Abstract
Objective:
On November 23, 1992, the first endovascular stent graft (ESG) repair of an aortic aneurysm was performed in North America. Following the treatment of this patient, we have continued to evaluate ESG over the past 10 years in the treatment of 817 patients.
Summary and Background Data:
Abdominal (AAA) or thoracic (TAA) aortic aneurysms are a significant health concern traditionally treated by open surgical repair. ESG therapy may offer protection from aneurysm rupture with a reduction in procedure morbidity and mortality.
Methods:
Over a 10-year period, 817 patients were treated with ESGs for AAA (723) or TAA (94). Patients received 1 of 12 different stent graft devices. Technical and clinical success of ESGs was reviewed, and the incidence of procedure-related complications was analyzed.
Results:
The mean age was 74.3 years (range, 25–95 years); 678 patients (83%) were men; 86% had 2 or more comorbid medical illnesses, 67% of which included coronary artery disease. Technical success, on an intent-to-treat basis was achieved in 93.8% of patients. Primary clinical success, which included freedom from aneurysm-related death, type I or III endoleak, graft infection or thrombosis, rupture, or conversion to open repair was 65 ± 6% at 8 years. Of great importance, freedom from aneurysm rupture after ESG insertion was 98 ± 1% at 9 years. There was a 2.3% incidence of perioperative mortality. One hundred seventy five patients died of causes not related to their aneurysm during a mean follow-up of 15.4 months.
Conclusions:
Stent graft therapy for aortic aneurysms is a valuable alternative to open aortic repair, especially in older sicker patients with large aneurysms. Continued device improvements coupled with an enhanced understanding of the important role of aortic pathology in determining therapeutic success will eventually permit ESGs to be a more durable treatment of aortic aneurysms.
On November 23, 1992, the first stent graft repair of an aortic aneurysm was performed in North America. Over the past 10 years, we have continued to apply this technique to the treatment of 817 patients with aortic aneurysms. Stent graft therapy for the treatment of aneurysms continues to evolve as an alternative to open aortic repair.
Endovascular Stent Grafts (ESG) are evolutionary medical devices that blend the vessel wall fixation properties of metallic intravascular stents with the arterial conduit properties of prosthetic vascular grafts. One of the first proposals for a minimally invasive intraluminal bypass was included with the initial clinical developments of catheter-based vascular intervention in the early 1960s.1–2 The landmark work by Dotter et al using arterial angioplasty and vascular stents suggested the application of these newly developed devices to the treatment of traumatic arterial injuries and aneurysms.3
Preclinical developments followed in several centers around the world focused predominantly on 3 areas: determining the best mechanism to provide graft fixation to the vessel wall; identifying an ideal conduit to bridge the vascular defect; and developing techniques to reliably deliver an ESG to a target vessel.4–8 The first published clinical application of ESG technology was by Volodos et al in the Ukraine.9–10 Shortly thereafter, Parodi et al repaired the first abdominal aortic aneurysm.11 The logical extension of the ESG concept to long segment arterial occlusive disease, aneurysms of the thoracic aorta and peripheral arteries and vascular trauma soon followed.12–19 The first endovascular aortic aneurysm repair in the Unites States was performed on November 23, 1992.20 This report summarizes a single experience that begins with that first patient treated in the United States and continues with the ESG treatment of 817 patients over a 10-year period.
PATIENTS AND METHODS
Patient Selection and Clinical Trials
Patients included in this study underwent endovascular repair of abdominal or thoracic aortic aneurysms between November 1992 and December 2002. ESG repairs were performed at 1 of 2 institutions under the care of the senior investigator (MLM). Twenty-six patients had grafts implanted at the Montefiore Medical Center in New York together with Juan Parodi, MD, and Frank Veith, MD, between November 1992 and November 1996. Seven hundred and ninety-one patients underwent ESG at Mount Sinai Medical Center in New York between December 1996 and December 2002. Each patient was enrolled in an Institutional Review Board approved clinical trial, which was either investigator sponsored, or performed under the supervision of the manufacturer of an ESG device (Table 1). Patients treated later in this investigation received FDA approved devices. All patients who received ESG repairs for abdominal or thoracic aortic aneurysm had a ≥10 mm implantation zone proximal and distal to the aneurysm for device attachment, depending on the specific inclusion and exclusion criteria of the clinical trial. Each patient was prospectively enrolled and followed at 1 month, 6 months, 1 year, and annually thereafter. Several clinical protocols also required an additional 3-month postoperative evaluation.
TABLE 1. Clinical Trials of ESG Repair of Aortic Aneurysms
Database and Patient Demographics
From November 1992 to December 2002, a consecutive series of 817 patients with abdominal or thoracic aortic aneurysms were enrolled in 1 of 15 distinct protocols for endovascular stent graft repair. All data regarding each patient, their ESG procedure, and follow-up studies were entered into a computerized vascular registry (Vascubase, Consensus Medical System Inc., 1993–2001). Access to the raw data of patients permitted confirmation prior to statistical analysis. Patient demographics and related comorbid medical illnesses of this study group are outlined in Table 2. Preoperative assessment of each patient as well as follow-up protocols are described elsewhere.11,26–29,55
TABLE 2. Patient Demographics and Comorbid Illnesses

Endovascular Stent Graft Devices
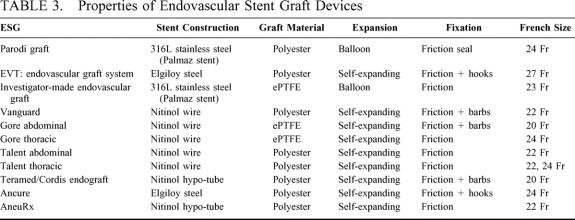
All ESG devices used in this experience required either balloon expansion for deployment or used self-expandable stents to achieve permanent fixation to the aortic wall. Metal fixation stents for ESGs were fabricated from Nitinol (Nickel-Titanium alloy), elgiloy steel, or 316L stainless steel metal. These ESG fixation stents are permanently attached to either polytetrafluoroethylene (ePTFE) or polyester fabric prosthetic conduits prior to loading them into custom introducer sheaths for intra-arterial insertion (Fig. 1). Fully supported and unsupported ESGs were used. ESG introducers included both passive and mechanical sheath retraction devices, or in some instances, sheath-less systems (Table 3). Techniques for ESG insertion are described in detail elsewhere.11,26–29,55

FIGURE 1. Endovascular stent graft devices used in this study. (A) Parodi Graft; (B) EVT Endograft; (C) Investigator ESG; (D) Boston Scientific Vanguard stent graft; (E) W.L. Gore Excluder stent graft; (F) W.L. Gore thoracic stent graft; (G) Medtronic/World Medical Talent abdominal aortic stent graft; (H) Medtronic/World Medical Talent thoracic aortic stent graft; (I) Teramed/Cordis abdominal aortic stent graft; (J) Guidant Ancure stent graft; (K) Medtronic AneuRx stent graft.
TABLE 3. Properties of Endovascular Stent Graft Devices
Definitions and Statistical Analysis
Definitions used to evaluate outcome in this study follow previously reported standards that are outlined below.21–22 Life tables analyses was performed using the Kaplan-Meier method.
Technical Success
Primary technical success was defined on an intent-to-treat basis and required: successful insertion and deployment of the ESG without the need for surgical conversion; no perioperative mortality; absence of type I or III endoleak; and freedom from limb obstruction or occlusion, up to 24 hours postoperatively.
Clinical Success
Clinical success of ESG is defined as technical success in conjunction with freedom from aneurysm-related death, type I or III endoleak, graft infection or thrombosis, aneurysm rupture, conversion to open repair, or graft migration during the life of a patient.
Endoleak
Endoleak is defined as blood flow outside the lumen of the ESG and contained within the aneurysm sac on spiral CT, MRA, duplex ultrasonography, or contrast arteriography.23,24 Type I endoleaks include those caused by inadequate proximal or distal ESG fixation. Type II endoleaks are defined by retrograde flow originating from aneurysm sac side branches back into the aneurysm sac. Type III endoleaks include those grafts either that developed prosthetic graft holes after implantation or ESGs that sustained a separation of 1 or more of the modular components that formed the completed graft.
Mechanical Device Failure
Over the 10-year period of this study, device mechanical integrity was assessed predominantly by evaluating the metal framework of ESGs using abdominal and chest radiographs. When available, explanted ESGs were also analyzed to evaluate graft metal failure. Complete details and techniques for fatigue analysis have been reviewed elsewhere.25 Device integrity failure, which resulted in an aneurysm-related death, type I or III endoleak, ESG migration, or aneurysm rupture would be classified as clinical failures.
RESULTS
Over a 10-year period, 817 patients received an ESG repair of an aortic aneurysm. There were 723 patients with abdominal aortic and 94 patients with thoracic aortic aneurysms. During the first 5 years of this experience, investigator-fabricated and first generation commercial endografts were predominantly used. During the second 5 years of this investigation, more advanced, second-generation systems were employed.
Technical Success
The combined technical success for ESG repair of AAA and TAA was 93.8%.
Abdominal Aortic Aneurysms (AAA): Technical Success
Seven hundred twenty-three patients were brought to the operating room with the intention of repairing an AAA using an ESG device. Five patients (0.7%) underwent immediate conversion to open surgical therapy, and 11 patients (1.5%) could not have an ESG successfully advanced through the iliac arteries to the target vessel for deployment. An additional 19 patients (2.6%) had ESG devices deployed within the aorta, resulting in an immediate type I endoleak that could not be eliminated prior to completion of the procedure (Fig. 2). There were 2 patients who sustained an acute limb occlusion in the immediate postoperative period (30 days). The overall technical success rate of ESG in AAA was 94.9%.

FIGURE 2. CT scan and an aortogram of a 76-year-old patient following endovascular AAA repair. (A) This CT scan demonstrates contrast within the lumen of the ESG and opacification of the aneurysmal sac. (B) Selective angiography clearly identifies the source of the endoleak from the proximal attachment site (Type I).
Thoracic Aortic Aneurysms: Technical Success
The treatment of thoracic aortic aneurysms (TAA) was initiated during the second 5-year period of this study, and 94 patients were enrolled. A total of 2 patients (2.9%) underwent immediate conversion to open surgical therapy for their TAA following an unsuccessful ESG attempt. An additional 3 patients (3.2%) could not have an ESG advanced and deployed within the target vessel. In 9 patients (9.6%), the thoracic aneurysm could not be completely excluded at the time of ESG insertion, resulting in a type I endoleak. The overall technical success rate for ESG of TAA was 85.1%.
Clinical Success
Primary initial success for ESG repair of all patients was 65 ± 6% at 8 years. Freedom from secondary interventions to maintain a successful repair was 62 ± 7% at 8 years. However, freedom from abdominal or thoracic aortic aneurysm rupture was achieved in 98 ± 1% at 9 years (Fig. 3). Successful aneurysm exclusion free from major complication (defined as limb occlusion, graft infection, or rupture) or major reintervention (defined as graft explant, distal revascularization, or conversion to open repair) was achieved in 85 ± 2% of patients at 9 years (Fig. 4).

FIGURE 3. Life table analysis demonstrating freedom from aneurysm rupture.

FIGURE 4. Life table analysis demonstrating successful aneurysm exclusion free from major complications or major reinterventions.
Abdominal Aortic Aneurysms: Clinical Success
There were 39 technical failures, 50 total type I or III endoleaks, 2 graft infections, 25 limb occlusions, 5 ruptured aneurysms with ESGs (all of which had type I endoleaks), and 7 patients converted to open aortic repair.
Thoracic Aortic Aneurysm: Clinical Success
There were 5 technical failures, 14 total type I or III endoleaks, 5 aneurysm ruptures with ESGs (all of which had type I endoleaks), and 1 late conversion to open repair.
Complications
Postoperative complications following ESG repair of AAA and TAA are summarized in Table 4. Overall survival following ESG repair at 5 years is 47 ± 4%. The majority of these non-aneurysm related late deaths were due to cardiac-related events and cancer.
TABLE 4. Major Adverse Events Following ESG Repair

Endoleak
Type I or III endoleaks occurred in 6.99% of patients following AAA ESG repair. There was a 21% incidence of type II endoleak after AAA treatment. Immediately following TAA repair, 15% of patients had type I or III endoleaks and 10% had type II endoleaks.
Mechanical Failure
AAA and TAA patients who received ESG procedures demonstrated device fatigue in 64 implants (7.8%). The most common form of device fatigue was longitudinal metal bar fractures.
DISCUSSION
Endovascular repair of aortic aneurysms continues to be evaluated as an alternative to open surgical repair. Properly selected candidates for open aortic surgery treated in centers of excellence may expect to have relatively low morbidity and surgical mortality in association with a durable repair.30 The primary allure of less invasive endovascular aneurysm surgery is the promise of marked reduction in perioperative morbidity, a shorter in-hospital stay, and a rapid postoperative recovery.
Basic research, while limited, has directed the clinical development of useful endovascular grafts.3–8 Further understanding of the complexity of aneurysm and human arterial anatomy has allowed for an evolution in device design toward developing systems with the capacity to accommodate to variable aortic pathology.31 Reviewing this single experience with this technique in 817 patients over 10 years provides data to support future efforts for aortic endografting as well as identifies significant limitations of these procedures.
The primary goal of endovascular aortic aneurysm repair is to prevent the risk of aneurysm rupture. To this end, several studies have examined this technique and have presented data that support that successful endovascular aneurysm exclusion, in properly selected patients, protects against the risk of aneurysm rupture.28,33,56 While randomized prospective studies comparing ESG techniques to standard open surgical therapy have not been performed, carefully performed prospectively controlled trials have supported the clinical equivalence of open and ESG approaches to aortic repair. The present study also supports these findings, with a freedom from aneurysm rupture at 9 years equal to 98 ± 1%. Of note, all patients who sustained a confirmed rupture in this study had a documented type I endoleak, stressing the grave danger associated with this type of problem. The reported possibility for developing a late failure with a type I endoleak of a previously excluded aneurysm further emphasizes the need for lifelong surveillance.34 Following open surgical repair of aortic aneurysms, late failures can also occur, with anastomotic aneurysm and graft occlusion presenting as significant problems, further emphasizing the need for lifelong surveillance for all patients with aortic aneurysm repairs.35,36
Anatomic challenges continue to be the most important reason for early and late endograft failure.37–39 Aortic neck length, neck and iliac artery angulation, and the coexistence of iliac occlusive disease have repeatedly been identified as primary causes for procedural failure. In the present experience, these troublesome anatomies were responsible for many of the early and late failures. Occlusive disease precluding passage of the endograft delivery system or disruption of an access artery complicated deployment of the relatively rigid, noncompliant catheter systems of early generation stent graft devices. More flexible catheters in conjunction with a reduction in the overall profile of these systems may be expected to reduce this limitation. In the present series, the W.L. Gore Excluder and Cordis Quantum devices were able to access the aorta with uniform success, even in the presence of small or diseased iliac arteries. Both of these systems employ very flexible 20 French outer diameter catheter delivery systems.
In patients in whom rigid ESGs are employed the presence of aortic angulation in all locations is thought to contribute to early and late type I endoleaks. Twelve type I endoleaks occurred in the present study where rigid ESGs were deployed in the setting of marked aortic neck angulation. The etiology of this problem may be related to the relative rigidity of these endograft prostheses and their inability to accommodate to the vessel wall geometry. Such devices, by design, allow for the development of “columnar strength” to assist in the support of the prosthesis within the aorta.40 Reports from other centers have also described experiences where rigid prostheses may increase the risk of late failure when deployed in the setting of an angulated aortic neck.41,42 Clearly, neck angulation in the infrarenal aorta is quite common, and accommodation to this type of anatomy will be essential to achieving durable fixation of an endograft and preventing late device migration and endoleak. In our experience, balloon-expandable Parodi ESG designs with malleable 316 L stainless steel attachment devices accommodate well to such neck angulation.
Controversy continues with regard to the significance of some endoleaks and the clinical approach to their management. While there is relative consensus of opinion on the uniform dangers of untreated type I endoleaks, the significance and management of type II endoleaks remains open to debate.43 When present, many type II endoleaks can be defined and treated by catheter-based techniques.44 However, the long-term value of this form of treatment is unclear. Several investigations have described the lack of endoleak resolution or the reduction in aneurysm diameter following percutaneous coil embolization of aortic aneurysm sac side branches.45,46 The relationship between persistent type II endoleaks and eventual procedural clinical failure with aneurysm rupture remains unknown.
Death from perioperative complications or late aneurysm rupture in this study was uncommon. However, death secondary to comorbid medical illnesses was substantial. Survival at 8 years was a dismal 47 ± 4%, with cardiovascular events, including myocardial infarction, being a leading cause. To some extent, the high follow-up mortality could be anticipated relative to the overall medical condition of the patient population treated in this study. Three quarters of patients with aortic aneurysms in this investigation were treated under protocols designed to evaluate endografts in the setting of significant medical/surgical high-risk comorbidity. Accordingly, the distribution of medical comorbidities in this patient population was significant, with 86% having ≥ 2 severe medical illnesses with an expectantly high follow-up mortality. All patients who received aortic endografts were evaluated preoperatively by a cardiologist, and all underwent, at minimum, a cardiac stress test and echocardiogram analysis. Select patients were referred for cardiac catheterization and revascularization. Despite this approach, death from cardiac disease continued. The use of a policy for global diagnostic cardiac catheterization prior to open aortic procedures has not been found in previous studies to decrease the risk of subsequent cardiac events and would not be expected to substantially improve long-term survival in this study population.47,48
The use of ancillary surgical procedures to facilitate ESG repair of aortic aneurysms was necessary in 35 patients. These maneuvers permitted ESG procedures to be successfully performed in settings that would have otherwise prevented endovascular aneurysm exclusion. These ancillary procedures appear particularly useful for thoracic aortic repair, largely because fusiform thoracic aneurysms often encroach on the proximal and distal limits of potential tube graft reconstruction. To this end, extra-anatomic visceral artery bypass has also been suggested as a means to permit endovascular reconstruction of the aorta in the setting of thoracoabdominal aneurysms.49,50 It is likely that creative combined techniques will be required to expand the population of patients who could benefit from ESG procedures.
Finally, the long-term integrity of the endovascular implant itself has been called into question, and its ability to continue to function throughout a patient’s life has raised some concern. Problems associated with metallic-based and fabric-based components of the prosthesis have been well-described.35,51–53 A tremendous amount of technical and engineering knowledge has been accumulated regarding the design and manufacture of these devices, which are typically exposed to significant intravascular stress.54 The complex interface between aortic pathology and the varied materials used to fabricate ESGs will likely undergo multiple iterative changes before an ideal, functional and stable product is identified. While significant advances have occurred since the initial experiences, additional improvements are essential before ESG becomes the standard of care.55
Discussion
Dr. Gregorio A. Sicard (St. Louis, Missouri): I would like to congratulate Dr. Marin for this excellent presentation and also for bringing to our attention some of the issues that have basically hampered this technology and the development of it. There is no question that since Juan Parodi performed the first clinical successful aortic aneurysm by endoluminal technique in September of 1990 that this field has exploded. This contribution by Dr. Parodi is considered the main advancement in aneurysm surgery since Charles DuBost and the contributions of Drs. Vorhees and DeBakey.
You have presented a ten-year experience with multiple devices of different generations. And obviously that hampers some of the evaluation, just like the Eurostar registry does. The Eurostar registry initially showed poor results of the first generations and improvement as the devices get a little bit smaller and easier to use. So I have a few questions for you.
In this extensive experience over the last 10 years from both abdominal and thoracic aneurysm endoluminal repair, your technical success was 93.8%. Most of the current devices both in the FDA trials as well as large personal series, including our own, shows that the technical success can be brought fairly close to 98 to 99%. Have you noticed a difference in your first 5 years experience in terms of your technical success with the early devices compared with the new second and third generation devices?
There is also a concern of your clinical success rate of 65% at 8 years. Have you noticed a difference with the new devices in terms of the clinical success rate? If you look at the graph of your clinical success rate, the main decrease in results occurs initially. Has that also changed as you have gone to lower profile devices?
Regarding Type 2 endoleaks, we recently reviewed our large experience of over 500 endografts and found that the use of selective embolization of Type 2 endoleaks – I think everybody agrees that Type 1 and Type 3 need to be treated aggressively because of their high risk of rupture – the Type 2 endoleak has been a large controversial area: Should they be treated? If so, when should they be treated? We found that with a follow-up of almost 26 months in over 500 cases taking a selective approach of embolization of the Type 2 endoleaks, if the aneurysm sac grows, that it is safe and cost effective. We had no ruptures in that group of patients. Could you address what is the protocol that you use for Type 2 endoleaks both in the abdominal but even more importantly and a little bit more complicated in the thoracic sector.
I really enjoyed your paper. It is an important contribution to this field.
Dr. Michael L. Marin (New York, New York): Thank you, Dr. Sicard.
With regard to technical success, there is no question that there has been a learning curve both with regard to the devices and the operator experience with the use of these prosthesis. If I could only – and I think that is everybody’s cry if they review a ten-year experience – if I could only drop off those first 2 years and eliminate those from my analysis, I would be much more proud of standing up and presenting it.
But your point is well taken. And I think that we have learned a tremendous amount. And it is not just the evolution of the devices but is the evolution of our own understanding of what is doable and what is not with this new and complex technology. Which vessels will accommodate to and accept the implantation of the prosthesis? Which will not? Which devices work better in different environments? All this is a product of the initial experience. And when we do segment away that, we see a dramatic rise in both technical success and clinical success.
Getting to your second question, our clinical success was low. And I think this parallels what is seen around the world with these devices as we go through these learning curves. The devices themselves, some of them were not designed for the rigors of sitting inside the aortic environment and decomposition and detachment of the device in the aortic wall was seen with several of the first generation systems, also now eliminated with the development and the engineering of more complex systems. So I think that those have contributed to an improvement in clinical success in the latter part of our study.
Another important issue reviewed by us and others has been the use of transrenal implantation which allows fixation to the more healthy segment of the aorta above and around the renal arteries, giving more durable attachment as well as the reduction in the perfusion of the sac from a Type 1 leak.
Finally, your statements about selective embolization I think are being adhered to by all around the world now. We still don’t understand the impact of these Type 2 leaks and how they affect long-term clinical outcome. Can retrograde flow through a lumbar artery, pressurize the sac adequately enough in isolation of any other attachment system failure to produce aneurysm rupture and the sequelae of that problem? This is unknown.
And we also adhere to a program whereby those aortas that increase as best as we can determine greater than or equal to 5 millimeters in diameter and who have the presence of these Type 2 leaks undergo catheter-based selective embolization and sealing. Although I hasten to add that a fair percentage of our patients over time, despite successful embolization, reperfuse those lumbars to other collateral vessels and the sac remains open. This area remains unknown.
Dr. Richard P. Cambria (Boston, Massachusetts): Dr. Marin, congratulations on a great series. At the MGH, pioneered largely by David Brewster of this Association, we have done about 700 abdominal and about 50 thoracic endografts. My question relates really to how we select this technology for our patients in contemporary practice.
Similar to your series, we have a total 8% failure rate if one defines that by either rupture or the need to convert or thrombosis of a graft limb. And your late survival clearly runs 15 to 20% less than what we are used to seeing for most surgical series of abdominal aortic aneurysms. My presumption, of course, is that this relates to patient selection.
So in contemporary practice, are you recommending endografting for any patients whose anatomy is suitable? What is your posture towards the so-called younger, good risk patient?
Dr. Michael L. Marin (New York, New York): Thank you. I think the point of selection is probably key in many people’s mind will begin to use this technology.
And 1 of the things we have learned looking at this ten-year experience is that anatomy is probably the most important feature to determine successful exclusion. When the iliac access vessels are small and diseased, when the implantation site in the perirenal or in the proximal descending thoracic aorta is short, the risk of persistent perfusion of the aneurysm sac becomes high.
So our inclusion criteria include those patients with aneurysms large enough to constitute repair using open therapy. For example, 5.5 in the infrarenal position and between 6 and 6.5 in the descending thoracic position and the anatomy that is seen in those 2 locations. Age has not been a criteria that we have used to determine who should be treated.
Dr. D. Craig Miller (Stanford, California): Dr. Marin, that was an excellent presentation. You and your colleagues should all be congratulated for pushing the envelope in this field.
I would like to get away from stent-grafting a technological or anatomic problem. I think those can be overcome. More importantly, as Dr. Cambria just alluded to, why are we doing these stent-graft procedures in these elderly, sick patients? Dr. Aldo Castaneda was always fond of saying that ‘death is immutable …’, no matter what most of us in this room think.
We at Stanford have been sobered by looking at the 5 to 10 year results in our initial 103 patients (among approximately 300 thoracic aortic stent-graft cases performed to date) undergoing descending thoracic aortic stent-grafting between 1992 and 1997. Two-thirds of these 103 patients, similar to your fraction, were not judged suitable for a conventional open operation, or thoracotomy in our cases. Yes, you usually can deploy a stent graft successfully; and yes, it usually works and prevents aneurysm rupture. But, in the ‘inoperable’ cohort, only 50% were still alive 2 years later, and the actuarial survival estimate was only 31% at 5 years.
So, philosophically, just what are we trying to do here? I am on record somewhere as saying that rupture of an asymptomatic, incidentally discovered descending thoracic aortic aneurysm in an elderly patient where his or her quality of life has already waned may be the contemporary equivalent of the old saying that ‘pneumonia is the old man’s best friend.’ Philosophically, we must first ask ourselves ‘What are we doing?’ before the various thoracic aortic stent-graft devices are approved for general clinical use in the U. S.
Dr. Michael L. Marin (New York, New York): I think if we continue to evaluate that, perhaps in a year or 2 I will be up here like Eric Rose and talking about the cost of care and the cost of the implantation of these extremely expensive devices and how we should choose the use of them with the limited number of dollars available from Medicare.
However, those are issues that have not been crossed yet, mostly because we haven’t been sure up unto this point about the long-term function of these devices and how successfully we can place them into patients with aneurysms that are at risk for rupture.
It is very difficult to say to a patient who is not a candidate for open therapy who may be on a life curve and on their way to death within the next 2 to 3 years that there is no form of therapy when we can offer something which we can comfortably be able to implant, successfully exclude the risk of aneurysm rupture, and allow them to live out their natural life without the risk of complications associated with aneurysm rupture. The answer to your question is not available to us now.
Dr. William C. Krupski (Denver, Colorado): Again, Dr. Marin, a terrific presentation. And you and Dr. Hollier have certainly been pioneers in this area.
But I would like to follow up on what Dr. Miller just said. Most Americans, when asked how they would like to die, answer they would like to die quickly, with as little suffering as possible. And in general, ruptured aneurysms qualify for that sort of definition. Have you done any quality of life studies of the patients who survive and then die of chronic obstructive pulmonary disease, die of cancer and so on? How many patients did have a good quality of life for their remaining years? Have you looked at this?
Dr. Michael L. Marin (New York, New York): Quality of life studies that we have done and reported elsewhere deal with the actual implant itself and the procedure associated with the implantation. Some of the things that are missing in that study as well as those done around the world looking at this so-called dramatic improvement in quality of life after endograft and compared with open therapy is that we have not really integrated in the long-term follow-up that is required in these patients and the often common need for secondary interventions which can impact significantly on the patient’s life.
Although intuitively I think most patients, particularly in this high or elderly age group with multiple comorbid medical illnesses, would rather select 2 or 3 small procedures over 1 large procedure. That is purely my intuitive sense and we have no data to support that at this time.
Footnotes
Reprints: Michael L. Marin, MD, Henry Kaufmann Professor of Vascular Surgery Chief, Division of Vascular surgery Mount Sinai School of Medicine 5 East 98th St, Box 1273 New York, NY 10029. E-mail: michael.marin@msnyuhealth.org.
REFERENCES
- 1.Fogarty T, Cranley J, Krause R, et al. A method for extraction of Arterial Emboli and Thrombi. Surg Gynecol Obstet. 1963;116:241–244. [PubMed] [Google Scholar]
- 2.Dotter CT, Judkins MP. Transluminal treatment of arteriosclerotic obstruction. Description of a new technic and a preliminary report of its application. Circulation. 1964;30:654. [DOI] [PubMed] [Google Scholar]
- 3.Dotter CT. Transluminally-placed coil-spring endarterial tube grafts. Long-term patency in canine popliteal artery. Invest Radiol. 1969;4:329–332. [DOI] [PubMed] [Google Scholar]
- 4.Balko A, Piasecki GJ, Shah DM, et al. Transfemoral placement of intraluminal polyurethane prosthesis for abdominal aortic aneurysm. J Surg Res. 1986;40:305–309. [DOI] [PubMed] [Google Scholar]
- 5.Lazarus HM. Intraluminal graft device: system and method. US Patent No. 4-1988-787-899.
- 6.Mirich D, Wright KC, Wallace S, et al. Percutaneously placed endovascular grafts for aortic aneurysms: feasibility study. Radiology. 1989;170:1033–1037. [DOI] [PubMed] [Google Scholar]
- 7.Laborde JC, Parodi JC, Clem MF, et al. Intraluminal bypass of abdominal aortic aneurysm: feasibility study. Radiology. 1992;184:185–190. [DOI] [PubMed] [Google Scholar]
- 8.Chuter TAM, Green RM, Ouriel K, et al. Transfemoral endovascular aortic graft placement. J Vasc Surg. 1993;18:185–197. [DOI] [PubMed] [Google Scholar]
- 9.Volodos NL, Shekhanin VE, Karpovich IP, et al. Self-fixing synthetic prosthesis for endoprosthetics of the vessels. Vestn Khir. 1986;137:123–125. [PubMed] [Google Scholar]
- 10.Volodos NL, Karpovich IP, Troyan VI, et al. Clinical experience of the use of self-fixing synthetic prostheses for remote endoprosthetics of the thoracic and the abdominal aorta and iliac arteries through the femoral artery and as intraoperative endoprosthesis for aorta reconstruction. Vasa Suppl. 1991;33:93–95. [PubMed] [Google Scholar]
- 11.Parodi JC, Palmaz JC, Barone HD. Transfemoral intraluminal graft implantation for abdominal aortic aneurysms. Ann Vasc Surg. 1991;5:491–499. [DOI] [PubMed] [Google Scholar]
- 12.Marin ML, Veith FJ, Panetta TF, et al. Percutaneous transfemoral insertion of a stented graft to repair a traumatic femoral arteriovenous fistula. J Vasc Surg. 1993;18:299–302. [PubMed] [Google Scholar]
- 13.Cragg AH, Dake MD. Percutaneous femoropopliteal graft placement. J Vasc Interven Radiol. 1993;4:455–463. [DOI] [PubMed] [Google Scholar]
- 14.May J, White G, Waugh R, et al. Transluminal placement of a prosthetic graft-stent device for treatment of subclavian artery aneurysm. J Vasc Surg. 1993;18:1056–1059. [PubMed] [Google Scholar]
- 15.Marin ML, Veith FJ, Panetta TF, et al. Transfemoral endoluminal stented graft repair of a popliteal artery aneurysm. J Vasc Surg. 1994;19:754–757. [DOI] [PubMed] [Google Scholar]
- 16.Marin ML, Veith FJ, Cynamon J, et al. Transfemoral endovascular stented graft treatment of aorto-iliac and femoropopliteal occlusive disease for limb salvage. Am J Surg. 1994;168:156–162. [DOI] [PubMed] [Google Scholar]
- 17.Moore WS, Vescera CL. Repair of abdominal aortic aneurysm by transfemoral endovascular graft placement. Ann Surg. 1994;220:331–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marin ML, Veith FJ. Transfemoral repair of abdominal aortic aneurysm. New Engl J Med. 1994;331:1751. [DOI] [PubMed] [Google Scholar]
- 19.Dake MD, Miller DC, Semba CP, et al. Transluminal placement of endovascular stent-grafts for the treatment of descending thoracic aortic aneurysms. New Engl J Med. 1994;331:1729–1734. [DOI] [PubMed] [Google Scholar]
- 20.Parodi JC, Marin ML, Veith FJ. Transfemoral, endovascular stented graft repair of an abdominal aortic aneurysm. Arch Surg. 1995;130:549–552. [DOI] [PubMed] [Google Scholar]
- 21.Chaikof EL, Blankensteijn JD, Harris PL, et al. Reporting standards for endovascular aortic aneurysm repair. J Vasc Surg. 2002;35:1048–1060. [DOI] [PubMed] [Google Scholar]
- 22.Chaikof EL, Fillinger MF, Matsumura JS, et al. Identifying and grading factors that modify the outcome of endovascular aortic aneurysm repair. J Vasc Surg. 2002;35:1061–1066. [DOI] [PubMed] [Google Scholar]
- 23.White GH, Yu W, May J. “Endoleak”: a proposed new terminology to describe incomplete aneurysm exclusion by an endoluminal graft. J Endovasc Surg. 1996;3:124–125. [DOI] [PubMed] [Google Scholar]
- 24.White GH, Yu W, May J, et al. Endoleak as a complication of endoluminal grafting of abdominal aortic aneurysms: classification, incidence, diagnosis and management. J Endovasc Surg. 1997;4:152–168. [DOI] [PubMed] [Google Scholar]
- 25.Jacobs TS, Won J, Gravereaux EC, et al. Mechanical failure of human implants: a 10-year experience with aortic stent grafts. J Vasc Surg. 2003;37:16–26. [DOI] [PubMed] [Google Scholar]
- 26.Matsumura JS, Brewster DC, Makaroun MS, et al. A multicenter controlled clinical trial of open versus endovascular treatment of abdominal aortic aneurysm. J Vasc Surg. 2003;37:262–271. [DOI] [PubMed] [Google Scholar]
- 27.Moore WS, Rutherford RB. Transfemoral endovascular repair of abdominal aortic aneurysms: results of the North American EVT phase 1 trial. EVT Investigators. J Vasc Surg. 1996;23:543–553. [DOI] [PubMed] [Google Scholar]
- 28.Zarins CK, White RA, Moll FL, et al. The AneuRx stent graft: four-year results and worldwide experience. J Vasc Surg. 2000;33:S135–S145. [DOI] [PubMed] [Google Scholar]
- 29.Criado FJ, Wilson EP, Fairman RM, et al. Update on the talent aortic stent-graft: a preliminary report from Unites States phase I and II trials. J Vasc Surg. 2001;33:S146–S149. [DOI] [PubMed] [Google Scholar]
- 30.Hertzer NR, Mascha EJ, Karaga MS, et al. Open infrarenal abdominal aortic aneurysm repair: The Cleveland Clinic Experience from 1989–1998. J Vasc Surg. 2002;35:1145–1154. [DOI] [PubMed] [Google Scholar]
- 31.Brener BJ, Faries P, Connelly T, et al. An in situ adjustable endovascular graft for the treatment of abdominal aortic aneurysms. J Vasc Surg. 2002;35:114–119. [DOI] [PubMed] [Google Scholar]
- 32.Thompson CS, Gaxotte VD, Rodriguez JA, et al. Endoluminal stent grafting of the thoracic aorta: initial experience with the Gore Excluder. J Vasc Surg. 2002;35:1163–1170. [DOI] [PubMed] [Google Scholar]
- 33.Makaroun MS. The Ancure Endovascular System: an update. J Vasc Surg. 2001;33:S129–S134. [DOI] [PubMed] [Google Scholar]
- 34.Alimi YS, Chakfe N, Rivoal E, et al. Rupture of an abdominal aortic aneurysm after endovascular graft placement and aneurysm size reduction. J Vasc Surg. 1998;28:178–183. [DOI] [PubMed] [Google Scholar]
- 35.Plate G, Hollier LA, O’Brien P, et al. Recurrent aneurysm and late vascular complications following repair of abdominal aortic aneurysms. Arch Surg. 1985;120:590–594. [DOI] [PubMed] [Google Scholar]
- 36.Hallett JW Jr, Marsall DM, Petterson TM, et al. Graft-related complications after abdominal aortic aneurysm repair. J Vasc Surg. 1997;25:277–286. [DOI] [PubMed] [Google Scholar]
- 37.Carpenter JP, Neschis DG, Fairman RM, et al. Failure of endovascular abdominal aortic aneurysm graft limbs. J Vasc Surg. 2001;33:296–303. [DOI] [PubMed] [Google Scholar]
- 38.Laheij RJ, Buth J, Harris PL, et al. Need for secondary interventions after endovascular repair of abdominal aortic aneurysms. Intermediate-term follow-up results of a European collaborative registry (EUROSTAR). Br J Surg. 2000;87:1666–1673. [DOI] [PubMed] [Google Scholar]
- 39.Carroccio A, Faries PL, Morrissey NJ, et al. Predicting iliac limb occlusions after bifurcated aortic stent grafting: anatomic and device related causes. J Vasc Surg. 2002;36:679–684. [PubMed] [Google Scholar]
- 40.Zarins CK, White RA, Schwartzen D, et al. AneuRx stent graft versus open surgical repair of abdominal aortic aneurysms: multicenter prospective clinical trial. J Vasc Surg. 1999;29:292–308. [DOI] [PubMed] [Google Scholar]
- 41.Conners MS 3rd, Sternbergh WC 3rd, Carter G, et al. Endograft migration one to four years after endovascular abdominal aortic aneurysm repair with the AneuRx device: a cautionary note. J Vasc Surg. 2002;36:476–484. [DOI] [PubMed]
- 42.Cao P, Verzini F, Zannetti S, et al. Device migration after endoluminal abdominal aortic aneuerysm repair: analysis of 113 cases with a minimum follow up period of 2 years. J Vasc Surg. 2002;35:229–235. [DOI] [PubMed] [Google Scholar]
- 43.Veith FJ, Baum RA, Ohki T, et al. Nature and significance of endoleaks and endotension: summary of opinions expressed at international conference. J Vasc Surg. 2002;35:1029–1035. [DOI] [PubMed] [Google Scholar]
- 44.Haulon S, Tyazi Z, Willoteaux S, et al. Embolization of type II endoleaks after aortic stent graft implantation: technique and immediate results. J Vas Surg. 2001;34:600–605. [DOI] [PubMed] [Google Scholar]
- 45.Solis MM, Ayerdi J, Babcock GA. Mechanism of failure in the treatment of type II endoleak with percutaneous coil embolization. J Vasc Surg. 2002;36:485–491. [DOI] [PubMed] [Google Scholar]
- 46.Arko FR, Filis KA, Siedel SA, et al. Intrasac flow velocities predict sealing of type II endoleaks after endovascular abdominal aortic aneurysm repair. J Vasc Surg. 2003;37:8–15. [DOI] [PubMed] [Google Scholar]
- 47.Massie MT, Rohrer MJ, Leppo JA, et al. Is coronary angiography necessary for vascular surgery patients who have positive results of dipyridamole thallium scans? J Vasc Surg. 1997;25:975–983. [DOI] [PubMed] [Google Scholar]
- 48.Suggs WD, Smith RB 3rd, Weintraub WS, et al. Selective screening for coronary artery disease in patients undergoing elective repair of abdominal aortic aneurysms. J Vasc Surg. 1993;18:349–357. [PubMed] [Google Scholar]
- 49.Quinones-Baldrich WJ, Panetta TF, Vescera CL, et al. Repair of type IV thoracoabdominal aneurysm with a combined endovascular and surgical approach. J Vasc Surg. 1999;30:555–560. [DOI] [PubMed] [Google Scholar]
- 50.Yano OJ, Faries PL, Morrissey N, et al. Ancillary techniques to facilitate endovascular repair of aortic aneurysms. J Vasc Surg. 2001;34:69–75. [DOI] [PubMed] [Google Scholar]
- 51.Riepe G, Heilberger P, Umscheid T, et al. Frame dislocation of body middle rings in endovascular stent tube grafts. Eur J Vasc Endovasc Surg. 1999;17:28–34. [DOI] [PubMed] [Google Scholar]
- 52.Matsumura JS, Ryu RK, Ouriel K. Identification and implications of transgraft microleaks after endovascular repair of aortic aneurysms. J Vasc Surg. 2001;34:190–197. [DOI] [PubMed] [Google Scholar]
- 53.Najibi S, Steinberg J, Katzen BT, et al. Detection of isolated hook fractures 36 months after implantation of the Ancure endograft: a cautionary note. J Vasc Surg. 2001;34:353–356. [DOI] [PubMed] [Google Scholar]
- 54.Umscheid T, Stelter WJ. Time-related alterations in shape, position, and structure of self-expanding, modular aortic stent-grafts: a 4-year single-center follow-up. J Endovasc Surg. 1999;6:17–32. [DOI] [PubMed] [Google Scholar]
- 55.Marin ML, Veith FJ, Cynamon J, et al. Initial experience with transluminally placed endovascular grafts for the treatment of complex vascular lesions. Ann Surg. 1995;222:449–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sicard GA, Rubin BG, Sanchez LA, et al. Endoluminal graft repair for abdominal aortic aneurysms in high-risk patients and octogenarians: is it better than open repair? Ann Surg. 2001;234:427–435. [DOI] [PMC free article] [PubMed] [Google Scholar]