Summary
Nephronophthisis, an autosomal-recessive cystic kidney disease, is the most frequent monogenic cause for renal failure in childhood. Infantile and juvenile forms of nephronophthisis are known to originate from separate gene loci. We describe here a new disease form, adolescent nephronophthisis, that is clearly distinct by clinical and genetic findings. In a large, 340-member consanguineous Venezuelan kindred, clinical symptoms and renal pathology were evaluated. Onset of terminal renal failure was compared with that in a historical sample of juvenile nephronophthisis. Onset of terminal renal failure in adolescent nephronophthisis occurred significantly later (median age 19 years, quartile borders 16.0 and 25.0 years) than in juvenile nephronophthisis (median age 13.1 years, quartile borders 11.3 and 17.3 years; Wilcoxon test P=.0069). A total-genome scan of linkage analysis was conducted and evaluated by LOD score and total-genome haplotype analyses. A gene locus for adolescent nephronophthisis was localized to a region of homozygosity by descent, on chromosome 3q22, within a critical genetic interval of 2.4 cM between flanking markers D3S1292 and D3S1238. The maximum LOD score for D3S1273 was 5.90 (maximum recombination fraction .035). This locus is different than that identified for juvenile nephronophthisis. These findings will have implications for diagnosis and genetic counseling in hereditary chronic renal failure and provide the basis for identification of the responsible gene.
Introduction
Nephronophthisis, a hereditary cystic kidney disease, is the most frequent monogenic cause of chronic renal failure in children (Kleinknecht 1989; Fivush et al. 1998). Fanconi et al. introduced the term “familial juvenile nephronophthisis” (NPH1 [MIM 256100]) to describe a disease characterized by autosomal-recessive inheritance, a defect in urinary concentrating capacity, severe anemia, and progressive renal failure that leads to death before puberty (Smith and Graham 1945; Fanconi et al. 1951). Renal histology is characterized by disintegrated tubular basement membranes, tubular atrophy and cyst formation, and a sclerosing tubulointerstitial nephropathy (Zollinger et al. 1980; Waldherr et al. 1982). Corticomedullary cysts occur late in the disease process (Blowey et al. 1996). In NPH1, the responsible gene (NPHP1), which is localized on chromosome 2q12-q13 (Antignac et al. 1993; Hildebrandt et al. 1993b), has recently been identified by positional cloning (Hildebrandt et al. 1997a; Saunier et al. 1997). Its gene product, nephrocystin, is novel and encodes an src homology 3 (SH3) domain. In ∼85% of patients with NPH1, large homozygous deletions of this gene are found (Konrad et al. 1996). In contrast, infantile nephronophthisis (NPH2 [MIM 602088]) leads to terminal renal failure within the first 3 years of life (Bodaghi et al. 1987; Gagnadoux et al. 1989; Haider et al. 1998). Morphologically, this type of hereditary tubulointerstitial nephropathy differs from NPH1 by the presence of cortical microcysts and by the absence of medullary cysts and typical tubular–basement membrane changes. A gene locus for NPH2 has been localized to 9q22-q31 (Haider et al. 1998).
Only in the recessive form of the disease have occasionally extrarenal associations—such as tapetoretinal degeneration, cerebellar ataxia, skeletal involvement, and liver disease—been described (Loken et al. 1961; Senior et al. 1961; Mainzer et al. 1970; Boichis et al. 1973; Antignac et al. 1998; Hildebrandt 1999). A gene locus for nephronophthisis associated with extrarenal disease manifestation has not yet been found.
The term “autosomal-dominant medullary cystic disease” (ADMCKD) has been used for a disease indistinguishable, by pathological means, from recessive nephronophthisis (Goldman et al. 1966; Strauss and Sommers 1967). Two loci for the autosomal-dominant disease, ADMCKD1 (MIM 174000) on chromosome 1q21 (Christodoulou et al. 1998) and ADMCKD2 (MIM 603860) on chromosome 16p12 (Scolari et al. 1999), have been described. Besides the different inheritance pattern, the main feature distinguishing between recessive and dominant disease conditions has been a different age at onset of terminal renal failure. Because of the morphological similarities of the above-described diseases, the term “nephronophthisis/medullary cystic disease complex” has been used to summarize this group of diseases (Gardner 1971).
We here describe a large Venezuelan kindred with a hereditary kidney disease of the nephronophthisis/medullary cystic disease complex. Inheritance appeared to be autosomal recessive, and renal failure was reached, in many cases, at age >25 years. This newly identified disease form was termed “adolescent nephronophthisis,” since it is clearly distinct from NPH1, by age at onset and by a separate gene locus.
Subjects and Methods
Pedigree and Clinical Evaluation
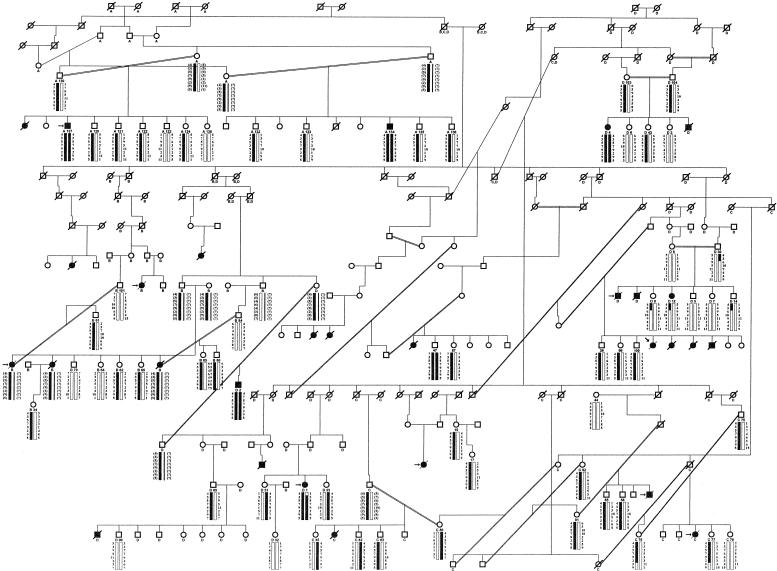
In a large, 340-member Venezuelan pedigree (fig. 1), blood samples were obtained, after informed consent was given, from 112 family members, including 6 affected individuals. Medical records were reviewed for all patients from the kindred who had received treatment <12 years previously at the Hospital of the Universidad de Los Andes, Merida, Venezuela. Specifically, family history, presenting symptoms, serum creatinine measurements at the first medical visit, results from renal ultrasound, and age at start of first renal replacement therapy were noted.
Figure 1.
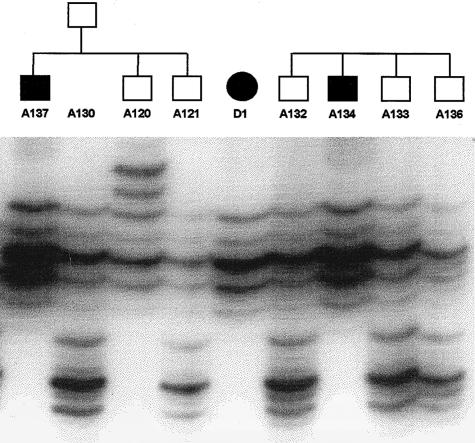
Haplotypes and recombination in the Venezuelan kindred with adolescent nephronophthisis. Genotypes are shown in centromere-to-telomere order (top to bottom) for microsatellite markers D3S1292, D3S1587, D3S1273, D3S1290, D3S3713, D3S3657, D3S1238, and D3S3684 on chromosome 3q22. Inferred alleles are shown in parentheses. For genotypes represented by a question mark (?), no data were available. Haplotypes were assembled by minimization of recombinants, and the chromosomal region cosegregating with the disease locus is represented by a blackened bar. No attempt has been made to show crossovers on the nondisease chromosome, depicted by an unblackened bar. Vertical positioning of pedigree members does not represent generational order, because of extended consanguinity within the pedigree. Consanguineous relationships are depicted by a double line. Individuals whose numerical designations are below their symbols were available for genotyping. In the index case (left, above arrow) a nephrectomy specimen, and in seven individuals a renal biopsy (left arrow), were available. For LOD-score analysis, the pedigree was fragmented into four subsets (A–D). Characters below symbols identify the individuals who were used for linkage calculation in each subset. Circles denote females, squares males, blackened symbols affected individuals, unblackened symbols unaffected individuals, and symbols with a slash deceased family members. Note that D3S1587, D3S1273, D3S1290, D3S3713, and D3S3657 are cosegregating markers, which are compatible with linkage in five affected individuals, as well as in all facultative and obligate carriers.
Statistical Analysis of Age at Renal Failure
For life-table analysis, “renal death” was defined as start of renal replacement therapy, occurrence of a serum creatinine >5.5 mg/dl, or death caused by uremia. The cumulative probability of terminal renal failure was determined for adolescent nephronophthisis and was compared with that in a historical sample of genetically proved NPH1 (Hildebrandt et al. 1997b), by use of the life-table method (“survival analysis”) of Kaplan and Meier (1958). The data were compared by use of the Wilcoxon test (Gretz 1994).
Histopathology
Renal biopsy specimens from eight affected individuals, including one nephrectomy specimen (index case), which had been obtained previously for diagnostic purposes, were reevaluated by use of standard histopathology staining procedures.
Total-Genome Scan
Genomic DNA was isolated by standard methods, directly from blood samples (Sambrook et al. 1989) or from peripheral blood lymphocytes after Epstein-Barr virus transformation (Steel et al. 1977). Total-genome linkage analysis was performed by use of 297 microsatellite markers from the MDC-microsatellite set, with an average spacing of 11 cM and, for further fine mapping, with 20 additional microsatellites from the region of 3q22 (Dib et al. 1996; Broman et al. 1998). Microsatellite markers were amplified individually by use of an MJ Research Multicycler PTC225 Tetrad (Biozym). After pooling, markers were separated on an ABI377XL DNA sequencer and were evaluated by use of GENESCAN software, as described elsewhere (Saar et al. 1997).
The total-genome scan was performed in a subset of the kindred (fig. 1), containing 6 affected (individuals A137, A134, D4, D2, D1, and D12) and 18 unaffected individuals (individuals A130, A121, A138, A132, A133, A135, A136, D103, D104, D43, D3, D8, D30, D9, D5, D7, D31, and D33). The results were evaluated by analyses of two-point LOD scores between the disease locus and microsatellite markers, by use of the MLINK subroutine of the computer program package FASTLINK (Lathrop et al. 1984; Schaffer 1996) and with help of the program LODVIEW (Hildebrandt et al. 1993a). The ILINK component of the FASTLINK package was used to calculate maximum LOD scores (Zmax) at various recombination fractions (θ). A high number of inbreeding loops increased the computational time enormously. Therefore, LOD-score analysis was performed after fragmentation of the large pedigree into four subsets (A–D) with preservation of most of the inbreeding loops (fig. 1). To test for the potential influence of fragmentation of the pedigree, we performed linkage analyses in various subsets of the pedigree, thus demonstrating the robustness of the strategy used. For linkage analysis, minimal diagnostic criteria for affected status were defined on the basis of a priori clinical criteria. An individual was regarded as affected by adolescent nephronophthisis only if the following diagnostic criteria were fulfilled: (1) terminal renal failure was present, (2) the individual belonged to the kindred, (3) results of urine analysis were normal, and (4) urinary-tract obstruction was not seen on sonography. The diagnosis was supported by the availability of eight biopsy specimens compatible with nephronophthisis in members from the same pedigree. An individual was considered to be likely not affected by adolescent nephronophthisis if the serum creatinine measurement was normal. Because affected status is age dependent, for these individuals an age-dependent risk was assigned. In accordance with data on age-related penetrance in this pedigree (fig. 2), liability classes were assigned as follows: affected subjects with adolescent nephronophthisis were assigned liability class 1 with penetrance vectors {.0000; .0000; 1.0000}; unaffected individuals age >47 years were assigned liability class 2 {.0000; .0000; .9900}, age 26–46 years liability class 3 {.0000; .0000; .9000}, age 20–25 years liability class 4 {.0000; .0000; .7000}, age 16–19 years liability class 5 {.0000; .0000; .5000}, age 13–15 years liability class 6 {.0000; .0000; .3000}, and age 11–13 years liability class 7 {.0000; .0000; .1000}. Unaffected individuals age <11 years were not included in the linkage analysis because their disease status was unknown. The gene frequency of adolescent nephronophthisis was set at .001. Allele frequencies of microsatellite markers were arbitrarily assigned a value of 1/n, where n refers to the number of alleles observed. Because the use of incorrect values for linkage analysis can cause false-positive results, LOD scores were calculated in a second model, using only data on affected individuals and thus testing for robustness of linkage. Linkage analysis and total-genome haplotype analysis were performed with the program GENEHUNTER (Kruglyak et al. 1996). For graphic representation of pedigree and haplotype data, the program CYRILLIC, version 2.0, was used (Cherwell Scientific).
Figure 2.
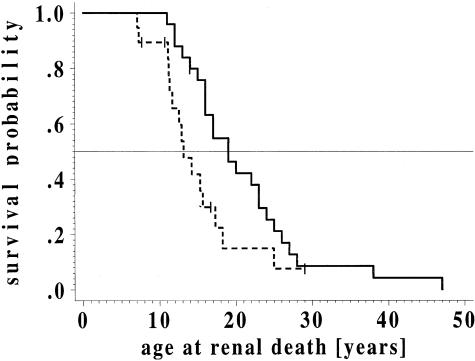
Cumulative probability of terminal renal failure. The cumulative probability of terminal renal failure was determined for 24 patients from the large kindred with adolescent nephronophthisis (solid lines) and was compared with that in a historical sample of genetically proved NPH1 (dashed lines [Hildebrandt et al. 1997b]), by use of the life-table method of Kaplan and Meier (1958). The data were compared by use of the Wilcoxon test. In the group with adolescent nephronophthisis, one patient, and, in the group with NPH1, four patients, were censored (vertical strokes), because terminal renal failure did not occur during the observation period.
Results
Pedigree and Clinical Characteristics
The large 340-member kindred with a hereditary kidney disease originates from a remote area in the Venezuelan Andes. A 247-member subset of this pedigree is shown in fig. 1. Fifty-five affected individuals were descendants of known consanguineous relationships, with 20 consanguinity loops identified altogether. Sex distribution among affected individuals showed a preponderance of females, with a ratio of 2.2:1 (20 females and 9 males). The percentage of affected siblings was 29.4% when only families with at least one affected child and more than two children were considered. This finding, together with the lack of direct disease transmission and the high degree of consanguinity, strongly suggests an autosomal-recessive mode of inheritance.
Twenty-three of 24 patients presented with signs of terminal renal failure and showed a constellation of symptoms typical of nephronophthisis: most of the patients suffered from anemia when they first came to medical attention. Eight of 24 patients had a history typical of nephronophthisis, with symptoms of polydipsia, polyuria, and secondary enuresis. Urine microscopy revealed no leukocyturia or hematuria at admission. No proteinuria was detected. Renal ultrasound showed no urinary-tract anomalies. Fundoscopic examination did not reveal signs of retinitis pigmentosa. No other associated extrarenal disease manifestation, including gout, was noted.
Life-table analysis for onset of terminal renal failure yielded a median age at “renal death” of 19.0 years (quartiles are 25%, 16.0 years; and 75%, 25.0 years), whereby “renal death” was defined as the start of renal replacement therapy, serum creatinine >5.5 mg/dl, or death due to uremia. The resulting survival curve showed no major plateau formation, indicating homogeneity within the group of patients with adolescent nephronophthisis (fig. 2). Comparison of life-table data from this pedigree versus those for a historical sample of molecular-genetically proved NPH1 revealed a significant difference, in the age at onset of terminal renal failure, for both diseases (Wilcoxon test, P=.0069). For comparison, life-table analysis for NPH1 yielded, for onset of terminal renal failure, a median age of 13.1 years (quartiles are 25%, 11.3 years; and 75%, 17.3 years [Hildebrandt et al. 1997b]). Thus, median age at onset of terminal renal failure occurred 5.9 years later in this kindred than in patients with NPH1. To distinguish between the two diseases, we use the term “adolescent nephronophthisis” for the disease variant observed in this kindred.
Pathology
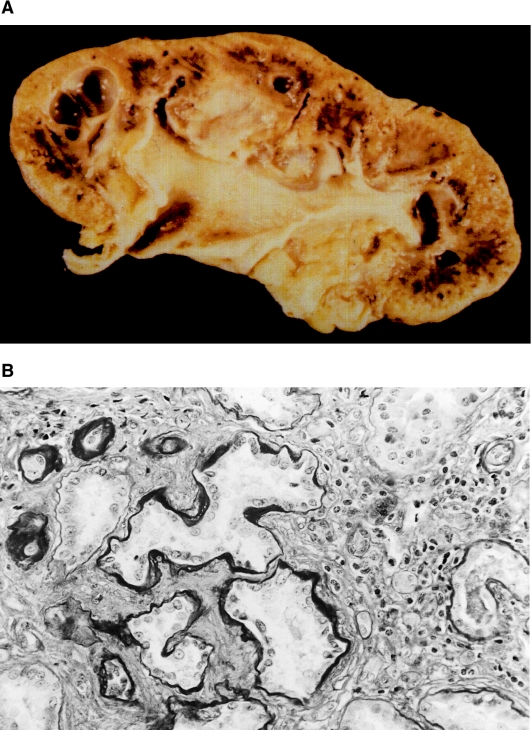
Affected individuals of the kindred exhibited typical macroscopic and microscopic features of nephronophthisis. In the index case, a nephrectomy specimen was examined (fig. 3, top). The small end-stage kidney has a pitted, granular surface. The cortex is thin and fibrotic. The cut surface shows multiple rounded cysts localized mainly at the corticomedullary border of the kidney. Previous histological examination of renal biopsies by a local pathologist had described a severe sclerosing tubulointerstitial nephropathy in all cases. Renal histology for three individuals was reviewed. All specimens showed alterations typical of nephronophthisis (fig. 3, bottom). Tubular basement membranes are altered, with segments of thickening, thinning, and folding and a multilayered appearance. Tubules are atrophic and are lined by a flattened dedifferentiated epithelium. Distal tubules seem to be predominantly affected, whereas proximal tubules are well preserved in many areas. Tubules are surrounded by mild to moderate mononuclear infiltrates and by diffuse interstitial fibrosis with an increase in collagen fibers (fig. 3, bottom). Many glomeruli are completely obsolescent. Nonsclerosed glomeruli frequently disclose both retraction of their tufts and concentric periglomerular fibrosis with thickening of Bowman's capsule. Glomerular mesangial cellularity is within the normal range, and basement membranes of nonsclerotic glomeruli appear normal. In all specimens, prominent vascular changes are present and consist of thickening of arteriolar and arterial walls, which is due to medial hypertrophy. Concentric intimal fibrosis with narrowing of the arterial lumina is prominent in two biopsies and in the nephrectomy sections. We conclude that histological findings in adolescent nephronophthisis are generally not distinguishable from those in NPH1.
Figure 3.
Macroscopic and microscopic pathology in adolescent nephronophthisis. A, Shrunken kidney with cyst formation predominantly at the corticomedullary junction in a nephrectomy specimen from the index case. The cortex is thin and fibrotic. B, PAS-stained renal biopsy specimen from the same individual, with irregularity in thickness of tubular basement membranes, with tubular atrophy and ectasia. In addition, there are mononuclear interstitial infiltrates and marked interstitial fibrosis. Original magnification x100.
Linkage Analysis
Before performing a genomewide search for linkage in the large Venezuelan kindred, we evaluated potential candidate genes. Haplotype analysis was performed by use of markers within and flanking the critical regions for infantile and NPH1 on chromosomes 9q22-q31 and 2q12-q13, respectively. These loci were excluded from linkage, indicating that adolescent nephronophthisis is not allelic with either of the two diseases (data not shown).
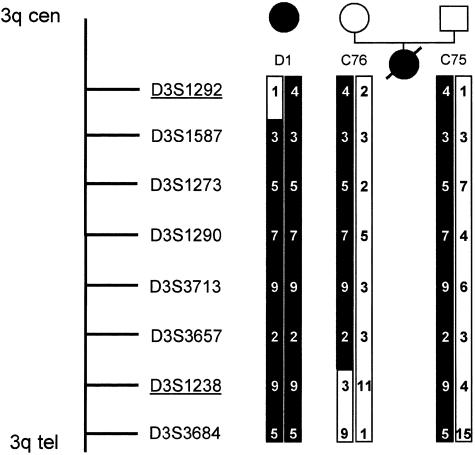
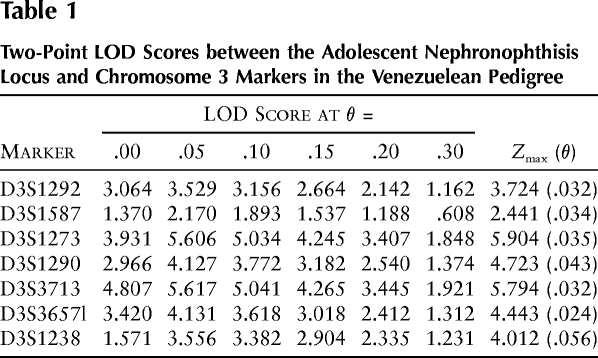
The total-genome scan was performed in a small subset of the kindred, containing 6 affected and 18 unaffected individuals. This screening procedure yielded a two-point Zmax of 1.60 (θ=.05) at marker D3S1267 on the long arm of chromosome 3, as calculated by GENEHUNTER. Genotyping data were consistent with a region of homozygosity by descent in three affected individuals. By fine mapping of a 15.1-cM region flanked by markers D3S1267 and D3S3554, a homozygous region was identified in five of six affected individuals. When haplotype analyses were evaluated, a proximal recombination event was detected for marker D3S1292 in individual D1, who is affected by adolescent nephronophthisis, and a distal recombination event was detected for marker D3S1238 in individual C76, who is an obligate heterozygous carrier of the disease haplotype (fig. 4). These two flanking markers define a critical interval of 2.4 cM of sex-averaged genetic distance (Collins et al. 1996). To illustrate homozygosity by descent, we showed the PCR products for the marker D3S1238, for a subset of the examined pedigree (fig. 5). Markers D3S1587, D3S1273, D3S1290, D3S3713, and D3S3657 are cosegregating markers compatible with linkage in five affected individuals and in all facultative and obligate carriers of the Venezuelan kindred (fig. 1). Proband 47 homozygously carries the haplotype associated with affected status. However, at age 10 years, affected status was uncertain. Only in affected individual D12 was no cosegregating marker identified, but individual D12 is partially heterozygous for the disease-associated allele. When reviewing the clinical findings on individual D12 and other close relatives, we found development of hearing deficit and intermittent hematuria in one of the deceased brothers and in individual D12. Data on the other affected sibling were not available. Since individual D12 was diagnosed as affected on the basis of a priori criteria, she was considered to be affected in all LOD-score calculations. Parametric two-point LOD-score analysis yielded a Zmax of 5.90 (θ=.035) with marker D3S1273, giving strongly significant evidence for linkage. When, as a test for robustness, only haplotype data on affected individuals were used, the Zmax was 3.57 (θ=.050) with marker D3S1273, which is still significant for linkage. The LOD-score results for markers D3S1292, D3S1587, D3S1273, D3S1290, D3S3713, D3S3657, and D3S1238 are summarized in table 1.
Figure 4.
Genetic map of markers of the critical region for adolescent nephronophthisis. The haplotype, depicted as a blackened bar, cosegregates with the disease. The disease interval is flanked by markers D3S1292 and D3S1238 and contains a 2.4-cM interval because of recombination events that occurred in individual D1 and individual C76, an obligate heterozygote.
Figure 5.
Autoradiograph of PCR products of marker D3S1238, for a subset of individuals of the presented pedigree. Affected individuals (A137, D1, and A134) are homozygous for the disease-associated allele, which they inherited through homozygosity by descent. One parent (A130) and unaffected siblings are heterozygous for this allele.
Table 1.
Two-Point LOD Scores between the Adolescent Nephronophthisis Locus and Chromosome 3 Markers in the Venezuelean Pedigree
Discussion
This clinical, pathological, and molecular-genetic study identifies adolescent nephronophthisis as a novel disease entity of the nephronophthisis/medullary cystic disease complex. Histopathological features and clinical symptoms are found to be virtually identical to those of NPH1. However, in adolescent nephronophthisis, median age at onset of terminal renal failure is significantly retarded, by 6 years, in comparison with that in NPH1. Through identification of a distinct gene locus on chromosome 3q22, we clearly defined adolescent nephronophthisis as a separate disease entity.
If adolescent nephronophthisis is considered within the context of other entities of the nephronophthisis/medullary cystic disease complex, three groups of diseases must be taken into account. The first group of diseases comprises recessive, purely renal forms of nephronophthisis. Since clinical symptoms, renal histology, and mode of inheritance in adolescent nephronophthisis are virtually indistinguishable from those in NPH1, it is justifiable to classify this new disease entity as belonging to this disease group. On the other hand, we were able to clearly distinguish adolescent nephronophthisis from NPH2 and NPH1 by exclusion of both gene loci, demonstration of a significantly different age at onset, and identification of a distinct gene locus for adolescent nephronophthisis. The second group of diseases of the nephronophthisis/medullary cystic disease complex consists of recessive nephronophthisis with extrarenal associations, such as retinitis pigmentosa (MIM 266900), hepatic fibrosis (Boichis et al. 1973), oculomotor apraxia (MIM 257550), or cerebellar ataxia (MIM 243910). Since extrarenal involvement was not seen in the large Venezuelan kindred studied, the type of nephronophthisis observed does not belong to this group. The third disease group, ADMCKD, shares with adolescent nephronophthisis identical renal pathology (Goldman et al. 1966; Strauss and Sommers 1967) and lack of extrarenal involvement (Gardner 1971). However, adolescent nephronophthisis can be clearly distinguished from this group, since our prior assumption of recessive inheritance, as judged on the basis of pedigree structure, was confirmed by demonstration of homozygosity by descent. In addition, the locus identified on 3q22 does not coincide with either the locus for ADMCKD1, on chromosome 1q21 (Christodoulou et al. 1998), or the locus for ADMCKD2, on chromosome 16p12 (Scolari et al. 1999). Gout, which is frequently found in ADMCKD, was absent in individuals with adolescent nephronophthisis. Formerly, the main distinguishing feature between autosomal-dominant and -recessive forms was believed to be a difference in onset of terminal renal failure, because median onset age was 13 years in NPH1 (Antignac et al. 1993; Hildebrandt et al. 1997b) and 31–62 years in ADMCKD, respectively (Christodoulou et al. 1998; Scolari et al. 1998).
In a review of the literature, only a few familial cases of nephronophthisis compatible with an autosomal-recessive mode of inheritance and onset of terminal renal failure at age >22 years were identified (Meier and Hess 1965; Fillastre et al. 1976; Steele et al. 1980; Zollinger et al. 1980; Grateau et al. 1986). However, all the reported affected individuals suffer from retinitis pigmentosa and most likely represent cases of late-onset Senior-Løken syndrome. Some authors used the occurrence of chronic renal failure at age >25 years as an exclusion criterion for recessive nephronophthisis of the purely renal form. Sporadic reports of adult patients were thought to represent ADMCKD or other diagnoses (Kleinknecht 1989). In light of the identification of adolescent nephronophthisis as a late-onset recessive form, classification of these cases has to be reconsidered. The identification of adolescent nephronophthisis as a novel disease entity will therefore have implications for genetic counseling in families with hereditary chronic renal failure. The observation of severe vascular changes consisting of thickening of arteriolar and arterial walls that is due to medial hypertrophy and concentric intimal fibrosis, with narrowing of the arterial lumina, is usually not a common feature of nephronophthisis. Whether vascular changes are a specific feature of the disease process or result from chronic renal failure cannot be determined, because no morphological data on the early disease course are available.
An important aspect in mapping of the disease gene for adolescent nephronophthisis was the consanguineous nature of the Venezuelan kindred, which allowed us to follow a strategy of homozygosity mapping (Lander and Botstein 1987). An assumption of identity by descent was made on the basis of pedigree information, cultural isolation, and the rarity of the disease in the general population. On the basis of data of total-genome haplotyping, a search for homozygous regions shared by affected patients led to the identification of the responsible gene locus, as indicated by a significant LOD score. Further evidence of linkage was demonstrated by extensive allele sharing among obligate and facultative carriers and affected individuals. The only exception was affected individual D12, in whom no cosegregating marker was identified. This individual carried the disease-associated haplotype only partially and in the heterozygous state. This finding might be explained by false identity or phenocopy of individual D12. The concomitant occurrence of intermittent microscopic hematuria and hearing deficit in individual D12 and in one of her affected siblings might indicate the presence of a different disease in these individuals. Since, in all LOD-score calculations, individual D12 was classified as affected, this discrepancy does not alter the highly significant LOD scores calculated.
The critical region for adolescent nephronophthisis, on 3q22, contains RYK, a gene encoding a receptor tyrosine kinase–related molecule with two leucine-rich motifs in the extracellular domain (Stacker et al. 1993). These elements appear to be implicated in highly specific protein/protein interactions, as well as in cell-cell adhesion (Rothberg et al. 1990). A similar function has been proposed for nephrocystin, which is mutated in NPH1, on the basis of its SH3 domain (Hildebrandt 1998). A potential role of RYK in kidney disease is also suggested by its expression in isolated glomeruli, mesangial cells, and glomerular endothelial cells and during rat kidney development (Takahashi et al. 1995; Kee et al. 1997). Identification of the gene for adolescent nephronophthisis might help to elucidate disease mechanisms of tubulointerstitial fibrosis and renal cyst development.
Acknowledgments
The cooperation of the members of the Venezuelan kindred is gratefully acknowledged. H.O. and F.H. were supported by grants DFG Om 6/1-1 and DFG Hi 381/3-3 from the German Research Foundation and by grant ZKF-A1 from the Zentrum Klinische Forschung, Freiburg. The Microsatellite Center is supported by a grant-in-aid from the German Genome Project to A.R.
Electronic-Database Information
Accession numbers and URL for data in this article are as follows:
References
- Antignac C, Arduy CH, Beckmann JS, Benessy F, Gros F, Medhioub M, Hildebrandt F, et al (1993) A gene for familial juvenile nephronophthisis (recessive medullary cystic kidney disease) maps to chromosome 2p. Nat Genet 3:342–345 [DOI] [PubMed]
- Antignac C, Kleinknecht C, Habib R (1998) Nephronophthisis. In: Cameron J (ed) Textbook of clinical nephrology. Oxford University Press, Oxford, pp 2417–2426 [Google Scholar]
- Blowey DL, Querfeld U, Geary D, Warady BA, Alon U (1996) Ultrasound findings in juvenile nephronophthisis. Pediatr Nephrol 10:22–24 [DOI] [PubMed]
- Bodaghi E, Honarmand MT, Ahmadi M (1987) Infantile nephronophthisis. Int J Pediatr Nephrol 8:207–210 [PubMed]
- Boichis H, Passwell J, David R, Miller H (1973) Congenital hepatic fibrosis and nephronophthisis: a family study. Q J Med 42:221–233 [PubMed]
- Broman KW, Murray JC, Sheffield VC, White RL, Weber JL (1998) Comprehensive human genetic maps: individual and sex-specific variation in recombination. Am J Hum Genet 63:861–869 [DOI] [PMC free article] [PubMed]
- Christodoulou K, Tsingis M, Stavrou C, Eleftheriou A, Papapavlou P, Patsalis PC, Ioannou P, et al (1998) Chromosome 1 localization of a gene for autosomal dominant medullary cystic kidney disease. Hum Mol Genet 7:905–911 [DOI] [PubMed]
- Collins A, Frezal J, Teague J, Morton NE (1996) A metric map of humans: 23,500 loci in 850 bands. Proc Natl Acad Sci USA 93:14771–14775 [DOI] [PMC free article] [PubMed]
- Dib C, Faure S, Fizames C, Samson D, Drouot N, Vignal A, Millasseau P, et al (1996) A comprehensive genetic map of the human genome based on 5,264 microsatellites. Nature 380:152–154 [DOI] [PubMed]
- Fanconi G, Hanhart E, Albertini A, Uhlinger E, Dolivo G, Prader A (1951) Die familiäre juvenile Nephronophthise. Helv Paediatr Acta 6:1–49 [PubMed] [Google Scholar]
- Fillastre JP, Guenel J, Riberi P, Marx P, Whitworth JA, Kunh JM (1976) Senior-Loken syndrome (nephronophthisis and tapeto-retinal degeneration): a study of 8 cases from 5 families. Clin Nephrol 5:14–19 [PubMed]
- Fivush BA, Jabs K, Neu AM, Sullivan EK, Feld L, Kohaut E, Fine R (1998) Chronic renal insufficiency in children and adolescents: the 1996 annual report of NAPRTCS. North American Pediatric Renal Transplant Cooperative Study. Pediatr Nephrol 12:328–337 [DOI] [PubMed]
- Gagnadoux MF, Bacri JL, Broyer M, Habib R (1989) Infantile chronic tubulo-interstitial nephritis with cortical microcysts: variant of nephronophthisis or new disease entity? Pediatr Nephrol 3:50–55 [DOI] [PubMed]
- Gardner KDJ (1971) Evolution of clinical signs in adult-onset cystic disease of the renal medulla. Ann Intern Med 74:47–54 [DOI] [PubMed]
- Goldman SH, Walker SR, Merigan TCJ, Gardner KDJ, Bull JM (1966) Hereditary occurrence of cystic disease of the renal medulla. N Engl J Med 274:984–992 [DOI] [PubMed]
- Grateau G, Grunfeld JP, Droz D, Noel LH (1986) Adult nephronophthisis: a single disease or 2 diseases? Nephrologie 7:104–108 [PubMed]
- Gretz NM (1994) How to assess the rate of progression of chronic renal failure in children? Pediatr Nephrol 8:499–504 [DOI] [PubMed]
- Haider NB, Carmi R, Shalev H, Sheffield VC, Landau D (1998) A Bedouin kindred with infantile nephronophthisis demonstrates linkage to chromosome 9 by homozygosity mapping. Am J Hum Genet 63:1404–1410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrandt F (1998) Identification of a gene for nephronophthisis. Nephrol Dial Transplant 13:1334–1336 [DOI] [PubMed]
- ——— (1999) Nephronophthisis. In: Avner E, Barrat T, Harmon W (eds) Pediatric nephrology. Williams & Wilkins, Baltimore, pp 453–458 [Google Scholar]
- Hildebrandt F, Otto E, Rensing C, Nothwang HG, Vollmer M, Adolphs J, Hanusch H, et al (1997a) A novel gene encoding an SH3 domain protein is mutated in nephronophthisis type 1. Nat Genet 17:149–153 [DOI] [PubMed]
- Hildebrandt F, Pohlmann A, Omran H (1993a) LODVIEW: a computer program for the graphical evaluation of lod score results in exclusion mapping of human disease genes. Comput Biomed Res 26:592–599 [DOI] [PubMed]
- Hildebrandt F, Singh-Sawhney I, Schnieders B, Centofante L, Omran H, Pohlmann A, Schmaltz C, et al (1993b) Mapping of a gene for familial juvenile nephronophthisis: refining the map and defining flanking markers on chromosome 2. APN Study Group. Am J Hum Genet 53:1256–1261 [PMC free article] [PubMed]
- Hildebrandt F, Strahm B, Nothwang HG, Gretz N, Schnieders B, Singh-Sawhney I, Kutt R, et al (1997b) Molecular genetic identification of families with juvenile nephronophthisis type 1: rate of progression to renal failure. APN Study Group. Arbeitsgemeinschaft fur Padiatrische Nephrologie. Kidney Int 51:261–269 [DOI] [PubMed]
- Kaplan E, Meier P (1958) Nonparametric estimation from incomplete observations. J Am Stat Assoc 53:457–481 [Google Scholar]
- Kee N, McTavish AJ, Papillon J, Cybulsky AV (1997) Receptor protein tyrosine kinases in perinatal developing rat kidney. Kidney Int 52:309–317 [DOI] [PubMed]
- Kleinknecht C (1989) The inheritance of nephronophthisis. In: Spitzer A, Avner E (eds) Kluwer Academic, Boston, pp 277–294 [Google Scholar]
- Konrad M, Saunier S, Heidet L, Silbermann F, Benessy F, Calado J, Le Paslier D, et al (1996) Large homozygous deletions of the 2q13 region are a major cause of juvenile nephronophthisis. Hum Mol Genet 5:367–371 [DOI] [PubMed]
- Kruglyak L, Daly MJ, Reeve-Daly MP, Lander ES (1996) Parametric and nonparametric linkage analysis: a unified multipoint approach. Am J Hum Genet 58:1347–1363 [PMC free article] [PubMed]
- Lander ES, Botstein D (1987) Homozygosity mapping: a way to map human recessive traits with the DNA of inbred children. Science 236:1567–1570 [DOI] [PubMed]
- Lathrop GM, Lalouel JM, Julier C, Ott J (1984) Strategies for multilocus linkage analysis in humans. Proc Natl Acad Sci USA 81:3443–3446 [DOI] [PMC free article] [PubMed]
- Loken A, Hanssen O, Halvorse S, Jolster N (1961) Hereditary renal dysplasia and blindness. Acta Paediatr 50:177–184 [DOI] [PubMed] [Google Scholar]
- Mainzer F, Saldino RM, Ozonoff MB, Minagi H (1970) Familial nephropathy associated with retinitis pigmentosa, cerebellar ataxia and skeletal abnormalities. Am J Med 49:556–562 [DOI] [PubMed]
- Meier D, Hess J (1965) Familial nephropathy with retinitis pigmentosa: a new oculo renal syndrome in adults. Am J Med 39:58–69 [DOI] [PubMed] [Google Scholar]
- Rothberg JM, Jacobs JR, Goodman CS, Artavanis-Tsakonas S (1990) slit: an extracellular protein necessary for development of midline glia and commissural axon pathways contains both EGF and LRR domains. Genes Dev 4:2169–2187 [DOI] [PubMed]
- Saar K, Chrzanowska KH, Stumm M, Jung M, Nurnberg G, Wienker TF, Seemanova E, et al (1997) The gene for the ataxia-telangiectasia variant, Nijmegen breakage syndrome, maps to a 1-cM interval on chromosome 8q21. Am J Hum Genet 60:605–610 [PMC free article] [PubMed]
- Sambrook J, Fritsch E, Maniatis T (1989) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY [Google Scholar]
- Saunier S, Calado J, Heilig R, Silbermann F, Benessy F, Morin G, Konrad M, et al (1997) A novel gene that encodes a protein with a putative src homology 3 domain is a candidate gene for familial juvenile nephronophthisis. Hum Mol Genet 6:2317–2323 [DOI] [PubMed]
- Schaffer AA (1996) Faster linkage analysis computations for pedigrees with loops or unused alleles. Hum Hered 46:226–235 [DOI] [PubMed]
- Scolari F, Puzzer D, Amoroso A, Caridi G, Ghiggeri GM, Maiorca R, Aridon P, et al (1999) Identification of a new locus for medullary cystic disease, on chromosome 16p12. Am J Hum Genet 64:1655–1660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senior B, Friedmann A, Braudo J (1961) Juvenile familial nephropathy with tapetoretinal degeneration: a new oculo-renal syndrome. Am J Ophthalmol 52:625–633 [DOI] [PubMed] [Google Scholar]
- Smith C, Graham J (1945) Congenital medullary cysts of kidneys with severe refractory anemia. Am J Dis Child 69:369–377 [Google Scholar]
- Stacker SA, Hovens CM, Vitali A, Pritchard MA, Baker E, Sutherland GR, Wilks AF (1993) Molecular cloning and chromosomal localisation of the human homologue of a receptor related to tyrosine kinases (RYK). Oncogene 8:1347–1356 [PubMed]
- Steel CM, Philipson J, Arthur E, Gardiner SE, Newton MS, McIntosh RV (1977) Possibility of EB virus preferentially transforming a subpopulation of human B lymphocytes. Nature 270:729–731 [DOI] [PubMed]
- Steele BT, Lirenman DS, Beattie CW (1980) Nephronophthisis. Am J Med 68:531–538 [DOI] [PubMed]
- Strauss MB, Sommers SC (1967) Medullary cystic disease and familial juvenile nephronophthisis. N Engl J Med 277:863–864 [DOI] [PubMed]
- Takahashi T, Shirasawa T, Miyake K, Yahagi Y, Maruyama N, Kasahara N, Kawamura T, et al (1995) Protein tyrosine kinases expressed in glomeruli and cultured glomerular cells: Flt-1 and VEGF expression in renal mesangial cells. Biochem Biophys Res Commun 209:218–226 [DOI] [PubMed]
- Waldherr R, Lennert T, Weber HP, Fodisch HJ, Scharer K (1982) The nephronophthisis complex: a clinicopathologic study in children. Virchows Arch A Pathol Anat Histol 394:235–254 [DOI] [PubMed]
- Zollinger HU, Mihatsch MJ, Edefonti A, Gaboardi F, Imbasciati E, Lennert T (1980) Nephronophthisis (medullary cystic disease of the kidney): a study using electron microscopy, immunofluorescence, and a review of the morphological findings. Helv Paediatr Acta 35:509–530 [PubMed]