Abstract
Objective:
To guide treatment and clinical follow-up by defining the natural history of thin melanomas and identifying negative prognostic characteristics that may delineate high-risk patients.
Summary Background Data:
In following > 10,000 patients with cutaneous melanoma over the past 30 years, our institution has observed nodal or metastatic disease in approximately 15% of patients with a thin (<1 mm) primary lesion.
Methods:
A database query of patients with cutaneous melanoma returned 1158 patients with primary lesion ≤ 1 mm thick and who received their initial treatment at a single institution. Median follow-up was 11 years (range, 1 to 34 years). Patient and melanoma characteristics as well as outcomes were recorded and statistically analyzed.
Results:
6.6% of patients had nodal or distant disease at presentation. Over time, an additional 9.4% developed metastases, including nodal and distal recurrences. Overall incidence of advanced disease was 15.3%. Univariate analysis identified male gender (P = 0.01), advanced age (>45 years; P = 0.05), and Breslow thickness (>0.75 mm; P = 0.008) as significant negative prognostic characteristics. Of patients with these 3 high-risk characteristics, 19.7% developed advanced disease (likelihood ratio 6.3; P = 0.007 versus nonhigh-risk patients). This group had more than twice the incidence of nodal recurrences. Patients with recurrence had significantly decreased 10-year survival (82% versus 45%; P < 0.0001). Surprisingly, neither ulceration nor Clark level predicted advanced disease.
Conclusions:
Thin melanomas are potentially lethal lesions. Long-term follow-up identified a high-risk population of older males with tumors between 0.75 mm and 1.0 mm whose risk of recurrent disease approaches 20%. Traditionally accepted negative prognostic factors such as ulceration and discordant Clark levels are not predictive for metastasis in this population. Given the poor prognosis associated with recurrent disease, we recommend close clinical evaluation and follow-up to maximize accurate staging and therapeutic options.
Although most patients with thin cutaneous melanoma treated by surgical resection have an excellent prognosis, approximately 15% of patients will develop distant disease. This paper reviews 1158 patients with melanomas < 1.0 mm thick over a 30-year period and identifies patient and tumor characteristics associated with worse outcomes.
Tumor thickness is the dominant prognostic factor in determining risk of metastasis and prognosis for cutaneous melanoma.1–3 For cutaneous malignant melanoma < 1.0 mm in depth, wide local excision is appropriate and adequate treatment, resulting in 5-year survival of {mt] 90%.4 There is a subset of patients with thin melanoma, however, who develop nodal or distant metastases and thus suffer a worse prognosis. Although several patient and histologic characteristics, including gender, age, site, thickness, ulceration, regression, and vertical growth phase,5–11 have been associated with more aggressive disease, clearly identifying a high-risk population with thin melanomas remains a challenge.
The ability to correctly select a subgroup of melanoma patients with a poor prognostic profile from among a patient population with expected excellent outcome would have 2 major implications. First, the group at increased risk may warrant aggressive efforts to detect metastatic disease. Secondly, vigilant long-term clinical follow-up may be essential to detect recurrent disease in a group of patients who otherwise would be thought cured. This study reviews a large population with a 30-year longitudinal follow-up that defines the natural history of thin melanoma, the timing and patterns of recurrence, and identifies negative prognostic histologic and patient characteristics.
PATIENTS AND METHODS
More than 10,000 patients with malignant melanoma have been evaluated at Duke University Comprehensive Cancer Center since 1972. Patient and melanoma histologic characteristics were prospectively collected and maintained in a melanoma database; 1563 patients had cutaneous melanomas with thickness < 1.0 mm. Broadly defined, there were 2 different groups of patients evaluated: those who received their initial treatment at Duke University Medical Center (n = 1158) and those who were referred after receiving at least 1 intervention or therapy prior to referral (n = 405). The secondary treatment population tended to be referred to our institution after initial treatment failure elsewhere. The recurrence rate for the secondary treatment population was nearly 8-fold that of the initial therapy group. Thus, combining both groups introduced an analysis bias. We believe that the typical experience with thin melanoma is represented by the former group. Therefore, the final study population consisted of 1158 patients with thin melanomas who received their initial treatment at a single institution.
Patients were treated by wide local excision with margins of at least 1 cm. Therapeutic lymph node dissections were performed if nodes were clinically palpable on examination. In recent years, a small percentage of patients underwent sentinel lymph node mapping and biopsy based on clinical and histologic suspicion of aggressive disease.
Patients were followed by clinical examination every 6 months for 5 years after diagnosis and then yearly thereafter. In addition, all patients underwent annual chest radiography. Both mean and median follow-up was 11 years (range, 1 to 34 years). Overall survival and disease-free survival were evaluated as outcomes. Disease recurrence was measured as time and site of first recurrence after initial treatment. Site of first recurrence was categorized by the most advanced recurrence after resection. For example, if both systemic and nodal metastases were detected at the time of first recurrence, that patient was categorized as having distant disease as the site of first recurrence. Overall survival was defined by death from any cause. Survival probabilities for 25 years were developed by the Kaplan-Meier method. Univariate variables were tested for influence on disease-free and overall survival by Cox regression analysis. P < 0.05 was considered significant.
RESULTS
Patient and Tumor Characteristics
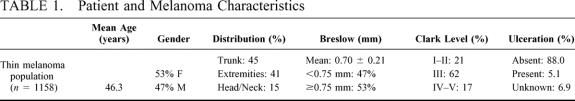
Patient and melanoma characteristics are displayed in Table 1. As noted, the mean patient age was 46 years, with a slightly higher proportion of females. The lesions were nearly equally distributed between trunk and extremities, with a small proportion located on the head or neck. The mean tumor thickness was 0.70 mm, with 47.4% < 0.75 mm. The majority of lesions were Clark level III. Ulceration was present in 5.1% of patients. Histologically, most lesions were superficial, spreading malignant melanomas (83.9%). Five percent of lesions were lentigo maligna, 2.7% were nodular melanomas, 1.4% were acral-lentiginous, 1.6% were characterized as other histologic patterns, and 5.4% of lesions were unknown in the database.
TABLE 1. Patient and Melanoma Characteristics
Advanced Disease
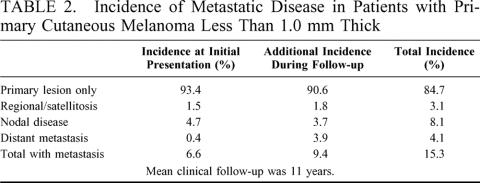
There were 1158 patients with melanoma < 1.0 mm who were initially treated at our institution included in this analysis. Most of patients (93.4%) presented with the primary cutaneous lesion as the only evidence of disease. Fifty-four patients (4.7%) had clinically palpable nodes at the time of presentation, 17 patients (1.5%) had additional local cutaneous disease or satellitosis, and 5 patients (0.4%) were found to have distant metastatic disease at initial evaluation (Table 2). Of the 1082 patients who presented with only a single cutaneous lesion and no evidence of metastasis, an additional 101 (9.4%) developed recurrent disease over a mean clinical follow-up of 11 years. Thus, 15.3% of all patients with thin melanoma either presented with metastatic disease, or developed recurrence at some point in their clinical course.
TABLE 2. Incidence of Metastatic Disease in Patients with Primary Cutaneous Melanoma Less Than 1.0 mm Thick
Patterns of Recurrence
The incidence and distribution of metastatic sites for the 140 patients (12.1%) who developed disease recurrence are given in Table 2. This set of 140 patients includes the subset who presented with disease beyond their primary and underwent surgical resection of all known disease and then subsequently developed recurrence. There were 76 patients with an advanced presentation who were treated surgically to achieve no evidence of disease. Thirty-nine (51.3%) of these patients developed recurrent disease. The majority of these recurrences were systemic failures (25 patients, 64.1%). Eight patients (20.5%) recurred at regional nodal basins, and 6 patients (16.4%) had local skin recurrence. Of the 67 total patients with systemic recurrence, lung, brain, and distant skin were the most common metastatic sites.
To help predict which patients with thin melanoma would develop recurrent or metastatic disease, we analyzed the subgroup of 1082 patients who presented only with a cutaneous primary melanoma. They were followed clinically for local, regional nodal, and distant recurrence. Within this group, 101 patients (9.3%) developed recurrent disease. Thirty-eight patients (3.5% overall, 38% of recurrences) had recurrent disease in nodal basins. Forty-two patients (3.9% overall, 42% of recurrences) recurred with distant disease. Nineteen patients (1.8% overall, 19% of recurrences) developed local skin recurrence, and 2 patients were found to have in-transit nodal disease.
Disease-Free Survival
As stated above, 140 patients (12.1%) with thin melanoma developed recurrent or metastatic disease during follow-up. The overall disease-free survival at 5, 10, 15, and 20 years was 86%, 78%, 71%, and 59%, respectively. There is a continued steady decline in disease-free survival throughout the entire period observed. These data are shown in Figure 1.
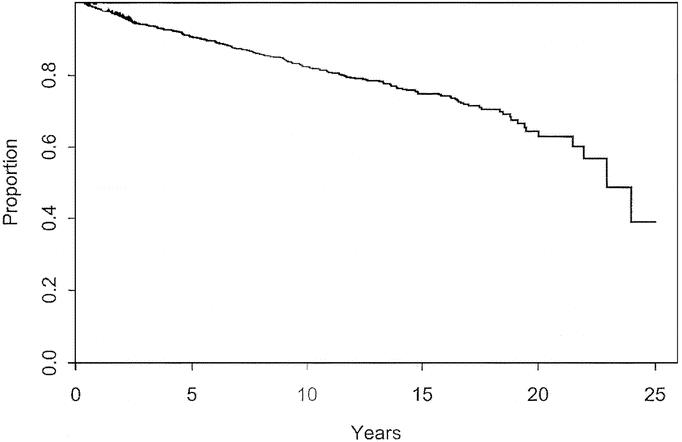
FIGURE 1. Disease-free survival for patients with cutaneous melanoma < 1.0 mm thick.
Survival
There were 230 deaths. The median survival was 22.9 years. The overall actuarial survival at 5, 10, 15, and 20 years was 90%, 82%, 75%, and 64%, respectively. These data are shown in Figure 2. Patients who were found to have locally advanced, nodal, or distant disease at the time of initial presentation had an overall survival of 46% at median follow-up of 11 years. Within the group of patients who initially presented with only a primary nonmetastatic lesion, 9.4% eventually developed recurrent advanced disease. As expected, all patients with recurrent disease, either nodal or systemic have a much worse prognosis than those patients without recurrence with an overall 10-year survival of 45% compared with 87%, and 20-year survival or 13% compared with 72% (Fig. 3).
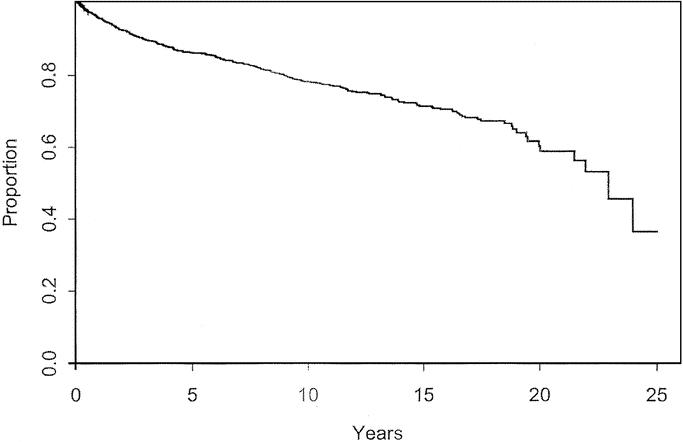
FIGURE 2. Overall survival for patients with cutaneous melanoma < 1.0 mm thick.
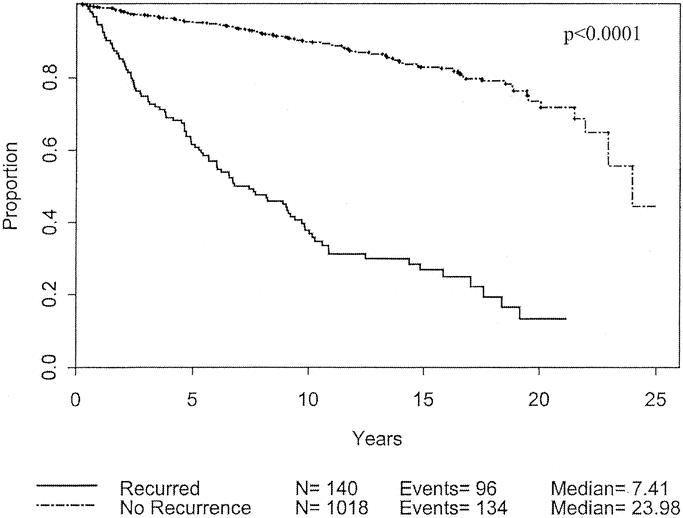
FIGURE 3. Overall survival based on tumor recurrence for patients with cutaneous melanoma < 1.0 mm thick.
Predictive Characteristics
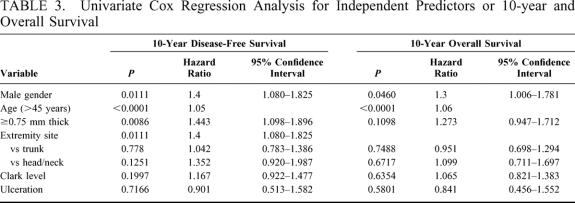
Univariate analysis was used to analyze patient and melanoma characteristics that might be associated with progression of disease, using recurrence or development of metastases as the endpoints. The impact on 10-year disease-free survival is displayed in Table 3. Male gender, advanced age, and tumor thickness > 0.75 mm were significant negative prognostic characteristics. Male gender and thicker melanomas each carry a hazard ratio of 1.4. The effects of these variables are charted through 25 years in Figures 3 to 5. Male patients’ disease-free survival was 76.6% at 10 years compared with 84.8% for female patients (P = 0.01). Advanced age was a significant negative prognostic factor as a continuous variable. For statistical analysis, age of 45 years was chosen as a break point at which melanoma recurrence and survival decrease (P < 0.0001). Patients with tumors < 0.75 mm had an 84.2% 10-year disease-free survival compared with 78.2% for those patients between 0.75 mm and 1.0 mm (P = 0.0086). Patients with primary lesions on the head or neck trended to do worse than counterparts with lesions on the extremities, but this was not statistically significant. Neither Clark level nor ulceration predicted recurrent disease.
TABLE 3. Univariate Cox Regression Analysis for Independent Predictors or 10-year and Overall Survival
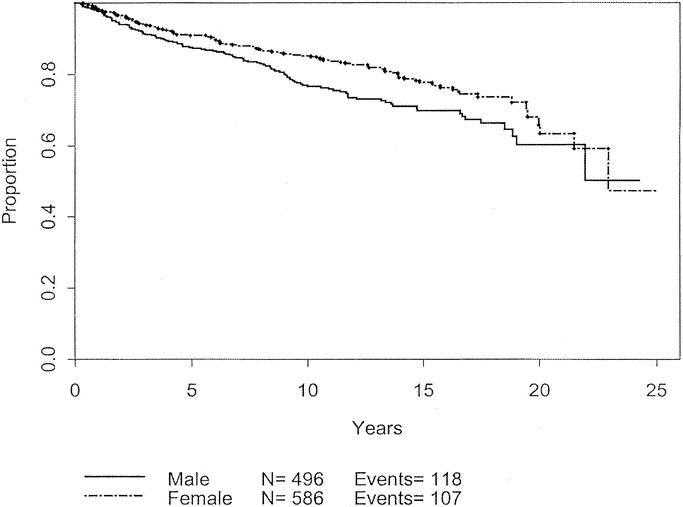
FIGURE 4. Disease-free survival based on gender.
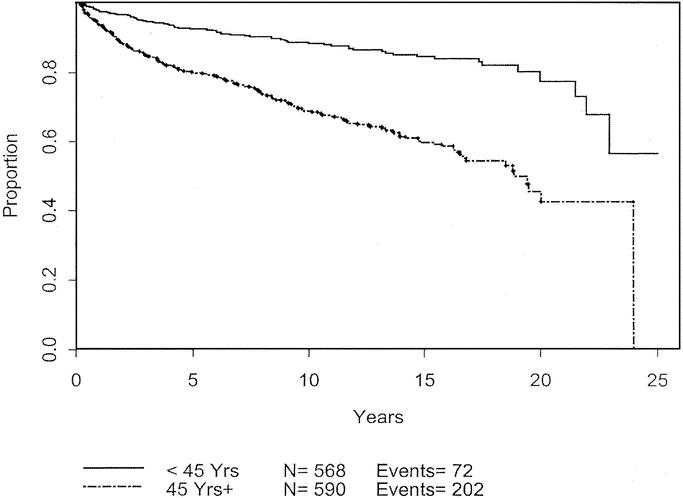
FIGURE 5. Disease-free survival based on age.
The same analysis was performed to determine effects of various characteristics on overall survival (Table 3). Again, male gender (P = 0.05) and advanced age (P < 0.0001) were associated with decreased survival. The effects of these variables are charted through 25 years in Figures 6 and 7. Although tumor thickness trended toward significance (P = 0.1098; HR, 1.273; CI, 0.947–1.72), it was not considered to influence long-term survival at a 5% significance level.
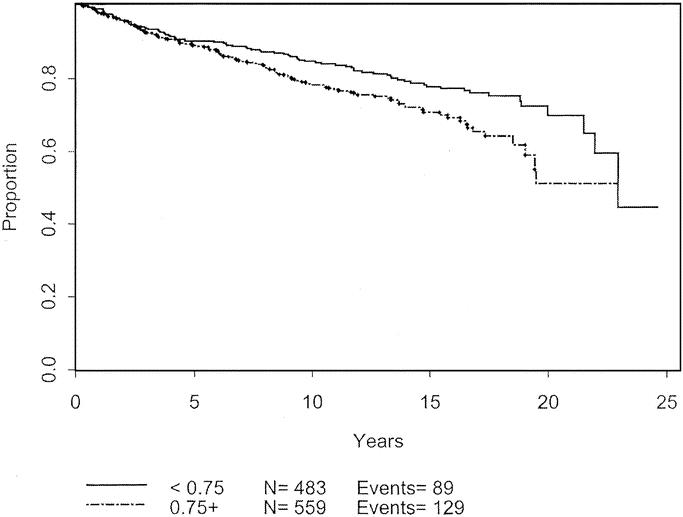
FIGURE 6. Disease-free survival based on primary tumor thickness.
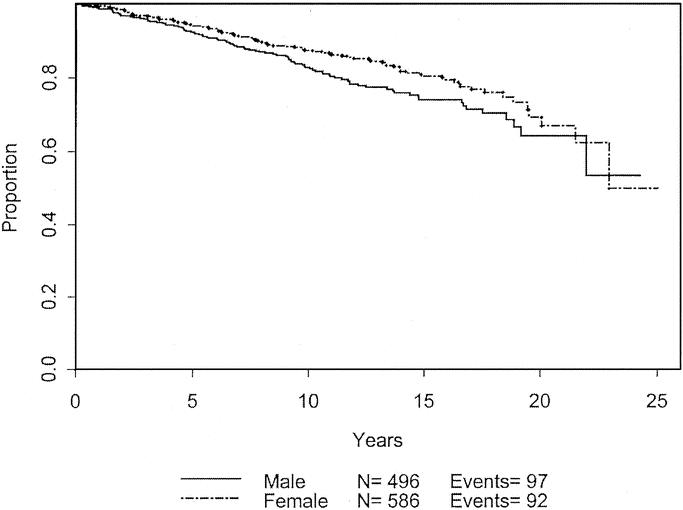
FIGURE 7. Overall survival based on gender.
Subgroup: “High-Risk”
Applying the above information, we defined a subgroup of patients with the 3 characteristics that were considered significant for poor prognosis by univariate analysis, ie, males > 45-year-old with tumor thickness between 0.75 and 1.0 mm. There were 157 patients (13.6%) that fit these characteristics. Six (3.8%) of the 157 patients had advanced disease at the time of presentation: 2 patients with regional skin metastases, and 4 patients with nodal involvement. This was not significantly different than the percentage of non–high-risk patients who presented with advanced disease.
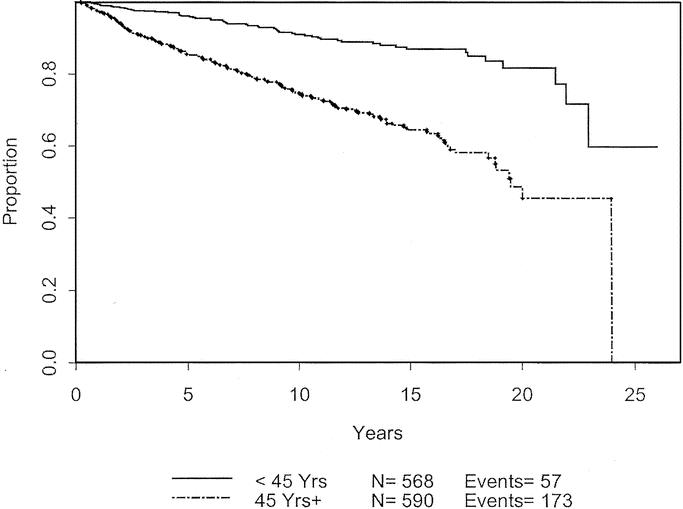
FIGURE 8. Overall survival based on age.
However, during clinical follow-up of the patients without advanced disease at initial presentation, 25 of the 151 patients (15.2%) developed recurrent melanoma. This number is significantly higher than the 8.4% of the non–high-risk population that progressed (P = 0.007) with a likelihood ratio was 6.3. In other words, patients with the high-risk characteristics were 6.3 times more likely to develop progression of disease than those without the high-risk characteristics. Of the high-risk patients with recurrent disease, 20% recurred locally, while 44% (6.6% of total) recurred in a nodal basin, and 36% (6.0% of total) recurred distally. For the high-risk group, the rate of nodal recurrence was more than double that than for those non–high-risk patients (6.6% versus 3.0%; P = 0.02). Among patients with these 3 high-risk characteristics, 19.7% either presented initially with advanced disease or developed recurrence of disease during follow-up. The high-risk group had significantly worse disease-free (Fig. 9) and overall survival (Fig. 10). The difference in recurrence and survival between the 2 groups begins after 5 years, suggesting that this may be a late recurrence phenomenon.
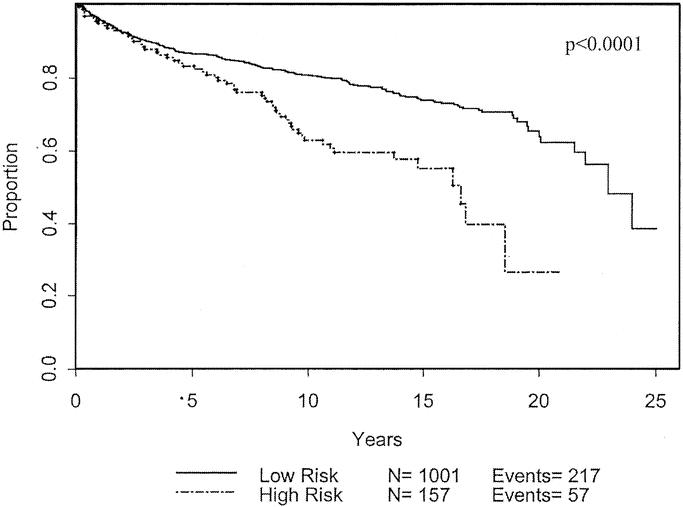
FIGURE 9. Disease-free survival based on patient population defined as high-risk compared with non–high-risk.
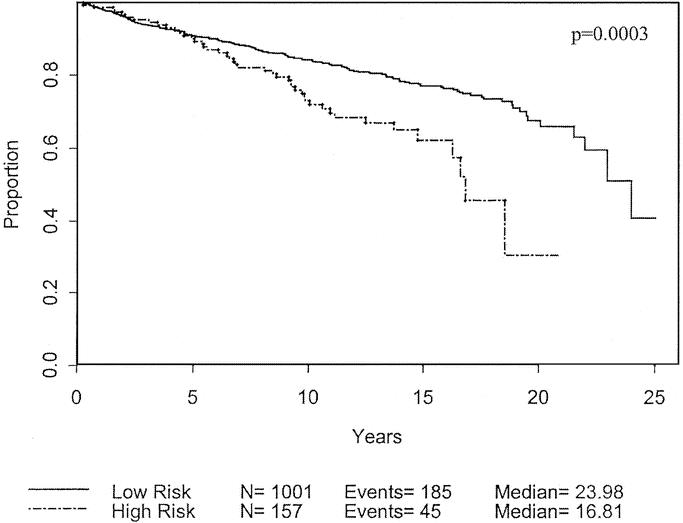
FIGURE 10. Overall survival based on patient population defined as high-risk compared with non–high-risk.
DISCUSSION
Thin melanomas treated by early detection and resection result in excellent long-term survival. Despite our best surgical efforts, however, approximately 10% to 15% of patients will develop recurrence and die of their disease. Many efforts have been made to help identify characteristics that may predict which patients will fail, but identifying these patients prospectively still remains difficult. Our study reviews a large series from a prospectively collected melanoma database with long-term follow-up in an effort to define the natural history of thin melanomas and to identify prognostic factors that may be used in patient management and education.
Previous work from this database by Slingluff et al7 nearly 15 years ago addressed the concept of lethal thin melanomas. They reviewed 681 patients with primary melanomas < 0.76 mm at a mean follow-up of 5.9 years and developed a predictive model using patient and histologic factors to identify high-risk patients. We have expanded on this work by further patient accrual and longer follow-up. We have included patients with melanomas up to 1.0 mm on the basis of the recent staging recommendations from the American Joint Committee on Cancer.4 Also differing from earlier work, we have excluded those patients who received initial care at a referring institution. Thus, our study focuses on a population who received their initial treatment at a single institution at a mean follow-up of 11 years. Furthermore, we analyze a subset of patients who are most likely the typical thin melanoma patient, those who present without evidence of metastatic disease, and follow them clinically for recurrence.
Most reports in the literature regarding melanoma recurrence and survival are truncated at end points of 5 to 10 years. Longer follow-up is particularly important for patients with thin melanoma, because > 10% of recurrences in this population may occur after 10 years after diagnosis,12 and the annual recurrence incidence remains constant for at least 15 years.13 Soong et al estimate that if a patient with a primary cutaneous melanoma < 1.5 mm in thickness is free from disease for 10 years, there is an 8% chance of recurrence and 3% chance of death from melanoma in the ensuing 10 years.14 Our study reports actuarial disease-free and overall survival of approximately 60% and 65%, respectively, at 20 years. As noted in Figure 3, patients with recurrent disease clearly have a worse prognosis. Although the majority of relapses occur within the first few years after diagnosis, the survival curves never completely plateau, suggesting that events of recurrence and death continue at long-term follow-up. As follow-up extends to 15 and 20 years, the disease-free and overall survival curves begin to run parallel, suggesting that most late events are deaths and not recurrences. Our database does not tabulate disease-specific death for all patients so it is difficult to comment on death due to melanoma at 25 years. Based on the slopes of our Kaplan-Meier curves, we can extrapolate that after approximately 15 to 20 years follow-up, most late events causing death are likely not related to melanoma.
In an attempt to predict which patients will have a worse prognosis, we identified patient and tumor characteristics that were statistically associated with worse prognosis. Male gender and advanced age were the predominant patient factors. These 2 characteristics have been well-studied and are known to have adverse effects on outcomes for patients with melanoma of any thickness.5,6,14 The site of primary tumor has been suggested by other groups to influence survival, with extremity lesions have better prognosis than lesions located on the trunk or head and neck.2,3,7,15 This may be attributed to differences in local control. In our experience, there is a trend for head and neck lesions to do worse; however, this trend was not statistically significant. This may be a true finding based on the possibly different biology of head and neck melanomas or may be secondary to the low total number of patients with head or neck lesions.
Tumor thickness was the main histologic feature that predicted decreased disease-free survival. Patients with tumors < 0.75 mm had a better prognosis, as demonstrated in Figure 6. Although it has been reported that there is no disease recurrence for lesions below 0.75 mm,16 approximately 10% of patients with lesions < 0.75 mm had a recurrence event within 5 years in our study. The thinnest melanoma that recurred in our series was 0.17 mm. Thus, one cannot rely on decreased thickness alone as a predictor of good outcome.
Surprisingly, neither Clark level nor ulceration were statistically significant factors in determining decreased prognosis for thin melanomas in our study. Theoretically, thin melanomas with Clark level IV and V have an increased potential for metastasis and recurrence due to the location of dermal lymphatics at the border between the papillary and reticular dermis. Recent reviews emphasize the priority of tumor thickness as opposed to Clark level for prognosis, with the exception of thin melanomas, where discordant Clark levels are associated with a worse outcome.4 Our group has reported on the superior predictive value of thickness compared with Clark level for lesions < 1.5 mm.17 One possible explanation for the differing results could be statistical. The AJCC recommendations were based on 5890 patients with thin melanomas, of which 1380 were classified as T1b due to ulceration or Clark level IV or V. Patients staged as T1b had an approximately 4% decreased survival at 5 and 10 years compared with patients with T1a staging. Perhaps the small numbers of patients in our study with discordant Clark level or ulceration could not provide enough statistical power to demonstrate the small survival difference. A second explanation for the different results in our population is the use of adjuvant therapy in that patient population. The majority of patients treated at our institution with Clark level IV or V disease or ulceration were enrolled in an immunotherapy protocol in which they were vaccinated with killed, irradiated melanoma tumor cells. The results of this program are still undetermined. The same 2 arguments above may provide the rationale to explain the lack of prognostic significance of ulceration in our study.
Based on our findings, we defined a subgroup of patients with negative characteristics and hypothesized that they would have worse outcomes. Within this subgroup of 157 patients, only 6 presented with advanced disease, which was similar to the percentage of patients without high-risk characteristics. Alternatively, when these high-risk patients were followed over time, there was a significant decrease in disease-free and overall survival, as shown in Figures 9 and 10.
One potential application of the data presented in this paper is the appropriateness of performing a sentinel lymph node biopsy for patients with melanomas < 1 mm thick. If one could predict which patients were predisposed to developing metastatic disease, then perhaps sentinel lymph node biopsy could be effective in improving outcomes. Studies have demonstrated the low positivity rate for routine sentinel lymph node biopsy for thin melanomas, ranging from 0% to 1% for < 1.0 mm,18,19 to 8% for lesions between 0.75 mm and 1.5 mm.19
The incidence of patients presenting with advanced disease or developing recurrent disease in our high-risk group approached 20%. We can project how this high-risk group could potentially benefit from intervention such as sentinel lymph node biopsy. Making the assumption that a sentinel node biopsy could identify all patients who develop nodal and distant disease, 19 of 151 patients in the high-risk group would have been detected earlier. Based on the low probability of metastatic disease in the non–high-risk group, we assume no indication for sentinel node biopsy. However, 61 of the 931 patients would have progression of disease to nodes or distally during follow-up. Thus, application of sentinel node biopsy to the high-risk group would identify only about 25% of the total number of patients with metastatic disease, Thus, tumor biology, not accounted for in our model and perhaps not yet understood, may direct the outcome. This analysis underscores the need to identify novel markers of aggressive disease for patients with thin melanoma in the hope of identifying a higher percentage of these individuals who will ultimately fail. Perhaps with advanced pathology and molecular biology techniques, rates of micrometastatic disease detection in the sentinel lymph node will improve. However, the importance of molecular detection of disease by RT-PCR remains to be determined.
In conclusion, thin melanomas are potentially lethal lesions and approximately 15% of patients will develop recurrent or metastatic disease. Prolonged clinical surveillance is necessary, as disease can recur in long-term follow-up. There is a subset of patients defined by male gender, advanced age, and tumor thickness who are at increased risk of recurrent disease. However, these characteristics do not account for all patients with aggressive tumor biology. Further research and analysis of thin melanomas is clearly needed to help detect new markers to assist in earlier detection in this patient population. The data presented in this study will help provide the natural history backdrop for designing future protocols that study tumor behavior.
ACKNOWLEDGMENTS
The authors thank Wilma Stanley for her assistance with database management.
Discussion
Dr. Craig L. Slingluff, Jr. (Charlottesville, Virginia): I thank the authors for giving me a copy of the manuscript in advance of this meeting. Dr. Kalady and his colleagues are to be congratulated on their very large review of clinical outcomes of patients with thin melanomas. Not only did they evaluate over 1,000 patients, the median follow-up of 11 years defines this as a very remarkable series.
Dr. Seigler and his colleagues have established Duke as a major referral center for melanoma, so it is expected that patients with advanced disease may be referred there in preference to some of those with less high risk disease; therefore, inclusion of the patients referred there with advanced disease may bias the population towards those with recurrence. However, the authors have been careful to select only those patients for analysis that had their treatment at Duke. This is very helpful for the study design in helping to avoid referral bias.
I had the pleasure of reviewing the Duke data on thin melanomas in 1988 when I was a resident at Duke, but we limited our evaluation to those patients who had thicknesses of melanoma less than 0.76 millimeters, in accord with Dr. Breslow’s initial definition and the staging criteria in place at the time. However, with the new AJCC staging criteria and staging system in place, it is particularly appropriate and timely to review the expanded data set, including patients with melanomas up to 1 millimeter in thickness.
The authors have identified thickness, age, and gender as prognostic factors that are observed, together, in about 14% of patients who were at higher risk. I have several questions. A particularly striking finding in this study is the steadily increasing mortality over 2 to 3 decades of follow-up, with overall risk that is not trivial in this population traditionally considered to have a good prognosis. Though high risk patients defined in this population had a poorer outcome when all 3 negative prognostic features were present, the overall outcome for those without all 3 negative prognostic features was still quite poor, with approximately 20% mortality at 10 years and about 40% at 20 years, based on the Kaplan-Meier curves. I would ask whether there are identifiable patient subsets who had much better prognosis? It would be expected that the opposite criteria, ie, young women with thinner melanomas would have the best prognosis. Was this subgroup assessed? And were other published prognostic models compared, on this data set, to the 1 generated?
Secondly, in the prognostic model, it is surprising, as mentioned by the authors, that ulceration and Clark’s level did not predict outcome, as they are both included in the new AJCC staging system for thin melanomas, based on the large experience in multiple institutions. The authors acknowledge that the low percent of patients with ulcerated thin melanomas may make it difficult to identify this as a significant prognostic factor. Did they consider doing their prognostic modeling with the help of neural network systems, which may overcome this issue?
Also, in tables 3 and 4, it is unclear whether prognostic modeling with Clark’s level was done as a continuous variable. It may be more appropriate to evaluate it as a dichotomous variable (i.e.; Clark I-III versus Clark IV-V; as this is the cutoff defined for the AJCC criteria). It would be helpful to clarify which was done for this analysis.
Also, many surgeons consider severe regression to be associated with a higher risk of metastasis, and to be a basis for sentinel node biopsy for patients with thin melanomas. We and others have observed metastasis associated with clinical and/or histologic evidence of completely regressed melanomas. Was this assessed in the development of prognostic models?
Also, vertical growth phase has been considered important, and I wondered whether this was included in the evaluation.
Overall, it is very surprising that age alone is such a dominant prognostic feature for recurrence in these patients. I wonder whether this persisted on multivariate analysis? My impression is this was primarily a univariate analysis described.
The proportion of patients with clinically evident metastases, mostly palpable nodes, was greater than 5%, and I think overrepresents the experience generally observed. I suspect that these represent some referral bias as well because of Duke being a major referral center. And I wonder whether the prognostic modeling was done with those excluded or included in the assessment?
Again, the authors are to be congratulated for this work. I appreciate the opportunity to review the manuscript and to discuss it before the Association.
Dr. Matthew F. Kalady (Durham, North Carolina): Thank you, Dr. Slingluff for your comments and your questions. I will do my best to address each of these in turn.
The first question if there was a subgroup of people with an excellent prognosis. The only group we looked at specifically was the patients with high-risk characteristics. We compared them as a single entity versus the nonhigh-risk group. I do believe that there probably are subgroups as you have reported in your earlier work such as younger women with thin melanomas that do particularly well. We did not specifically address that group, but that is something we could look at. As far as the discrepancy with some of the AJCC data, I think it is important to look at those numbers in perspective. That study was derived from data on more than 17,000 patients. Within the T1 subgroups, I believe there were about 4,500 patients with T1A and an additional 1,300 with T1B. Between the 2 groups, there was about a 5% survival difference at both 5 and 10 years with those patients with ulceration or advanced Clark level doing worse. In our population, only 5% of patients had ulceration and 17% had advanced Clark level. I’m not certain of the overlap between these characteristics, but at most 250 people in our study would be staged T1B. So trying to find that small of a difference, a 5% survival benefit at 10 years, might be difficult with our lower numbers.
In addition, our patients at Duke who have advanced or discordant Clark levels or have ulceration, generally undergo an immunotherapy vaccine protocol. The impact of this program is not yet fully known, but it may be that it improves outcomes. That could be another explanation why the Clark level is not as important for prognosis in our study.
Again, in terms of Clark level, you asked how we had treated this variable. We did look at each individual level. Patients with level I and II were grouped, and IV and V were grouped, and IIIs were their own group. A log-rank analysis was done on the survival distributions for these groupings and we did not find a difference.
In terms of regression and vertical growth, there is an extensive list of histologic criteria in our database which unfortunately have some varying degrees of completeness. We looked at regression as 1 of the variables to include, but it was an incomplete data set and we felt it might not give us a true finding. There is great variability in the literature as to the importance of regression and therefore it was our choice not to include it in this study. We have actually been pulling the pathology slides and retrospectively reviewing some of the incomplete data. That is something we hope to address in the future along with other factors such as vertical growth phase.
You asked about age being a dominant factor. On multivariate analysis, age was actually the factor that turned out to be most significant. Again, that was analyzed as a continuous variable, suggesting that with advancing age at all levels, people have a worse prognosis. When we used 45 years as the cutoff, as that was our mean age, it was statistically significant as a discrete variable.
Another question dealt with which patient group was used to develop our prognostic model. We separated patients according to their extent of disease at the time of presentation, weeding out those who had metastatic disease at the time of presentation. We then analyzed the patients who presented only with their primary lesion. The prognostic factors were then developed based on the roughly 1,000 patients who had no evidence of nodal or metastatic disease at first evaluation. We felt that this was more appropriate for the typical melanoma patient seen and would be more clinically useful.
Dr. Charles M. Balch (Alexandria, Virginia): The Duke investigators have contributed through the decades both in terms of natural history of the disease through their data analysis community prospectively and also their studies of immunology and immunotherapy.
This study is quite different than those that we have recently published from the Melanoma Staging Committee of the American Joint Committee on Cancer, which had 5,300 patients with thin melanomas. In that study, the most important predictors of outcome were the presence or absence of tumor ulceration or deep levels of invasion; that is, level IV, specifically.
I worried in our database about how representative it was, because in practice 70% of melanomas present at T1 or thin melanomas whereas in our study 35% of the patients referred into the 13 cancer centers that contributed this data were T1 melanomas and in your study only 11% were T1 melanomas. And even comparing your distribution of patients, your patients were thicker and had a greater incidence of level III and IV. So there may be some referral bias of patients who stayed at home and never got to Duke that may influence some of your results and may contribute to the differences between our 2 data sets.
My question is this, given your findings and the AJCC findings, how do you use this information in deciding which patients should undergo sentinel node excisions and entry into some of your vaccine protocols?
For example, a 0.7 millimeter level IV melanoma, would that be an indication for a sentinel node excision in your practice or not? Certainly in ours at Johns Hopkins, those with level IV or ulcerated would be an indication in our group of patients. Thank you very much.
Dr. Matthew F. Kalady (Durham, North Carolina): Thank you, Dr. Balch. Our indications for sentinel lymph node biopsy primarily follow the AJCC recommendations and every patient with a melanoma greater than 1 millimeter thick will be offered a sentinel node biopsy. As far as looking at the patients with a primary less than that, we do use the high-risk criteria that we have presented here, and we still consider ulceration and regression as important factors. For patients with those characteristics, we will discuss the data with the patient, and recommend a sentinel node biopsy. Those are our main indications. Unfortunately, even within the high-risk group, as 1 of my last slides showed, we are not picking up all patients who are going to develop advanced disease. I think there are still some other characteristics out there - other factors defining the biology of thin melanomas that are not fully understood, and we can’t fully predict which patients will do poorly.
Overall, I think given the best information we have available to us, young males with melanomas greater than 0.75 mm thick or with ulceration or regression do worse. We try to be aggressive with this group, particularly patients on the younger side, who may have another 30 to 40 years to live.
Dr. Harold J. Wanebo (Providence, Rhode Island): This is a very important contribution. There is a great deal of literature, including what was just mentioned by Dr. Balch relating the effect of Clark levels IV and V in thin level with known prognostic risk factors. My question is, why doesn’t your data show that? One question I would ask is, is it possible that the level IV and level V thin lesions are submerged in this large group of high risk male patients of older age. Is it possible that there are certain risk sites – for example, head and neck – where thin level Clark level IV and V show a prognostic effect? So my question is, are there some unique sites with level IV and level V thin level lesions where it does show significance?
Dr. Matthew F. Kalady (Durham, North Carolina): Thank you, Dr. Wanebo. We did not look at that specifically. We looked at sites by themselves and head and neck locations tended to have a worse prognosis, although in our database it was not statistically significance. We believe that a lot of the poor prognosis is probably due to issues of local control at the time of excision. However, we did not analyze that group specifically in terms of Clark level.
Footnotes
Presented at the 123rd Annual Meeting of the American Surgical Association, April 25, 2003, Washington, DC.
Reprints: Hilliard F. Seigler, MD, Professor of Surgery and Immunology, Clinical Director, Duke Cancer Center, Mailing: Box 3966, Duke University Medical Center, Durham, NC 27710. E-mail: seigl001@mc.duke.edu
REFERENCES
- 1.Balch CM, Soong SJ, Gershenwald JE, et al. Prognostic factors analysis of 17, 600 melanoma patients: validation of the American Joint Committee on Cancer melanoma staging system. J Clin Oncol. 2001;19:3622–3634. [DOI] [PubMed] [Google Scholar]
- 2.Masback A, Olsson H, Westerdahl J, et al. Prognostic factors in invasive cutaneous malignant melanoma: a population-based study and review. Melanoma Res. 2001;11:435–445. [DOI] [PubMed] [Google Scholar]
- 3.Soong SJ, Shaw HM, Balch CM, et al. Predicting survival and recurrence in localized melanoma: a multivariate approach. World J Surg. 1992;16:191–195. [DOI] [PubMed] [Google Scholar]
- 4.Balch CM, Buzaid AC, Soong SJ, et al. Final version of the American Joint Committee on Cancer staging system for cutaneous melanoma. J Clin Oncol. 2001;19:3635–3648. [DOI] [PubMed] [Google Scholar]
- 5.Austin PF, Cruse CW, Lyman G, et al. Age as a prognostic factor in the malignant melanoma population. Ann Surg Oncol. 1994;1:487–494. [DOI] [PubMed] [Google Scholar]
- 6.Unger JM, Flaherty LE, Liu PY, et al. Gender and other survival predictors in patients with metastatic melanoma on Southwest Oncology Group trials. Cancer. 2001;91:1148–1155. [DOI] [PubMed] [Google Scholar]
- 7.Slingluff CL Jr, Vollmer RT, Reintgen DS, et al. Lethal “thin” malignant melanoma. Identifying patients at risk [comment]. Ann Surg. 1988;208:150–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cook MG, Clarke TJ, Humphreys S, et al. The evaluation of diagnostic and prognostic criteria and the terminology of thin cutaneous malignant melanoma by the CRC Melanoma Pathology Panel [comment]. Histopathol. 1996;28:497–512. [DOI] [PubMed] [Google Scholar]
- 9.Balch CM, Soong SJ, Milton GW, et al. A comparison of prognostic factors and surgical results in 1,786 patients with localized (stage I) melanoma treated in Alabama, USA, and New South Wales, Australia. Ann Surg. 1982;196:677–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kelly JW, Sagebiel RW, Blois MS. Regression in malignant melanoma. A histologic feature without independent prognostic significance. Cancer. 1985;56:2287–2291. [DOI] [PubMed] [Google Scholar]
- 11.Clark WH Jr, Elder DE, Guerry DT, et al. Model predicting survival in stage I melanoma based on tumor progression. J Nat Cancer Inst. 1989;81:1893–1904. [DOI] [PubMed] [Google Scholar]
- 12.Crowley NJ, Seigler HF. Relationship between disease-free interval and survival in patients with recurrent melanoma. Arch Surg. 1992;127:1303–1308. [DOI] [PubMed] [Google Scholar]
- 13.Slingluff CL Jr, Dodge RK, Stanley WE, et al. The annual risk of melanoma progression. Implications for the concept of cure. Cancer. 1992;70:1917–1927. [DOI] [PubMed] [Google Scholar]
- 14.Soong S, Weiss H. Predicting outcome in patients with localized melanoma. In: Balch CM, ed. Cutaneous melanoma. St. Louis: Quality Medical Publishing, Inc., 1998; 51–61. [Google Scholar]
- 15.Garbe C, Buttner P, Bertz J, et al. Primary cutaneous melanoma. Prognostic classification of anatomic location. Cancer. 1995;75:2492–2498. [DOI] [PubMed] [Google Scholar]
- 16.Statius Muller MG, van Leeuwen PA, van Diest PJ, et al. No indication for performing sentinel node biopsy in melanoma patients with a Breslow thickness of less than 0.9 mm. Melanoma Res. 2001;11:303–307. [DOI] [PubMed] [Google Scholar]
- 17.Owen SA, Sanders LL, Edwards LJ, et al. Identification of higher risk thin melanomas should be based on Breslow depth not Clark level IV. Cancer. 2001;91:983–991. [PubMed] [Google Scholar]
- 18.Nguyen CL, McClay EF, Cole DJ, et al. Melanoma thickness and histology predict sentinel lymph node status. Am J Surg. 2001;181:8–11. [DOI] [PubMed] [Google Scholar]
- 19.Lens MB, Dawes M, Newton-Bishop JA, et al. Tumour thickness as a predictor of occult lymph node metastases in patients with stage I and II melanoma undergoing sentinel lymph node biopsy. Br J Surg. 2002;89:1223–1227. [DOI] [PubMed] [Google Scholar]