Abstract
Objective:
To identify predictors of graft and recipient survival from a single-institution series of in situ split-liver transplantations and compare outcomes to living donor and whole organs for adults and children.
Summary Background Data:
Split-liver transplantation is a surgical technique that creates 2 allografts from a single cadaver donor. We have applied split-liver transplantation to all indications and categories of medical urgency for initial as well as retransplantation to expand the current donor pool and decrease reliance upon living donation.
Methods:
A retrospective analysis was conducted of 100 consecutive in situ split-liver transplantations yielding a left lateral segment and right trisegment graft that were performed at the University of California Los Angeles between 9/91 and 02/03. These 100 transplantations generated 190 allografts for transplantation into 105 children and 60 adults, with the sharing of 25 allografts among transplant centers across the United States. Outcomes and incidence of complications were compared with living donor and whole organ recipients receiving liver transplantation during the same time period with independent predictors of split-liver graft and recipient survival identified by multivariate analysis.
Results:
The incidence of biliary and vascular complications observed in recipients of left lateral segment grafts created by split-liver transplantation was not statistically different from recipients of left lateral segment grafts created from living donation or children receiving whole-organ grafts from pediatric donors. Kaplan-Meier survival estimations of left lateral segment graft and recipient survival also demonstrated no statistical difference among split-liver, living donor, and whole-organ recipients. Right trisegment grafts from split-liver transplantation demonstrated a 10% incidence of biliary and 7% incidence of vascular complications. Long-term graft function was excellent with patient and graft survival equal to 1086 recipients of cadaver whole-organ grafts from donors ages 10–40 years who underwent transplant operations during the same time period. Predictors of split-liver transplantation graft and recipient survival included United Network for Organ Sharing status at transplantation, indication, occurrence of a complication, donor creatinine, and donor length of hospitalization.
Conclusions:
Split-liver transplantation is an effective mechanism for immediate expansion of the cadaver donor pool that can reduce dependence upon living donation in adults and children.
Split-liver transplantation creates 2 allografts from a single cadaver donor. This single-institution series of 100 split-liver transplantations identifies predictors of graft and recipient survival and compares outcomes to living donor and whole organ transplantation in adults and children.
Split-liver transplantation (SLT) is a surgical technique that creates 2 allografts from 1 cadaver/donor organ. Simultaneously reported by Pichlmayr et al1 and Bismuth et al2 in 1989, SLT was a response to cadaver organ scarcity. The first series of 9 SLT procedures performed upon 18 recipients at the University of Chicago lead the authors to conclude SLT was “feasible and could have a substantial impact in transplant practice.”3 The following decade has endured an exponential increase in cadaver organ scarcity,4 resulting in expansion of “marginal” cadaver organs5–9 and increasing the application of live donations to children and adults.10–14 In the climate of increasing donor scarcity, SLT remains an infrequently performed procedure.15,16
The University of California Los Angeles has pursued SLT as an avenue to expand the cadaver/donor pool, increase availability of allografts to children and small adults, and decrease dependence upon living donations. This series of 100 in situ SLT identifies predictors of graft and recipient outcomes and compares SLT to living donor and whole cadaver organ transplantation.
METHODS
Surgical Techniques
The anatomic classification of the liver by Couinaud17 and refined by Bismuth18 is the universal reference system for describing allografts created by SLT. Several SLT techniques are recognized that generate a variety of grafts.2,19–25 This report is limited to the performance of the “conventional technique”,26 where parenchyma transection is performed at the falciform ligament to create a Couinaud segment II/III graft, termed a left lateral segment graft (LLS), of approximately 250 mL volume for pediatric recipients, and a Couinaud segment I, IV-VIII right trisegment graft (RTS) of approximately 1000 mL volume for transplantation of older children and adults.19,21,27 Detailed surgical descriptions of SLT have been published.19–21,26,28,29 All SLT procedures were performed in situ at the donor hospital without additional equipment or staff and concomitant with thoracic and other abdominal organ procurements. Living-donor (LD) liver recipients in this study were restricted to LLS grafts.30
Donor Selection
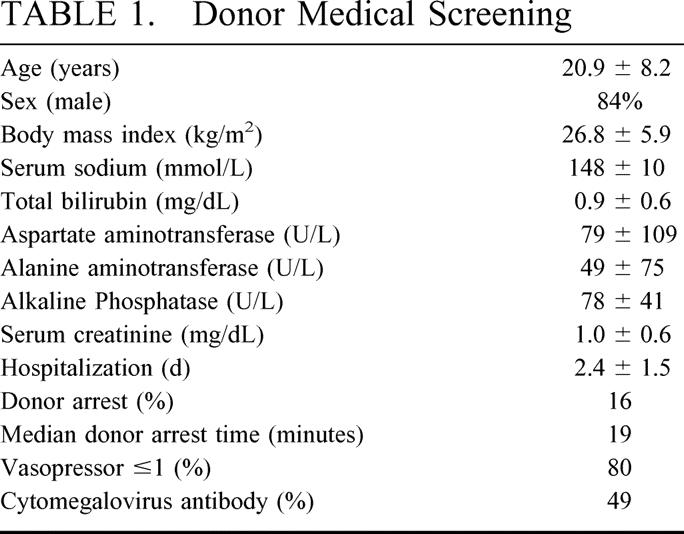
SLT donor selection involves rigorous medical screening combined with accurate organ assessment at the donor hospital. Previous data from our group21,31,32 have restricted SLT donor selection to optimal candidates as demonstrated in Table 1. Accurate donor assessment by the procurement team is paramount because SLT outcomes are affected by vascular and biliary anatomy as well as parenchyma quality and quantity.26 The liver is assessed for normal color and texture, equal perfusion, and sharp edges. The left lateral segment is assessed to assure fit into the recipient abdominal cavity, and the volume is estimated to provide a graft fraction of at least 1% ([graft volume (L)/total body weight (kg)] × 100).33
TABLE 1. Donor Medical Screening
Recipient Data Collection
Retrospective review included an internal scientific registry, patient charts, and the United Network for Organ Sharing (UNOS) Scientific Registry of Transplant Recipients (SRTR). SLT was broadly applied to a variety of indications and medical urgency, including initial grafts, rescue grafts for primary nonfunction (PNF), retransplantation grafts, and as components of multivisceral grafts. All SLT recipients were fully informed of the risks and benefits of SLT as well as our institutional experience with the procedure and signed specific consents for SLT in addition to standard consents for liver transplantation. A specific indication that was biased against SLT of LLS grafts was hepatoblastoma/hepatocellular carcinoma as our practice is to include the retrohepatic vena cava in the native hepatectomy to achieve adequate surgical margins.
Demographic, operative, clinical, and pathologic data were collected for all SLT recipients before and after transplantation. Specific recipient variables included date of transplantation, gender, age, indication, UNOS status, weight, intensive care unit hospitalization, ventilator dependency, and dialysis requirement. Serum chemistries and hematologic parameters (serum albumin, serum creatinine, total bilirubin, and prothrombin time expressed as international normalization ratio) at transplantation as well as use of a “T-tube” choledochostomy were recorded. Recipient graft variables included graft type, graft mass, graft fraction, requirement for ex vivo reconstruction of the hepatic artery prior to transplantation, multiple duct biliary anastomoses, use of a Roux limb for biliary drainage, cold ischemia period, and warm ischemia period.
Outcomes analysis included graft loss, retransplantation, reoperation, length of initial hospitalization, occurrence of a complication, and death. Complications were initially categorized as biliary, vascular, and PNF. Number and type of interventions necessary to treat a complication were recorded. Biliary complications diagnosed subsequent to the diagnosis of hepatic artery thrombosis (HAT) were not treated as independent events. Follow-up was terminated on the date of last known visit for patients lost to follow-up, death, or 03/31/2003, whichever is earlier. Comparative study populations included 43 LD-LLS pediatric recipients and 207 children ≤7 years of age who received pediatric whole cadaver organs during the study period. An age of ≤7 years was defined as the upper limit of the 95% confidence interval of the SLT-LLS population. This generated 3 very similar groups for comparison.
RTS graft and recipient survival were compared with adults receiving orthotopic whole-organ transplantation at UCLA from donors who were 10–40 years between 09/91 and 02/03. A total of 2134 liver transplant procedures were performed at UCLA over the study period of which donor information was available on 1991. Of this group, 905 grafts were from donors >40 years whereas 1086 grafts were from donors ages 10–40 years.
Immunosuppression
Immunosuppressive therapy was administered equally to all graft types. The standard regimen for children and adults consists of tacrolimus (Prograf, Fujisawa USA, Deerfield, IL) and corticosteroids with the selective use of mycophenolate mofetil (Cellcept, Roche, Nutley, NJ).34 Induction therapy is not routinely applied.
Statistical Analysis
Means, medians, standard deviations, and ranges summarize data distribution. Unless indicated, all means are expressed as mean ± standard deviation. Comparisons of continuous measures were assessed by one-way analysis of variance followed by the t test for parametric data or the nonparametric Wilcoxon rank sum test for data not approximated by a Gaussian distribution. Categorical variables were analyzed by the χ2 test. Survival was estimated by the Kaplan-Meier method35 for (1) time to recipient death and (2) time to recipient graft loss or death, whichever occurs earlier. Kaplan-Meier survival estimations were compared by the log-rank test for nonparametric data. Eighteen categorical factors and 16 continuously valued factors were studied as predictors of SLT graft and recipient survival. Predictors of SLT graft and recipient survival were identified by univariate survival analysis with continuous variables dichotomized at the median value, followed by multivariate logistic regression analysis to confirm independent significance. Statistical significance is assumed for P < 0.05.
RESULTS
Graft Use
One-hundred SLT procedures yielded 190 grafts for transplantation. Of this group, 165 grafts (LLS: 94, RTS: 71) were transplanted into 105 children and 60 adults at UCLA. The impact of SLT upon pediatric graft use over a 10-year period is depicted in Figure 1. Twenty-five grafts (LLS: 3, RTS: 22) were shared with 6 transplant centers within our UNOS region and 2 centers across the country (UNOS regions 2 and 9). Three LLS and one RTS recipient received a concomitant kidney from the same donor and one RTS graft was a component of a multivisceral liver-small bowel-kidney transplantation. Ten grafts (LLS: 3, RTS: 7) were not used most frequently because of unexpected dropout of a potential recipient during the SLT procedure and inability to recruit another recipient within a reasonable time period. Two LLS intraoperative deaths that occurred after allograft revascularization but before completion of the transplant procedure were censored.
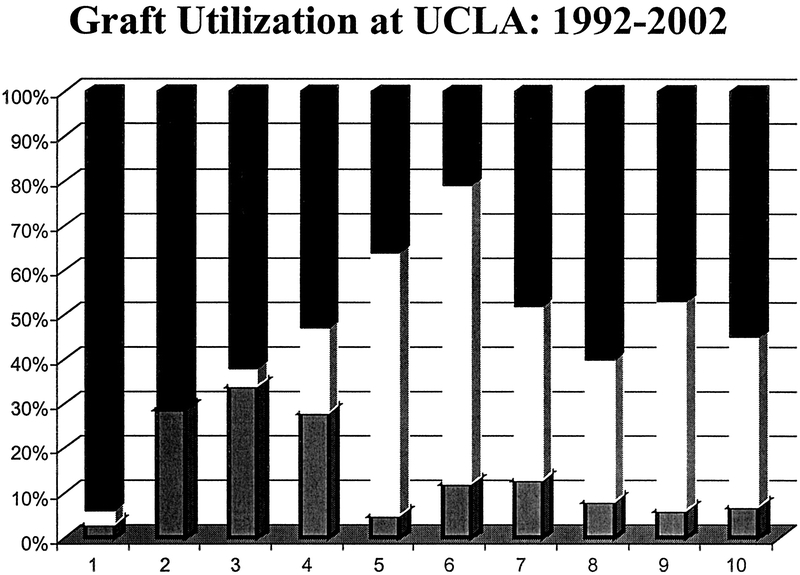
FIGURE 1. Graft use at the University of California Los Angeles between 1992 and 2002 for children ≤7 years of age at transplantation. The application of split-liver transplantation (clear) has dramatically reduced our need for living-donation (hatched). Since 1997, greater than 90% of allografts have been from cadaver donors. Pediatric whole-organs are solid bar.
LLS Grafts
Recipient Population
SLT-LLS recipients included 1 small adult (age 45, 49 kg) and 2 teenagers ages 13 (29 kg) and 15 years (50 kg) with the remaining recipients <10 years in age and less than 30 kg in weight. Thirty-eight percent of SLT-LLS recipients required intensive care hospitalization while awaiting transplantation with 21% ventilator dependent. Although 83% of SLT-LLS recipients were initial transplants, each of the 16 SLT-LLS grafts used in the setting of retransplantation were urgent status “rescue grafts” for graft failure secondary to PNF (n = 8) or vascular thrombosis (hepatic artery: 7, portal vein: 1). Comparison of SLT-LLS demographic data to LD-LLS and pediatric, whole cadaver-organ recipients demonstrates similarity with respect to physical characteristics and graft utilization (Table 2). The significant differences observed in the length of follow-up reflect the increasing application of SLT during the study period with reduced dependence upon LD and cadaver whole-organs (Fig. 1). The result is a bimodal distribution of shorter average SLT follow-up and longer average follow-up of LD and cadaver whole-organ recipients.
TABLE 2. Split-LLS, Living-Donor-LLS, and Pediatric Whole-Organ Recipient Demographic Data

Graft Data
LLS grafts predispose to unique complications resulting from anatomic variations as well as recipient physiology. Each of these variables was considered independently by graft type in Table 3. Ex vivo reconstruction to establish a single arterial inflow was required in 10% of SLT-LLS recipients. Multiple bile ducts requiring separate anastomoses was observed in 15% of recipients. The higher incidence of arterial reconstruction and multiple bile ducts observed in LD-LLS recipients may reflect a slightly different procurement technique of less left hepatic arterial dissection and parenchyma transection closer to the falciform ligament.29,30 In addition, our early practice in living donation was to apply donor-derived venous conduit to increase arterial length.30 Pediatric whole-organ grafts only require ex vivo arterial reconstruction in the event of a replaced right hepatic artery that is observed in approximately 10% of cadaver donors.36,37 The volume of SLT-LLS and LD-LLS grafts are relatively equal and their application, represented by graft fraction, is also equal. Warm ischemia times tend to be longer in LLS grafts than whole-organs because arterial anastomoses are frequently included prior to reperfusion.31 As expected, the cold ischemia time for living donor grafts is significantly lower than cadaver-derived organs.
TABLE 3. Split-LLS, Living-Donor-LLS, and Pediatric Whole-Organ Graft Data
Complications
Complications were categorized as biliary, vascular, PNF, and incidence of reoperation after SLT. The incidence of biliary complications among SLT-LLS, LD-LLS, and pediatric whole-organ recipients was 9%, 12%, and 10%, respectively (P = 0.43). However, unique patterns of complications were observed. SLT-LLS grafts incurred 5 parenchyma bile leaks, 2 anastomotic leaks, and 1 anastomotic stricture. Interestingly, multiple bile ducts were not identified in any graft where a biliary complication was observed. Early biliary complications were treated by surgical intervention. LD-LLS grafts demonstrated anastomotic strictures at 81 to 953 days posttransplant with each treated successfully by balloon dilatation. Of the 207 pediatric whole-organ recipients, a total of 21 biliary complications were observed between 4 days and 10 years posttransplantation including16 anastomotic strictures and 5 anastomotic bile leaks. All biliary leaks occurred within the immediate postoperative period and were treated by surgical intervention. In addition, 2 early anastomotic strictures, diagnosed at 24 and 38 days posttransplantation were treated with surgical revision. The occurrence of a biliary complication did not correlate with initial transplantation (P = 0.74) or UNOS status (P = 0.62) in any graft type.
The overall incidence of vascular complications among SLT-LLS, LD-LLS, and pediatric whole-organs did not demonstrate statistical significance (P = 0.34). Vascular complications, when subdivided into HAT and portal vein thrombosis (PVT), did reveal differences between groups. HAT was observed in 7 SLT-LLS recipients between 1 and 780 days posttransplantation. The majority of HAT (n = 5) were diagnosed within 7 days of SLT prompting surgical exploration, thrombectomy, and attempted revascularization. One late HAT resulted in a bile leak that also required surgical intervention. Two of the 5 early diagnosed HAT occurred in grafts that required ex vivo arterial reconstruction. Among LD-LLS recipients, HAT was observed in 8 recipients between 1 and 245 days posttransplantation. Two recipients were diagnosed within 7 days posttransplantation and were treated with surgical exploration, thrombectomy, and revascularization. Ex vivo arterial reconstruction was performed on 7 of the 7 grafts with HAT including both early thromboses. HAT was observed in 13% of pediatric whole-organ transplant recipients with 40% occurring within 7 days posttransplantation. As above, early HAT occurrence was treated by surgical intervention with later occurrences managed expectantly. There was no correlation among pediatric whole-organ recipients to hepatic artery reconstruction.
PVT occurred in 8% SLT-LLS, 11% of LD-LLS, and <2% of cadaver whole-organ recipients. Diagnosis within 30 days occurred in 6 of the 8 SLT recipients and 3 of the 5 LD recipients. Management of PVT was similar between groups with early diagnosis treated by surgical exploration and thrombectomy. Later complications were managed expectantly. Only 1 of the 3 PVT observed in pediatric whole-organ recipients occurred early. There was no correlation between vascular complication, initial transplantation, or UNOS status in any graft type.
Early reoperation is necessitated by the occurrence of a biliary complication, vascular complication, hemorrhage, or sepsis. Reoperation was required in 52% of SLT-LLS recipients versus 42% of LD-LLS and 39% of pediatric whole organ recipients (P = 0.02). There was no significant difference in the total number of posttransplant operations among recipients who underwent surgery (P = 0.47) with the mode and median number of surgical procedures required in each group equal to one. The requirement for surgical intervention did not correlate to initial transplant, arterial reconstruction, multiple bile ducts, or UNOS status in any group.
PNF was significantly increased among SLT-LLS recipients: 8 SLT-LLS grafts versus 1 LD-LLS graft and 7 pediatric whole-organs (P = 0.01). The incidence of PNF decreased with institutional experience. Only 3 incidences of PNF occurred in the last 50 SLT procedures and no incidence of PNF in the last 25 SLT-LLS grafts. All grafts required retransplantation. The distribution of PNF in SLT-LLS grafts by UNOS status was equal. Four UNOS status I and 4 semi-urgent UNOS status SLT-LLS recipients were affected with all the 4 UNOS status I SLT-LLS recipients expiring during their hospitalization despite retransplantation. The length of hospitalization for the transplant procedure was evaluated as a marker of outcomes. Median hospital stay was 28 days for SLT-LLS, 31 days for LD-LLS, and 28 days for pediatric whole-organ recipients (P = 0.68).
Graft and Recipient Survival
Kaplan-Meier estimations35 of SLT-LLS, LD-LLS, and pediatric whole-organ graft and recipient survival were compared by log rank analysis. Six-month, 1-, and 3-year SLT-LLS graft survival were 72%, 68%, and 64%, respectively. Pediatric whole-organ graft survival at the same time points was 79%, 77%, and 73%, versus 76%, 74%, and 71%, respectively, for recipients of LD-LLS (Fig. 2A). A total of 33 SLT-LLS grafts were lost during the study period: 15 within the first 7 days post-SLT, 11 between 1 week and 6 months post-SLT, and 7 grafts later than 6 months post-SLT. PNF and vascular complications accounted for all immediate graft failures within 7 days post-SLT. Of these 15 recipients, 11 underwent successful retransplantation with 1 SLT-LLS, 1 LD-LLS, and 9 pediatric whole grafts. Two biliary and 3 vascular complications occurring after the first week post-SLT contributed to sepsis and additional graft failure; however, the remainder of grafts lost were the result of rejection, recurrent viral infection, or sepsis not attributable to graft function.
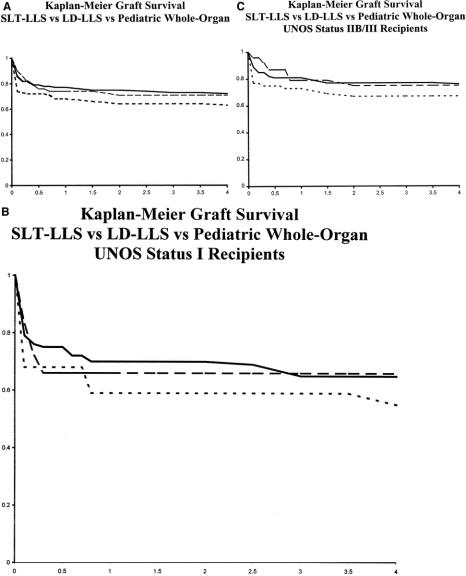
FIGURE 2. A, Kaplan-Meier graft survival of SLT-LLS (dash), LD-LLS (interrupted), and pediatric whole-organ recipients (solid line). P = 0.12. B, Kaplan-Meier graft survival of SLT-LLS (dash), LD-LLS (interrupted), and pediatric whole-organ recipients (solid line) stratified by UNOS Status I. P = 0.46. C, Kaplan-Meier graft survival of SLT-LLS (dash), LD-LLS (interrupted), and pediatric whole-organ recipients (solid) stratified by UNOS Status IIB/III. P = 0.29.
Each graft type achieved acceptable long-term graft survival with early graft function demonstrating the most significant impact upon survival. The slightly increased incidence of acute complications among SLT-LLS recipients resulted in a lower graft survival curve; however, the differences between each graft type in overall graft survival did not achieve statistical significance (P = 0.12). Graft-survival of each graft type strongly correlated to urgent status at SLT with significantly lower survival observed in UNOS status I recipients versus nonurgent UNOS status IIB and status III recipients. Further comparison of graft survival for each graft-type, stratified by UNOS status at transplantation, demonstrated no statistically significant difference in graft survival suggesting equal performance of the individual graft types (Fig. 2B and C).
Recipient survival for each graft type followed a similar trend with 6-month, 1-, and 3-year SLT-LLS recipient survival of 79%, 78%, and 75% versus 83%, 83%, and 81% for pediatric whole-organ and 86%, 86%, and 84% for LD-LLS, respectively. As observed above, recipient survival of each graft type correlated with UNOS status at transplantation (P = 0.01). Comparison of overall recipient survival between graft types, when stratified by UNOS status at transplantation, revealed no significant differences.
Right Trisegment Grafts
Recipient Population
Seventy-one SLT-RTS grafts were allocated to 57 adults and 14 children at UCLA. An additional 22 grafts were shared with other centers. RTS grafts were principally allocated to older children and small adults, 2 recipient populations with historically long waiting periods. The 14 children (male, 5; female, 9) receiving SLT-RTS grafts ranged from 2.1 to 17.9 years of age (median, 13 years) with weights of 10.6 to 85 kg (median, 34.7kg). Adult SLT-RTS recipients (male, 23; female, 34) ranged from 18.0 to 76.2 years of age and weighed from 41.8 to 93 kg with a median age of 47.9 years and a median weight of 63 kg. Thirty-four recipients met criteria for urgent transplantation (48%) with 21 acute liver failure UNOS status I recipients and 13 acutely decompensated cirrhotics requiring intensive care hospitalization as UNOS status IIA recipients. The most frequent indications for liver transplantation were: hepatitis C (24%), fulminant hepatic failure (14%), alcoholic liver disease (11%), autoimmune hepatitis (10%), and primary biliary cirrhosis (10%). Sixty-one SLT-RTS grafts (86%) were initial transplants, 9 of the 10 retransplant grafts were second grafts, and 1 was a fourth graft. All grafts were ABO blood group compatible (12%) or identical (88%). Mean follow-up was 2.9 ± 2.4 years.
Graft Data
SLT-RTS graft mass ranged from 420–1600 mL with a mean of 1213 ± 335 mL and a median of 1040 mL yielding graft fractions of 0.8 to 5.1% (mean, 2.0 ± 1.0%). Ex vivo reconstruction to provide a single arterial inflow was only required in the presence of a replaced right hepatic artery originating from the superior mesenteric artery which occurred in 14% of SLT-RTS grafts. Use of SLT-RTS grafts does not require a specific biliary drainage procedure. Biliary drainage by a Roux-en-Y hepatojejunostomy was performed in 17% of patients while the remainder received a choledocho-choledochostomy with (57%) or without a T-tube. Mean cold ischemia time for SLT-RTS grafts was 6.2+2.5 hours with a warm ischemia time of 44+13minutes.
Complications
Biliary complications were observed in 10% of SLT-RTS recipients. The 7 complications included 3 parenchyma cut surface leaks, 2 anastomotic leaks, 1 late anastomotic stricture, and a single graft with prolonged cholestasis from intrahepatic duct disease. Four of the 7 biliary complications were diagnosed within 8 days post-SLT with the remainder diagnosed at >55 days. Surgery was required for both anastomotic leaks and a parenchyma surface leak; however, all other biliary complications were treated without reoperation. The occurrence of a biliary complication did not correlate with UNOS status, initial transplant, indication, or requirement for hepatic artery reconstruction. Furthermore, the biliary complications did not significantly impact graft outcomes.
Vascular complications were diagnosed in 5 SLT-RTS recipients (7%); however, no vascular complications occurred within 7 days of SLT. HAT occurred in 3 recipients at 10, 18, and 303 days post-SLT whereas PVT was observed at 425 and 1897 days. Notably, 1 HAT recipient (10 days) and 1 PVT recipient (425 days) were diagnosed with thrombotic disease. All vascular complications were observed in urgent status recipients; however, the data are too small to permit statistical correlation.
Reoperation was required in 17 SLT-RTS recipients (24%). Exploration for hemorrhage (n = 8), followed by sepsis (n = 4) and biliary complication (n = 3) were the most common indications. Twelve of the 17 SLT-RTS recipients requiring reoperation had a single procedure performed, 4 required 2 procedures, and 1 required 3 procedures for continuing sepsis. The requirement for surgical intervention did not correlate to initial transplant, arterial reconstruction, multiple bile ducts, or UNOS status. The necessity for reoperation did not significantly increase the length of initial hospitalization which was 25 days for SLT-RTS recipients not requiring surgical intervention versus 28 days for recipients requiring reoperation.
PNF was the most frequent complication observed in 7 UNOS status I, 2 status IIA, and 4 nonurgent recipients. Of the 7 UNOS status I recipients, 4 were retransplantation rescue grafts. SLT-RTS recipients diagnosed with PNF were heavier as a group with a median weight of 62 kg; further suggesting the allocation of these grafts to individuals with a grim prognosis and no alternative organs available. SLT-RTS grafts demonstrating PNF also experienced a longer warm ischemia period (mean, 55 ± 13 minutes), which may reflect increased technical difficulty. PNF of the corresponding SLT-LLS graft was observed in 2 SLT-RTS grafts (SLT number 1 and 34), each transplanted into nonurgent recipients suggesting PNF from graft-associated variables. As with SLT-LLS grafts, the majority of PNF was observed within the first fifty procedures with no observed RTS PNF in the last twenty procedures. There was no correlation between PNF and cold ischemia time or requirement of arterial reconstruction.
Graft and Recipient Survival
RTS grafts demonstrated overall 6-month and 1-year graft survival of 72% and 69%. A total of 32 SLT-RTS grafts were lost during the study period: 8 within the first week post-SLT, 8 between 1 week and 1 month post-SLT, and 16 beyond 1 month post-SLT. The most frequent cause of graft loss within the first week post-SLT was PNF with no vascular complications observed. Six of these 8 recipients underwent successful retransplantation. Between 1 week and 1 month post-SLT, the most common cause of graft loss was sepsis with 1 HAT diagnosed in a prothrombotic recipient. Sepsis during this period most frequently resulted in death rather than retransplantation. Lastly, graft loss in the remaining 16 SLT-RTS recipients beyond 1-month post-SLT was not related to graft function except for the occurrence of 1 HAT and 1 PVT. Overall SLT-RTS recipient survival at 6 months and one-year was 83% and 78%, respectively.
As the intense screening process of donors for SLT could introduce a selection bias, SLT-RTS graft and recipient survival were compared with 1086 cadaver whole-organ recipients from donors ages 10 to 40 years undergoing liver transplantation at UCLA during the same time period. This represented a surrogate “optimal liver” group to attempt to determine if SLT had a negative impact on graft and patient survival. Comparison of these 2 groups revealed no significant difference in graft or recipient survival as well as no significant difference in the performance of SLT-RTS grafts.
DISCUSSION
Over a decade has passed since the initial descriptions of SLT1,2 and the first series of outcomes.3 During that period, SLT has been principally applied by select centers in the United States21 and Europe19,27,38 that have periodically updated their series.39,40 Overall outcomes were acceptable but despite an exponential increase in the discrepancy between donor supply and recipient demand, SLT remains an infrequently performed procedure.15 Multiple factors, including personnel and logistic demands, a potentially restrictive organ allocation policy, as well as the perception of inferior results have contributed to the under utilization of SLT.41
This series of 100 consecutive in situ SLT culminates over 10 years of experience by the authors and the evolution of techniques to optimize outcomes. The successful application of partial-liver grafts, whether derived from living donors or split-liver transplantation, mandates unique surgical and medical considerations. The principal surgical challenges include the creation of a graft with sufficient liver volume to meet the metabolic needs of the recipient, positioning of the graft to optimize vascular inflow, venous outflow, and biliary drainage, as well an appreciation of anatomic variations that necessitate complex biliary or vascular reconstruction. Acute partial-graft complications most often require surgical correction with the number of reoperations higher among partial-graft recipients versus pediatric whole-organ recipients as demonstrated above. Early surgical intervention optimizes graft and recipient outcomes with little effect on length of hospitalization.42 This is the nature of these highly technical procedures and surgeons embarking on partial-graft liver transplantation should be prepared for, and accept, a higher incidence of reoperation among partial-graft recipients to maintain optimal outcomes.
Medical considerations include the management of biliary complications, complications from bleeding of the cut-liver surface, acute and chronic hepatic venous outflow obstruction, hepatic arterial complications, and initial graft dysfunction secondary to insufficient hepatic volume, termed “small-for-size syndrome.”43–46 The reward for application of these techniques is earlier “timing” of transplantation with avoidance of patient deterioration while awaiting a whole cadaver graft, decreased hospitalization, and the provision of parenchyma quality that has received intense medical screening with minimal ischemia times.39,40
Comparison of 92 SLT-LLS recipients, 43 LD-LLS recipients, and 207 pediatric whole-organ recipients identifies the unique considerations applicable to each graft type. The 3 populations were remarkably similar with respect to demographic data; however, partial-organ grafts were more frequently applied to UNOS status I recipients, reflecting our institutional commitment to these procedures as a mechanism to minimize wait-times for urgent status recipients. Although the overall incidence of biliary and vascular complications among the 3 graft types did not achieve statistical significance, the specific patterns of complications as well as the incidence of PNF reveals parenchyma quality as the fundamental difference between partial-grafts derived from LD and SLT. Parenchyma quality may explain the higher occurrence of surface bile leaks and PNF observed in SLT-LLS grafts.47 Inferior SLT-LLS performance affected graft survival but did not impact overall patient survival. The observation of slightly inferior outcomes with respect to graft and recipient survival that are not great enough to achieve statistical significance between SLT, LD, and whole organs is demonstrated in our previous32 as well as present data.
Parenchyma injury within SLT grafts originates from the process of brain death as well as the procedure itself. Hypotension, shock, sepsis, and previous cardiac arrest initiate a cascade of events leading to direct organ injury. This is compounded by significant hemodynamic and neuroendocrine changes resulting from brain death. The result is a potentially injured organ that then undergoes a surgical procedure inciting further injury through parenchyma destruction. The community performing SLT has overcome this fragility of donor parenchyma through optimal donor selection to achieve acceptable outcomes. Currently, we limit SLT donors to heartbeating, hemodynamically stable individuals age 10 to 50 years with minimal vasopressor requirements, liver function tests within 3X upper normal limits, normal renal function, negative virologic serologies (except cytomegalovirus) and minimal or absent period of cardiac arrest who receive an equally vigorous assessment by the procurement team for vascular and biliary anatomy as well as parenchyma quality and quantity.
The performance of SLT in situ limits potential parenchyma injury by minimizing cold ischemia time and averting unintentional graft rewarming during the ex vivo split procedure. Realizing the fragility of parenchyma quality may explain the decreased results observed in ex vivo versus in situ SLT. Longer cold ischemia and extended parenchyma injury incurred during ex vivo SLT contribute to the inferior outcomes of ex vivo SLT grafts applied to urgent status recipients.38 Parenchyma perfusion is maintained during the in situ SLT while biliary and vascular structures are readily identifiable. Hemostasis is achieved prior to graft revascularization, which lowers transfusion requirements, potential disseminated intravascular coagulopthy, and graft stress after reperfusion. In addition, a reduced potential for biliary complications facilitated by easier identification of biliary structures reduces the chance of later sepsis and graft dysfunction. Lastly, the authors maintain in situ SLT facilitates graft sharing by permitting direct allocation from the donor hospital with minimal cold ischemia time.
The increased blood loss and volume replacement incurred during in situ SLT has prompted concerns that the quality of thoracic organs may be affected.48 Data from centers with a commitment to in situ SLT as well as our own data suggest the impact of in situ SLT is negligible.49 We allocate approximately 90 minutes of additional time for a conventional in situ SLT. While longer procurement times may occasionally be necessary, in situ SLT has not been an excessive burden in our experience, provided adequate communication has occurred between all procurement teams.
Recipient selection mandates unique considerations in the performance of SLT. Several authors have attempted to define a “minimal mass” or threshold for the prediction of graft dysfunction secondary to insufficient hepatic mass to meet the recipient's metabolic requirements.43–46 These studies have been exclusively performed in the context of living donation. With respect to SLT, our preference is to require a greater amount of hepatic mass for an individual SLT recipient than would be required in living-donation21 with a graft fraction of at least 1% recipient body mass. In our experience, this has provided satisfactory function for recipients across indications and medical urgency.21,32
SLT-LLS grafts yield similar outcomes with respect to biliary and vascular complications as LD-LLS grafts and pediatric whole-organs with a higher incidence of PNF. Our experience suggests PNF may be reduced by careful donor and recipient selection and has yet to significantly contribute to lower graft or recipient survival. This option can substantially reduce reliance upon living donation and donor complications that have become apparent since the wide scale application of LD. Furthermore, detailed studies have demonstrated application of living-donation to only a minority of potential recipients, a limitation not applicable to SLT.50–52 The immediate potential of the existing cadaver donor pool could substantially reduce the need for LD-LLS grafts in all but emergency situations and has lead the authors to assume a policy of SLT over living donation when at all possible.
SLT-RTS outcomes are more controversial. In our series, RTS grafts demonstrated a 10% incidence of biliary complications and a 7% incidence of vascular complications. The requirement for reoperation and the incidence of PNF were high, particularly among urgent UNOS status recipients. RTS grafts were applied frequently to critically ill recipients who were heavier than their nonurgent status RTS counterparts. Although 9 of the 13 graft failures were in Status I and IIA recipients with 4 failures in Status I patients suffering from acute graft failure necessitating emergency retransplantation, the failure of the remaining 5 grafts is unaccountable. Similarly, 2 of the RTS failures in nonurgent recipients occurred as a result of donor selection as evidenced by concurrent SLT-LLS primary nonfunction. Each of these failures occurred early in our series with no concurrent PNF observed over the last 65 SLT procedures. Nonetheless, our experience with SLT-RTS has lead to our application of these grafts in much the same manner as right lobe grafts derived from living donation. Examination of SLT-RTS outcomes by UNOS status demonstrates graft and recipient survival that do not statistically differ from cadaver whole-organ grafts with a similar incidence of complications encountered in the routine applications of LD right lobe grafts. We have used RTS grafts to reduce our right lobe living donor pool and believe much greater reductions in living donation could be achieved with a re-evaluation of allocation policy.
In conclusion, SLT is a significant surgical achievement with ultimate acceptance and application in the field of liver transplantation yet to be defined. We have used SLT across the spectrum of liver transplantation for adults and children as an effective mechanism to alleviate the current cadaver-organ shortage and reduce dependency upon living-donation with outcomes that rival cadaver whole-organs. Further application of SLT can result in immediate expansion of the current cadaver-donor pool with excellent outcomes if the limitations outlined above are recognized.
Discussion
Dr. Henri Bismuth (Villejuif, France): You must be congratulated, Dr. Busuttil and colleagues, for your excellent paper which reports the largest experience of in situ split-liver transplants which allowed you to transplant 188 patients with 100 liver grafts.
I opened the field of liver resection in liver transplantation more than 20 years ago by the reduced size graft and today I am very pleased to see to which extent liver surgery is acting in liver transplantation.
At a time where there is a so important shortage of grafts and when patients with end-stage liver disease are dying on the waiting list, the split transplantation is surely the best solution to increase the liver graft pool.
You are right to say that developing the split liver will decrease the demand for living donation. Even if the risk for the living donor is low, it cannot be, of course, equal to the split cadaveric one, and this advantage is definitive in favor of the split.
However, the transplantation does not achieve the same results that the usual cadaveric whole organ, and if not significant, there are differences in complications and also in survival. And you said, and I agree totally with you, that the failures of the split are mainly due to the quality of the graft and to the condition of the recipient.
So my first question is how to improve these results. If you don’t succeed to achieve that, opponents, that is all the transplant groups who are reluctant to do the split, will not be convinced.
In my country, facing the same problem, we have convinced our organ-sharing organization to distribute the best liver graft as 2 livers. Anatomically the liver is a pair organ. For 1 donor ideally we have to distribute 2 livers, as 2 kidneys.
A second point, we select patients as candidates for split-liver. It is not easy. It is a heavy organization. But 1 liver gained is 1 life gained. What is at balance is the ethics of living donation versus the organization of the split. So my question is, are you ready in this country, which is the leading country, to adopt this policy? If do you that, all the world will follow you.
I have another question. You said some years ago that about 30% of the liver grafts may be split. As liver transplantation for children is about 10 to 15% of the total liver transplantation program, to develop fully the split you have to develop the split transplantation for 2 adults. In my country, there are no more children dying on the waiting list, but only adults. So what do you think on the split for 2 adults? You are lucky to have access to an in situ liver procurement program which allows you to gain for the hepatic reserve for the 2 hemigrafts.
Now I have a small final remark on anatomy. You know I like anatomy. You adopt the segmental anatomy. But you say for the left graft 2 segments, and you call it left lateral segment. And the right graft, which is 6 segments, you call it tri-segments. You are in between the old and the new.
Really, to conclude, I enjoyed very much your paper, and as a long-time admirer of the UCLA Group, I congratulate you, Dr. Busuttil, Dr. Yersiz, and Dr. Renz, for your really excellent work on a huge experience.
Dr. Ronald W. Busuttil (Los Angeles, California): Thank you very much, Dr. Bismuth. I certainly have to acknowledge your pioneering work in liver surgery which is all-inclusive. You have asked 4 very good questions.
How do we improve the results? First of all, split-liver transplantation has a steep learning curve. It is a very technically demanding operation and it takes a lot of experience with a lot of cases to get the complication rate down. If we look at the results that we have achieved in our second 50 cases compared with those that we achieved in our first 50 cases, there is a startling difference both with a decreased incidence of complication and decreased incidence of graft failure. So our role is to try to convince our colleagues that it is not going to be easy at the beginning but you have got to pursue it, because it certainly has, I believe, a very important role in expanding the donor pool.
The second question, about a policy on split liver in the United States. We have tried to present a mandatory policy to split ideal donor livers. UNOS looked at that, but it has never really been implemented. I think the reason that it not been implemented is that there is still skepticism on the success of operation and if it really works as well as does whole organ cadaveric grafting.
Your third question about adult to adult. I think there is a much greater pool of people that we would be helping if we were to consistently and successfully do true right-left splits. However, we are having a hard enough time convincing the transplant community that we should be doing conventional splits, as I have described. I think most people still consider the true right-left splits as experimental. Thus it is unlikely that this latter procedure will be used to any great extent.
Dr. Nancy L. Ascher (San Francisco, California): Dr. Busuttil, you have previously published that patients who are more gravely ill (higher status) do worse with the right lobe grafts than less gravely ill patients or with whole cadaveric grafts. You indicated that a number of your patients were urgent and received the right lobe grafts. The way we are distributing these right lobe grafts in California is that the most gravely ill patients dictate graft distribution; so an urgent child would get segment 2 and segments 5-8 graft would go to the most gravely ill adult patient next on the list. So I would like you to comment on whether you feel this distribution scheme works in light of the poorer success of right lobe grafts in gravely ill recipients.
A second question involves an analysis of a given patient’s chance upon entry to the transplant list of being alive at the end of the year and beyond. how do we help a given patient make a decision to accept a right lobe graft, to take their chance and wait for a whole organ, or to convince their brother or sister or spouse that they have got to donate their right lobe? Can you comment on that?
Dr. Ronald W. Busuttil (Los Angeles, California): Thank you, Dr. Ascher. First of all, you are correct that in our early experience we thought that split grafts worked equivalently well between urgent patients and nonurgent patients. We now clearly know that this is not the case. If we look at our right lobe grafts in a nonurgent patient, they have 85% survival long term. If you look at them in an urgent patient, it is 64%. So you definitely pay a penalty in the urgent recipient.
To answer your second question, I think that a policy of shipping out these grafts is not going to work. I think that they ought to be retained with the center that splits them. This will allow maximum utilization of the right lobe grafts.
To answer your last question, I think that one has to be very open and look at your own center and see what your chances are of dying on the waiting list. When we evaluate a patient for live transplant, we give them 3 options: 1) living related, 2) split-liver option, and 3) whole organ cadaveric option. There are a set of complications that go with each one of those. And we inform our patients up front so that they know which option is for them and the attendant risks.
Dr. Leslie H. Blumgart (New York, New York): Dr. Busuttil, congratulations. Just a very brief question. I saw that your major biliary complications for right-sided grafts had a 10% complication rate. How much does that relate to abnormal biliary anatomy on the right side, particularly the posterior sectoral ducts coming around onto the left side? Has that been a problem?
Dr. Ronald W. Busuttil (Los Angeles, California): It is very little. Because the posterior sectorial duct is not touched. Most of these complications with the right-side segmental graft are surface leaks.
Dr. J. Michael Henderson (Cleveland, Ohio): Dr. Busuttil, wonderful paper, as always. I had 2 questions. You answered the first 1 on consent for the recipients: you sign them up up front. I think that is very important.
The second question is, you had 100 grafts out of how many total donors in your OPO over the same period of time? So what is the percentage of donors that fulfilled your criteria? How many weren’t even considered for splitting?
Dr. Ronald W. Busuttil (Los Angeles, California): Our efficiency is two-thirds – meaning when we have identified on paper a donor that is suitable for splitting we go out and look at it. The final decision is not made until we see it. And in our series, two-thirds of those grafts are suitable for splitting and we split them. I would say that probably 20% of all donors would be suitable.
REFERENCES
- 1.Pichlmayr R, Ringe B, Gubernatis G. Transplantation of a donor liver to 2 recipients (splitting transplantation)—a new method in the further development of segmental liver transplantation. Langenbecks Archiv Chir. 1989;373:127–130. [PubMed] [Google Scholar]
- 2.Bismuth H, Morino M, Castaing D, et al. Emergency orthotopic liver transplantation in two patients using one donor liver. Br J Surg. 1989;76:722–4. [DOI] [PubMed] [Google Scholar]
- 3.Emond JC, Whitington PF, Thistlethwaite JR, et al. Transplantation of two patients with one liver. Analysis of a preliminary experience with ’split-liver’ grafting. Ann Surg. 1990;212:14–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.UNOS. United Network for Organ Sharing. http://www.UNOS.org 03/31/2003.
- 5.Yersiz H, Shaked A, Oltoff K, et al. Correlation between donor age and the pattern of liver graft recovery after transplantation. Transplantation. 1996;60:790–794. [PubMed] [Google Scholar]
- 6.Alexander J, Zola J. Expanding the donor pool: use of marginal donors for solid organ transplantation. Clin Transplant. 1996;10:1–19. [PubMed] [Google Scholar]
- 7.Grande L, Matus D, Rimola A, et al. Expanded liver donor age over 60 years for hepatic transplantation. Clin Transplant. 1998:297–301. [PubMed]
- 8.Melendez H, Heaton N. Understanding “marginal” liver grafts. Transplantation. 1999;68:469–471. [DOI] [PubMed] [Google Scholar]
- 9.Loinaz C, Gonzalez E. Marginal donors in liver transplantation. Hepatogastroenterology. 2000;47:256–263. [PubMed] [Google Scholar]
- 10.Renz JF, Busuttil RW. Adult-to-adult living-donor liver transplantation: a critical analysis. Semin Liver Dis. 2000;20:411–424. [DOI] [PubMed] [Google Scholar]
- 11.Reding R, Chardot C, Paul K, et al. Living-related liver transplantation in children at Saint-Luc University Clinics: a seven year experience in 77 recipients. Acta Chir Belg. 2001;101:17–19. [PubMed] [Google Scholar]
- 12.Brown R, Russo M, Lai M, et al. A survey of liver transplantation from living adult donors in the United States. N Engl J Med. 2003;348:818–825. [DOI] [PubMed] [Google Scholar]
- 13.Broelsch C, Frilling A, Testa G, et al. Living donor liver transplantation in adults. Eur J Gastroenterol Hepatol. 2003;15:3–6. [DOI] [PubMed] [Google Scholar]
- 14.Broering D, Sterneck M, Rogiers X. Living donor liver transplantation. J Hepatol. 2003;38:S119–135. [DOI] [PubMed] [Google Scholar]
- 15.Renz JF, Emond JC, Yersiz H, et al. Split-liver transplantation in the United States: Outcomes of a national survey. Ann Surg, in press. [DOI] [PMC free article] [PubMed]
- 16.Emond JC, Freeman RB, Renz JF, et al. Optimizing the use of donated cadaver livers: analysis and policy development. Liver Transplant. 2002;8:863–872. [DOI] [PubMed] [Google Scholar]
- 17.Couinaud C. Le Foie: Etudes Anatomiques et Chirurgicales. Paris: Masson; 1957. [Google Scholar]
- 18.Bismuth H. Surgical anatomy and anatomical surgery of the liver. World J Surg. 1982;6:3–9. [DOI] [PubMed] [Google Scholar]
- 19.Azoulay D, Astarcioglu I, Bismuth H, et al. Split-liver transplantation. The Paul Brousse policy. Ann Surg. 1996;224:737–746; discussion 746–748. [DOI] [PMC free article] [PubMed]
- 20.Rogiers X, Malago M, Gawad K, et al. In situ splitting of cadaveric livers. The ultimate expansion of a limited donor pool. Ann Surg. 1996;224:331–339; discussion 339–341. [DOI] [PMC free article] [PubMed]
- 21.Goss JA, Yersiz H, Shackleton CR, et al. In situ splitting of the cadaveric liver for transplantation. Transplantation 1997;64:871–877. [DOI] [PubMed] [Google Scholar]
- 22.Humar A, Ramcharan T, Sielaff T, et al. Split liver transplantation for two adult recipients: an initial experience. Am J Transplant. 2001;1:366–372. [DOI] [PubMed] [Google Scholar]
- 23.Yersiz H, Renz JF, Hisatake G, et al. Technical and logistical considerations of in situ split-liver transplantation for two adults: part I. Creation of left segment II, III, IV and right segment I, V-VIII grafts. Liver Transplant. 2001;7:1077–1080. [DOI] [PubMed] [Google Scholar]
- 24.Oike F, Sakamoto S, Kasahara M, et al. Monosegment graft in living donor liver transplantation, Transplant 2001, Chicago, IL, May 12–16, 2001.
- 25.Yersiz H, Renz JF, Hisatake G, et al. Technical and logistical considerations of in situ split-liver transplantation for two adults: Part II. Creation of left segment I-IV and right segment V-VIII grafts. Liver Transplant. 2002;8:78–81. [DOI] [PubMed] [Google Scholar]
- 26.Yersiz H, Renz J, Hisatake G, et al. The conventional technique of in-situ split-liver transplantation. J Hepatobil Pancreatic Surg. 2003;10:11–15. [DOI] [PubMed] [Google Scholar]
- 27.Rogiers X, Malago M, Habib N, et al. In situ splitting of the liver in the heart-beating cadaveric organ donor for transplantation in two recipients. Transplantation 1995;59:1081–1083. [PubMed] [Google Scholar]
- 28.Rela M, Vougas V, Muiesan P, et al. Split liver transplantation: King's College Hospital experience. Ann Surg. 1998;227:282–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reichert PR, Renz JF, D'Albuquerque LA, et al. Surgical anatomy of the left lateral segment as applied to living-donor and split-liver transplantation: a clinicopathologic study. Ann Surg. 2000;232:658–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jurim O, Shackleton C, McDiarmid S, et al. Living-donor liver transplantation at UCLA. Am J Surg. 1995;169:529–532. [DOI] [PubMed] [Google Scholar]
- 31.Busuttil RW, Goss J. Split liver transplantation. Ann Surg. 1999;229:313–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ghobrial RM, Yersiz H, Farmer DG, et al. Predictors of survival after in vivo split liver transplantation: analysis of 110 consecutive patients. Ann Surg. 2000;232:312–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Urata K, Kawasaki S, Matsunami H, et al. Calculation of child and adult standard liver volume for liver transplantation. Hepatology. 1995;21:1317–1321. [PubMed] [Google Scholar]
- 34.Goss J, Shackleton CR, McDiarmid S, et al. Long-term results of pediatric liver transplantation: An analysis of 569 transplants. Ann Surg. 1998;228:411–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaplan GL, Meier P. Nonparametric estimation from incomplete observation. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 36.Emond JC, Renz JF. Surgical anatomy of the liver and its application to hepatobiliary surgery and transplantation. Semin Liver Dis. 1994;14:158–168. [DOI] [PubMed] [Google Scholar]
- 37.Yersiz H, Busuttil RW. Surgical techniques in liver transplantation. In: Norman DJ, Turka LA, eds. Primer on Transplantation. Mt. Laurel: American Society of Transplantation; 2001:544–550. [Google Scholar]
- 38.Noujaim HM, Mayer DA, Buckels JAC, et al. Division of vascular medical and vascular complications after ex vivo split liver transplantation. Report of the European Liver Transplant Registry. Joint Meeting of the International Liver Transplantation Society, European Liver Transplantation Association, and the Liver Intensive Care Group of Europe, Berlin, Germany, July 11–13, 2001.
- 39.Azoulay D, Marin-Hargreaves G, Castaing D, et al. Ex situ splitting of the liver: the versatile Paul Brousse technique. Arch Surg. 2001;136:956–961. [DOI] [PubMed] [Google Scholar]
- 40.Broering D, Mueller L, Ganschow R, et al. Is there still a need for living-related liver transplantation in children. Ann Surg. 2001;234:713–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Emond JC, Ascher NL, Busuttil RW. Split liver transplantation in the United States: Creation of a national registry and preliminary outcomes. Am J Transplant. 2000;1:260. [Google Scholar]
- 42.Renz JF, Rosenthal P, Roberts JP, et al. Planned exploration of pediatric liver transplant recipients reduces posttransplant morbidity and lowers length of hospitalization. Arch Surg. 1997;132:950–955; discussion 955–956. [DOI] [PubMed]
- 43.Emond JC, Renz JF, Ferrell LD, et al. Functional analysis of grafts from living donors. Implications for the treatment of older recipients. Ann Surg. 1996;224:544–552; discussion 552–554. [DOI] [PMC free article] [PubMed]
- 44.Kiuchi T, Kasahara M, Uryuhara K, et al. Impact of graft size mismatching on graft prognosis in liver transplantation from living donors. Transplantation. 1999;67:321–327. [DOI] [PubMed] [Google Scholar]
- 45.Lo CM, Fan S, Liu CL, et al. Minimum graft size for successful living donor liver transplantation. Transplantation. 1999;68:1112–1116. [DOI] [PubMed] [Google Scholar]
- 46.Sugawara Y, Makuuchi M. Small-for-size graft problems in adult-to-adult living-donor liver transplantation. Transplantation. 2003;75:S20–S22. [DOI] [PubMed] [Google Scholar]
- 47.Farmer DG, Yersiz H, Ghobrial RM, et al. Early graft function after pediatric liver transplantation: comparison between in situ split liver grafts and living-related liver grafts. Transplantation. 2001;72:1795–1802. [DOI] [PubMed] [Google Scholar]
- 48.Karbe T, Rogiers X, Malago M, et al. Technical procedures and logistics of split-liver transplantation. Transplant Proc. 1996;28:3345–3346. [PubMed] [Google Scholar]
- 49.Ramcharan T, Glessing B, Lake JR, et al. Outcome of other organs recovered during in situ split-liver procurements. Liver Transplant. 2001;7:653–657. [DOI] [PubMed] [Google Scholar]
- 50.Renz JF, Mudge CL, Heyman MB, et al. Donor selection limits use of living-related liver transplantation. Hepatology. 1995;22:1122–1126. [DOI] [PubMed] [Google Scholar]
- 51.Marcos A, Ham JM, Fisher RA, et al. Single-center analysis of the first 40 adult-to-adult living donor liver transplants using the right lobe. Liver Transpl. 2000;6:296–301. [DOI] [PubMed] [Google Scholar]
- 52.Trotter JF. Selection of donors and recipients for living donor liver transplantation. Liver Transpl. 2000;6:S52–S58. [DOI] [PubMed] [Google Scholar]