Abstract
Objective:
This study examined the relationship of surgeon subspecialty training and interests to in-hospital mortality while controlling for both hospital and surgeon volume.
Summary Background Data:
The relationship between volume of surgical procedures and in-hospital mortality has been studied and shows an inverse relationship.
Methods:
A large Statewide Planning and Research Cooperative System was used to identify all 55,016 inpatients who underwent gastrectomy (n = 6434) or colectomy (n = 48,582) between January 1, 1998 and December 31, 2001. Surgical subspecialty training and interest was defined as surgeons who were members of the Society of Surgical Oncology (training/interest; n = 68) or the Society of Colorectal Surgery (training; n = 61) during the study period. The association of in-hospital mortality and subspecialty training/interest was examined using a logistic regression model, adjusting for demographics, comorbidities, insurance status, and hospital and surgeon volume.
Results:
Overall mortality for colectomy patients was 4.6%; the adjusted mortality rate for subspecialty versus nonsubspecialty-trained surgeons was 2.4% versus 4.8%, respectively (adjusted odds ratio [OR] = 0.45; 95% confidence interval [CI] = 0.34, 0.60; P < 0.0001). Gastrectomy patients experienced an overall mortality rate of 8.4%; the adjusted mortality rate for patients treated by subspecialty trained surgeons was 6.5%, while the adjusted mortality rate for nonsubspecialty trained surgeons was 8.7% (adjusted OR = 0.70; 95% CI = 0.46, 1.08; P = 0.10).
Conclusions:
For gastrectomies and colectomies, risk-adjusted mortality is substantially lower when performed by subspecialty interested and trained surgeons, even after accounting for hospital and surgeon volume and patient characteristics. These findings may have implications for surgical training programs and for regionalization of complex surgical procedures.
This study examines the relationship of surgeon subspecialty training and interests to in-hospital mortality after colectomy or gastrectomy, controlling for hospital and surgeon volume and patient characteristics. Risk-adjusted mortality is substantially lower when performed by subspecialty interested and trained surgeons, even after accounting for both hospital and surgeon volume.
The relationship between hospital and surgeon volume and outcomes of care has been documented in numerous studies.1-11 Outcomes, including mortality, complications, and resource use, have been shown to be lower for high-volume hospitals and surgeons.3-6 This observed association has led to calls for surgical care programs providing complex operative procedures to be regionalized at high-volume centers as a way to improve the quality of care.3 For example, the Leapfrog Group has recommended that insurance carriers contract with hospitals meeting certain volume standards for coronary artery bypass, coronary angioplasty, and several other complex procedures as a way to improve outcomes.12,13
Despite the large number of studies documenting improved outcomes associated with higher surgical volumes, the causal links in this relationship are not well understood.2,3 Many studies have accounted for patient characteristics (including comorbidities, age, and insurance status) that may underlie the observed differences, and recent work has shown that surgeon volume in addition to hospital volume is associated with hospital mortality rates.3,6 To further understand the relationship between volume and surgical outcomes, we sought to evaluate whether having surgical subspecialty training or a special surgical interest had an impact on treatment-related mortality beyond the volume relationship.
To address this question, we developed a multivariate model to evaluate the independent effect of having subspecialty surgical training or major surgical interest on the outcome of hospital mortality for patients undergoing gastrectomy or colectomy. We identified surgeons in New York State with subspecialty training or major surgical interest (defined through membership in surgical subspecialty societies) and compared patient outcomes after treatment by these physicians for colectomy and gastrectomy procedures to surgeons without subspecialty training or major surgical interest.
METHODS
The New York State Department of Health's Statewide Planning and Research Cooperative System (SPARCS) was used to identify all patients discharged alive or deceased from acute care nonfederal hospitals in New York State during the time period January 1, 1998 through December, 31, 2001, who underwent colectomy or gastrectomy. SPARCS contains automated discharge data that records the following information: patient age, sex, race, admission status, principal diagnosis, up to 14 secondary diagnoses, principal procedure, up to 14 secondary procedures, permanent facility (hospital) identifier, unique surgeon identifier (operating physician state license number), and discharge status. The Department of Health has the responsibility for verifying the accuracy of the assembled data, which is abstracted from medical records by trained hospital personnel.
Colectomy principal procedures were defined by ICD-9-CM codes 45.73–45.76, which include right hemicolectomy, resection of transverse colon, left hemicolectomy, and sigmoidectomy. Gastrectomy principal procedures were defined by ICD-9-CM codes 43.5–43.99, which include partial gastrectomy with anastomosis to esophagus, partial gastrectomy with anastomosis to duodenum, partial gastrectomy with anastomosis to jejunum, other partial gastrectomy, and total gastrectomy. We did not restrict these procedures exclusively to cancer-related principle diagnoses.
Surgical subspecialty training and/or major surgical interest was defined as surgeons who were members of the Society of Colorectal Surgery (relevant for colectomy) or the Society of Surgical Oncology (relevant for gastrectomy) in New York State during the four-year study period. The state license numbers (issued by the New York State Department of Education) of the subspecialty-trained surgeons were identified from surgical subspecialty society lists and matched to the identical surgeon identifier as reported in the SPARCS data. We then de-identified the data before analysis.
All of the following methods were performed separately for colectomy and gastrectomy procedures. In-hospital mortality was defined as the patient dying in the hospital during or after the procedure was performed versus the patient being discharged from the hospital alive. The prevalences of potential patient risk factors for mortality were calculated, and the χ2 test was used to assess the bivariate association between each risk factor and in-hospital mortality. The following patient characteristics and comorbidities were evaluated with respect to mortality: age, gender, race, Medicaid status (yes/no), ischemic heart disease, airway obstruction, congestive heart failure, organ metastasis, peripheral vascular disease, chronic obstructive pulmonary disease, diabetes, and dysrhythmia. Comorbidities were defined by ICD-9-CM diagnosis codes (available from authors upon request). Patient characteristics and comorbidity prevalences were also stratified by subspecialty-trained surgeons versus nonsubspecialty trained surgeons.
Four-year hospital volumes for colectomy and gastrectomy were calculated by summing the number of procedures performed in each hospital during the study period. Four-year surgeon volumes for colectomy and gastrectomy were calculated by summing the number of procedures performed by each surgeon during the study period. Categorical variables for hospital and surgeon volume for each procedure (ie, separate analyses for colectomy and gastrectomy) were defined by patient volume quartiles and the χ2 test for trend was used to assess the relationship between in-hospital mortality and each quartile category of 4-year hospital and surgeon volume. The correlation between 4-year hospital and surgeon volume was assessed by the Spearman-rank correlation coefficient.
Indicator variables for a combined hospital and surgeon volume measure were constructed by dichotomizing each volume measure at the median value and creating intersecting high/low categories. The combined hospital/surgeon volume measure had the following 4 categories: low hospital volume/low surgeon volume; low hospital volume/high surgeon volume; high hospital volume/low surgeon volume; and high hospital volume/high surgeon volume. This approach allows for a combined hospital and surgeon volume measure to be included in a multivariate model (see below), whereas the 2 separate volume measures cannot be included simultaneously in a multivariate model due to collinearity between the 2 volume measures. Hospital and surgeon volume distributions were also stratified by subspecialty trained surgeons versus nonsubspecialty surgeons.
The relationship between subspecialty training/interest and in-hospital mortality for colectomy and gastrectomy was assessed by the χ2 test. Differences in the median hospital length of stay for patients undergoing colectomy and gastrectomy performed by subspecialty versus nonsubspecialty surgeons were assessed by the Wilcoxon rank sum test. A multivariate generalized linear model for binary outcomes was used (separate models for colectomy and gastrectomy) to examine the risk-adjusted relationship between subspecialty training/interest and in-hospital mortality. Specifically, a generalized estimating equations model14 was used to account for clustering by hospital and surgeon (ie, nonindependent observations nested within surgeon and nested within hospital; SAS Institute, Inc., Cary, NC). This type of model generated robust (conservative) standard error estimates that account for the nonindependence (clustering) of the observations in the SPARCS database.
The unit of analysis in the model was the patient with the mortality outcome defined as dying in the hospital versus being discharged alive. The independent variables included in the model, defined a priori, included subspecialty training/interest status, age, gender, race, Medicaid status, all comorbidities as defined above, and hospital and surgeon volume measures. Various models were run accounting separately for hospital volume, surgeon volume, and the combined hospital/surgeon volume measure. Because all of these models yielded consistent results for the risk-adjusted effect of subspecialty training/interest on in-hospital mortality for colectomy and gastrectomy, we present the subspecialty-training/interest effect estimates (odds ratios) and associated P values from models where hospital volume was entered as a log-transformed continuous covariate. This technique, with respect to the subspecialty-training effect estimate, reduces residual confounding in the estimate that may be introduced by including only volume quartiles in the models. It also eliminates the opportunity for selecting volume cut points that would optimize the P values for the specialty-training effect estimates.
RESULTS
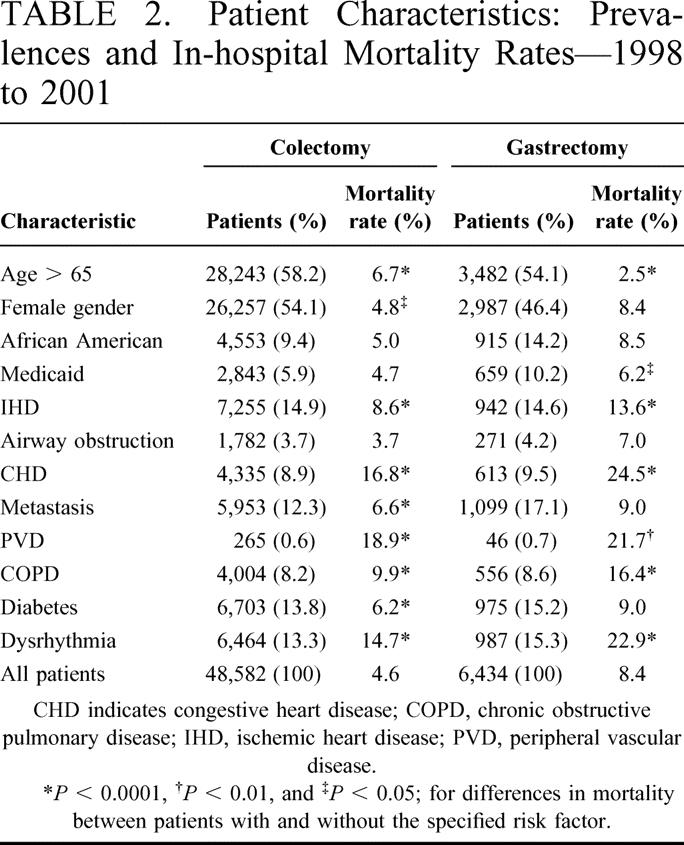
Table 1 presents, for all colectomy and gastrectomy procedures performed in New York State during 1998 through 2001, the number of patients, in-hospital mortality rate, number of hospitals where procedures were performed, number of surgeons who performed the procedures, number of subspecialty trained and nonsubspecialty trained surgeons who performed the procedures, and quartiles for both hospital and surgeon volume. The number of colectomy and gastrectomy procedures performed was 48,582 and 6434, respectively, and the associated in-hospital mortality rates were 4.6% and 8.4%, respectively. More hospitals (n = 223) performed colectomies than gastrectomies (n = 213). Sixty-one of 2651 surgeons who performed colectomies during the 4-year period had surgical subspecialty training (membership in Society of Colorectal Surgery) and 68 of 1387 surgeons who performed gastrectomies during the period had surgical subspecialty training (membership in Society of Surgical Oncology). For both procedures, there was a large difference between the 25th percentile of hospital volume and the maximum volume for the 4-year period. For colectomy, 25% of all patients during the 4-year period attended hospitals performing 191 or fewer procedures, whereas 1725 procedures (3.6%) where performed in 1 hospital. For gastrectomy, 25% of all patients attended hospitals performing 27 or fewer procedures, whereas 496 procedures (7.7%) were performed in 1 hospital. The same pattern for both procedures was evident with respect to surgeon volume (Table 1).
TABLE 1. Mortality and Provider Characteristics for Colectomy and Gastrectomy Procedures in New York State—1998 to 2001
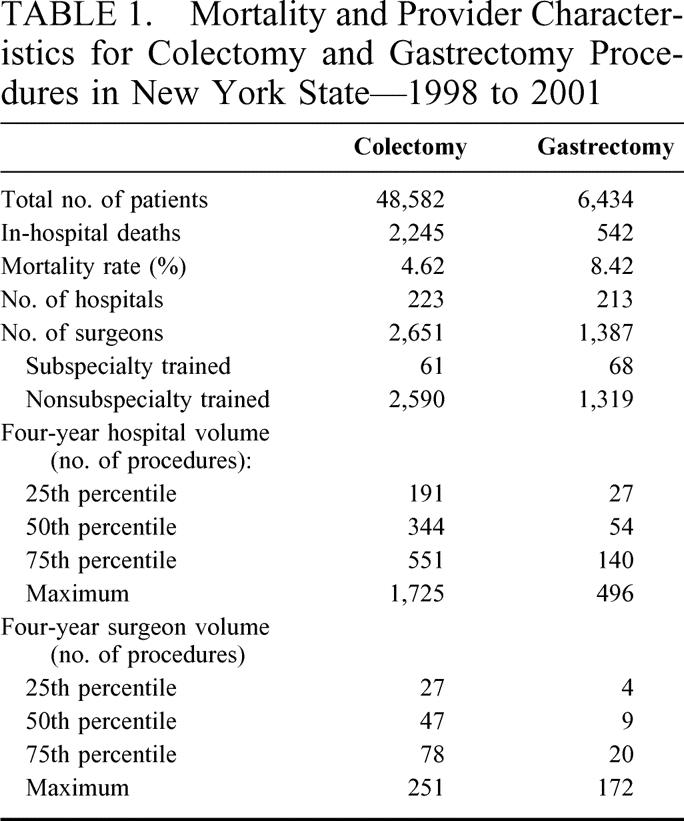
Table 2 presents in-hospital mortality rates for patients undergoing colectomy and gastrectomy stratified by a number of potential patient risk factors for mortality. As indicated, 9 of the 12 patient risk factors and 7 of the 12 patient risk factors were significantly associated with in-hospital mortality for colectomy and gastrectomy patients, respectively. Among colectomy patients, mortality for significant risk factors ranged from 4.8% for patients who were female to 18.9% for patients with peripheral vascular disease. For gastrectomy patients, mortality for significant risk factors ranged from 6.2% for Medicaid patients to 24.5% for patients with congestive heart disease.
TABLE 2. Patient Characteristics: Prevalences and In-hospital Mortality Rates—1998 to 2001
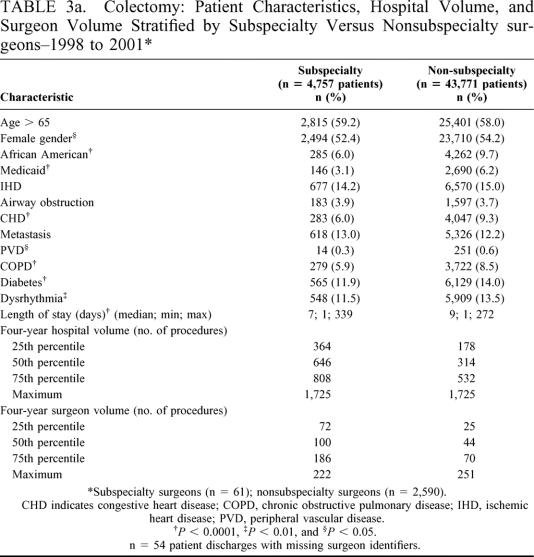
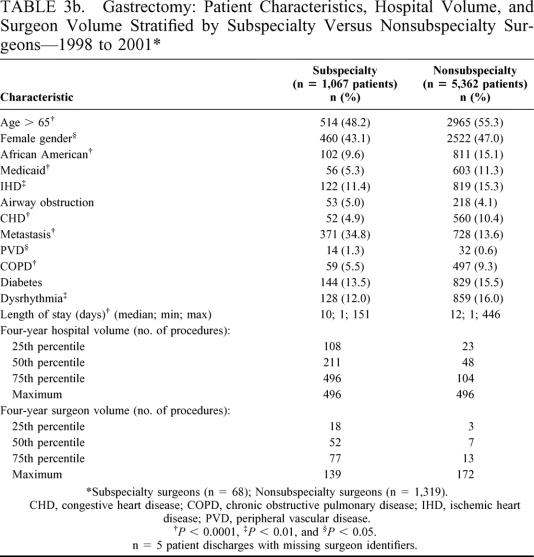
Tables 3a and 3b present patient characteristics, hospital volume, and surgeon volume stratified by the number of colectomy and gastrectomy procedures performed by subspecialty surgeons (colectomy n = 4757 procedures; gastrectomy n = 1067 procedures) and nonsubspecialty surgeons (colectomy n = 43,771 procedures; gastrectomy n = 5362 procedures). In general, subspecialty surgeons treated patients with lower rates of comorbidities compared with patients treated by nonsubspecialty surgeons. Subspecialty trained surgeons were associated with shorter patient lengths of stay but also treated more patients with organ metastasis than nonsubspecialty trained surgeons (eg, gastrectomy: 34.8% metastasis versus 13.6% metastasis, respectively, P < 0.0001). Subspecialty surgeons performed colectomies and gastrectomies in hospitals with higher four-year hospital volumes as compared with nonsubspecialty surgeons for both colectomy and gastrectomy. Likewise, subspecialty surgeons performed more of both types of procedures than nonsubspecialty surgeons for all quartile categories of surgeon volume (Tables 3a and 3b).
TABLE 3a. Colectomy: Patient Characteristics, Hospital Volume, and Surgeon Volume Stratified by Subspecialty Versus Nonsubspecialty surgeons–1998 to 2001
TABLE 3b. Gastrectomy: Patient Characteristics, Hospital Volume, and Surgeon Volume Stratified by Subspecialty Versus Nonsubspecialty Surgeons—1998 to 2001
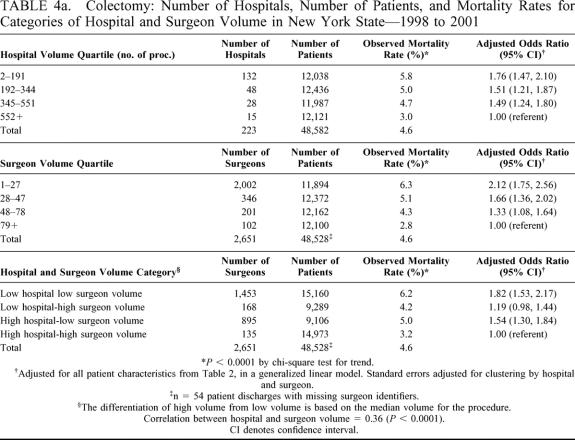
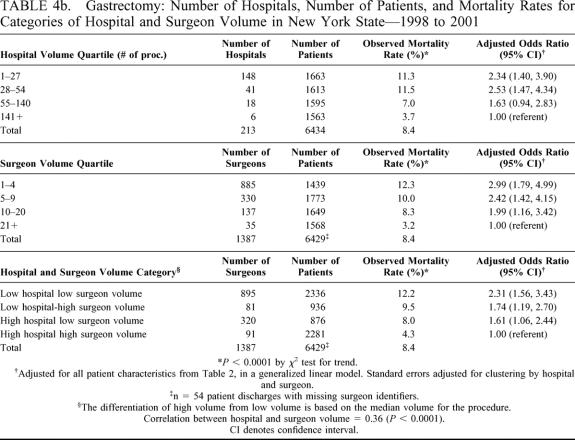
Tables 4a and 4b present the number of providers and patients, observed mortality rate, and risk-adjusted odds ratio (and 95% CI) relative to the highest volume category for each of the hospital and surgeon volume categories for colectomy and gastrectomy. Odds ratios were adjusted for all 12 patient risk factors as described in Table 2 in a multivariate generalized linear model, which accounted for clustering by hospital and surgeon. Observed mortality rates decreased with increasing quartiles of both hospital and surgeon volume (colectomy [hospital volume, 5.8% to 3.0%, P < 0.0001; surgeon volume, 6.3% to 2.8%, P < 0.0001]; gastrectomy [hospital volume, 11.3% to 3.7%, P < 0.0001; surgeon volume, 12.3% to 3.2%, P < 0.0001]). The adjusted odds ratios for both procedures revealed the same mortality pattern with increasing volume even after adjustment for patient risk factors. Similar results were demonstrated for the combined hospital and surgeon volume measure. Patients undergoing operations performed by below-median-volume surgeons in below-median-volume hospitals had the highest mortality rates even after risk adjustment (Tables 4a and 4b).
TABLE 4a. Colectomy: Number of Hospitals, Number of Patients, and Mortality Rates for Categories of Hospital and Surgeon Volume in New York State—1998 to 2001
TABLE 4b. Gastrectomy: Number of Hospitals, Number of Patients, and Mortality Rates for Categories of Hospital and Surgeon Volume in New York State—1998 to 2001
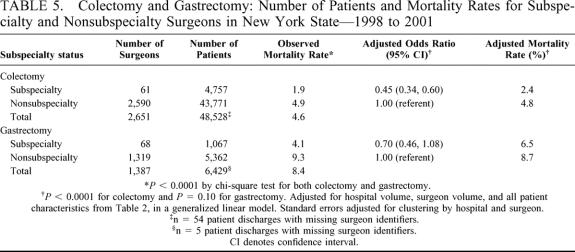
Table 5 presents the number of patients, observed mortality rate, risk-adjusted odds ratio (and 95% CI), and risk-adjusted mortality rate, for subspecialty and nonsubspecialty surgeons performing colectomies and gastrectomies during the four-year period. As indicated, the observed mortality rate for subspecialty versus nonsubspecialty surgeons was 1.9% versus 4.9% for colectomy (P < 0.0001) and 4.1% versus 9.3% for gastrectomy (P < 0.0001). For colectomy, after adjustment for hospital volume, surgeon volume, and all patient risk factors as described in Table 2, the adjusted odds ratio for subspecialty surgeons demonstrated a 55% reduction in hospital mortality relative to nonsubspecialty surgeons (reference category) (adjusted OR = 0.45; 95% CI = 0.34, 0.60; P < 0.0001). The associated risk-adjusted mortality rate was 2.4% relative to 4.8% among nonsubspecialty surgeons.
TABLE 5. Colectomy and Gastrectomy: Number of Patients and Mortality Rates for Subspecialty and Nonsubspecialty Surgeons in New York State—1998 to 2001
Likewise, for gastrectomy, the adjusted odds ratio for subspecialty surgeons demonstrated a 30% reduction in hospital mortality relative to nonsubspecialty surgeons (adjusted OR = 0.70; 95% CI = 0.46, 1.08; P = 0.10), and the associated risk-adjusted mortality rate was 6.5% relative to 8.7% among nonsubspecialty surgeons. Although the adjusted odds ratio for gastrectomy is not significant at the 0.05 alpha level, the associated 95% confidence limits are compatible with a modest association. All risk-adjusted models accounted for clustering by hospital and surgeon and, as a result, produced conservative standard error estimates. For example, if clustering is not accounted for in the gastrectomy model, the P value for the subspecialty training effect is 0.048 and the associated 95% confidence interval is (0.49, 0.99), also consistent with a modest effect of subspecialty training on hospital mortality for gastrectomy.
DISCUSSION
Gastrectomy and colectomy performed by subspecialist surgeons yields adjusted mortality rates that ranged from approximately half to 75% of those for nonsubspecialists. The adjusted mortality rate for colectomy is 4.8% for patients treated by nonsubspecialty surgeons, but only 2.4% if treated by subspecialty-focused surgeons (P < 0.0001). A similar trend was observed for gastrectomy patients who have an adjusted mortality rate of 8.7% if treated by nonsubspecialty surgeons compared with 6.5% for subspecialty surgeons (P = 0.10). These findings take into account subspecialty training/interest, demographics, comorbidities, insurance status, and both hospital and surgeon volume.
If all patients eligible for the studied procedures could obtain care from subspecialist surgeons rather than from nonspecialists, among colectomy patients 1073 hospital deaths (of 2245 deaths) could potentially have been avoided in New York State during the 4 year study period. Similarly, we estimate a potential reduction of 122 hospital deaths (of 542 deaths) among gastrectomy patients during this same period if all patients were treated by subspecialty surgeons. Whether this is feasible in practice is uncertain, because these patients may not live within a reasonable radius of subspecialist surgeons who also tend to be more likely to perform such procedures at higher volume hospitals. To implement such a referral approach, some organization would have to develop a broad-scale information dissemination program to tell patients where best to seek care. If patients require emergency surgery, it might not be realistic to transport them to more distant surgeons.
We attempt to address limitations of previous studies by incorporating physician and hospital volume and using population-based data. The interaction of physician and hospital volume in our study shows that care provided by a low-volume surgeon in a low-volume hospital increases one's probability of mortality with an odds ratio of 1.82 for colectomy (95% CI = 1.53, 2.17) and 2.31 for gastrectomy (95% CI = 1.56, 3.43) compared with the reference case (high-volume surgeon and hospital), whereas the other combinations of high surgeon volume and low hospital volume or low surgeon volume and high hospital volume fall between 1.0 and the estimate for low-volume surgeons in low-volume hospitals. Besides including hospital and physician volume, another strength of our approach is that it includes all patients undergoing these procedures during the study period for the entirety of New York State rather than for a selected subgroup, which enhances the generalizability of our work.
An important limitation of this study is that we used administrative data to assess the volume-specialty-mortality relationship. This did not allow us to account for the appropriateness of patient selection or for detailed comorbid conditions, nor does it give us long-term follow-up. We reduced the likelihood of confounding, however, by adjusting for risk based on comorbidities ascertained in the claims data and controlling for other demographic variables.
Regardless, this study adds to the literature in an important way by elucidating the impact of variation in surgical training and subspecialty focus, and probably indirectly, surgical judgment, technique, and operative experience. Not only do we account for and demonstrate the significance of hospital and physician volume, which are associated with short-term mortality for colectomy, gastrectomy, and lung lobectomy,15 and esophageal, pancreatic, colorectal, gastric, and lung cancer surgeries among others,3 but we also illustrate that surgeon subspecialty focus is important independent of the impact of hospital and surgeon volume. This suggests that there is a fixed quality effect of care provided by subspecialists.
Our findings have implications for elective surgeries in that individuals, employers, or health plans may want to avoid not only lower-volume surgeons, but also surgeons not subspecializing in such challenging procedures as colectomy and gastrectomy. These provider-specific characteristics appear to affect the quality of care and in-hospital mortality. Further work to understand predictors and influences on procedure-related mortality rates may help to understand better the relationship of volume to surgical outcomes. It remains critical to identify specific processes of care that affect patients’ outcomes.
Discussion
Dr. Timothy J. Eberlein (St. Louis, Missouri): This is an excellent presentation. This Association has had several papers demonstrating improved outcomes for either high volume surgeons or high volume hospitals actually presented by several of the past presidents of this Association. However, the manuscript that opened this meeting demonstrated the difficulty in interpreting administrative databases and the fact that thoughtful analysis is required to use these databases in establishing substantive norms and setting standards.
In the present study the authors use an administrative database to assess the relationship of volume (both surgeon and hospital) and specialty training as well as with an end-point of in-hospital mortality. Therefore, patient selection and detailed comorbidities are not well assessed.
My first question deals with the issue of the criteria for defining subspecialty training.
The authors found 61 of 2,651 surgeons were subspecialty trained for colectomy as defined as membership in the Society of Colorectal Surgeons. How did they define members of the Society of Surgical Oncology who also perform colectomies?
Similarly for gastrectomy. 68 of 1,387 surgeons were members of the Society of Surgical Oncology. But what about other subspecialty organizations such as SAGES?
Similarly, what is the accuracy of the comorbidity data used in this administrative database? For example, in my own institution, we have had extensive difficulty having the hospital document comorbidities accurately for our billing purposes.
While intuitively I agree with the authors that subspecialty training or specialization as well as volume clearly makes a difference in outcome, why wouldn’t gastrectomy have shown a greater significant difference?
Finally, how much impact did the 1 highest volume hospital have in the overall results? We saw from Dr. Brennan’s Presidential Address the outstanding results his institution demonstrated having high volume surgeons, a high volume institution, as well as subspecialty training.
I want to congratulate the authors for presenting a very well-written manuscript and thank the Association for the privilege of discussing this paper.
Dr. Mark A. Callahan (New York, New York): Let me first address the issue of defining subspecialty training. We agree there are limitations with our definition. We used the database of membership in these societies to assign subspecialty versus nonsubspecialty, realizing there is some overlapping in both the skill set and the training for these 2 groups. However, that served as a proxy for our analysis.
That relates to your third question about why we didn’t see a more significant difference in the gastrectomy data. Gastrectomy data was limited by the fact that, number one, we did have a relatively limited number of surgeons performing that we called subspecialty surgeons. And secondly, there are many fewer of those procedures done annually in New York state and therefore the number of mortality events was lower, and that affects our statistical power. Now, the odds ratio we found was .75, which says that you have about a one-third less chance of dying if a subspecialty trained surgeon does your operation by our definition, and had we had more observations I think we would have achieved significance statistically.
As far as the accuracy of comorbidity data, I am shocked to hear that a hospital doesn’t accurately code that, actually. We have never seen that in our institution. The reality is that all these data sets suffer from the same problem, all these administrative sets. There are biases to overcoding and undercoding for comorbidities obviously driven by staffing issues, accuracy issues, and payment pressures.
All administrative data sets do suffer from those issues. The fact that we had a fairly large data set with 55,000 collective patients across 200-some-odd hospitals, you hope that to some degree you can wash out the over- and the undercoding within the data set.
That being said, there were a couple of interesting things that came out. Subspecialty trained surgeons tend to operate on more patients with metastases in our data set. And that came through very clearly and I think that is probably a real observation.
Now, that may be driven by the issue of high volume hospitals and how do they impact on these outcomes? I am at Cornell, and right next to us is Sloan-Kettering. Obviously that is a very high quality institution which is doing a number of these operations.
It turns out that the high volume hospitals, which there is a limited number of them across New York state, which tend to be university hospitals, had the best outcomes, had the most subspecialty trained surgeons. So there is a clearly effect there of being at those institutions and having those surgeons operate on you.
Dr. Carlos A. Pellegrini (Seattle, Washington): I am still troubled by the same segue that Dr. Eberlein brought up. I am not convinced that you can isolate in your study the training issue from the volume issue. Instead I see subspecialty training as 1 component of outcome where volume, dedication to a disease process and other factors also play a role. The only way that you can demonstrate your point would be if you had somebody with subspecialty training who was working in a low-volume hospital, was doing a low volume of operations and still had excellent outcomes. Did you find any such situation in your study?
I believe that when you look at a high volume surgeon in a high volume hospital, if you add subspecialty training you may observe better results but I think that is a function of the volume. Were you ever able to see an independent effect of subspecialty training when an individual had not frequently practiced a given operation?
Dr. Mark A. Callahan (New York, New York): That is a very important question that Dr. Pellegrini is raising. It goes to the heart of the type of analysis that we were doing statistically. And in a multivariate analysis, you have got subspecialty trained physicians – not a lot of them, but we have some in our database – who are doing low volumes of these procedures. And you can compare that individual statistically to a nonsubspecialty trained physician also doing low volume and see a difference in their mortality.
You have also got the additional from high volume people and they are getting even better outcomes because of the subspecialty training. That is the advantage of the type of analytic approach that we took, because you have got surgeons who cross all our volume domains and who also cross all our training domains, if you will, and you are allowed to sort of tease that out statistically within the data set.
We are limited by the number of surgeons who can go into each of these categories statistically. And there was a very good paper presented earlier this morning where the discussion was we can only put 8 or 9 variables into our model because of the limited number of patients. We had a larger number of patients and we still were limited by the number of variables we can analyze statistically.
Dr. Henry A. Pitt (Milwaukee, Wisconsin): When we looked at the volume effects for liver resection in the state of Maryland a number of years ago, we subdivided our patients into major versus minor operations, which was important in showing differences. Therefore, my question is whether you have data on the subset of total gastrectomy or the subset of proctocolectomy patients where you would anticipate worse outcomes? What was the effect of subspecialty training in those patients undergoing even higher risk operations?
Dr. Mark A. Callahan (New York, New York): An important question. We did do a subgroup analysis based on some of the codes but not all of the codes. We did see the effect in some of the more complicated operations. The problem, of course, is that you lose numbers in those, you lose power statistically, and therefore our confidence intervals got wider.
Dr. Paris Tekkis (Cleveland, Ohio): I would like to congratulate the authors for this excellent presentation. The Association of Coloproctology of Great Britain and Ireland have recently performed a similar study and I would like to share the similarities of their results in relation to this presentation.
A total of 8,077 major colorectal procedures were evaluated with similar outcomes, however in addition to the risk factors used in your presentation, 2 further variables were found to be important in the risk stratification process. The first risk factor was cancer staging. Stage IV disease was associated with a 2-fold increase in operative mortality in comparison with Stage I, II or III. The second risk factor was mode of presentation. Emergency surgery was associated with a doubling of the operative mortality over elective procedures.
With regards to specialist and nonspecialist surgeons there was no difference in operative mortality for the emergency group of operated patients, but a significant difference in operative mortality was evident in a selective group of elective colorectal resections. I wonder whether similar results were obtained in your study?
Dr. Mark A. Callahan (New York, New York): Thank you. An important question.
We don’t have cancer staging because of the database limitations. You cannot get cancer stage out of administrative data that we have. So I can’t answer the question. Although I suspect that your observation would hold true in our data as well if we had that.
As far as the urgent versus nonurgent, we did separate out the urgent versus the nonurgent at 1 point in time. We didn’t see a difference. We saw an effect in both. Now, on the collections, we have 55,000. And if you had 8,000, then whatever your subgroup of urgents was with some fraction, you may not have had enough power to be able to do the observations because of the number of patients.
Dr. Martin S. Litwin (New Orleans, Louisiana): I felt obligated to rise and ask a question which seems obvious to me from both the abstract and the manuscript. The overall mortality for gastrectomy patients was 8.4%, with the adjusted for subspecialty-trained surgeons of 5.4%. For the colectomy patients it was 4.6%.
These seem like exceedingly high mortality rates to me. I wonder if you have had the opportunity to go into the data a little further and determine why those mortalities are so high. Those are high mortalities for these patients. Those are mortalities that are even high for congenital heart surgery. I wonder if you could comment on that?
Dr. Mark A. Callahan (New York, New York): I really can’t tell you the ‘why.’ I can tell you that unfortunately this is the state of the art of surgery in our state at this point in time, or actually the observation of what is actually going on in our state at this point in time.
Most of us here are at very sophisticated academic medical centers where our experiences are quite different. And indeed when you stratify your data out and look at the high volume academic medical centers, we are running 2 to 3% mortality rates for these types of procedures, considerably lower than the statewide average.
The reality is that the folks in this room do not do the majority of procedures in their states. There are a lot of procedures being done at other institutions where the experience is quite different.
Dr. Marshall Z. Schwartz (Philadelphia, Pennsylvania): I want to expand on Dr. Pellegrini’s question further and address the issue of not just mortality but the cause of death. Were you able to evaluate the cause of death and whether the mortality was directly related to a surgical problem such as an anastomotic leak or perhaps unrelated such as a myocardial infarction.
The reason for addressing the cause of death is that in a higher volume environment you are likely to have better ICU care, better cardiology, pulmonology, et cetera. Thus, it is possible that the decreased mortality in higher volume environments may be related to better ancillary support and have less to do with surgical skills.
Dr. Mark A. Callahan (New York, New York): Thank you. That is a very important question.
We cannot differentiate the cause of death from the administrative records. However, again, by doing the analytic approach that we did, we can look at a low volume place where you have got a high volume surgeon – those types of places exist – or a low volume place where you have got a subspecialty surgeon and statistically tease out the independent effect of that specialty training or of that volume for that individual surgeon on the outcomes.
Now, the presumption is that that low volume place doesn’t have the rich ancillary support, ICU care, et cetera, that the higher volume place does. And that is probably true. We can at least mathematically pull it out, but I can’t tell you for sure – we haven’t gone to each hospital and said: What services do you not have? And also, what is the actual cause of death of these patients?
Dr. Michael G. Sarr (Rochester, Minnesota): Let’s come back to specialty. You talked about specialty training. But yet your definition is membership in a society. That doesn’t always equate. Why don’t you go back and look at specialty training and then redo your calculations and not base your argument just on ‘membership’ in a society?
Dr. Mark A. Callahan (New York, New York): A very important point. In the manuscript we refer to specialty training or interest. I think that is reflected by being a member of 1 of these societies. But it is not a 100% definition that you have had specialty training. We agree with that. That is 1 of the limitations we acknowledge in our paper.
Dr. Ronald V. Maier (Seattle, Washington): One last quick question. As you know, at the American Board of Surgery there is currently ongoing significant debate about reengineering the training of the general surgeon and where subspecialty training fits.
As an extension of the previous question, is the improvement in outcome due to subspecialty training or is it merely additional training? In other words, if you take your proxy group with advanced training in surgical oncology and looked their outcomes following colectomy instead of gastrectomy, do you find the same improvement in outcome? That may not be the best crossover procedure to compare, but can you use your database to analyze the impact of the additional year of training on improving survival across the board and that it doesn’t matter necessarily what you did the additional training in, just that you did it? Are the current traditionally trained surgeons inadequately trained?
Dr. Mark A. Callahan (New York, New York): We haven’t looked at our data that way. I think that is an excellent suggestion for the next step in this work is to sort of see.
There are 2 points there. One is that the extra training makes you better. The second thing is there is sort of an issue of lifetime volume of procedures. Is it just another year of training means you just have that many more procedures? We can’t really tease that out at this point in time. My own gut feeling is that there is a role for that extra training in improving technique specifically. And that is, I think, part of the results that we are observing.
Footnotes
Funding for this study was provided solely by salary support from the authors’ respective institutions.
Reprints: Mark A. Callahan, MD, Department of Public Health, Weill Medical College of Cornell University, 411 E. 69th Street, New York, NY 10021. E-mail: macallah@med.cornell.edu.
REFERENCES
- 1.Luft HS, Bunker JP, Enthoven AC. Should operations be regionalized? The empirical relation between surgical volume and mortality. N Engl J Med. 1979;301:1364-1369. [DOI] [PubMed] [Google Scholar]
- 2.Luft HS. The relation between surgical volume and mortality: an exploration of causal factors and alternative models. Med Care. 1980;18:940-959. [DOI] [PubMed] [Google Scholar]
- 3.Halm EA, Lee C, Chassin MR. Is volume related to outcome in health care? A systematic review and methodologic critique of the literature. Ann Intern Med. 2002;137:511-520. [DOI] [PubMed] [Google Scholar]
- 4.Pearce WH, Parker MA, Feinglass J, et al. The importance of surgeon volume and training in outcomes for vascular surgical procedures. J Vasc Surg. 1999;29:768-776; discussion 777-768. [DOI] [PubMed]
- 5.Hannan EL, Popp AJ, Tranmer B, et al. Relationship between provider volume and mortality for carotid endarterectomies in New York state. Stroke. 1998;29:2292-2297. [DOI] [PubMed] [Google Scholar]
- 6.Birkmeyer JD, Siewers AE, Finlayson EV, et al. Hospital volume and surgical mortality in the United States. N Engl J Med. 2002;346:1128-1137. [DOI] [PubMed] [Google Scholar]
- 7.Nallamothu BK, Saint S, Ramsey SD, et al. The role of hospital volume in coronary artery bypass grafting: is more always better? J Am Coll Cardiol. 2001;38:1923-1930. [DOI] [PubMed] [Google Scholar]
- 8.Luft HS, Hunt SS, Maerki SC. The volume-outcome relationship: practice-makes-perfect or selective-referral patterns? Health Serv Res. 1987;22:157-182. [PMC free article] [PubMed] [Google Scholar]
- 9.Flood AB, Scott WR, Ewy W. Does practice make perfect? Part I: the relation between hospital volume and outcomes for selected diagnostic categories. Med Care. 1984;22:98-114. [PubMed] [Google Scholar]
- 10.Flood AB, Scott WR, Ewy W. Does practice make perfect? Part II: the relation between volume and outcomes and other hospital characteristics. Med Care. 1984;22:115-125. [PubMed] [Google Scholar]
- 11.Hannan EL. The relation between volume and outcome in health care. N Engl J Med. 1999;340:1677-1679. [DOI] [PubMed] [Google Scholar]
- 12.Purchasing Principles. The Leapfrog Group. Available at: www. leapfroggroup. org/purchase1.htm. Accessed March 31, 2003.
- 13.Birkmeyer JD, Finlayson EV, Birkmeyer CM. Volume standards for high-risk surgical procedures: potential benefits of the Leapfrog initiative. Surgery. 2001;130:415-422. [DOI] [PubMed] [Google Scholar]
- 14.Zeger SL, Liang KY. An overview of methods for the analysis of longitudinal data. Stat Med. 1992;11:1825-1839. [DOI] [PubMed] [Google Scholar]
- 15.Hannan EL, Radzyner M, Rubin D, et al. The influence of hospital and surgeon volume on in-hospital mortality for colectomy, gastrectomy, and lung lobectomy in patients with cancer. Surgery. 2002;131:6-15. [DOI] [PubMed] [Google Scholar]