Abstract
Influenza virions bud preferentially from the apical plasma membrane of infected epithelial cells, by enveloping viral nucleocapsids located in the cytosol with its viral integral membrane proteins, i.e., hemagglutinin (HA), neuraminidase (NA), and M2 proteins, located at the plasma membrane. Because individually expressed HA, NA, and M2 proteins are targeted to the apical surface of the cell, guided by apical sorting signals in their transmembrane or cytoplasmic domains, it has been proposed that the polarized budding of influenza virions depends on the interaction of nucleocapsids and matrix proteins with the cytoplasmic domains of HA, NA, and/or M2 proteins. Since HA is the major protein component of the viral envelope, its polarized surface delivery may be a major force that drives polarized viral budding. We investigated this hypothesis by infecting MDCK cells with a transfectant influenza virus carrying a mutant form of HA (C560Y) with a basolateral sorting signal in its cytoplasmic domain. C560Y HA was expressed nonpolarly on the surface of infected MDCK cells. Interestingly, viral budding remained apical in C560Y virus-infected cells, and so did the location of NP and M1 proteins at late times of infection. These results are consistent with a model in which apical viral budding is a shared function of various viral components rather than a role of the major viral envelope glycoprotein HA.
The first step in viral invasion of a multicellular organism involves the infection of a superficial layer of polarized epithelial cells. The progeny virions resulting from this infection are usually released in a polarized fashion from the epithelial cell surface (50, 63). It is believed that the ability of a virus to bud apically or basolaterally from epithelial cells plays an important role in the pathogenicity and invasiveness of the virus (for a review, see reference 63). Although viruses which bud apically from infected epithelial cells, such as influenza viruses, might still cause systemic infections, viruses that bud basolaterally may more easily reach the underlying tissues and establish faster systemic infections. In fact, the budding site of Sendai virus in polarized epithelial cells, in addition to the cleavage-activation of the fusion glycoprotein by ubiquitous proteases, has been shown to be one of the determinants for organ tropism and pathogenicity in mice (60).
Viral budding at specific membrane locations requires the transport of all structural viral components to these specific membrane domains. Accordingly, viruses have evolved mechanisms for the polarized transport of their proteins to the apical or basolateral surfaces of epithelial cells, characterized by different protein and lipid compositions, segregated by tight junctions (47, 48). Integral viral envelope proteins are segregated immediately after their synthesis in the endoplasmic reticulum, in the trans-Golgi network, by incorporation into different post-Golgi vesicles that fuse with either apical or basolateral plasma membrane domains (46, 65). Segregation into different post-Golgi vesicles is directed by apical and basolateral sorting motifs present in the transported proteins. Influenza virus hemagglutinin (HA) and neuraminidase (NA) proteins have apical targeting information in their transmembrane domains (23, 52), whereas vesicular stomatitis virus G protein and human immunodeficiency virus type 1 (HIV-1) gp160 have basolateral targeting signals in their cytoplasmic domains (11, 44, 50, 61). The signals and mechanisms utilized by viral glycoproteins to reach apical and basolateral domains are identical to those used by endogenous plasma membrane proteins (for reviews, see references 24, 38, and 49).
In contrast to the wealth of information on the sorting of viral integral membrane proteins, considerably less is known about the mechanism responsible for the localization of internal viral components, such as the matrix and capsid proteins. It has been hypothesized that specific interactions between the polarized viral glycoproteins and the capsid or matrix components of the virus may mediate the transport of the latter to the budding surfaces in infected cells. Evidence for an interaction between the viral glycoprotein HN and the viral matrix M protein of Newcastle disease virus has been obtained (12). For Sendai virus, expression of the viral glycoproteins HN or F appears to enhance the association of the viral M protein with membranes (3, 54, 55). For HIV-1, it has been shown that the location of the envelope protein determines the site of virus budding in polarized cells (9, 32, 45). However, there are examples of polarized viral budding occurring independently of the polarized envelope viral glycoproteins. Budding of measles virus in MDCK cells occurs at apical surfaces even though its surface glycoproteins H and F are transported in a nonpolarized fashion and to the basolateral membrane domain, respectively (33). Similarly, the spike protein of coronavirus is not involved in the polarized sorting of this virus (51). Moreover, Marburg virus glycoprotein is transported in MDCK cells mainly to the apical surface, while progeny viruses are released exclusively at the basolateral surface (56).
In the case of influenza A virus, viral assembly and budding occurs at the apical surface of infected cells (50), the site of accumulation of its three viral integral membrane proteins, the HA, NA, and M2 proteins, which is mediated by specific sorting signals that promote their association with sphingoglycolipid rafts (5, 19, 23, 30, 52). In contrast, the mechanism responsible for the transport of the viral proteins which form the inside of the virion to the apical budding surfaces of the infected cells is poorly understood. For example, the role of the HA, NA, and M2 proteins in the localization of the viral matrix protein, M1, which lies underneath the viral envelope, is unclear (10, 25, 67). Interestingly, it appears that M1 interactions with HA and NA mediate the association of M1 with detergent-resistant membranes (2, 4, 69).
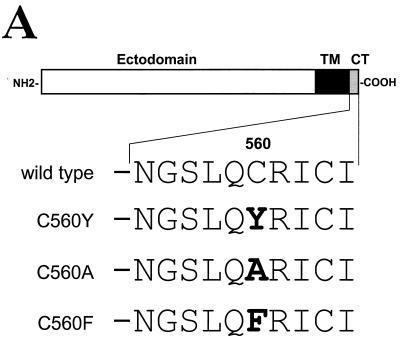
Here, we report experiments to characterize the role of influenza virus HA, the major envelope protein of influenza virus, in the polarized assembly of the virus. The experiments are based on an observation by Brewer and Roth (7) that a single amino acid change at the cytoplasmic tail of the HA is responsible for a dramatic change in its localization from the apical to basolateral membranes when stably expressed from a cytomegalovirus promoter in polarized MDCK cells. We have investigated the influence of this amino acid mutation in viral capsid and matrix protein transport and virus budding by using a recombinant influenza virus expressing this mutant HA protein. The transfectant influenza A/WSN/33 virus (C560Y, H1 subtype) was previously generated (71). Its HA was mutated at amino acid position 560 by substituting the wild-type cysteine residue with a tyrosine (71). This amino acid position lies within the 10-amino-acid cytoplasmic tail of the HA protein (Fig. 1A). An identical substitution was previously shown by Brewer and Roth (7) to be responsible for basolateral transport of the HA in cells stably expressing the corresponding HA mutant protein of an H2 subtype. Although the H1 and H2 HAs are antigenically different, their 10-amino-acid cytoplasmic tails are identical. Therefore, it was expected that transfectant C560Y virus-infected cells will express the viral HA at the basolateral surface. Transfectant influenza A/WSN/33 viruses with phenylalanine and alanine amino acid substitutions at C-560 (C560F and C560A) had also been constructed previously. The mutations generated in these viruses are represented in Fig. 1A.
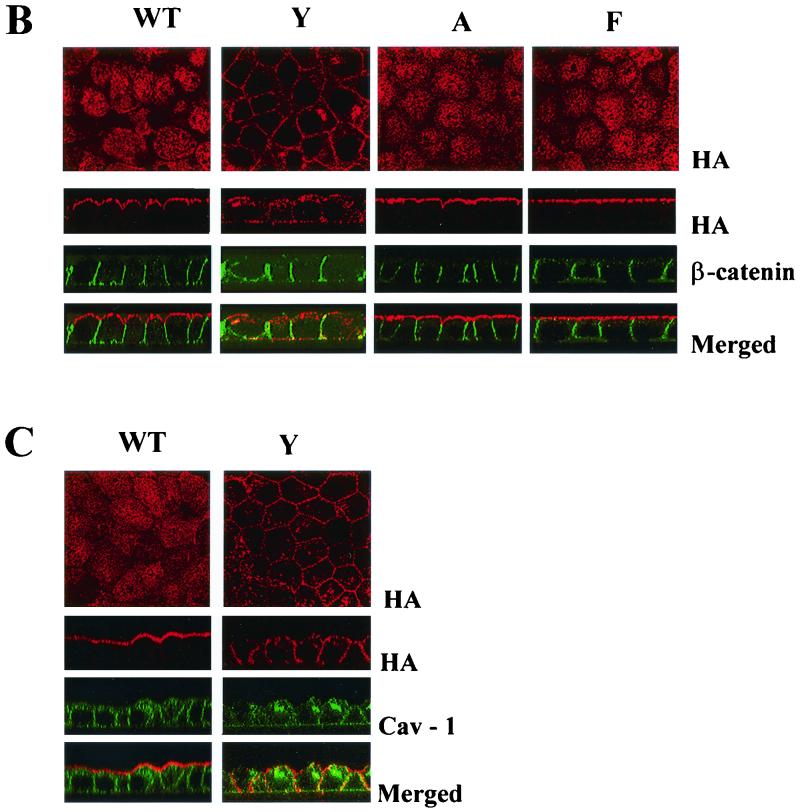
FIG. 1.
(A) Schematic representation of wild-type and mutant HA proteins of influenza A/WSN/33 virus used in our studies. Amino acid residues located in the cytoplasmic tail (CT) following the transmembrane domain (TM) of the HA protein are indicated. Mutated amino acid residue at position 560 is shown in bold for each mutant HA. (B) Nonpolar sorting of the HA protein in MDCK cells infected with C560Y transfectant influenza virus. MDCK monolayers grown in filters were infected with wild-type (WT), C560Y (Y), C560A (A), and C560F (F) viruses. At 11 h postinfection, cells were fixed and immunostained with HA-specific monoclonal antibody (red) and with β-catenin-specific polyclonal antibody (green). The localization of the HA and β-catenin was visualized by confocal microscopy. Representative HA staining pictures taken from the top are shown in the upper panels. The lower panels are reconstructed side views of the immunostained cells. β-catenin is distributed on the lateral membrane. (C) Nonpolar sorting of C560Y HA protein in a constitutively HA expressing MDCK cell line. MDCK monolayers expressing wild-type HA (WT) and C560Y HA (Y) were grown on filters, fixed, and immunostained with HA-specific monoclonal antibody (red) and with caveolin-1 specific polyclonal antibody (green). The distribution of the HA and caveolin-1 was visualized by confocal microscopy. Caveolin-1 is localized to the apical and basolateral plasmalemma and to the Golgi complex.
We first investigated the polarized HA expression in virus-infected MDCK cells by indirect immunofluorescence confocal microscopy. MDCK cells were grown on 0.4-μm-pore-size polycarbonate Transwell R chambers (Corning Costar Corporation, Cambridge, Mass.) in Dulbecco modified Eagle medium supplemented with 5% fetal bovine serum, penicillin (50 mU/ml), and streptomycin (50 μg/ml). At 3 to 5 days postconfluency, the cells were infected as previously described (50) with WSN wild-type virus or with C560Y, C560A, or C560F viruses at a multiplicity of infection (MOI) of 5. In these experiments the intactness of the monolayers prior to infections was monitored by measuring the transepithelial electrical resistance. At 11 h postinfection, cells were fixed with 2% paraformaldehyde, quenched with NH4Cl, permeabilized with 0.075% saponin in phosphate-buffered saline with 0.5 mM Ca and 0.5 mM Mg (PBS-CM), and incubated with H15-C5-1R1, an HA-specific monoclonal antibody (6), and with a rabbit polyclonal antibody against β-catenin (Sigma, St. Louis, Mo.). HA and β-catenin bound antibodies were visualized by using Alexa Fluor 594 (red)- and Alexa Fluor 488 (green)-conjugated secondary antibodies, respectively (Molecular Probes, Eugene, Oreg.). Labeled monolayers were examined and scans were obtained either as x-y (en face) or x-z (transverse) sections in a dual-channel laser scanning confocal microscope (Sarastro; Molecular Dynamics, Sunnyvale, Calif.). The results shown in Fig. 1B indicate that, while the HA protein is mainly located at the apical surface in wild-type, C560A, and C560F virus-infected cells, a significant fraction of the HA is located at the basolateral surface in C560Y virus-infected cells. In all cases, β-catenin was located at the lateral (cell-cell interface) surfaces, a finding consistent with the known distribution pattern of this cellular protein in adherens junctions (1).
Brewer et al. previously reported that the equivalent C560Y mutation in the H2 protein of influenza A/Japan/305/57 virus (C543Y in H2 nomenclature) was responsible for a change from apical to basolateral localization upon stable expression of the HA in the absence of other viral components (7). While the C543Y Japan HA was ca. 90% basolateral in stably transfected MDCK cells, expression of C560Y WSN HA in infected MDCK cells was observed at both apical and basolateral surfaces (Fig. 1B). These results may be explained by differences in polarized transport of H1 and H2 subtype HA containing the Y mutation at the cytoplasmic tail. In order to explore this possibility, MDCK cells were cotransfected with plasmid pCB7, encoding for hygromicin resistance and with plasmids pCAGGS-HAwt or pCAGGS-HAC560Y expressing wild-type and C560Y HA of WSN virus under the control of a chicken actin promoter (42). Stable MDCK cell clones expressing HA wild-type or HA C560Y mutant were selected in medium containing 0.2 mg of hygromycin/ml. Independent cell clones were expanded, and the distribution of the HA at the cell surface in these clones was studied by immunofluorescence with HA monoclonal antibody and rabbit polyclonal antibody against caveolin-1 (BD Transduction Laboratories), a protein with nonpolar distribution within the cell (37). Results from two representative clones are shown in Fig. 1C. Wild-type HA-expressing MDCK cells showed apical distribution of HA at the cell surface, as expected. By contrast, the C560Y HA was distributed at both apical and basolateral surfaces in MDCK cells.
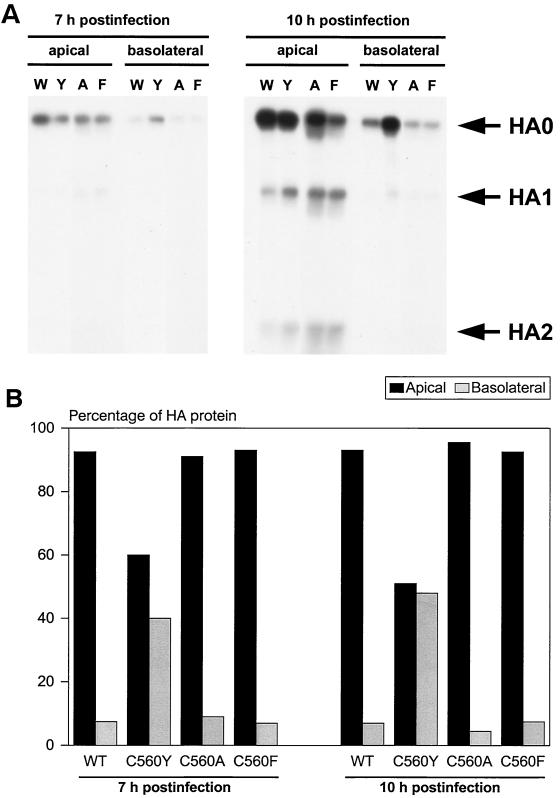
Previously, we found that the total levels of viral protein expression were similar among cells infected with wild-type, C560Y, C560A, and C560F viruses (data not shown). In order to quantitate the altered steady-state distribution of the HA protein seen by immunofluorescence in C560Y virus-infected cells, we performed biotinylation studies. Filter-grown MDCK cells were infected with WSN wild-type or with C560Y, C560A, or C560F influenza viruses at an MOI of 10, metabolically labeled with [35S]methionine and [35S]cysteine, and biotinylated from the apical or the basolateral side at 7 or 10 h postinfection, as previously described (28). Briefly, steady-state metabolic labeling was performed by the incubation of filters for 4 or 7 h (prior to biotinylation) with 200 μCi of Express 35S35S-label (Perkin-Elmer Life Sciences, Inc., Boston, Mass.)/filter in methionine-cysteine-free Dulbecco modified Eagle medium-0.2% bovine serum albumin supplemented with 0.1 volume of complete medium. Metabolically labeled monolayers were then biotinylated at 4°C by the addition of sulfo-NHS-biotin (0.5 mg/ml in PBS-CM) to the upper (apical) or the lower (basolateral) compartments of the filter chamber. The other compartment received an equivalent volume of PBS-CM. Biotinylation of monolayers on Transwells with biotin was carried out twice in a row for 15 min each. After removal of the biotin solution, the excess reactive biotin was blocked with 50 mM NH4Cl-PBS-CM. After extraction, immune precipitation, and streptavidin precipitation, the amount of apically or basolaterally biotinylated HA was measured by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (Fig. 2A), followed by densitometry (Fig. 2B). As expected, most surface HA protein (> 90%) was delivered to the apical membrane in cells infected with wild-type virus. In contrast, approximately equal amounts of HA were delivered to both apical and basolateral surfaces in cells infected with C560Y virus. Monolayers infected with C560A and C560F mutant viruses had a distribution of HA similar to that of cells infected with wild-type virus. The results demonstrate that a single amino acid change in the cytoplasmic tail of the HA protein results in altered (nonpolar) delivery of HA in cells infected with the C560Y virus. Interestingly, the percentage of basolateral HA (H1 subtype) was ca. 50% rather than the ca. 90% observed upon stable expression of the protein (H2 subtype) in the absence of other viral components (7). Analysis of MDCK cell clones stably expressing C560Y HA showed that, when this mutant H1 HA was expressed in the absence of other viral proteins, between 48 and 56% of the HA is located at the basolateral membrane (data not shown).
FIG. 2.
HA expression at apical and basolateral surfaces of infected MDCK cells. Cells were infected with the indicated wild-type (W) and mutant viruses C560Y (Y), C560A (A), and C560F (F). (A) Proteins expressed in infected cells were 35S labeled. At the indicated time point, proteins at the apical or basolateral membranes were biotinylated, cell extracts were made, and the HA was immunoprecipitated. After immunoprecipitation, the biotinylated HA was recovered with immobilized streptavidin and subjected to SDS-PAGE and autoradiography in order to determine the HA fraction present at apical or basolateral surfaces. Signals corresponding to uncleaved (HA0) and cleaved (HA1 and HA2) HA are indicated by the arrows. (B) The relative intensity of the HA present at apical (black columns) and basolateral (gray columns) surfaces was quantified by densitometry.
The nonpolar HA distribution caused by the C560Y mutation could affect the polarized budding of new virions from infected cells. To determine whether this is the case, we studied virus particle formation from the basolateral and apical membranes of cells infected with transfectant influenza viruses. As shown in Table 1, most infectious virus particles were released from the apical surface of cells infected with all three transfectant viruses, including C560Y. Thus, the mistargeting of significant amounts of HA to the basolateral membrane did not result in a redistribution of the areas of infectious influenza viruses assembly from the apical to the basolateral cell surfaces. The overall decrease in virus titers observed with C560Y virus might be explained by lower virus release due to lower levels of HA in the apical surface or to a decrease in viral budding due to the C560Y mutation. However, we cannot exclude that some other HA functions have been affected by the C560Y mutation resulting in decreased infectivity.
TABLE 1.
Infectious influenza virus particles released from the apical and basolateral membranes of polarized MDCK cellsa
| Virus | Particles (PFU/ml)
|
|
|---|---|---|
| Apical | Basolateral | |
| Wild type | 3 × 107 | 3 × 104 |
| C560A | 1 × 107 | 5 × 103 |
| C560F | 1 × 107 | 6 × 103 |
| C560Y | 4 × 106 | 4 × 103 |
MDCK cells were infected with an MOI of 2, and the numbers of infectious viruses released into the apical or basolateral chamber were determined 12 h postinfection by measuring the PFU in MDBK cells.
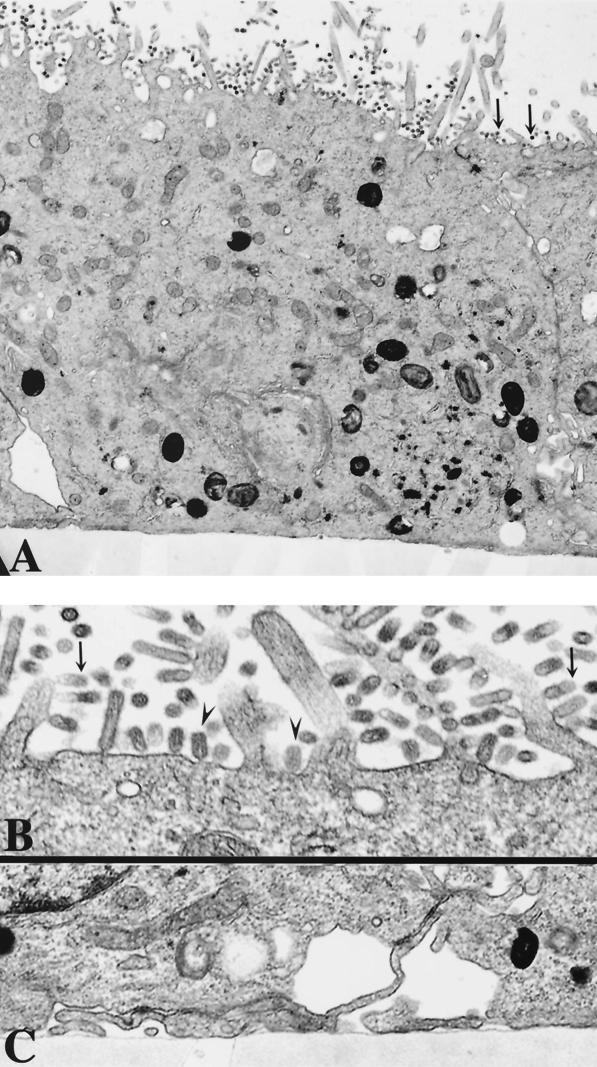
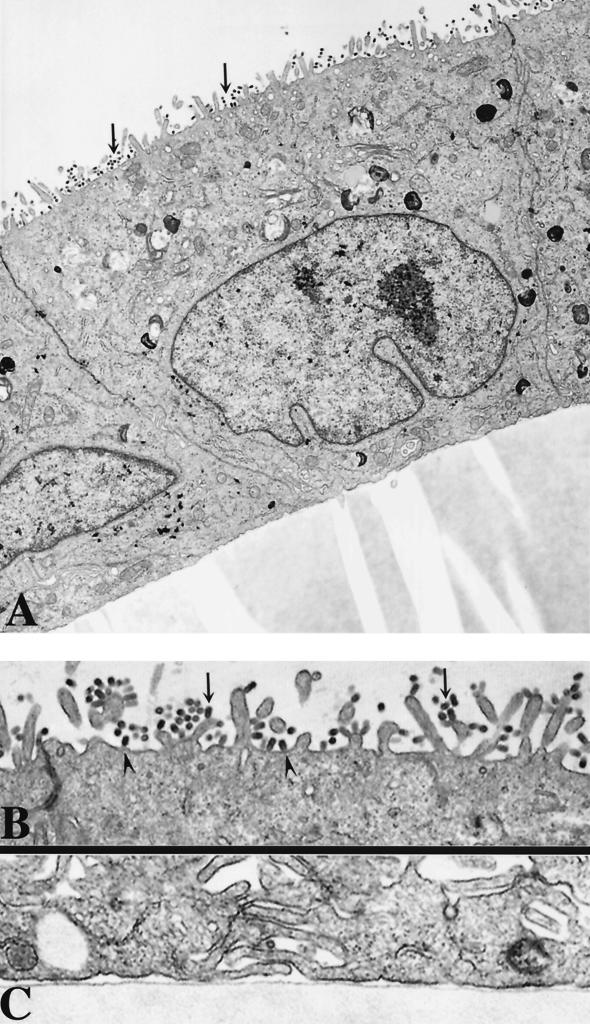
It might be possible that the transport of a significant percentage of the HA of C560Y virus to the basolateral membrane results in the basolateral relocation of several viral components sufficient for the budding of viruses which are noninfectious. In order to test this possibility, we performed electron microscopy studies of virus-infected cells. Polarized MDCK monolayers on polycarbonate filters were inoculated with wild-type or transfectant C560Y viruses at an MOI of 5. After 10 h of infection, the cells were analyzed by electron microscopy. Briefly, after three rinses with PBS-CM, the cells were fixed with 2% glutaraldehyde plus 1% paraformaldehyde in PBS-CM for 30 min. After three 0.1 M cacodylate buffer (pH 7.4) rinses, the samples were postfixed with 1% OsO4 in cacodylate buffer, block stained with 1% aqueous uranyl acetate, dehydrated in graded alcohols, and embedded in Epon 812. Specimens were examined with a JEOL 100 CX electron microscope. As shown in Fig. 3 and 4, budding influenza viruses were mainly detected at the apical surfaces in both wild-type and C560Y virus-infected cells. C560F and C560A viruses also budded mainly from apical surfaces of MDCK-infected cells (data not shown).
FIG. 3.
Electron micrographs of wild-type-virus-infected cells. Polarized MDCK cell monolayers were infected with wild-type virus and processed for transmission electron microscopy at 10 h postinfection. (A) Infected cells at ×11,000 magnification. Arrows indicate viruses at late stages of budding. (B and C) Apical (B) and basolateral (C) surfaces of infected cells at ×54,000 and ×22,000 magnifications, respectively. Virus buds (particles) are found assembled both on the microvillar (arrows) and on the planar areas (arrowheads) of the apical plasmalemma. Note that the viral particles are vectorially budding only on the apical surface of MDCK cells.
FIG. 4.
Electron micrographs of C560Y virus-infected cells. Polarized MDCK cell monolayers were infected with C560Y transfectant virus and processed for transmission electron microscopy at 10 h postinfection. (A) Infected cells at ×11,000 magnification. Arrows indicate viruses at late stages of budding. (B and C) Apical (B) and basolateral (C) surfaces of infected cells at ×26,500 and ×30,000 magnifications, respectively. Virus buds (particles) are found assembled both on the microvillar (arrows) and on the planar areas (arrowheads) of the apical plasmalemma. Note that the viral particles are vectorially budding only on the apical surface of MDCK cells.
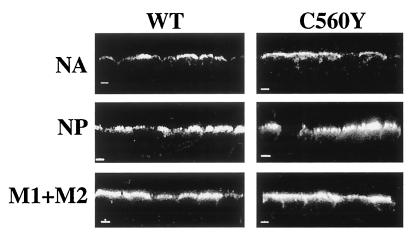
Incorporation of the HA in the virus particles may occur through specific interactions with other structural virus proteins. These interactions could lead to the colocalization of the interacting proteins in the same subcellular location. Thus, the distribution of different viral proteins at late times of infection was analyzed by confocal microscopy in C560Y-infected cells (Fig. 5) with monoclonal antibodies 10C9, HT103, and E10 directed against the NA, NP, and M1/M2 proteins of influenza A/WSN/33 virus, respectively (53, 70). E10 is a monoclonal antibody raised against the shared amino-terminal domain of M1 and M2 proteins of influenza A viruses, and it recognizes both M1 and M2 proteins (53). Although almost half of the HA protein was transported to the basolateral membrane in these cells (Fig. 1), all other viral structural proteins which were analyzed (NA, M1 + M2, and NP) were mainly located at the apical surface where virus budding takes place. As expected, these proteins were also apically transported in wild-type virus-infected cells. In these experiments we used MDCK cells at 11 h postinfection, when most of the structural viral proteins are distributed at the viral budding areas. Of interest, since the NA protein of WSN is known to facilitate the cleavage of the HA into HA1 and HA2 subunits (15, 29), apical location of NA may be responsible for the impaired HA cleavage at the basolateral surface of C560Y virus-infected cells (Fig. 2A).
FIG. 5.
Polarized sorting of the NA, NP, and M1+M2 proteins in MDCK cells infected with wild-type (WT) and C560Y viruses. MDCK monolayers grown on filters were infected with the indicated viruses. At 11 h postinfection, cells were fixed and immunostained with NA-specific, NP-specific, or M1-M2-specific monoclonal antibodies. The localization of these viral proteins was visualized by confocal microscopy. Representative reconstructed side views of immunostained cells are shown. Bars, 5 μm.
We have investigated the transport of the HA protein in polarized epithelial MDCK cells infected with the transfectant influenza virus C560Y. This virus contains a single amino acid change in the HA protein of influenza A/WSN/33 virus (H1 subtype) which results in the presence of a novel tyrosine residue in the cytoplasmic tail of the protein. An identical change was found to be responsible for the basolateral delivery of the HA protein of influenza A/Japan/305/57 virus (H2 subtype) in polarized MDCK cells which were constitutively expressing the mutated HA (7). In agreement with this observation, we found that the apical transport of the HA in C560Y virus-infected cells was decreased, and a significant proportion of the HA was now delivered to the basolateral membranes. However, apical delivery of the HA was not totally eliminated, and ca. 50% of this protein was still transported to the apical surface of the plasma membrane. Similar results were obtained when the C560Y WSN HA was expressed in MDCK cells in the absence of other viral proteins. The polarized apical transport of the constitutively expressed H2 HA mutant protein was previously shown to be more drastically reduced (7). The differences in polarized transport between the H1 and H2 HA mutant proteins might be due to differences in the amino acid composition and/or structure outside the cytoplasmic tails of these HA molecules.
The cysteine residue 560 in the cytoplasmic tail of the HA of influenza viruses has previously been shown to be palmitoylated (40, 41, 57, 58, 64). As HA was apically transported in cells infected with transfectant viruses C560A and C560F, the altered transport of the HA of C560Y virus was due to the presence of a new tyrosine residue in the cytoplasmic tail rather than to the loss of a palmitoylation site. A critical tyrosine residue is a common feature of basolateral transport signals in the cytoplasmic tail of cellular and viral glycoproteins (18, 20, 31, 32, 34, 36, 43, 61, 62). The tyrosine mutation has been found to mediate the internalization and cell surface recycling of the H2 HA through coated pits (27); however, we have not yet explored whether this is also the case for the H1 HA protein of the transfectant C560Y virus.
The glycoproteins of several enveloped viruses, when expressed in the absence of other viral structural proteins, are transported to the membrane domains where budding takes place (23, 48, 52, 59). This observation led to the hypothesis that the place of insertion of the viral envelope proteins determines the site of viral assembly. Mechanistically, this hypothesis requires that specific interactions between the envelope proteins and internal components of the virus are established in infected cells. In the case of influenza A virus there are three different integral viral envelope proteins: HA, NA, and M2. All of them are independently transported to the apical membrane and represent potential key elements in the determination of the membrane domain for virus assembly and budding in polarized epithelial cells. The altered transport of the HA protein of the transfectant influenza virus C560Y provided us with a unique opportunity to investigate the role of the subcellular HA localization in the viral budding process. We have found that, despite the accumulation of almost 50% of the surface-associated HA in the basolateral compartment of virus-infected cells, viral assembly and budding continues to be restricted to the apical surface. These results suggest that the HA is not a major determinant for the localization of viral budding in influenza virus-infected cells. However, we cannot exclude the possibility that interactions of the C560Y mutant HA with the cell-sorting machinery prevents its interaction with other viral components. It will be interesting to generate influenza virus mutants containing basolateral transport signals in their NA and M2 proteins in order to know the influence of these two viral integral membrane proteins in the viral budding localization.
It is possible that redundant signals in the HA, NA, and M2 cytoplasmic tails might mediate interactions with internal viral components, resulting in the targeted apical release of influenza viruses. Both the HA and NA cytoplasmic tails have been reported to stimulate the membrane association of the M1 protein (10), most likely by stimulating association of this protein with detergent-resistant membranes or lipid rafts (2, 4, 69). Although it was possible to generate infectious influenza viruses which lack the cytoplasmic tails of the HA, NA, or M2 proteins (13, 21, 35, 66), an influenza virus lacking the cytoplasmic tails of both HA and NA proteins showed altered virion morphology (22) and genome packaging (68). These results suggest that the cytoplasmic tail domains of the HA and NA proteins contain redundant signals involved in viral budding. In addition, a role of the transmembrane domains of the viral envelope proteins in promoting protein-protein interactions leading to apical virus budding cannot be excluded.
Consistent with our virus budding results, NA, M2, M1, and NP proteins are targeted to the apical membranes in wild-type and C560Y virus-infected cells. M1 and NP proteins are the major internal components of influenza viruses. NP is associated with the genomic RNA in form of ribonucleoprotein (RNP) complexes, while the M1 appears to be enwrapping the RNPs (39). Although it is known that the M1 protein can interact with membranes (8, 16, 17, 25) and that this protein appears to provide the budding force required for virus particle formation (14, 26), the signals responsible for the transport of the M1-RNP complexes to the apical surfaces in polarized cells infected with influenza viruses are unknown. Here we provide evidence supporting that the HA protein, which is the most abundant viral envelope protein, does not provide such a signal. Our experiments suggest that the responsibility for polarized influenza virus budding is shared by several of its membrane and matrix components. Whether the delivery of the M1-RNP complexes to the apical inner surface of infected cells is due to an intrinsic signal within this complex or to the establishment of interactions with the NA or M2 proteins is a subject that requires future experimentation.
Acknowledgments
We gratefully acknowledge Lee Cohen-Gould for the expert assistance with electron microscopy and Estanislao Nistal-Villán for excellent technical assistance.
This work was partially supported by NIH grants to A.G.-S. and P.P. and NIH grant GM 34107 (E.R.-B). E.R.-B. was supported by a Jules and Doris Stein Professorship from the Research to Prevent Blindness Foundation.
REFERENCES
- 1.Aberle, H., H. Schwartz, and R. Kemler. 1996. Cadherin-catenin complex: protein interactions and their implications for cadherin function. J. Cell. Biochem. 61:514-523. [DOI] [PubMed] [Google Scholar]
- 2.Ali, A., R. T. Avalos, E. Ponimaskin, and D. P. Nayak. 2000. Influenza virus assembly: effect of influenza virus glycoproteins on the membrane association of M1 protein. J. Virol. 74:8709-8719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ali, A., and D. P. Nayak. 2000. Assembly of Sendai virus: M protein interacts with F and HN proteins and with the cytoplasmic tail and transmembrane domain of F protein. Virology 276:289-303. [DOI] [PubMed] [Google Scholar]
- 4.Barman, S., A. Ali, E. K. Hui, L. Adhikary, and D. P. Nayak. 2001. Transport of viral proteins to the apical membranes and interaction of matrix protein with glycoproteins in the assembly of influenza viruses. Virus Res. 77:61-69. [DOI] [PubMed] [Google Scholar]
- 5.Barman, S., and D. P. Nayak. 2000. Analysis of the transmembrane domain of influenza virus neuraminidase, a type II transmembrane glycoprotein, for apical sorting and raft association. J. Virol. 74:6538-6545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonilha, V. L., A. D. Marmorstein, L. Cohen-Gould, and E. Rodriguez-Boulan. 1997. Apical sorting of influenza hemagglutinin by transcytosis in retinal pigment epithelium. J. Cell Sci. 110:1717-1727. [DOI] [PubMed] [Google Scholar]
- 7.Brewer, C. B., and M. G. Roth. 1991. A single amino acid change in the cytoplasmic domain alters the polarized delivery of influenza virus hemagglutinin. J. Cell Biol. 114:413-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bucher, D. J., I. G. Kharitonenkov, J. A. Zakomirdin, V. B. Grigoriev, S. M. Klimenko, and J. F. Davis. 1980. Incorporation of influenza virus M-protein into liposomes. J. Virol. 36:586-590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deschambeault, J., J. P. Lalonde, G. Cervantes-Acosta, R. Lodge, E. A. Cohen, and G. Lemay. 1999. Polarized human immunodeficiency virus budding in lymphocytes involves a tyrosine-based signal and favors cell-to-cell viral transmission. J. Virol. 73:5010-5017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Enami, M., and K. Enami. 1996. Influenza virus hemagglutinin and neuraminidase glycoproteins stimulate the membrane association of the matrix protein. J. Virol. 70:6653-6657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fantini, J., S. Baghdiguian, N. Yahi, and J. C. Chermann. 1991. Selected human immunodeficiency virus replicates preferentially through the basolateral surface of differentiated human colon epithelial cells. Virology 185:904-907. [DOI] [PubMed] [Google Scholar]
- 12.García-Sastre, A., J. A. Cabezas, and E. Villar. 1989. Proteins of Newcastle disease virus envelope: interaction between the outer hemagglutinin-neuraminidase glycoprotein and the inner non-glycosylated matrix protein. Biochim. Biophys. Acta 999:171-175. [DOI] [PubMed] [Google Scholar]
- 13.García-Sastre, A., and P. Palese. 1995. The cytoplasmic tail of the neuraminidase protein of influenza A virus does not play an important role in the packaging of this protein into viral envelopes. Virus Res. 37:37-47. [DOI] [PubMed] [Google Scholar]
- 14.Gómez-Puertas, P., C. Albo, E. Pérez-Pastrana, A. Vivo, and A. Portela. 2000. Influenza virus matrix protein is the major driving force in virus budding. J. Virol. 74:11538-11547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goto, H., and Y. Kawaoka. 1998. A novel mechanism for the acquisition of virulence by a human influenza A virus. Proc. Natl. Acad. Sci. USA 95:10224-10228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gregoriades, A., and B. Frangione. 1981. Insertion of influenza M protein into the viral lipid bilayer and localization of site of insertion. J. Virol. 40:323-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hay, A. J. 1974. Studies on the formation of the influenza virus envelope. Virology 60:398-418. [DOI] [PubMed] [Google Scholar]
- 18.Höning, S., and W. Hunziker. 1995. Cytoplasmic determinants involved in direct lysosomal sorting, endocytosis, and basolateral targeting of rat lgp120 (lamp-I) in MDCK cells. J. Cell Biol. 128:321-332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hughey, P. G., R. W. Compans, S. L. Zebedee, and R. A. Lamb. 1992. Expression of the influenza A virus M2 protein is restricted to apical surfaces of polarized epithelial cells. J. Virol. 66:5542-5552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hunziker, W., C. Harter, K. Matter, and I. Mellman. 1991. Basolateral sorting in MDCK cells requires a distinct cytoplasmic domain determinant. Cell 66:907-920. [DOI] [PubMed] [Google Scholar]
- 21.Jin, H., G. P. Leser, and R. A. Lamb. 1994. The influenza virus hemagglutinin cytoplasmic tail is not essential for virus assembly or infectivity. EMBO J. 13:5504-5515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jin, H., G. P. Leser, J. Zhang, and R. A. Lamb. 1997. Influenza virus hemagglutinin and neuraminidase cytoplasmic tails control particle shape. EMBO J. 16:1236-1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jones, L. V., R. W. Compans, A. R. Davis, T. J. Bos, and D. P. Nayak. 1985. Surface expression of influenza virus neuraminidase, an amino-terminally anchored viral membrane glycoprotein, in polarized epithelial cells. Mol. Cell. Biol. 5:2181-2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Keller, P., and K. Simons. 1997. Post-Golgi biosynthetic trafficking. J. Cell Sci. 110:3001-3009. [DOI] [PubMed] [Google Scholar]
- 25.Kretzschmar, E., M. Bui, and J. K. Rose. 1996. Membrane association of influenza virus matrix protein does not require specific hydrophobic domains or the viral glycoproteins. Virology 220:37-45. [DOI] [PubMed] [Google Scholar]
- 26.Latham, T., and J. M. Galarza. 2001. Formation of wild-type and chimeric influenza virus-like particles following simultaneous expression of only four structural proteins. J. Virol. 75:6154-6165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lazarovits, J., and M. Roth. 1988. A single amino acid change in the cytoplasmic domain allows the influenza virus hemagglutinin to be endocytosed through coated pits. Cell 53:743-752. [DOI] [PubMed] [Google Scholar]
- 28.Le Bivic, A., F. X. Real, and E. Rodriguez-Boulan. 1989. Vectorial targeting of apical and basolateral plasma membrane proteins in a human adenocarcinoma epithelial cell line. Proc. Natl. Acad. Sci. USA 86:9313-9317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li, S., J. Schulman, S. Itamura, and P. Palese. 1993. Glycosylation of neuraminidase determines the neurovirulence of influenza A/WSN/33 virus. J. Virol. 67:6667-6673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin, S., H. Y. Naim, A. C. Rodriguez, and M. G. Roth. 1998. Mutations in the middle of the transmembrane domain reverse the polarity of transport of the influenza virus hemagglutinin in MDCK epithelial cells. J. Cell Biol. 142:51-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lodge, R., L. Delamarre, J. P. Lalonde, J. Alvarado, D. A. Sanders, M. C. Dokhelar, E. A. Cohen, and G. Lemay. 1997. Two distinct oncornaviruses harbor an intracytoplasmic tyrosine-based basolateral targeting signal in their viral envelope glycoprotein. J. Virol. 71:5696-5702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lodge, R., J. P. Lalonde, G. Lemay, and E. A. Cohen. 1997. The membrane-proximal intracytoplasmic tyrosine residue of HIV-1 envelope glycoprotein is critical for basolateral targeting of viral budding in MDCK cells. EMBO J. 16:695-705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maisner, A., H. Klenk, and G. Herrler. 1998. Polarized budding of measles virus is not determined by viral surface glycoproteins. J. Virol. 72:5276-5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matter, K., W. Hunziker, and I. Mellman. 1992. Basolateral sorting of LDL receptor in MDCK cells: the cytoplasmic domain contains two tyrosine-dependent targeting determinants. Cell 71:741-753. [DOI] [PubMed] [Google Scholar]
- 35.Mitnaul, L. J., M. R. Castrucci, K. G. Murti, and Y. Kawaoka. 1996. The cytoplasmic tail of influenza A virus neuraminidase (NA) affects NA incorporation into virions, virion morphology, and virulence in mice but is not essential for virus replication. J. Virol. 70:873-879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Monlauzeur, L., A. Rajasekaran, M. Chao, E. Rodriguez-Boulan, and A. Le Bivic. 1995. A cytoplasmic tyrosine is essential for the basolateral localization of mutants of the human nerve growth factor receptor in Madin-Darby canine kidney cells. J. Biol. Chem. 270:12219-12225. [DOI] [PubMed] [Google Scholar]
- 37.Mora, R., V. L. Bonilha, A. Marmorstein, P. E. Scherer, D. Brown, M. P. Lisanti, and E. Rodriguez-Boulan. 1999. Caveolin-2 localizes to the golgi complex but redistributes to plasma membrane, caveolae, and rafts when coexpressed with caveolin-1. J. Biol. Chem. 274:25708-25717. [DOI] [PubMed] [Google Scholar]
- 38.Mostov, K. E., M. Verges, and Y. Altschuler. 2000. Membrane traffic in polarized epithelial cells. Curr. Opin. Cell Biol. 12:483-490. [DOI] [PubMed] [Google Scholar]
- 39.Murti, K. G., P. S. Brown, W. J. Bean, Jr., and R. G. Webster. 1992. Composition of the helical internal components of influenza virus as revealed by immunogold labeling/electron microscopy. Virology 186:294-299. [DOI] [PubMed] [Google Scholar]
- 40.Naeve, C. W., and D. Williams. 1990. Fatty acids on the A/Japan/305/57 influenza virus hemagglutinin have a role in membrane fusion. EMBO J. 9:3857-3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Naim, H. Y., B. Amarneh, N. T. Ktistakis, and M. G. Roth. 1992. Effects of altering palmitylation sites on biosynthesis and function of the influenza virus hemagglutinin. J. Virol. 66:7585-7588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Niwa, H., K. Yamamura, and J. Miyazaki. 1991. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene 108:193-199. [DOI] [PubMed] [Google Scholar]
- 43.Ohno, H., J. Stewart, M. C. Fournier, H. Bosshart, I. Rhee, S. Miyatake, T. Saito, A. Gallusser, T. Kirchhausen, and J. S. Bonifacino. 1995. Interaction of tyrosine-based sorting signals with clathrin-associated proteins. Science 269:1872-1875. [DOI] [PubMed] [Google Scholar]
- 44.Owens, R. J., and R. W. Compans. 1989. Expression of the human immunodeficiency virus envelope glycoprotein is restricted to basolateral surfaces of polarized epithelial cells. J. Virol. 63:978-982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Owens, R. J., J. W. Dubay, E. Hunter, and R. W. Compans. 1991. Human immunodeficiency virus envelope protein determines the site of virus release in polarized epithelial cells. Proc. Natl. Acad. Sci. USA 88:3987-3991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rindler, M. J., I. E. Ivanov, H. Plesken, E. Rodriguez-Boulan, and D. D. Sabatini. 1984. Viral glycoproteins destined for apical or basolateral plasma membrane domains traverse the same Golgi apparatus during their intracellular transport in doubly infected Madin-Darby canine kidney cells. J. Cell Biol. 98:1304-1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rodriguez-Boulan, E., and W. J. Nelson. 1989. Morphogenesis of the polarized epithelial cell phenotype. Science 245:718-725. [DOI] [PubMed] [Google Scholar]
- 48.Rodriguez-Boulan, E., and M. Pendergast. 1980. Polarized distribution of viral envelope proteins in the plasma membrane of infected epithelial cells. Cell 20:45-54. [DOI] [PubMed] [Google Scholar]
- 49.Rodriguez-Boulan, E., and S. K. Powell. 1992. Polarity of epithelial and neuronal cells. Annu. Rev. Cell Biol. 8:395-427. [DOI] [PubMed] [Google Scholar]
- 50.Rodriguez-Boulan, E., and D. D. Sabatini. 1978. Asymmetric budding of viruses in epithelial monlayers: a model system for study of epithelial polarity. Proc. Natl. Acad. Sci. USA 75:5071-5075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rossen, J. W., R. de Beer, G. J. Godeke, M. J. Raamsman, M. C. Horzinek, H. Vennema, and P. J. Rottier. 1998. The viral spike protein is not involved in the polarized sorting of coronaviruses in epithelial cells. J. Virol. 72:497-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Roth, M. G., R. W. Compans, L. Giusti, A. R. Davis, D. P. Nayak, M. J. Gething, and J. Sambrook. 1983. Influenza virus hemagglutinin expression is polarized in cells infected with recombinant SV40 viruses carrying cloned hemagglutinin DNA. Cell 33:435-443. [DOI] [PubMed] [Google Scholar]
- 53.Salvatore, M., C. F. Basler, J.-P. Parisien, C. M. Horvath, S. Bourmakina, H. Zheng, T. Muster, P. Palese, and A. García-Sastre. Effects of influenza A virus NS1 protein on protein expression: the NS1 protein enhances translation and is not required for shutoff of host protein synthesis. J. Virol., in press. [DOI] [PMC free article] [PubMed]
- 54.Sanderson, C. M., N. L. McQueen, and D. P. Nayak. 1993. Sendai virus assembly: M protein binds to viral glycoproteins in transit through the secretory pathway. J. Virol. 67:651-663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sanderson, C. M., H. H. Wu, and D. P. Nayak. 1994. Sendai virus M protein binds independently to either the F or the HN glycoprotein in vivo. J. Virol. 68:69-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sanger, C., E. Muhlberger, E. Ryabchikova, L. Kolesnikova, H. D. Klenk, and S. Becker. 2001. Sorting of Marburg virus surface protein and virus release take place at opposite surfaces of infected polarized epithelial cells. J. Virol. 75:1274-1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schmidt, M. F., and B. Lambrecht. 1985. On the structure of the acyl linkage and the function of fatty acyl chains in the influenza virus haemagglutinin and the glycoproteins of Semliki Forest virus. J. Gen. Virol. 66:2635-2647. [DOI] [PubMed] [Google Scholar]
- 58.Steinhauer, D. A., S. A. Wharton, D. C. Wiley, and J. J. Skehel. 1991. Deacylation of the hemagglutinin of influenza A/Aichi/2/68 has no effect on membrane fusion properties. Virology 184:445-448. [DOI] [PubMed] [Google Scholar]
- 59.Stephens, E. B., R. W. Compans, P. Earl, and B. Moss. 1986. Surface expression of viral glycoproteins is polarized in epithelial cells infected with recombinant vaccinia viral vectors. EMBO J. 5:237-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tashiro, M., J. T. Seto, S. Choosakul, M. Yamakawa, H. D. Klenk, and R. Rott. 1992. Budding site of Sendai virus in polarized epithelial cells is one of the determinants for tropism and pathogenicity in mice. Virology 187:413-422. [DOI] [PubMed] [Google Scholar]
- 61.Thomas, D. C., C. B. Brewer, and M. G. Roth. 1993. Vesicular stomatitis virus glycoprotein contains a dominant cytoplasmic basolateral sorting signal critically dependent upon a tyrosine. J. Biol. Chem. 268:3313-3320. [PubMed] [Google Scholar]
- 62.Thomas, D. C., and M. G. Roth. 1994. The basolateral targeting signal in the cytoplasmic domain of glycoprotein G from vesicular stomatitis virus resembles a variety of intracellular targeting motifs related by primary sequence but having diverse targeting activities. J. Biol. Chem. 269:15732-15739. [PubMed] [Google Scholar]
- 63.Tucker, S. P., and R. W. Compans. 1993. Virus infection of polarized epithelial cells. Adv. Virus Res. 42:187-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Veit, M., E. Kretzschmar, K. Kuroda, W. Garten, M. F. Schmidt, H. D. Klenk, and R. Rott. 1991. Site-specific mutagenesis identifies three cysteine residues in the cytoplasmic tail as acylation sites of influenza virus hemagglutinin. J. Virol. 65:2491-2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wandinger-Ness, A., M. K. Bennett, C. Antony, and K. Simons. 1990. Distinct transport vesicles mediate the delivery of plasma membrane proteins to the apical and basolateral domains of MDCK cells. J. Cell Biol. 111:987-1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Watanabe, T., S. Watanabe, H. Ito, H. Kida, and Y. Kawaoka. 2001. Influenza A virus can undergo multiple cycles of replication without M2 ion channel activity. J. Virol. 75:5656-5662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang, J., and R. A. Lamb. 1996. Characterization of the membrane association of the influenza virus matrix protein in living cells. Virology 225:255-266. [DOI] [PubMed] [Google Scholar]
- 68.Zhang, J., G. P. Leser, A. Pekosz, and R. A. Lamb. 2000. The cytoplasmic tails of the influenza virus spike glycoproteins are required for normal genome packaging. Virology 269:325-334. [DOI] [PubMed] [Google Scholar]
- 69.Zhang, J., A. Pekosz, and R. A. Lamb. 2000. Influenza virus assembly and lipid raft microdomains: a role for the cytoplasmic tails of the spike glycoproteins. J. Virol. 74:4634-4644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zheng, H., P. Palese, and A. García-Sastre. 1996. Nonconserved nucleotides at the 3′ and 5′ ends of an influenza A virus RNA play an important role in viral RNA replication. Virology 217:242-251. [DOI] [PubMed] [Google Scholar]
- 71.Zürcher, T., G. Luo, and P. Palese. 1994. Mutations at palmitylation sites of the influenza virus hemagglutinin affect virus formation. J. Virol. 68:5748-5754. [DOI] [PMC free article] [PubMed] [Google Scholar]