Abstract
Objective:
Although the Asymptomatic Carotid Atherosclerosis Study (ACAS) reported that carotid endarterectomy (CEA) is beneficial for patients with asymptomatic ≥60% carotid stenosis (ACS), several other studies have reported mixed results. Our prospective study analyzed the natural history of ≥60% ACS in patients with contralateral carotid occlusion (CCO).
Patient Population and Methods:
During a 10-year period, patients with 60-<70% ACS with CCO were entered into a protocol of clinical examination and duplex surveillance every 6 months. All patients underwent maximum medical therapy. Late CEAs were considered if lesions became symptomatic or progressed to ≥70% stenosis. A Kaplan-Meier lifetable analysis was performed to estimate the freedom from both ipsilateral strokes and all strokes.
Results:
Eighty-two patients were enrolled with a mean follow-up of 59.5 months (range, 7–141 months). Late strokes were noted in 27 of 82 patients (33%); 19 (23%) were ipsilateral and 8 (10%) were contralateral (side of CCO). Late transient ischemic attacks (TIAs) were noted in 22 of 82 (27%, 7 ipsilateral and 15 contralateral). The combined neurologic event (TIA/stroke) rate was 60% (49 of 82, 32% ipsilateral and 28% contralateral). Kaplan-Meier lifetable analysis showed that the rates of freedom from ipsilateral strokes, all strokes, and progression to ≥70% stenosis at 1, 2, 3, 4, and 5 years were 94%, 90%, 85%, 80%, 73%; 94%, 89%, 84%, 77%, 67%; and 99%, 96%, 92%, 86%, and 82%, respectively. The ipsilateral stroke-free survival rates at l, 2, 3, 4, and 5 years were 94%, 88%, 78%, 70%, and 63%. Twenty-one late CEAs were performed with no perioperative stroke/deaths (5 for ipsilateral TIAs, 9 for ipsilateral strokes, and 7 for ≥70% ACS). Overall, 20 (24%, 11 with symptoms and 9 asymptomatic) progressed to ≥70% stenosis.
Conclusions:
Patients with 60-<70% ACS and CCO with maximal medical therapy carry a higher incidence of ipsilateral strokes and all strokes than what was reported by the ACAS study; therefore, prophylactic CEA may be justified in these patients.
Eighty-two patients with 60-<70% asymptomatic carotid stenosis with contralateral carotid occlusion were enrolled into a protocol of clinical examination and duplex surveillance with a mean follow-up of 59.5 months. Late strokes were noted in 27 (33%); 19 (23%) were ipsilateral and 8 (10%) were contralateral. The Kaplan-Meier lifetable analysis showed that the freedom from ipsilateral strokes and all strokes at 1, 2, 3, 4, and 5 years was 94%, 90%, 85%, 80%, 73%; and 94%, 89%, 84%, 77%, and 67%, respectively. These stroke rates were higher than that reported by the Asymptomatic Carotid Atherosclerosis Study; therefore, prophylactic carotid endarterectomy may be justified in these patients.
Although the Asymptomatic Carotid Atherosclerosis Study (ACAS) concluded that a combination of carotid endarterectomy (CEA) and best medical therapy significantly reduced the risk of stroke when compared with medical therapy alone in patients with ≥60% asymptomatic carotid stenosis, some clinicians are still reluctant to recommend CEA to these patients.1 Several other studies have reported on this subject with mixed results.2-6
The progression of carotid artery stenosis has also been well-established7-11; however, no studies have examined the natural history of ≥60% asymptomatic carotid artery stenosis (ACS) in the presence of contralateral carotid occlusion. Therefore, this study analyzes the natural history of ≥60% ACS in the presence of contralateral carotid occlusion in regards to the rate of progression of stenosis and freedom from neurologic events (transient ischemic attacks [TIA] and strokes).
PATIENT POPULATION AND METHODS
Patients with 60-<70% ACS, based on duplex ultrasonography, with contralateral carotid occlusion during a 10-year period were entered into a protocol of clinical examination and duplex surveillance every 6 months. All patients were instructed to call their physician if they became symptomatic. All patients had maximal medical therapy and were given 1 adult aspirin (325 mg) daily and if contraindicated, they were given Plavix, 150 mg initially, and then 75 mg daily. Hemispheric ipsilateral TIA/stroke symptoms were defined as symptoms that were referable to the territory appropriate to the affected ipsilateral carotid artery stenosis, and contralateral TIA/stroke symptoms were defined as symptoms that were referable to the territory appropriate to the contralateral carotid artery occlusion. Only a new onset of neuro events from the date of enrollment were included in this analysis, that is, past remote neuro events attributed to the contralateral occlusion prior to enrollment were not included in this analysis. All strokes were documented using computed tomography scanning and were felt to be ischemic strokes and not hemorrhagic strokes. There was no delineation regarding the character of the thrombotic events, whether thromboembolic or hypoperfusion
CEA was considered if the lesion progressed to ≥70% stenosis, based on duplex ultrasound, or if the patient became symptomatic (ipsilateral hemispheric TIAs or stroke). The 70% stenosis cutoff was used because our duplex severity classification was changed to <30% stenosis, 30%-<50% stenosis, 50%-<60% stenosis, 60%-<70%, and 70%-99% stenosis to be compatible with the North American Symptomatic Carotid Endarterectomy Trial Collaborators (NASCET) and ACAS trials.
Duplex Ultrasound Protocol
A carotid color duplex ultrasound examination was performed on all patients at their initial visit using Advanced Technology Laboratory equipment (ATL, HDI 3000 or HDI 5000 system, Bothwell, WA). Cross-sectional and longitudinal images were taken throughout the common carotid, external carotid, and the extracranial portion of the internal carotid artery, bilaterally. The severity of stenosis was determined based on our previously described criteria.12 Because surgery was based on duplex ultrasound criteria, the following selected optimum criteria with the best positive predictive value (≥95%) were used: for ≥60%-70% internal carotid artery stenosis—a peak systolic velocity of ≥220 cm/s, an end diastolic velocity of ≥80 cm/s, or an internal carotid/common carotid artery peak systolic velocity ratio of ≥4.25; for ≥70% stenosis—a peak systolic velocity of ≥300 cm/s or an end diastolic velocity of ≥110 cm/s. The measurement of carotid stenosis in these previously published criteria were similar to the ACAS study.
Statistical Analysis
A Kaplan-Meier lifetable analysis was used to estimate the freedom from ipsilateral strokes, contralateral strokes, all strokes (ipsilateral and contralateral), stroke-free survival, and progression to ≥70% stenosis.
RESULTS
Eighty-two patients were enrolled in this study with a mean follow-up of 59.5 months (range, 7 to 141 months). All of these patients were asymptomatic ipsilateral to the ≥60–70% stenosis; however, 31 of these had a past history of neuro events (TIA/stroke) attributed to the contralateral carotid occlusion at the time of their enrollment. Table 1 summarizes the demographic/clinical characteristics of these patients. These characteristics were comparable in patients who developed neuro events versus those who did not in regards to mean age (66.2 vs. 65.7 years), gender, smoking, hypertension, hypercholesterolemia, diabetes mellitus, and coronary artery disease. Table 2 summarizes the incidence of neurologic events. Late strokes were noted in 27 of 82 (33%); 19 (23%) were ipsilateral and 8 (10%) were contralateral strokes. Late TIAs were noted in 22 of 82 (27%); 7 were ipsilateral and 15 were contralateral TIAs. The combined neurologic event (TIA/stroke) rate was 60% (49 of 82; 32% ipsilateral and 28% contralateral). Of the 26 patients who developed ipsilateral TIAs and strokes, 11 had progressed at the time of symptoms (nine presented with stroke and 2 with TIA), that is, 15 of 82 (18%) had ipsilateral TIA or stroke without progression. Overall, there were a total of 20 patients (24%) who progressed to ≥70% stenosis to 100% occlusion; 11 were associated with symptoms (strokes/TIA), and 9 were asymptomatic, 2 of which were 100% occlusions.
TABLE 1. Demographic and Clinical Characteristics

TABLE 2. Incidence of Neurological Events

Twenty-one late CEAs were performed with no perioperative stroke/death; 5 for ipsilateral TIAs, 9 for ipsilateral strokes, and 7 for ≥70%-99% asymptomatic carotid stenosis. Overall, 19 patients developed ipsilateral strokes: 4 of whom died of the stroke, 2 had major strokes with minimal recovery, 2 refused CEAs, 2 had 100% carotid occlusions, and 9 underwent ipsilateral CEAs. Of the 7 patients with ipsilateral TIAs, 5 underwent late CEA, 1 refused surgery, and 1 was associated with a total occlusion.
Tables 3, 4, and 5 (Figs. 1, 2, and 3) summarize the Kaplan-Meier lifetable analysis of freedom from ipsilateral strokes, contralateral strokes, and all strokes. As noted, the freedom from ipsilateral strokes at 1, 2, 3, 4, and 5 years was 94%, 90%, 85%, 80%, and 73%, respectively. The freedom from contralateral stroke and all strokes at 1, 2, 3, 4, and 5 years was 100%, 99%, 99%, 97% and 92%; and 94%, 89%, 84%, 77%, and 67%, respectively. Tables 6 and 7 (Figs. 4 and 5) summarize the freedom from ipsilateral stroke/death and all strokes and death. As noted, the ipsilateral stroke-free survival and all stroke-free survival rates at 1, 2, 3, 4, and 5 years were 94%, 88%, 78%, 70%, and 63%; and 94%, 87%, 77%, 68%, and 57%, respectively.
TABLE 3. Kaplan-Meier Lifetable Analysis of Freedom From Ipsilateral Strokes
TABLE 4. Kaplan-Meier Lifetable Analysis of Freedom From Contralateral Strokes
TABLE 5. Kaplan-Meier Lifetable Analysis of Freedom From All Strokes (Ipsilateral and Contralateral)
FIGURE 1. Kaplan-Meier lifetable analysis of freedom from ipsilateral strokes.
FIGURE 2. Kaplan-Meier lifetable analysis of freedom from contralateral strokes.
FIGURE 3. Kaplan-Meier lifetable analysis of freedom from all strokes.
TABLE 6. Kaplan-Meier Lifetable Analysis for Freedom From Ipsilateral Stroke or Death
TABLE 7. Kaplan-Meier Lifetable Analysis of Freedom From All Strokes or Death
FIGURE 4. Kaplan-Meier lifetable analysis of freedom from ipsilateral stroke and death.
FIGURE 5. Kaplan-Meier lifetable analysis of freedom from all strokes and death.
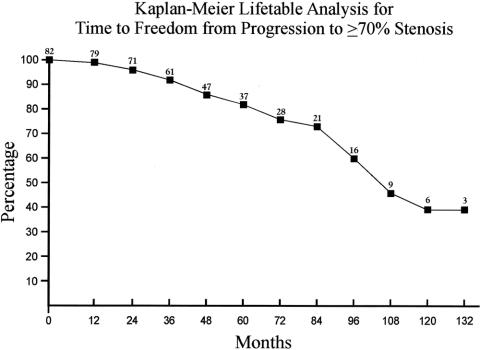
There were 15 late deaths in this series: 6 secondary to myocardial infarction, 4 secondary to strokes, 2 secondary to malignancy, 1 caused by pneumonia, 1 caused by sepsis, and 1 of unknown cause. Table 8 (Fig. 6) summarizes the overall survival rates. Freedom from progression to ≥70% stenosis at 1, 2, 3, 4, and 5 years was 99%, 96%, 92%, 86%, and 82%, respectively (Table 9 and Fig. 7). The rates of freedom from all strokes, death, or progression to ≥70% stenosis are noted in Table 10 and Figure 8.
TABLE 8. Kaplan-Meier Lifetable Analysis of Overall Survival Rates
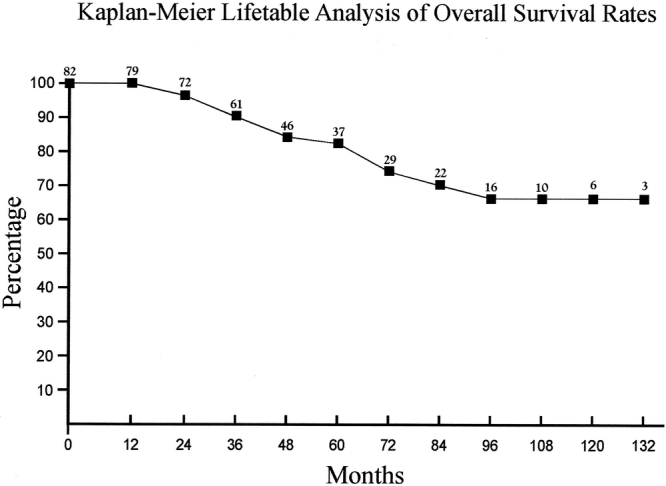
FIGURE 6. Kaplan-Meier lifetable analysis of overall survival rates.
TABLE 9. Kaplan-Meier Lifetable Analysis of Freedom From Progression to ≥70% Stenosis
FIGURE 7. Kaplan-Meier lifetable analysis of freedom from progression to ≥70% stenosis.
TABLE 10. Kaplan Meier Lifetable Analysis of Freedom From All Strokes, Death, or Progression to ≥70% Stenosis
FIGURE 8. Kaplan-Meier lifetable analysis of freedom from all strokes, death, or progression to ≥70% stenosis.
DISCUSSION
The estimated annual stroke rate for significant ACS has been reported to range from 2% to 5%.1,13-15 Cerebral infarctions without the warning symptoms of TIA can be the first initial presentation of ACS. Further progression of ACS to occlusion can be disastrous. Several studies have reported disabling stroke rates of 20% at the time of occlusion, and a 1.5% to 5% stroke rate annually thereafter.16,17 In view of this, several studies have compared the benefit of CEA versus medical therapy in treating patients with ACS with mixed results.1-6 The most widely quoted study by proponents of CEA for patients with ≥60% ACS is the ACAS study.1 The ACAS study demonstrated that the aggregate risk over 5 years for ipsilateral strokes and any perioperative death or stroke was estimated to be 5.1% for patients undergoing CEA, in contrast to 11% for patients treated medically (aggregate risk reduction of 53%). This study concluded that patients with ≥60% ACS, and whose general health makes them a good candidate for elective CEA, would have a reduced 5-year risk of ipsilateral stroke if CEA was performed with less than 3% perioperative morbidity and mortality rate with aggressive management of modifiable risk factors. The ACAS has also established that men with a good life expectancy who have ≥60% ACS are protected from stroke by CEA, whereas, the results for women are less certain. The 5-year reduction in stroke risk among men was 66% and 17% among women. This can be partially attributed to a higher perioperative complication rate in women.
The ACAS study also concluded that there was no increased risk of stroke among patients with 60-<80% stenosis compared with those with 80–99% stenosis; this is in direct opposition to the results published in the North American Symptomatic Carotid Endarterectomy Trial (NASCET).18 The NASCET study demonstrated an obvious increase in the incidence of stroke as stenosis progressed. However, the ACAS study was not designed to address this issue. Despite the conclusions of the ACAS study, some clinicians are still reluctant to recommend prophylactic CEA for patients with ≥60% ACS.2-5 Rockman et al6 concluded that prophylactic CEA should be offered to patients with ≥60% ACS when the degree of stenosis reaches 80% or more. However, it should be noted that this study used the duplex criteria that was published by the University of Washington that defined moderate stenosis to range between 50–79%.13 These criteria differ from the criteria used by the ACAS study.1 Inzitari et al5 confirmed other studies that found the increasing degree of stenosis in asymptomatic patients increased the risk of stroke; however, this was lower than the risk in symptomatic patients. Similar findings were reported in the European Carotid Surgery Trialists’ Collaborative (ECST) study.19 Inzitari et al5 also concluded that 45% of strokes in asymptomatic patients were attributed to lacunar infarcts or secondary to cardioembolic events.
Our current study specifically examines patients with 60-<70% ACS who have contralateral carotid occlusion. Late strokes were noted in 33% of our patients; 23% were ipsilateral and 10% were contralateral. Late TIAs were also noted in 27% of patients; 9% ipsilateral and 18% contralateral. The combined neurologic event (stroke/TIA) rate was 60% (32% ipsilateral and 28% contralateral). The rate of freedom from ipsilateral strokes at 1, 2, 3, 4, and 5 years was 94%, 90%, 85%, 80%, and 73%. The rate of freedom from all strokes (contralateral and ipsilateral) was 94%, 89%, 84%, 77%, and 67%, respectively. The ipsilateral stroke-free survival rates at 1, 2, 3, 4, and 5 years were 94%, 88%, 78%, 70%, and 63%. As noted earlier, 21 late CEAs were performed in this group of 82 patients with no perioperative stroke or death. Our experience confirms that patients with 60-<70% ACS and contralateral carotid occlusion, even with maximum medical therapy, carry a higher incidence of ipsilateral stroke and all strokes than that reported by the ACAS study.
This study also evaluates the natural progression of ≥60% ACS in patients with contralateral carotid occlusion. To the best of our knowledge, there is no published study on the natural history of ≥60% ACS with contralateral carotid occlusion. Of the 82 patients enrolled in this study, 24% progressed to ≥70% (20 patients); 11 were associated with symptoms (TIA/stroke) and 9 were asymptomatic. A few studies have reported on the progression of ≥50% ACS with mixed results. Rockman et al6 reported on the natural history of asymptomatic, moderately stenotic internal carotid arteries. Seventeen percent of their patients had documented progression. They could not recommend prophylactic CEA for asymptomatic moderately stenotic lesions, however they concluded that prophylactic CEA is recommended if the lesion progresses to ≥80% stenosis.
In a previously published study,20 we concluded that patients with 60% to 69% asymptomatic carotid stenosis with heterogeneous plaques had a higher incidence rate of late ipsilateral stroke, TIA, and progression to ≥70% stenoses than patients with homogeneous plaques.
Other studies have concluded that patients with ≥50%-80% are more likely to have disease progression and are significantly more likely to develop stenoses of >80% or carotid occlusion than patients with <50% stenosis.7-11 Johnson et al8 reported that 20 out of 94 (27%) patients with initial ≥50%-79% stenosis progressed to >80% within 7 years, and 20% progressed to complete occlusion. The annual progression in their series was 3.7%, which is significantly higher than for patients with initial stenoses of <50%, in whom only 4% progressed to >50% stenosis, and none progressed to carotid occlusion. Muluk11 projected an 11% annual incidence of carotid artery stenosis progression, and he also reported on the risk correlated with initial carotid artery stenosis. The relative risk of progression for patients with >50% stenosis, compared with <50% stenosis, was 3.34, and the difference in the risk of progression was statistically significant.
In conclusion, patients with 60-<70% ACS and contralateral carotid occlusion, even with maximum medical therapy, carry a higher incidence of ipsilateral strokes and all strokes (ipsilateral and contralateral) than what was reported by the ACAS study. Therefore, prophylactic CEA may be justified in these patients.
Discussion
Dr. John J. Ricotta (Stony Brook, New York): Thank you, Dr. AbuRahma, for allowing me to review your manuscript in advance.
Dr. AbuRahma presented data on patients with moderate 60 to 70% carotid stenosis in the face of contralateral occlusion. His results suggest risk of ipsilateral stroke in this cohort was approximately double that reported in ACAS. Why does this discrepancy exist? Are these patients with contralateral occlusion different? This leads to a series of questions.
First, do you have similar data on patients with 60 to 70% stenosis with a patent contralateral artery or do you have data on patients with 60 to 70% stenosis who have had a contralateral endarterectomy?
Second, how were your neurologic events adjudicated? Did all the symptomatic patients have CAT scans? In the patients who had strokes, could you tell whether these were large vessel atherosclerotic, cardioembolic, or watershed?
Third, are data available on plaque character in your patients and the risk of stroke with respect to plaque character?
Fourth, was there routine surveillance protocol to detect progression, or did these patients only come back if they were symptomatic?
Fifth, many of your patients were female. Was stroke risk equally distributed by gender? Did you perform a multivariate analysis to determine risk factors for symptoms or progression?
Sixth, progression occurred in 20% of cases, over half of which were symptomatic. What was the incidence of developing symptoms in plaques which were stable or did not progress?
Dr. AbuRahma, you have shown that patients with bilateral carotid disease are at a high risk of stroke and are well served by a meticulously conducted operation. You are to be congratulated on your results. I would like to thank the Society for the privilege of the floor.
Dr. Ali F. Aburahma (Charleston, West Virginia): Thank you, Dr. Ricotta, for your nice comments.
I am going to try to answer most of your questions. In regards to whether or not we looked at bilateral disease in the presence of 60% stenosis, this was not the subset of patients that we analyzed. Specifically, we looked into patients with about 60% asymptomatic carotid stenosis in the presence of contralateral carotid occlusion. The symptoms of TIA were defined as given by a patient’s history, however, CT scanning was used to define strokes. This study did not analyze plaque morphology, however, in a previous study that we published over a year ago, we analyzed the role of carotid plaque morphology in patients with about 60% stenosis. Our findings in that study indicated that heterogeneous plaques were more dangerous than homogeneous plaques. In regards to follow-up, all patients were enrolled in a protocol of clinical follow-up and duplex ultrasound every 6 months until the end of the follow-up. If any of these patients developed neuro events, they were seen at that time. A specific risk analysis was not done, simply because this was not the objective of this study, and because the number of patients included was not high enough to give us conclusive information.
Dr. Anthony J. Comerota (Toledo, Ohio): Dr. AbuRahma, a very nice presentation, and congratulations on election this year to the Society. A couple of comments:
I think most of us would have a lower threshold for operating on an asymptomatic stenosis in a patient with contralateral occlusion. I agree with your comments in your introduction regarding surgeons’ reluctance to operate on an asymptomatic 60% stenosis.
Your criteria for diagnosis were based upon the velocities of your duplex examination. When you have a contralateral occlusion, there are compensatory increased velocities in the side that is open, would you take that into consideration? Based upon data from a number of centers, our own included, we would ordinarily compensate by about a 15 to 20% increase in velocity, which would reduce the degree of stenosis on duplex if this were not considered.
We have also found gender differences in regard to carotid velocities, especially in females who have a 50 to 75% stenosis. Did you make such an observation?
Finally, in older patients, we have found that generally older patients have lesser degrees of progression and their plaque seems to be more stable, whereas the same degree of stenosis in the younger patient worries us considerably. Did you make such an observation?
Thank you for the privilege of the floor.
Dr. Ali F. Aburahma (Charleston, West Virginia): Thank you, Dr. Comerota.
In regards to your question as to whether or not we could have overestimated the carotid stenosis due to the presence of contralateral carotid occlusion, this was avoided because we relied on criteria we published previous in the Journal of Vascular Surgery several years ago. Because of this, our velocity data were modified to address this issue, and we only used criteria with the highest positive predictive value (>95%). Gender and age were not analyzed because this was not included in the original objective of this study.
Footnotes
Presented at the 123rd Annual Meeting of the American Surgical Association, April 24–26, 2003, Washington, DC.
Reprints: Ali F. AbuRahma, MD, 3100 MacCorkle Ave., SE, Suite 603, Charleston, WV 25304. E-mail: Ali.aburahma@camc.org.
REFERENCES
- 1.The Executive Committee for the Asymptomatic Carotid Athereosclerosis (ACAS) Study: Endarterectomy for asymptomatic carotid artery stenosis. JAMA. 1995;273:1421. [PubMed] [Google Scholar]
- 2.Perry JR, Szalai JP, Norris JW. Consensus against both endarterectomy and routine screening for asymptomatic carotid artery stenosis. Arch Neurol. 1997;54:25-28. [DOI] [PubMed] [Google Scholar]
- 3.Olin JW, Fonseca C, Childs MB, et al. The natural history of asymptomatic moderate internal carotid artery stenosis by duplex ultrasound. Vasc Med. 1998;3:101-108. [DOI] [PubMed] [Google Scholar]
- 4.Barnett HJM, Meldrum HE, Eliasziw M. The dilemma of surgical treatment for patient with asymptomatic carotid disease. Ann Intern Med. 1995;123:723-725. [DOI] [PubMed] [Google Scholar]
- 5.Inzitari D, Eliasziw M, Gates P, et al. The causes and risk of stroke in patients with asymptomatic internal carotid artery stenosis. N Engl J Med. 2000;342:1693-1700. [DOI] [PubMed] [Google Scholar]
- 6.Rockman CB, Riles TS, Lamparello PJ, et al. Natural history and management of the asymptomatic, moderately stenotic internal carotid artery. J Vasc Surg. 1997;25:423-431. [DOI] [PubMed] [Google Scholar]
- 7.Shanik GD, Moore DJ, Leahy A, et al. Asymptomatic carotid stenosis: a benign lesion? Eur J Vasc Surg. 1992;6:10-15. [DOI] [PubMed] [Google Scholar]
- 8.Johnson BE, Verlato F, Bergelin RO, et al. Clinical outcome in patients with mild and moderate carotid artery stenosis. J Vasc Surg. 1995;21:120-126. [DOI] [PubMed] [Google Scholar]
- 9.Nehler MR, Moneta GL, Lee RW, et al. Improving selection of patients with less than 60% asymptomatic internal carotid artery stenosis for follow-up carotid artery duplex scanning. J Vasc Surg. 1996;24:580-587. [DOI] [PubMed] [Google Scholar]
- 10.Mansour MA, Mattos MA, Faught WE, et al. The natural history of moderate (50% to 79%) internal carotid artery stenosis in symptomatic, nonhemispheric and asymptomatic patients. J Vasc Surg. 1995;21:346-358. [DOI] [PubMed] [Google Scholar]
- 11.Muluk SC, Muluk VS, Sugimoto H, et al. Progression of asymptomatic carotid stenosis: a natural history study in 1004 patients. J Vasc Surg. 1999;29:208-216. [DOI] [PubMed] [Google Scholar]
- 12.AbuRahma AF, Robinson PA, Stickler DL, et al. Proposed new duplex classification for threshold stenoses used in various symptomatic and asymptomatic carotid endarterectomy trials. Ann Vasc Surg. 1998;12:349-358. [DOI] [PubMed] [Google Scholar]
- 13.Norris JW, Zhu CZ, Bornstein NM, et al. Vascular risks of asymptomatic carotid stenosis. Stroke. 1991;22:1485-1490. [DOI] [PubMed] [Google Scholar]
- 14.Hennerici M, Hulsbomer H-B, Hefter H, et al. Natural history of asymptomatic extracranial arterial disease. Brain. 1987;110:777-791. [DOI] [PubMed] [Google Scholar]
- 15.O'Hollerhan LW, Kennelly MM, McClurken M, et al. Natural history of asymptomatic carotid plaque. Am J Surg. 1987;154:659-662. [DOI] [PubMed] [Google Scholar]
- 16.Cote R, Barnett HJM, Taylor DW. Internal carotid occlusion. Stroke. 1983;14:898-902. [DOI] [PubMed] [Google Scholar]
- 17.Nicholls SC, Kohler TR, Bergelin RO, et al. Carotid artery occlusion. J Vasc Surg. 1986;4:479-485. [PubMed] [Google Scholar]
- 18.North American Symptomatic Carotid Endarterectomy Trial Collaborators. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade stenosis. N Engl J Med. 1991;325;445-453.1852179 [Google Scholar]
- 19.European Carotid Surgery Trialists’ Collaborative Group. European Carotid Surgery Trial. Interim results for symptomatic patients with severe (70–99%) or with mild (0–29%) carotid stenosis. Lancet. 1991;337:1235-1243. [PubMed] [Google Scholar]
- 20.AbuRahma AF, Thiele SP, Wulu JT. Prospective controlled study of the natural history of asymptomatic 60% to 69% carotid stenosis according to ultrasonic plaque morphology. J Vasc Surg. 2002;36:437-442. [DOI] [PubMed] [Google Scholar]