Abstract
An effective vaccine against human immunodeficiency virus (HIV) should protect against mucosal transmission of genetically divergent isolates. As a safe alternative to live attenuated vaccines, the immunogenicity and protective efficacy of a DNA vaccine containing simian immunodeficiency virus (SIV) strain 17E-Fr (SIV/17E-Fr) gag-pol-env was analyzed in rhesus macaques. Significant levels of cytotoxic T lymphocytes (CTL), but low to undetectable serum antibody responses, were observed following multiple immunizations. SIV-specific mucosal antibodies and CTL were also detected in rectal washes and gut-associated lymphoid tissues, respectively. Vaccinated and naive control monkeys were challenged intrarectally with SIV strain DeltaB670 (SIV/DeltaB670), a primary isolate whose env is 15% dissimilar to that of the vaccine strain. Four of seven vaccinees were protected from infection as determined by the inability to identify viral RNA or DNA sequences in the peripheral blood and the absence of anamnestic antibody responses postchallenge. This is the first report of mucosal protection against a primary pathogenic, heterologous isolate of SIV by using a commercially viable vaccine approach. These results support further development of a DNA vaccine for protection against HIV.
A vaccine capable of controlling human immunodeficiency virus type 1 (HIV-1) infection is urgently needed to stem the AIDS pandemic. A significant challenge in the development of a vaccine for HIV is the enormous diversity of HIV-1 isolates encountered by the population at risk (23). At a minimum, an AIDS vaccine should protect against intraclade exposure (e.g., isolates with a genetic heterogeneity of 15 to 20%), and since sexual exposure is the primary route of HIV transmission (44), an effective vaccine should provide mucosal protection. A number of candidate AIDS vaccines have been tested in nonhuman primate models for the ability to protect against persistent infection or disease, with several candidate vaccines conferring protection against intravenous, nonpathogenic challenges (21). Protection against intrarectal (2, 11) or intravaginal (12) challenges with a homologous, pathogenic simian-human immunodeficiency virus or the homologous, primary simian immunodeficiency virus (SIV) isolate SIVmac251 has also been observed. These studies are encouraging in that protection against mucosal exposure to a homologous virus has been achieved using commercially viable vaccine approaches. The ability of an experimental vaccine tested in an animal model to protect against homologous challenge, however, argues little for its success in stemming the AIDS epidemic, in which individuals are routinely exposed to highly divergent genotypes. To date, only a live attenuated vaccine has been shown to protect the mucosa against the establishment of a chronic infection by a genetically distinct primary isolate of SIV (30). Several studies, however, have raised serious questions regarding the safety of live attenuated vaccines, particularly for wide-scale use (5, 35), so safer alternatives must be developed.
DNA vaccination results in the intracellular expression of encoded antigens (14, 43) and the induction of antigen-specific humoral and T-cell responses (13, 14, 41). The endogenous production of antigen following DNA delivery in the host cell mimics live attenuated vaccines without the safety concerns associated with administration of an infectious virus.
Substantial evidence now exists that supports a significant role for cytotoxic T lymphocytes (CTL) in the containment of HIV and SIV infections (1, 9, 33, 36). DNA vaccines, unlike live recombinant virus vaccine strategies, can induce high-frequency CTL responses against both dominant and subdominant epitopes (6), making this strategy an attractive, commercially viable alternative to live attenuated HIV vaccines. Studies in nonhuman primates have shown that DNA vaccines afford significant protection from challenge with avirulent or homologous, pathogenic AIDS viruses (2, 7, 10, 22, 24, 32). However, the ability of a DNA vaccine to provide mucosal protection against a heterologous, pathogenic AIDS virus has not been shown. In this study, we tested DNA vaccination for the ability to overcome critical obstacles encountered in human exposure to HIV by mucosally challenging vaccinated monkeys with a pathogenic, heterologous primary SIV isolate. Four of seven vaccinated monkeys were protected against this rigorous challenge, demonstrating the potential for DNA vaccination to induce broad-spectrum mucosal protection against AIDS.
MATERIALS AND METHODS
Animals.
Rhesus macaques were cared for in accordance with the National Institutes of Health's Guide for the Care and Use of Laboratory Animals, the Animal Welfare Act, and protocols approved by the University of Pittsburgh Institutional Animal Care and Use Committee.
DNA vaccine.
SIV strain 17E-Fr (SIV/17E-Fr) gag sequences were isolated using StuI and BamHI sites and cloned into pCMV-BGHpA/AMP, a vector containing the pUC19 origin of replication, the ampicillin resistance gene, the cytomegalovirus (CMV) immediate-early promoter, and the polyadenylation signal from the bovine growth hormone gene (BGHpA). This cloning yielded intermediate plasmid p185-31. The CMV-gag-BGHpA sequences from p185-31 were then subcloned into a plasmid containing the pBR322 origin of replication, resulting in intermediate plasmid WRG7132. pol-env sequences were then isolated from SIV/17E-Fr and ligated into WRG7132 by using BsiEI and DraIII sites to generate vaccine plasmid WRG7135 carrying SIV/17E-Fr gag-pol-env. This cloning fully deleted the 5′ long terminal repeat (LTR) and truncated the 3′ LTR by 360 bp. SIV nef was truncated at amino acid 93 by the insertion of a stop codon.
Immunizations.
Plasmid DNA was precipitated onto 1- to 3-μm-diameter gold particles, as previously described (34), at a rate of 2.0 μg of DNA per mg of gold. Abdominal and inner-leg fur was clipped from the monkeys, and DNA-coated gold particles were introduced into the epidermis near and over the inguinal lymph node by using the PowderJect XR1 gene delivery device (PowderJect Vaccines, Inc., Madison, Wis.) at a helium pressure of 500 lb/in2. Each delivery consisted of 1.0 mg of gold and 2.0 μg of DNA. Administration of DNA into 10 sites resulted in a dose of 20 μg of DNA per immunization. Consecutive DNA immunizations were spaced 12 to 14 weeks apart.
ELISAs.
SIV env- and gag-specific immunoglobulin G (IgG) and SIV env-specific mucosal IgA and IgG end-point titers were measured by enzyme-linked immunosorbent assay (ELISA) as previously described (19). Rectal washes were collected by gentle infusion and aspiration of 1.0-ml volumes of sterile saline. Enzyme immunoassay plates were coated with 0.3 to 0.4 μg of recombinant SIVmac251 gp120 or p28 protein (Intracel Corporation, Issaquah, Wash.) per well. Serially diluted monkey sera or rectal washes (clarified by centrifugation) were added. Antibodies bound to the coated antigen were detected using a rabbit anti-rhesus monkey IgG (heavy plus light chains) or goat anti-rhesus IgA conjugated to alkaline phosphatase (Accurate Chemical, Westbury, N.Y.).
Cytotoxicity assays.
Peripheral blood and mucosal CTL were measured as previously described (28). Peripheral blood mononuclear cells (PBMC) were purified by Ficoll-HyPaque (Pharmacia, Milwaukee, Wis.) density centrifugation. Jejunum and mesenteric lymph nodes (MLN) were collected from macaques by ventral abdominal midline celiotomies and purified by density gradient centrifugation. PBMC and mucosal mononuclear cells were stimulated with recombinant vaccinia viruses (0.1 PFU/cell) containing either SIVmac251 env (gp160), gag, or pol (kind gifts of G. P. Mazzara and D. Panicali, Therion Biologic Corp., Cambridge, Mass.) in the presence of recombinant interleukin-7 (IL-7) (R&D Systems, Minneapolis, Minn.) and IL-2 (Hoffman-La Roche, Nutley, N.J.). Following stimulation, CD8+ effector T cells were positively selected by using Dynabeads M450 with Detachabead (Dynal, Great Neck, N.Y.). Cytolytic activity was measured against 51Cr-labeled autologous B-cell target lines infected with either wild-type (wt) or recombinant SIV gag, pol, or env vaccinia virus.
Cytokine secretion analysis.
To measure SIV-specific T-helper cell cytokine secretion, macaque PBMC were cultured at 106 cells/ml in 24-well plates for 3 days in RPMI 1640 medium supplemented with antibiotics and 10% human AB serum. PBMC were cultured alone (negative control) or in the presence of 10 μg of recombinant SIVmac251 p28 or gp120 protein/ml (Intracel Corporation). The amount of rhesus monkey IL-4 or gamma interferon (IFN-γ) secreted in supernatants was measured using a rhesus monkey IFN-γ immunoassay kit (Biosource International, Camarillo, Calif.) or a rhesus IL-4 ELISA (U-CyTech BV; Utrecht University, Utrecht, The Netherlands) according to the manufacturer's instructions.
Viral challenge.
Macaques were fasted 12 h and sedated with ketamine (Parke-Davis) (10 mg/kg). Animals were positioned to provide sternal recumbancy and inoculated via atraumatic insertion of a 3-ml syringe approximately 5 cm into the rectum. The inoculum consisted of 1.0 ml of undiluted cryopreserved stock of SIV strain DeltaB670 (SIV/DeltaB670) (104 50% tissue culture infective dose) and 1.0 ml of 15% RPMI.
Diagnostic PCR.
Systemic infection was determined by detection of SIV-specific sequences in DNA lysates from PBMC by using a nested PCR and primers specific for the viral LTR as previously described (25).
Plasma viral loads.
Quantitation of virion-associated RNA in plasma was performed by real-time PCR in a Prism 7700 sequence detection system (Applied Biosystems, Inc., Foster City, Calif.). Virions were pelleted from 1 ml of plasma by centrifugation at 14,000 × g for 1 h. Total RNA was extracted from the virus pellet by using Trizol reagent (Life Technologies, Rockville, Md.), and 20 μl of each sample was analyzed in a 96-well plate. Synthesis of cDNA was accomplished in triplicate reactions in a mixture containing 50 mM MgCl, 1× PCR buffer II (50 mM KCl, 10 mM Tris-HCl, pH 8.3), 0.75 mM dGTP, 0.75 mM dATP, 0.75 mM dCTP, 0.75 mM dTTP, 1 U of RNase inhibitor, 1.2 U of murine leukemia virus reverse transcriptase (RT), 2.5 μM random hexamers, and 10% total viral RNA. Samples were mixed and incubated at room temperature for 10 min followed by 42°C for 12 min. The reaction was terminated by heating at 99°C for 5 min and incubating at 4°C for 5 min. The PCR was initiated immediately after the addition of RT by adding 30 μl of a PCR master mix containing 1× PCR buffer A, 5.5 mM MgCl2, 2.5 U of Amplitaq Gold, 200 mM deoxyribonucleoside triphosphates (dNTPs), 450 nM each primer, and 200 nM probe. The primers used were 5′AGGCTGGCAGATTGAGCCCTGGGAGGTTTC3′ and 5′CCAGGCGGCGACTAGGAGAGATGGGAACAC3′, and the probe used was 5′TTCCCTGCTAGACTCTCACCAGCACTTGG3′. The probe was labeled in the 5′ position with the fluorescent reporter dye 6-carboxyfluorescein and in the 3′ position with the quencher dye 6-carboxymethylrhodamine.
The amplification was carried out in the Prism 7700 sequence detection system by heating the specimens at 95°C for 10 min to activate Amplitaq Gold (Perkin-Elmer, Foster City, Calif.), prior to subjecting them to 40 cycles of 95°C for 15 s, 55°C for 15 s, and 72°C for 30 s. Serial dilutions of RNA obtained by in vitro transcription of an LTR-containing plasmid, ranging from 108 to 100 copies/reaction, were subjected to reverse transcription-PCR in triplicate, along with the samples, to generate a standard curve with a sensitivity threshold of 10 copies/reaction. RNA copy numbers from the unknown plasma samples were calculated from the standard curve and expressed as RNA copies per milliliter of plasma.
Viral sequence analysis.
Viral quasispecies infecting naive controls following rectal challenge were determined by sequencing the 477 bp spanning the first and second hypervariable regions of gp120 in proviral DNA from PBMC collected 14 days postinoculation as described previously (3). Full-length gp120 envelope sequences from isolated proviruses were generated by nested PCR amplification, resulting in a 1.6-kb final PCR product spanning the gp120 env region. The PCR-amplified product was cloned into the pcDNA3.1/V5-His-Topo eukaryotic TA vector (Invitrogen, Carlsbad, Calif.). Clones containing appropriately sized inserts were selected and sequenced in an ABI 370 automated DNA sequencer (Applied Biosystems, Inc.). Consensus amino acid sequences were derived for the SIV/DeltaB670 clones and aligned with the 17E/Fr vaccine strain sequence by using CLUSTALW (39). The average percentage of amino acid diversity between the vaccine and challenge strains was calculated with PROTODIST (16).
RESULTS
Vaccine-induced peripheral blood immune responses.
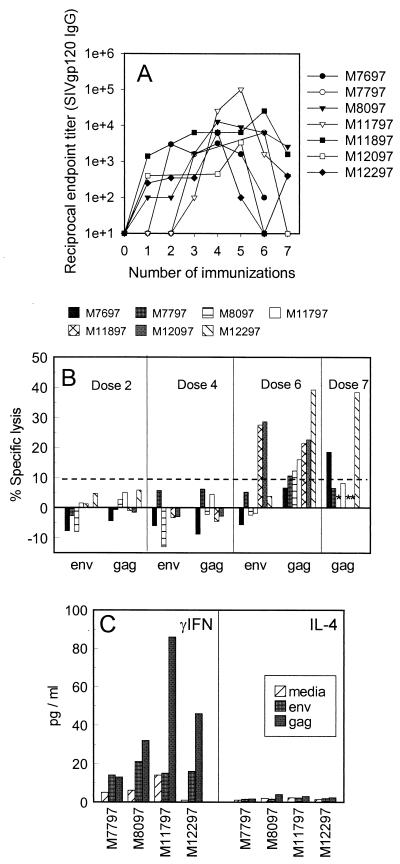
Seven rhesus macaques were immunized with a total of seven doses, spaced 12 to 14 weeks apart, by using the PowderJect gene delivery system to deliver the DNA into the skin (34). Each dose consisted of 20 μg of plasmid DNA carrying SIV/17E-Fr gag-pol-env. SIV gp120 (env)-specific and SIV p28 (gag)-specific serum antibody responses were measured by capture ELISA 2 weeks after each immunization. SIV p28-specific antibody titers were low or undetectable throughout the course of the immunizations (data not shown). However, SIV gp120-specific antibody titers in most animals peaked at moderate levels following the fourth or fifth DNA dose and then progressively declined to low or undetectable levels by the seventh DNA dose (Fig. 1A). The mean SIV gp120-specific antibody titer for all monkeys after the seventh DNA dose (1:774) was significantly lower than the mean titer following the fourth DNA dose (1:9,142; P < 0.05). The suppression of antibody responses following repeated DNA immunizations reported here is consistent with earlier findings in macaques immunized with a more accelerated spacing of 4 to 6 weeks between each of seven DNA doses (19). CTL responses against SIVmac239 gag, pol, and env were measured in PBMC from each monkey following the second, fourth, sixth, and seventh DNA immunizations. Significant CTL activity was not detected until after the sixth or seventh DNA immunization. Interestingly, to various degrees in each animal, the progressive decline in antibody titer following the fifth DNA dose occurred just before or immediately after significant CTL responses were first detected (Fig. 1B).
FIG. 1.
SIV-specific antibody and cellular immune responses in rhesus macaques vaccinated with DNA consisting of SIV/17E-Fr gag-pol-env. (A) SIV gp120-specific serum antibody titers were determined 2 weeks after each immunization by capture ELISA. (B) CD8+ CTL responses were measured 3 weeks after the second, fourth, sixth, and seventh DNA immunizations. PBMC were stimulated in vitro, and cytolytic activity was measured against Epstein-Barr virus-transformed B-lymphocyte cell lines (BLCLs) infected with vaccinia virus containing gag or env. For each monkey, background lysis measured against control targets (vaccinia virus wt-vaccinated autologous BLCLs) was subtracted from the test value. Background lysis values were between 1.4 and 6.0% in each case. A net value of ≥10% lysis after control subtraction is considered significant and is indicated by the dashed line. ∗, not tested. Monkeys M8097, M11897, and M12097 were not tested after the seventh dose. (C) SIV-specific T-helper cytokine secretion responses were measured 3 weeks after the seventh DNA immunization in four vaccinated macaques. PBMC were stimulated in vitro with recombinant SIV gag or env, and levels of secreted IFN-γ or IL-4 were determined.
This pattern is consistent with previous findings in studies of mice, in which immunization with multiple doses of DNA carrying HIV gp120 resulted in an inverse induction of antigen-specific antibody and CTL responses (18). In mice, this pattern corresponded to a switch between Th2- and Th1-like cytokine patterns, suggesting that the inverse relationship between CTL and antibody responses may be due to cytokine cross-regulation. The cytokine secretion response was similarly assessed in four monkeys in this study, following the sixth DNA immunization, when antibodies declined and significant levels of CTL were first detected. Significant levels of IFN-γ, but undetectable levels of IL-4, were observed in supernatants of SIV Gag and/or Env protein-stimulated PBMC from all four monkeys tested, indicating the dominance of a Th1-like response following the sixth DNA immunization (Fig. 1C).
Vaccine-induced mucosal immune responses.
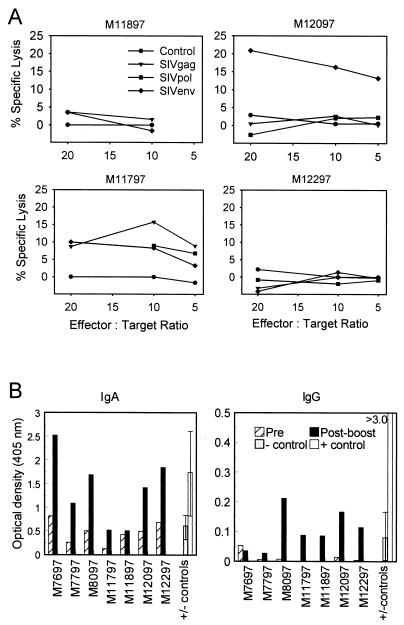
To determine if skin immunization with DNA induced mucosal immune responses, SIV-specific IgG and IgA antibody responses were evaluated in rectal washes before and 3 weeks after the final DNA immunization, when serum antibody responses were low. Rectal washes from three naive monkeys (negative controls) and three additional monkeys that had been challenged intrarectally with SIV 4 to 8 weeks before specimen collection (positive controls) were also tested for comparison. Background levels detected in the vaccinated monkeys prior to the booster immunization were comparable to levels detected in naive monkeys. Following the boost, significant 3- to 4-fold elevations in mucosal SIV gp120-specific IgA antibodies were observed in all seven macaques, and 9- to 20-fold elevations in mucosal SIV gp120-specific IgG antibodies were observed in six of seven macaques (Fig. 2B). The levels of rectal SIV-specific IgA detected in most of the vaccinated monkeys were comparable to levels detected in mucosally infected controls, whereas levels of rectal SIV-specific IgG in the vaccinees were significantly lower.
FIG. 2.
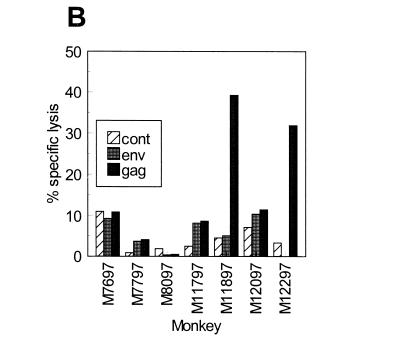
Mucosal immune responses in rhesus macaques following skin immunization with SIV DNA. (A) Gut-specific CD8+ CTL responses were measured 3 weeks after the fifth DNA immunization. CD8+ T cells were isolated from the lamina propria and MLN, and cytolytic activity against vaccinia virus gag-, pol-, env-, or wt (control)-vaccinated autologous BLCL was measured following in vitro stimulation. A net value of ≥10% lysis after control subtraction was considered significant. (B) Mucosal SIV gp120-specific IgG and IgA responses were measured in rectal washes by ELISA 1 or 2 weeks after the final DNA immunization. Postboost responses with optical density values >2-fold over preboost levels were considered significant. Negative controls (− cont) represent averages of background levels measured in rectal washes from three naive macaques. Positive controls (+ cont) represent averages of SIV-specific IgG or IgA levels measured in rectal washes from three macaques intrarectally infected with SIV/DeltaB670 4 to 8 weeks before the collection of rectal washes.
SIV-specific CTL responses were also measured in the gut-associated lymphoid tissue (GALT) in four of the vaccinees 3 weeks after the fifth DNA immunization. A 20-cm section of the jejunum and an accompanying mesenteric lymph node were surgically removed from monkeys M11897, M11797, M12097, and M12297. CD8+ T cells were isolated from the tissues and assayed for CTL activity against SIV gag-, pol-, and env-expressing autologous targets (Fig. 2A). Significant SIV-specific CTL activity (≥10% specific lysis) was detected in two of four macaques tested (M11797 and M12097). In monkey M11797, significant CTL activities of 15.8 and 10.1% were detected against SIV Gag and Env, respectively, and borderline activity of 9.2% was detected against SIV Pol. Only Gag-specific CTL were detected in M12097. Taken together, these results demonstrate that the skin-administered DNA vaccine induced SIV-specific immune responses in the gut-associated mucosa.
Mucosal protection against primary, heterologous SIV.
Six weeks after the final immunization, the seven vaccinated monkeys and eight naive controls were challenged intrarectally with 104 tissue culture infectious doses of cell-free SIV/DeltaB670. Following challenge, three of the seven vaccinated monkeys and seven of the eight naive controls became persistently infected, as determined by the identification of proviral DNA in PBMC by PCR using a nested reaction with sensitivities of 1 and 10 copies/reaction for LTR and env primers, respectively. The remaining four vaccinees and one of the controls remained PCR negative (Table 1). The one control animal that did not become infected is the only one of 20 animals that failed to become infected following mucosal exposure to this dose of the cryopreserved inoculum (data not shown). The failure to infect four of seven vaccinated monkeys compared to one of eight naive animals was not statistically significant (P = 0.119; 95% confidence interval; two-sided Fisher's exact test). However, three other animals from a separate study (M7097, M7297, and M8497) were challenged concurrently with the same dose and inoculum. These animals were part of a larger study intended to optimize delivery of DNA vaccines into mucosal tissues. They had received three intrarectal administrations of the DNA vaccine, but due to suboptimal delivery conditions, the vaccine failed to penetrate into the mucosal tissue. As a result, SIV-specific responses were never detected in either the mucosal tissues or peripheral blood in any of the monkeys at any time point prior to challenge (data not shown). Thus, we consider these animals naive with respect to SIV, and as expected, all three of these monkeys became infected as a result of intrarectal challenge (Table 1). The addition of these animals to this study for the purposes of evaluating statistical significance (4 of 7 protected vaccinated animals versus 1 of 11 unprotected naive animals) achieved statistical significance (P = 0.047).
TABLE 1.
PCR detection of SIV DNA in PBMC from macaques postchallengea
| Group | Monkey | Wk postinfectionb
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 4 | 11 | 16 | 20 | 24 | 28 | 32 | 36 | 40 | 44 | 48 | ||
| Control | M7097 | +/+ | +/+ | +/+ | +/+ | NT | NT | +/+c | ||||||
| M7297 | +/+ | +/+ | +/+ | +/+ | NT | NT | +/+c | |||||||
| M8497 | +/+ | +/+ | +/+ | +/+ | NT | NT | +/+c | |||||||
| M0698 | +/+ | +/+ | +/+ | +/+ | NT | NT | +/+c | |||||||
| M1798 | +/+ | +/+ | +/+ | +/+ | NT | NT | +/+ | +/+ | +/+ | +/+ | +/+ | +/+ | +/+ | |
| M2298 | +/+ | +/+ | +/+ | +/+ | NT | NT | +/+d | |||||||
| M1698 | −/− | −/− | −/− | −/− | NT | NT | −/− | −/− | −/− | −/− | −/− | −/− | −/− | |
| M12597 | +/+ | +/+ | +/+ | +/+ | NT | NT | +/+ | +/+ | +/+ | +/+ | +/+ | +/+ | +/+ | |
| M12897 | +/+ | +/+ | +/+ | +/+ | NT | NT | +/+ | +/+ | +/+ | +/+ | +/+ | +/+ | +/+ | |
| M13197 | +/+ | +/+ | +/+ | +/+ | NT | NT | +/+ | +/+ | +/+ | +/+ | +/+ | +/+ | +/+ | |
| M13097 | +/+ | +/+ | +/+ | +/+ | NT | NT | +/+ | +/+ | +/+ | +/+ | +/+ | +/+ | +/+ | |
| Vaccinee | M8097 | +/+ | +/+ | +/+ | +/+ | +/+ | +/+ | +/+ | +/+ | +/+ | +/+ | +/+ | +/+ | +/+ |
| M11797 | +/+ | +/+ | +/+ | +/+ | +/+ | +/+ | +/+ | +/+ | +/+d | |||||
| M11897 | +/+ | +/+ | +/+ | +/+ | +/+ | +/+ | +/+ | +/+ | +/+ | +/+ | +/+ | +/+ | +/+ | |
| M12097 | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | |
| M12297 | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | |
| M7697 | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | |
| M7797 | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | |
PCR was performed on PBMC samples in duplicate using nested primers spanning the LTR of the V1-V5 region of env. env-specific primers were used for PBMC from vaccinated monkeys on weeks 1 to 4. All other specimens were amplified with both env and LTR primers. Both reactions routinely detected 10 copies/reaction.
NT, not tested.
Indicates the transfer of the animal to another study.
Indicates the week the animal was euthanized due to AIDS.
Virus loads postchallenge.
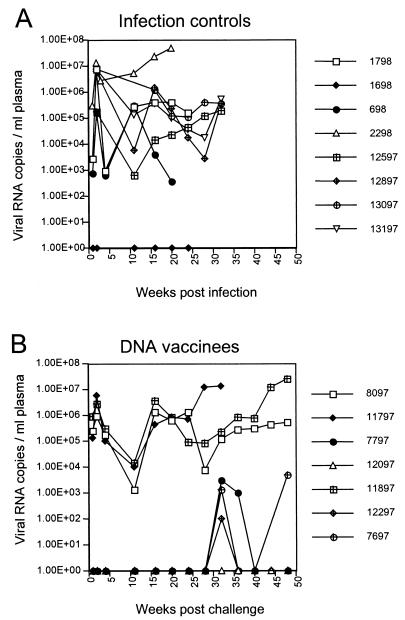
Postchallenge plasma virus loads were also determined by real-time PCR (Fig. 3). The plasma viremia observed in the control monkeys (Fig. 3A) is consistent with the peak and threshold levels previously observed in naive animals infected with this dose of the inoculum (42). As expected, the control monkey (M1698) that was PCR negative had no detectable virus in the plasma. The three persistently infected vaccinees (Fig. 3B) displayed a pattern of plasma viremia indistinguishable from that of the infected controls (Fig. 3A). In contrast, virus loads in the four PCR-negative vaccinees remained below the threshold level of detection for 28 weeks postchallenge (Fig. 3B). Interestingly, after 28 weeks, three of these monkeys (M12297, M7797, and M7697) had low, intermittent levels of plasma viremia at several time points. It is unlikely that these results were due to sample contamination, because the same values were obtained from independently stored plasma aliquots taken at these time points and omission of RT in the reaction resulted in negative values (data not shown). The lack of detectable infected cells in the periphery indicated that the source of viral RNA sporadically detected in the circulation was not PBMC.
FIG. 3.
Virus burden in infection controls (A) and DNA-vaccinated animals (B) following intrarectal challenge with SIV/DeltaB670. Numbers of RNA copies/ml of plasma were determined in an ABI Prism 7700 apparatus using probe primers specific for the viral LTR. This assay reliably detects and quantifies 50 copies/ml of plasma.
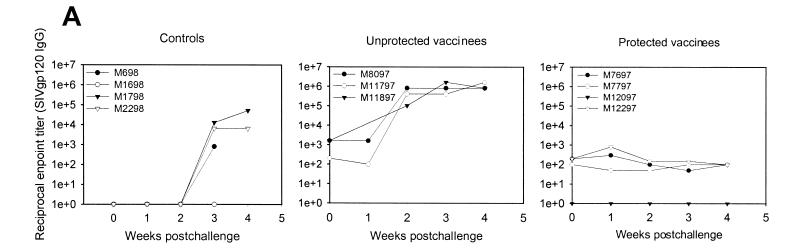
Postchallenge serum antibody responses in vaccinated and control monkeys were consistent with plasma viremia. Antibody responses before challenge were low to undetectable (1:1,600 to <1:10) in all monkeys (Fig. 4A). As expected, unprotected vaccinees showed strong anamnestic responses following challenge. In contrast, anamnestic antibody responses were undetectable in the protected vaccinees within the first 4 weeks postchallenge (Fig. 4A), and titers remained below the limit of detection (<1:100) until death (data not shown), suggesting that the vaccine contained the virus within the mucosal compartment.
FIG. 4.
Postchallenge antibody and CTL responses. Monkeys were challenged with SIV/DeltaB670 by the intrarectal route on week 0. (A) Postchallenge SIV gp120-specific serum antibody titers were determined by capture ELISA. (B) CTL responses were measured against vaccinia virus gag- or env-vaccinated autologous BLCLs following in vitro stimulation of PBMC.
CTL responses against SIV Gag and Env were also measured in the peripheral blood of vaccinated monkeys 3 weeks postchallenge. Similar analysis of the GALT tissue was left unperformed to avoid compromising the challenge by performing invasive surgery postinfection (p.i.). SIV Gag-specific CTL responses were detected in the peripheral blood in one of the four protected monkeys and in one of the three unprotected monkeys (Fig. 4B). The lack of significant CTL responses in the peripheral blood at a single time point postchallenge may be due to vaccine-primed CTL remaining sequestered in the mucosal compartment in a manner analogous to previous observations following low-dose rectal (28) or vaginal (42) exposure to replication-competent SIV.
Heterogeneity of the virus challenge.
Significant genetic divergence exists not only among different isolates of HIV but also among members of the quasispecies infecting a single individual. Thus, a working vaccine for HIV must protect from exposure to genetically distinct strains of virus. It has previously been shown that multiple genotypes contained within the SIV/DeltaB670 challenge stock successfully enter the circulation via various mucosal portals of entry (e.g., vagina, rectum, and mouth) (40). To confirm that the vaccinated monkeys were indeed exposed to a genetic mixture of genotypes found in the stock, as well as to identify the genetic relationship of these viruses to that used to construct the vaccine, the virus present in the plasma of control animals 2 weeks p.i. was genetically analyzed. Of the >20 different replication-competent genotypes identified in the inoculum (3, 40), 7 were found in two of the controls. Consistent with earlier studies (40), the two dominant variants in the inoculum were found in the greatest abundance. An alignment of the gp120 consensus sequences of the variants detected in the infected controls with the vaccine sequence is shown in Fig. 5. A sequence comparison of the gp120 region of the SIV/DeltaB670 variants revealed a deduced amino acid diversity of less than 6%, whereas similar comparisons of each variant in the challenge strain to the vaccine sequence (SIV/17E-Fr) revealed a diversity of over 15%. Most of the heterogeneity observed among these variants was found within the V1 and V4 hypervariable regions, with single amino acid differences noted through the remaining gp120 sequence. Although the significance of variable regions of the viral env with respect to immune function is not clear, these comparisons demonstrate a degree of genetic difference between the challenge and vaccine sequences analogous to that observed among different strains of HIV-1 residing within a single clade (17, 23). These data confirm that the DNA vaccine induced broad-spectrum protection.
FIG. 5.
gp120 sequence alignments of vaccine (17E/Fr) and challenge virus (SIV/DeltaB670) genotypes. The average percentage amino acid diversity between vaccine and challenge sequences was 15.2%. Sequences were aligned with CLUSTALW and diversities were calculated with PROTODIST.
Tissue-specific viral reservoirs.
The intermittent appearance of low levels of plasma viremia in three of the four protected vaccinees late in their infection prompted us to search for a reservoir of virus replication in peripheral and mucosal lymph nodes and tissues. Thirteen months postchallenge, both controls and vaccinees were sacrificed, and DNAs from lysates of PBMC, axillary lymph nodes and MLN, and spleen, jejunum, and colon were analyzed for the presence of viral DNA by PCR (Table 2). As expected, viral DNA was readily detected in all tissues examined in the persistently infected animals. Representative data from one infected vaccinee (M8097) are shown for comparison. In contrast, all tissue samples from three of the four protected vaccinees were uniformly PCR negative. However, one of the two PCR amplifications of the MLN from the fourth protected vaccinee (M7797) was positive. This result suggests that infected cells in the GALT may have served as a reservoir for the viral RNA sporadically detected in the peripheral blood of protected vaccinees (Fig. 3). These results further suggest that mucosal challenge of these animals resulted in a localized infection that was contained in the mucosal compartment by virus-specific immune responses present in the mucosa.
TABLE 2.
SIV PCR analysis of tissue DNA at sacrifice 48 weeks postchallenge
| Monkey | Tissue viral DNAa
|
|||||
|---|---|---|---|---|---|---|
| PBMC | ALN | MLN | Jej | Spl | Cln | |
| M7697 | −/− | −/− | −/− | −/− | −/− | −/− |
| M7797 | −/− | −/− | +/− | −/− | −/− | −/− |
| M12097 | −/− | −/− | N/A | −/− | −/− | −/− |
| M12297 | −/− | −/− | −/− | −/− | −/− | −/− |
| M8097 | +/+ | +/+ | +/+ | +/+ | +/+ | +/+ |
PBMC, peripheral blood mononuclear cells; ALN, axillary lymph node; MLN, mesenteric lymph node; Jej, jejunum; Spl, spleen; Cln, colon; N/A, not available.
Disease progression.
SIV/DeltaB670 is a highly pathogenic, primary isolate that causes AIDS in untreated rhesus macaques, with a mean time to death of 11 months (37). Furthermore, disease progression is identified by a significant decline in CD4+ T lymphocytes that is often evident by 3 months p.i. In this study, one infected vaccinee (M11797) and two infected controls (M2298 and M698) showed early declines in levels of CD4+ lymphocytes and died of AIDS by 36 weeks postchallenge. Among the remaining animals, T-cell subset changes correlated with the presence of viremia in each case, with the two surviving infected vaccinees and most of the infected controls showing declines in CD4+ lymphocytes by 1 year p.i. In contrast, the four protected vaccinees remained clinically and immunologically normal throughout the duration of the study (data not shown). These results were not surprising, because with the rare exception of long-term nonprogressors who fail to develop disease as a result of their intrinsic ability to ward off AIDS (37), it has been observed that vaccinated monkeys that become persistently infected with SIV/DeltaB670 following challenge eventually develop disease (19).
DISCUSSION
A growing body of evidence from studies of infected humans and nonhuman primates supports a role for CTL (1, 9, 33, 36), mucosal antibodies (4, 26) and mucosal CTL (28, 42) in the containment or prevention of HIV or SIV infection. No single measured immunological parameter was identified in the periphery at the time of challenge that could be correlated with mucosal protection in this study. However, the lack of detectable serum antibodies in the protected monkeys before and after challenge, coupled with the significant CTL responses identified in both the GALT and the periphery before challenge, suggests a potential role for CTL. In addition, mucosal antibody responses and T-helper cell responses induced by the vaccine may have also played a role in protection.
The inability to correlate CTL responses detected in the peripheral blood to protection was not surprising, given previous observations following low-dose virus exposure, in which SIV-specific CTL responses associated with mucosal protection were often detectable only in mucosal tissues (28, 42). In fact, the absence of mucosal CTL was shown in these studies to correlate with an uncontrolled persistent infection following mucosal challenge (28, 42). This result is similar to the outcome in the study reported here, in which four of the seven vaccinees were protected from a persistent infection while the remaining three vaccinees showed no evidence of protection. A similar dichotomy in the challenge outcome has also been observed in other SIV vaccine trials in which mucosal challenges with homologous viruses were performed (8, 31). In these studies, protected vaccinees showed no evidence of infection while unprotected vaccinees developed a persistent, uncontrolled infection with no evidence of virus load reduction. In the study reported here and in previous studies, the prevention of a persistent systemic infection following mucosal SIV challenge in some but not all vaccinated macaques may be analogous to the findings for some African prostitutes who fail to become systemically infected despite repeated mucosal exposures (33).
In this study, viral DNA was not detected in the PBMC of any of the four protected vaccinees but was detected in the mesenteric lymph node of one protected vaccinee. This result suggests that the GALT may have served as a reservoir for virus that was sporadically detected in the peripheral circulation. Whether the protected vaccinees that showed low-level, sporadic plasma viremia would have eventually developed a full-blown persistent infection and succumbed to disease remains an open question. However, the DNA vaccine employed in this study induced significant control of virus infection in the protected vaccinees for more than a year postchallenge. Taken together, these results support the possibility that mucosal immune responses induced by the vaccine may have promoted considerable control of the infection by containing the virus within the mucosal compartment and preventing systemic dissemination.
Upon first inspection, the induction of immune responses in the rectal and gut-associated compartments following DNA vaccination to the skin is unexpected, because peripheral immunization does not generally induce mucosal immune responses (27). However, studies have shown that under certain conditions the skin can function as an effective inductive site for both mucosal and systemic immune responses (15, 20), indicating that there is cross talk between the skin-associated lymphoid tissue and mucosal immune systems. The results from this study suggest that particle-mediated DNA vaccination to the skin may be an effective strategy to elicit mucosal immunity against HIV.
In experimental models, both the route of challenge and the challenge strain employed can have important implications in the selection of candidate vaccines for human trials. Experimental challenge by the mucosal route is imperative to assess the potential for a vaccine to protect from sexual exposure, the primary route of HIV transmission. However, vaccines that are highly effective at inducing immune responses in the periphery may not induce mucosal immunity, a component that may be essential for vaccine protection against mucosal exposure. Furthermore, mucosal immune responses are not always reflected by the responses identified in the peripheral blood. Therefore, it is imperative that vaccines tested in nonhuman primate models for the ability to protect from mucosal challenge be evaluated for their ability to induce responses in the mucosa.
With respect to the challenge strain employed, multiple genotypes can successfully penetrate the mucosal barrier and infect the host (19, 29, 38, 40). Thus, the genetic composition of challenge stocks used in these studies must be known. In this study, we sought to test DNA vaccination for the ability to overcome critical obstacles encountered in human exposure by mucosally challenging with a heterologous, primary viral isolate. The ability of the DNA vaccine tested in this study to protect four of seven monkeys against this rigorous challenge supports further development and clinical testing of a DNA vaccine for HIV.
Acknowledgments
We gratefully acknowledge A. Schultz, F. Vogel, and L. Payne, whose helpful discussions and continuous support made this study possible. We thank J. Mich-Bisso, L. Malicki, S. Wilson, J. Bruhn, and L. Fresh for technical support.
This work was supported by grants NO1-AI-65300 (M.M.C.) and RO1 AI35550 (M.M.C.) and Department of the Army grant DAMD-17-94-J-4426 (J.R.H.).
REFERENCES
- 1.Allen, T. M., D. H. O'Connor, P. Jing, J. L. Dzuris, B. R. Mothe, E. Dunphy, M. E. Liebl, T. U. Vogel, C. Emerson, N. Wilson, K. J. Kunstman, X. Wang, A. L. Hughes, R. C. Desrosiers, J. D. Altman, S. M. Wolinsky, A. Sette, and D. I. Watkins. 2000. Tat-specific cytotoxic T lymphocytes select for SIV escape variants during resolution of primary viremia. Nature 407:386-390. [DOI] [PubMed] [Google Scholar]
- 2.Amara, R. R., F. Villinger, J. D. Altman, S. L. Lydy, S. P. O'Neil, S. I. Staprans, D. C. Montefiori, Y. Xu, J. G. Herndon, L. S. Wyatt, M. A. Candido, N. L. Kozyr, P. L. Earl, J. M. Smith, H. L. Ma, B. D. Grimm, M. L. Hulsey, J. Miller, H. M. McClure, J. M. McNicholl, B. Moss, and H. L. Robinson. 2001. Control of a mucosal challenge and prevention of AIDS by a multiprotein DNA/MVA vaccine. Science 292:69-74. [DOI] [PubMed] [Google Scholar]
- 3.Amedee, A. M., N. Lacour, J. L. Gierman, L. N. Martin, J. E. Clements, R. Bohm, Jr., R. M. Harrison, and M. Murphey-Corb. 1995. Genotypic selection of simian immunodeficiency virus in macaque infants infected transplacentally. J. Virol. 69:7982-7990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baba, T. W., V. Liska, R. Hofmann-Lehmann, J. Vlasak, W. Xu, S. Ayehunie, L. A. Cavacini, M. R. Posner, H. Katinger, G. Stiegler, B. J. Bernacky, T. A. Rizvi, R. Schmidt, L. R. Hill, M. E. Keeling, Y. Lu, J. E. Wright, T. C. Chou, and R. M. Ruprecht. 2000. Human neutralizing monoclonal antibodies of the IgG1 subtype protect against mucosal simian-human immunodeficiency virus infection. Nat. Med. 6:200-206. [DOI] [PubMed] [Google Scholar]
- 5.Baba, T. W., V. Liska, A. H. Khimani, N. B. Ray, P. J. Dailey, D. Penninck, R. Bronson, M. F. Greene, H. M. McClure, L. N. Martin, and R. M. Ruprecht. 1999. Live attenuated, multiply deleted simian immunodeficiency virus causes AIDS in infant and adult macaques. Nat. Med. 5:194-203. [DOI] [PubMed] [Google Scholar]
- 6.Barouch, D. H., A. Craiu, S. Santra, M. A. Egan, J. E. Schmitz, M. J. Kuroda, T. M. Fu, J. H. Nam, L. S. Wyatt, M. A. Lifton, G. R. Krivulka, C. E. Nickerson, C. I. Lord, B. Moss, M. G. Lewis, V. M. Hirsch, J. W. Shiver, and N. L. Letvin. 2001. Elicitation of high-frequency cytotoxic T-lymphocyte responses against both dominant and subdominant simian-human immunodeficiency virus epitopes by DNA vaccination of rhesus monkeys. J. Virol. 75:2462-2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barouch, D. H., S. Santra, J. E. Schmitz, M. J. Kuroda, T. M. Fu, W. Wagner, M. Bilska, A. Craiu, X. X. Zheng, G. R. Krivulka, K. Beaudry, M. A. Lifton, C. E. Nickerson, W. L. Trigona, K. Punt, D. C. Freed, L. Guan, S. Dubey, D. Casimiro, A. Simon, M. E. Davies, M. Chastain, T. B. Strom, R. S. Gelman, D. C. Montefiori, and M. G. Lewis. 2000. Control of viremia and prevention of clinical AIDS in rhesus monkeys by cytokine-augmented DNA vaccination. Science 290:486-492. [DOI] [PubMed] [Google Scholar]
- 8.Benson, J., C. Chougnet, M. Robert-Guroff, D. Montefiori, P. Markham, G. Shearer, R. C. Gallo, M. Cranage, E. Paoletti, K. Limbach, D. Venzon, J. Tartaglia, and G. Franchini. 1998. Recombinant vaccine-induced protection against the highly pathogenic simian immunodeficiency virus SIVmac251: dependence on route of challenge exposure. J. Virol. 72:4170-4182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Borrow, P., H. Lewicki, B. Hahn, G. Shaw, and M. Oldstone. 1994. Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J. Virol. 68:6103-6110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boyer, J. D., K. E. Ugen, B. Wang, M. Agadjanyan, L. Gilbert, M. L. Bagarazzi, M. Chattergoon, P. Frost, A. Javadian, W. V. Williams, Y. Refaeli, R. B. Ciccarelli, D. McCallus, L. Coney, and D. B. Weiner. 1997. Protection of chimpanzees from high-dose heterologous HIV-1 challenge by DNA vaccination. Nat. Med. 3:526-532. [DOI] [PubMed] [Google Scholar]
- 11.Cranage, M. P., A. M. Whatmore, S. A. Sharpe, N. Cook, N. Polyanskaya, S. Leech, J. D. Smith, E. W. Rud, M. J. Dennis, and G. A. Hall. 1997. Macaques infected with live attenuated SIVmac are protected against superinfection via the rectal mucosa. Virology 229:143-154. [DOI] [PubMed] [Google Scholar]
- 12.Crotty, S., C. J. Miller, B. L. Lohman, M. R. Neagu, L. Compton, D. Lu, F. X. Lu, L. Fritts, J. D. Lifson, and R. Andino. 2001. Protection against simian immunodeficiency virus vaginal challenge by using Sabin poliovirus vectors. J. Virol. 75:7435-7452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Donnelly, J. J., J. B. Ulmer, J. W. Shiver, and M. A. Liu. 1997. DNA vaccines. Annu. Rev. Immunol. 15:617-648. [DOI] [PubMed] [Google Scholar]
- 14.Eisenbraun, M. D., D. H. Fuller, and J. R. Haynes. 1993. Examination of parameters affecting the elicitation of humoral immune responses by particle bombardment-mediated genetic immunization. DNA Cell Biol. 12:791-797. [DOI] [PubMed] [Google Scholar]
- 15.Enioutina, E. Y., D. Visic, and R. A. Daynes. 2000. The induction of systemic and mucosal immune responses to antigen-adjuvant compositions administered into the skin: alterations in the migratory properties of dendritic cells appear to be important for stimulating mucosal immunity. Vaccine 18:2753-2767. [DOI] [PubMed] [Google Scholar]
- 16.Felsenstein, J. 1996. Inferring phylogenies from protein sequences by parsimony, distance, and likelihood methods. Methods Enzymol. 266:418-427. [DOI] [PubMed] [Google Scholar]
- 17.Fonjungo, P. N., E. N. Mpoudi, J. N. Torimiro, G. A. Alemnji, L. T. Eno, J. N. Nkengasong, F. Gao, M. Rayfield, T. M. Folks, D. Pieniazek, and R. B. Lal. 2000. Presence of diverse human immunodeficiency virus type 1 viral variants in Cameroon. AIDS Res. Hum. Retrovir. 16:1319-1324. [DOI] [PubMed] [Google Scholar]
- 18.Fuller, D. H., and J. R. Haynes. 1994. A qualitative progression in HIV type 1 glycoprotein 120-specific cytotoxic cellular and humoral immune responses in mice receiving a DNA-based glycoprotein 120 vaccine. AIDS Res. Hum. Retrovir. 10:1433-1441. [DOI] [PubMed] [Google Scholar]
- 19.Fuller, D. H., L. Simpson, K. S. Cole, J. E. Clements, D. L. Panicali, R. C. Montelaro, M. Murphey-Corb, and J. R. Haynes. 1997. Gene gun-based nucleic acid immunization alone or in combination with recombinant vaccinia vectors suppresses virus burden in rhesus macaques challenged with a heterologous SIV. Immunol. Cell Biol. 75:389-396. [DOI] [PubMed] [Google Scholar]
- 20.Glenn, G. M., T. Scharton-Kersten, R. Vassell, C. P. Mallett, T. L. Hale, and C. R. Alving. 1998. Transcutaneous immunization with cholera toxin protects mice against lethal mucosal toxin challenge. J. Immunol. 161:3211-3214. [PubMed] [Google Scholar]
- 21.Johnston, M. I. 2000. The role of nonhuman primate models in AIDS vaccine development. Mol. Med. Today 6:267-270. [DOI] [PubMed] [Google Scholar]
- 22.Kent, S. J., A. Zhao, S. J. Best, J. D. Chandler, D. B. Boyle, and I. A. Ramshaw. 1998. Enhanced T-cell immunogenicity and protective efficacy of a human immunodeficiency virus type 1 vaccine regimen consisting of consecutive priming with DNA and boosting with recombinant fowlpox virus. J. Virol. 72:10180-10188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Korber, B. T., E. E. Allen, A. D. Farmer, and G. L. Myers. 1995. Heterogeneity of HIV-1 and HIV-2. AIDS 9:S5-S18. [PubMed] [Google Scholar]
- 24.Letvin, N. L., D. C. Montefiori, Y. Yasutomi, H. C. Perry, M. E. Davies, C. Lekutis, M. Alroy, D. C. Freed, C. I. Lord, L. K. Handt, M. A. Liu, and J. W. Shiver. 1997. Potent, protective anti-HIV immune responses generated by bimodal HIV envelope DNA plus protein vaccination. Proc. Natl. Acad. Sci. USA 94:9378-9383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martin, L. N., K. F. Soike, M. Murphey-Corb, R. P. Bohm, E. D. Roberts, T. J. Kakuk, S. Thaisrivongs, T. J. Vidmar, M. J. Ruwart, S. R. Davio, et al. 1994. Effects of U-75875, a peptidomimetic inhibitor of retroviral proteases, on simian immunodeficiency virus infection in rhesus monkeys. Antimicrob. Agents Chemother. 38:1277-1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mascola, J. R., G. Stiegler, T. C. VanCott, H. Katinger, C. B. Carpenter, C. E. Hanson, H. Beary, D. Hayes, S. S. Frankel, D. L. Birx, and M. G. Lewis. 2000. Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat. Med. 6:207-210. [DOI] [PubMed] [Google Scholar]
- 27.McGhee, J. R., M. E. Lamm, and W. Strober. 1999. Mucosal immune responses, p. 485-506. In P. L. Ogra, J. Mestecky, M. E. Lamm, W. Strober, J. Bienenstock, and J. R. McGhee (ed.), Mucosal immunology. Academic Press, San Diego, Calif.
- 28.Murphey-Corb, M., L. A. Wilson, A. M. Trichel, D. E. Roberts, K. Xu, S. Ohkawa, B. Woodson, R. Bohm, and J. Blanchard. 1999. Selective induction of protective MHC class I-restricted CTL in the intestinal lamina propria of rhesus monkeys by transient SIV infection of the colonic mucosa. J. Immunol. 162:540-549. [PubMed] [Google Scholar]
- 29.Neildez, O., R. Le Grand, P. Caufour, B. Vaslin, A. Cheret, F. Matheux, F. Theodoro, P. Roques, and D. Dormont. 1998. Selective quasispecies transmission after systemic or mucosal exposure of macaques to simian immunodeficiency virus. Virology 243:12-20. [DOI] [PubMed] [Google Scholar]
- 30.Nilsson, C., B. Makitalo, R. Thorstensson, S. Norley, D. Binninger-Schinzel, M. Cranage, E. Rud, G. Biberfeld, and P. Putkonen. 1998. Live attenuated simian immunodeficiency virus (SIV)mac in macaques can induce protection against mucosal infection with SIVsm. AIDS 12:2261-2270. [DOI] [PubMed] [Google Scholar]
- 31.Polacino, P., V. Stallard, D. C. Montefiori, C. R. Brown, B. A. Richardson, W. R. Morton, R. E. Benveniste, and S. L. Hu. 1999. Protection of macaques against intrarectal infection by a combination immunization regimen with recombinant simian immunodeficiency virus SIVmne gp160 vaccines. J. Virol. 73:3134-3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Robinson, H. L., D. C. Montefiori, R. P. Johnson, K. H. Manson, M. L. Kalish, J. D. Lifson, T. A. Rizvi, S. Lu, S.-L. Hu, G. P. Mazzara, S. L. Lydy, M. S. Wyand, and H. M. McClure. 1999. Neutralizing antibody-independent containment of immunodeficiency virus challenges by DNA priming and recombinant pox virus booster immunizations. Nat. Med. 5:526-534. [DOI] [PubMed] [Google Scholar]
- 33.Rowland-Jones, S., et al. 1995. HIV-specific cytotoxic T-cells in HIV-exposed but uninfected Gambian women. Nat. Med. 1:59-64. [DOI] [PubMed] [Google Scholar]
- 34.Roy, M. J., M. S. Wu, L. J. Barr, J. T. Fuller, L. G. Tussey, S. Speller, J. Culp, J. K. Burkholder, W. F. Swain, R. M. Dixon, G. Widera, R. Vessey, A. King, G. Ogg, A. Gallimore, J. R. Haynes, and D. H. Fuller. 2000. Induction of antigen-specific CD8+ T cells, T helper cells, and protective levels of antibody in humans by particle-mediated administration of a hepatitis B virus DNA vaccine. Vaccine 19:764-778. [DOI] [PubMed] [Google Scholar]
- 35.Sawai, E. T., M. S. Hamza, M. Ye, K. E. Shaw, and P. A. Luciw. 2000. Pathogenic conversion of live attenuated simian immunodeficiency virus vaccines is associated with expression of truncated Nef. J. Virol. 74:2038-2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schmitz, J. E., M. J. Kuroda, S. Santra, V. G. Sasseville, M. A. Simon, M. A. Lifton, P. Racz, K. Tenner-Racz, M. Dalesandro, B. J. Scallon, J. Ghrayeb, M. A. Forman, D. C. Montefiori, E. P. Rieber, N. L. Letvin, and K. A. Reimann. 1999. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science 283:857-860. [DOI] [PubMed] [Google Scholar]
- 37.Seman, A. L., W. F. Pewen, L. F. Fresh, L. N. Martin, and M. Murphey-Corb. 2000. The replicative capacity of rhesus macaque peripheral blood mononuclear cells for simian immunodeficiency virus in vitro is predictive of the rate of progression to AIDS in vivo. J. Gen. Virol. 81:2441-2449. [DOI] [PubMed] [Google Scholar]
- 38.Sodora, D. L., F. Lee, P. J. Dailey, and P. A. Marx. 1998. A genetic and viral load analysis of the simian immunodeficiency virus during the acute phase in macaques inoculated by the vaginal route. AIDS Res. Hum. Retrovir. 14:171-181. [DOI] [PubMed] [Google Scholar]
- 39.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Trichel, A. M., E. D. Roberts, L. A. Wilson, L. N. Martin, R. M. Ruprecht, and M. Murphey-Corb. 1997. SIV/DeltaB670 transmission across oral, colonic, and vaginal mucosae in the macaque. J. Med. Primatol. 26:3-10. [DOI] [PubMed] [Google Scholar]
- 41.Ulmer, J., J. Donnelly, S. Parker, G. Rhodes, P. Felgner, V. Dwarki, S. Gromkowski, R. Deck, C. DeWitt, A. Friedman, L. Hawe, K. Leander, D. Martinez, H. Perry, J. Shiver, D. Montgomery, and M. Liu. 1993. Heterologous protection against influenza by injection of DNA encoding a viral protein. Science 259:1745-1748. [DOI] [PubMed] [Google Scholar]
- 42.Wilson, L. A., M. Murphey-Corb, L. N. Martin, R. M. Harrison, M. S. Ratterree, and R. P. Bohm. 2000. Identification of SIV env-specific CTL in the jejunal mucosa in vaginally exposed, seronegative rhesus macaques (Macaca mulatta). J. Med. Primatol. 29:173-181. [DOI] [PubMed] [Google Scholar]
- 43.Wolff, J. A., R. W. Malone, P. Williams, W. Chong, G. Acsadi, A. Jani, and P. L. Felgner. 1990. Direct gene transfer into mouse muscle in vivo. Science 247:1465-1468. [DOI] [PubMed] [Google Scholar]
- 44.World Health Organization. 1998. WHO report on the global HIV/AIDS epidemic. World Health Organization, Geneva, Switzerland.