients who required pharmacologic management. For insulin using patients, the mean insulin dosage requirement (mixed regular and NPH insulin) in 52 insulin-using patients before surgery was 96 units per patient per day and 33 of the 52 patients (64%) also required oral agents (1.3 oral medications per patient per day). After surgery, 11 patients required daily insulin (79% reduction) and their daily insulin dose requirement decreased from 146 units/day to 45 units/day (P = 0.019). Only 13 of 33 patients on both oral agents and insulin before surgery still required oral medications (73% reduction), and the quantity of agents required per day remained constant (1.2 oral medications per patient per day). A total of 4 patients only required both insulin and oral agents after surgery.
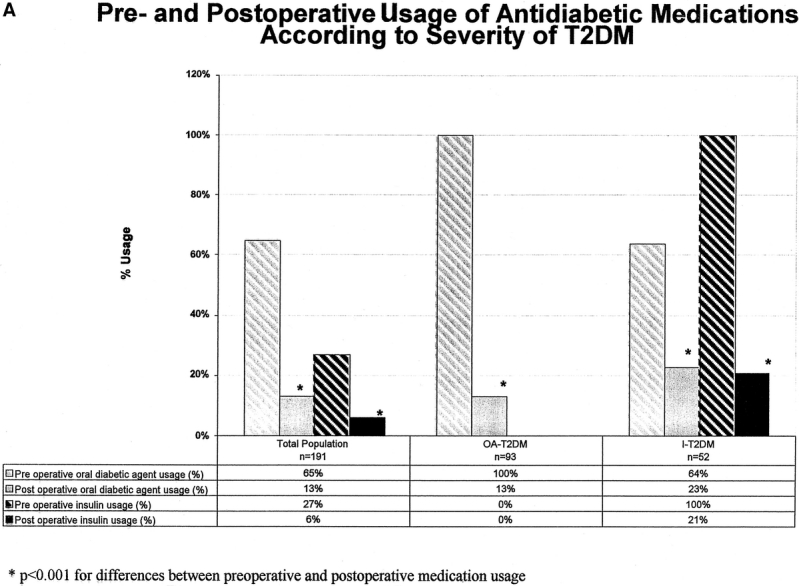
FIGURE 6A. Pre- and postoperative usage of antidiabetic medication according to severity of TLDM.
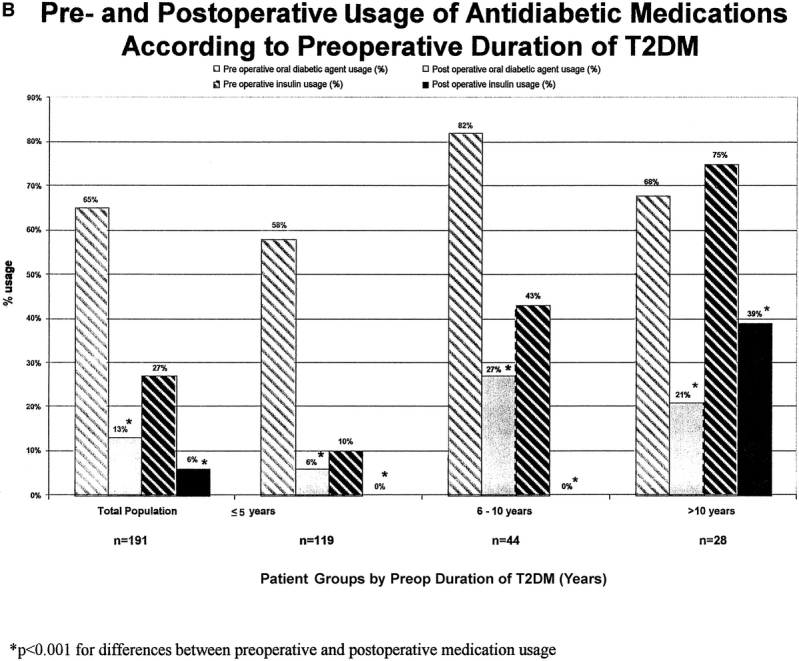
FIGURE 6B. Pre- and postoperative usage of antidiabetic medications according to preoperative duration of T2DM. P < 0.001 for differences between preoperative and postoperative medication usage.
For patients on oral agents only (not on insulin) there were 93 patients who required a total of 149 oral antidiabetic agents prior to surgery (mean of 1.6 agents per patient). After surgery, only 12 of the 93 patients required oral agents (87% reduction) and for these patients the number of oral agents required after surgery decreased from 28 agents (2.1 per patient) to 14 agents (1.1 per patient; P = 0.003).
In 30% of patients requiring pharmacologic therapy preoperatively (oral agents or insulin), all diabetic medications were discontinued immediately after hospital discharge (before significant weight loss), that is, immediate medication independence. A higher rate of immediate medication independence was observed in patients with the shortest duration of T2DM (34% and 30% for T2DM < 5 years and T2DM 6–10 years respectively versus 18% for patients with > 10 history of T2DM, P = 0.003). This occurrence of early medication withdrawal was also more pronounced in those patients taking oral hypoglycemics alone (57%) as compared with those who required insulin (23%) (P < 0.001).
Clinical Resolution of T2DM
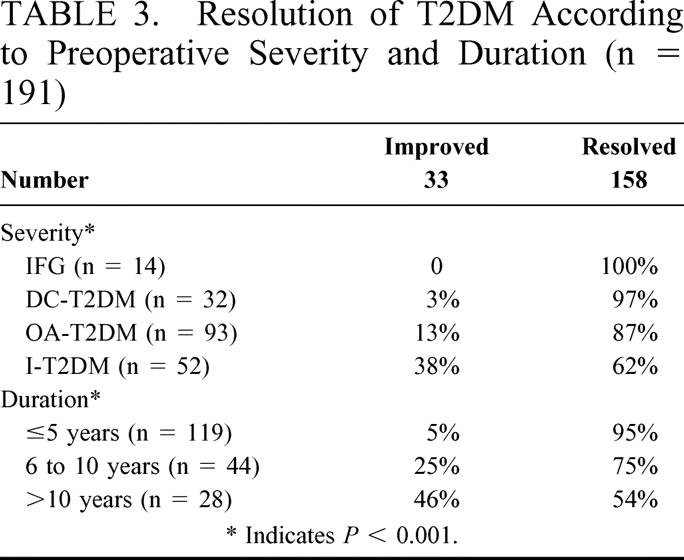
Clinical resolution or improvement in diabetes occurred in all patients evaluated (no patients remained the same) with the most dramatic changes occurring in patients with a short duration of diabetes or less severe diabetes (Table 3). Patients with a long history of DM (>10 years) or with severe DM (insulin using) were less likely to achieve complete resolution (P < 0.001 and P = 0.001, respectively). During the study period there were no new occurrences or reoccurrences of T2DM in 310 patient-years follow-up.
TABLE 3. Resolution of T2DM According to Preoperative Severity and Duration (n = 191)
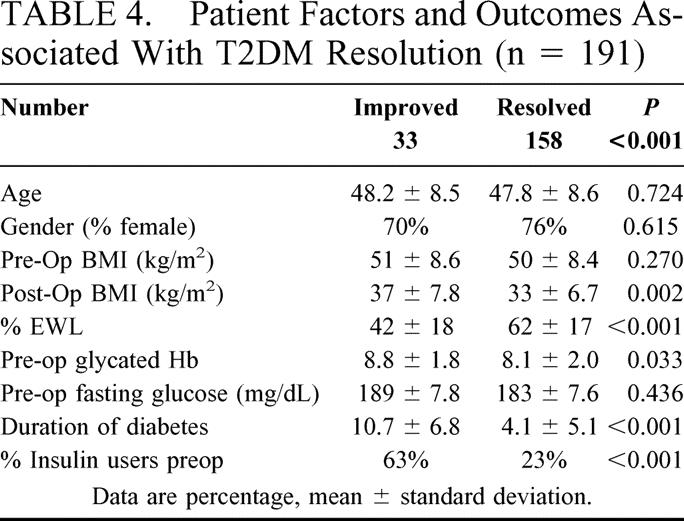
Patient factors and outcomes that were negatively associated with T2DM resolution versus improvement included preoperative duration and severity of T2DM; whereas net weight loss (EWL) and preoperative degree of glycemic control, were positively associated with T2DM resolution (Table 4).
TABLE 4. Patient Factors and Outcomes Associated With T2DM Resolution (n = 191)
Impact on Obesity Comorbidities and Complications of T2DM
Complete resolution or improvement in major comorbidities was noted in the majority of patients. Hypertension (n = 134) resolved in 48 patients (36%), improved in 71 patients (53%) remained unchanged in 12 patients (9%) and worsened in 3 patients (2%). Hypercholesterolemia (n = 107) resolved in 40 patients (37%), improved in 44 patients (41%), remained unchanged in 3 patients (3%) and worsened in one patient (1%). Obstructive sleep apnea (n = 109) resolved in 36 patients (33%), improved in 51 patients (47%), remained unchanged in 11 patients (10%) and worsened in 1 patient (1%). Diabetic neuropathy was present in 47 patients preoperatively (25%), and symptomatic improvement was reported by 50% of patients after surgery: 33% much improved, 17% improved, 39% no change, 7% worse, and 4% unknown. Diabetic erectile dysfunction was present in 11 of 48 males (23%) preoperatively: improvement was reported by 18% of patients, whereas 82% were unchanged.
DISCUSSION
The problem of obesity and T2DM in the United States is legion. Obesity and T2DM are currently 2 of the most common chronic, debilitating diseases of Western society; both have experienced epidemic growth in the last few decades. Thirty-four percent of the US adult population is overweight (BMI 25–29.9), and another 27% is obese (BMI >30).20 The prevalence of obesity has increased by more than 75% since 1980.21 In the US there are 800,000 new cases of diabetes per year (almost all are type 2) and almost 8% of the adult population and 19% of the population older than the age of 65 years have diabetes.22 The understanding that obesity is an etiologic factor in the development of T2DM is well established.23 There is no medical cure for T2DM, and despite treatment with antidiabetic medication, the natural course of the disease is characterized by progression to microvascular complications, leading to neuropathy, nephropathy, erectile dysfunction, retinopathy, and cardiovascular disease.24 T2DM in the United States is the most common cause of blindness, renal failure, and amputation, and 70% of diabetic patients die of cardiovascular disease.24,25 The cost for treating diabetes and its complications in the US is estimated to be 100 billion dollars per year.26 Although surgical correction of morbid obesity has been shown to significantly improve or resolve T2DM,2,5–13 rarely is bariatric surgery mentioned in the management algorithm for T2DM or even considered as a therapeutic option in the management of T2DM in general practice.24
In this study of 191 patients with T2DM and IFG, we demonstrated that during this 5-year period, LRYGBP resulted in significant excess weight loss (mean 60%), restoration of mean FPG (187 mg/dL preoperatively and 100 mg/dL postoperative; P < 0.001) and mean HbA1c (preoperatively 8.2% to 5.5%, P < 0.001) to normal levels, and profound reduction in the percentage of patients using antidiabetic medication (oral agent usage 65% to 13%; insulin usage 27% to 6%, P < 0.001). The major morbidity and mortality rate of 13.6% and 0.5% respectively appears reasonable for these relatively high risk patients.
T2DM in Morbidly Obese Patients Seeking Bariatric Surgery
Demographics of the patient population is noteworthy in that patients (Table 1) were predominately female (white) with an average age in the late 40s and mean BMI of 50. In this study population there was a vast spectrum of T2DM from prediabetes (IFG) to severe disease requiring large doses of insulin (>100 units/day). Additionally, many patients were recently diagnosed with T2DM whereas others had the disease for 17 years or more. Contrary to the perception that T2DM is a mild disease, the disease severity was quite significant overall, with a majority of patients requiring oral agents (65%) and approximately 1 quarter (27%) requiring insulin as well. The mean preoperative FPG (180 mg/dL) and HgA1c (8%) were significantly beyond normal range despite attempts to optimize glycemic control before surgery. As might be expected, oral agent usage (68%) and insulin usage (75%) in patients with longstanding T2DM (>10 years) was much higher than in patients with early disease (58% and 10%, respectively, P < 0.02;P < 0.001). This finding is consistent with the known gradual loss of glycemic control occurring in T2DM patients that is thought to result from beta cell deterioration over time related to chronic hyperglycemia.24 Interestingly, as T2DM severity increased (Table 2) so did patient age, % males, American Society of Anesthesia class (operative risk), and preoperative glycemic control according to FPG and HbA1c. These findings suggest that patients with severe T2DM are not only higher operative risk but also may be more refractory to any treatment (medical or surgical).
Weight Loss After LRYGBP in Patients With Diabetes
Weight loss is the direct goal of LRYGBP and appears to account for most of the clinical benefit. The mean 60% EWL and decrease in BMI from 50 to 34 achieved in this series is comparable to reported results for the major open and laparoscopic bariatric operations in diabetic patients (Table 5). 2,5–13,27 Our data suggest that net weight loss in diabetic patients is inferior to nondiabetics because our initial study (78% nondiabetic) demonstrated 83–77% EWL at 2 to 2–1/2 years follow-up, whereas this study shows 67–59% EWL for patients with diabetes at 2 to 3 years follow-up.2 Dixon et al10 for LASGB and Wittgrove et al5 for LRYGBP demonstrated a similar pattern of inferior weight loss for patients with diabetes compared with their total population of patients (Table 5). Adding further strength to this argument, our study is the first to show that increasing severity (but not preoperative duration) of T2DM also negatively affects net weight loss independent of preop BMI (Fig. 3). A plausible explanation for diabetic patients’ apparent resistance to weight loss may be due to inherent, unexplained metabolic differences between patients with diabetes and nondiabetics. Alternatively, antidiabetic medications, including oral agents and insulin, have been known to cause weight gain and thus may infer resistance at least in patients who remain on these agents after surgery.24 The weight loss achieved in this series is largely sustained out to 4 years, but the patients with longer follow-up do appear to have some slight weight regain to a BMI of 36 and net EWL of 52%. Others similarly have shown some weight regain after LRYGBP over time but most of the weight loss is maintained out to 14 years.7 Whether patients with diabetes are prone to greater weight regain than nondiabetics is not known.
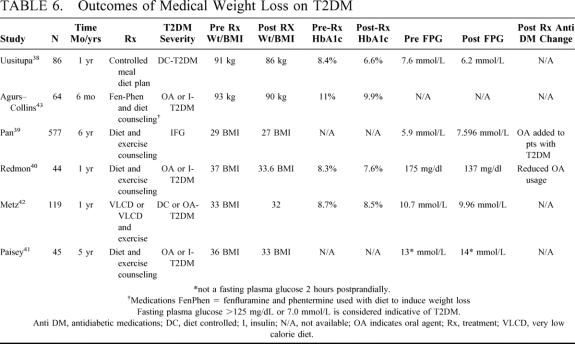
TABLE 5. Outcomes of Surgical Weight Loss on T2DM
Summary of Outcomes of Bariatric Surgery on T2DM
Resolution of T2DM
Our results are consistent with recent studies demonstrating significant and sustained improvement in T2DM (up to 10–20 years) after the major types of bariatric operations, including vertical banded gastroplasty, laparoscopic adjustable silicone gastric banding (LASGB), RYGBP, and biliopancreatic diversion (Table 5). Direct comparison of these studies is problematic because they are quite variable in terms of the distribution of severity of T2DM within the study population (ie, %IFG vs. DC-T2DM vs. OA-T2DM vs. I-T2DM) and methodology of evaluating improvement by biochemical or clinical assessment. Nevertheless, clinical resolution, usually defined as independence from all antidiabetic medication, was reported to occur in 47–80% of patients after restrictive procedures, 80–98% of patients after RYGBP, and 96–100% of patients after BPD procedures. More reliable biochemical evidence of improvement was available in few studies. Dixon et al and Pontiroli et al independently showed restoration of mean FPG to normal levels (6.2 and 5.4 mmol/L, respectively) and HbA1c to near normal levels (6.2% and 5.3%, respectively) after LASGB in predominantly prediabetic patients (IFG).6,10 The higher rate of diabetes resolution achieved by Pontiroli et al (80%) versus Dixon et al (64%) is most readily explained by the higher rate of prediabetes (71% vs. 55%) in the Pontiroli study. The Pories study (50% T2DM, 50% IGT) and the present study (93%T2DM, 7% IFG) showed that FPG reduces to normal or near normal levels (117 mg/dL vs. 98 mg/dL, respectively) and HbA1c improves to normal or near normal levels (6.6% vs. 5.6%, respectively) after RYGBP. The differences in diabetes resolution between the Pories study and our study (89% vs. 82%, respectively), like the LASGB series differences, are likely explained by study population differences in rates of T2DM versus IFG or IGT. For the BPD procedure, Scopinaro et al showed that it perhaps achieved even more durable normalization of plasma glucose and glycosylated hemoglobin (actual levels not reported) in 80–100% of patients with T2DM than LASGB or RYGBP.9 The impact of theses changes in glycemic control after bariatric surgery are amplified further when one considers that preoperative levels of HbA1c (mostly above 8%) were usually taken in the setting of medical management with the intent to optimize glycemic control before surgery. Thus most patients after surgery were able to simultaneously wean from medical therapy and achieve normalization of glycemic control.
Although not the primary focus of this study, proposed mechanisms that may explain the relative differences in diabetes resolution between the various restrictive versus bypass procedures include (1) weight loss (reduced fat cell mass), (2) reduced calorie intake (negative energy balance), and (3) changes in gut hormone secretion due to foregut bypass.28–30 Most agree that although there appears to be a correlation between procedure specific weight loss (less for restrictive, more for bypass procedures), net weight loss may not necessarily be the dominant mechanism driving T2DM resolution since many patients after RYGBP are rendered euglycemic before significant weight loss occurs.28 In our study for example, nearly one third (30%) of patients permanently discontinued antidiabetic medications after discharge from the hospital before significant weight loss could occur. Reduced calorie intake, which does occur immediately after all the procedures, may partially explain both the immediate and long-term improvement because it has been shown that very low-calorie diets (800 calories/day) can also result in immediate improvement in T2DM.31 Perhaps a more promising explanation, however, unique to the bypass procedures (RYGBP and BPD) is the alteration in gut hormone secretion manifested by plasma concentration increases in glucagon-like peptide-132 and PYY,33,34 and or decreases in ghrelin.37 Many other gut hormones, “incretins,” may play a role in T2DM pathophysiology. The effect of these and other gut hormones on glycemic control, insulin resistance, and beta cell function are largely unknown and ripe for future investigation.
Clinical Improvement in Diabetes: an “Asymptomatic Disease”
Biochemical improvement and resolution of glycemic control are the means to achieve the end goal of clinical improvement of T2DM and decreased disease progression. Because treated diabetes is primarily an asymptomatic disease (rarely does hyperglycemia cause debilitating symptoms) factors other than symptom control must be used to measure clinical improvement. In this study we used medication reduction as one indicator of clinical improvement. We showed that LRYGBP resulted in an impressive 80% reduction in oral agent usage and 79% reduction in insulin usage (65% to 13% and 27% to 6%, respectively, P < 0.001; Figs. 6A and 6B). The quantity of oral agents required decreased by 84% from 189 oral agents per day in 126 patients (1.5 oral agent/patient/day) to 30 agents per day in 25 patients (1.2 oral agents/patient/day; P < 0.0.001) The quantity of insulin required decreased by 90% from 4992 units per day in 52 patients (96 units/patient/day) to 495 units per day in 11 patients (45 units/patient/day; P < 0.001). Most of the patients who remained dependent on antidiabetic medications had either long-standing disease (>10 years) or severe disease (high insulin requirement) before surgery. Similarly, MacDonald et al35 showed that morbidly obese patients with T2DM who underwent RYGBP decreased their diabetic medication requirement (oral agents or insulin) from 31% to 8.6% (P = 0.001) whereas an unoperated control group of severely obese patients with diabetes increased their diabetic medication requirement from 56.4% to 87.5% (P = 0.003) during the 6-year follow-up period. Sugerman et al27 found a similar reduction in oral agent usage (35% to 9%) and insulin usage (39% to 20%) after RYGBP. After LASGB, Dixon et al also showed a significant reduction in oral medication usage at 1 year after surgery. Preoperatively, 29 patients were on 58 medication whereas postoperatively 8 patients were on 16 medications. Three of 4 patients who were on insulin preoperatively required insulin postoperatively but had lower postoperative insulin dosages.10 Diabetic medication reduction may be an under emphasized significant clinical benefit to the diabetic patient in terms of associated improvement in quality of life and reduction in associated costs and side effects of these agents which may include pain and discomfort from insulin injection, dangerous hypoglycemia, gastrointestinal symptoms, and weight gain.24
Other measures of clinical improvement may include assessment of obesity comorbidities and secondary complications of diabetes. Our results show significant improvement or resolution in hypertension, hypercholesterolemia, and obstructive sleep apnea for the majority of our diabetic patients. Others have demonstrated similar changes in comorbidities for nondiabetics and patients with diabetes after bariatric surgery.2,5–7,9–17 Furthermore, we found that 50% of patients with neuropathy noted improvement and 18% of patients with erectile dysfunction noted improvement following surgery and subsequent weight loss. To our knowledge this is the first report of potential improvement in secondary complications of diabetes after bariatric surgery. We do, however, acknowledge that accuracy of theses results are, of course, limited by the fact that they represent self-assessment as opposed to direct measures of neuropathy and erectile function. A placebo effect of surgery cannot be excluded as an explanation for these findings.
Predictors of T2DM Resolution
As shown in Table 4, factors that appear to negatively correlate with T2DM resolution include preoperative duration of T2DM, elevated HbA1c, and insulin usage. Both elevated HbA1c and insulin usage are measures of increased T2DM severity. The magnitude of weight loss (EWL) and postsurgery BMI, measures of reduced fat cell mass, positively correlate with T2DM resolution. Other investigators have found similar correlations with diabetes remission. Dixon et showed that EWL and preoperative duration of T2DM were independent predictors of diabetes remission after LASGB.36,37 Sugerman found that younger age and EWL were predictors of T2DM resolution, although age was not a predictor of resolution in our study or the Dixon study.27 To our knowledge, our study is the first to show that both preoperative duration of T2DM and severity negatively influence the likelihood of T2DM resolution.
Medical Versus Surgical Management
Currently best medical management of T2DM in severely obese patients consists of 1) conventional (low risk) weight loss strategies including diet modification (low calorie diet 1200 cal/d versus very low calorie diet 800 calories/d) along with exercise and pharmacologic agents and 2) “tight” glycemic control with intensive medical therapy to achieve an end point HbA1c < 7%. Table 6 summarizes results of contemporary studies employing these nonsurgical weight loss strategies in patients with obesity but not morbid obesity (BMI generally < 35 kg/m2).38–43 Weight loss is modest varying from 3–5 Kg or 2–3 BMI units and sustained weight loss beyond 1 year is demonstrated in only 2 of the studies.39,41 Correspondingly modest improvement in biochemical glycemic control was achieved, but the mean HbA1c remained above 7% in most series despite fairly intense medical therapy. Minimal data is available regarding reduction in medication requirement with medical weight loss. In 1 study, however, oral agents were added over time as patients progressed from IFG to T2DM despite modest weight loss,39 and in another study oral agents were reduced slightly as the mean HbA1c decreased from 8.3% to 7.6%.40
TABLE 6. Outcomes of Medical Weight Loss on T2DM
Tight glycemic control with medication (HbA1c less than 7%) has been shown to decrease the risk of microvascular complications (retinopathic, nephropathic, and neuropathic) associated with diabetes in 2 large randomized studies (United Kingdom prospective diabetes study, UKPDS, and diabetes control and complications trial, DCCT).44,45 These studies both showed that for every drop of 1% (ie, from 9% to 8% HbA1c) there was a relative risk reduction of 25% to 45%. Glycemic control to achieve a HbA1c ≤ 7 in some of these patients was only possible with insulin doses > than 100 units per day and even then for only short periods of time.45 It is believed that in community settings where >95% of diabetic patients are treated, that HbA1c levels in I-T2DM patients typically vary from 8.5 to 9% suggesting that tight control with medication is difficult to achieve.45 Furthermore, intensive medical therapy required to achieve a HbA1c ≤ 7% has a corresponding 2%-4% yearly incidence of severe hypoglycemia.45
Direct comparison of medical versus surgically induced weight loss in the management of T2DM demonstrates not surprisingly that surgery yields superior weight loss both in terms of magnitude and sustainability. Perhaps more importantly, our data and others show that bariatric operations consistently result in superior glycemic control with restoration of HbA1c to normal levels. The only treatment thus far that has been proven to alter the natural progression of T2DM and improve microvascular related disease is treatment that restores patients to euglycemia. Unequivocally, surgery is superior to medical management of T2DM. Clearly though, surgery carries a higher risk, but the major morbidity and mortality rate of 13.6% and 0.5%, respectively, in our series appears to be a reasonable risk for the immediate clinical benefit and promising long-term consequence of minimizing the progression of this very debilitating disease. Thus our study and others suggest that bariatric surgery should strongly be considered an option for patients with severe obesity and T2DM.
The Case for Early Surgical Intervention
The present study results suggest that not only should bariatric surgery be considered an option for obese T2DM patients, but also early surgical intervention is warranted. Patients with less severe T2DM have better weight loss from surgery and the magnitude of weight loss appears to increase the likelihood of resolution. Sugerman et al showed that younger age patients are more likely to resolve their T2DM.28 This study and others show that the likelihood of complete resolution is increased in patients with T2DM of short duration (<5 years) and less severe disease (noninsulin-requiring T2DM). These findings suggests that B cell deterioration as shown in other studies is reversible at least early in the course of the disease24 and that this may be the best time to intervene before permanent damage occurs. Our data also shows that patients with more severe disease are higher operative risks, thus earlier surgical intervention should decrease major complications. Finally, medical management is not as effective in achieving glycemic control nor is it likely to alter the course of the disease. Younger diabetic patients with less severe disease stand to gain more from surgery by circumventing years of progressive, debilitating disease.
CONCLUSION
It appears that weigh loss is the most effective treatment of patients with T2DM and severe obesity regardless of how it is achieved. Currently, bariatric surgery is the most effective weight loss treatment resulting in significant weight loss that is sustained for many years. Most patients with T2DM undergoing LRYGBP achieve excellent biochemical glycemic control and are able to reap the clinical benefits of withdrawing from most if not all antidiabetic medications including insulin. These significant benefits of surgery appear to justify the reasonable increased risks over conventional medical management. Although not proven, improved glycemic control resulting from surgically induced weight loss is likely to halt the progression of microvascular complications of T2DM. The impressive effect of LRYGBP on morbidly obese patients (BMI ≥ 35) with T2DM raises the argument for lowering the threshold for surgical intervention to moderate or mild obesity (BMI 25–35). Further investigation demonstrating risk versus benefit for patients with moderate obesity is warranted. Finally, this study suggests that bariatric surgery should be considered standard treatment of T2DM in morbidly obese patients who are appropriate surgical candidates.
Discussion
Dr. Michael G. Sarr (Rochester, Minnesota): Currently, obesity is possibly the greatest health crisis facing us in the United States. One need only consider diabetes mellitus, hypertension, coronary artery disease, hyperlipidemia, and degenerative joint disease. These diseases are all directly weight-related and potentially reversible with bariatric surgery. Thus we need to pay special attention to this paper by Schauer and colleagues as well as the paper on Saturday by Gary Anthone from USC.
Frighteningly, some investigators have speculated that bariatric surgery may become the most common general surgical procedure performed in the next decade. We in the American Surgical Association should remember that as surgeons, we continue to be internists who can also operate.
Dr. Schauer, I have 2 questions. First, patients with morbid obesity have insulin resistance. Did you measure serum insulin levels to correlate the extent of insulin resistance with outcome? Second, some investigators have suggested that gastric bypass has effects on weight loss independent of absolute caloric intake. Have you any thoughts on this as relates to the correction of diabetes mellitus?
Dr. Philip R. Schauer (Pittsburgh, Pennsylvania): Thank you, Dr. Sarr. Two very good questions.
First of all, we did not specifically measure postoperative insulin levels or insulin resistance. Our study mainly focused on outcome parameters looking at fasting plasma glucose and hemoglobin A1C. However, others have looked at these levels with gastric bypass surgery with some of the malabsorption operations as well as restrictive procedures. And they have shown with these operations a reduction in postoperative insulin and an improvement in insulin resistance. And these may be very, very important factors in terms of the mechanistic reasons why patients get better.
Your other question dealt with caloric intake, whether or not changing the anatomy, that is a diversionary procedure versus a restrictive procedure, has a difference in resolution of diabetes independent of weight loss. The best way to look at this is to compare results of restrictive operations, such as the adjustable banding procedures, and gastric bypass.
We know certainly that the benefit is primarily weight-loss driven. The restrictive procedures in general do not ultimately result in as large an amount of weight loss as the gastric bypass operations.
Some authors have hypothesized that other intestinal or gut hormones such as gastrointestinal peptide may be altered by the rerouting of the GI tract and this may affect insulin utilization and resistance. Our study did not specifically look at that mechanism. But I think more endocrine-based metabolic studies need to be performed on these operations with a better idea as to exactly why these operations work and it may give us a better insight into the pathophysiology of Type 2 diabetes.
Dr. Michael A. Meguid (Syracuse, New York): This is a very interesting study. I should like to thank Dr. Schauer and his colleagues for giving me the privilege of reviewing their manuscript. It is reassuring to see in this age of fiscal limitations that clinical research continues to flourish. The authors are to be congratulated for their excellent follow-up of the 1,160 patients who underwent laparoscopic Roux-en-Y gastric bypass (RYGB) of whom 240 had impaired fasting glucose or Type II diabetes associated with morbid obesity; 192 were available for follow up. I have 3 questions interspersed by a comment. My first question: Of the 192 patients, 16 had a gastrojejunal leak postoperatively. Such a leak, even if contained, would be associated with physiological stress and hyperglycemia. Therefore, were these patients included in your results? If so, how were they managed and did they affect the outcome of your study. My second question: Not all the 192 patients improved their impaired fasting glucose or Type II diabetes. Does he or his co-authors believe that this may be related to varying the length of the afferent small bowel limb?
My comment is designed to provide some insight as to the mechanisms of your results. Your data confirms that the best method for treating Type II diabetes, hyperlipidemia and other metabolic abnormalities associated with morbid obesity is a RYGB, which limits food intake. The limited food intake is diverted from the gastric atrium, duodenum and the approximately 100 cm of proximal jejunum; known powerful neuroendocrine organs. However, your data was not designed to show that the normalization of hyperglycemia and hyperinsulinemia occurs within the first few postoperative days, long before a significant weight loss has occurred. Based on this observation, Pories et al1 proposed that Type II diabetes and obesity are 2 separate disease entities because there are Type II patients with diabetes who are slim, and as your data show, only 21% of the 1,160 morbidly obese patients had diabetes. Pories postulated “RYGB changes the intestinal plumbing, preventing the foregut neuroendocrine organs from being stimulated by food.”
This hypothesis was tested in an RYGB model in diet-induced obese rats.2 Hypercalcemia and hyperinsulinemia disappeared within 10 days after RYGB. The model allows the mechanism of diabetes and other metabolic disorders to be examined using gene array profiling to identify candidate genes in the appetite centers of the hypothalamus in which the carbohydrate and lipid metabolic pathways were found to be up-regulated, eg, pro-gastrin and pro-insulin are increased by 5-7 fold. These act as a negative feedback to most of the same genes of carbohydrate and lipid metabolic pathways in peripheral fat and muscle. Thus these genes and their protein products are down-regulated. This model permits us to postulate that the hyperglycemia is due to the over stimulation by food of the foregut inducing pro-inflammatory cytokines especially TNF, IL-1, IL-6 and leptin. The cytokines stimulate the hypothalamic-pituitary-adrenal axis inducing up-regulation of ACTH and cortisol secretion in blood, which in turn contributes to the hyperglycemia.3
These metabolic abnormalities are reversed remarkably quickly after RYGB, long before weight loss occurs; merely by diverting the stream of nutrients away from the proximal foregut. In essence, we conclude, “too much food is a metabolic stressor.” My third question therefore emanates from my comment: Have you had the opportunity to measure cytokines and cortisol in either blood or fat tissues of your patients?
I should like to thank the Association for giving me the privilege of making these comments.
REFERENCES
- 1.Pries WJ, Albrecht RJ. Etiology of type II diabetes mellitus: role of the foregut. World J Surg. 2001;25:52731. [DOI] [PubMed] [Google Scholar]
- 2.Xu Y, Ohinata K, Meguid MM, Marx W, Tada T, Chen C, Quinn R, Inui A, Gastric bypass model in the obese rat to study metabolic mechanisms of weight loss. J Surg Res. 2002;107:5663. [DOI] [PubMed] [Google Scholar]
- 3.Ramos EJB, Xu Y, Romanova I, Middleton F, Chen C, Quinn R, Inui A, Das U, Meguid M. Is obesity an inflammatory disease? Surgery 2003, In press. [DOI] [PubMed]
Dr. Philip R. Schauer (Pittsburgh, Pennsylvania): The first question dealt with intestinal leaks after surgery. Yes, we did report our results on those patients. Most of those were contained leaks. I believe there were only 3 leaks that actually had any sort of sepsis parameters at all and those were managed operatively and resolved. Those did not appear to affect the long-term outcome in these patients.
Number 2, we did not specifically measure any of these cortisol or other hormones in these patients. I think that is very interesting hypothesis that should require further investigation.
Dr. Henry Buchwald (Minneapolis, Minnesota): I would like to thank Dr. Schauer for giving me an advance copy of his paper. This is the second landmark paper presented by this group before this Association. It confirms the Pories’ conclusion, makes it conclusive, and divides it into cohorts of analysis. Basically, it states the less severe the disease and the shorter the duration, the better are the results. This is intuitive. But it needed to be demonstrated. The 73% overall response rate is sensational. No diet or no drug therapy can do this.
I would like to caution the authors about contributing the effect on hypertension, dyslipidemia, and sleep apnea to a decrease in diabetes. We see these outcomes in nondiabetics as well. They are cobenefits, not necessarily cause and effect.
I have 2 questions: Do you have any data on your Type 1 patients with diabetes whom you have operated on? And, you discussed the neuropathy and the penile dysfunction in your Type 2s. You did not discuss the retinopathy, nephropathy, and enteropathy. Do you have any data with respect to these entities, especially the nephropathy? This information would be of interest to our transplant surgeons, who routinely refer their obese patients to us preoperatively.
Thank you for this paper. Thank you for the privilege of the floor.
Dr. Philip R. Schauer (Pittsburgh, Pennsylvania): Thank you, Dr. Buchwald.
Regarding Type 1 patients, in our entire series of almost 2,000 patients now we have only had just a handful of patients who were Type 1. So we can’t make any strong conclusions about their outcome. I can say that in general they got better. Their insulin requirement decreased significantly. I can’t say anything about their end-organ function over the long-term. But in most of them, their insulin requirement did decrease significantly. All of them still required insulin after the weight loss.
The second question regarded other end-organ parameters of diabetes resolution. We specifically looked at neuropathy and erectile dysfunction because these are fairly easy to substantiate at least according to symptoms or patient response. All the others are quite a bit more difficult.
We did look at nephropathy. We measured pre and postoperative BUN creatinine in these patients. First of all, a fairly small percentage, only 5% of our patients, had a true diabetic necropathy. So it was a fairly small group to compare. Some of them BUN creatinine improved, some of them stayed the same, some got worse. The population was so small that we didn’t feel it was appropriate to make any strong conclusions. So that definitely needs to be done. And it will probably take a follow-up period of several years, 5 years or more, to really see if there is significant improvement in those patients.
Footnotes
Presented at the annual meeting of The American Surgical Society, Thursday, April 24, 2003, Washington DC.
Reprints: Philip R. Schauer, MD, Director of Bariatric Surgery, The University of Pittsburgh, Magee-Women's Hospital, Suite 5500, 300 Halket Street, Pittsburgh, PA. E-mail: schauerpr@msx.upmc.edu; Website: www.upmc.edu/obesitysurgery.
REFERENCES
- 1.Higa KD, Boone KB, Ho T. Complications of the laparoscopic Roux-en-Y gastric bypass: 1, 040 patients—what have we learned? Obes Surg. 2000;10:509–513. [DOI] [PubMed] [Google Scholar]
- 2.Schauer PR, Ikramuddin S, Gourash W, et al. Outcomes after laparoscopic Roux-en-Y gastric bypass for morbid obesity. Ann Surg. 2000;232:515–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DeMaria EJ, Sugarman HJ, Kellum JM, et al. Results of 281 consecutive total laparoscopic Roux-en-Y gastric bypasses to treat morbid obesity. Ann Surg. 2002;235:640–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nguyen NT, Ho HS, Palmer LS, et al. A comparison study of laparoscopic versus open gastric bypass for morbid obesity. J Am Coll Surg. 2000;191:149–155. [DOI] [PubMed] [Google Scholar]
- 5.Wittgrove A, Clark G. Laparoscopic gastric bypass, Roux-en-Y 500 patients: technique and results, with 3–60 month follow-up. Obes Surg. 2000;10:233–239. [DOI] [PubMed] [Google Scholar]
- 6.Pontiroli A Pizzocri P, Librenti M, et al. Laparoscopic adjustable gastric banding for the treatment of morbid (grade 3) obesity and its metabolic complications: a three year study. J Clin Endocrinol Metab. 2002;87:3555–3561. [DOI] [PubMed] [Google Scholar]
- 7.Pories WJ, Swanson MS, MacDonald KG, et al. Who would have thought it? An operation proves to be the most effective therapy for adult-onset diabetes mellitus. Ann Surg. 1995;222:339–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marceau P Hould FS, Simard S, et al. Biliopancreatic diversion with duodena switch. World J Surg. 1998;22:947–954. [DOI] [PubMed] [Google Scholar]
- 9.Scopinaro N, Gianetta E, Adani GF, et al. Biliopancreatic diversion for obesity at eighteen years. Surgery. 1996;119:261–268. [DOI] [PubMed] [Google Scholar]
- 10.Dixon JB, O'Brien PE. Health outcomes of severely obese type 2 diabetic subjects 1 year after laparoscopic adjustable gastric banding. Diabetes Care. 2002;25:358–363. [DOI] [PubMed] [Google Scholar]
- 11.Sjostrom C, Lissner L, Wedel H, et al. Reduction in incidence of diabetes, hypertension and lipid disturbances after intentional weight loss induced by bariatric surgery: the SOS Intervention Study. Obes Res. 1999;7:477–484. [DOI] [PubMed] [Google Scholar]
- 12.Karason K, Lindroos A, Stenlof K, et al. Relief of cardiorespiratory symptoms and increased physical activity after surgically induced weight loss: results from the Swedish Obese Subjects study. Arch Intern Med. 2000;160:1797–1802. [DOI] [PubMed] [Google Scholar]
- 13.Pasquali R, Vicennati V, Scopinaro N, et al. Achievement of near-normal body weight as the prerequisite to normalize sex hormone-binding globulin concentrations in massively obese men. Int J Obes. 1997;21:1–5. [DOI] [PubMed] [Google Scholar]
- 14.NIH Conference. Gastrointestinal surgery for severe obesity; consensus development conference panel. Ann Int Med. 1991;115:956–961. [PubMed] [Google Scholar]
- 15.Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 2003;26:S5–S20. [DOI] [PubMed]
- 16.Schauer PR, Ikramuddin S. Laparoscopic surgery for morbid obesity. Surg Clin North Am. 2001;81:1145–1179. [DOI] [PubMed]
- 17.Schauer P, Ikramuddin S, Hamad G, et al. The learning curve for laparoscopic Roux-en-Y gastric bypass is 100 cases. Surg Endosc. 2003;17:212–215. [DOI] [PubMed] [Google Scholar]
- 18.Schauer P. Laparoscopic Roux-en-Y gastric bypass at the University of Pittsburgh Evolution of Techniques. Op Tech Gen Surg.Surg Endosc. 2003;5:114–124. [Google Scholar]
- 19.Kilpatrick E. Glycated haemoglobin in the year 2000. J Clin Pathol. 2000;53:335–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prevalence of overweight and obesity among adults: United States, 1999. National Center for Health Statistics, Health E-Stats, 2000 April 10, 2003; http://www.cdc.g v/nchs/pr ducts/pubs/pubd/hestats/bese/bse99.htm.
- 21.Flegal K, Carroll M, Kuczmarski R, et al. Overweight and obesity in the United States: prevalence and trends, 1960–1994. Int J Obes Relat Metab Dis. 1998;22:39–47. [DOI] [PubMed] [Google Scholar]
- 22.Harris M, Flegal K, Cowie C, et al. Prevalence of diabetes, impaired fasting glucose, and impaired glucose tolerance in U.S. adults. Diabetes Care. 1998;21:518–524. [DOI] [PubMed] [Google Scholar]
- 23.National Task Force on the Prevention and Treatment of Obesity. Over-weight, obesity, and health risk. Arch Int Med. 2000;160:898–904.10761953 [Google Scholar]
- 24.Nathan D. Initial management of glycemia in type 2 diabetes mellitus. N Engl J Med. 2002;347:1342–1349. [DOI] [PubMed] [Google Scholar]
- 25.Panzram G. Mortality and survival in type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia. 1998;30:123–131. [DOI] [PubMed] [Google Scholar]
- 26.Rubin R, Altman W, Mendelson D. Health care expenditures for people with diabetes mellitus, 1992. J Clin Endocrinol Metab. 1994;78:809A–809F. [DOI] [PubMed] [Google Scholar]
- 27.Sugerman H, Wolfe L, Sica D, et al. Diabetes and hypertension in severe obesity and effects of gastric bypass induced weight loss. Annals of Surgery. 2003;237:751–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pories W, Albrecht R. Etiology of type II diabetes mellitus: role of the foregut. World J Surg. 2001;25:527–531. [DOI] [PubMed] [Google Scholar]
- 29.Kellum J, Kuemmerle J, O'Dorisio R, et al. GI hormone response to meals before and after gastric bypass and vertical banded gastroplasty. Ann Surg. 1990;211:763–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Greenway S, Greenway F, Klein S. Effects of obesity surgery on non-insulin-dependent diabetes mellitus. Arch Surg. 2002;137:1109–1117. [DOI] [PubMed] [Google Scholar]
- 31.Wing R, Marcus M, Salata R, et al. Effects of a very-low-calorie diet in long-term glycemic control in obese type II diabetic subjects. Arch Intern Med. 1991;151:1331–1340. [PubMed] [Google Scholar]
- 32.Mason E. Ileal transposition and enteroglucagon/GLP-1 in obesity (and diabetic? ) surgery. Obes Surg. 1999;9:223–228. [DOI] [PubMed] [Google Scholar]
- 33.Alvarez B, Borque M, Martinez-Sarmiento J, et al. Peptide YY secretion in morbidly obese patients before and after vertical banded gastroplasty. Obes Surg. 2002;12:324–327. [DOI] [PubMed] [Google Scholar]
- 34.Cummings D, Weigle D, Frayo R, et al. Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. N Engl J Med. 2002;346:1623–1630. [DOI] [PubMed] [Google Scholar]
- 35.MacDonald K, Long S, Swanson M, et al. The gastric bypass operation reduces the progression and mortality of non-insulin-dependent diabetes mellitus. Gastrointest Surg. 1997;1:213–220. [DOI] [PubMed] [Google Scholar]
- 36.Dixon J, O'Brien P. Changes in comorbidities and improvements in quality of life after LAP-BAND placement. Am J Surg. 2002;184:51S–54S. [DOI] [PubMed] [Google Scholar]
- 37.Dixon J, Dixon A, O'Brien P. Improvements in insulin sensitivity and beta-cell function (HOMA) with weight loss in the severely obese. Homeostatic model assessment. Diabetic Med. 2003;20:127–134. [DOI] [PubMed] [Google Scholar]
- 38.Uusitupa MIJ. Early lifestyle intervention in patients with non-insulin dependent diabetes mellitus and impaired glucose tolerance. Ann Med. 1996;28:445–449. [DOI] [PubMed] [Google Scholar]
- 39.Pan X, Li G, Hu Y, et al. Effects of diet and exercise in preventing NIDDM in people it impaired glucose tolerance: the Da Qing IGT and diabetes study. Diabetes Care. 1997;20:537–544. [DOI] [PubMed] [Google Scholar]
- 40.Redmon J, Raatz S, Kwong C, et al. Pharmacologic induction of weight loss to treat type 2 diabetes. Diabetes Care. 1999;22:896–903. [DOI] [PubMed] [Google Scholar]
- 41.Paisey R, Frost J, Harvey A, et al. Five year results of a prospective very low calorie diet or conventional weight loss programme in type 2 diabetes. J Hum Nutr Dietet. 2002;15:121–127. [DOI] [PubMed] [Google Scholar]
- 42.Metz J, Stern JS, Kris-Etherton P, et al. A randomized trial of improved weight loss with a prepared meal plan in overweight and obese patients: impact on cardiovascular risk reduction. Arch Intern Med. 2000;160:2150–2158. [DOI] [PubMed] [Google Scholar]
- 43.Agurs-Collins T, Kumanyika S, Ten Have T, et al. A randomized controlled trial of weight reduction and exercise for diabetes management in older African American subjects. Diabetes Care. 1997;20:1503–1511. [DOI] [PubMed] [Google Scholar]
- 44.The relationship of glycemic exposure (HbA sub1c) to the risk of development and progression of retinopathy in the diabetes control and complications trial. Diabetes 1995;44:968–983. [PubMed] [Google Scholar]
- 45.Intensive blood glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352:837–853. [PubMed] [Google Scholar]