Abstract
Bombesin is an endogenous gut peptide that is prominent in the stomach. In addition to its effects on modulating acid and gut peptide secretion, recent evidence indicates that bombesin is a potent gastroprotective agent. This review article examines the ability of bombesin to prevent gastric injury. Its protective actions appear to be mediated primarily via the release of endogenous gastrin, as gastroprotection is negated by blockade of gastrin receptors. Bombesin-induced gastroprotection and gastrin release are modified by somatostatin. Immunoneutralization of endogenous somatostatin increases the ability of bombesin to prevent gastric injury by increasing gastrin release. In mechanistic studies, ablation of capsaicin-sensitive afferent neurons abolishes bombesin-induced gastroprotection while cyclo-oxygenase inhibition partially reverses this effect. Nitric oxide synthase inhibition also negates bombesin-induced gastroprotection as well as the ability of bombesin to increase gastric mucosal blood flow. Taken together, the available evidence indicates that bombesin causes release of endogenous gastrin that activates sensory neurons located in the gastric mucosa. Activation of sensory neurons causes increased production of nitric oxide through activation of constitutive nitric oxide synthase, which leads to a resultant increase in gastric mucosal blood flow and renders the stomach less susceptible to damage from luminal irritants.
Bombesin is a gastrointestinal neuropeptide that modulates acid and gut peptide secretion. In addition, recent evidence indicates that bombesin is a potent gastroprotective agent. This review focuses on bombesin's ability to render the stomach more resistant to injury from luminal insults and summarizes the possible mechanisms involved.
Bombesin was first discovered in 1970 in extracts taken from the skin of two European amphibians, Bombina bombina and Bombina variegata.1 This 14-amino acid peptide was discovered to have a variety of pharmacologic effects in mammals, including the stimulation of both gastrin and cholecystokinin (CCK) release within the gastrointestinal tract.2 Bombesin was later noted to have a mammalian counterpart that was named gastrin-releasing peptide (GRP).3 Bombesin or GRP has since been cloned and is present in almost every species. Bombesin staining immunoreactivity has been detected throughout the digestive tract but is particularly prominent in both the acid- and the gastrin-secreting portions of the stomach.4,5 In the stomach, bombesin-containing neurons modulate acid secretion as well as the secretion of gastrin and somatostatin, which are functionally linked in the antrum.6–8 Interestingly, bombesin has recently been shown to increase the resistance of the gastric mucosa to injury.9,10 Consequently, this article reviews the ability of bombesin to act as a gastroprotective agent and examine possible gastroprotective mechanisms through its interaction with other gut peptides.
GUT PEPTIDES
Gastrin and CCK are endogenous gut peptides that participate in a variety of physiologic functions within the gastrointestinal tract.11 In addition to their other well-known effects, both gastrin and CCK have been shown to prevent gastric injury from luminal irritants when given exogenously in physiologic doses.9,11–14 The protective actions of gastrin and CCK are negated by administration of type B and type A CCK receptor antagonists, respectively.13–16 Because these receptor antagonists are highly selective in their effects, they provide powerful investigational tools to study the effects of exogenous, as well as endogenous, gastrin and CCK in a variety of biologic matrices.15,16
As previously mentioned, bombesin prevents gastric injury when given exogenously and is a potent stimulus for gastrin as well as CCK release.9,10,17 Thus, it was logical to hypothesize that the ability of bombesin to prevent gastric injury was linked to the release of endogenous gastrin or CCK. As a result, studies were undertaken in a conscious rat model of gastric injury to examine the role of endogenous gastrin and CCK in bombesin-induced gastroprotection by using the selective type A and type B CCK receptor antagonists.
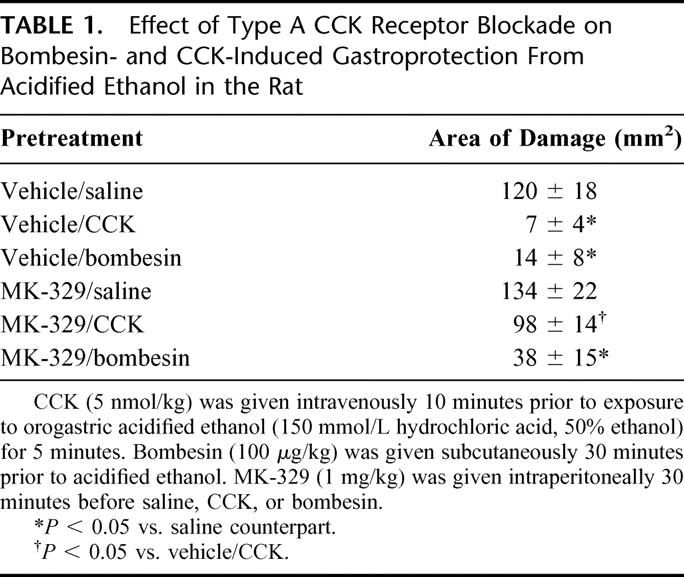
Bombesin was found to dose-dependently prevent acidified ethanol-induced gastric injury according to macroscopic and morphologic criteria.10 Administration of the type B CCK receptor antagonist l-365,260 was able to almost completely abolish bombesin-induced gastroprotection as well as exogenous gastrin-17-induced gastroprotection, as shown in Figure 1. In contrast, the type A CCK receptor antagonist MK-329 failed to reverse or significantly diminish the protective actions of bombesin but did negate CCK-induced gastroprotection (Table 1). Taken together, the receptor antagonist studies suggested that bombesin-induced gastroprotection is mediated primarily via the release of endogenous gastrin.10
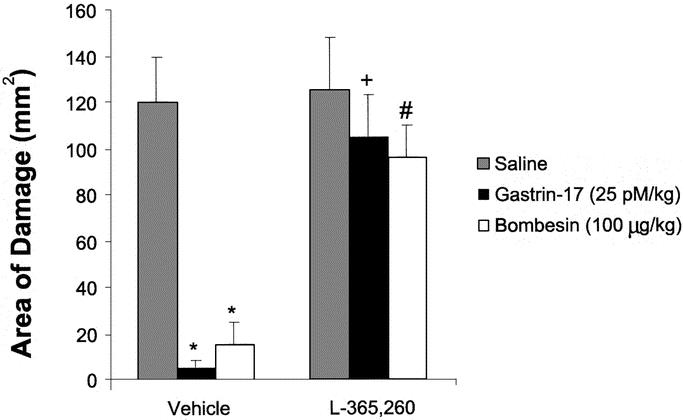
FIGURE 1. Effect of intraperitoneal type B CCK/gastrin receptor blockade (L-365,260) given 30 minutes before a 30-minute subcutaneous pretreatment with saline or bombesin (100 μg/kg) or a 10-minute intravenous treatment with gastrin-17 (25 pmol/kg) on macroscopic gastric injury from acidified ethanol; expressed as mean ± standard error of mean, n ≥ 5 for all groups. *P < 0.05 versus saline counterpart. +P < 0.05 versus vehicle/gastrin. #P < 0.05 versus vehicle/bombesin.
TABLE 1. Effect of Type A CCK Receptor Blockade on Bombesin- and CCK-Induced Gastroprotection From Acidified Ethanol in the Rat
More recent studies have demonstrated both endogenous and exogenous bombesin have a protective effect on intestinal microcirculation during ischemia-reperfusion injury in rats.18 In addition, bombesin has been shown to improve maintenance of gut mucosal integrity after severe burn by decreasing burn-induced gut mucosal atrophy and epithelial cell apoptosis.19,20 These studies demonstrate that bombesin may play a role in the intrinsic gastric mucosal defense system against a variety of luminal irritants.
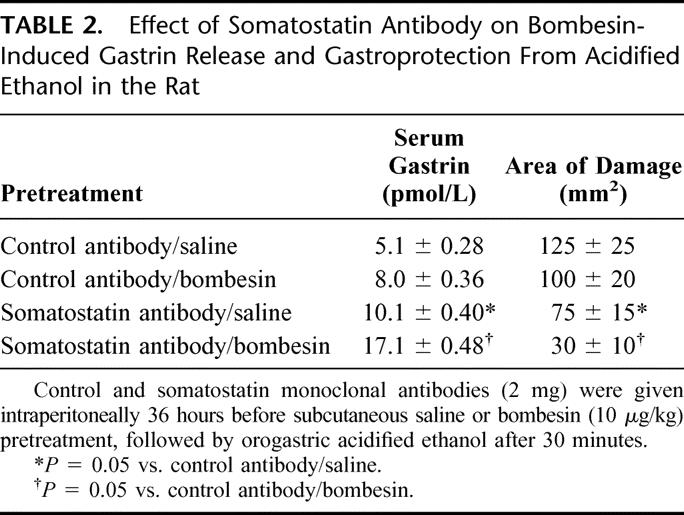
Because endogenous somatostatin exerts a tonic inhibitory effect on gastrin release,21 and because bombesin-induced gastrin released is enhanced in the presence of somatostatin monoclonal antibody,22 additional studies were undertaken to examine what effect immunoneutralization of endogenous somatostatin had on both bombesin-induced gastroprotection and gastrin release. It was hypothesized that if the protective actions of bombesin are dependent upon release of gastrin, then bombesin-induced gastroprotection and gastrin release should be augmented in the presence of somatostatin monoclonal antibody. This study clearly demonstrated that low-dose bombesin (10 μg/kg) had minimal, if any, gastroprotective actions and resulted in an insignificant increase in gastrin release.10 However, after somatostatin antibody, this same dose of bombesin resulted in significant gastroprotection and a more than 2-fold increase in serum gastrin levels (Table 2). These data indicate that bombesin-induced gastroprotection involves release of endogenous gastrin and that both the gastroprotective effects, as well as the release of endogenous gastrin, are modified by somatostatin. Under control conditions, endogenous somatostatin exerts a restraint on gastrin release such that low-dose bombesin is unable to prevent gastric injury from luminal irritants. However, when the tonic inhibitory restraint of somatostatin is removed, bombesin is able to maximally stimulate gastrin release and, as a result, even low doses of bombesin are able to prevent gastric injury.10
TABLE 2. Effect of Somatostatin Antibody on Bombesin-Induced Gastrin Release and Gastroprotection From Acidified Ethanol in the Rat
PROSTAGLANDINS AND SENSORY NEURONS
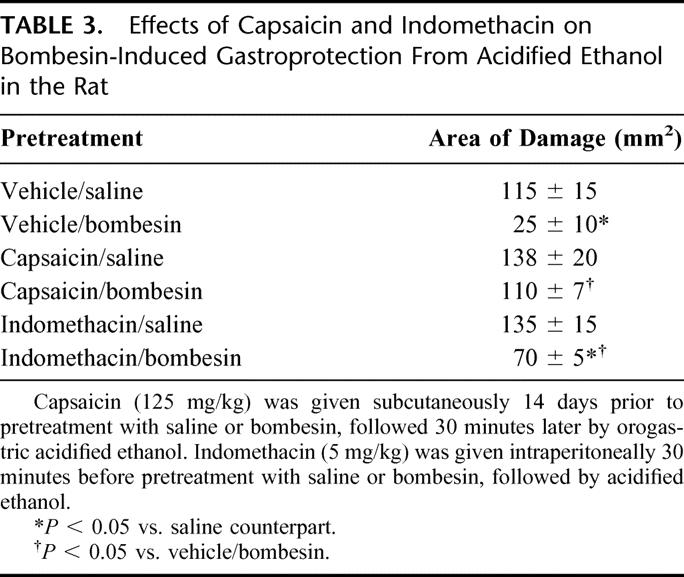
Sensory neurons and endogenous prostaglandins play an important role in gastric mucosal defense.23,24 The activation of sensory neurons in the gastric mucosa has been proposed to play a pivotal role in gastric mucosal defense through release of vasoactive neuropeptides with a resultant increase in gastric mucosal blood flow.25 Similarly, prostaglandins have been shown to prevent gastric injury and increase gastric mucosal blood flow.26 Because bombesin causes release of endogenous gastrin, which in turn increases gastric mucosal blood flow,27 the role of sensory neurons and prostaglandins as potential mediators of bombesin-induced gastroprotection was assessed. The role of capsaicin-sensitive afferent neurons was studied by functionally ablating these neurons with the selective neurotoxin capsaicin given in a desensitizing dose 2 weeks prior to experimentation. The role of prostaglandins was assessed by utilizing the cyclo-oxygenase inhibitor indomethacin. In these studies, functional ablation of capsaicin-sensitive afferent neurons negated bombesin-induced gastroprotection. In contrast, cyclo-oxygenase inhibition with indomethacin only partially reversed the protective effects of bombesin (Table 3). These findings indicated that bombesin requires intact capsaicin-sensitive afferent neurons as well as the presence of endogenous prostaglandins to fully exert its gastroprotective actions.28,29
TABLE 3. Effects of Capsaicin and Indomethacin on Bombesin-Induced Gastroprotection From Acidified Ethanol in the Rat
Because the gastroprotective actions of gastrin are also inhibited by capsaicin desensitization13 and because the gastroprotective actions of bombesin are primarily mediated via release of endogenous gastrin, it seems reasonable to conclude that bombesin elicits the release of endogenous gastrin, activating sensory neurons located locally within the gastric mucosa. In comparison, the incomplete reversal of bombesin-induced gastroprotection with indomethacin suggests that, in addition to prostaglandins, other mediators are also involved in this process. One logical candidate is nitric oxide. Both nitric oxide and prostaglandins share similar effects that may be related to their ability to maintain mucosal integrity. Moreover, evidence suggests that there is an interaction between endogenous prostaglandins and nitric oxide in gastric mucosal defense.30 Consequently, additional studies were undertaken to study the role of nitric oxide in bombesin-induced gastroprotection.
NITRIC OXIDE AND BLOOD FLOW
The importance of nitric oxide in mucosal defense has been illustrated by numerous investigators. It is a potent vasodilator and not surprisingly is involved with regulation of the gastric mucosal microcirculation.31 Nitric oxide, whether it be given exogenously or produced endogenously, has been shown to protect the gastric mucosa against injury from luminal irritants.32,33 It is produced by both constitutive and inducible isoforms of nitric oxide synthase (NOS). Most evidence suggests that the constitutive isoforms of NOS (endothelial and neural) primarily function to maintain homeostasis and have protective roles in the gastric mucosa.30,34–36 However, recent evidence suggests that the inducible isoforms of NOS may play a protective role in the stomach under certain conditions.37 Interestingly, there are considerable data indicating that the protective actions of sensory neurons in the gastric mucosa are mediated via increased production of nitric oxide.38,39 Because bombesin-induced gastroprotection is mediated primarily via release of endogenous gastrin and subsequent activation of capsaicin-sensitive sensory neurons, it was hypothesized that nitric oxide likely plays a role in mediating bombesin-induced gastroprotection. It was further hypothesized that bombesin-induced gastroprotection requires functioning constitutive neural or endothelial isoforms of NOS.
To study the role of NOS in bombesin-induced gastroprotection, studies were undertaken with the nonselective NOS inhibitor NG-nitro-l-arginine methyl ester (l-NAME) as well as the selective inducible NOS inhibitor aminoguanidine. In these studies, l-NAME, but not aminoguanidine, reversed the gastroprotective actions of bombesin (Table 4) and bombesin-induced increases in gastric mucosal blood flow (Fig. 2). The effects of l-NAME on bombesin-induced gastroprotection as well as gastric mucosal blood flow were reversed by l- but not d-arginine. These studies suggested that bombesin-induced gastroprotection and gastric hyperemia are mediated via increased release of nitric oxide produced from the constitutive isoforms of NOS as opposed to the inducible isoform because aminoguanidine failed to reverse or attenuate the effects of bombesin. When the role of NOS isoforms was further examined, with Western immunoblot analysis of gastric NOS isoforms, bombesin increased endothelial NOS immunoreactivity but not neural or inducible NOS immunoreactivity. These latter findings suggested that it is nitric oxide produced from the endothelial isoform of NOS that participates in the ability of bombesin to increase gastric mucosal blood flow and maintain the integrity of the gastric epithelium in the face of a damaging luminal insult.40
TABLE 4. Effects of Nitric Oxide Synthase Inhibition on Bombesin-Induced Gastroprotection From Acidified Ethanol in the Rat
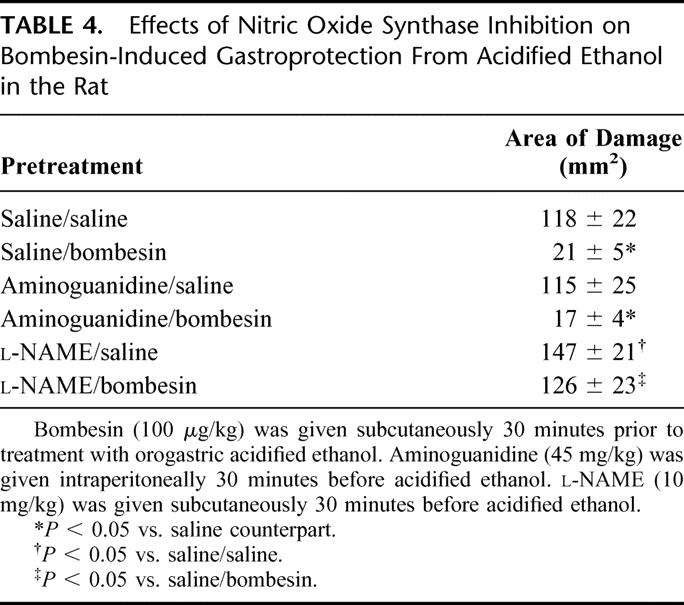
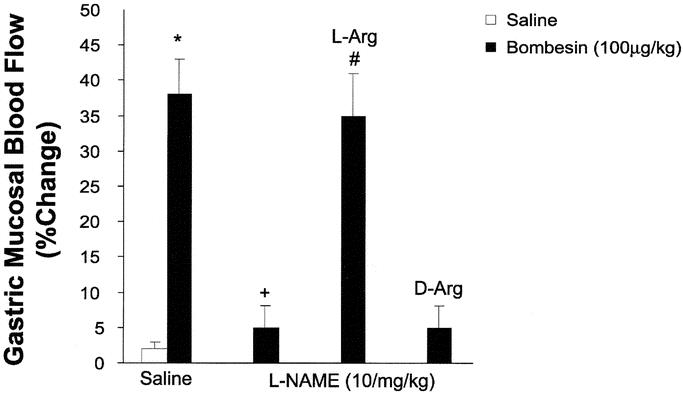
FIGURE 2. Effect of nitric oxide synthase inhibition with subcutaneous l-NAME (10 mg/kg) and the effect of l-NAME combined with intraperitoneal l-arginine (l-Arg; 300 mg/kg) or d-arginine (d-Arg; 300 mg/kg) on bombesin (100 μg/kg)-induced gastric hyperemia. Data are presented as percent change from baseline blood flow determinations and represent the mean ± SEM; n ≥ 5/group. *P < 0.05 versus saline counterpart. +P < 0.05 versus saline/bombesin. #P < 0.05 versus l-NAME/bombesin.
CONCLUSION
Bombesin, gastrin, somatostatin, sensory neurons, prostaglandins, and nitric oxide, among many substances, interact and have been implicated in mucosal defense. Interestingly, most of these peptides or mediators cause an increase in gastric mucosal blood flow. In addition to supplying nutrients and oxygen to the epithelium, the microcirculation functions to dilute or remove toxic substances that diffuse into the mucosa from the gastric lumen.41,42 Increases in blood flow also assure that those intracellular processes that underlie mucosal resistance to injury can proceed unabated.
The proposed mechanism responsible for bombesin-induced hyperemia and gastroprotection is shown schematically in Figure 3. Bombesin causes the release of endogenous gastrin, the subsequent activation of sensory neurons, and perhaps increases cyclo-oxygenase activity as well. Activation of sensory neurons in turn increases production of nitric oxide through activation of constitutive NOS isoforms, and increased cyclo-oxygenase activity leads to increased production of prostaglandins. Both nitric oxide and prostaglandins then act upon the gastric microcirculation to cause an increase in gastric mucosal blood flow. Thus, it appears that bombesin-induced gastroprotection involves the release of gut peptides that interact with sensory neurons, nitric oxide, and prostaglandins to augment gastric mucosal blood flow that enables the gastric mucosa to withstand damaging luminal insults.
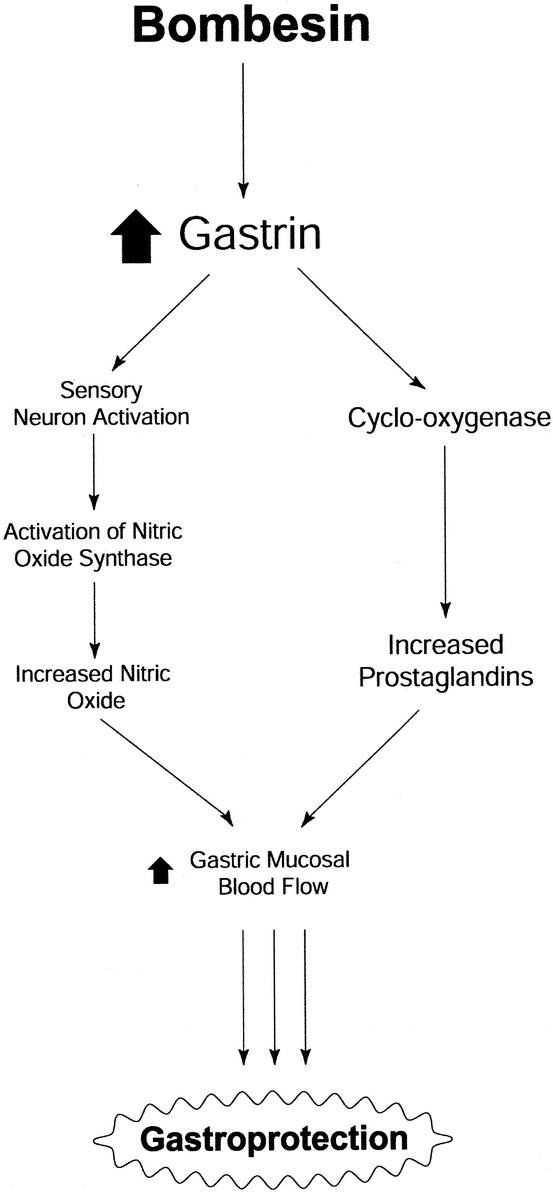
FIGURE 3. Schematic representation of mechanism(s) responsible for bombesin-induced gastroprotection.
Footnotes
Supported by NIH grant no. DK50445 and NIGMS grant no. GM 08792.
Reprints: David W. Mercer, MD, UT-Houston Medical School/LBJ General Hospital, 5656 Kelley Street, Suite 3-OS 62008, Houston, TX 77026. E-mail: david.w.mercer@uth.tmc.edu.
REFERENCES
- 1.Erspamer V, Erspamer GF, Inselvini M. Some pharmacological actions of alytesin and bombesin. J Pharm Pharmacol. 1970;22:875–876. [DOI] [PubMed] [Google Scholar]
- 2.Erspamer V, Erspamer GF, Inselvini M, et al. Occurrence of bombesin and alytesin in extracts of the skin of three European discoglossid frogs and pharmacological actions of bombesin on extravascular smooth muscle. Br J Pharmacol. 1972;45:333–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McDonald TJ, Nilsson G, Vagne M, et al. A gastrin releasing peptide from the porcine nonantral gastric tissue. Gut. 1978;19:767–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Greeley GH Jr, Partin M, Spannage A, et al. Distribution of bombesin-like peptides in the alimentary canal of several vertebrate species. Regul Pept. 1986;16:169–181. [DOI] [PubMed] [Google Scholar]
- 5.Richelsen B, Rehfeld JF, Larsson LI. Antral gland cell column: a method for studying release of gastric hormones. Am J Physiol. 1983;245:G463–G469. [DOI] [PubMed] [Google Scholar]
- 6.Dockray GJ, Vaillant C, Walsh JH. The neuronal origin of bombesin-like immunoreactivity in the rat gastrointestinal tract. Neuroscience. 1979;4:1561–1568. [DOI] [PubMed] [Google Scholar]
- 7.Walsh JH. Why does the gastric oxyntic gland mucosa contain gastrin-releasing peptide nerve fibers? Gastroenterology. 1989;97:793–795. [DOI] [PubMed] [Google Scholar]
- 8.Saffouri B, Weir GC, Bitar KN, et al. Gastrin and somatostatin secretion by perfused rat stomach: functional linkage of antral peptides. Am J Physiol. 1980;238:G495–G501. [DOI] [PubMed] [Google Scholar]
- 9.Evangelista S, Maggi CA, Meli A. Influence of peripherally administered peptides on ethanol-induced gastric ulcers in the rat. Gen Pharmacol. 1987;18:647–649. [DOI] [PubMed] [Google Scholar]
- 10.Mercer DW, Cross JM, Chang LK, et al. Bombesin prevents gastric injury in the rat: role of gastrin. Dig Dis Sci. 1998;43:836–833. [DOI] [PubMed] [Google Scholar]
- 11.Walsh JH. Gastrointestinal hormones. In: Johnson LR, ed. Physiology of the Gastrointestinal Tract, vol. 1, 2nd ed. New York: Raven, 1987:181–253. [Google Scholar]
- 12.Mercer DW, Klemm K, Cross JM, et al. Cholecystokinin-induced protection against gastric injury is independent of endogenous somatostatin. Am J Physiol. 1996;271:G692–G700. [DOI] [PubMed] [Google Scholar]
- 13.Mercer DW, Cross JM, Smith GS, et al. Protective action of gastrin-17 against alcohol-induced gastric injury in the rat: role in mucosal defense. Am J Physiol. 1997;273:G365–G373. [DOI] [PubMed] [Google Scholar]
- 14.Mercer DW, Cross JM, Barreto JC, et al. Cholecystokinin is a potent protective agent against alcohol-induced gastric injury in the rat: role of endogenous prostaglandins. Dig Dis Sci. 1995;40:651–660. [DOI] [PubMed] [Google Scholar]
- 15.Chang RSL, Lotti VJ. Biochemical and pharmacological characterization of an extremely potent and selective non-peptide cholecystokinin antagonist. Proc Natl Acad Sci USA. 1986;83:4923–4926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lotti VJ, Chang RSL. A new potent and selective non-peptide gastrin antagonist and brain cholecystokinin receptor (CCK-B) ligand: L-365,260. Eur J Pharmacol. 1989;162:273–280. [DOI] [PubMed] [Google Scholar]
- 17.Mercer DW, Cross JM, Smith GS, et al. Do endogenous gut peptides play a role in gastric mucosal defense? Gastroenterology. 1996;110:A194. [Google Scholar]
- 18.Heuser M, Pfaar O, Gralla O, et al. Impact of gastrin-releasing peptide on intestinal microcirculation after ischemia-reperfusion in rats. Digestion. 2000;61:172–180. [DOI] [PubMed] [Google Scholar]
- 19.Wu XW, Spies M, Chappell VL, et al. Effect of bombesin on gut mucosal impairment after severe burn. Shock. 2002;18:518–522. [DOI] [PubMed] [Google Scholar]
- 20.Alican I, Unluer E, Yegen C, et al. Bombesin improves burn-induced intestinal injury in the rat. Peptides. 2000;21:1265–1269. [DOI] [PubMed] [Google Scholar]
- 21.Chiba TS, Kadowaki S, Taminato T, et al. Effect of antisomatostatin gammaglobulin on gastrin release in rats. Gastroenterology. 1981;81:321–326. [PubMed] [Google Scholar]
- 22.DuVal JW, Saffouri B, Weir GC, et al. Stimulation of gastrin and somatostatin secretion from the isolated rat stomach by bombesin. Am J Physiol. 1981;241:G242–G247. [DOI] [PubMed] [Google Scholar]
- 23.Evangelista S, Maggi CA, Meli A. Involvement of capsaicin-sensitive mechanism(s) in the antiulcer defense of intestinal mucosa in rats. Proc Soc Exp Biol Med. 1987;184:264–266. [DOI] [PubMed] [Google Scholar]
- 24.Miller TA. Protective effects of prostaglandins against gastric mucosal damage: current knowledge and proposed mechanisms. Am J Physiol. 1983;245:G601–G623. [DOI] [PubMed] [Google Scholar]
- 25.Holzer P, Peskar BM, Peskar BA, et al. Release of calcitonin gene-related peptide induced by capsaicin in the vascularly perfused rat stomach. Neurosci Lett. 1990;108:195–200. [DOI] [PubMed] [Google Scholar]
- 26.Ritchie WP, Mercer DW. Mediators of bile acid-induced alterations in gastric mucosal blood flow. Am J Surg. 1991;161:126–130. [DOI] [PubMed] [Google Scholar]
- 27.Stroff T, Plate S, Respondek M, et al. Protection by gastrin in the rat stomach involves afferent neurons, calcitonin gene-related peptide, and nitric oxide. Gastroenterology. 1995;109:89–97. [DOI] [PubMed] [Google Scholar]
- 28.Mercer DW, Cross JM, Castaneda AA, et al. Gastroprotective actions of bombesin, L-DOPA, and mild irritants: roles of prostaglandins and sensory neurons. Surgery. 1998;124:864–870. [PubMed] [Google Scholar]
- 29.Peskar BM. Neural aspects of prostaglandin involvement in gastric mucosal defense. J Physiol Pharmacol. 2001;52:555–568. [PubMed] [Google Scholar]
- 30.Whittle BJR, Lopez-Belmonte J, Moncada S. Regulation of gastric mucosal integrity by endogenous nitric oxide: interactions with prostanoids and sensory neuropeptides in the rat. Br J Pharmacol. 1990;99:607–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Whittle BJR. Neuronal and endothelium-derived mediators in the modulation of the gastric microcirculation: integrity in the balance. Br J Pharmacol. 1993;110:3–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Masuda E, Kawano S, Nagano K, et al. Endogenous nitric oxide modulates ethanol-induced gastric mucosal injury in rats. Gastroenterol Jpn. 1995;108:58–64. [DOI] [PubMed] [Google Scholar]
- 33.Lopez-Belmonte J, Whittle BJR, Moncada S. The actions of nitric oxide donors in the prevention or induction of injury to the rat gastric mucosa. Br J Pharmacol. 1993;108:73–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.MacNaughton WK, Cirino G, Wallace JL. Endothelium derived relaxing factor (nitric oxide) has protective actions in the stomach. Life Sci. 1989;45:1869–1876. [DOI] [PubMed] [Google Scholar]
- 35.West SD, Helmer KS, Chang LK, et al. Cholecystokinin secretagogue-induced gastroprotection: role of nitric oxide and blood flow. Am J Physiol. 2003;284:G399–G410. [DOI] [PubMed] [Google Scholar]
- 36.Helmer KS, West SD, Shipley GL, et al. Gastric nitric oxide synthase expression during endotoxemia: implications in mucosal defense. Gastroenterology. 2002;123:173–186. [DOI] [PubMed] [Google Scholar]
- 37.Tepperman BL, Soper BD. Nitric oxide synthase induction and cytoprotection of rat gastric mucosa from injury by ethanol. Can J Physiol Pharmacol. 1994;72:1308–1312. [DOI] [PubMed] [Google Scholar]
- 38.Tepperman BL, Whittle BJ. Endogenous nitric oxide and sensory neuropeptides interact in the modulation of the rat gastric microcirculation. Br J Pharmacol. 1992;105:171–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lambrecht N, Burchert M, Respondek M, et al. Role of calcitonin gene-related peptide and nitric oxide in the gastroprotective effect of capsaicin in the rat. Gastroenterology. 1993;104:1371–1380. [DOI] [PubMed] [Google Scholar]
- 40.Castaneda AA, Kim YS, Chang LK, et al. Nitric oxide synthase inhibition negates bombesin-induced gastroprotection. Surgery. 2000;128:422–428. [DOI] [PubMed] [Google Scholar]
- 41.Menguy R, Desbaillets L, Masters YF. Mechanisms of stress ulcer: influence of hypovolemic shock on energy metabolism in the gastric mucosa. Gastroenterology. 1974;66:46–55. [PubMed] [Google Scholar]
- 42.Starlinger M, Schiessel R, Hung CR, et al. H+ back diffusion stimulating gastric mucosal blood flow in the rabbit fundus. Surgery. 1981;89:232–236. [PubMed] [Google Scholar]


