Abstract
Objective:
To determine the safety and efficacy of the duodenal switch procedure as surgical treatment of morbid obesity.
Summary Background Data:
The longitudinal gastrectomy and duodenal switch procedure as performed for morbid obesity involves a 75% subtotal greater curvature gastrectomy and long limb suprapapillary Roux-en-Y duodenoenterostomy. This results in a restricted caloric intake and diversion of bile and pancreatic secretions to induce fat malabsorption. Broad acceptance of this procedure has been impeded because of concerns that the malabsorptive component may produce serious nutritional complications.
Methods:
Review of data collected prospectively from all patients who underwent duodenal switch as the primary surgical treatment of morbid obesity at a single institution during the 10-year period beginning September 1992. Operative morbidity and mortality, weight loss, volume of food intake, and bowel function were recorded. Sequential measurements of serum albumin, hemoglobin, and calcium levels were obtained to assess metabolic function and nutrient absorption.
Results:
Duodenal switch was performed as the primary operation in 701 (81%) of a total 863 patients undergoing bariatric surgery during the period of study. The average body mass index (BMI) was 52.8 (range, 34–95). Perioperative mortality was 1.4%, and morbidity (including leaks, wound dehiscence, splenectomy, and postoperative hemorrhage) occurred in 21 patients (2.9%). Weight loss averaged 127 pounds at 1 year, 131 at 3 years, and 118 at 5 or more years (% EBWL of 69%, 73%, and 66%, respectively). The mean number of bowel movements was fewer than 3 per day. Patients reported and maintained a mean restriction of 63% of their preoperative intake (approximately 1600 calories), with no specific food intolerance, at 3 or more years follow-up. At 3 years, serum albumin remained at normal levels in 98% of patients, hemoglobin in 52%, and calcium in 71%. No patients reported dumping, and marginal ulcers were not seen.
Conclusions:
The longitudinal gastrectomy with duodenal switch is a safe and effective primary procedure for the treatment of morbid obesity. It has the advantage of allowing acceptable alimentation with a minimum of side effects while producing and maintaining significant weight loss. These results are achieved without developing significant dietary restrictions or clinical metabolic or nutritional complications.
The longitudinal gastrectomy with duodenal switch operation was performed as the primary treatment of morbid obesity in 701 patients. The mean loss of excess body weight exceeded 65% at 5 or more years. Perioperative mortality was 1.4% and morbidity was 2.9%. The procedure is a safe and effective treatment of morbid obesity.
Morbid obesity is defined as having a body mass index (BMI) of greater than 40 kg/m2 or being 100 lbs over ideal body weight as defined by the 1983 Metropolitan Life Insurance tables.1 Although environmental factors are clearly important, identical twin studies2 and ongoing elucidation of the functional roles of hormones such as leptin3,4 and ghrelin5 have shown that genetic and physiologic factors have a major role in the etiology of this disease. In the United States, morbid obesity has reached epidemic proportions. Two percent of the adult male and 6% of the adult female population, a total of approximately 12 million Americans, are morbidly obese. The disease is associated with an increased risk of serious comorbidities, including type II diabetes, sleep apnea, cardiovascular diseases, and orthopedic disabilities. It has recently been estimated that individuals with morbid obesity have a mortality risk similar to that of smokers.6 A 25-year-old male with a body mass index of 45 can expect to lose approximately 14 years of life.7
Nonoperative treatments for patients with morbid obesity have not been shown to produce reliable long-term benefit, consequently, surgical therapy has become the preferred treatment.8 It is estimated that 98,000 surgical procedures for the treatment of obesity will be performed in 2003, compared with 63,000 in 2002 and 47,000 in 2001.9 The 1991 National Institutes of Health consensus conference on morbid obesity recommended that surgery be considered for well-informed patients with acceptable operative risks.10 The intention of the conference, although often construed to be otherwise, was not to recommend or condemn a particular surgical procedure. Historically, the first surgical procedure designed to produce weight loss was the jejunoileal bypass in 1953.11 The severe hepatic, metabolic, and nutritional complications of this procedure12 led to the development of the loop gastric bypass,13 the vertical banded gastroplasty,14 and the Roux-en-Y gastric bypass.15 In 1979, Scopinaro described the biliopancreatic diversion procedure, combining a distal gastrectomy with a long-limb Roux-en-Y reconstruction to induce fat malabsorption.16
The duodenal switch procedure was described by DeMeester and colleagues in 1987 as a surgical solution for primary bile reflux gastritis or to decrease the postgastrectomy symptoms seen in patients after distal gastrectomy and gastroduodenostomy.17 Later that year, Hess adapted this procedure to the treatment of morbid obesity by adding a 75% longitudinal gastrectomy to reduce gastric capacity and acid production and extending the Roux limb to a length similar to that recommended by Scopinaro to induce fat malabsorption (Fig. 1). 18 Despite favorable reports on the use of the duodenal switch procedure for the treatment of morbid obesity,18–23 it has been slow to gain widespread popularity. There are 3 reasons for this. First, there is a perception that its malabsorptive component may be associated with metabolic complications, protein calorie malnutrition, or other nutrient deficiencies. Second, the procedure is longer and more technically demanding than other bariatric operations. Third, the procedure is difficult to perform laparoscopically. The aim of the current study is to report the results obtained over a 10-year period on the use of the duodenal switch procedure for the treatment of morbid obesity at a single institution, with a specific focus on its safety and efficacy.
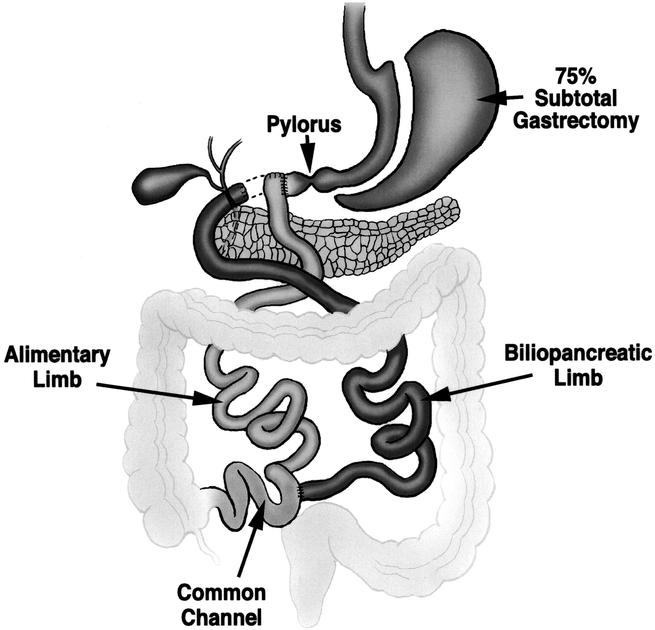
FIGURE 1. The duodenal switch procedure as performed at the University of Southern California for the treatment of morbid obesity. The operation consists of a 75% longitudinal gastrectomy, creation of an alimentary limb approximately 50% of total small bowel length, and a common channel length of 100 cm. A cholecystectomy is routinely performed.
METHODS
The study population consisted of 701 patients who underwent a duodenal switch operation for weight loss at the University of Southern California during the 10-year period beginning September 1992. Patients who had prior weight loss surgeries or underwent another type of bariatric operation were excluded (n = 162). All patients were informed about the various surgical procedures for weight loss at an educational seminar prior to their individual evaluation in the clinic. Preoperative testing in all patients included pulmonary function studies with room air arterial blood gas analysis, a cardiac stress evaluation, a barium upper gastrointestinal contrast study, ultrasound of the liver and gallbladder, and serum hematologic and chemical metabolic profile. Psychologic assessment was mandatory. Prior to obtaining informed consent, patients were given a multiple-choice examination to test their understanding of weight loss surgery in general and the duodenal switch operation in particular.
The majority of patients were admitted on the day of surgery. An epidural catheter was placed preoperatively for postoperative pain management. Central venous and arterial lines, a urinary catheter, sequential compression stockings, and special operating table padding precautions were used routinely. The surgery was performed via an upper midline incision using the Gomez retractor (Pilling Surgical, Horsham, PA.). The triangular ligament of the left lateral liver segment was incised to expose the area of the hiatus. After ligating all gastroepiploic and short gastric vessels, a 75% longitudinal gastrectomy was performed leaving a tubularized stomach of approximately 100 mL (Fig. 2). The duodenum was divided proximal to the ampulla, about 4 cm beyond the pylorus at the level of the gastroduodenal artery. The small bowel length was measured using a 15-cm umbilical tape placed sequentially along the unstretched antimesenteric border. The small bowel was divided at its midpoint, and the distal end (alimentary limb) was brought through a window created in the transverse mesocolon and anastomosed to the proximal duodenum (Fig. 3). The proximal end of the divided small bowel, now the distal end of the biliopancreatic limb, was anastomosed to the ileum 100 cm from the ileocecal valve to create a 100-cm common channel (Fig. 1). A cholecystectomy and feeding jejunostomy were performed. On postoperative day three, a video swallow was performed to exclude anastomotic stenosis; if normal, a clear liquid diet was started. Most patients were taking a solid diet at the time of their discharge on the fifth postoperative day.
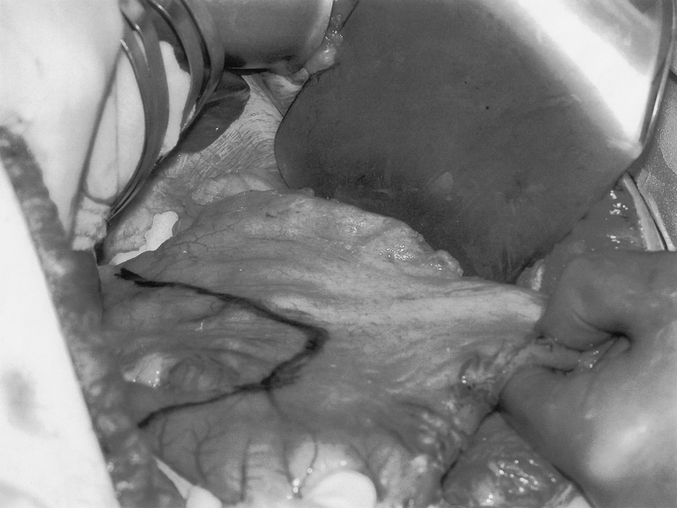
FIGURE 2. The longitudinal gastrectomy is performed by resecting along a line parallel to, and approximately 2 cm from, the lesser curvature. This produces a tubularized stomach of approximately 100 mL.
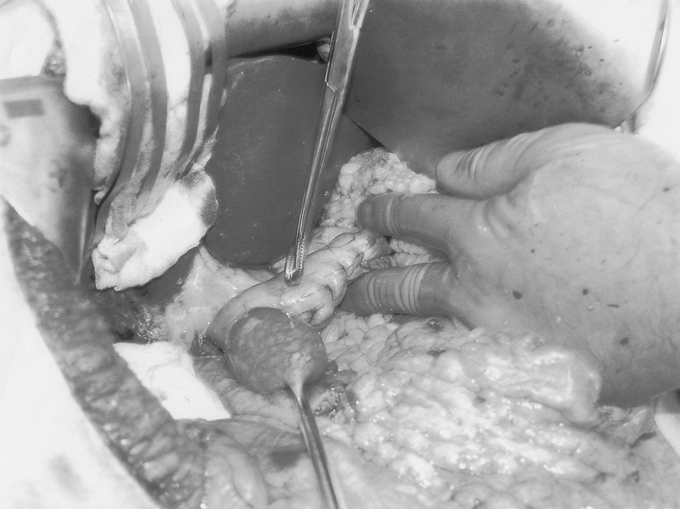
FIGURE 3. Air insufflation of the retrocolic duodenoenterostomy anastomosis. Note the lack of tension at the suture line.
Postoperatively, patients were seen initially at 3 and 6 weeks, and then every 3 months during the first year, 18 and 24 months during the second year, and yearly thereafter. At each visit, the patients were weighed, the volume of food and calories ingested was estimated using a questionnaire and a dietician interview, and the number of daily bowel movements was recorded. The serum albumin, hemoglobin, and calcium were measured. Patients were repeatedly instructed to take daily vitamin and mineral supplementation.
The Student t test was used for all continuous demographic and follow-up data. The paired t test was used for preoperative and sequential follow-up data analysis within the same groups of patients. The Mann-Whitney U test was used to test for associations between continuous factors and the outcome perioperative mortality. Fisher exact test was used to compare proportions between 2 groups. All P values are 2-sided. SPSS version 11.5 software (SPSS Inc., Chicago IL) was used for all statistical analyses.
RESULTS
Demographic Details
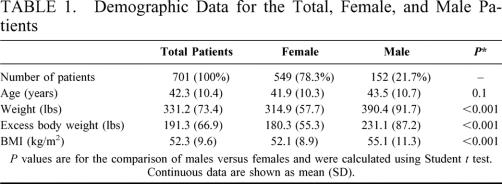
Demographic data for the total 701 patients who underwent the duodenal switch procedure for weight loss, and for male and female patients separately, are shown in Table 1. The preoperative weight, excess body weight (EBW), and BMI were all significantly higher in male patients. Fifty-eight percent of the patients (407) were supermorbidly obese, with a BMI ≥ 50 kg/m2. Twenty-two percent of the patients (155) had a preoperative BMI ≥ 60 kg/m2.
TABLE 1. Demographic Data for the Total, Female, and Male Patients
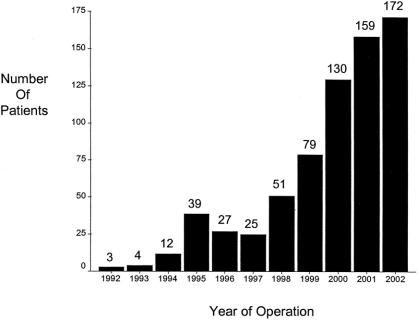
Accrual of patients increased progressively over the 10-year period, with most patients (66%) operated on during the last 3 years (Fig. 4).
FIGURE 4. Bar graph showing the yearly accrual rate of patients who had a duodenal switch procedure as their primary weight loss operation during the period of the study. The number of patients per year is shown above the bar.
Weight Loss
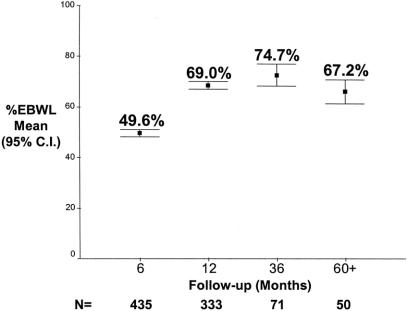
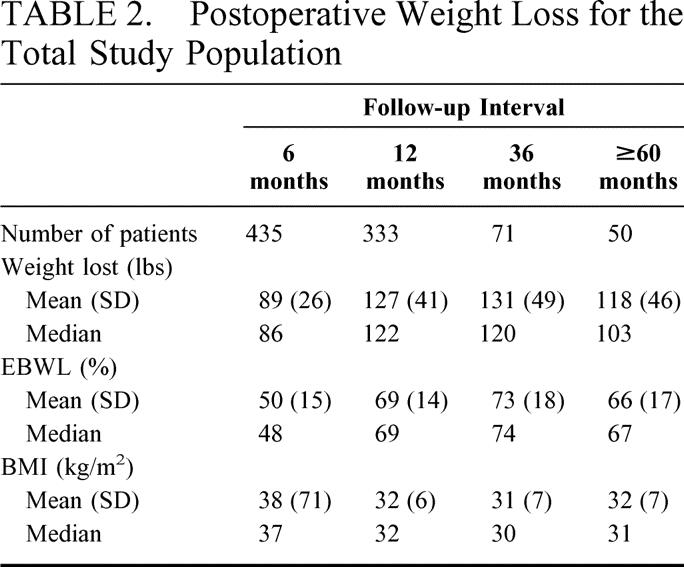
Figure 5 shows the mean and 95% CI for percent excess body weight loss (%EBWL) for all patients at 6, 12, 36, and ≥ 60 months after operation. Table 2 shows the mean and median for weight loss, %EBWL, and BMI for the total study population. Patient weight and body mass index were significantly lower, and the %EBWL was significantly higher, at all postoperative intervals compared with the preoperative values (all P < 0.001, all paired samples t test).
FIGURE 5. Percent excess body weight loss (%EBWL) for all patients. Graph shows mean and 95% CI for the mean.
TABLE 2. Postoperative Weight Loss for the Total Study Population
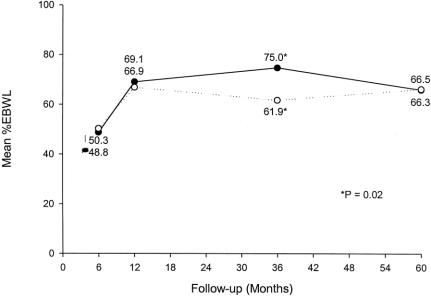
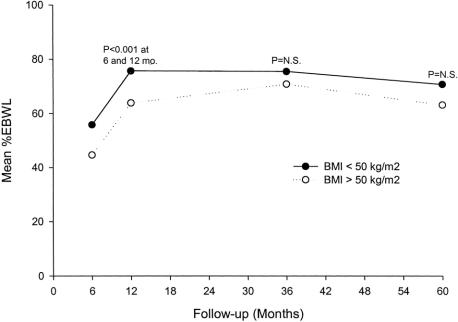
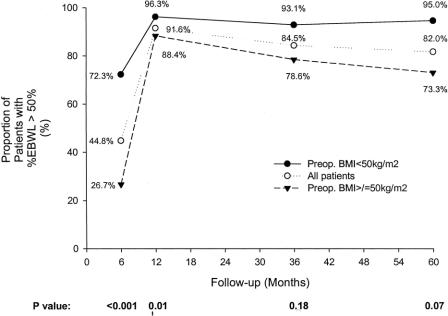
The %EBWL for male and female patients was similar (Fig. 6). Patients with a preoperative BMI ≥ 50 kg/m2 had a slightly lower %EBWL at all postoperative intervals compared with patients with a preoperative BMI < 50 kg/m2, but this difference was not significant after the first postoperative year (Fig. 7). Table 3 shows the mean weight loss, the BMI, and the %EBWL for patients with a preoperative BMI < 50 kg/m2 and ≥ 50 kg/m2. The success rate of the procedure, using the criteria of a loss of at least 50% of excess body weight (%EBWL ≥ 50) for the total patients and those with a preoperative BMI < and ≥ 50 kg/m2, is shown in Figure 8. Even in superobese patients (preoperative BMI ≥ 50 kg/m2), the success rate was 78.6% and 73.3% at 3 and 5 or more years, respectively.
FIGURE 6. %EBWL for female patients (solid line) versus male patients (dotted line).
FIGURE 7. %EBWL and preoperative BMI < 50 kg/m2 (solid line) or ≥ 50 kg/m2 (dotted line).
TABLE 3. Weight Loss in Patients with a Preoperative BMI <50 kg/m2 and BMI ≥50 kg/m2
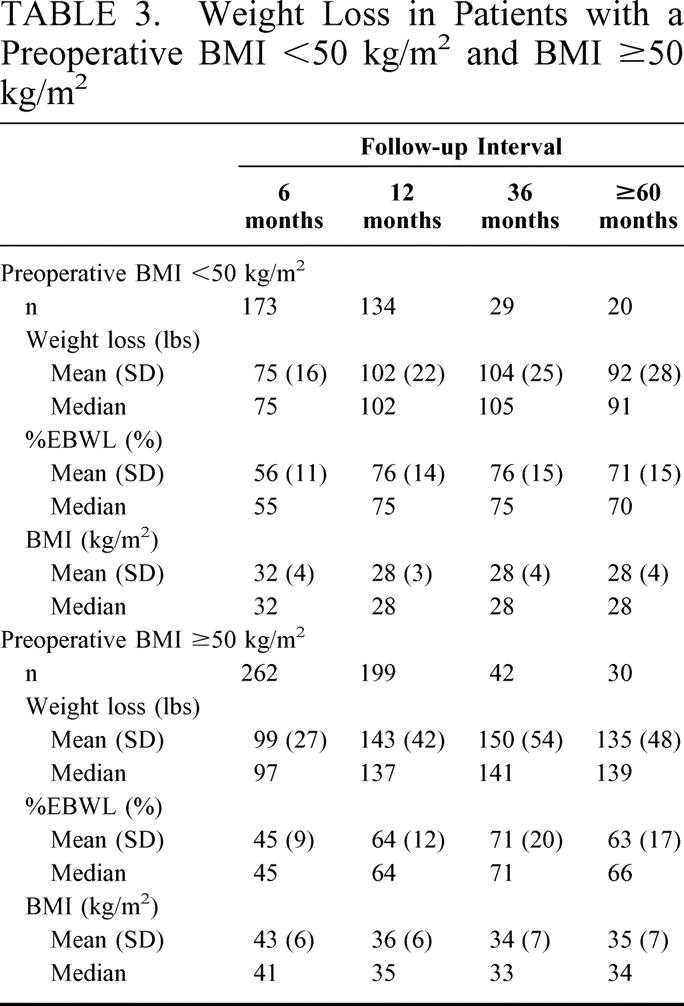
FIGURE 8. Proportion of patients with a successful outcome, defined as %EBWL ≥ 50%. The solid line shows patients with a preoperative BMI < 50 kg/m2 (morbidly obese), the dotted line shows the total patients, and the dashed line shows patients with a preoperative BMI ≥ 50 kg/m2 (supermorbidly obese). P values for the comparison of the super morbidly obese patients with the morbidly obese patients are shown at the bottom of the graph at the specific follow-up intervals (Fisher exact test).
Nutritional and Metabolic Parameters, Dietary Volume, and Bowel Function
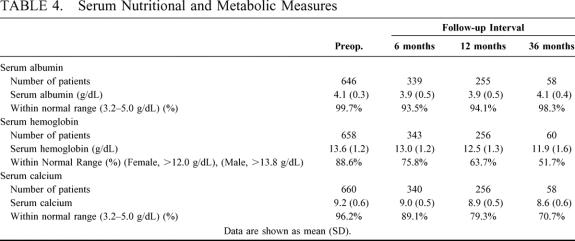
Maximum weight loss occurred by 3 years after operation and represents the time of maximal metabolic and nutritional challenge. At this time interval, the percentage of patients with normal serum values was 98.3% for albumin (normal range, 3.2–5.0 g/dL), 70.7% for total calcium (normal range, 8.5–10.3 mg/dL), and 51.7% for hemoglobin (normal range, 13.8–17.2 g/dL (male patients), 12.0–15.6 g/dL (female patients) (Table 4). Apart from the 40 patients who required readjustment of the length of the common channel, no clinical sequelae have occurred from hypocalcemia or anemia. Of importance, there was no evidence of hepatic dysfunction or liver failure manifested by either elevated serum transaminases or bilirubin levels.
TABLE 4. Serum Nutritional and Metabolic Measures
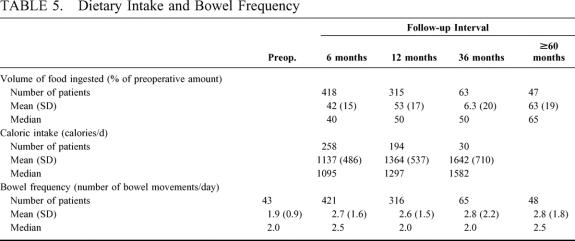
Table 5 shows (a) the patients’ assessment of ingested volume as a percentage of their preoperative volume, (b) the dietician’s estimate of daily caloric intake, and (c) the mean number of bowel movements per day. Despite a gradual increase in dietary volume and caloric intake, weight loss was maintained, indicating that the malabsorptive component of the procedure is effective. The observation that the mean caloric intake was limited to less than 1700 calories per day indicates that the restrictive component was working as well. These benefits were achieved with minimal to no restrictions on the types of food ingested. Bowel habits were minimally altered despite a 100-cm common channel. Dumping did not occur, indicating that, in addition to preservation of the pylorus, vagal control of gastrointestinal function was maintained.
TABLE 5. Dietary Intake and Bowel Frequency
Morbidity and Mortality
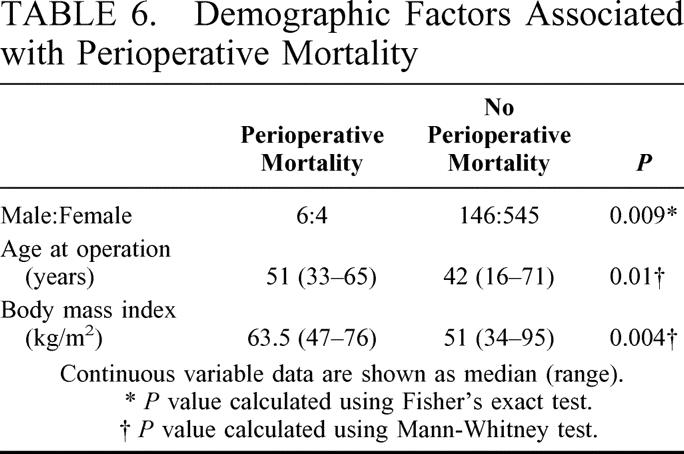
Perioperative (in-hospital and 30-day postoperative) deaths occurred in 10 of the 701 (1.4%) patients. The causes of death were pulmonary embolus in 4 patients, rhabdomyolysis in 2, duodenal stump leak in 1, gastric leak in 1, cecal necrosis in 1, and aspiration pneumonia in 1. Demographic data for the 10 patients who died are shown in Table 6. Male gender, older age, and higher preoperative BMI were significantly associated with perioperative mortality.
TABLE 6. Demographic Factors Associated with Perioperative Mortality
Significant perioperative morbidity occurred in 21 patients (2.9%). Nonfatal leaks occurred at the duodenoenteric anastomosis in 1 patient, at the enteroenterostomy anastomosis in 1, duodenal stump in 1, and at the gastric staple line in 2. Splenectomy to control bleeding was necessary in 3 patients. Four patients required postoperative exploratory laparotomy: 2 for bleeding, 1 for small bowel obstruction, and 1 for presumed abdominal sepsis. Four of six patients recovered from gluteal rhabdomyolysis. Wound dehiscence occurred in 5 patients.
Revisional surgery to increase the length of the common channel was necessary in 40 patients (5.7%). Reoperation was driven by evidence of malnutrition reflected in hypoalbuminemia, peripheral edema, or continued weight loss (34 patients); by persistent diarrhea despite a restriction of fat intake and medication use (4 patients); or by chronic unexplained abdominal pain (2 patients).
DISCUSSION
The cardinal finding of this study is that the duodenal switch is a safe and effective primary operation for the treatment of morbid obesity. The safety of the procedure is evident by the low operative mortality comparable to other large series,24,25 and satisfactory weight loss without significant side effects. Similar to Livingston et al, we found that male gender, age, and superobesity are all significant risk factors for mortality. Several large studies of both open and laparoscopic bariatric surgery have reported lower mortality rates, but these series included a lower proportion of superobese patients (BMI > 50 kg/m2; 58% of patients in our series). The morbidity of the duodenal switch procedure is also similar to reports on the open gastric bypass series.24,25 Of interest is the observation that our anastomotic leak rate is low compared with other reports. This is likely due to the close proximity of the bowel and thus the lack of tension on the duodenoenteric anastomosis compared with the gastroenteric anastomosis performed near the cardia in the standard gastric bypass procedure. The risk of leakage from the longer longitudinal gastric suture line is minimal and is comparable to the leak rate from the gastric staple line in the gastric bypass procedure.
The duodenal switch operation includes restrictive and a malabsorptive components. This has resulted in conflicting opinions regarding the side effects of the malabsorptive component, with some investigators believing that severe metabolic and nutritional complications are frequent after the operation. This is likely due to a presumed similarity of the currently performed malabsorptive operations, ie, biliopancreatic diversion and duodenal switch, to the old and now-discredited jejunoileal bypass. Unlike the jejunoileal bypass, the duodenal switch does not have a blind enteric limb. Rather, both limbs are stimulated, one with food and the other with biliopancreatic secretions, thus minimizing mucosal atrophy, bacterial overgrowth, and liver injury. Indeed, we have not seen instances of liver failure following the duodenal switch operation. We recently reported the results of the duodenal switch procedure on liver histology by studying biopsy specimens before and after weight loss.26 An activity score, based on lobular inflammation or necrosis, fatty change, and other factors, and a portal fibrosis score, were both decreased or unchanged in most patients.
A modification of gastric bypass, by lengthening the Roux limb, has been proposed for patients with more extreme degrees of obesity or when the routine Roux-en-Y bypass has failed. This long-limb or distal gastric bypass has been associated with significant metabolic and nutritional complications that have been inappropriately assumed to occur with the duodenal switch procedure.27–29 The hypoalbuminemia and other nutritional deficits observed in patients with a long-limb gastric bypass may be secondary to the combination of extreme gastric restriction imposed by the procedure along with a malabsorption component. In contrast, the duodenal switch procedure allowed patients to ingest approximately two thirds of their preoperative dietary volume without specific food intolerances, and more than 98% had a serum albumin within the normal range 3 years after surgery.
A certain percentage of patients will develop anemia and hypocalcemia after any operation that bypasses the duodenum, including Roux-en-Y gastric bypass and the duodenal switch. This is because iron and calcium are preferentially absorbed in the distal duodenum and proximal jejunum. The frequency of anemia in our study was 48% and is comparable to that reported by Brolin et al after Roux-en-Y gastric bypass, where it varied from 41% after a short limb to 74% after a long Roux limb.29 After duodenal switch procedure, the degree of anemia was usually mild, but it proved refractory to oral iron supplementation in a small percentage of patients, even though a portion of duodenum remains. These patients do respond to intramuscular or intravenous iron injections. Further, we have not observed Vitamin B12 deficiencies, in contrast to the 35% incidence reported after Roux-en-Y gastric bypass.29 This is likely due to the preservation of more gastric mucosa with the duodenal switch procedure.
We recently reported the effect of the duodenal switch operation on calcium and parathyroid hormone metabolism (presented at Pacific Coast Surgery Association, February 2003). No patient has developed clinical evidence of hypocalcemia or bone loss, a finding consistent with other reports of calcium metabolism following the duodenal switch procedure.30
The magnitude of weight loss that occurs after the duodenal switch procedure is comparable to that achieved by biliopancreatic diversion and better than that achieved by Roux-en-Y gastric bypass. Criteria for satisfactory weight loss are not well defined in the literature, but loss of 50% of EBWL is broadly accepted as a measure of success. It is notable that in the study by MacLean et al on isolated gastric bypass, only 57% of patients with supermorbid obesity (BMI > 50) achieve this goal.31 In contrast, 73.3% of patients with BMI ≥ 50 achieved success by this criterion at 5 or more years after duodenal switch operation in the present study, with even higher success rates reported by Hess et al.18
Each subsequent follow-up period saw a decrease in the number of evaluable patients such that only 46% of patients eligible for follow-up at 5 or more years were included in this study. Every effort was made to contact patients by mail, by phone, or by invitation to our monthly support group meetings. However, we feel the number of patients included at each follow-up interval was large enough to be representative of the group as a whole. In addition, because we are the only center in the Southern California area performing the duodenal switch procedure, patients with postoperative problems are generally referred back to us for their care. We stress the need for long-term follow-up to all patients before their surgery especially the need for iron and calcium supplementation.
If the mortality and morbidity of the duodenal switch is comparable to other common weight loss procedures, what advantage does it have to offset the longer operative time and technical difficulty? In our view, the answer relates to quality of life. While it is unquestioned that morbidly obese patients must adopt some behavioral change of their eating habits, most bariatric procedures that employ a small proximal gastric pouch and Roux-en-Y limb are characterized by a much more extreme restriction of intake and the development of the dumping syndrome. Indeed it is commonly stated that the dumping symptoms following carbohydrate-rich foods act as a deterrent to eating and are an integral component of the mechanism of action of the Roux-en-Y gastric bypass. After the duodenal switch procedure, patients can consume a wide variety of foods and can ingest a volume approximately half of their preoperative intake without the fear of dumping. This permits restoration of social functioning and good quality of life.
In conclusion, the duodenal switch provides excellent weight loss with preservation of good alimentation, even in the superobese. This is accomplished with acceptable operative mortality and minimal dietary limitations and metabolic sequelae. The results of this study should remove the inhibitions that exist about the use of the duodenal switch procedure as treatment of patients with morbid obesity.
Discussion
Dr. Michael G. Sarr (Rochester, Minnesota): Twenty years ago a paper on bariatric surgery would have been an oddity at this meeting In contrast, this is the second paper on this year’s program, and I doubt that anyone in the audience comes from a place where there isn’t a bariatric program either in place or in development. What a change!
But bariatric surgery does, regretfully, have a dark history. Witness the appropriate, but ignominious fate of the jejunoileal bypass. Hopefully we have learned the lesson that newly devised “wonder” operations, no matter how theoretically attractive, require serious study prior to disseminating their use.
I would like to put Dr. Anthone’s presentation in perspective. The best results of an intense, nonoperative medical approach to weight loss in patients with morbid obesity yield success rates of less than 5% at 5 years. Compare this 5% rate with a medical approach to the 82% success rate reported by Dr. Anthone. Yes, we are internists who can also operate.
Dr. Anthone, I have 3 short questions. First, do these patients all have steatorrhea? Second, the major worry is metabolic bone disease. Serum calcium levels lack sensitivity in detecting this problem. Have you measured bone alkaline phosphatase, serum PTH levels, or have you measured bone density in enough patients to make a sensitive and specific comment? Third, my calculations from your paper suggest a follow-up success of much less than 50%. This of course raises many other questions.
Dr. Gary J. Anthone (Los Angeles, California): Thank you, Dr. Sarr, for your comments and questions.
Addressing the scheaterrhia issue, since we have gone to the standard length of common channel of 100 cm we rarely see any problems with bowel movements. So scheaterrhis in essence is not a problem with the 100 cm common channel.
Obviously our main concern is calcium metabolism. And we have just presented our work on calcium metabolism at the Pacific Coast Society a few months ago. You are right, serum calcium levels, by the time they are low it is almost too late to do something. So we routinely check serum parathyroid hormone levels in all our patients.
Surprisingly, we have found that serum parathyroid hormone levels are elevated in about 15% of morbidly obese patients preoperatively and then we see it elevated in about 30% of patients postoperatively. So we follow the parathyroid hormone levels very closely and then give megadoses of vitamin B-2, 50,000 units once a week, to try to prevent calcium problems.
We have not seen any bone density loss. And that is similar to another report in the literature.
As far as follow-up, we get a great follow-up up to about 2 years, about 80% follow-up. After 2 years it drops tremendously down to about 40 or 50%, you are right. And that is with stringent phone follow-up with our patients. We just can’t keep phone contact with them. However, because this is a unique operation and we are the only ones that really perform it in the southern California area, we hope that if there were problems, the patients would be showing up at our institution again.
Dr. Carlos A. Pellegrini (Seattle, Washington): I have not had a chance to read the manuscript, but based on the pictures you showed during your presentation I am not sure how the stomach of these patients empties after your 75% gastrectomy. As you know the integrity of the antrum is most important in the emptying of solids from the stomach. When you divide a portion of it as you indicated you do, I presume you compromise emptying. Have you measured it? Have your patients developed abnormal gastroesophageal reflux?
Dr. Gary J. Anthone (Los Angeles, California): As far as gastric emptying, we did some initial studies with transcutaneous gastric emptying probes and found that there was really no delay in gastric emptying. As a matter of fact, we think that over time gastric emptying is probably even speeded up even without the antrum.
As far as heartburn, most weight loss operations state that they cure heartburn, although we find that after the duodenal switch with this type of gastrectomy heartburn is a problem for about 6 to 12 months after surgery. We are not sure it is heartburn per se, but my theory is that because the stomach is actually now made even smaller than the diameter of the esophagus, it is more of an esophageal spasm or motility problem. But this almost always goes away after 12 months.
Dr. Robert J. Fitzgibbons, Jr. (Omaha, Nebraska): Dr. Anthone, I don’t do this operation but I think I know a sentinel paper when I have heard one, and I think we have heard one here today. Several questions come to mind, however.
Why do you perform the gastrectomy? Has this procedure been done without the gastrectomy? It seems to me that the major reason for the weight loss would be the diversion.
Second, it is becoming increasingly popular to do this procedure laparoscopically with the robot. Do you have any experience with this? Do you think that is a reasonable idea?
And then finally, could you explain to us what gluteal rhabdomyolysis is?
Dr. Gary J. Anthone (Los Angeles, California): I will explain. Gluteal rhabdomyolysis is when a patient has been described in some transplantation in aortic aneurysm patients when they are on the operating table for an extended period and basically you get a compartment syndrome of your gluteal muscles and it is going to extend up into your paraspinal muscles so that basically your muscles die of lack of blood flow.
And it is very serious. It doesn’t show up even externally until about a week to 10 days after surgery. So you have to follow it by CPK levels to determine the extent. Once we see CPK levels rising above 5,000 we know there is probably some muscle injury and start appropriate alkalinization of the urine and diuresis.
The question about the laparoscopic, there are some centers that have been performing this procedure, the duodenal switch, laparoscopically. We believe that we have approximately a four-day to five-day hospital stay. It is my personal belief that at this time the benefits of laparoscopic don’t outweigh the open procedure.
The first question was the gastrectomy. The first question I asked Doug Hess when I saw this operation described: Why even do it? The diversion of bile and pancreatic juices should be enough to induce a weight loss. But we know for a fact that there are 2 components of this operation: a gastric restrictive phase and a malabsorptive phase. And the 2 in combination help the operation work the best. It has been done without the gastrectomy for patients with hyperlipidemia with good results. But as far as achieving good weight loss results, you have to add that restrictive component.
Footnotes
Presented at American Surgical Association, Washington DC, April 24–26, 2003.
Reprints: Gary J. Anthone, MD, Director, USC Bariatric Surgery Program, 1450 San Pablo Street, Suite 5501, Los Angeles, CA 90033-4612. E-mail: ganthone@surgery.usc.edu.
REFERENCES
- 1.Anthone GJ. Surgery for morbid obesity. In: Zuidema GD, Yeo CJ (eds). Shackelford’s surgery of the alimentary tract. Philadelphia, PA: W. B. Saunders; 2002:437–447. [Google Scholar]
- 2.Stunkard AJ, Harris JR, Pedersen NL, et al. The body-mass index of twins who have been reared apart. New Engl J Med. 1990;322:1483–1487. [DOI] [PubMed] [Google Scholar]
- 3.Zhang Y, Proenca R, Maffei M, et al. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. [DOI] [PubMed] [Google Scholar]
- 4.Friedman JM. Obesity in the new millennium. Nature. 2000;404:632–634. [DOI] [PubMed] [Google Scholar]
- 5.Cummings DE, Weigle DS, Frayo RS, et al. Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. New Engl J Med. 2002;346:1623–1630. [DOI] [PubMed] [Google Scholar]
- 6.Peeters A, Barendregt JJ, Willekens F, et al. NEDCOM The Netherlands Epidemiology and Demography Compression of Morbidity Research Group. Obesity in adulthood and its consequences for life expectancy: a life-table analysis. Ann Intern Med. 2003;138:24–32. [DOI] [PubMed] [Google Scholar]
- 7.Fontaine KR, Redden DT, Wang C, et al. Years of life lost due to obesity. JAMA. 2003;289:187–193. [DOI] [PubMed] [Google Scholar]
- 8.Patterson EJ, Urbach DR, Swanstrom LL. A comparison of diet and exercise therapy versus laparoscopic Roux-en-Y gastric bypass surgery for morbid obesity: a decision analysis model. J Am Coll Surg. 2003;196:379–384. [DOI] [PubMed] [Google Scholar]
- 9.Mitka M. Surgery for obesity: demand soars amid scientific, ethical questions. JAMA. 2003;289:1761–1762. [DOI] [PubMed] [Google Scholar]
- 10.NIH Consensus Development Conference Draft Statement on Gastrointestinal Surgery for Severe Obesity. Obes Surg. 1991;257–265.
- 11.Kremen AJ, Linner JH, Nelson C. An experimental evaluation of the nutritional importance of proximal and distal small intestine. Ann Surg. 1954;140:439–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DeWind LT, Payne JH. Intestinal bypass surgery for morbid obesity. Long-term results. JAMA. 1976;236:2298–2301. [PubMed] [Google Scholar]
- 13.Mason EE, Ito C. Gastric bypass. Ann Surg. 1969;170:329–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Printen KJ, Mason EE. Gastric surgery for relief of morbid obesity. Arch Surg. 1973;106:428–431. [DOI] [PubMed] [Google Scholar]
- 15.Griffin WO, Young VL, Stevenson CC. A prospective comparison of gastric and jejunoileal bypass procedures for morbid obesity. Ann Surg. 1977;186:500–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scopinaro N, Gianetta E, Civalleri D, et al. Bilio-pancreatic bypass for obesity: II. Initial experience in man. Br J Surg. 1979;66:618–620. [DOI] [PubMed] [Google Scholar]
- 17.DeMeester TR, Fuchs KH, Ball CS, et al. Experimental and clinical results with proximal end-to-end duodenojejunostomy for pathologic duodenogastric reflux. Ann Surg. 1987;206:414–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hess DS, Hess DW. Biliopancreatic diversion with a duodenal switch. Obes Surg. 1998;8:267–282. [DOI] [PubMed] [Google Scholar]
- 19.Lagace M, Marceau P, Marceau S, et al. Biliopancreatic diversion with a new type of gastrectomy: some previous conclusions revisited. Obes Surg. 1995;5:411–418. [DOI] [PubMed] [Google Scholar]
- 20.Marceau P, Biron S, Bourque RA, et al. Biliopancreatic diversion with a new type of gastrectomy. Obes Surg. 1993;3:29–35. [DOI] [PubMed] [Google Scholar]
- 21.Marceau P, Hould FS, Simard S, et al. Biliopancreatic diversion with duodenal switch. World J Surg. 1998;22:947–954. [DOI] [PubMed] [Google Scholar]
- 22.Baltasar A, Bou R, Bengochea M, et al. Duodenal switch: an effective therapy for morbid obesity–intermediate results. Obes Surg. 2001;11:54–58. [DOI] [PubMed] [Google Scholar]
- 23.Rabkin RA. Distal gastric bypass/duodenal switch procedure, Roux-en-Y gastric bypass and biliopancreatic diversion in a community practice. Obes Surg. 1998;8:53–59. [DOI] [PubMed] [Google Scholar]
- 24.Pories WJ, Swanson MS, MacDonald KG, et al. Who would have thought it? An operation proves to be the most effective therapy for adult-onset diabetes mellitus. Ann Surg. 1995;222:339–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Livingston EH, Huerta S, Arthur D, et al. Male gender is a predictor of morbidity and age a predictor of mortality for patients undergoing gastric bypass surgery. Ann Surg. 2002;236:576–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abramyan L, Kanel G, Anthone GJ, et al. Severity of nonalcoholic fatty liver disease (NAFLD) after surgical weight loss in patients with morbid obesity [abstract]. Gastroenterology. 2003;124:A825. [Google Scholar]
- 27.Sugerman HJ, Kellum JH, DeMaria EJ. Conversion of proximal to distal gastric bypass for failed gastric bypass for superobesity. J Gastrointest Surg. 1997;1:517–524. [DOI] [PubMed] [Google Scholar]
- 28.Murr MM, Balsiger BM, Kennedy FP, et al. Malabsorptive procedures for severe obesity: comparison of pancreaticobiliary bypass and very long limb Roux-en-Y gastric bypass. J Gastrointest Surg. 1999;3:607–612. [DOI] [PubMed] [Google Scholar]
- 29.Brolin RE, LaMarca LB, Kenler HA, et al. Malabsorptive gastric bypass in patients with superobesity. J Gastrointest Surg. 2002;6:195–203. [DOI] [PubMed] [Google Scholar]
- 30.Marceau P, Biron S, Lebel S, et al. Does bone change after biliopancreatic diversion? J Gastrointest Surg. 2002;6:690–698. [DOI] [PubMed] [Google Scholar]
- 31.MacLean LD, Rhode BM, Nohr CW. Late outcome of isolated gastric bypass. Ann Surg. 2000;231:524–528. [DOI] [PMC free article] [PubMed] [Google Scholar]