Abstract
The human immunodeficiency virus type 1 transmembrane glycoprotein (TM) is efficiently endocytosed in a clathrin-dependent manner. Internalization is mediated by a tyrosine-containing motif within the cytoplasmic domain, and replacement of the cytoplasmic tyrosine by cysteine or phenylalanine increased expression of mutant glycoprotein on the surface of transfected cells by as much as 2.5-fold. Because interactions between the cytoplasmic domain of Env and the matrix protein (MA) have been suggested to mediate incorporation of Env in virus particles, we examined whether perturbation of endocytosis would alter incorporation. Proviruses were constructed to contain the wild-type or mutant Env in conjunction with point mutations in MA that had previously been shown to block Env incorporation. These constructs were used to evaluate the effect of glycoprotein endocytosis on incorporation into virus particles and to test the necessity for a specific interaction between Env and MA to mediate incorporation. Viruses produced from transfected 293T cells were used to infect various cell lines, including MAGI, H9, and CEMx174. Viruses encoding both a disrupted endocytosis motif signal and mutations within MA were significantly more infectious in MAGI cells than their counterparts encoding a mutant MA and wild-type Env. This complementation of infectivity for the MA incorporation mutant viruses was not due to increased glycoprotein incorporation into particles but instead reflected an enhanced fusogenicity of the mutated Env proteins. Our findings further support the concept that a specific interaction between the long cytoplasmic domain of TM and MA is required for efficient incorporation of Env into assembling virions. Alteration of the endocytosis signal of Env, and the resulting increase in cell surface glycoprotein, has no effect on incorporation despite demonstrable effects on fusion, virus entry, and infectivity.
The expression of many glycoproteins on the surfaces of mammalian cells is regulated by endocytosis. Glycoproteins are internalized either through an interaction with components of the endocytic machinery of the cell, such as the adaptin family of proteins associated with clathrin-coated pits (6-8, 13, 26, 42-44, 50, 70), or via a nonspecific mechanism whereby proteins are taken up at the same rate as the lipid components of the membrane. Nonspecific uptake may also utilize clathrin-coated pits, but the proteins are not concentrated specifically in these structures on the plasma membrane.
Once a protein is internalized, the clathrin-coated vesicle with its glycoprotein contents fuses with a sorting endosome. From this endosome compartment, glycoproteins are either directed to lysosomes for proteolytic degradation, recycled back to the plasma membrane, or, in polarized cells, retargeted to a different surface. Many of these processes are thought to be directed through the interaction of sequences in the cytoplasmic or membrane-spanning domain of the cargo protein with cellular protein transport and localization components (1, 8, 38, 39, 48, 69-71).
Thus, glycoprotein expression on the cell surface depends upon specific signal sequences contained within the cytoplasmic and membrane-spanning domains. Two cytoplasmic internalization motifs have been particularly well characterized. The first is a tyrosine-based YXXΦ sequence, where X is any amino acid and Φ represents an amino acid with a bulky aliphatic side chain (8, 38, 53, 70, 71). The motif is usually located within 20 amino acids of the membrane-spanning domain and has been modeled as a tyrosine residue projecting from a tight turn, although recent crystallization data indicate that the motif is bound by an adaptin molecule in an extended conformation (1, 8, 48, 70). The second motif associated with internalization is a dileucine or Leu-Ile sequence which can be transferred to heterologous proteins but lacks any predicted structural characteristics to distinguish it from dileucine residues which do not participate in internalization (25, 28).
The transmembrane protein (TM) subunits of the human immunodeficiency virus type 1 (HIV-1), HIV-2, and simian immunodeficiency virus (SIV) Env glycoproteins, as well as the cytoplasmic domains of a number of other virus fusion proteins, contain cytoplasmic domain sequences that are highly conserved and consistent with proposed tyrosine-based internalization signals (YXXΦ) (3, 4, 14, 45, 46, 58, 62, 68, 69). Recently, the functionality of these signals in SIV and HIV-1 Env internalization has been demonstrated (14, 58, 62). Egan et al. and Rowell et al. have shown that the HIV-1 glycoprotein is efficiently endocytosed into clathrin-coated pits at a rate consistent with its concentration and that such internalization is attenuated in the presence of the viral Gag protein (14, 58). Thus, it appears that there is competition between the endocytic machinery and Gag for interactions with the HIV TM. The rapid endocytosis of the HIV-1 glycoprotein is dependent upon maintenance of a tyrosine residue at position 712 in Env, and the clathrin adapter protein AP-2 has been shown to bind to the cytoplasmic domain of TM at this Tyr712 motif (2, 3, 45).
The Gag polyprotein is the primary building block in the assembly of retroviral particles. It is both necessary and sufficient for assembly and release of virions (5, 10, 19, 20, 54, 59, 63, 64). The amino-terminal matrix domain (MA) of Gag is closely associated with the viral membrane, with which it can be cross-linked in some viruses (51). MA is thought to target assembling Gag molecules to the plasma membrane through a myristic acid moiety, which modifies the N terminus, and through a high density of basic charges on the putative membrane association face of MA. Our understanding of the precise role of MA in HIV particle assembly is complicated by a recent study by Reil et al., which suggested that the entire MA domain of Gag was dispensable for HIV-1 replication (55). This result could be achieved, however, only when the cytoplasmic domain of Env was truncated.
We previously proposed that glycoprotein incorporation into virions could proceed by two mechanisms. In the first, glycoprotein is actively recruited into virions through protein-protein interactions among Gag, presumably MA, and the cytoplasmic domain of TM. The second possible mechanism for glycoprotein incorporation is that of active exclusion of cellular proteins with passive incorporation of Env. The latter mechanism requires that the accumulation and intermolecular interaction of Gag precursors during assembly and budding exclude cellular proteins that are tethered to the cytoskeleton or are interacting with cytoplasmic partners. As an extension of this hypothesized model, we suggested that interactions between Env and Gag might be required to prevent Env molecules from being sequestered from the assembly pathway by the endocytic system (24).
Incorporation of the full-length virus-encoded glycoprotein into HIV virions does appear to be dependent on a specific interaction between MA and the cytoplasmic domain of Env (15, 16). Mutagenesis and biochemical data have strongly suggested that such an interaction is likely, but direct evidence has proven difficult to obtain. It is clear, however, that single point mutations in HIV-1 MA (positions 12, 30, and 34) can block virus infectivity and do so by blocking glycoprotein incorporation (15, 16). Truncation of the HIV TM overcomes the block to incorporation and complements the loss of infectivity (17, 36, 72). Moreover, pseudotyping MA mutant HIV with heterologous glycoproteins that possess short cytoplasmic tails (those from amphotropic murine leukemia virus [MuLV] or human T-cell leukemia virus) or with some truncated HIV-1 glycoproteins results in viruses that retain infectivity (17, 36). These data imply that a specific interaction between MA and Env is required to incorporate HIV-1 glycoproteins with long cytoplasmic domains. Indeed, a recent report suggested that this MA-Env interaction requires the carboxy-terminal 60 amino acids of the HIV-1 TM (9). The significance of this interaction and its role in incorporating Env have yet to be fully investigated in viral constructs, but it is clear that some truncations in this region of the HIV-1 glycoprotein can attenuate infectivity by blocking incorporation (12, 17). Finally, while Env appears to play no direct role in Gag assembly and virus release, it can direct assembly of Gag to the basolateral surface for assembly in polarized epithelial cells. This directionality of virus assembly supports the concept of Env-Gag interactions and is dependent upon a tyrosine-containing targeting signal that overlaps the endocytosis signal described above (34, 35, 49).
Evidence supportive of the passive mechanism for incorporation of at least some forms of Env is equally compelling. Envelope proteins lacking cytoplasmic domains, heterologous viral and cellular glycoproteins, and even glycolipid-anchored derivatives of HIV Env are efficiently incorporated into virions; moreover, the entire cytoplasmic domain of SIV is dispensable for virus replication in vitro (27, 32, 52, 60, 65, 76). Additionally, we have shown that expression of an Env which is retained in the endoplasmic reticulum (ER) is insufficient to redirect HIV-1 budding to that compartment, suggesting that if an interaction between Gag and Env occurs, it must occur in a temporally and spatially specific manner (61).
To explore further the two possible mechanisms for incorporation of Env, we have examined the abilities of glycoprotein endocytosis mutants to complement MA mutations that block virus infectivity and glycoprotein incorporation. We found that mutation of Tyr712 in the dominant cytoplasmic endocytosis signal restored infectivity of the MA mutants in a cell-dependent manner. Surprisingly, however, this complementation of infectivity did not correlate with increases in glycoprotein incorporation, and viruses containing mutations in both MA and the TM endocytosis signals were incapable of multiple rounds of replication in T cells. Freeing the HIV glycoprotein from the endocytosis machinery and enhancing glycoprotein surface expression was thus insufficient to overcome defects in glycoprotein incorporation imposed by MA mutations. These findings not only support the concept that incorporation of HIV-1 glycoproteins with full-length cytoplasmic domains requires a specific interaction between MA and Env but also suggest an additional role for sequences around tyrosine 712 in modulating Env biological activity.
MATERIALS AND METHODS
Cell lines and culture.
COS-1 and 293T cells were obtained from the American Type Culture Collection (Manassas, Va.) and maintained in complete medium consisting of Dulbecco's modified Eagle medium (DMEM) supplemented with 10% fetal calf serum (FCS), 2 mM glutamine, and 100 U of penicillin G/ml-100 μg of streptomycin sulfate (Pen/Strep; Life Technologies, Rockville, Md.)/ml. The cells were passaged three times weekly and were transfected at 50 to 70% confluence. T-cell lines, H9 and CEMx174, were obtained from the National Institutes of Health (NIH) AIDS Reference and Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Disease, Bethesda, Md., and were propagated in RPMI 1640 with 15% FCS, 2 mM glutamine, and Pen/Strep. Viability was evaluated prior to infection by trypan blue dye uptake. If viability dropped below 50%, viable cells were recovered by Ficoll density gradient separation. HeLa-CD4-long terminal repeat (LTR)-β-galactosidase (X4 MAGI) cells were obtained from the NIH AIDS Reference and Reagent Program (29). This cell line was grown in DMEM with 10% FCS, Pen/Strep, and 2 mM glutamine, as described above, supplemented with 100 μg of hygromycin B (Calbiochem, La Jolla, Calif.)/ml and 200 μg of Geneticin-G418 sulfate (Calbiochem)/ml. The cells were maintained in a subconfluent state at all times and were disposed of after 20 passages.
Glycoprotein and proviral expression constructs.
HIV-1 Env endocytosis mutants were generated using a three-oligonucleotide PCR protocol employing thermostable Ampligase (Epicenter, Madison, Wis.) and Taq polymerase (Promega, Madison, Wis.). The 5′ oligonucleotide overlapped a BsmI site at position 7228. The Tyr712 mutagenic oligonucleotide was 5′ phosphorylated and overlapped the Tyr712 (TAT) codon at nucleotide positions 8348 to 8350. (All nucleotide numbers refer to the NL4.3 proviral sequence [GenBank accession no. M19921].) The Tyr-to-Cys mutation converted TAT to TGT, and the Tyr-to-Phe mutation converted TAT to TTT. The 3′ reverse-complement oligonucleotide overlapped the unique BamHI site at position 8475. Simultaneous DNA amplification and ligation were carried out using the above-mentioned primers and enzymes on a gel-purified, XhoI-linearized HIV-1 Env-containing expression vector, pSRH. The mutagenesis reaction mixture was as follows: 1 ng of pSRH template DNA, 100 pM (each) BsmI and BamHI primers, 1 nM phosphorylated mutagenic primer, 250 nM deoxynucleoside triphosphates, 1.0 mM NAD, 1.0 mM dithiothreitol, 1× Taq polymerase buffer (Promega), 5 U of Ampligase, 2 U of Taq polymerase, and H2O to 100 μl. The reaction mixtures were overlaid with mineral oil, and the following cycles were carried out on an MJ Research thermal controller: an initial denaturation at 94°C for 4 min, denaturation at 94°C for 1 min, annealing at 50°C for 1 min, and extension at 65°C for 6 min for 30 cycles. The products were digested with BsmI and BamHI and separated by agarose gel electrophoresis. A fragment corresponding to the mutant BsmI-BamHI 1,237 bp of Env was subcloned into the vector pSPEX, a pSP (Promega)-derived vector, containing the EcoRI-XhoI sequences of HIV-1 NL4-3. Mutations were verified in pSPEX by sequencing the entire region encompassed by the outer PCR primers. Subsequent to verification of the mutants, the NheI (nucleotide 7250)-XhoI (nucleotide 8887) (both unique sites) fragment was subcloned from the pSPEX derivatives into the mammalian expression vector pSRH, a simian virus 40 promoter-Mason-Pfizer monkey virus constitutive transport element-based expression system. The resulting clones are referred to as pSRH Y/C and pSRH Y/F. The Env sequence encoded by this vector also contains the reading frames for Tat and Rev, and the vector expresses all three proteins.
Proviruses were generated by subcloning the NheI-XhoI fragment from pSRH clones that had been sequenced and shown to express glycoprotein into pNL4.3wt or pNL4.3 MA12LE, MA30LE, or MA34VE, provided by Eric Freed at NIH. The presence of the mutations in the proviral constructs was verified by DNA sequencing. All plasmids were propagated in Escherichia coli DH5α cells and purified by alkaline lysis followed by cesium chloride gradient centrifugation.
Metabolic labeling and immunoprecipitation.
Endocytosis motif mutant pSRH constructs were transfected into COS-1 cells by standard calcium phosphate protocols as previously described. To verify protein expression, processing, and stability, the transfected cells were metabolically labeled. Glycoprotein-expressing cells were starved for 30 min with leucine-deficient DMEM (Sigma, St. Louis, Mo.). The cells were labeled with [3H]leucine (250 μCi/ml) (Perkin-Elmer NEN, Boston, Mass.) for 1 h at 37°C. The [3H]leucine was removed, and the radioactive protein was chased through the secretory pathway by incubating the cells for 4 h in complete medium. At the completion of the chase, the supernatants were removed and filtered through 0.45-μm-pore-size syringe filters. Lysis buffer was added to a final concentration of 1% Nonidet P-40-0.1% sodium dodecyl sulfate (SDS) and 0.5% deoxycholate, and the lysate was immunoprecipitated with AIDS patient sera at a dilution of 1:1,000. To prepare cell lysates, cells were washed once in ice-cold phosphate-buffered saline (PBS) and then lysed in the buffer described above. The nuclei were pelleted at 20,000 × g for 1 min in a microcentrifuge, and the cleared cell lysates were transferred to new tubes and immunoprecipitated with patient sera as described above for the supernatants. Immunoprecipitated proteins from the supernatants and the cell lysates were precipitated with formalin-fixed Staphylococcus aureus (protein A), and the complexes were washed three times with lysis buffer lacking deoxycholate. A final wash in 20 mM Tris (pH 6.8) was used to remove residual detergent, and the complex was denatured by heating it for 5 min at 95°C in protein-loading buffer (50 mM Tris [pH 6.8], 100 mM dithiothreitol, 2% SDS, 0.1% bromophenol blue, and 10% glycerol). Viral glycoproteins were separated by SDS-8% polyacrylamide gel electrophoresis (PAGE). The gels were fixed and impregnated with the fluorographic compound En3Hance (Dupont, NEN). The dried gels were fluorographed at −70°C.
Quantification of surface expression using biotinylation.
The initial steps for surface labeling of viral Env proteins on transiently transfected COS-1 cells were the same as those described above for metabolic labeling except that [35S]Cys-[35S]Met protein labeling mix (500 μCi/ml; Perkin-Elmer NEN) was used instead of [3H]leucine. The label was changed in order to facilitate quantitation. The cells were pulse-labeled for 30 min and chased for 4 h. The supernatants were removed, and the cells were washed twice with ice-cold PBS containing Mg2+ and Ca2+. Surface proteins were labeled for 30 min on ice with 2 ml of sulfo-NHS-SS-biotin (Pierce, Rockford, Ill.) (100 mg/ml in ice-cold PBS containing Mg2+ and Ca2+) according to modifications of the methods of Lisanti et al. and Salzwedel et al. (33, 61). The biotin solution was removed, and any excess biotin was quenched in three ice-cold washes with serum-free medium supplemented with 10 mM glycine. The biotinylated cells were then lysed on ice with the lysis buffer described above supplemented with 20 mM glycine. Immunoprecipitations were carried out as described above except that 20 mM glycine was present in all washes. Prior to the final Tris (pH 6.8) wash, 25% of the lysate was removed for total glycoprotein analysis and processed for gel analysis as described above. The remainder (75%) was subjected to surface protein analysis as follows: the immunoprecipitate was boiled for 4 min in 10% SDS to dissociate the antigen-antibody complex, and then the detergent-protein solution was diluted to 0.1% SDS in PBS with 20 mM glycine (to quench any released biotin) and precipitated with monomeric avidin-agarose beads (Pierce). The biotinylated protein-avidin complex was washed two times with lysis buffer lacking deoxycholate and then processed for electrophoresis. Proteins were separated by SDS-8% PAGE. The gels were fixed, dried, and exposed to phosphor screens for quantitation of the steady-state surface-to-total glycoprotein ratio using a PhosphorImager (Molecular Dynamics, Sunnyvale, Calif.) and ImageQuant software. Relative surface protein levels were calculated as {[(Msur − B)/(Mtot − B)]/[(WTsur − B)/(WTtot − B)] × 100%}, where Msur represents counts for the surface expression of the mutant glycoprotein, Mtot represents counts for the total glycoprotein fraction, WTsur represents counts for the surface expression of the wild-type glycoprotein, WTtot represents counts for the total wild-type glycoprotein fraction, and B is the lane background for each of the individual measures.
RT assays.
Reverse transcriptase (RT) activity from virus-containing supernatants was assayed as previously described. Briefly, 25 μl of filtered (0.45-μm pore size) culture supernatant was incubated with 75 μl of reaction cocktail (67 mM Tris [pH 8.0], 6.7 mM dithiothreitol, 6.7 mM MgCl2, 200 mM KCl, 0.133% Triton X-100, 0.67 mM EGTA, 5 μCi of 35S-TTP [10 mCi/ml; NEN]) and 1.25 μg of poly(A)·(dT)10 (Boehringer/Roche, Indianapolis, Ind.) in a 96-well plate at 37°C for 90 min, at which time the reaction was stopped by the addition of 50 μl of 200 mM sodium pyrophosphate. Reaction mixtures were dot blotted onto NA45 membranes (Schleicher & Schuell, Keene, N.H.), washed two times in 0.5 M sodium phosphate, and quantitated using a radioanalytic scanning system (AMBIS Systems-Scanalytics, Fairfax, Va.).
trans-complementation virus entry analysis.
To assess the abilities of endocytosis mutant glycoproteins to facilitate virus entry in a single round of infection, we created an HIV NL4-3 chloramphenicol acetyltransferase (CAT) reporter vector with Env deleted analogous to that developed by Helseth et al. (21). This vector, pNL4ΔEnv CAT, was cotransfected with pSRH plasmids expressing wild-type or mutant Env at a ratio of 2:1. Virus-containing supernatants from transiently transfected COS-1 cells were normalized for RT activity and adjusted for volume with complete medium. Supernatants were added to 107 CEMx174 cells and adsorbed for 4 h at 37°C. The cells were washed with complete medium to remove unbound virus and incubated for an additional 48 h. Cytoplasmic extracts of the infected cells were obtained by repeated freeze-thaw lysis. A portion of the cytoplasmic extract was evaluated for the presence of CAT as described previously (21). The products of the CAT assay were separated by thin-layer chromatography and quantitated using an AMBIS Systems-Scanalytics radioanalytic scanner. The percent conversion of [14C]chloramphenicol (Perkin-Elmer NEN) to its acetylated or diacetylated form was determined from radioanalytic scanning of the thin-layer plate and is presented as CAT activity.
Infectivity in HeLa-MAGI or T-cell lines.
Proviral constructs were transfected into 293T cells for virus production. The supernatants were filtered and assayed for RT activity 48 h posttransfection. Equal RT activity in volume-adjusted supernatants was added to the target cells and allowed to adsorb for 4 h. The cells were washed to remove unbound particles and then cultured at 37°C. X4 MAGI cells were stained as described below 48 to 60 h postinfection. To obtain replication kinetics data, T-cell lines were allowed to develop a multiple-round infection. Supernatants were harvested from the cultures on days 4, 8, 12, and 16. After removal of the supernatant, the H9 cells were split 1:4 at each time point. At the various time points, RT activity in the T-cell culture supernatant was evaluated by RT analysis as described above.
Sucrose density gradient analysis.
Subsequent to the removal of the culture supernatant for RT analysis and infectivity determinations, the proviral-DNA-transfected 293T cells were metabolically labeled with [35S]Cys-[35S]Met protein-labeling mix for 16 h to generate labeled virus particles for gradient analysis and to determine the level of glycoprotein incorporation. Briefly, the labeled, virus-containing supernatant was pelleted in a type 70.1ti rotor (Beckman, Palo Alto, Calif.) at 100,000 × g through a 23% (wt/wt) sucrose cushion for 60 min. The virus pellet was expanded in 400 μl of serum-free DMEM for 2 h at 4°C. The labeled virus was loaded onto continuous 24 to 48% (wt/wt) sucrose gradients and centrifuged in a Beckman SW41ti swinging-bucket rotor at 100,000 × g for 16 h (to equilibrium) at 4°C. The gradients were drip fractionated into 1-ml fractions, each fraction was evaluated for RT activity (see above), and the density of each fraction was determined using a refractometer (Bausch and Lomb, Rochester, N.Y.). Peak RT fractions, which coincided with those at approximately 1.16-g/ml density, were combined, diluted 1:2 in PBS, and immunoprecipitated with AIDS patient sera (see above). The precipitated protein was separated on SDS-10% PAGE, and the gels were autoradiographed and also exposed to phosphor screens for quantitation. The ratio of glycoprotein on gradient-banded particles was determined as follows: (counts gp120 − counts background)/(counts p24 − counts background). The background was determined for each sample from an equivalent area of each lane of the protein gel containing no distinct bands. Relative incorporation is expressed as a percentage of the wild-type Env:Gag ratio. As a control for nonspecific pelleting of Env, either through aggregation or because of microvesicle contamination of gradient fractions, 293T cells were transfected independently with an LTR-driven Env construct, pIII-Env (21), and pNL4ΔEnv CAT. The supernatants were pooled prior to the sucrose gradient separations and gel analyses described above.
Cell-cell fusion assays.
COS-1 cells were transiently transfected with pSRH glycoprotein expression constructs. Twenty-four hours subsequent to the medium change required by Ca2PO4 transfection, the cells were trypsinized and counted using a hemocytometer. Equivalent numbers of cells (105) for each mutant were added in triplicate to 50% confluent HeLa-CD4-LTR-β-galactosidase (X4 MAGI) cell monolayers in 12-well plates. The cells were incubated in DMEM for 24 h or less, as specified, to allow glycoprotein-mediated fusion to occur. Subsequent to the prescribed incubation, the cells were fixed in 1.0% formaldehyde-0.2% glutaraldehyde in PBS, washed two times in PBS, and stained for β-galactosidase. Syncytia were counted by visual microscopy at ×40 magnification. Two measures were utilized. The total number of syncytia in a random field was determined for up to 10 fields and reported as syncytia per field. Alternatively, a number of syncytia were randomly selected, and the total nuclei in each were counted to determine the number of nuclei per syncytium. The former assessment is useful at early time points due to the uniformity in size of the syncytia.
Two-color fusion assay for fusion kinetics.
A dual-expression plasmid was constructed for analysis of fusion by a two-color fluorescence-activated cell sorter (FACS) analysis assay. The enhanced blue fluorescent protein (EBFP) expression cassette was purified from pEBFP-N1 (Promega) by double digestion of the plasmid with the restriction enzymes AflII and AseI. The purified DNA fragment was inserted into the pSRH plasmid at the SacII site by blunt-end ligation. This new pSRH-EB construct supports high-level expression of the HIV-1 wild-type Env proteins from the simian virus 40 late promoter as well as EBFP expression from the human cytomegalovirus immediate-early promoter. The pSRH derivative plasmids expressing endocytosis mutants were also engineered in the same way to include the EBFP expression cassette. The pHTC-EB vector, which expresses a cleavage-defective, glycosylphosphatidylinositol-linked form of the HIV-1 Env, was used as a control for cell aggregation. COS-1 cells were transfected in triplicate with the dual-expression vectors (1 μg/35-mm-diameter well) using the Fugene 6 reagent (Roche, Indianapolis, Ind.) and were used as effector cells in fusion assays.
X4 MAGI cells were incubated with a 0.4 μM calcein-AM (excitation, 496 nm; emission, 517 nm) solution for 30 to 45 min at 37°C. The cells were then removed from the plate in cell dissociation buffer (PBS containing 25 mM EDTA) and resuspended in complete DMEM. A total of 106 target cells were added to COS-1 cells at room temperature for 30 min prior to incubation at 37°C for various times. The coculture was washed with PBS and dissociated with 1 ml of cell dissociation buffer/well, and the cells were resuspended in sorting tubes (BD Biosciences, San Jose, Calif.) for FACS analysis in a Vantage FACS workstation (BD Biosciences). A UV laser was used for EBFP, and a 488-nm laser was used for calcein-AM. In general 10,000 blue cells were gated and analyzed for blue-and-green double-positive cells. Additional details of this assay will be published elsewhere (X. Lin, C. A. Derdeyn, R. Blumenthal, and E. Hunter, submitted for publication).
RESULTS
Construction of mutants.
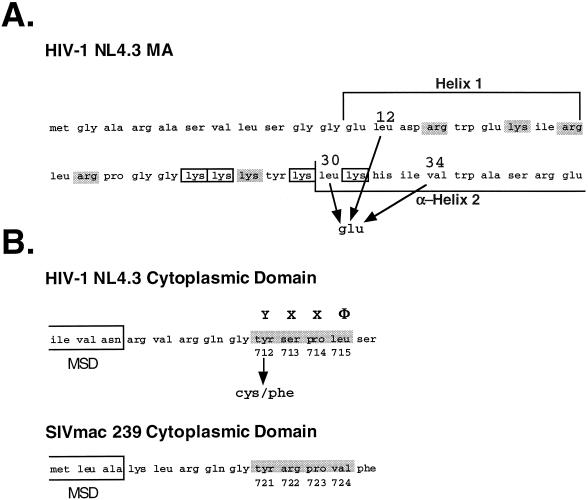
To investigate a possible adverse role for endocytosis signals in HIV glycoprotein incorporation, we constructed full-length glycoprotein expression constructs containing cysteine and phenylalanine substitutions for Tyr712 of the HIV-1 TM glycoprotein. In addition to Env, these glycoprotein expression constructs also express the viral Tat and Rev proteins. The Tyr712Cys and Tyr712Phe mutations were also cloned into proviruses containing single point mutations at position 12 (Leu to Glu), position 30 (Leu to Glu), or position 34 (Val to Glu) to generate MA-TM double mutants (Fig. 1). Freed et al. have shown that these MA point mutations attenuate incorporation of the wild-type glycoprotein and block virus infectivity (18). The endocytosis mutants were also introduced into proviruses containing the wild-type MA protein, and the proviruses containing the MA mutants with wild-type Env sequences were used as controls.
FIG. 1.
Locations of mutations in the HIV-1 (NL4-3) MA and Env domains. (A) Mutations in the MA protein that confer a block to infectivity. The numbers indicate the positions of the mutations subsequent to cleavage of the N-terminal Met residue. Charged residues, identified as essential for MA function, are indicated by boxes, whereas those residues which are mutable without consequence are shaded. Designation of the helices is according to the crystal structure determined by Hill et al. (23). (B) The membrane-spanning region (indicated by the boxes) and the immediately distal cytoplasmic domain sequences for the Env proteins of HIV-1 (NL4-3) and SIVmac 239 are aligned. The region predicted to form a consensus YXXΦ endocytosis signal is identified by shading. The numbering of amino acid residues is from the initiation of the Env precursor.
Synthesis and surface expression of mutant glycoproteins in COS-1 cells.
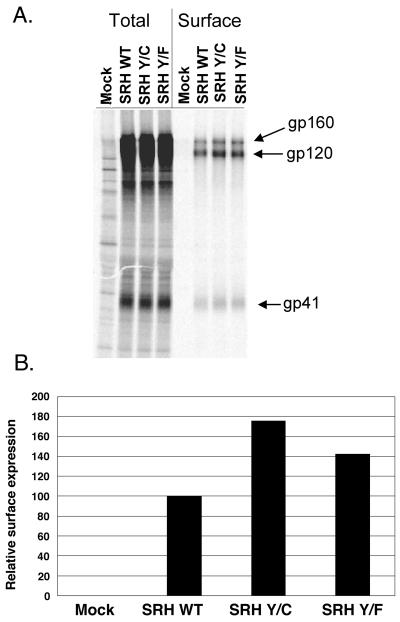
Mutant glycoprotein expression from the pSR vector in COS-1 cells was verified by metabolic labeling and immunoprecipitation of the labeled proteins with AIDS patient sera followed by SDS-PAGE. The glycoprotein mutants were synthesized and processed in a manner indistinguishable from that of the wild type over the course of a 4-h chase, as evidenced by the presence of gp120 and gp41 (Fig. 2A). To directly compare and quantify the levels of mutant and wild-type glycoproteins on the surfaces of transiently transfected cells, cell surface proteins were labeled with a membrane-impermeable biotinylation reagent following the 4-h chase and recovered from immunoprecipitates by streptavidin precipitation. The amounts of surface and total Env protein were determined, and ratios were calculated and then normalized to the wild-type level. Substitution of a cysteine residue for Tyr712 increased the steady-state surface expression by 1.75- to 2.5-fold compared to that of the wild type in multiple experiments, whereas the phenylalanine substitution for Tyr712 increased the surface expression by 1.3- to 1.4-fold (Fig. 2B). An HIV-1 glycoprotein construct encoding an Env molecule with a C-terminal ER retrieval signal was not detectable on the cell surface (data not shown). It is likely that both mutations enhanced cell surface expression of Env relative to the wild type by attenuating endocytosis, since previous work had shown that mutation of tyrosine 712 reduced endocytosis (58), and a detailed analysis of the internalization of the mutants described here confirmed this (S. Wyss and M. Thali, unpublished results). The conservative replacement of tyrosine 712 by another aromatic amino acid residue was not as detrimental to glycoprotein uptake as the cysteine mutation in these experiments. Serine, threonine, proline, and tryptophan substitutions all blocked endocytosis to various degrees (data not shown).
FIG. 2.
Surface expression of mutant glycoproteins in COS-1 cells. Surface biotinylation was used to label envelope glycoproteins on transiently transfected cells. (A) Total glycoprotein was immunoprecipitated from lysates of metabolically labeled cells. Three quarters of the immunoprecipitate was further subjected to precipitation with streptavidin reagents to recover that portion of the Env present on the cell surface. WT, wild type; mock, mock transfected. (B) The ratio of surface to total glycoprotein (gp120) was calculated from panel A using phosphorimaging analysis as described in Materials and Methods. Data for the mutants are presented relative to the wild-type ratio. Representative analyses are shown for panels A and B.
Examination of the amount of gp120 shed into supernatants from transiently transfected COS-1 cells revealed no enhanced instability of the SU and TM association due to the introduced mutations (data not shown).
Endocytosis-defective glycoproteins mediate virus entry into T-cell lines more efficiently than wild type.
The modest enhancement of surface expression of the endocytosis mutants over that of the wild-type glycoprotein prompted us to examine the abilities of the Cys and Phe mutant glycoproteins to complement virus entry using an HIV-1 trans-complementation system (21).
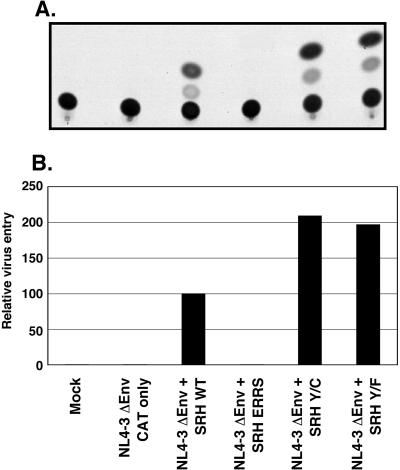
The vector with env deleted, pNL4ΔEnv CAT, was cotransfected with pSRH plasmids expressing wild-type or mutant Env; then virus-containing supernatants normalized for RT activity were used to infect CEMx174 cells as described in Materials and Methods. Cytoplasmic extracts of the infected cells were obtained by repeated freeze-thaw lysis and evaluated for the presence of CAT. As shown in Fig. 3, mutant glycoproteins enhanced NL4ΔEnv CAT entry by as much as 2.1-fold for the Tyr-to-Cys mutant and 1.9-fold for Tyr to Phe over the level mediated by the wild-type glycoprotein. An expressed but ER-retained HIV-1 Env construct (ERRS [61]) was unable to mediate entry of the env-defective provirus (Fig. 3). Similar results were obtained in Jurkat cells (not shown). Thus, the endocytosis mutant glycoproteins mediated enhanced virus entry at levels consistent with their relative levels of surface expression.
FIG. 3.
Virus entry mediated by mutant glycoprotein constructs. (A) A trans-complementation assay measuring expressed CAT activity was utilized (21). Subsequent to single-round infection with an Env-complemented defective NL4-3-CAT vector, CEMx174 cells were lysed and the CAT activity was measured. Acetylated and nonacetylated forms of [14C]chloramphenicol were resolved using thin-layer chromatography. (B) For each construct, CAT activity was calculated from the percent conversion of nonacetylated to acetylated forms. The CAT activity, relative to that in the wild type (WT), for the chromatogram in panel A is shown. Mock, mock-transfected supernatants.
Virus infectivity in CXCR4 MAGI cells.
To determine whether alteration of endocytosis could complement defects in incorporation imposed by point mutations in MA, we assessed the infectivity of full-length viral constructs containing mutations at Tyr712 in combination with wild-type or mutant MA. These experiments were carried out on the CD4+ CXCR4+ β-galactosidase indicator (X4 MAGI) cells. This assay allowed us to probe whether the failure of the MA point mutants (MA12LE, MA30LE, and MA34VE) to incorporate wild-type Env was a function of an inability of MA to interact specifically with sequences in the Env cytoplasmic domain or rather an inability of MA to effectively compete with the endocytosis machinery. At 48 h after transfection of proviral constructs into 293T cells, virus-containing supernatants were harvested and normalized for RT activity, and then equivalent numbers of RT units were used to infect CXCR4 MAGI cells.
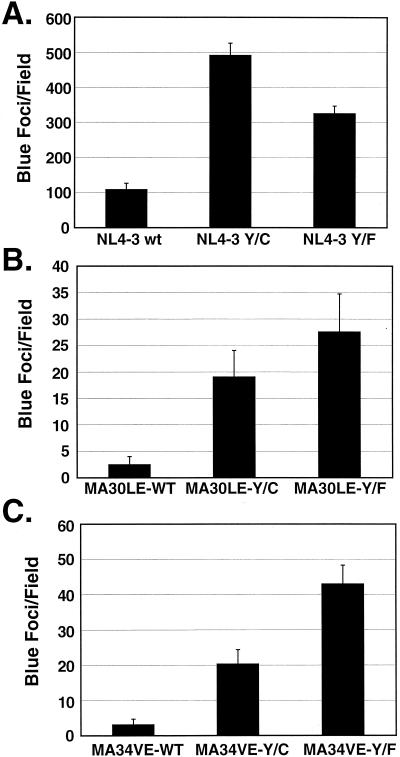
The infectivity of virus encoding wild-type MA was enhanced more than fourfold with the Y712C Env mutation and approximately threefold with the Y712F Env mutation (Fig. 4A). These findings are consistent with those for surface Env expression presented in Fig. 2. Virus encoding the MA30LE mutation and wild-type Env was 40-fold less infectious than constructs encoding wild-type MA (Fig. 4B). The Y712C and Y712F mutations increased infectivity of the MA30LE virions by 7.7- and 11.1-fold, respectively, corresponding to 18 and 25% of the level of fully wild-type NL4-3. A similar complementation of infectivity was observed with the MA34VE provirus, where the cysteine and phenylalanine substitutions resulted in 6.4- and 13.5-fold increases in infectivity, respectively (Fig. 4C), corresponding to 18.7% (Cys) and 39.5% (Phe) of the infectivity of the NL4-3 wild type. The levels of complementation with MA12LE proviruses encoding the Y712 mutations were similar (data not shown). Clearly, mutation of the HIV-1 envelope glycoprotein endocytosis signal can significantly complement (reaching up to 40% of the wild-type level) the loss of infectivity due to MA mutations.
FIG. 4.
Single-round virus infectivity in CXCR4 MAGI cells. Viruses were produced by transfection of 293T cells. The RT-normalized virus-containing supernatants were then added to monolayers of CXCR4 MAGI cells. Subsequently, the cells were fixed and stained in situ for β-galactosidase as an indicator of infection, and the blue cells were counted. The effects of mutant glycoproteins on the infectivities of virus containing the wild-type (wt, WT) MA (A), of virus containing the MA30LE mutation (B), and of virus containing the MA34VE mutation (C) are shown. For each panel, blue foci exhibiting nuclear staining were counted in 9 microscope fields by two individuals (total number of fields, 18). The error bars indicate standard deviations.
Quantitation of glycoprotein incorporation into MA mutant virions.
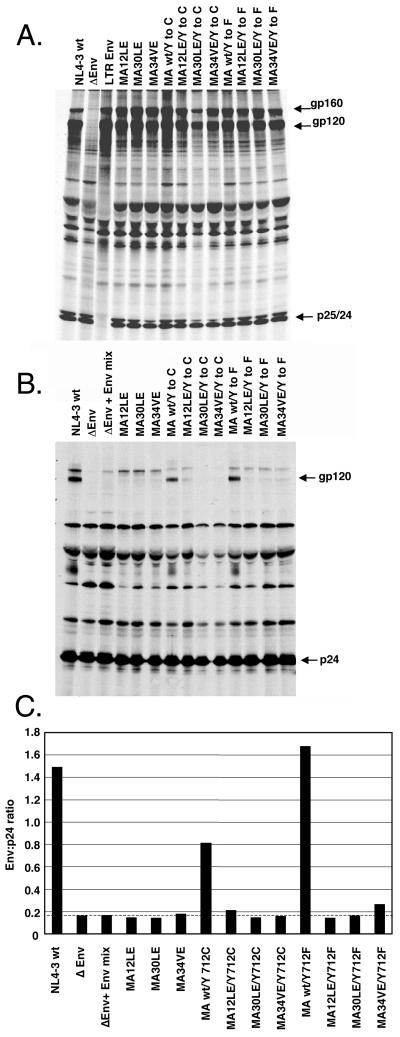
To establish whether the complementation of infectivity observed in the MAGI cells was a function of the level of glycoprotein present in virions, we evaluated the protein composition of highly purified virus particles. The 293T cells used to produce the supernatants for evaluating infectivity in the MAGI assay were metabolically labeled overnight, and the resulting supernatant was harvested and filtered. Virions were pelleted through a sucrose cushion and then subjected to equilibrium density gradient centrifugation. Despite similar expression of viral proteins in cell lysates (Fig. 5A), very little glycoprotein was incorporated into any of the MA mutant virions irrespective of the envelope genotype (Fig. 5B). A control gradient, in which supernatants from cells independently transfected with the pNL4ΔEnv CAT provirus or pIII-Env (Fig. 5B, lane ΔEnv + Env mix) were mixed, showed that low levels of gp160 copurified with the Env− virions. In contrast, no gp120 was observed in this control virus preparation, suggesting that intracellular vesicles containing uncleaved precursor gp160 from the Env-expressing cells cosedimented to the density of HIV particles. This is supported by the relative sharpness of the band, which is consistent with core-glycosylated gp160. Quantitative evaluation of the ratio of Env (gp41 plus gp120) to Gag in each of the lanes verified that there was no significant difference among the amounts of glycoprotein incorporated into MA12LE, MA30LE, and MA34VE virions with either the wild-type or Tyr712 mutant glycoprotein (Fig. 5C). Therefore, the complementation of infectivity described above must be the result of some mechanism other than enhancement of glycoprotein incorporation into virions. This conclusion is validated by the fact that, although the endocytosis mutants increased infectivity of the wild-type MA virions by 3.0- to 4.5-fold over that of wild-type Env, there was no evidence from the Gag/Env ratios of enhanced incorporation of Env, even in the context of a wild-type MA protein. The observation that a single point mutation in the cytoplasmic domain of TM alters infectivity independent of the level of glycoprotein incorporated into virions is a novel and unexpected finding.
FIG. 5.
Analysis of protein compositions of virions purified by density gradient centrifugation. (A) Immunoprecipitated cell lysates from COS-1 cells transiently transfected with proviral DNA constructs. wt, wild type. (B) Peak RT activity-containing fractions from sucrose density gradients of virions produced from the cells in panel A immunoprecipitated with AIDS patient sera. The Env (gp160 and gp120) and CA proteins are indicated. ΔEnv + Env mix is a control for comigration of unincorporated Env (microvesicles or nonspecific adherence) with sedimenting particles. (C) Quantification of the ratio of Env to CA from phosphorimaging analysis of the gel in panel B. A representative experiment is shown.
The Y721 mutations do not support multiple rounds of MA mutant virus replication.
Our analyses to this point focused on the abilities of mutant glycoproteins to facilitate virus entry or infectivity in a single round of replication. To examine the potential consequence of alteration of the HIV-1 Env endocytosis signal for multiple rounds of virus replication, we infected the CD4+ H9 T-cell line and monitored virus spread over time by measuring RT released into the culture medium. Virus-containing supernatants produced from 293T cells 48 h posttransfection with the mutant pNL4 proviruses were normalized for RT activity and used to infect H9 cells overnight. The following day, the media were removed, and the cells were washed to remove unadsorbed virus. Virus-containing supernatants were collected prior to medium changes as shown in Fig. 6.
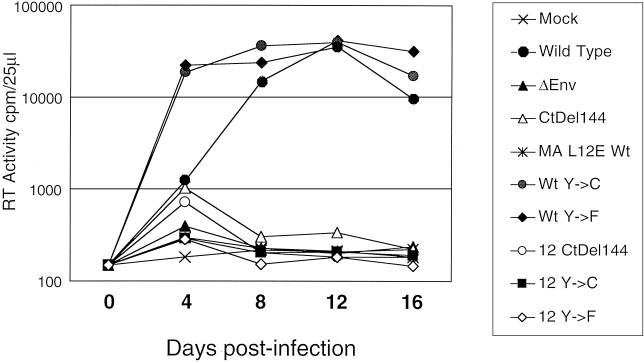
FIG. 6.
Replication of viruses containing mutations in Env and MA in H9 cells. RT activity in filtered culture supernatant was measured as described in Materials and Methods. Supernatants were assayed at 4-day intervals for 16 days. Wt, wild type; Mock, mock infected.
The kinetics of wild-type NL4.3 replication in H9 cells resembled those described previously (12), with a peak of RT activity approximately 12 days postinfection (Fig. 6). In contrast, viruses encoding the Y712C and Y712F mutations in the context of wild-type MA showed much faster replication kinetics, with peak RT values being observed as early as 4 days postinfection (Fig. 6). This result is consistent with the enhanced infectivity observed for the Y712 mutant viruses in MAGI cells.
Despite demonstrable virus entry in MAGI cells, the HIV MA12LE-Y712F/C double mutants were incapable of sustaining multiple rounds of virus replication in H9 cells (Fig. 6). Indeed, the level of RT activity remained at the level of the MA12LE single mutant throughout the experiment.
As a control for these experiments, we employed a truncation mutant of HIV-1 (Ct Del144) previously reported to complement infectivity of the MA12LE mutant in MAGI cells (17, 72). Expression of the MA12LE-Ct Del144 double mutant viral construct in 293T cells yielded virus that contained normal levels of Env and was as infectious as wild-type pNL4 virus in MAGI cells (data not shown). However, in H9 cells, despite an initial peak of RT activity 4 days postinfection, this Env-truncated double mutant was unable to undergo multiple rounds of infection. This is consistent with the recent findings of Murakami and Freed (41) showing that the requirements for Env incorporation are more stringent in T cells than in 293T cells and that in H9 cells the Ct Del144 mutant is not efficiently incorporated into virions. Interestingly, however, the MA-Env double mutants showed similar defects in virus propagation in MT4 cells, which are somewhat permissive for pNL4 encoding the Ct Del144 mutation (data not shown).
Alteration of the endocytosis motif enhances cell-cell fusion.
Clearly, introduction of the Y712 mutations into pNL4 resulted in a more infectious virus. Moreover, these same Env mutations restored the infectivity of the MA mutants (12LE, 30LE, and 34VE) to nearly 50% that of wild-type virus. The observation that complementation for infectivity did not correlate with surface expression levels of the mutant Env proteins or with increased Env incorporation suggested that the effect of the Tyr712 alteration was a result of changes in the intrinsic function of the Env proteins themselves. We therefore examined the potential roles that mutations in Tyr712 might play in altering the fusogenicity of the HIV-1 glycoprotein. Initial endpoint fusion assays indicated no significant difference in the fusion capacities of the different constructs, although there was a trend towards enhanced fusion with the Tyr712 mutants (data not shown).
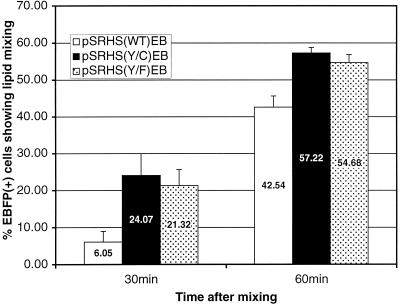
To investigate whether the mutant Env proteins might mediate fusion at a higher rate, fusion kinetics were analyzed in a two-color fusion assay in which COS cells were transfected with a vector (pSRH-EB) expressing both EBFP and wild-type or mutant Env. The transfected cells were mixed with X4 MAGI target cells labeled with the fluorescent dye calcein-AM. Fused cells, fluorescing at both blue and green wavelengths, were detected by flow cytometry 30 or 60 min after the mixed cell populations were incubated at 37°C. Figure 7 shows that as early as 30 min post-cell mixing, both Tyr712 mutant glycoproteins mediated significantly higher numbers of fusion events (approximately 4.0 and 3.5 times higher) than that observed with the wild type. At 60 min post-cell mixing, the mutants still demonstrated higher levels of fusion than the wild type, although the difference was less striking. This result is consistent with data from steady-state fusion assays.
FIG. 7.
Glycoprotein-mediated cell-cell fusion. COS-1 cells transiently transfected with the endocytosis mutant glycoprotein-EBFP coexpression constructs were mixed 1:5 with X4 MAGI cells and allowed to fuse for the indicated times. At the end of the incubation, the cells were removed from the plates and fusion was assessed by flow cytometric analysis of double-labeled cells as described in Materials and Methods. WT, wild type.
DISCUSSION
There is strong evidence in the literature that glycoprotein incorporation into HIV-1 virions is mediated through a specific interaction between the MA protein and the cytoplasmic domain of the TM glycoprotein (9, 12, 15, 17, 18, 37). We previously suggested that the endocytic pathway might compete for incorporation by removing Env from the pool of molecules that are available on the plasma membrane. Thus, in the absence of a strong endocytosis signal, Env might be passively incorporated into particles—even lacking a specific incorporation signal (24). To test this hypothesis, we utilized mutations in the HIV-1 MA protein that block infectivity by attenuating full-length-glycoprotein incorporation in combination with mutations in the cytoplasmic sequences of TM that constitute a strong endocytosis signal (14, 18, 58).
If a specific interaction between MA and Env was not essential for glycoprotein incorporation in the absence of endocytosis, then increasing the availability of glycoprotein on the surface of cells expressing MA mutations should restore infectivity and Env incorporation. We show here that alteration of the endocytosis signal within the cytoplasmic domain of TM by mutation of Tyr712 to either Cys or Phe yields glycoproteins exhibiting synthesis and processing equivalent to that of the wild-type protein but which are expressed at higher levels (2.5- and 1.33-fold, respectively) than the wild-type glycoprotein on the surfaces of transiently transfected cells. Increases in surface expression of the mutations led to a similarly small increase in the level of glycoprotein shed into the medium (data not shown), suggesting that the mutations have not altered the stabilities of glycoprotein complexes in the membrane. The levels of surface glycoprotein we report are in general agreement with those described by Rowell et al. (58) for an Ala substitution at position 712. The increase in surface accumulation observed is also similar to that described for a mutation in the cytoplasmic Tyr motif in a full-length SIVmac 239 Env (31). However, the reported levels for mutants in the analogous Tyr residue at position 721 of SIV CP-MAC, a cytopathic variant of SIVmac encoding a truncated form of the SIV Env, were estimated to be as much as 25-fold that of the wild type (30). The discrepancy in the SIV and HIV data, therefore, may be due to other sequences within the long cytoplasmic domains of both TMs that also function (inefficiently) as internalization or targeting signals. The HIV and SIV cytoplasmic domains contain a number of potential YXXΦ and dileucine motifs that can contribute to internalization (73). A recent examination of other Tyr-containing sequences in the HIV-1 TM suggested that Tyr712 represents the only functional Tyr-based endocytosis motif (2, 3). Nevertheless, it is clear that sequences in the cytoplasmic domain do confer a low level of endocytosis that reduces the steady-state level of Env on the cell surface, perhaps similar to that observed with Rous sarcoma virus Env (47).
Initial experiments in which mutant proviruses encoding both a mutant MA domain and a mutation of Tyr712 in TM were compared to those encoding single MA mutations indicated that the loss of a dominant endocytosis signal could substantially restore the infectivity of the MA mutants (6- to 14-fold). We initially expected that this enhanced infectivity resulted from a higher level of Env incorporation into the virions. However, comparison of the glycoprotein contents of the individual MA mutants (with wild-type Env) and the MA-Y712 double mutants revealed no detectable increase in incorporation. Even in the context of a wild-type Gag protein, where the Y712 mutation resulted in higher (three- to fourfold) levels of infectivity, this was not accompanied by higher levels of Env incorporation. It seems likely, therefore, that the increased infectivity of viruses containing either mutant or wild-type MA results from an enhancement in glycoprotein fusogenicity that is triggered by the introduced change in the cytoplasmic domain of the virus. This conclusion is supported by the results of multiple experiments in which the Y712F mutation, which enhanced surface expression by only 25%, resulted in the same increase in infectivity as the cysteine substitution with a higher surface expression level. Moreover, functional augmentation of the glycoprotein is consistent with the increase in the rate of fusion exhibited by the Y712 mutant glycoproteins in kinetic cell-cell fusion assays.
Previous work with SIV and HIV showed that truncations of the cytoplasmic domain could enhance the fusogenicity of the Env (TM) protein (27, 30, 31, 40, 57, 66, 67, 76), but in general, this was attributed to the higher levels of Env observed on the surfaces of the expressing cells (27). In the cases of MuLV and Mason-Pfizer monkey virus, a viral proteinase-mediated cleavage of the cytoplasmic domain results in enhancement of Env-mediated fusion that, in the case of MuLV, is important for virus infectivity (56). The C-terminal peptide (R peptide) released by proteolysis modulates fusogenicity by an undefined mechanism. Nevertheless, substitution of the MuLV R peptide sequence onto a truncated SIV cytoplasmic domain inhibited fusion by this protein (74). Mutation of a leucine residue (L627) in the R peptide region of MuLV Env resulted in increased fusogenicity, similar to that observed on removal of the R peptide, confirming that sequences in the cytoplasmic domain can modulate fusion in both a positive and a negative fashion (75). Although we have not observed an equally large increase in the fusogenicity of the HIV glycoprotein upon mutation of the Y712 residue, the mutant HIV glycoproteins do mediate cell fusion with faster kinetics. In addition, HIV virions containing these mutant glycoproteins also demonstrate a significantly higher specific infectivity with no increase in Env content.
The restoration of infectivity that we observed with the MA mutants when coexpressed with Y712-mutated Env suggests that there is a threshold level of glycoprotein function necessary to mediate fusion of the viral membrane with that of the cell. The data also suggest that this threshold is the product of the density of Env molecules on the virus surface and the fusogenic potential of the Env molecules themselves. In the case of influenza virus hemagglutinin, a minimum level of glycoprotein expression on the cell surface was shown to be required for cell-cell fusion (22). We postulate that in the case of the MA mutants, the level of wild-type Env is below the threshold required for virus entry and that the enhanced fusogenicity of the Y712 mutant glycoproteins raises a majority of these Env-deficient virions above the fusion threshold. While this effective complementation of the MA mutations is observed for virus produced from 293T cells and assayed on MAGI cells, it is clearly not sufficient to allow sustained replication of the virus in H9 cells. This finding is analogous to the results observed with the Ct Del144 mutant of HIV-1. Env is incorporated into both wild-type and MA mutant virions at levels equivalent to that in the wild-type protein and mediates wild-type levels of infectivity in MAGI cells, but the virus cannot sustain multiple rounds of infection in H9 cells. Recent evidence suggests that this defect is due to a defect in Ct Del144 Env incorporation in T cells and peripheral blood mononuclear cells (41). An additional cell-dependent decrease in the level of the Y712C/F Env in the MA mutant virions produced from H9 cells might be predicted to reduce glycoprotein function below the threshold necessary to initiate entry and infection.
It is clear from the studies described here that enhancement of the surface expression of HIV-1 Env through alteration of the cytoplasmic endocytosis signal in TM is insufficient to compensate for defects in Env incorporation imposed by point mutations in MA. The fact that freeing Env from the endocytic machinery failed to drive incorporation supports previous findings that a specific interaction between MA and TM is required to incorporate a glycoprotein with a full-length cytoplasmic tail. These results refute our previous hypothesis (24) that the absence of Env endocytic competence obviates the need for a specific interaction with Gag.
The conservation of an internalization signal in all HIV and SIV isolates described to date suggests a critical function for internalization in the virus life cycle. The maintenance of such a signal is seemingly at odds with the process of virus assembly and incorporation of Env. Moreover, given the fact that mutation of Tyr712 in the NL4-3 provirus results in more rapid virus replication kinetics, conservation of the tyrosine motif must yield an advantage in vivo that compensates for the slower replication phenotype. It is important to consider that the motif may not be retained only for glycoprotein internalization. Expression of the envelope glycoprotein in polarized cells has been shown to direct viral budding in a polarized manner in both epithelial and lymphocytic cells (11, 49). Studies have demonstrated that a polarized-transport signal overlaps the endocytosis signal (11, 34, 35). Since polarized assembly may have implications for virus transmission, such a signal might be essential for virus pathogenicity.
Acknowledgments
We thank Mike Sakalian for critical review of the manuscript. MA mutant constructs were kindly provided by Eric Freed. The CXCR4 MAGI cell line was obtained form the NIH AIDS Reagent and Reference Program and was provided by Michael Emerman, Fred Hutchinson Cancer Center, Seattle, Wash. We thank Scott Parker for AIDS patient sera from the Clinical Core of the UAB Center for AIDS Research.
This work was supported by U.S. Public Health Service grant R37AI33319 to E.H. J.T.W. was supported by NIH training grant T32AI07493. Experiments performed in the Central Virus Culture Core and Molecular Biology Core of the UAB Center for AIDS Research and acquisition of AIDS patient sera from the Clinical Core of the UAB Center for AIDS Research were supported by grant P30-AI27767.
REFERENCES
- 1.Bansal, A., and L. M. Gierasch. 1991. The NPXY internalization signal of the LDL receptor adopts a reverse-turn conformation. Cell 67:1195-1201. [DOI] [PubMed] [Google Scholar]
- 2.Berlioz-Torrent, C., B. L. Shacklett, L. Erdtmann, L. Delmarre, I. Bouchaert, P. Sonigo, M. C. Dokhelar, and R. Benarous. 1999. Interactions of the cytoplasmic domains of human and simian retroviral transmembrane proteins with components of the clathrin adaptor complexes modulate intracellular and cell surface expression on envelope glycoproteins. J. Virol. 73:1350-1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boge, M., S. Wyss, J. S. Bonifacino, and M. Thali. 1998. A membrane-proximal tyrosine-based signal mediates internalization of the HIV-1 envelope glycoprotein via interaction with the AP-2 clathrin adaptor. J. Biol. Chem. 273:15773-15778. [DOI] [PubMed] [Google Scholar]
- 4.Boll, W., H. Ohno, Z. Songyang, I. Rapoport, L. C. Cantley, J. S. Bonifacino, and T. Kirchhausen. 1996. Sequence requirements for the recognition of tyrosine-based endocytic signals by clathrin AP-2 complexes. EMBO J. 15:5789-5795. [PMC free article] [PubMed] [Google Scholar]
- 5.Campbell, S., and V. M. Vogt. 1997. In vitro assembly of virus-like particles with Rous sarcoma virus Gag deletion mutants: identification of the p10 domain as a morphological determinant in the formation of spherical particles. J. Virol. 71:4425-4435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang, C. P., C. S. Lazar, B. J. Walsh, M. Komuro, J. F. Collawn, L. A. Kuhn, J. A. Tainer, I. S. Trowbridge, M. G. Farquhar, and M. G. Rosenfeld. 1993. Ligand-induced internalization of the epidermal growth factor receptor is mediated by multiple endocytic codes analogous to the tyrosine motif found in constitutively internalized receptors. J. Biol. Chem. 268:19312-19320. [PubMed] [Google Scholar]
- 7.Collawn, J. F., L. A. Kuhn, L. F. Liu, J. A. Tainer, and I. S. Trowbridge. 1991. Transplanted LDL and mannose-6-phosphate receptor internalization signals promote high-efficiency endocytosis of the transferrin receptor. EMBO J. 10:3247-3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collawn, J. F., M. Stangel, L. A. Kuhn, V. Esekogwu, S. Q. Jing, I. S. Trowbridge, and J. A. Tainer. 1990. Transferrin receptor internalization sequence YXRF implicates a tight turn as the structural recognition motif for endocytosis. Cell 63:1061-1072. [DOI] [PubMed] [Google Scholar]
- 9.Cosson, P. 1996. Direct interaction between the envelope and matrix proteins of HIV-1. EMBO J. 15:5783-5788. [PMC free article] [PubMed] [Google Scholar]
- 10.Delchambre, M., D. Gheysen, D. Thines, C. Thiriart, E. Jacobs, E. Verdin, M. Horth, A. Burny, and F. Bex. 1989. The Gag precursors of simian immunodeficiency virus assemble into virus-like particles. EMBO J. 8:2653-2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deschambeault, J., J. P. Lalonde, G. Cervantes-Acosta, R. Lodge, E. A. Cohen, and G. Lemay. 1999. Polarized human immunodeficiency virus budding in lymphocytes involves a tyrosine-based signal and favors cell-to-cell viral transmission. J. Virol. 73:5010-5017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dubay, J. W., S. J. Roberts, B. H. Hahn, and E. Hunter. 1992. Truncation of the human immunodeficiency virus type 1 transmembrane glycoprotein cytoplasmic domain blocks virus infectivity. J. Virol. 66:6616-6625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eberle, W., C. Sander, W. Klaus, B. Schmidt, K. von Figura, and C. Peters. 1991. The essential tyrosine of the internalization signal in lysosomal acid phosphatase is part of a beta turn. Cell 67:1203-1209. [DOI] [PubMed] [Google Scholar]
- 14.Egan, M. A., L. M. Carruth, J. F. Rowell, X. Yu, and R. F. Siliciano. 1996. Human immunodeficiency virus type 1 envelope protein endocytosis mediated by a highly conserved intrinsic internalization signal in the cytoplasmic domain of gp41 is suppressed in the presence of the pr55 gag precursor protein. J. Virol. 70:6547-6556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Freed, E. O., and M. A. Martin. 1996. Domains of the human immunodeficiency virus type 1 matrix and gp41 cytoplasmic tail required for envelope incorporation into virions. J. Virol. 70:341-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Freed, E. O., and M. A. Martin. 1995. The role of human immunodeficiency virus type 1 envelope glycoproteins in virus infection. J. Biol. Chem. 270:23883-23886. [DOI] [PubMed] [Google Scholar]
- 17.Freed, E. O., and M. A. Martin. 1995. Virion incorporation of envelope glycoproteins with long but not short cytoplasmic tails is blocked by specific, single amino acid substitutions in the human immunodeficiency virus type 1 matrix. J. Virol. 69:1984-1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Freed, E. O., J. M. Orenstein, A. J. Buckler-White, and M. A. Martin. 1994. Single amino acid changes in the human immunodeficiency virus type 1 matrix protein block virus particle production. J. Virol. 68:5311-5320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gheysen, H. P., E. Jacobs, F. de Foresta, C. Thiriart, M. Francotte, D. Thines, and M. De Wilde. 1989. Assembly and release of HIV-1 precursor Pr55gag virus-like particles from recombinant baculovirus-infected insect cells. Cell 59:103-112. [DOI] [PubMed] [Google Scholar]
- 20.González, S. A., J. L. Affranchino, H. R. Gelderblom, and A. Burny. 1993. Assembly of the matrix protein of simian immunodeficiency virus into virus-like particles. Virology 194:548-556. [DOI] [PubMed] [Google Scholar]
- 21.Helseth, E., M. Kowalski, D. Gabuzda, U. Olshevsky, W. Haseltine, and J. Sodroski. 1990. Rapid complementation assays measuring replicative potential of human immunodeficiency virus type 1 envelope glycoprotein mutants. J. Virol. 64:2416-2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hernandez, L. D., L. R. Hoffman, T. G. Wolfsberg, and J. M. White. 1996. Virus-cell and cell-cell fusion. Annu. Rev. Cell Dev. Biol. 12:627-661. [DOI] [PubMed] [Google Scholar]
- 23.Hill, C. P., D. Worthylake, D. P. Bancroft, A. M. Christensen, and W. I. Sundquist. 1996. Crystal structures of the trimeric human immunodeficiency virus type 1 matrix protein: implications for membrane association and assembly. Proc. Natl. Acad. Sci. USA 93:3099-3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hunter, E. 1994. Macromolecular interactions in the assembly of HIV and other retroviruses. Semin. Virol. 5:71-83. [Google Scholar]
- 25.Hunziker, W., and C. Fumey. 1994. A di-leucine motif mediates endocytosis and basolateral sorting of macrophage IgG Fc receptors in MDCK cells. EMBO J. 13:2963-2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jing, S. Q., T. Spencer, K. Miller, C. Hopkins, and I. S. Trowbridge. 1990. Role of the human transferrin receptor cytoplasmic domain in endocytosis: localization of a specific signal sequence for internalization. J. Cell Biol. 110:283-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnston, P. B., J. W. Dubay, and E. Hunter. 1993. Truncations of the simian immunodeficiency virus transmembrane protein confer expanded virus host range by removing a block to virus entry into cells. J. Virol. 67:3077-3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kang, S., L. Liang, C. D. Parker, and J. F. Collawn. 1998. Structural requirements for major histocompatibility complex class II invariant chain endocytosis and lysosomal targeting. J. Biol. Chem. 273:20644-20652. [DOI] [PubMed] [Google Scholar]
- 29.Kimpton, J., and M. Emerman. 1992. Detection of replication-competent and pseudotyped human immunodeficiency virus with a sensitive cell line on the basis of activation of an integrated β-galactosidase gene. J. Virol. 66:2232-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.LaBranche, C. C., M. M. Sauter, B. S. Haggarty, P. J. Vance, J. Romano, T. K. Hart, P. J. Bugelski, and J. A. Hoxie. 1994. Biological, molecular, and structural analysis of a cytopathic variant from a molecularly cloned simian immunodeficiency virus. J. Virol. 68:5509-5522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.LaBranche, C. C., M. M. Sauter, B. S. Haggarty, P. J. Vance, J. Romano, T. K. Hart, P. J. Bugelski, M. Marsh, and J. A. Hoxie. 1995. A single amino acid change in the cytoplasmic domain of the simian immunodeficiency virus transmembrane molecule increases envelope glycoprotein expression on infected cells. J. Virol. 69:5217-5227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Le Guern, M., and J. A. Levy. 1992. Human immunodeficiency virus (HIV) type 1 can superinfect HIV-2-infected cells: pseudotype virions produced with expanded cellular host range. Proc. Natl. Acad. Sci. USA 89:363-367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lisanti, M. P., M. Sargiacomo, L. Graeve, A. R. Saltiel, and E. Rodriguez-Boulan. 1988. Polarized apical distribution of glycosyl-phosphatidylinositol-anchored proteins in a renal epithelial cell line. Proc. Natl. Acad. Sci. USA 85:9557-9561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lodge, R., H. Göttlinger, D. Gabuzda, E. A. Cohen, and G. Lemay. 1994. The intracytoplasmic domain of gp41 mediates polarized budding of human immunodeficiency virus type 1 in MDCK cells. J. Virol. 68:4857-4861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lodge, R., J.-P. Lalonde, G. Lemay, and E. A. Cohen. 1997. The membrane-proximal intracytoplasmic tyrosine residue of HIV-1 envelope glycoprotein is critical for basolateral targeting of viral budding in MDCK cells. EMBO J. 16:695-705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mammano, F., E. Kondo, J. Sodroski, A. Bukovsky, and H. G. Göttlinger. 1995. Rescue of human immunodeficiency virus type 1 matrix mutants by envelope glycoproteins with short cytoplasmic tails. J. Virol. 69:3824-3830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mammano, F., Å. Öhagen, S. Höglund, and H. G. Göttlinger. 1994. Role of the major homology region of human immunodeficiency virus type 1 in virion morphogenesis. J. Virol. 68:4927-4936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marsh, M., and H. T. McMahon. 1999. The structural era of endocytosis. Science 285:215-220. [DOI] [PubMed] [Google Scholar]
- 39.Matter, K., W. Hunziker, and I. Mellman. 1992. Basolateral sorting of LDL receptor in MDCK cells: the cytoplasmic domain contains two tyrosine-dependent targeting determinants. Cell 71:741-753. [DOI] [PubMed] [Google Scholar]
- 40.Mulligan, M. J., G. V. Yamshchikov, G. D. J. Ritter, F. Gao, M. J. Jin, C. D. Nail, C. P. Spies, B. H. Hahn, and R. W. Compans. 1992. Cytoplasmic domain truncation enhances fusion activity by the exterior glycoprotein complex of human immunodeficiency virus type 2 in selected cell types. J. Virol. 66:3971-3975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Murakami, T., and E. O. Freed. 2000. The long cytoplasmic tail of gp41 is required in a cell type-dependent manner for HIV-1 envelope glycoprotein incorporation into virions. Proc. Natl. Acad. Sci. USA 97:343-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Odorizzi, C. G., I. S. Trowbridge, L. Xue, C. R. Hopkins, C. D. Davis, and J. F. Collawn. 1994. Sorting signals in the MHC class II invariant chain cytoplasmic tail and transmembrane region determine trafficking to an endocytic processing compartment. J. Cell Biol. 126:317-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Odorizzi, G., and I. S. Trowbridge. 1997. Structural requirements for basolateral sorting of the human transferrin receptor in the biosynthetic and endocytic pathways of Madin-Darby canine kidney cells. J. Cell Biol. 137:1255-1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Odorizzi, G., and I. S. Trowbridge. 1997. Structural requirements for major histocompatibility complex class II invariant chain trafficking in polarized Madin-Darby canine kidney cells. J. Biol. Chem. 272:11757-11762. [DOI] [PubMed] [Google Scholar]
- 45.Ohno, H., R. C. Aguilar, M. C. Fournier, S. Hennecke, P. Cosson, and J. S. Bonifacino. 1997. Interaction of endocytic signals from the HIV-1 envelope glycoprotein complex with members of the adaptor medium chain family. Virology 238:305-315. [DOI] [PubMed] [Google Scholar]
- 46.Olson, J. K., and C. Grose. 1997. Endocytosis and recycling of varicella-zoster virus Fc receptor glycoprotein gE: internalization mediated by a YXXL motif in the cytoplasmic tail. J. Virol. 71:4042-4054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oschenbauer, C., S. R. Dubay, and E. Hunter. 2000. The Rous sarcoma virus Env glycoprotein contains a highly conserved motif homologous to tyrosine-based endocytosis signals and displays an unusual internalization phenotype. Mol. Cell. Biol. 20:249-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Owen, D. J., and P. R. Evans. 1998. A structural explanation for the recognition of tyrosine-based endocytotic signals. Science 282:1327-1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Owens, R. J., and R. W. Compans. 1989. Expression of the human immunodeficiency virus envelope glycoprotein is restricted to basolateral surfaces of polarized epithelial cells. J. Virol. 63:978-982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pelchen-Matthews, A., J. E. Armes, and M. Marsh. 1989. Internalization and recycling of CD4 transfected into HeLa and NIH3T3 cells. EMBO J. 8:3641-3649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pepinsky, R. B., and V. M. Vogt. 1979. Identification of retrovirus matrix proteins by lipid-protein cross-linking. J. Mol. Biol. 131:819-837. [DOI] [PubMed] [Google Scholar]
- 52.Perez, L. G., G. L. Davis, and E. Hunter. 1987. Mutants of the Rous sarcoma virus envelope glycoprotein that lack the transmembrane anchor and cytoplasmic domains: analysis of intracellular transport and assembly into virions. J. Virol. 61:2981-2988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pytowski, B., T. W. Judge, and T. E. McGraw. 1995. An internalization motif is created in the cytoplasmic domain of the transferrin receptor by substitution of a tyrosine at the first position of a predicted tight turn. J. Biol. Chem. 270:9067-9073. [DOI] [PubMed] [Google Scholar]
- 54.Rasmussen, L., J. K. Battles, W. H. Ennis, K. Nagashima, and M. A. Gonda. 1990. Characterization of virus-like particles produced by a recombinant baculovirus containing the gag gene of the bovine immunodeficiency-like virus. Virology 178:435-451. [DOI] [PubMed] [Google Scholar]
- 55.Reil, H., A. A. Bukovsky, H. R. Gelderblom, and H. G. Göttlinger. 1998. Efficient HIV-1 replication can occur in the absence of the viral matrix protein. EMBO J. 17:2699-2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rein, A., J. Mirro, J. G. Haynes, S. M. Ernst, and K. Nagashima. 1994. Function of the cytoplasmic domain of a retroviral transmembrane protein: p15E-p2E cleavage activates the membrane fusion capability of the murine leukemia virus Env protein. J. Virol. 68:1773-1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ritter, G. D., M. J. Mulligan, S. L. Lydy, and R. W. Compans. 1993. Cell fusion activity of the simian immunodeficiency virus is modulated by the intracytoplasmic domain. Virology 197:255-264. [DOI] [PubMed] [Google Scholar]
- 58.Rowell, J. F., P. E. Stanhope, and R. F. Siliciano. 1995. Endocytosis of the HIV-1 envelope protein: mechanism and role in processing for association with class II MHC. J. Immunol. 155:473-488. [PubMed] [Google Scholar]
- 59.Sakalian, M., S. D. Parker, R. A. Weldon, Jr., and E. Hunter. 1996. Synthesis and assembly of retrovirus Gag precursors into immature capsids in vitro. J. Virol. 70:3706-3715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Salzwedel, K., P. B. Johnston, S. J. Roberts, J. W. Dubay, and E. Hunter. 1993. Expression and characterization of glycophospholipid-anchored human immunodeficiency virus type 1 envelope glycoproteins. J. Virol. 67:5279-5288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Salzwedel, K., J. T. West, Jr., M. J. Mulligan, and E. Hunter. 1998. Retention of the human immunodeficiency virus type 1 envelope glycoprotein in the endoplasmic reticulum does not redirect virus assembly from the plasma membrane. J. Virol. 72:7523-7531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sauter, M. M., A. Pelchen-Matthews, R. Bron, M. Marsh, C. C. LaBranche, P. J. Vance, J. Romano, B. S. Haggarty, T. K. Hart, W. M. F. Lee, and J. A. Hoxie. 1996. An internalization signal in the simian immunodeficiency virus transmembrane protein cytoplasmic domain modulates expression of envelope glycoproteins on the cell surface. J. Cell Biol. 132:795-811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shioda, T., and H. Shibuta. 1990. Production of human immunodeficiency virus (HIV)-like particles from cells infected with recombinant vaccinia viruses carrying the gag gene of HIV. Virology 175:139-148. [DOI] [PubMed] [Google Scholar]
- 64.Smith, A. J., M.-I. Cho, M.-L. Hammerskjold, and D. Rekosh. 1990. Human immunodeficiency virus type 1 Pr55gag and Pr160gag-pol expressed from a simian virus 40 late replacement vector are efficiently processed and assembled into virus-like particles. J. Virol. 64:2743-2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Spector, D. H., E. Wade, D. A. Wright, V. Koval, C. Clark, D. Jaquish, and S. A. Spector. 1990. Human immunodeficiency virus pseudotypes with expanded cellular and species tropism. J. Virol. 64:2298-2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Spies, C. P., and R. W. Compans. 1994. Effects of cytoplasmic domain length on cell surface expression and syncytium-forming capacity of the simian immunodeficiency virus envelope glycoprotein. Virology 203:8-19. [DOI] [PubMed] [Google Scholar]
- 67.Spies, C. P., G. D. Ritter, M. J. Mulligan, and R. W. Compans. 1994. Truncation of the cytoplasmic domain of the simian immunodeficiency virus envelope glycoprotein alters the conformation of the external domain. J. Virol. 68:7840-7845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Thomas, D. C., C. B. Brewer, and M. G. Roth. 1993. Vesicular stomatitis virus glycoprotein contains a dominant cytoplasmic basolateral sorting signal critically dependent upon a tyrosine. J. Biol. Chem. 268:3313-3320. [PubMed] [Google Scholar]
- 69.Thomas, D. C., and M. G. Roth. 1994. The basolateral targeting signal in the cytoplasmic domain of glycoprotein G from vesicular stomatitis virus resembles a variety of intracellular targeting motifs related by primary sequence but having diverse targeting activities. J. Biol. Chem. 269:15732-15739. [PubMed] [Google Scholar]
- 70.Trowbridge, I. S., and J. F. Collawn. 1992. Structural requirements for high efficiency endocytosis of the human transferrin receptor. J. Inorg. Biochem. 47:209-217. [DOI] [PubMed] [Google Scholar]
- 71.Trowbridge, I. S., J. F. Collawn, and C. R. Hopkins. 1993. Signal-dependent membrane protein trafficking in the endocytic pathway. Annu. Rev. Cell Biol. 9:129-161. [DOI] [PubMed] [Google Scholar]
- 72.Wilk, T., T. Pfeiffer, and V. Bosch. 1992. Retained in vitro infectivity and cytopathogenicity of HIV-1 despite truncation of the C-terminal tail of the env gene product. Virology 189:167-177. [DOI] [PubMed] [Google Scholar]
- 73.Wyss, S., C. Berlioz-Torrent, M. Boge, G. Blot, S. Honing, R. Benarous, and M. Thali. 2001. The highly conserved C-terminal dileucine motif in the cytosolic domain of the human immunodeficiency virus type 1 envelope glycoprotein is critical for its association with the AP-1 clathrin adapter. J. Virol. 75:2982-2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yang, C., and R. W. Compans. 1996. Analysis of the cell fusion activities of chimeric simian immunodeficiency virus-murine leukemia virus envelope proteins: inhibitory effects of the R peptide. J. Virol. 70:248-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yang, C., and R. W. Compans. 1997. Analysis of the murine leukemia virus R peptide: delineation of the molecular determinants which are important for its fusion inhibition activity. J. Virol. 71:8490-8496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zingler, K., and D. R. Littman. 1993. Truncation of the cytoplasmic domain of the simian immunodeficiency virus envelope glycoprotein increases Env incorporation into particles and fusogenicity and infectivity. J. Virol. 66:2824-2831. [DOI] [PMC free article] [PubMed] [Google Scholar]