Abstract
Objective:
To assess our outcomes after minimally invasive esophagectomy (MIE).
Summary Background Data:
Esophagectomy has traditionally been performed by open methods. Results from most series include mortality rates in excess of 5% and hospital stays frequently greater than 10 days. MIE has the potential to improve these results, but only a few small series have been reported. This report summarizes our experience of 222 cases.
Methods:
From 1996 to 2002, MIE was performed in 222 patients. Indications for operation included high-grade dysplasia (n = 47) and cancer (n = 175). Neoadjuvant chemotherapy was used in 78 (35.1%) and radiation in 36 (16.2%). Initially, a laparoscopic transhiatal approach was used (n = 8), but subsequently our approach evolved to include thoracoscopic mobilization (n = 214).
Results:
There were 186 men and 36 women. Median age was 66.5 years (range, 39–89). Nonemergent conversion to open procedure was required in 16 patients (7.2%). MIE was successfully completed in 206 (92.8%) patients. The median intensive care unit stay was 1 day (range, 1–30); hospital stay was 7 days (range, 3–75). Operative mortality was 1.4% (n = 3). Anastomotic leak rate was 11.7% (n = 26). At a mean follow-up of 19 months (range, 1–68), quality of life scores were similar to preoperative values and population norms. Stage specific survival was similar to open series
Conclusions:
MIE offers results as good as or better than open operation in our center with extensive minimally invasive and open experience. In this single institution experience, we observed a lower mortality rate (1.4%) and shorter hospital stay (7 days) than most open series. Given these results, we are now developing an intergroup trial (ECOG 2202) to assess MIE in a multicenter setting.
This report summarizes outcomes in 222 patients who underwent minimally invasive esophagectomy. The median intensive care unit stay was 1 day; hospital stay was 7 days. The operative mortality rate was 1.4%. At a mean follow-up of 19 months, quality of life scores were similar to population norms.
Now that advanced minimally invasive surgical procedures are being performed more frequently, detailed results and outcomes must be reported to the surgical community to assess potential advantages and disadvantages. A number of open approaches are used to resect esophageal cancer. These operations are frequently associated with significant morbidity and a mortality rate reported from experienced centers in the range of 6–7%.1 In a report summarizing nationwide statistics, the mortality rates from esophagectomy ranged from 8% in high-volume centers to as high as 23% in low-volume centers.2 Given concerns over this high morbidity and mortality, patients with esophageal cancer may not even be referred for operation at all. Older patients and those with comorbid conditions may be referred for nonsurgical therapies; for example, photodynamic therapy is used in some centers to treat high-grade dysplasia. Photodynamic therapy, with its low mortality rate (which approaches 0%) is attractive to patients and referring physicians, even though its results have not been proven to equal those of surgical resection.3 Yet, because of the risk of incomplete ablation of Barrett's and the risk of missing an early stage occult cancer that could be potentially cured by resection, most surgeons continue to favor esophagectomy.4
Since the introduction of laparoscopic fundoplication in 1991,5 improvements in instrumentation and optics have allowed the development of minimally invasive approaches to esophageal diseases that have been traditionally managed by open operation. Such diseases include the treatment of achalasia,6 giant paraesophageal hernia,7 and other complex esophageal disorders.8-10 Minimally invasive esophagectomy (MIE) has the potential to lower the morbidity of open operation and allow quicker return to normal function. In addition if morbidity is lowered, a greater number of patients may be referred and benefit from MIE. We have previously reported on our initial experience of MIE in 77 patients.10 This report details our growing experience of MIE and describes outcomes in 223 patients.
MATERIALS AND METHODS
We store our data in a prospective institutional review board-approved database designed to assess outcomes after MIE. We entered and analyzed all data on an SPSS file (version 11 for Windows) and included two-tailed t-tests, χ2 and Kaplan-Meier survival analysis.
Over a 5-year period from June 1996 through August 2002, we performed MIE in 222 patients. The primary inclusion criterion for esophageal cancer patients fit for operation was the presence of a resectable lesion after evaluation with endoscopic ultrasound (EUS) and computerized tomography (CT). If we felt that the gastric tube was not suitable for reconstruction or a neck anastomosis, we used an open approach with an intrathoracic anastomosis. In addition to esophageal cancer, patients with high-grade dysplasia were included in this series.
Initially, we used a laparoscopic transhiatal approach for patients with smaller tumors or high-grade dysplasia (n = 8). This approach rapidly evolved with time to include the addition of a right video-assisted thoracoscopic (VATS) approach to mobilize the intrathoracic esophagus and to allow a more complete lymph node dissection. This combined thoracoscopic and laparoscopic approach remains our procedure of choice in most patients (n = 214). Our current technique is similar to our previous description previous description.10 But given the unfamiliarity of most surgeons with this technique along with the addition of important minor modifications we hereby include a complete description of our current operative technique below.
Operative Technique
We perform an on-the-table esophagogastroduodenoscopy (EGD) to make a final assessment of the tumor's location and the gastric conduit's suitability for reconstruction. If the EGD, EUS, or CT scan findings suggest gastric extension, T4 local extension or possible metastases, we perform a staging laparoscopy or a thoracoscopy or both. Patients are then intubated with a double-lumen tube and positioned in the left lateral decubitus position. The surgeon stands on the right and the assistant on the left. Four thoracoscopic ports are used (Fig. 1). A 10-mm camera port is placed at the 7th to 8th intercostal space, just anterior to the midaxillary line. A 5-mm port is placed at the 8th or 9th intercostal space, posterior to the posterior axillary line, for the ultrasonic coagulating shears (US Surgical, Norwalk, CT). A 10-mm port is placed in the anterior axillary line at the 4th intercostal space; this port is used to pass a fan shaped retractor to retract the lung anteriorly and allow exposure of the esophagus. The last 5-mm port is placed just posterior to the scapula tip; it is used to place instruments for retraction and countertraction. In most patients a single retracting suture (0-Endostitch, US Surgical, Norwalk, CT) is placed near the central tendon of the diaphragm and brought out through the inferior anterior chest wall through a 1-mm skin incision. Doing so provides downward traction on the diaphragm, allowing good exposure of the distal esophagus.
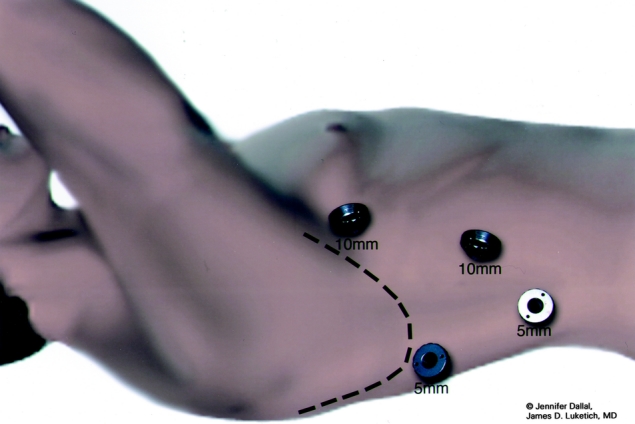
FIGURE 1. Video-assisted thoracoscopic surgical port sites.
Next, the inferior pulmonary ligament is divided. The mediastinal pleura overlying the esophagus is divided up to the level of the azygos vein to expose the thoracic esophagus. An endoscopic stapler (Endo-GIA II; US Surgical, Norwalk, CT) is used to divide the azygos vein. Care is taken to preserve the mediastinal pleura above the azygos vein. We believe this pleura helps to maintain the gastric tube in a mediastinal location and may also help seal the plane around the gastric tube near the thoracic inlet, thereby minimizing the downward extension of a cervical leak into the chest. Circumferential mobilization of the esophagus is performed up to the level of 1 to 2 cm above the carina, including all surrounding lymph nodes; periesophageal tissue and fat; the plane along the pericardium, aorta and contralateral mediastinal pleura up to but not including the thoracic duct and azygos vein laterally. Care is taken to clip any aortoesophageal vessels and to clip any lymphatic branches from the thoracic duct. A Penrose drain is placed around the esophagus to facilitate traction and exposure (Fig. 2). The entire intrathoracic esophagus is mobilized from the thoracic inlet to the diaphragmatic reflection. As the dissection proceeds toward the thoracic inlet, care is taken to stay near the esophagus to avoid trauma to the posterior membranous trachea and the recurrent laryngeal nerves. Care is also taken to avoid extending the distal dissection too low into the peritoneal cavity to avoid difficulty in maintaining pneumoperitoneum during the abdominal dissection.
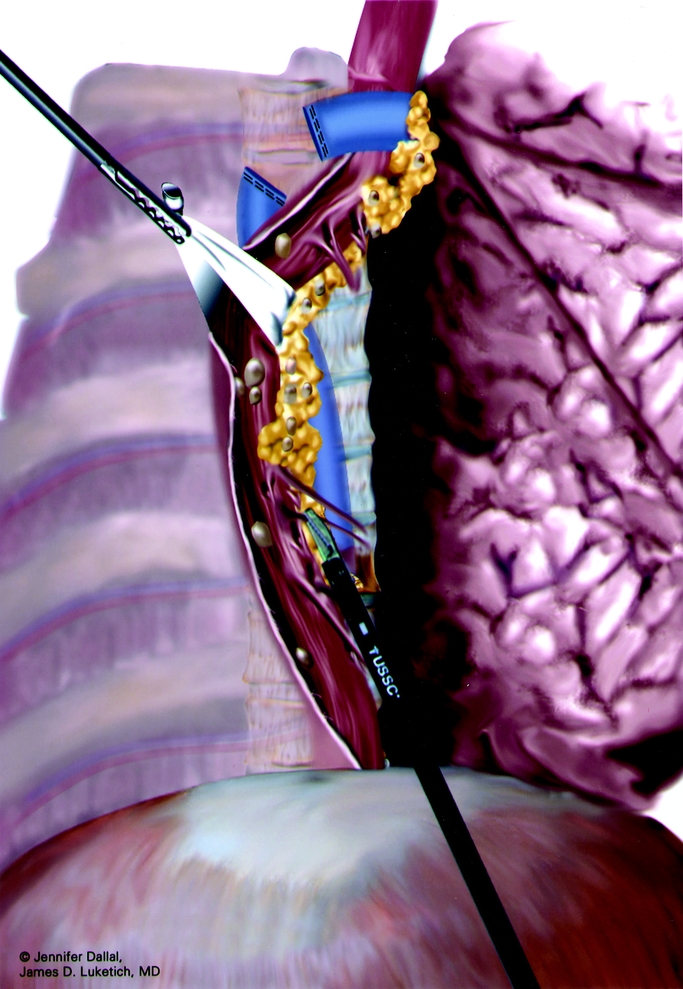
FIGURE 2. Penrose drain around thoracic esophagus.
After placement of a single 28-F chest tube, each intercostal space is injected with 1 to 2 mL of 0.5% bupivacaine with epinephrine. The lung is then inflated to search for any air leaks from the trachea, proximal bronchus, and re-expanded lung. We then close the thoracic ports and turn the patient to the supine position. The surgeon remains on the patient's right; the patient is positioned in steep reverse Trendelenburg. Five abdominal ports (four 5-mm and one 11-mm) are used for the dissection (Fig. 3). The gastrohepatic ligament is divided; the right and left crura of the diaphragm are dissected. At this stage of the operation we avoid dividing the phrenoesophageal membrane, as early entry into the mediastinum may lead to loss of pneumoperitoneum into the chest cavity and to technical difficulties. The stomach is mobilized by dividing the short gastric vessels using the ultrasonic coagulating shears. The gastrocolic omentum is divided with care taken to preserve the right gastroepiploic arcade. The stomach is retracted superiorly, and the left gastric vessels are identified. The left gastric artery and vein can be divided from the retrogastric or lessor curve view, depending on the anatomy, using the Endo-GIA stapler (vascular load). Currently, we perform pyloroplasty in all cases. In our experience, pyloromyotomy was not able to be performed satisfactorily. The ultrasonic shears are used to open the pylorus, and the Endo-stitch (2.0, US Surgical, Norwalk, CT) is used to close the pylorus transversely (Fig. 4). A gastric tube is then constructed by dividing the stomach starting at the lesser curve and preserving the right gastric vessels with the 4.8-mm stapler (Endo-GIA II, US Surgical, Norwalk, CT; Fig. 5). There is some variability in the construction of the gastric tube based on the characteristics of the tumor. It may be necessary to construct a slightly more narrow tube or to resect some of the proximal stomach in tumors with significant gastric extension, In general the need to resect more stomach is recognized during the preoperative EUS; the surgeons EGD or laparoscopic staging if performed. If gastric extension of the tumor is significant, we generally prefer to resect more stomach and to make an intrathoracic anastomosis. For most patients in this current report, there was minimal gastric involvement. Currently, we prefer a gastric tube of 5 to 6 cm in diameter. However, midseries we used a narrower tube (3 to 4 cm) in 58 patients and noted an increase in gastric tip necrosis and neck leaks with intrathoracic extension. This practice has now been modified so that we use a larger gastric tube (5 to 6 cm). Extreme caution must be used when manipulating the gastric tube during mobilization and stapling to avoid trauma. The most cephalad portion of the gastric tube is then attached to the esophageal and gastric specimen using two 2.O Endo-sutures. An additional superficial stitch may be placed on the anterior proximal gastric tube to facilitate orientation and prevent twisting as the tube is brought up into the neck.
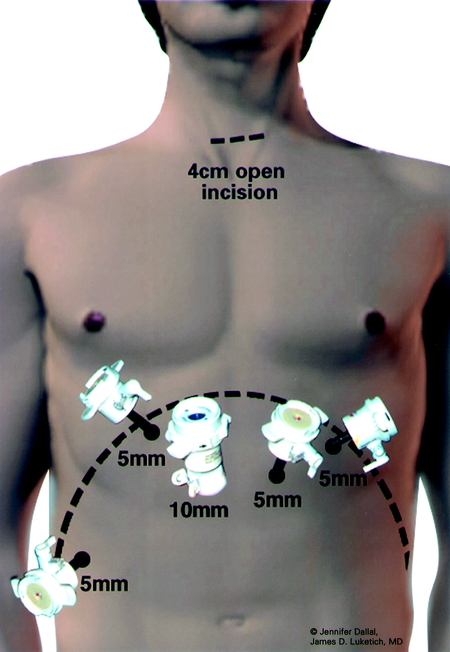
FIGURE 3. Abdominal port sites for laparoscopy.
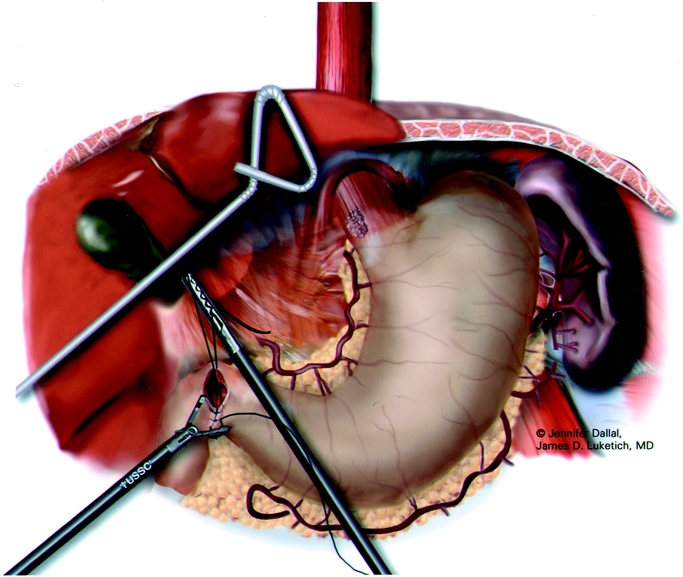
FIGURE 4. Laparoscopic pyloroplasty.
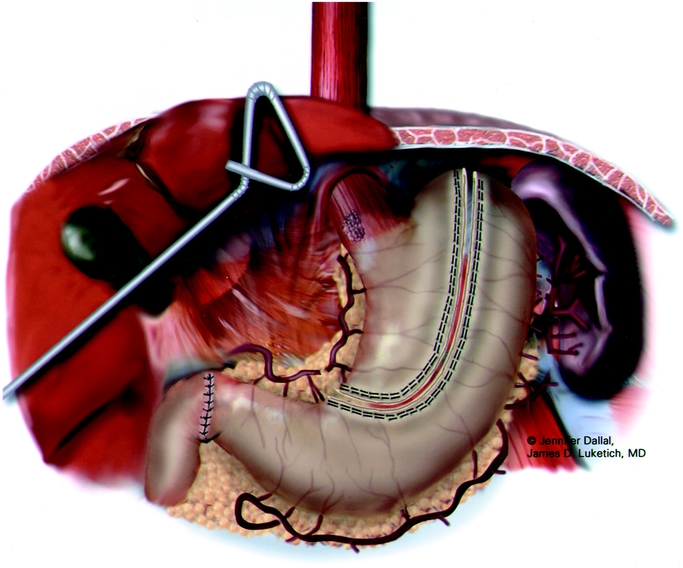
FIGURE 5. Laparoscopic gastric tubularization.
A feeding jejunostomy tube is placed laparoscopically by first attaching a limb of proximal jejunum (25 cm distal to the ligament of Treitz) to the anterior abdominal wall in the left lateral midquadrant with the Endo-stitch. In most cases, we add an additional 10-mm port in the right lower quadrant to facilitate suturing of the jejunum to the anterior abdominal wall. A needle catheter kit (Compact Biosystems, Minneapolis, MN) is placed percutaneously into the peritoneal cavity. Under direct laparoscopic vision, the guide wire and catheter are directed into the loop of jejunum that has been tacked to the anterior abdominal wall. The entry site of the needle catheter j-tube is tacked completely to the anterior abdominal wall for a distance of several centimeters.
The last step in the abdominal operation is the dissection of the phrenoesophageal membrane. Doing so at this stage helps to minimize the loss of pneumoperitoneum into the mediastinum during the earlier parts of the laparoscopic procedure. We also partially divide the right and left crura to allow easy passage of the gastric specimen and tube through the hiatus and to prevent later gastric outlet obstruction.
Next, a 4- to 6-cm horizontal neck incision is made. The cervical esophagus is exposed. Careful dissection is performed down until the thoracic dissection plane is encountered, generally quite easily since the VATS dissection is continued well into the thoracic inlet. In addition, we leave the penrose drain around the esophagus during the thoracic dissection and push the drain into the peri-esophageal plane at the thoracic inlet, so that it is easily visualized during the neck dissection and actually allows the surgeon to pull the penrose out through the neck to facilitate the neck dissection. The esophagogastric specimen is pulled out of the neck incision and the cervical esophagus divided high (1 to 2 cm below the cricopharyngeal muscle). The specimen is removed from the field. An anastomosis is performed between the cervical esophagus and gastric tube using standard techniques. Currently we prefer the 25-mm EEA stapler. Next, the surgeon returns to the laparoscopic view and gently pulls downward on the pyloroantral area to retrieve any excess gastric tube that may have been pulled up into the chest during the neck anastomosis and mobilization. The laparoscopic pull is performed gently, and only until the assistant at the neck observes the tube beginning to be pulled down at the level of the anastomosis. We strive to achieve a very high anastomosis just below the level of the cricopharyngeus to ensure adequate removal of any islands of Barrett's and to ensure that any anastomotic leaks, will be more likely to drain out via the neck.
The last step of the laparoscopic approach is to place tacking sutures between the gastric tube and the diaphragm to prevent hiatal herniation. Care must be taken during this step to maintain orientation of the greater curve vessels towards the left crus and to avoid compromise of these vessels during suturing. We usually place 3 tacking sutures; 1 between the left crus and stomach just anterior to the greater curve arcade; the second on the right side of the gastric tube just above the right gastric vessels to the right crus; the third suture is placed anteriorly between the stomach and the diaphragm. The completed reconstruction is shown in Figure 6.
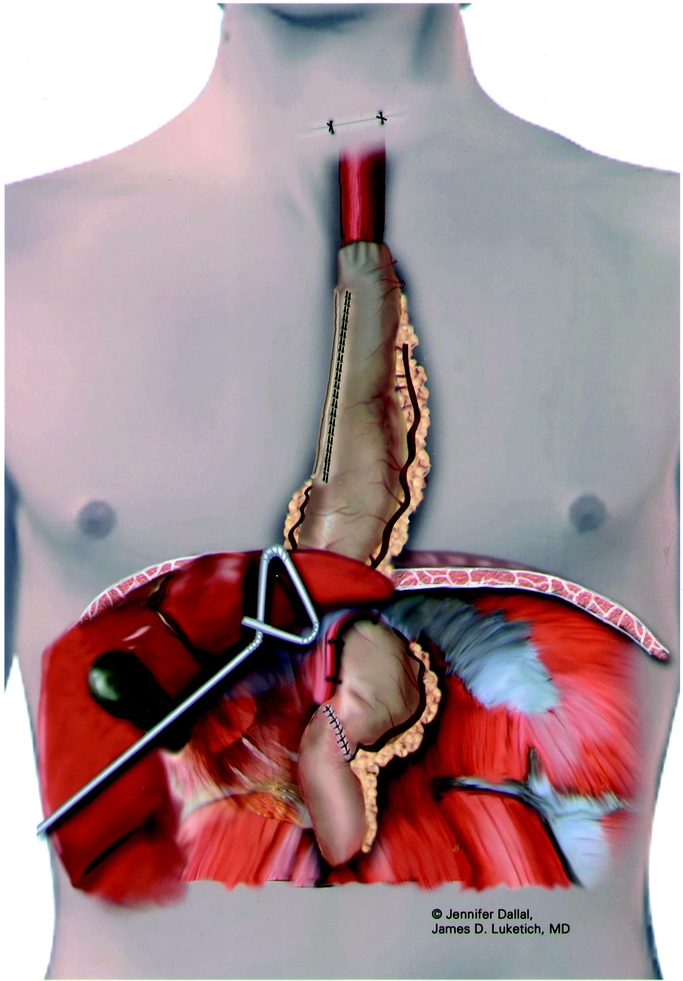
FIGURE 6. Completed reconstruction using gastric tube.
Subjective Measures of Outcome
In addition to standard reporting of outcome we also recorded subjective measures of outcome. We used of the Short-Form 36 (SF36) to measure quality of life (QOL). This instrument has been extensively validated and population normal values identified.11 Scores were expressed as physical component summary scores and mental component summary scores (MCS). In addition the Gastroesophageal Reflux disease-Health Related Quality of Life Scale (HRQOL) was used to measure postoperative reflux.12 This disease-specific instrument consists of 9 questions with responses from 0 to 5. The best possible score (no symptoms) is 0 and the worst possible score (most severe symptoms) is 45. We classified HRQOL scores as excellent (0–9), satisfactory (10–14), or poor (15–45). Dysphagia was measured using a scale from 1 (no dysphagia) to 5 (severe dysphagia).
RESULTS
Our 222 patients included 186 (83.8%) men and 36 (16.2%) women. Median age was 66.5 years (range, 39–89). Preoperative indications for operation included carcinoma in 175 (78.8%) and high-grade dysplasia in 46 (21.2%). Neoadjuvant chemotherapy was used in 78 (35.1%) patients and radiation in 36(16.2%) patients. Before MIE, expandable esophageal stents had been placed in 13 patients (5.9%), jejunostomy tubes in 4 patients (1.8%) and gastrostomy tubes in 3 patients (1.4%). Additionally 19 patients (8.6%) had undergone photodynamic therapy at some point in their preoperative care for severe dysphagia. Usually these procedures were performed to assist with nutritional intake during induction therapy but some were performed prior to referral to our institution. There was a history of previous open abdominal surgery in 55 (24.8%) of patients
Most of the MIE operations were performed at the university hospital (n = 166); 56 were performed at an affiliated tertiary care hospital by our thoracic surgical group. The stomach was used as a conduit in all cases. The esophageal bed was used for the gastric conduit in 213 cases and the substernal route selected in 9 cases to allow postoperative radiation to the esophageal bed without irradiation of the gastric pull-up. Pyloromyotomy was performed in 28 and pyloroplasty in 136 patients Midseries, our group used a narrower tube of 3 to 4 cm in diameter (n = 58) to avoid the need for a pyloric drainage procedure and in the hope that less reflux would be encountered subsequently. This narrow gastric tube was later abandoned because of an increased leak rate (see below). A laparoscopic feeding jejunostomy was placed in 202 patients at the time of MIE
MIE was successfully completed in 206 (92.8%) patients. Thoracotomy was required in 12 (5.4%) and laparotomy in 4(1.8%) patients. In 5 of these 16 cases, minithoracotomy was selected from the outset, 10 cases required nonemergent conversions due to adhesions and 1 nonemergent conversion was necessary for a persistent intercostal vessel that could not be satisfactorily controlled by VATS and required a minithoracotomy to oversew.
The 30-day operative mortality was 1.4% (n = 3). One of these 3 deaths occurred in a 66-year patient who developed pneumonia with septic hemodynamic parameters on the third postoperative day. Initial endoscopy showed no evidence of anastomotic leak and a viable gastric tube. However further deterioration occurred with multisystem organ failure, subsequent ischemia of the gastric tube, and leakage from his pyloroplasty site requiring reoperation. Ultimately support was withdrawn on the twentieth postoperative day. The second death was from a postoperative myocardial infarction occurring on the 5th postoperative day and the third death was from pericardial tamponade occurring 3 days after MIE.
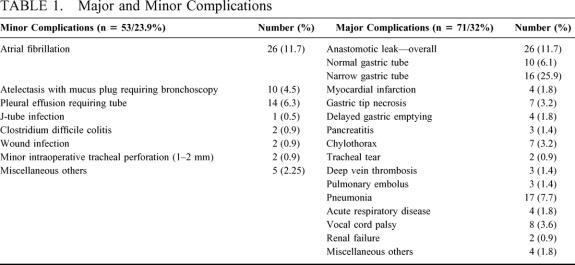
Major and minor morbidity are outlined in Table 1. The most common minor complications were atrial fibrillation (11.7%) and pleural effusions requiring a tube (6.3%). The anastomotic leak rate was affected by the size of the gastric tube. In those patients with our standard diameter gastric tube of 6 cm, anastomotic leaks occurred in 10 out of 164 (6.1%). In those patients where a narrow tube (3 to 4 cm) was used (n = 58) the leak rate was significantly increased (P < 0.001), occurring in 15 (n=27.6%) of patients.
TABLE 1. Major and Minor Complications
The median intensive care unit stay was 1 day (range, 1–30) time to oral intake was 4 days (range, 1–40), and hospital stay was 7 days (range, 3–75). The mean follow-up was 19 months (range, 1–68). Kaplan-Meier survival curves based on cancer stage are shown in Figure 7.
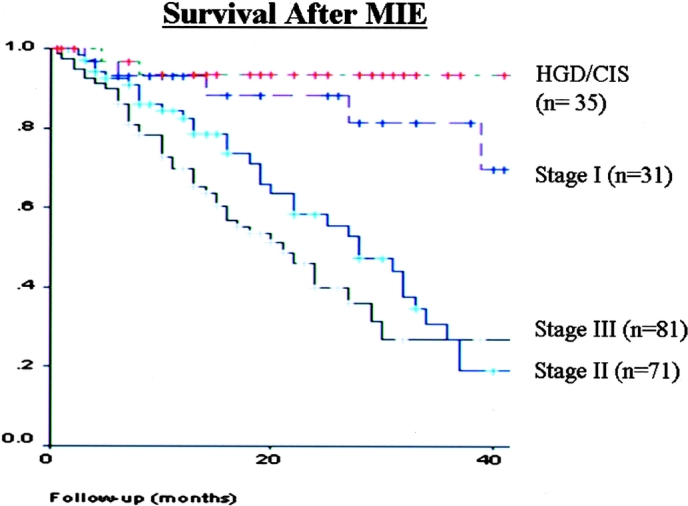
FIGURE 7. Kaplan-Meier Survival Curve after MIE based on cancer stage
Follow-up dysphagia scores were excellent with a mean score of 1.4. The mean HRQOL scores was 4.6, which represents a normal (no reflux) score. Only 4% of the patients questioned complained of significant reflux (HRQOL of 15 or more). Since the median age of our patients was 66.5 years, SF36 scores were compared with US norms for the 65–74 year age group. Mean physical component summary score was 44.1, which was not significantly different from a normal value of 43.33. The mean MCS score was 49.67. This was slightly but statistically (P = 0.001) lower than the US norm of 52.68. Preoperative and follow-up scores were only available in 57 patients. In this subgroup, pre- and postoperative scores were 46.5 and 43.7 (P = 0.431), respectively. Preoperative and postoperative MCS scores were 51.07 and 50.68 (P = 0.145), indicating preservation of quality of life after operation.
DISCUSSION
The optimal approach to esophagectomy remains controversial. Recently a randomized study was performed in the Netherlands comparing an extended transthoracic approach (with en bloc lymphadenectomy) to a transhiatal approach in 220 patients.13 At a median follow-up of 4.2 years, no significant difference in survival was noted between the 2 groups of patients. The overall mortality in this series was 3%, again with no difference noted between the 2 groups.
Probably of more importance than the approach used is the case volume and experience of the centers involved that perform esophagectomy. This was demonstrated in a report from Texas where mortality rates ranged from 12.2% in low volume centers to 3% in high volume centers after esophagectomy.13 Total morbidity rates including all minor and major complications are often not documented, although appear to be around 60% when reported.14,15 Most authors tend to report specific complications such as anastomotic leak rate. The series by Orringer et al of 1085 patients is one of the largest reported and serves as a standard to compare with.16 In their series, the overall anastomotic leak rate was 13% with a perioperative mortality of 4%. Fifty-three percent of patients were discharged by the 10th postoperative day.
More recently the results from a prospective VA database of 1777 patients undergoing esophagectomy were published.17 In this series perioperative mortality was 10% and the incidence of 20 predefined complications was 50%. Our mortality of 1.3% and median hospital stay of 7 days compare well with these results of open esophagectomy. In the VA series the most frequent reported complications were pneumonia (21%), respiratory failure (16%) and prolonged ventilator support (22%). In our series the low incidence of pneumonia (7.6%) and ARDS (5%) suggest an advantage for the minimally invasive approach.
MIE was first described by DePaula et al18 from Brazil in a series that included many patients with end-stage achalasia from Chagas’ Disease. Swanstrom et al's series19 of totally laparoscopic esophagectomy in 9 patients was the first report of MIE in North America. Both authors described a laparoscopic transhiatal approach to MIE. The advantages of the transhiatal approach are that no repositioning of the patient or single lung ventilation is required. We initially tried this approach.20 Because of the limitations of working through the small esophageal hiatus, impairing dissection of the middle and upper esophagus and thoracic lymph nodes our preferred approach evolved to include a right VATS mobilization.
Our approach is similar to the 3-stage open technique described by Swanson et al.21 In their single institution report of 342 patients, a right thoracotomy was used in conjunction with laparotomy and a cervical anastomosis. A major difference from our series was a higher percentage (81%) of induction therapy. Mortality and length of stay were 3.6% and 13 days respectively. The 3 year survivals for their stage I, IIa, IIb, and III patients were 65%, 41%, 45% and 17% respectively which is similar to the survival seen in our series
Only 1 report has compared open esophagectomy with minimally invasive esophagectomy.22 This study favored the role of MIE with shorter operating times, blood loss, intensive care unit and hospital stays in this group. The study groups however were not comparable. The open group had more advanced cancers, and operations were performed in an earlier period by 4 surgeons with variable experience. The MIE operations were performed by 1 surgeon with specific expertise in esophageal surgery.
Previous studies have demonstrated superior gastric emptying with the use of a narrow gastric tube without a pyloroplasty.23 Midseries we adopted this approach to avoid the additional operative step of a pyloroplasty and the potential problems associated with dumping. Our data, however, demonstrated a significant increase in our anastomotic leak rate, with several leaks traveling down the narrow gastric tube into the right hemothorax using this approach. Other studies have also demonstrated impaired vascularization of the stomach and increased anastomotic complications with the use of a narrow conduit.24,25 In light of these complications we have abandoned this approach and now favor the use of a wider gastric conduit (6 cm diameter) with a pyloroplasty.
QOL is a key factor when treating esophageal cancer. One study reviewed SF36 scores in a cohort of 54 patients after esophagectomy for high-grade dysplasia.26 At a median follow-up of 63 months there was no impairment of quality of life compared with normal patients. Another study assessed dysphagia and QOL using a disease specific instrument, (the QLQ-C30) before and after esophagectomy.27 Initially QOL deteriorated in all patients but improved back to baseline in those patients surviving at least 2 years. The QOL data seen in our study support the role of MIE. In those patients where pre and postoperative scores were available, QOL was seen to be preserved. Additionally the mean postoperative QOL scores were similar to population normal values. One advantage of a neck anastomosis is that the incidence of reflux is low compared with an intrathoracic anastomosis. The excellent HRQOL scores that were seen in our study support the continued use of a neck anastomosis when possible.
We have demonstrated that MIE is feasible and can produce therapeutic outcomes comparable to and in our experience, better than those reported in most open series. In a previous review, we did not find advantage for hybrid operations, that is where parts of the esophagectomy were performed open (laparotomy) and parts were performed minimally invasively (thoracoscopic).28 It is important to note that our results of MIE originate from center with extensive experience in both benign and malignant esophageal surgery and daily exposure to advanced minimally invasive surgical techniques. It will be important to determine whether MIE can be developed in other centers with similar outcomes. Additionally the effect on QOL needs to be investigated further. A phase 2 intergroup study (E2202) is currently being developed with plans to study this very issue. If MIE is indeed found to be feasible and reproducible in a multicenter setting, any advantages to open operation will need to be confirmed in further randomized studies.
Discussion
Dr. Michael J. Zinner (Boston, Massachusetts): Dr. David Sugarbaker was asked to lead the discussion here; unfortunately, because of a family emergency he wasn’t able to come, and he faxed me his discussion, which he asked me to present.
Dr. Luketich, you and your colleagues should be congratulated on an excellent presentation. This represents the largest series of minimally invasive gastrectomy in the world. You and your associates continue to have a leadership role in standardizing and refining the technique of minimally invasive esophagectomy.
As with other minimally invasive operations, some have withstood the test of time and become adopted as the gold standard; for example, VAX. Similarly, laparoscopic antireflux procedures have supplanted open antireflux procedures. Only time will tell in this operation.
But Ford has widely adopted several questions he would like you to answer: Does this operation result in improved survival over conventional esophagectomy, or at least result in equivalent survival? A randomized trial comparing open against minimally invasive approach would answer this question. Two, you report short ICU and total hospital stays and imply a potential cost savings. Have you performed a cost analysis comparing open with the minimally invasive approach taking into account the higher cost of the instruments? Three, what are your current indications for open esophagectomy?
In addition, there were 3 technical questions: One, with a minimally invasive approach you are not able to palpate the tumor to assess margins. How do you ensure that both lateral and distal margins are adequate? Two, since you remove the specimen through the cervical incision, do you encounter any difficulty getting a larger bulky tumor through the thoracic inlet? Do you have any tricks to share in this procedure? And three, and finally, how much do you think laparoscopy adds to this operation? Many of the concerns about margins in removing tumors through the neck could be laid to rest by using a small upper midline decision, which is the approach they take at the Brigham.
Dr. Luketich, thank you very much for the opportunity to comment.
Dr. James D. Luketich (Pittsburgh, Pennsylvania): I don’t think we can make any claims of improved survival. I think we can hope to lower the surgical mortality. The survival curve was very similar stage for stage to the open literature. So I don’t know that there are hopes for any improved survival, though some minimally invasive reports have suggested perhaps there might be based on less immune damage at the time of surgery.
Randomized trial open to minimally invasive. Well, the next step is our multicenter trial to assess feasibility of this operation. It will be not a randomized trial, it will be 125 patients at 5 centers that have demonstrated some experience in this operation. And perhaps the next step beyond that might be randomization.
Cost analysis. We have looked at that briefly with some of the other complex minimally invasive operations, not esophagectomy. What we found is that the up-front costs clearly are higher because the instruments are disposable. We can approach cost savings only if we can significantly shorten that hospital stay. Perhaps in this prospective study we will be able to assess the costs, but we have not demonstrated improved cost analysis.
Current indications for open. Well, I would say that most of these patients are coming to minimally invasive operations now. If we have a question of a large bulky tumor that won’t come out or perhaps there’s been multiple previous upper abdominal surgeries or any question that the gastric conduit will not be usable and might require a colon interstitium, we would prefer to open.
Margins we assess preoperatively pretty good by endoscopic ultrasound in terms of our concerns about the margins and where we think the problem areas might be. We have a very good endoscopic ultrasonographer who does about 90% of these himself, and that is very helpful. We also do an on-the-table endoscopy – EGD, that is – by the surgeon in every single case, and we get pretty good at looking for little dimpling or polymers and retroflexions that might suggest a cardia extension that may lead to a problem with your margin in your gastric tube. Any question at that site we either biopsy or we would do it open.
Surgical incision for large tumors. By and large, most of these tumors will come out through the neck. And most of us are familiar with the hands that transhiatal down the neck, you get a pretty good 3 or 4 fingers of your hands down into that inlet during a transhiatal, and we found that by opening up the mediastinal pleura, the tumor usually slides right up the inlet. My own experience with our thyroid surgeons has shown them to get the very large goiters out of that inlet, and perhaps that experience has helped us slide the gastric tube with the tumor out. We have not had any problems at that level. Although very large tumors that are bulky, we would approach through an open incision in the abdomen. That decision is based on the surgeon at the time of surgery. But very few are open.
And I think your last question was, should we do a small upper midline incision? We looked at some of this data early on in a summary, we published this, looking at the series that were out there that did what I call a hybrid operation; that is, either laparoscopic with an open thoracotomy or a thoracoscopic with an open laparotomy. There weren’t any advantages demonstrated in those small series back in the late ’90s. But again, those were early in the experience. And I think in experienced hands maybe a hybrid operation would work very well. But we haven’t compared them head to head.
Dr. Luketich, your leadership in this field is well-recognized. The 1 remarkable number in your presentation was the 1.4 operative mortality. Yet I was surprised to see that 62% of your patients were early-stage disease. Could you comment on that patient selection bias?
Dr. James D. Luketich (Pittsburgh, Pennsylvania): Yes. The early mortality rate – we had an MIE, and there was another patient that wound up with peritonitis and sepsis, and 1 died of ARDS. And those results – esophagectomy, I think the thing that will increase your morbidity is that more patients with this undergo perhaps neoadjuvant therapy up front. We think that is going to be a little bit higher.
But our early experience – by our own choice we would not take on difficult tumors early on, we were looking for high grade and early-stage tumors. And we had a pretty aggressive screening trial ongoing at our institution. So we had some of these patients to deal with. So we chose the easy ones intentionally, which I would recommend for anyone considering this operation. Don’t pick the tough ones first. I hope that answers your question.
Footnotes
Presented at the American Surgical Association, April 24, 2003, Washington, DC.
Correspondence: Hiran C. Fernando, MD, UPMC Presbyterian, Suite C800 200 Lothrop Street, Pittsburgh, PA 15213. E-mail: fernandohc@msx.upmc.edu.
REFERENCES
- 1.Kelsen DP, Ginsberg R, Pajak TF, et al. Chemotherapy followed by surgery compared with surgery alone for localized esophageal cancer. N Engl J Med. 1998;339:1979-1984. [DOI] [PubMed] [Google Scholar]
- 2.Birkmeyer JD, Siewers AE, Finlayson EVA, et al. Hospital volume and surgical mortality in the United States. N Engl J Med. 2002;346:1128-1137. [DOI] [PubMed] [Google Scholar]
- 3.Overholt BF, Panjehpour M, Ayres M. Photodynamic therapy for Barrett's esophagus: cardiac effects. Lasers Surg Med. 1997;21:317-320. [DOI] [PubMed] [Google Scholar]
- 4.Fernando HC, Luketich JD, Beunaventura PO, et al. Outcomes of minimally invasive esophagectomy (MIE) for high-grade dysplasia of the esophagus EJCTS. 2002;22:1-6. [DOI] [PubMed] [Google Scholar]
- 5.Dallemagne B, Weerts JM, Jehaes C, et al. Laparoscopic Nissen fundoplication: preliminary report. Surg Laparosc Percut Tech. 1991;1:138-143. [PubMed] [Google Scholar]
- 6.Luketich JD, Fernando HC, Christie NA, et al. Outcomes after minimally invasive esophagomyotomy. Ann Thorac Surg. 2001;72:1909-1913. [DOI] [PubMed] [Google Scholar]
- 7.Pierre A, Luketich JD, Fernando HC, et al. Results of laparoscopic repair of giant paraesophageal hernia: 200 consecutive patients. Ann Thorac Surg. 2002;74:1909-1915. [DOI] [PubMed] [Google Scholar]
- 8.Krasna MJ, Jiao X. Thoracoscopic and laparoscopic staging for esophageal cancer. Sem Thorac Cardiovasc Surg. 2000;12:186-194. [DOI] [PubMed] [Google Scholar]
- 9.Luketich JD, Schauer P, Landreneau R, et al. Minimally invasive surgical staging is superior to endoscopic ultrasound in detecting lymph node metastases in esophageal cancer. J Thorac Cardiovasc Surg. 1997;114:817-823. [DOI] [PubMed] [Google Scholar]
- 10.Luketich JD, Schauer PR, Christie NA, et al. Minimally invasive esophagectomy. Ann Thorac Surg. 2000;70:906-912. [DOI] [PubMed] [Google Scholar]
- 11.Ware JE, Scherbourne CD. The MOS 36-item short form health survey (SF36). Med Care. 1992;30:473-483. [PubMed] [Google Scholar]
- 12.Velanovich V, Vallance SR, Gusz JR, et al. Quality of life scale for gastroesophageal reflux disease. J Am Coll Surg. 1996;183:217-224. [PubMed] [Google Scholar]
- 13.Hulscher JB, Van Sandick JW, de Boer AG, et al. Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the esophagus. N Eng J Med. 2002;347:1662-1669. [DOI] [PubMed] [Google Scholar]
- 14.Swisher SG, Deford L, Merriman KW, et al. Effect of operative volume on morbidity, mortality, and hospital use after esophagectomy for cancer. J Thorac Cardiovasc Surg. 2000;119:1126-1132. [DOI] [PubMed] [Google Scholar]
- 15.Zaninotto G, Parenti AR, Ruol A, et al. Oesophageal resection for high-grade dysplasia in Barrett's oesophagus. Br J Surg. 2000;87:1102-1105. [DOI] [PubMed] [Google Scholar]
- 16.Orringer MB, Marshall B, Iannettoni MD. Transhiatal esophagectomy: clinical experience and refinements. Ann Surg. 1999;230:392-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bailey SH, Bull DA, Harpole DH, et al. Outcomes after esophagectomy: a ten-year prospective cohort. Ann Thorac Surg. 2003;75:217-222. [DOI] [PubMed] [Google Scholar]
- 18.Depaula AL, Hashiba K, Ferreira EA, et al. Laparoscopic transhiatal esophagectomy with esophagogastroplasty. Surg Laparosc Endosc Percut Tech. 1995;5:1-5. [PubMed] [Google Scholar]
- 19.Swanstrom LL, Hanson P. Laparoscopic total esophagectomy. Arch Surg. 1997;132:943-949. [DOI] [PubMed] [Google Scholar]
- 20.Luketich JD, Nguyen NT, Schauer PR. Laparoscopic transhiatal esophagectomy for Barrett's esophagus with high-grade dysplasia. JSLS. 1998;2:75-77. [PMC free article] [PubMed] [Google Scholar]
- 21.Swanson SJ, Batirel HF, Bueno R, et al. Transthoracic esophagectomy with radical mediastinal and abdominal lymph node dissection for esophageal carcinoma. Ann Thorac Surg. 2001;72:1918-1924. [DOI] [PubMed] [Google Scholar]
- 22.Nguyen NT, Follette DM, Wolfe BM, et al. Comparison of minimally invasive esophagectomy with transthoracic and transhiatal esophagectomy. Arch Surg. 2000;135:920-925. [DOI] [PubMed] [Google Scholar]
- 23.Bemelman WA, Taat CW, Slors JF, et al. Delayed postoperative emptying after esophageal resection is dependent on the size of the gastric substitute. J Am Coll Surg. 1995;180:461-464. [PubMed] [Google Scholar]
- 24.Pierie JP, de Graaf PW, von Vroonhoven TJ, et al. The vascularization of a gastric tube as a substitute for the esophagus is affected by its diameter. Dis Esophagus. 1998;11:231-235. [DOI] [PubMed] [Google Scholar]
- 25.Collard JM, Tinton N, Malaise J, et al. Esophageal replacement: gastric tube or whole stomach? AnnThorac Surg. 1995;60:261-266. [DOI] [PubMed] [Google Scholar]
- 26.Headrick JR, Nichols FC, Miller DL, et al. High-grade dysplasia: long-term survival and quality of life after esophagectomy. Ann Thorac Surg. 2002;73:1697-1702. [DOI] [PubMed] [Google Scholar]
- 27.Blazeby JM, Farndon JR, Donovan J, et al. A prospective longitudinal study examining the quality of life of patients with esophageal carcinoma. Cancer. 2000;88:1781-1787. [PubMed] [Google Scholar]
- 28.Luketich JD, Schauer P, Urso K, et al. Future directions in esophageal cancer. Chest. 1998;113:120S-122S. [DOI] [PubMed] [Google Scholar]