Abstract
The ability of the immune system to respond by ridding a pathogen without debilitating the host depends upon the ability of the effector Th (eTh) to make a discrimination between ‘self’ and ‘nonself’ antigens. This ability is somatically learned and involves The sorting of The somatically generated random repertoire of initial state Th (iTh) into two classes of specificity: one, anti-self, the functional expression of which must be inactivated; The other, anti-non-self, the functional expression of which must be activated. We propose a model for The origin of a sufficiency of eTh anti-non-self and an insufficiency of eTh anti-self based on two postulates. (i) An antigenindependent pathway to a priming level of eTh anti-non-self under conditions where iTh anti-self are effectively deleted by interaction with self. This state is established during a window of fetal development and maintained throughout life because self is persistent. (ii) Associative recognition of antigen (peptide-MHC class II) on an antigen-presenting cell between iTh and ‘primer’ eTh that results in The rapid induction of an effective level of helper activity to non-self antigen. A computer simulation is provided that enables evaluation of this model.
Keywords: computer simulation, origin of effector Th, self—non-self discrimination, tolerance, two-signal model
Introduction
The vertebrate immune system is a biodestructive ridding mechanism for The elimination of pathogens. The immune system is unique in That The antigen-specific receptors are generated at random by a somatic diversification process. Whether by somatic mutation, gene conversion, gene rearrangement or The combinatorial complementation of subunits, the somatic diversification of a few germline gene segments results in many new specificities, some of which recognize components of The host. When recognition leads to The biodestructive elimination of host components, the results are deleterious. Thus, the immune system requires a regulatory apparatus that is able to uncouple The immediate consequences of The recognition of antigen from The subsequent biodestructive ridding responses. The mechanism that allows The repertoire of randomly generated specificities to be sorted into Those that will not become biodestructive effectors and those that will is generally termed The self—non-self discrimination.
Several mechanisms for The self—non-self discrimination have been proposed over The years, many of which were discussed in a recent publication (1). As a result of this exchange of views we were prompted to develop a simulation tool that would allow an unbiased analysis of The common kinetic processes required by these postulated sorting mechanisms.
This first of two papers aims to illustrate The simulation tool that is computer based and is available via an interactive Internet server (http://www.cig.salk.edu, click on Th genesis). Here we provide The outline of the Theoretical foundation leaving a full description of The mathematics and simulation methods as part of our Internet server link.
All models of The self—non-self discrimination require a mechanism for the sorting of the somatically generated random repertoire that is based on distinguishing the ‘self’ and ‘non-self’ classes of antigen. In the end, this sorting mechanism is the responsibility of the Th cell that must learn which antigens are self and which are non-self. Upon encountering antigen, the Th cell must decide if it will proceed down one or another pathway of differentiation. One pathway leads to the inactivation of that cell, the other pathway leads to its activation and the subsequent expression of effector function (eTh). However, no sorting mechanism can be ‘perfect’ and the sorting mechanism of the self—non-self discrimination has to have a failure rate such that some individuals die, either because they fail to rid a non-self antigen (e.g. a pathogen) or because they in fact rid a self antigen. Evolution then selects on the failure rate such that the probability of a mutation lowering the failure rate is selectable above the combined probabilities of the individual being eliminated by some other pathway (e.g. the failure to run faster than a predator). Our goal when developing this simulation tool was to give quantitative precision to the key steps required by any internally coherent mechanism for the sorting of paratopes. These variables can then be evaluated for experimental reasonableness.
In order to use a random paratopic repertoire to identify nonself antigens in a pool of self antigens, the paratopes have to be sorted according to some property that enables them to distinguish these two classes of antigens. We recall that we are discussing the ‘adaptive’, not the ‘innate’ immune system. The sorting of a somatically generated and selected paratopic repertoire requires a somatic selection mechanism that must be based on the behavior, not some physico-chemical property of antigens as classes.
Conceptual framework
A somatically selected self—non-self discrimination is simply one that acts to sort a large and random repertoire of somatically generated paratopes. The simulation tool that we have developed is designed to investigate the combined effects of the various sources of error in sorting systems that make a self—non-self discrimination. While the simulation tool can be adapted to all of the presently proposed models, we have been encouraged by the reviewers to present this revised version in which we concentrate on our Minimal Model (2). We will deal with ‘other views’ in a second paper (Langman, R. E., Mata, J. J. and Cohn, M., in preparation).
The Minimal Model treats ‘self’ and ‘non-self’ as being indistinguishable except for their behaviors. It is assumed that the behavior which is unique to self antigens is their constant presence, whereas the unique behavior of non-self antigens is their variable presence. In principle, the immune system had two choices, either to remember the antigens that are always present or to remember the antigens that are always absent. While the idea of remembering constantly present or persistent antigens seems intuitively easy and attractive, it turns out that the minimal condition is that the immune system must remember the antigens that have been absent—no memory being needed for persistent antigens. The repository for this memory of unseen or absent antigens is the eTh cell anti-nonself.
Under the Minimal Model, all T and B cells are ‘born’ with an antigen receptor of a single random specificity. The cells are in a decision or i-state (initial state) of differentiation that is incapable of expressing effector function. The usual way for istate cells to become e-state effectors is for their receptors to first bind antigen and then be informed by eTh cells whether that particular antigen is non-self. This eTh cell passes on its information via an antigen-specific link to i-state cells, which then utilize this information and differentiate to the e-state. In the absence of such information (i.e. the absence of eTh cells with specificity for a persistent self antigen), these i-state cells having bound antigen then proceed to death. Of course, since the informative Th cells are effectors (eTh), the next step is to determine where these ‘primer’ eTh cells come from. This is a non-trivial problem, given that the model at this stage requires eTh in order to convert iTh that have bound ligand into new eTh. The solution to this conundrum depends on the postulate that an antigen-independent pathway exists uniquely for the conversion of iTh to eTh. This pathway operates in the extended absence of antigenic encounter, thereby ensuring that only eTh specific for non-self accumulate as primer. The sorting process is initiated during an embryonic window when the immune system arises in the presence of all self antigens, no non-self antigens and no eTh. Once initiated, the window closes and the continued presence of self antigens maintains the insufficiency of eTh anti-self while allowing a priming level of eTh anti-non-self to arise in the absence of non-self. The immune system is responsive when a priming repertoire of eTh anti-non-self is established.
These steps can be formulated as a series of rate equations where the rate constants are chosen to be both biologically reasonable and consistent with the requirements of the Minimal Model. Finding solutions to these equations is not difficult and we have programmed one of our web-based servers to find solutions in response to a straightforward set of questions. Indeed we encourage any interested reader to log on to the website (http://www.cig.salk.edu, click on Th genesis) and test it out. The only part of the mathematics and computerese we need to deal with here is how the rate equations are set up.
The Minimal Model in terms of the simulation tool
The Th cells uses an antigen receptor (TCR), which identifies peptides bound to MHC class II molecules expressed on the surface of the antigen-presenting cells (APC). Under the Minimal Model, interaction between antigen and an iTh cell results in its being converted into an anticipatory a-state (i.e. antigen delivers Signal[1] that drives the iTh to become an aTh). Cells in the a-state await information (i.e. Signal[2]) from the eTh. If Signal[2] fails to arrive in a given time, then the aTh cell dies. Only if Signal[2] arrives before that time limit is reached does the aTh become an eTh cell. Thus, only iTh that do not have specificity for self peptides and, therefore, do not encounter antigen can, after a sufficient period of waiting, progress to become eTh in what we term an antigenindependent step. The result is a series of timed steps that can be converted into rates, which are then used to compute the consequences of choosing various parameters that control these rates.
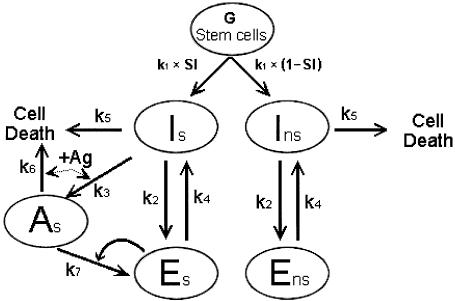
Referring to Fig. 1, the symbols we chose for simplicity in these equations are I for the number of iTh, A for the number of aTh and E for the number of eTh cells. The secondary symbols ‘s’ and ‘ns’ denote the specificities of the various cells with respect to self and non-self respectively. Thus we can write:
k3 as the rate constant for iTh + Ag → aTh or Is + Ag → As
k2 as the rate constant for iTh → eTh or Is → Es and Ins →Ens
k6 as the rate constant for aTh + Ag → death or As + Ag → death (the antigen in this case is self)
k7 as the rate constant for aTh, eTh/Ag > eTh, where eTh anti-non-self delivers Signal[2] via associated recognition of antigen (we refer to this as the ‘autocatalytic’ step)
Fig. 1.
The pathway (Th genesis) generating ‘primer’ effector Th (see text).
Coupled with the turnover rate constant k5, as well as the rate constant (k4) for reversion from E to I, these rates of reactions cover the key regulatory events needed to make a self—non-self discrimination.
Since There are no non-self antigens present at the stages of ontogeny that occur under maternal immune protection, there can be no Ans, although the antigen-independent pathway does allow Ens to eventually appear. Overall, we assume homeostasis of the number of total cells per milliliter so that the rate of Th genesis at the level of iTh is exactly equal to the combined rates of death of all cells from all causes.
These relationships are illustrated in Fig. 1, which also shows the various rate constants that govern each conversion in the state of the cells. Briefly, a pool of precursors (G) generates I cells at k1G cells per hour. They are split into the anti-self (Is) and anti-non-self (Ins) pathways according to the value SI (specificity index), which is simply the proportion of anti-self in the repertoire. Thus k1G × SI is the number of cells per hour that become Is and k1G × (1 - SI) is the number per hour that become Ins. Then, governed by rate constant k2, I cells, whether Is or Ins, are converted to Es and Ens cells respectively as required under the rules of the antigenindependent pathway. In the case of Is cells, interaction with self antigen drives them to As at a rate k3. Cells in the a-state cannot enter the antigen-independent pathway, only i-state cells can do this. Cells in the a-state require interaction with eTh (delivery of Signal[2]) to proceed down the pathway to effectors. The Es and Ens can revert to the Is and Ins states respectively at a rate k4. Is and Ins cells die at a rate k5 that maintains homeostasis, and As cells die at a rate k6 if they fail to receive Signal[2]. We will deal in a second paper (3) with the ‘autocatalytic’ antigen-driven conversion of aTh to eTh by ‘primer’ eTh shown in Fig. 1 (The k7 step). Here, we consider the state of the system before encounter with non-self antigen. As a consequence, we will use an approximation to establish the limit conditions for permissible levels of Es and Ens.
What Is the SI, why Is it essential and what values can it take?
Given that the paratopic repertoire of T cells Is random with respect to the antigens that are recognized, it follows that in practice some will be anti-self while others are anti-non-self. The goal of the sorting mechanism that we are simulating Is to minimize the number of Es (because these have the potential to kill the host) and maximize the number of Ens (because these are required to rid the pathogen). Our simulation is based on real numbers of cells in each compartment and this makes it necessary to determine the actual numbers of anti-self and anti-non-self cells that are present in the population before sorting. Our choices for the values for the rate constants have to be such that we have an effective sorting mechanism. The SI is a definition of the relative numbers of T cells that enter the sorting pathway as anti-self and anti-nonself. SI is defined as the proportion of anti-self in the total repertoire (i.e. anti-self + anti-non-self) and can take values in The range from 1 to near 0 (in principle the smallest value of SI would be 1/repertoire when there is only one anti-self in the total repertoire).
Since we are dealing with the genesis of Th cells, there has to be a source of iTh that enter the sorting system after having passed through positive selection as well as a certain amount of negative selection in the thymus. Without having to specify just how much the antigen-specific repertoire has been selected intrathymically we can use SI as a parameter that is independent of the size of the repertoire. The value of SIthat is chosen is a variable that has to be determined by a meld of trial and error plus a measure of common sense. In other words, the value chosen for SI establishes the magnitude of the sorting problem; if, for example, SI = 0.5, then half of the repertoire is anti-self and the magnitude of the sorting problem is large, whereas if SI = 0.0001, then there would be only one anti-self in 10,000 receptors and the sorting problem would be small. The value of SI is a measure of the uniqueness of an antigen in the context of a random paratopic repertoire. Whether the size of the repertoire is such that it divides the antigenic universe into 104 or 108 slices, the value of SIwould be a constant because it measures the relative ratios of the two classes of antigen, self and non-self.
Based on our previous analysis of specificity in T and B cells (4-6), we estimate the value of SI to be 0.01 (i.e. <0.1 and >0.001). However, the simulation tool will accept any value between 0 and 1 chosen by the user.
The principle of a modular T-Protecton is needed
We recall that the B-Protecton arose from our realization that the immune system must have evolved as a modular structure. The ‘B-Protecton’ is defined as the minimum sized sample of B cells from an individual that would have all of the protective properties of the whole (3,4). The Protecton allowed us to resolve the paradox that, as individuals, a mouse with 108 B cells is as well-protected as a human with 1012 B cells. This means that a human is no different from a pool of B cells made from 104 mice. Our best estimate for the size of the B-Protecton is ∼107 total B cells; meaning that, as an order of magnitude, a mouse has 10 Protectons and a human 105 Protectons.
The concept of a Protecton must also apply to the T cell compartment and we use the symbols Tt and Tb to denote the total number of cells in one T- or B-Protecton respectively. In the case of B cells, their secreted antibodies function concentration dependent and it is relatively straightforward to calculate the structure of the B-Protecton. While T cells must also show a density- or concentration-dependent behavior, the relationship between concentration and function is less well characterized.
While we have at present no way to independently calculate the value of Tt, it seems on a priori grounds that this should not be very different from Tb. It is reasonable, however, to estimate the upper limit to the size of a Protecton at ∼107 cells on the grounds that this is the total number of lymphocytes in the smallest animals with functional immune systems (e.g. the pygmy shrew and humming bird). The tadpole does have an immune system with ∼106 total cells, but it is not clear that this is > 10% effective in protecting the tadpole during its rather short period of developmental existence. Therefore, as an initial guess we started with Tt = 107, keeping in mind that we could always check the effects of different values of Tt in the simulation tool.
Choosing an initial set of rate constants, k2, k3, k4, k5 and k6
Starting with a value for k5 we appreciated that the half-life of an iTh must be long enough to have a good chance of its being properly sorted and stably functional. A value of 10 h for the half-life seemed too short when we thought about the time (value of k5) needed to control the antigen-independent generation of eTh, so we started with k5 expressed as t1/2 = 100 h and found after several simulations an optimized value of k5 expressed as t1/2 = 128 h. with the slowest rate constant k5 set at around t1/2 = 100-150 h we then looked at the fastest rate constant, i.e. k3, which is the rate of conversion of iTh to aTh in the presence of antigen and found that a half-life of 1 h seemed to be of the right order. The remaining rate constants were arrived at by running a range of values for each in the simulator, and selecting for those that optimized the criteria of having few eTh anti-self (i.e. Es) and sufficient eTh anti-non-self (i.e. Ens).
We arrived at the following rate constants, expressed for convenience in terms of half-lives in hours: k2 = 40, k3 = 1, k4 = 12, k5 = 128 and k6 = 5. We recall that k = 0.693/t1/2.
Homeostasis is a required assumption
T cells are produced throughout the life of the individual. Thus, there must be a homeostatic control of the number of T cells per gram or per milliliter of animal. From this it follows that the input number of T cells must equal the output. The rate constant k1 deals with the input of I cells and k5 deals with the predominant output pathway of I cells with a minor output via k6. Under conditions of homeostasis we need to assign values to either k5 and k6, or k1—not both. The small additional effect of k6 makes it easier to assign k5 and k6, which then sets the value of k1. Figure 1 also illustrates that if k5 is set to zero, then the only pathway for I cells to leave is via k3 and then k6. If the half-life of I cell turnover is set at 128 h (i.e. k5 = 5.42 × 10-3), then the corresponding thymic output computes to be 106 iTh per day per T-Protecton or 107 iTh per day per mouse. We use the notation G to symbolize the concentration of precursor iTh cells so that k1G gives the number of iTh per hour, after positive selection, that enters the pool of cells that follows the various pathways indicated in Fig. 1. These inputs and outputs are independent of the actual number of cells in the total measurable pool of I, A and E cells which is set to a total Tt.
What exactly is being simulated?
At this point we have a system of equations and a starting set of values for the variables. However, the results consist of steady-state numbers of all the various cell types and in most cases these are not the kinds of numbers that can be presently measured experimentally. What we would really like to know is whether these numbers are compatible with a protective immune response to non-self antigen without anti-self debilitating the host. While it is evident that at least one Ens per T-Protecton is required, it is not obvious how many Ins would be needed to ensure the initiation of a protective response, and the best that can be said about the Es and Is populations is that they have to be acceptably small, and certainly less than the Ens and Ins populations.
The data in Table 1 are taken directly from the raw numbers obtained from the Internet interface (www.cig.salk.edu, click on Th genesis) and are based on a set of values for the rate constants that we regard as near optimal.
Table 1.
Assigning values to the parameters of Th genesis: the reference Protecton
| Is | Es | As | Ins | Ens | Thymic output | |
|---|---|---|---|---|---|---|
| (k1G per hour) | ||||||
| Cells per T-Protecton (Tt) | 603 | 181 | 3015 | 7689386 | 2306816 | 42113 |
| Cells per epitope per Tt (Ct) | 0.60 | 0.18 | 3.0 | 77.7 | 23.3 |
The values of the rate constants were: k2 = 0.017325 (40 h), k3 = 0.693 (1 h), k4 = 0.05775 (12 h), k5 = 0.005422 (128 h) and k6 = 0.1386 (5 h), where the times in parenThesis are the half-lives, and k = 0.693/t1/2; SI = 0.01, Tt = 107,Rt = 105 (Rt is the size of the repertoire, see later).The numbers of self and non-self epitopes are calculated As follows: total self epitopes = SI × Rt = 103, total non-self epitopes = (1 - SI)× Rt = 9.9 × 104. the half-life of the T-Protecton turnover when the rate of input of T cells (k1 × G) is 42113 computes to be 164 h or 6.8 days (42113 × 10-7 = 1 - e-k; k= 0.00422, t1/2 = 164 h).
The values in Table 1 are artificially significant but they are given in order to show the exact numbers as they appear in the simulator on the Internet server. The calculated values for the actual number of Es per self epitope per T-Protecton and Ens per non-self epitope per T-Protecton show a 130-fold difference between the two. While this seems intuitively satisfying, we found ourselves in need of a new parameter that gives a more realistic dimension to the system.
A new parameter, the Priming Inductive Event (PIE)
We calculate the probability of a PIE. PIE is the probability that an Es and As cell will associatively recognize antigen (peptide) in a priming inductive event, the consequence of which is that the As becomes an Es. This initial A—E interaction is an essential component of associative recognition of antigen which requires that only A and E cells specific for the same antigen be allowed to productively interact. For the purposes of this simulation we assume that one antigen produces one peptide which is bound to MHC class II molecules on the surface of the APC. Thus, PIE will depend on the number of APC available to mediate effective presentation of peptide from a given antigen.
The number of PIE per T-Protecton is a function of the number of APC that present a given epitope per T-Protecton. PIE values are calculated as follows. If X is set to equal E cells per epitope per APC and Y is set to equal A cells per epitope per APC, then the probability of an A-E interaction per APC is (1 - e -X)(1 - e -Y). This is the probability for each APC and, when multiplied by the number of APC per T-Protecton, becomes the value of PIE. When computed for As and Es we obtain PIEs for anti-self cells and similarly PIEns for the antinon-self cells.
The calculation of PIEns needs a comment. As we are interested here in the status of the immune system prior to the appearance of non-self antigens, there are no Ans present. However, to approximate the priming readiness of the system, we used the values for Ins to make the calculation of PIEns. This approximation is reasonable as the conversion of I → A in the presence of antigen is rapid. The second paper (3) will formalize this ‘autocatalytic’ step.
If the number of APC presenting a given self epitope is much below the copy number (Ct) of either As or Es, then all APC will be occupird with one or more As or Es, or both. Under these conditions our calculation treats the APC as producing a single PIE. While this assumption may not be valid, there must be some limit to the maximum number of PIE per APC and at present there is no clear indication of what the limit might be. We will assume it to be one.
There is a trade-off to consider at low and high numbers of APC per epitope per T-Protecton. At low numbers of APC the time it takes for the rare E and A cells to find each other becomes limiting. At high numbers of APC the probability of an A and an E cell finding the peptides from one antigen on a given APC becomes limiting.
In conclusion, we should emphasize that There may be other ways of formulating a PIE, but the one element that seems unavoidable under all models is the critical role of the APC. The Minimal Model only considers APC that present antigen at a functional threshold level as would be expected for self antigens. The limiting factor in this case is the eTh having to find a corresponding iTh on the same APC.
Establishing limits to immunity in terms of PIEs and PIEns
The minimal, least stringent boundary conditions would be <1 PIEs and >1 PIEns. However, we would imagine that such a singular dividing line is unrealistic, in part because the risk of inducing immunity toward a self antigen would be unacceptably high. The most stringent yet reasonable value of the autoimmune boundary in terms of PIEs is <0.01, which means that there would be less than one priming inductive event in 100 T-Protectons per self epitope. The most stringent yet reasonable value of the immune boundary in terms of PIEns is >10, which means that There would be >10 priming inductive events per Protecton. We will use these stringent boundary conditions in evaluating the parameters, i.e. PIEs <0.01 and PIEns >10.
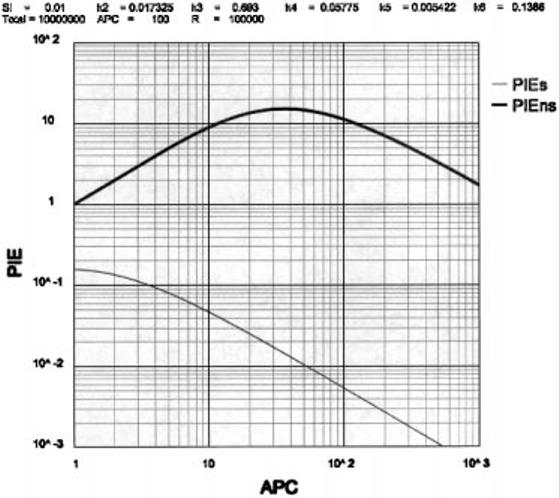
The data in Fig. 2 shows the effect of varying the number of APC per T-Protecton on the values of PIEs and PIEns using our optimal values for the rate constants. In order to meet the PIEs < 0.01 autoimmune boundary, the number of APC presenting a given self peptide must exceed 50 per T-Protecton. In order to meet the PIEns > 10 immune boundary, the number of APC presenting a given non-self peptide must be 15 < APC < 120 per T-Protecton. The result then is that the window of function in this case is rather narrow (50 < APC < 120), but it must be remembered that we have set boundary conditions that are quite stringent. By inspection the two curves suggest that there is unlikely to be a window of joint compliance with stringent boundary conditions over a >2-to4-fold difference in APC numbers separating the upper and lower bounds. We will return to this point later.
Fig. 2.
The relationship between PIEs or PIEns and the numbers of APC presenting a given peptide per T-Protecton.
Estimating limits to the variables under stringent PIEs and PIEns boundary conditions
Based on what we considered to be the optimal set of values in the simulation, we next searched for the upper and lower limits of each parameter. The results are summarized under individual headings.
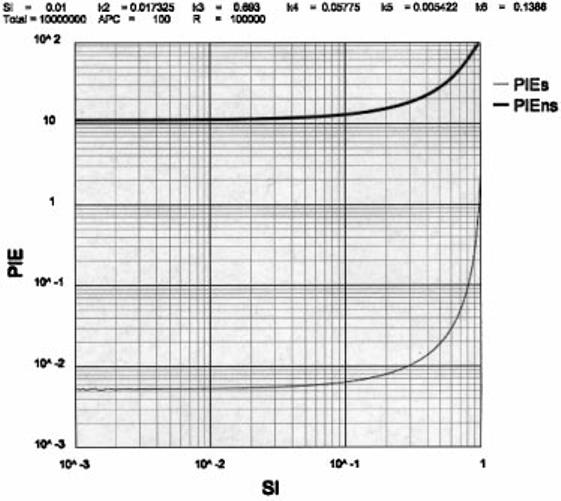
Limits to the value of SI
Figure 3 shows a plot of the effect of various values of SI on the number of PIEs and PIEns. Even if we consider stringent values for the autoimmune and immune boundaries (i.e. <0.01 PIEs and >10 PIEns), there is little effect on these boundaries at values of SI < 0.1. Several effects combine to make values of SI > 0.1 unreasonable. When SI >> 0.1 the input of cells (k1G) increases to result in a half-life of the Protecton (Tt) that decreases sharply from 6.8 days at SI = 0.01 to 6.2 days at SI = 0.1, to 2.9 days at SI = 0.6. At SI > 0.3 the PIEs boundary of 0.01 is breached. The increase in PIEns as SI increases >0.1 is due to the increase in copy number (Ct) of the anti-non-self repertoire. We consider values of SI > 0.1 beyond biological expectation.
Fig. 3.
The relationship between PIEs or PIEns and SI.
Values of SI < 0.001 reduce the numbers of anti-self cells per epitope per Protecton to such low levels (Is = 0.6, As = 3.0, Es = 0.2) that it becomes unselectable (5). To illustrate this point, Es = 0.2 means that there would be two effector Th anti-‘a-given-self peptide’ per mouse (equivalent to 10 T-Protectons).
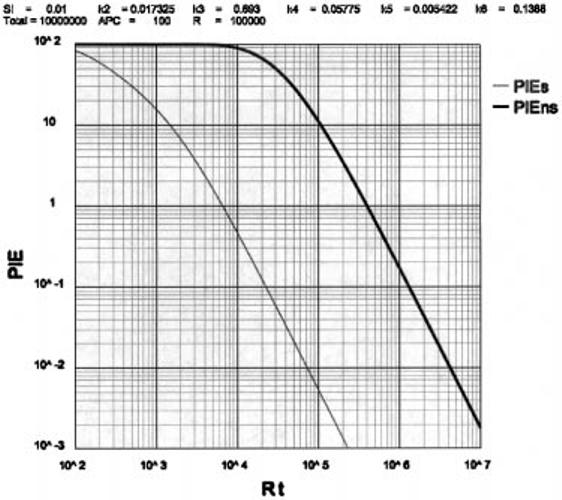
Limits to the size of the TCR repertoire
The size of the TCR repertoire, Rt, can be roughly estimated from the amount of information available to TCR recognition in the peptide that binds to MHC. If There are ∼5 amino acid residues that are available to TCR recognition after fixing the anchor residues and if at each position any of 20 amino acids could be inserted, then the maximum number of possible peptides is 205 or ∼3 × 106. Since only about half of the amino acid replacements change recognition in a functional way, a reasonable range would place Rt at > 104 and < 106. In Fig. 4 we plot the PIEs and PIEns for various values of Rt. Using 100 APC per T-Protecton as the standard condition we find that as Rt falls <7 × 104, the upper limit of 0.01 PIEs is reached, and when Rt rises > 1.3 × 105 the boundary of 10 PIEns is breached because the number of cells per epitope is too low to permit sufficient induction on 100 APC and decreasing the number of APC runs into the breaching of the PIEs < 0.01 autoimmune boundary. Thus, to find such a narrow range for the optimal value of Rt was unexpected when viewed from the uncritical perspective of more-is-always-better.
Fig. 4.
The relationship between PIEs or PIEns and Rt.
Effects due to the size of the T-Protecton
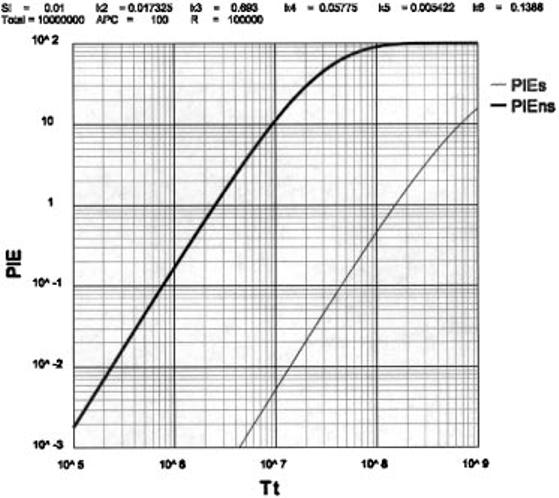
Although homeostasis keeps the number of T cells constant, the actual number of T cells in the T-Protecton (Tt) is largely determined by the size of the repertoire Rt. Setting Rt = 105 and the total number of APC per Tt = 100, the results in Fig. 5 show the effect of varying Tt on the boundary conditions, PIEs < 0.01 and PIEns > 10. There is a surprisingly sharp upper limit of Tt = 1.3 × 107 when the PIEs boundary exceeds 0.01 and a lower limit of Tt = 9 × 106 when the immune boundary PIEns drops <10.
Fig. 5.
The relationship between PIEs or PIEns and the size of the protection (Tt)
Choice of the rate constants
The rate constants show a general pattern of broad limits even under the very restrictive conditions of PIEs < 0.01 and PIEns > 10. This surprisingly broad spectrum of rate constants suggests that evolution did not need a finely tuned set of reactions to establish a functional self—non-self discrimination mechanism. However, the more exacting test of the Minimal Model using the simulator is to use ranges of random numbers and select for systems of parameters that satisfy the PIE boundary conditions.
Using random values of the variables to search for unexpected solutions
The intuitive hunt-and-peck method of searching is usually good enough to identify optimal values for the variables within the bounds of a moderately transparent set of assumptions. However, this does not guarantee that other solutions may not also exist and should be examined in the light of the assumptions that would need to be made. We therefore set up a set of searches based on the use of random values for most of the variables while searching for the largest acceptable repertoire. The rationale for searching for solutions that give the largest possible repertoire that remains functional was that this seemed to be the most likely evolutionary selection pressure and this would reveal those parameters that most severely limit the size of the repertoire.
The results were somewhat surprising in the sense that the values of the rate constants were not a major factor in limiting the size of the repertoire. While this kind of result is often put forward as evidence of the ‘robustness’ of a model, we have interpreted this as simply showing the validity of the principle of the Minimal Model because it provides a mechanism that is largely independent of the size of the repertoire.
One obvious limiting factor in the size of the repertoire is the total number of cells Tt in the T cell Protecton and the copy number (Ct) of Th cells specific for a given non-self antigen. It is self evident that a value of Tt = 107 and a Ct = 10 would limit The functional repertoire to < 106.
Summarizing the relationship between parameters and boundaries
The limits to the parameters of the Th-Protecton determined under various boundary conditions are shown in Table 2. Under stringent conditions they are surprisingly narrow and, unsurprisingly, relaxing the boundary conditions broadens the limits, which nonetheless remain quite restricted.
Table 2.
The limits to the parameters of the Th genesis pathway
| Parameter | Autoimmune boundary (PIEs) |
Immune boundary (PIEns) |
|||
|---|---|---|---|---|---|
| <0.01 | <0.1 | <1 | >10 | >1 | |
| APC per antigen | >50 | >4 | >1b | <120 | <2000 |
| Specificity index (SI) | <0.1a | <0.1a | <0.1a | <0.1a | <0.1a |
| Size of Protecton (Tt) | <1.3 × 107 | <4.5 × 107 | <1.5 × 108 | >9 × 106 | >1.5 × 106 |
| Size of repertoire (Rt) | >7 × 104 | >2 × 104 | >6 × 103 | <1.3 × 105 | <4 × 105 |
SI > 0.1 results in biologically meaningless effects discussed in text. Under stringent conditions PIEs < 0.01 and SI < 0.1, best estimate SI = 0.01.
The maximum PIEs is only 0.15 when There is 1 APC per antigen.
The ‘autoimmune boundary’ is the value of PIEs above which the individual would be debilitated.
The ‘immune boundary’ is the value of PIEns below which the response to a pathogen would be ineffective.
It might be added that as a result of the detailed search using random variables as described above, there is an added factor based on the number of APC and the choice of PIEns boundary that determines the maximum size of the T cell anti-peptide repertoire (Rt). as the stringency of PIEns is decreased, the permissible size of the repertoire increases provided there is a corresponding decrease in the number of APC that can present antigen.
Discussion
The cells of the ‘adaptive’ immune system are born as initial state or i-cells without effector activity. They undergo a self—non-self discrimination, a decision mechanism that sorts the somatically generated paratopic repertoire expressed by i-cells into anti-self, which is purged leaving an anti-non-self repertoire to protect the animal. This decision mechanism is dependent on the sufficiency or insufficiency of eTh specific for a given antigen. As Th are born as iTh, and their ability to make a self—non-self discrimination is dependent on an insufficiency of eTh anti-self and a sufficiency of eTh antinon-self, the question arises, what is the origin of these two eTh states of sufficiency?
Unique to our answer is (i) an antigen-independent pathway from iTh to eTh and (ii) the induction of iTh to eTh requires associative recognition of antigen. This latter means that antigen (defined as the unit of elimination by the ridding effector function) must be processed to peptide and presented on an APC as a (peptide-class II MHC) complex.
An iTh and an eTh must recognize the presented peptides from the single antigen (unit of elimination) and the eTh must deliver Signal[2] to the iTh, which then goes on to differentiate to an eTh (the ‘autocatalyic’ step). The iTh interacting with an APC in the absence of an eTh delivered Signal[2] dies. In order for this interaction of associative recognition to take place, the peptides derived from the given antigen must be presented in a ‘signaling patch’, which is the only pathway permitting an eTh to deliver a signal to the iTh.
The question of the origin of the priming level of eTh anti-nonself and the insufficiency of eTh anti-self is dealt with here by analyzing in detail an antigen-independent pathway from iTh to eTh that itself undergoes a self—non-self discrimination.
While there is little disagreement that associative recognition of antigen by i-cells and eTh operates in the case of B cells and cytotoxic T cells, there is lack of acceptance of this assumption for Th where an iTh—APC—eTh interaction is denied.
The acceptance or denial of associative recognition of antigen for the induction of iTh is what distinguishes the models (Langman, R. E., Mata, J. J. and Cohn, M.; in preparation). As each APC presents peptides of both self and non-self origin, APC cannot sort the Th repertoire. The sorting must be antigen specific and dependent solely on the recognition elements of the Th repertoire itself.
It is the associative recognition of antigen on the APC (iTh—APC—eTh) that gives the APC a unique role in regulating the magnitude of the response. Too few or too many APC presenting a given antigen become rate limiting. Too few are limiting because iTh and eTh sit around awaiting their turn; too many are limiting because iTh and eTh find themselves on different APC unable to communicate. No other model predicts an optimum number of APC because it is assumed that iTh + APC → eTh. This latter response would be proportional to the number of APC presenting antigen, of course until it saturates.
Despite a strong intuitive belief that the rates of reaction would place sharp limits on the values of the parameters of the Minimal Model of the self—non-self discrimination, we were wrong. The rate constants of the reactions illustrated in Fig. 1 show a wide range of possible values, limited primarily by the PIEs and PIEns boundary conditions that are chosen. Rather than tout the ‘robustness’ of the model, as if this were an a priori desirable feature of any model, we emphasize the evolutionary advantage of allowing an even quite sloppy range of rate constants to control key elements in the selection process. In other words, once there are two pathways for the iTh to take, one being antigen-driven inactivation (Signal[1]) and the other being antigen-independent activation, the relative rates can differ several fold and yet permit an adequate self—non-self discrimination to be made. Thus, evolution did not have to get all the different parts and set the rate constants of each just right before a somatic self—non-self discrimination could be made. Certainly over time evolution will optimize these reactions and their rates, but these were not necessarily critical to get the process started.
The effect of the ‘autocatalytic’ step (k7 in Fig. 1) will be analyzed in a second paper (Langman, R. E., Mata, J. J. and Cohn, M.; in preparation) where the response to non-self antigens will be considered, as will also the other views.
Acknowledgement
This investigation has been supported by NIH grant RR07716.
Abbreviations
- APC
antigen-presenting cell
- aTh
Anticipatory Th
- Ct
cells specific for a given epitope per Tt (i.e. copy number)
- eTh
Effector Th
- iTh
Initial state ‘antigen-responsive’ Th
- ns
non-self
- PIE
priming inductive event
- Rt
size of paratopic T cell repertoire
- s
self
- Tb
total number of cells per B-Protecton
- Tt
total number of cells per T-Protecton
References
- 1.Langman RE, editor. Self—non-self discrimination revisited. Semin. Immunol. 2000;12:344. doi: 10.1006/smim.2000.0227. [DOI] [PubMed] [Google Scholar]
- 2.Langman RE, Cohn M. A minimal model for the self— non-self discrimination: a return to the basics. Semin. Immunol. 2000;13:189. doi: 10.1006/smim.2000.0231. [DOI] [PubMed] [Google Scholar]
- 3.Langman RE, Cohn M. The E—T (elephant—tadpole) paradox necessitates the concept of a unit of B-cell function: the Protecton. Mol. Immunol. 1987;24:675. doi: 10.1016/0161-5890(87)90050-2. [DOI] [PubMed] [Google Scholar]
- 4.Cohn M, Langman RE. The Protecton: the evolutionarily selected unit of humoral immunity. Immunol. Rev. 1990;115:1. doi: 10.1111/j.1600-065x.1990.tb00783.x. [DOI] [PubMed] [Google Scholar]
- 5.Cohn M. A new concept of immune specificity emerges from a consideration of the self—non-self discrimination. Cell. Immunol. 1997;181:103. doi: 10.1006/cimm.1997.1212. [DOI] [PubMed] [Google Scholar]
- 6.Langman RE. The specificity of immunological reactions. Mol. Immunol. 2001;37:555. doi: 10.1016/s0161-5890(00)00083-3. [DOI] [PubMed] [Google Scholar]