Abstract
Objective:
To establish outcome and optimal timing of local control for patients with nonmetastatic Ewing sarcoma/primitive neuroectodermal tumor (ES/PNET) of the chest wall.
Methods:
Patients ≤30 years of age with ES/PNET of the chest wall were entered in 2 consecutive protocols. Therapy included multiagent chemotherapy; local control was achieved by resection, radiotherapy, or both. We compared completeness of resection and disease-free survival in patients undergoing initial surgical resection versus those treated with neoadjuvant chemotherapy followed by resection, radiotherapy, or both. Patients with a positive surgical margin received radiotherapy.
Results:
Ninety-eight (11.3%) of 869 patients had primary tumors of the chest wall. Median follow-up was 3.47 years and 5-year event-free survival was 56% for the chest wall lesions. Ten of 20 (50%) initial resections resulted in negative margins compared with 41 of 53 (77%) negative margins with delayed resections after chemotherapy (P = 0.043). Event-free survival did not differ by timing of surgery (P = 0.69) or type of local control (P = 0.17). Initial chemotherapy decreased the percentage of patients needing radiation therapy. Seventeen of 24 patients (70.8%) with initial surgery received radiotherapy compared with 34 of 71 patients (47.9%) who started with chemotherapy (P = 0.061). If a delayed operation was performed, excluding those patients who received only radiotherapy for local control, only 25 of 62 patients needed radiotherapy (40.3%; P = 0.016).
Conclusion:
The likelihood of complete tumor resection with a negative microscopic margin and consequent avoidance of external beam radiation and its potential complications is increased with neoadjuvant chemotherapy and delayed resection of chest wall ES/PNET.
The extent of resection and disease-free survival was assessed in patients with chest wall Ewing sarcoma/primitive neuroectodermal tumors treated in 2 consecutive studies. When compared with initial resection, neoadjuvant chemotherapy and delayed resection increased the likelihood of complete tumor resection with negative microscopic margins and avoidance of radiotherapy.
Ewing sarcoma/primitive neuroectodermal tumor (ES/PNET) is the most frequent malignant tumor of the chest wall in children and young adults. It is an aggressive tumor in which distant micrometastasis must be assumed to be present, and all patients receive adjuvant chemotherapy to control the distant disease. One major clinical decision at presentation of ES/PNET of the chest wall is whether to resect the primary tumor initially or after neoadjuvant chemotherapy. Complete resection is the goal, and if this is accomplished with adequate margins, it allows the patient to avoid radiation therapy with its potential long-term complications, including pulmonary fibrosis, an increased incidence of coronary artery disease, and a significant incidence of secondary tumors.1–4 A previous analysis of the first protocol in this study suggested that initial treatment with adjuvant chemotherapy might increase the frequency of complete resection of the tumor and result in a decreased need for radiotherapy to the chest.5 We repeated this analysis for the combined protocols to both increase the number of subjects available for review as well as to extend the interval of follow-up.
MATERIALS AND METHODS
Patient Population and Therapeutic Plan
The initial study (INT-0091) was open for enrollment from December 1988 to November 1992. The subsequent study (POG 9354) was open from May 1995 to September 1998. Eligibility required an age of 30 years or less at diagnosis and a tumor with the histologic diagnosis of Ewing sarcoma or primitive neuroectodermal tumor. The initial study limited enrollment to patients with tumors of bone, and included metastatic patients. The second study (POG 9354) included patients with tumors of soft tissue as well as those of bone, but excluded patients with documented metastases at diagnosis. All tumors received central pathology review and confirmation of diagnosis. The current analysis reviews the records of nonmetastatic patients only from both intergroup protocols. Patients who received chemotherapy and/or radiation therapy prior to diagnosis and study registration were excluded. Eligibility required the initiation of protocol chemotherapy within 1 month of the diagnostic biopsy. Informed written consent according to institutional guidelines was required prior to study entry.
In each study patients were randomized at study entry to receive 1 of 2 chemotherapy treatments. Patients in INT-0091 were randomized to receive either the then standard regimen of vincristine, cyclophosphamide, and doxorubicin (or actinomycin-D) or the standard regimen alternating by course with courses of ifosfamide and etoposide. The group that received ifosfamide and etoposide had a better outcome, and thus that regimen served as the standard arm of the second protocol.6 The experimental therapy for the subsequent protocol employed the same chemotherapeutic agents and the same total doses as the standard arm, but delivered them in a dose intensified manner. Details of the chemotherapy regimens are presented elsewhere.6,7
Local control measures consisted of either radiation therapy, an operation, or both; physicians at treating institutions decided on local control methods for each patient. Local control was to occur at 12 weeks, and surgery was allowed for tumors deemed to be resectable. For patients who did not have resection attempts, radiotherapy ports included the initial tumor volume (soft tissue and osseous extent of tumor) with a 3-cm margin to 4500 cGy followed by a reduction of the port to the postchemotherapy, preradiotherapy extent of tumor for an additional 1080 cGy. The total dose was 5580 cGy. Guidelines for radiation therapy were the same for patients with gross residual disease after surgery. If only microscopic disease remained after surgery, the total dose was 5040 cGy with a 1-cm margin of the initial tumor volume. The protocol allowed for attempted surgical resection before the start of chemotherapy.
Statistical Analysis
Event-free survival was defined as the time from study entry until disease progression, diagnosis of a second malignant neoplasm, or death. Otherwise, the patient was censored at the date of last contact. For INT-0091, data were current as of 8/31/00 for CCG patients, and 8/21/02 for POG patients. For POG 9354 patients, data were current as of 8/20/02.
For a patient to be considered eligible for evaluation for local control, the individual must have started maintenance after all local interventions were completed. Event-free survival after local control was defined to be the time from the start of maintenance therapy until disease progression, diagnosis of a second malignant neoplasm, or death whichever came first. Otherwise, the patient was censored at the date of last contact. The log-rank test was used to determine which factors were independently prognostic for outcome.
The standard errors of the Kaplan-Meier-product-limit survival estimates, were computed using the method of Peto et al.8 Risk for adverse event was compared across groups using the log-rank test.9 The Fisher exact test was used to test for association between pairs of categorical variables.
RESULTS
Ninety-eight (11.3%) of 869 patients enrolled in these 2 protocols had primary chest wall tumors (INT-0091: 53 of 393 patients and POG 9354: 45 of 476 patients). Median follow-up time for patients with chest wall lesions was 3.47 years. The 5-year event-free survival was 56% (SE 7%) for the chest wall lesions compared with 64% (SE 2.4%) for the entire cohort of patients. Patients with primary lesions of the chest wall achieved an event-free survival equivalent to that of patients with primary lesions of the humerus/femur and pelvis (P = 0.26), but patients with primary lesions of the distal extremities or soft tissue had better event-free survival (P < 0.025; Fig. 1).
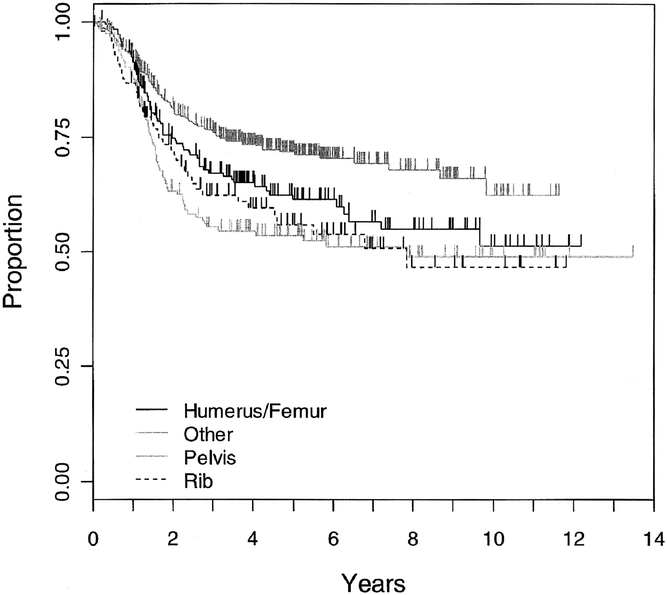
FIGURE 1. Kaplan-Meier estimate of event-free survival for patients according to site of primary tumor: ribs (n = 98), humerus or femur (n = 188), pelvis (n = 159), and all other sites (n = 424). No difference was seen between the rib, pelvis and humerus/femur cohorts (P = 0.26), but a clear difference existed between the “other” lesions when compared with rib (P < 0.01), pelvis (P < 0.01), and humerus/femur (P = 0.02) cohorts.
Tumor resection was attempted in 25 patients before the start of chemotherapy. In the initial study 16 of 53 patients had an initial resection. In the second study 9 of 45 patients had a primary resection (P = 0.35). The size of the primary tumor (largest dimension <8 cm or ≥8 cm) did not appear to effect whether patients were managed with primary resection versus adjuvant chemotherapy with delayed resection (P = 0.26).
Three patients were not evaluatable for local control. One patient developed progressive disease during the local control interval, the second developed progressive disease at week 25, and the third went off study at week 20 when further treatment was refused, all before they entered the maintenance phase of therapy.
Margins as assessed by the institutional pathologists were available for 20 of 25 patients with primary resection and 53 of 62 delayed resections performed after initial treatment with chemotherapy. Ten of 20 (50%) initial resections resulted in negative margins compared with 41 of 53 (77%) delayed resections after chemotherapy (P = 0.043). Seventeen of 24 patients (70.8%) with initial surgery received radiotherapy compared with 34 of 71 patients (47.9%) who started with chemotherapy (P = 0.061).
We attempted to exclude patients for whom surgery was not an option, thus eliminating patients who would always need radiation independent of timing of local control, such as those with apical or spinal extensions. We assumed that anyone who could have a surgical excision had surgery either upfront or after induction chemotherapy. Only patients who had a resection (either primary or after chemotherapy) were included in the analyses. Nine patients who had only radiotherapy for local control were excluded. Only 25 of 62 patients (40.3%) who had delayed operations needed radiation therapy, a lower proportion compared with those patients who had primary resections (P = 0.016).
Event-free survival did not differ by timing of surgery computed as time elapsed from date of resection (P = 0.69; Fig. 2) or type of local control (P = 0.17; Fig. 3). The local recurrence rate did not depend on the type of local control (P = 0.95), but the number of patients and the frequency of events was small. Similarly, event-free survival for patients with tumor free pathologic margins did not differ between patients whose local control was surgery alone and those who had surgery and radiotherapy (P = 0.97; Fig. 4); however, sample sizes were small (31 had surgery alone, and 18 had surgery and radiotherapy).
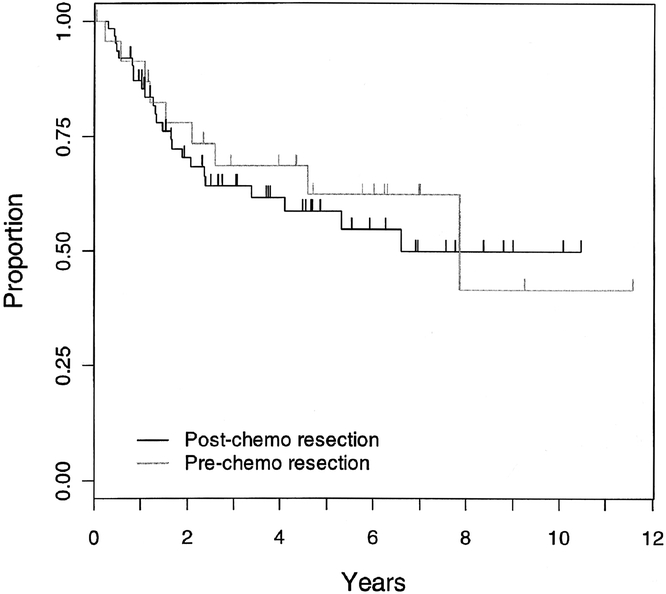
FIGURE 2. Kaplan-Meier estimate of event-free survival for patients shown according to whether they had primary resection or post chemotherapy resection (P = 0.69).

FIGURE 3. Kaplan-Meier estimate of event-free survival for patients shown by method of local control: surgery and radiotherapy, radiotherapy alone, and surgery alone (P = 0.17).
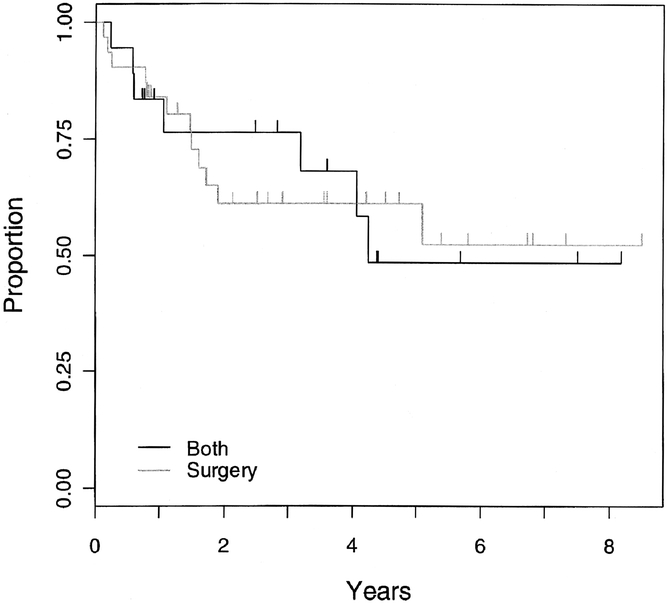
FIGURE 4. Kaplan-Meier estimate of event-free survival by method of local control (surgery alone or surgery and radiotherapy) (P = 0.97) for all patients who had complete surgical resection with pathologic margins negative for tumor.
DISCUSSION
Complete resection of the primary tumor of the chest wall was accomplished more frequently in resections performed after initial adjuvant chemotherapy compared with primary resections. Although thoracic tumors are frequently large and similar to the size of tumors of the pelvis, a primary resection is often attempted for chest wall lesions while secondary resection is generally the approach for tumors of this size at other sites.5 The probability of initial resection attempt was independent of tumor size in this study. Delayed resection resulted in a significant decrease in the proportion of patients requiring radiation therapy to the chest. Radiation therapy in doses recommended for the treatment of Ewing sarcoma/PNET has been associated with a significant incidence of secondary tumors in these patients (10–30%).1–4 Furthermore, radiation therapy to the thorax causes specific and significant toxicity: pulmonary fibrosis may occur if large volumes of lung are in the radiation field.10,11 Radiation to the heart increases the likelihood for doxorubicin induced cardiomyopathy, and patients with Hodgkin disease have developed coronary artery disease from radiation to their heart at significantly lower doses than received for this disease.12,13
We found no survival benefit caused by primary resection of the tumor. However, patients undergoing definitive surgery after initial chemotherapy had a higher frequency of successful complete resection of the tumor, eventuating in the avoidance of radiation therapy to the chest. These findings lead us to agree with others who have concluded based on much smaller series that the preferred sequence of therapy for these patients is initial biopsy followed by adjuvant chemotherapy with delayed resection after 4 courses of treatment.5,14–16 This will not avoid the need for radiation in all patients, particularly those with paravertebral or apical involvement or those with extremely large tumors. But in the majority of patients, adjuvant chemotherapy will significantly shrink the tumor as well as decrease its friability and vascularity, which will enhance the surgeon’s ability to define the margins of the lesion and achieve its complete resection. The data showed no benefit caused by the addition of radiotherapy to the local control of patients with complete resection and pathologic margins free of tumor, however the number of patients in this analysis who had surgery and radiotherapy was small.
ACKNOWLEDGMENTS
The authors thank the investigators of the Pediatric Oncology Group and the Children’s Cancer Group and their successor the Children’s Oncology Group and the many pathologists, surgeons, pediatricians, radiation oncologists, and other health professionals who managed the children entered into these studies.
Discussion
Dr. Richard J. Andrassy (Houston, Texas): Dr. Shamberger and his colleagues have reported their results from 2 consecutive cooperative trials on the management of chest wall small round cell tumors, specifically Ewing’s sarcoma/primitive neural ectodermal tumors. Chest wall sites have continued to have a poorer prognosis than other sites, but recent changes in neoadjuvant chemotherapy provide some hope of improved outcome.
The hypothesis of this review is that neoadjuvant chemotherapy followed by resection would result in better local control and decreased distant relapse compared with primary surgery followed by chemo and/or radiation. They did show that the number of patients achieving negative margins improved in the patients receiving neoadjuvant therapy.
Although my bias is to provide neoadjuvant therapy and then resection, the results of this study do not provide a statistical difference in that event-free survival and local recurrence were no different in the group receiving neoadjuvant therapy or primary resection. The number of patients in the neoadjuvant group receiving postoperative radiation was reduced, which is admirable in light of the complications of radiation in children. I have 2 questions.
Number 1, even though you didn’t see much difference in the size of the tumor between the group receiving radiation and the group not receiving radiation, is it possible that the group receiving radiation had more involved or extensive tumors and despite that they had the same incidence of local control? Is it possible that patients with reportedly negative margins may have had less local recurrence if they were radiated as well?
Number 2, some authors, including Rao and others, have stated that neoadjuvant chemotherapy allows the surgeon to remove only the involved rib and that a chest wall resection is not necessary for local control. It appears that many patients in this study had a more formal chest wall resection. What is your recommendation for the extent of resection?
Thank you for your continued interest in this group of difficult patients and your fine presentation. Thank you.
Dr. Robert C. Shamberger (Boston, Massachusetts): Thank you, Dr. Andrassy, for your kind comments. In response to the first question, we did an analysis to look at the size of the tumors included in the primary resection and secondary resection groups. We found, surprisingly enough, that there was an equal frequency of large lesions in both groups. We could not demonstrate a preference for preoperative chemotherapy in the patients with large tumors. This was well-demonstrated, however, in the extremity lesions treated primarily by our orthopedic colleagues. We found no basis for the administration of radiotherapy to patients who had completely negative resection margins. In searching through the literature, we could find no evidence to support this widely prevalent practice.
In response to the question regarding what extent of resection is necessary, we follow the policy that you must resect anything that looks like residual or scarred tumor. In the very fibrotic tissue resulting from chemotherapy you can have microscopic islands of residual tumor demonstrated.
There was an effort by St. Jude’s Children’s Research Hospital reported by Arai to decrease the dose of radiation in patients who had a very good response to chemotherapy. They decreased dosage to 30 to 36 Gy. They had a marked increase in the local recurrence rate to 38% within the radiation field, which has led us to believe that despite the apparent efficacy of chemotherapy, you cannot decrease the extent of local control measures.
Dr. Murray F. Brennan (New York, New York): Is that true, Dr. Shamberger? Does local recurrence change survival?
Dr. Robert C. Shamberger (Boston, Massachusetts): In both of these protocols patients who have suffered local relapse have done poorly. They have been treated with all of the known effective agents, so have very few therapeutic options.
Dr. Murray F. Brennan (New York, New York): Distant relapse kills you. That is true. Metastasis kills patients. Does local recurrence kill patients?
Dr. Robert C. Shamberger (Boston, Massachusetts): Local recurrence has been a very unfavorable prognostic indicator. Of 10 patients with local relapse, 9 have died including all 6 who initially had only local recurrence.
Dr. Murray F. Brennan (New York, New York): That is not the question I asked. Local recurrence is a very bad factor, but is it a cause of death?
Dr. Robert C. Shamberger (Boston, Massachusetts): Yes.
Dr. Murray F. Brennan (New York, New York): Some of us might disagree about that.
Dr. Robert C. Shamberger (Boston, Massachusetts): If you have local recurrence and you are not able to control the local recurrence in those cases, that leads to metastatic disease. The recurrent tumor is often not resectable.
Dr. Murray F. Brennan (New York, New York): Uncontrolled local recurrence causes death. I accept that.
Dr. Luis O. Vasconez (Birmingham, Alabama): May I ask Dr. Shamberger a question? On the patients who received neoadjuvant chemotherapy, what was the interval of time between the chemotherapy and institution of surgery, and have you had any delayed wound healing problems for such patients?
Dr. Robert C. Shamberger (Boston, Massachusetts): Administering chemotherapy within a 7 to 10 day period of a surgical incision in an experimental model produces a significant impairment in wound healing. The agents administered determine the extent of impairment, but Adriamycin produces 1 of the greatest adverse impacts. In this study, the complications of the primary and secondary surgical resections were limited and no significant difference in complications could be demonstrated between the 2 cohorts.
Dr. Thomas R. Weber (St. Louis, Missouri): Dr. Shamberger, you mentioned second malignancies early in your presentation. Did any of these patients have second malignancies, and were they the cause of death in any of them?
Dr. Robert C. Shamberger (Boston, Massachusetts): In these protocols there have been second malignancies that have occurred, Dr. Weber. I do not believe any have occurred in the patients with chest wall primaries. Second malignancies generally first appear between three-and-a-half and 5 years after exposure, so we are still relatively early in the follow-up interval for these complications. We have been unable, as Dr. Andrassy pointed out, to show increased survival for patients with complete resection in our current studies. We are fearful, however, that patients who have received radiation in the thorax as part of their therapy will over the long term have significant morbidity and mortality, particularly cardiomyopathy from the combination of Adriamycin and irradiation of the heart. Alkalating agents are associated with secondary tumors, and the addition of radiation therapy, particularly at doses above 4,000 cGy significantly increases the incidence of second malignancies.
Footnotes
Reprints: Robert C. Shamberger, MD Department of Surgery, Children’s Hospital, 300 Longwood Avenue, Boston, MA 02115. E-mail: Robert.Shamberger@TCH.Harvard.edu.
Supported in part by the following grants from the National Institutes of Health: CA-02649, CA-02971, CA-03161, CA-03526, CA-03750, CA-03888, CA-05436, CA-045587, CA-07306, CA-07431, CA-10198, CA-10382, CA-11233, CA-11796, CA-13539, CA-13809, CA-14560, CA-15525, CA-17829, CA-20320, CA-20549, CA-25408, CA-26044, CA-26126, CA-26270, CA-27678, CA-28383, CA-28439, CA-28476, CA-28851, CA-28882, CA-29013, CA-29139, CA-29293, CA-29314, CA-29691, CA-30969, CA-32053, CA-33587, CA-33603, CA-33625, CA-36015, CA-41573, CA-42764, CA-53128, CA-69177, CA-69428, CA-07431, CA-29233, CA-31566, CA-15989, and CA-05587.
REFERENCES
- 1.Strong LC, Herson J, Osborne BM, et al. Risk of radiation-related subsequent malignant tumors in survivors of Ewing’s sarcoma. J Natl Cancer Inst. 1979;62:1401–1406. [PubMed] [Google Scholar]
- 2.Tucker MA, D’Angio GJ, Boice JD Jr, et al. Bone sarcomas linked to radiotherapy and chemotherapy in children. N Engl J Med. 1987;317:588–593. [DOI] [PubMed] [Google Scholar]
- 3.Kuttesch JF Jr, Wexler LH, Marcus RB, et al. Second malignancies after Ewing’s sarcoma: radiation dose-dependency of secondary sarcomas. J Clin Oncol. 1996;14:2818–2825. [DOI] [PubMed] [Google Scholar]
- 4.Paulussen M, Ahrens S, Lehnert M, et al. Second malignancies after Ewing tumor treatment in 690 patients from a cooperative German/Austrian/Dutch study. Ann Oncol. 2001;12:1619–1630. [DOI] [PubMed] [Google Scholar]
- 5.Shamberger RC, Laquaglia MP, Krailo MD, et al. Ewing sarcoma of the rib: results of an intergroup study with analysis of outcome by timing of resection. J Thorac Cardiovasc Surg. 2000;119:1154–1161. [DOI] [PubMed] [Google Scholar]
- 6.Grier HE, Krailo MD, Tarbell NJ, et al. Addition of ifosfamide and etoposide to standard chemotherapy for Ewing’s sarcoma and primitive neuroectodermal tumor of bone. N Engl J Med. 2003;348:694–701. [DOI] [PubMed] [Google Scholar]
- 7.Granowetter L, Womer R, Devidas M, et al. Comparison of dose intensified and standard dose chemotherapy for the treatment of non-metastatic Ewing’s sarcoma (ES) and primitive neuroectodermal tumor (PNET) of bone and soft tissue: a Pediatric Oncology Group-Children’s Cancer Group phase III trial. SIOP XXXIII meeting, Brisbane. Med Pediatr Oncol. 2001;37:172. [Google Scholar]
- 8.Peto R, Pike MC, Armitage P, et al. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. II. Analysis and examples. Br J Cancer. 1977;35:1–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lawless JF. Statistical Models and Methods for Lifetime Data. New York: John Wiley & Sons; 1982. [Google Scholar]
- 10.Horning SJ, Adhikari A, Rizk N, et al. Effect of treatment for Hodgkin’s disease on pulmonary function: results of a prospective study. J Clin Oncol. 1994;12:297–305. [DOI] [PubMed] [Google Scholar]
- 11.Marks LB. The pulmonary effects of thoracic irradiation. Oncology. 1994;8:89–106; discussion 100, 103–104. [PubMed]
- 12.Corn BW, Trock BJ, Goodman RL. Irradiation-related ischemic heart disease. J Clin Oncol. 1990;8:741–750. [DOI] [PubMed] [Google Scholar]
- 13.Boivin JF, Hutchison GB, Lubin JH, et al. Coronary artery disease mortality in patients treated for Hodgkin’s disease. Cancer. 1992;69:1241–1247. [DOI] [PubMed] [Google Scholar]
- 14.Rao BN, Hayes FA, Thompson EI, et al. Chest wall resection for Ewing’s sarcoma of the rib: an unnecessary procedure. Ann Thorac Surg. 1988;46:40–44. [DOI] [PubMed] [Google Scholar]
- 15.Rao BN, Hayes FA, Thompson EI, et al. Chest wall resection for Ewing’s sarcoma of the rib: an unnecessary procedure. 1988. Updated in 1995. Ann Thorac Surg. 1995;60:1454–1455. [DOI] [PubMed] [Google Scholar]
- 16.Shamberger RC, Tarbell NJ, Perez-Atayde AR, et al. Malignant small round cell tumor (Ewing’s-PNET) of the chest wall in children. J Pediatr Surg. 1994;29:179–184; discussion 184–185. [DOI] [PubMed]


