Abstract
Nectin-1 is a receptor for herpes simplex virus (HSV), a member of the immunoglobulin superfamily, and a cellular adhesion molecule. To study domains of nectin-1α involved in cell fusion, we measured the ability of nectin-1α/nectin-2α chimeras, nectin-1α/CD4 chimeras, and transmembrane domain and cytoplasmic tail mutants of nectin-1α to promote cell fusion induced by HSV-1 glycoproteins. Our results demonstrate that only chimeras and mutants containing the entire V-like domain and a link to the plasma membrane conferred cell-fusion activity. The transmembrane domain and cytoplasmic tail of nectin-1 were not required for any viral receptor or cell adhesion function tested. Cellular cytoplasmic factors that bind to the nectin-1α cytoplasmic tail, therefore, did not influence virus entry or cell fusion. Interestingly, the efficiency of cell fusion was reduced when membrane spanning domains of nectin-1α and gD were replaced by glycosylphosphatidylinositol tethers, indicating that transmembrane domains may play a modulatory role in the gD/nectin-1α interaction in fusion.
Keywords: glycosylphosphatidylinositol, fusion, syncytium, nectin-1, herpes simplex, transmembrane domain, cytoplasmic tail
INTRODUCTION
Herpes simplex virus (HSV) entry into susceptible cells and virus-induced cell fusion require glycoproteins B (gB), gD, gH, and gL (Cai et al. 1988a; Cai et al. 1988b; Highlander et al. 1988; Huff et al. 1988; Johnson and Ligas 1988; Ligas and Johnson 1988; Forrester et al. 1992; Hutchinson et al. 1992; Roop et al. 1993; Balan et al. 1994; Davis-Poynter et al. 1994; Wilson et al. 1994). HSV particles can fuse directly with the plasma membrane in a pH-independent manner (Wittels and Spear 1991). However, recent studies suggest that a cell-type or cell-line dependent internalization of viral particles and a reduction in pH may increase efficiency of virus entry (Nicola et al. 2003; Gianni et al. 2004; Nicola and Straus 2004). Regardless, HSV glycoprotein-induced cell fusion occurs at neutral pH. Fusion of HSV-infected cells with adjacent uninfected cells can result in the formation of large multi-nucleated cells called syncytia (Spear 1993; Pertel and Spear 1998). Because syncytia survive for a relatively short period of time, syncytium formation may represent a mechanism of virus-induced cell killing. To study the fusion events that occur during virus entry and virus-induced cell fusion, plasmid-based expression systems have been developed. Cells expressing a gD receptor form syncytia when transfected with plasmids expressing gB, gD, gH, and gL (Connolly et al. 2003; Jones and Geraghty 2004). Also, when mixed together, cells expressing gB, gD, gH, and gL fuse with cells expressing a gD receptor, and the extent of fusion can be measured by reporter gene expression (Turner et al. 1998; Pertel et al. 2001; Jones and Geraghty 2004; Tiwari et al. 2004).
Efficient fusion requires the interaction of viral gD with a cell surface receptor from one of three classes of receptors (Spear et al. 2000). HVEM (herpesvirus entry mediator) is a member of the tumor necrosis factor receptor family. HVEM is expressed on lymphocytes and other cells and can mediate the entry of most HSV-1 and HSV-2 isolates (Montgomery et al. 1996; Kwon et al. 1997). A second class of gD receptor, 3-O-sulfotransferase-modified heparan sulfate present on cell surface proteoglycans, mediates the entry of many HSV-1 strains (Shukla et al. 1999). The final class of receptors contains the immunoglobulin (Ig) superfamily members nectin-1 and nectin-2. Nectin-1 expression in normally resistant cells allows entry of all viable isolates of HSV-1 and -2 tested thus far (Cocchi et al. 1998b; Geraghty et al. 1998; Krummenacher et al. 2004). Nectin-2 mediates the entry of laboratory isolates of HSV-1 with a mutation in the amino-terminus of gD and some strains of HSV-2 (Warner et al. 1998; Lopez et al. 2000; Yoon and Spear 2004). Once gD binds its receptor, a region of gD, the “pro-fusion” domain, may interact with the other viral fusion glycoproteins to promote membrane fusion (Cocchi et al. 2004; Zago et al. 2004).
Nectins are members of a growing family of related Ca2+-independent cell adhesion molecules that localize to E-cadherin-based adherens junctions (Takahashi et al. 1999; Miyahara et al. 2000; Satoh-Horikawa et al. 2000; Reymond et al. 2001). To promote cell adhesion, dimers of nectin-1 interact in trans with nectin-1, nectin-3, or nectin-4 dimers on adjacent cells (Lopez et al. 1998; Miyahara et al. 2000; Satoh-Horikawa et al. 2000; Sakisaka et al. 2001; Momose et al. 2002). Trans interactions occur via binding of respective V-like domains and are required for efficient localization of nectins to areas of cell-cell contact (Miyahara et al. 2000; Fabre et al. 2002). Mutagenesis, monoclonal antibody binding, and in vitro binding studies have identified the V-like domain of nectin-1 as the gD-binding region (Cocchi et al. 1998a; Krummenacher et al. 1999; Krummenacher et al. 2000; Cocchi et al. 2001; Geraghty et al. 2001; Martinez and Spear 2002; Struyf et al. 2002a). The gD-binding region overlaps with the region involved in the trans interactions necessary for cell adhesion (Fabre et al. 2002; Krummenacher et al. 2002). The V-like domain alone, when engineered to be expressed on the surface of the cell, can mediate virus entry although at a level significantly reduced from wild-type nectin-1 (Cocchi et al. 1998a). Upon addition of the V-like domain to CD4, nectin-2, or the poliovirus receptor, these chimeric molecules display wild-type nectin-1 virus-entry activity (Cocchi et al. 2001; Geraghty et al. 2001). However, a nectin-1/CD4 chimera, called 1/1/1/4/4, containing the entire extracellular domain of nectin-1 (including an intact V-like domain) binds gD but does not allow entry of HSV-1 (Geraghty et al. 2001), indicating that the V-like domain is necessary but not sufficient for virus entry. The lack of 1/1/1/4/4 entry activity may be due to the extended distance of the V-like domain from the plasma membrane (Jones and Geraghty 2004). The two C-like extracellular domains of nectin-1 have as of yet not been assigned a specific role in virus entry.
The cytoplasmic tail (CT) of many nectins, including the nectin-1αisoform, binds the PDZ domains of the F-actin-binding protein afadin (Mandai et al. 1997; Takahashi et al. 1999; Satoh-Horikawa et al. 2000) and the cell polarity protein PAR-3 (Takekuni et al. 2003). Trans interactions between nectins result in the activation of Rac and Cdc42 small G proteins, independent of afadin or PAR-3, via a mechanism involving activation of c-Src (Kawakatsu et al. 2002; Fukuhara et al. 2003; Honda et al. 2003; Fukuhara et al. 2004; Hoshino et al. 2004). It has not been reported whether Cdc42/Rac activation occurs upon gD binding but since the gD-binding region overlaps with the region involved in trans interactions (Fabre et al. 2002; Krummenacher et al. 2002), gD binding could result in small G protein activation. Manipulation of the actin cytoskeleton or other cytoskeletal elements by afadin or activation of Rac and Cdc42 could impact nectin-dependent processes such as virus entry and cell fusion. Actin remodeling facilitates membrane fusion during cellular processes such as intracellular trafficking (Eitzen 2003). Previous reports found that HSV-1 “fusion from without” and “fusion from within” were inhibited by the addition of the actin filament-disrupting agent cytochalasin D, suggesting a role for actin in syncytium formation (Heeg et al. 1986; Walev et al. 1991; Yura et al. 2000). It has yet to be determined whether association of nectin-1α and cellular cytoplasmic proteins influences the efficiency of virus entry or cell fusion.
Nectin-1α, therefore, has multiple extracellular and intracellular domains that function in virus entry and cell adhesion. To better understand the process of nectin-1α-mediated cell fusion and to compare and contrast the requirements for fusion in virus entry and cell fusion, we analyzed nectin-1α mutants and chimeras to identify the domains important for cell fusion. We found that only nectin-1α mutants and chimeras with an intact V-like domain and a link to the plasma membrane, such as a transmembrane domain (TM) or a glycosylphosphatidylinositol (GPI) link, functioned in cell fusion. The efficiency of cell fusion was reduced when membrane spanning domains of nectin-1α and gD were replaced by a GPI tether, indicating that TMs play a modulatory role in the gD/nectin-1 interaction in fusion. Recently, a nectin-1gpi mutant was demonstrated to mediate virus entry and cell fusion (Gianni et al. 2004). Here we confirm and extend those results to quantify virus entry, cell fusion, and trans interactions for a nectin-1gpi mutant and also a nectin-1α mutant lacking a CT. The nectin-1α CT, and therefore interactions with cytoplasmic cellular factors, were not important for virus entry or cell fusion.
RESULTS
Nectin-1 chimeras
The major domains of nectin-1 (3 Ig-like folds in the extracellular domain, the transmembrane domain, and the cytoplasmic tail) have functions assigned to them such as gD binding, trans interactions for cell adhesion, cellular localization, association with the actin cytoskeleton, and activation of small G proteins. Our goal was to determine the importance of each domain/function in cell fusion to better understand the process of nectin-1-mediated cell fusion. To study general domains important for cell fusion involving nectin-1, we utilized nectin-1α/nectin-2α and nectin-1α/CD4 chimeras. These chimeras have been previously characterized for their ability to bind gD and to mediate virus entry (Geraghty et al. 2001) allowing us to compare and contrast the receptor requirements for fusion occurring during virus entry and cell-cell fusion.
Diagrams of nectin-1α, nectin-2α, CD4, and the general positions where the chimeras were constructed are depicted in Figures 1A and B. Details on the construction of the chimeras and exact amino acid composition of each chimera have been described previously (Geraghty et al. 2001). Nectin-1α and nectin-2α are closely related (35% amino acid identity) and have the same general domain organization. The 1/2/2 chimera contains all of the predicted nectin-1α V-like domain replacing the homologous region of nectin-2α. The 1*/2/2 chimera is nearly identical to 1/2/2 except the final 22 amino acids of the V-like domain were derived from nectin-2α. Chimera 1/1*/2 contains the entire nectin-1 V-like domain and most of the first C-like domain joined to nectin-2α at the equivalent amino acid after the fourth conserved Cys. Figure 1B depicts the nectin-1α/CD4 chimeras containing single (1/4/4) or multiple (1/1/4/4 and 1/1/1/4/4) Ig-like domains of nectin-1α fused to a hinge region in CD4 located N-terminal to the two membrane-proximal Ig domains. The nectin-1α region in chimera 1/4/4 consisted of the entire V-like domain except for a 22 amino acid deletion between the second conserved Cys and the predicted end of the V-like domain.
Figure 1.
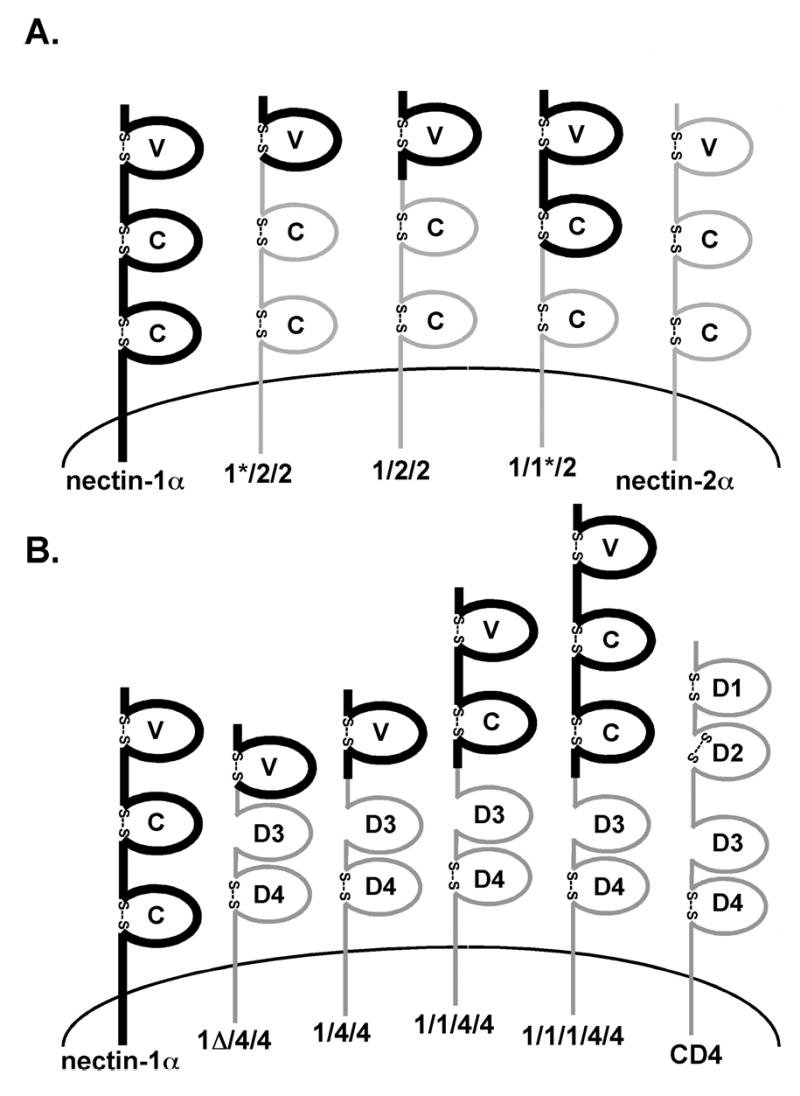
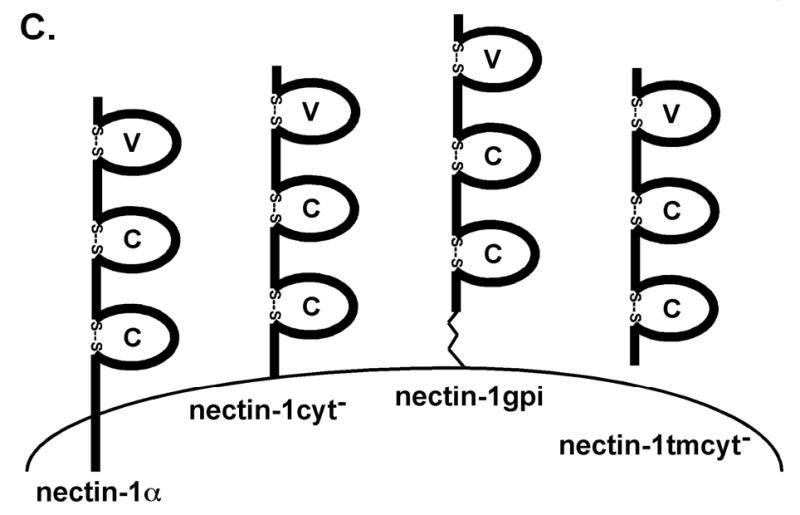
Nectin-1α/nectin-2α chimeras and nectin-1α/CD4 chimeras. (A) Regions of nectin-2α (thin gray lines) were replaced with the homologous nectin-1α regions (thick black lines). The names indicate origin of first, second and third Ig-like domains. (B) Nectin-1α sequence indicated by thick black lines and CD4 sequence indicated by the thin gray lines. (C) Nectin-1α transmembrane and cytoplasmic domain mutants. Nectin-1cyt−, cytoplasmic tail deleted after arginine 382. Nectin-1gpi, transmembrane domain and cytoplasmic tail removed after threonine 354 and GPI addition sequence from DAF added. Nectin-1tmcyt−, transmembrane domain and cytoplasmic tail deleted after phenylalanine 333. Amino acid numbering starts with the initiator methionine residue for the nectin-1α sequence (GenBank accession number AF060231). Characteristic disulfide bonds predicted for many Ig domains indicated by “S—S”. V= variable-like domain, C= constant-like domain, D= Ig-like domain. Drawing not to scale. (A) and (B) reprinted from (Geraghty et al. 2001) with permission from Elsevier.
All chimeras were previously shown to be expressed at the cell surface at levels equivalent to wild-type nectin-1α with two exceptions, 1/1/4/4 and 1/1/1/4/4, for which expression was reduced but significantly above background (Geraghty et al. 2001). When gD binding was related to cell surface expression, all chimeras bound HSV-1 gD at approximately equivalent levels compared to wild-type nectin-1α (Geraghty et al. 2001). The one exception was the 1/4/4 chimera which displayed no detectable gD-binding activity (Geraghty et al. 2001).
Cell fusion activity of nectin-1 chimeras
Chinese hamster ovary (CHO) cells (K1 clone) do not express a functional gD receptor and therefore do not support HSV-1-glycoprotein-induced cell fusion, making them ideal for the study of gD receptors in cell fusion. We employed a cell-mixing fusion assay to assess the ability of nectin-1α and chimeras to promote cell fusion induced by HSV-1 glycoproteins. CHO K1 cells were transfected with plasmids expressing gB, gD, gH, gL, and T7 polymerase (1:1:1:1:1 molar ratio). The glycoprotein-transfected cells were mixed (at a 1:1 ratio) with CHO K1 cells transfected with plasmids expressing nectin-1α (or chimera) and pG1NT7β-gal (3:1 molar ratio). The plasmid G1NT7β-gal contains the lacZ gene under the control of the T7 promoter (Feng et al. 1996). Upon cell fusion, the cell contents mix and T7 polymerase induces the synthesis of β-galactosidase (β-gal). The amount of β-gal produced was measured as an indication of cell fusion. To determine cell surface expression of the nectin-1 chimeras during the fusion assay, the transfected cells were analyzed by CELISA in parallel with each fusion assay. The CELISA technique has been previously described (Geraghty et al. 2000; Geraghty et al. 2001; Jones and Geraghty 2004). Briefly, transfected cells were incubated with an anti-nectin-1 monoclonal antibody (mAb) CK6, washed, and fixed prior to the addition of secondary antibody and an antibody detection system. CK6 recognizes a linear epitope in the V-like domain of nectin-1 (Krummenacher et al. 2000) present in all the chimeras.
The relative results for the nectin-1α/nectin-2α chimeras are shown in Figure 2A. The chimeras with an intact nectin-1α V-like domain, 1/2/2 and 1/1*/2, mediated cell fusion via the HSV-1(KOS) glycoproteins at levels just below wild-type nectin-1α. The 1*/2/2 chimera, however, mediated cell fusion at a greatly reduced level when compared to nectin-1α (Fig. 2A), despite a higher cell surface expression (Fig. 2B). These results directly paralleled those obtained for gD binding and virus entry (Geraghty et al. 2001). A low but significant level of cell fusion was mediated by nectin-2α despite the use of HSV-1(KOS) envelope glycoproteins (Fig. 2B). To determine whether the low fusion activity was caused by low expression of nectin-2α compared to nectin-1α, tagged version of the two receptors were created by fusing the yellow fluorescent protein (YFP) to the C-terminus of the CT of the respective receptors. The fusion results with the YFP-tagged versions of nectin-1α and nectin-2α were identical to those in Figure 2A (data not shown). Nectin-1YFP and nectin-2YFP were expressed at the same level in cells used in the fusion assay based upon western blot analysis using an anti-YFP antibody (data not shown). Therefore, when nectin-1α and nectin-2α were expressed at equivalent levels, nectin-2α was much less efficient at mediating cell fusion with HSV-1(KOS) glycoproteins. Our results are consistent with previous observations for nectin-2α using a similar cell fusion assay (Yoon et al. 2003; Zago and Spear 2003), but differed from the negligible activity of nectin-2α for HSV-1(KOS) entry described previously (Warner et al. 1998).
Figure 2.
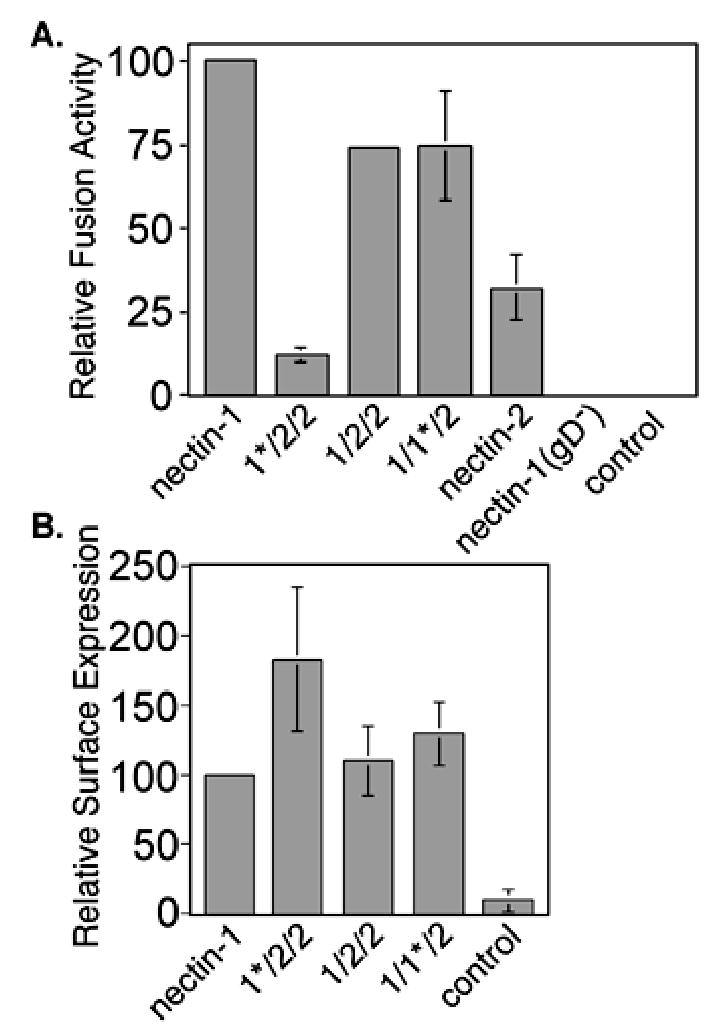
Fusion activity of nectin-1α/nectin-2α chimeras in a cell-mixing fusion assay. (A) Fusion assay. CHO-K1 cells expressing gB, gD, gH, gL, and T7 polymerase, or control plasmid pCAGGS substituted for gD plasmid (gD−), or all control plasmid (control) were mixed with CHO-K1 cells expressing nectin-1α or chimera and pG1NT7β-gal. Within each experiment, all values were expressed as a percentage of the value obtained for the positive control (nectin-1). (B). Cell-surface expression of chimeras in the fusion assay. CELISA analysis of receptor expressing cells from (A) using the nectin-1 mAb CK6. Within each experiment, all values were expressed as a percentage of the value obtained for the positive control (nectin-1). The absence of error bars for relative values given is due to standard deviations too small to generate a visible error bar. The fusion/CELISA experiments were performed three times and the mean values plus standard deviations for the combined relative results are depicted.
Results obtained for the nectin-1α/CD4 chimeras are shown in Figure 3. The 1/4/4, 1/1/4/4, and 1/1/1/4/4 chimeras all mediated HSV-1-induced cell fusion (Fig. 3A). The relative fusion activity (percent of nectin-1α) for 1/4/4 and 1/1/4/4 (Fig. 3A) was higher than the relative cell surface expression (percent of nectin-1α) shown in Figure 3B. The ratio of relative fusion activity to relative surface expression for 1/4/4 and 1/1/4/4 was 147%/71% and 55%/23%, respectively, approximately a value of 2. The ratio for 1/1/1/4/4 was 43%/61%, approximately 0.75. Those values indicate that the 1/4/4 and 1/1/4/4 chimeras demonstrated an enhanced fusion activity over nectin-1α (which would by definition have a value of 1) if the relationship between cell surface expression of nectin-1α and cell fusion activity was linear for the cell mixing assay. The positive fusion result for the 1/1/1/4/4 chimera was surprising because although 1/1/1/4/4 bound HSV-1(KOS) gD with wild-type nectin-1 efficiency, it did not mediate HSV-1(KOS) entry (Geraghty et al. 2001). Therefore, the virus entry and cell fusion results with the 1/1/1/4/4 chimera suggest a difference in the receptor requirements for the two processes. The 1Δ/4/4 chimera failed to mediate cell fusion despite being expressed at high levels at the cell surface (Fig. 3A and B). This result was consistent with the inability of 1Δ/4/4 to bind HSV-1 gD and an inability to mediate virus entry (Geraghty et al. 2001).
Figure 3.
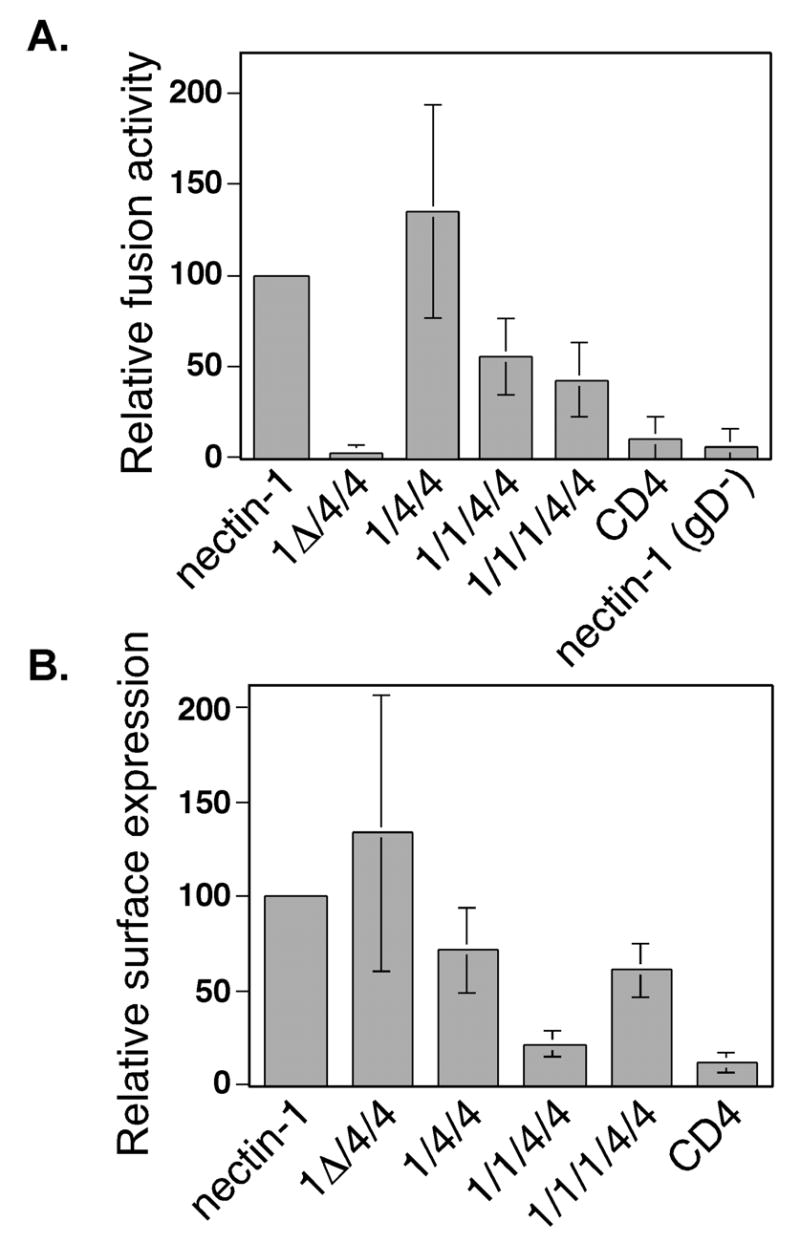
Fusion activity of nectin-1α/CD4 chimeras in a cell-mixing fusion assay. (A) Fusion assay. CHO-K1 cells expressing gB, gD, gH, gL, and T7 polymerase, or control plasmid pCAGGS instead of gD plasmid (gD−), or all control plasmid pCAGGS (control) were mixed with CHO-K1 cells expressing nectin-1α or chimera and pG1NT7β-gal. Within each experiment, all values were expressed as a percentage of the value obtained for the positive control (nectin-1). (B). Cell-surface expression of chimeras in the fusion assay. CELISA analysis of cells from (A) using the nectin-1 mAb CK6. Within each experiment, all values were expressed as a percentage of the value obtained for the positive control (nectin-1). The 1Δ/4/4 chimera, however, was not efficiently recognized by CK6 so another anti-nectin-1 V-like domain mAb, CK5, was used and the resulting values were expressed as a percentage of the results obtained for nectin-1-expressing cells with CK5. The fusion/CELISA experiments were performed three times and the mean values plus standard deviations for the combined relative results are depicted.
Taken together, the results with the nectin-1α chimeras indicate that like virus entry, efficient cell fusion requires an intact V-like domain. The domains of nectin-1α other than the V-like domain, the two C-like extracellular domains and the TM and CT, are not required for efficient cell fusion.
Nectin-1 mutants promote trans interactions and virus entry
Because the specific nectin-1α TM and CT were not necessary for efficient cell fusion, we next sought to determine whether any TM or CT was required for cell fusion. The nectin-1cyt− mutant was created to examine the importance of the nectin-1α CT to cell fusion (Fig. 1C). The entire CT in nectin-1cyt− was deleted except for the four Arginine anchor just after the TM. To examine the importance of the nectin-1α TM and CT for cell fusion, we constructed a nectin-1α molecule lacking those regions but still tethered to the outer leaflet of the plasma membrane via a GPI anchor. This mutant, nectin-1gpi, was created using polymerase chain reaction (PCR) to replace the TM and CT of nectin-1α with the 29 aminoacid-GPI-addition sequence from decay-accelerating factor (DAF) (Zhou et al. 1997) (Fig. 1C). To further examine whether a link to the plasma membrane was required for cell fusion, we constructed a nectin-1α mutant lacking the entire TM and CT domains, nectin-1tmcyt− (Fig. 1C).
Nectin-1gpi expression was detected at the surface of transfected CHO K1 cells by CELISA using an anti-nectin-1 mAb and the expression was comparable to wild-type nectin-1α (Fig. 4A). We treated CHO K1 cells expressing nectin-1gpi or nectin-1α with phosphatidylinositol phospholipase C (PIPLC), which cleaves GPI-anchored proteins from the surface of cells, to verify that nectin-1gpi was attached to the plasma membrane by a GPI anchor. As depicted in Figure 4A, PIPLC treatment reduced cell-surface expression of nectin-1gpi approximately 95% while the cell-surface expression of nectin-1α was not reduced.
Figure 4.
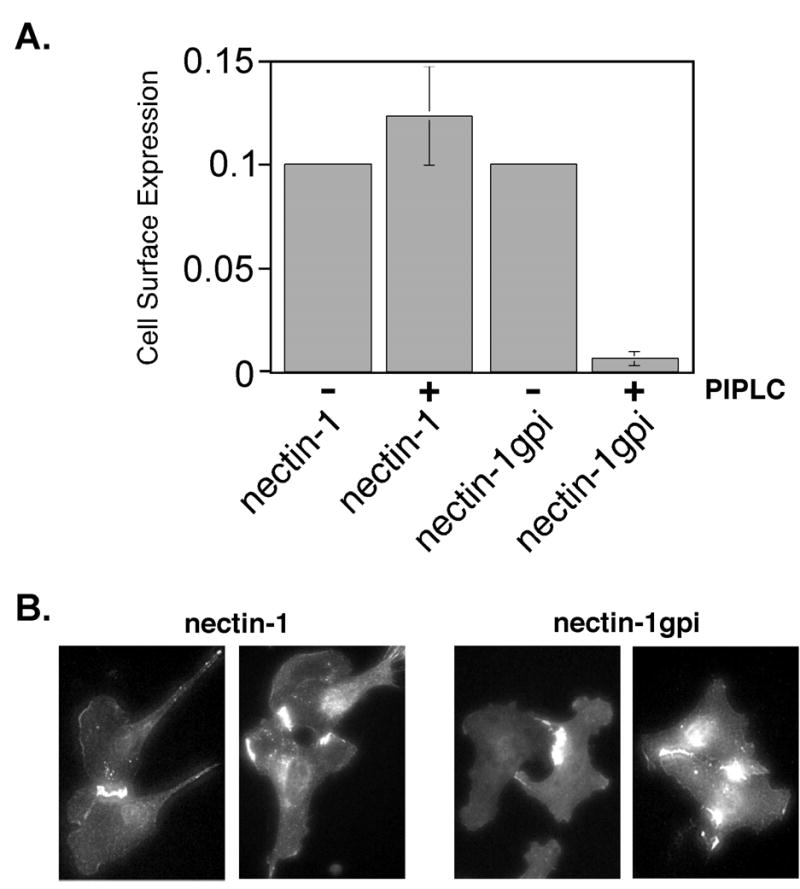
Cell surface expression and PIPLC sensitivity of GPI-linked nectin-1. (A) CELISA analysis. CHO-K1 cells expressing nectin-1 or nectin-1gpi were treated with PIPLC or mock treated, incubated with mAb CK6, followed by an antibody detection system. The assays were performed in triplicate and repeated two times with similar results. The mean values plus standard deviations for a representative experiment are depicted. The absence of error bars for mean values given is due to standard deviations too small to generate visible error bars. (B) Indirect immunofluorescence of cells expressing nectin-1α or nectin-1gpi. B78H1 cells expressing nectin-1α, B78H1 CJ4E, or nectin-1gpi, B78H1 n1gpiA, were fixed with ice-cold methanol and stained with anti-nectin-1 mAb CK41 followed by an FITC-conjugated secondary antibody.
Since nectin-1α is a cell-surface adhesion molecule, we investigated whether the nectin-1gpi mutant was capable of undergoing the trans interactions associated with cell adhesion. Trans interactions between molecules on adjacent cells cause protein accumulation at areas of cell-cell contact (Takahashi et al. 1999; Struyf et al. 2002b; Yoon and Spear 2002; Yoon et al. 2003). Indirect immunofluorescence analysis of B78H1 cell lines expressing nectin-1gpi or nectin-1α using an anti-nectin-1 mAb demonstrated increased staining at areas of cell-cell contact (Fig. 4B). B78H1 cells do not express endogenous nectin-1 or any other gD receptor (Miller et al. 2001). Another way to demonstrate trans interactions is to measure the ability to bind a secreted form of a nectin. Nectin-1 and nectin-3 undergo a heterotypic trans interaction important for cell adhesion (Satoh-Horikawa et al. 2000; Fabre et al. 2002). For the nectin binding experiments, we used a secreted version of nectin-3 with its transmembrane domain and cytoplasmic tail replaced by the constant region of human IgG (Fabre et al. 2002). Cells expressing nectin-1α bound nectin-3:Fc more efficiently than a nectin-1:Fc (data not shown) (Fabre et al. 2002), so nectin-3:Fc was used here. When analyzed by CELISA for ability to bind nectin-3:Fc, nectin-1cyt− and nectin-1gpi bound nectin-3:Fc approximately as well as nectin-1 (Fig. 5A). Taken together, these studies indicate that the nectin-1α TM and CT, and therefore an association with cytoplasmic factors such as afadin, are not required for trans interactions. Both the B78H1 and CHO K1 cells were positive for afadin expression by indirect immunofluorescence (data not shown).
Figure 5.
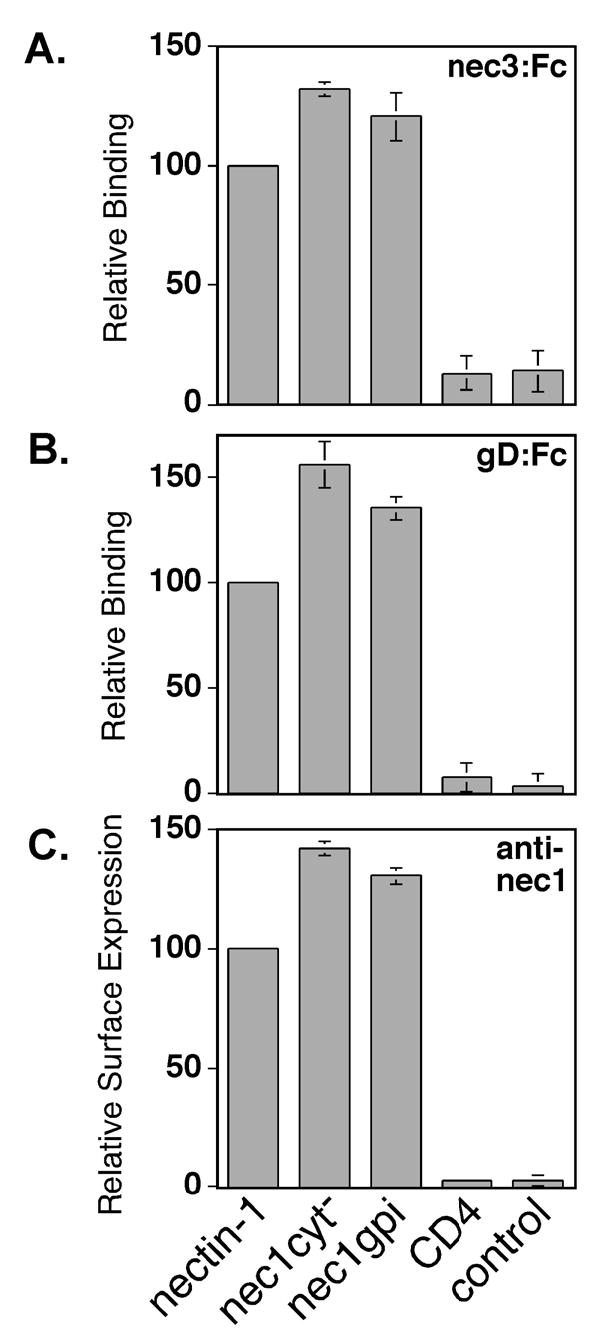
Binding of nectin-3:Fc, HSV-1(KOS) gD:Fc, or an anti-nectin-1 mAb to CHO-K1 cells expressing nectin-1α and nectin-1cyt−, or nectin-1gpi. CHO-K1 cells were transfected with plasmids expressing the receptors indicated, replated in 96 well plates and incubated in triplicate with nectin-3:Fc (A), gD:Fc (B), or Prr1-PE anti-nectin-1 mAb (C). The cells were then washed, fixed, incubated with biotinylated secondary antibodies, and an avidin-HRP detection system. Within each experiment, all values were expressed as a percentage of the value obtained for the positive control (nectin-1). The values graphed are the means and standard deviations of the relative results from three independent experiments.
We next characterized the virus receptor properties of nectin-1cyt− and nectin-1gpi. The gD-binding activity of nectin-1cyt− and nectin-1gpi was analyzed by CELISA using a secreted form of gD, gD:Fc. The extracellular domain of HSV-1(KOS) gD was fused to the constant region of rabbit IgG to create gD:Fc (Geraghty et al. 2000). As shown in Figure 5B, cells expressing nectin-1cyt− and nectin-1gpi bound soluble HSV-1(KOS) gD:Fc with wild-type nectin-1α efficiency. The slight increase in gD-binding efficiency, as well as nectin-3-binding efficiency, may be explained by an increased surface expression of nectin-1cyt− and nectin-1gpi as determined by CELISA analysis using the anti-nectin-1 mAb, Prr1-PE (Fig. 5C). We further examined the ability of nectin-1cyt− and nectin-1gpi to mediate virus entry. CHO K1 cells transiently expressing nectin-1α, nectin-1cyt−, nectin-1gpi, or control cells were inoculated with an HSV-1(KOS) isolate capable of expressing β-gal upon virus entry, HSV-1(KOS)tk12 (Warner et al. 1998). Six hours later, the cells were lysed and β-gal activity was measured as an indication of virus entry. The nectin-1cyt− mutant consistently displayed entry-mediating activity slightly higher than wild-type nectin-1α, most likely due to cell-surface expression that was slightly higher than wild-type nectin-1α (Fig. 6A). The nectin-1gpi mutant also mediated virus entry at a higher level than wild-type nectin-1α while also being expressed at a higher level than wild-type nectin-1α (Fig. 6B). When corrected for cell-surface expression, both nectin-1cyt− and nectin-1gpi displayed wild-type nectin-1α activity in mediating HSV-1 entry. Identical results were obtained when cells were inoculated for a two hour period, citrate-treated to inactivate bound virus, and incubated for four further hours in cell culture media (data not shown). The lack of a nectin-1α TM and CT, and corresponding lack of signaling through the nectin-1α CT, did not influence the ability of nectin-1α to mediate virus entry. The absence of a link to the plasma membrane altogether, as occurred with the nectin-1tmcyt− mutant, resulted in undetectable virus entry (data not shown).
Figure 6.
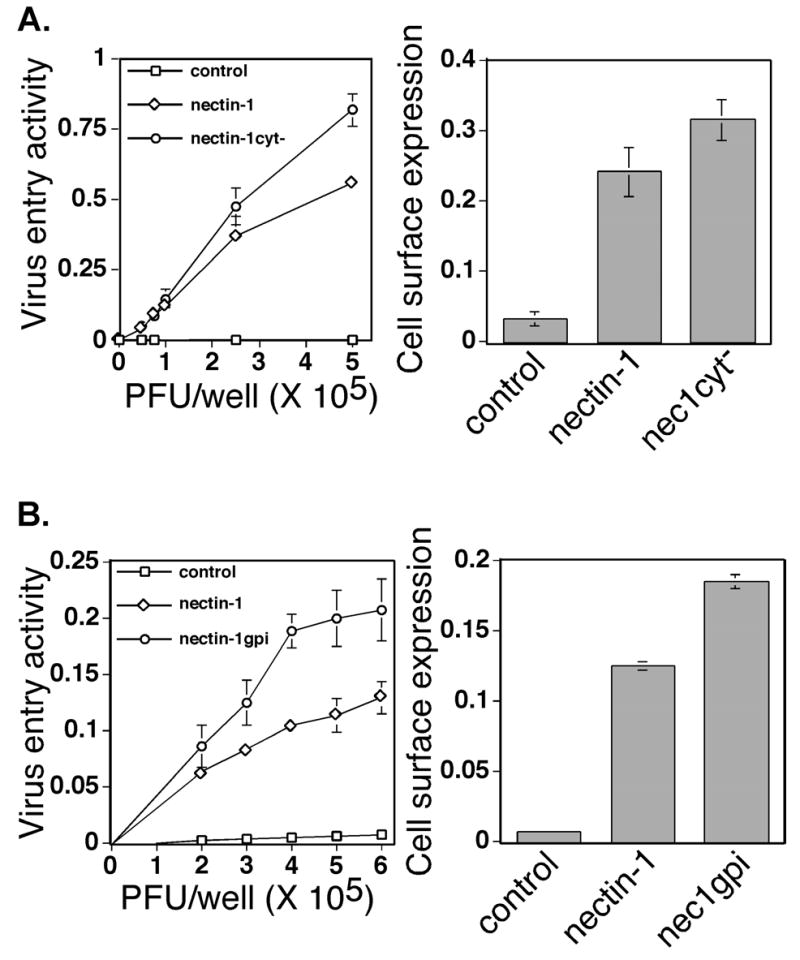
HSV-1 entry activity and surface expression of the nectin-1α mutants nectin-1cyt− (A) and nectin-1gpi (B). CHO-K1 cells were transfected with plasmids expressing the proteins indicated or with a control plasmid and then replated in 96 well plates. The next day the cells were inoculated with an HSV-1 recombinant expressing β-galactosidase. Six hrs after inoculation, cells were lysed and β-galactosidase activity determined as a measure of virus entry. The assays were performed in triplicate and repeated three times with similar results. The mean values plus standard deviations for representative experiments are depicted. The graphs on the right represent CELISA analysis with anti-nectin-1α mAb CK6 to detect cell surface expression of nectin-1 or mutant on transfected cells used in the entry assay. The assays were performed in triplicate and repeated three times with similar results. The mean values plus standard deviations for representative experiments are depicted.
Cell fusion mediated by nectin-1 transmembrane domain and cytoplasmic tail mutants
To determine whether the nectin-1α TM and CT were important for cell fusion, we mixed CHO K1 cells expressing nectin-1cyt−, nectin-1gpi, or nectin-1tmcyt− with cells expressing the four HSV-1 fusion glycoproteins. Both the nectin-1cyt− and nectin-1gpi mutants were expressed at the cell surface at higher levels than wild-type nectin-1α and both mutants mediated cell fusion at correspondingly elevated levels (Fig. 7A and C). The lack of a TM and CT did not diminish the fusion activity of nectin-1gpi, indicating that the absence of an interaction with cellular cytoplasmic factors did not affect cell fusion in the cell-mixing assay. Because GPI-linked proteins do not span the lipid bilayer but are tethered to the outer leaflet, our results with the nectin-1gpi mutant indicate that a membrane spanning domain was not required for efficient cell fusion with the wild-type HSV-1 fusion glycoproteins. Again, similarly to virus entry, a link to the plasma membrane was required for cell fusion because cells expressing the nectin-1tmcyt− mutant were unable to fuse with cells expressing the HSV-1 fusion glycoproteins above background levels (Fig. 7A). We were unable to detect nectin-1tmcyt− expression by CELISA probably because the mutant is secreted and not stably expressed on the cell surface. The number of CHO K1 cells expressing nectin-1tmcyt− in the fusion assay was similar to the number of CHO K1 cells expressing nectin-1α when examined by indirect immunofluorescence (data not shown).
Figure 7.
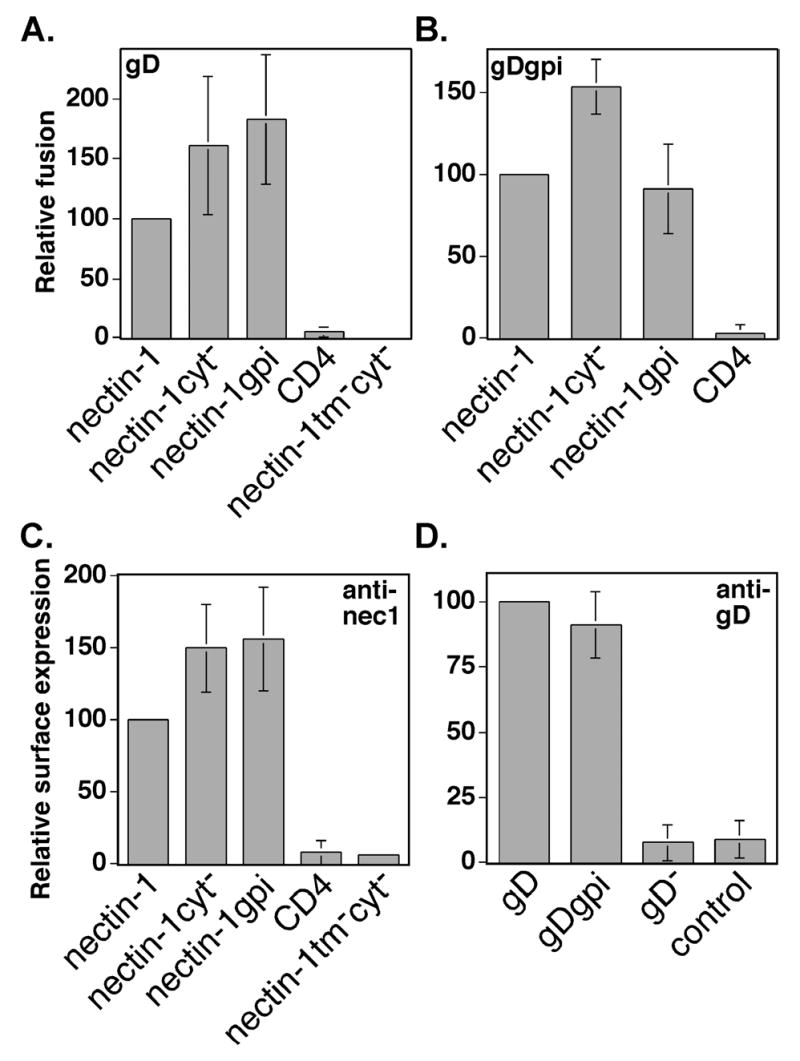
Fusion activity of nectin-1α mutants using gD or gDgpi. (A) Fusion assay using wild-type gD. CHO-K1 cells expressing gB, gD, gH, gL, and T7 polymerase were mixed with CHO-K1 cells transiently expressing nectin-1α or mutant and pG1NT7β-gal. Within each experiment, all values were made relative to the value obtained for the positive control (nectin-1). (B) Fusion assay using gDgpi. CHO-K1 cells expressing gB, gDgpi, gH, gL, and T7 polymerase were mixed with CHO-K1 cells expressing nectin-1α or mutant and pG1NT7β-gal. Within each experiment, all values were expressed as a percentage of the value obtained for the positive control (nectin-1). (C) Cell-surface expression of nectin-1α mutants in the fusion assay. CELISA analysis of cells from (A) and (B) using the nectin-1 mAb Prr1-PE (anti-nec1). Within each experiment, all values were expressed as a percentage of the value obtained for the positive control, nectin-1. (D) Cell-surface expression of gD and gDgpi in the fusion assay. CELISA analysis of cells from (A) and (B) using the rabbit polyclonal anti-gD serum R7 (anti-gD). Cells transfected with plasmids expressing gB, gD, gH, gL, and T7 (labeled gD), or gB, gDgpi, gH, gL, and T7 (labeled gDgpi), or gB, gH, gL, control plasmid pCAGGS, and T7 (labeled gD−) or control plasmid pCAGGS (labeled control). Within each experiment, all values were expressed as a percentage of the value obtained for the positive control, gD. The fusion/CELISA experiments were performed at least three times and the mean values plus standard deviations for the combined relative results are depicted.
Previously, we have shown that a GPI-linked version of gD, gDgpi, retained nearly wild-type gD function in the cell mixing fusion assay (Jones and Geraghty 2004). To our knowledge, gDgpi is the only reported fusion glycoprotein that still retains function when the TM and CT are replaced by a GPI tether. We were thus presented with a unique opportunity to examine cell fusion when both gD and nectin-1α lacked a TM and CT but were linked to the plasma membrane via a GPI tether. Cell fusion using gDgpi occurred with nearly wild-type gD efficiency when envelope glycoprotein-expressing cells were mixed with cells expressing nectin-1α and nectin-1cyt− (Fig. 7B). A reduction in cell fusion occurred when cells expressing nectin-1gpi were mixed with cells expressing the fusion glycoproteins including gDgpi (Fig. 7B). This reduction is best documented by comparing the ratio of the mean fusion activity observed when gDgpi was used versus the mean fusion activity observed when gD was used. There was approximately equivalent expression of gD and gDgpi in the fusion experiments (Fig. 7D). The ratio of gDgpi-mediated to gD-mediated fusion for nectin-1α and nectin-1cyt− was 0.90 and 0.95, respectively, whereas the ratio of gD-mediated fusion to gDgpi-mediated fusion for nectin-1gpi was 0.50. The slight reduction in wild-type nectin-1α-dependent fusion with gDgpi, when compared to gD, agreed with our previous findings (Jones and Geraghty 2004). Our results in the cell mixing assay indicate that the gD/nectin-1 interactions required to promote cell fusion were less efficient when both membrane spanning domains were replaced by GPI links.
Syncytium formation mediated by nectin-1 transmembrane and cytoplasmic domain mutants
The cell-mixing fusion assay described above most likely measures fusion of two or a few cells. Another assay to measure cell fusion, the syncytium formation assay, involves fusion of many cells and may, more so than the cell-mixing assay, require an extensive rearrangement of actin or other cytoskeletal elements. To examine syncytium formation mediated by the HSV-1 envelope glycoproteins, we used B78H1 cells. B78H1 cells readily form syncytia when engineered to express a gD receptor such as nectin-1α (Connolly et al. 2003; Jones and Geraghty 2004). We created B78H1 cell lines expressing nectin-1α (CJ4E), nectin-1cyt− (cyt−A3-2), or nectin-1gpi (n1gpiA) to examine the importance of the nectin-1α TM and CT in syncytium formation. The cell-surface expression of nectin-1cyt− on the cyt−A3-2 cells and nectin-1gpi on the n1gpiA cells was approximately equivalent to wild-type nectin-1α when examined by flow cytometry using an anti-nectin-1 mAb (Fig. 8A and B).
Figure 8.
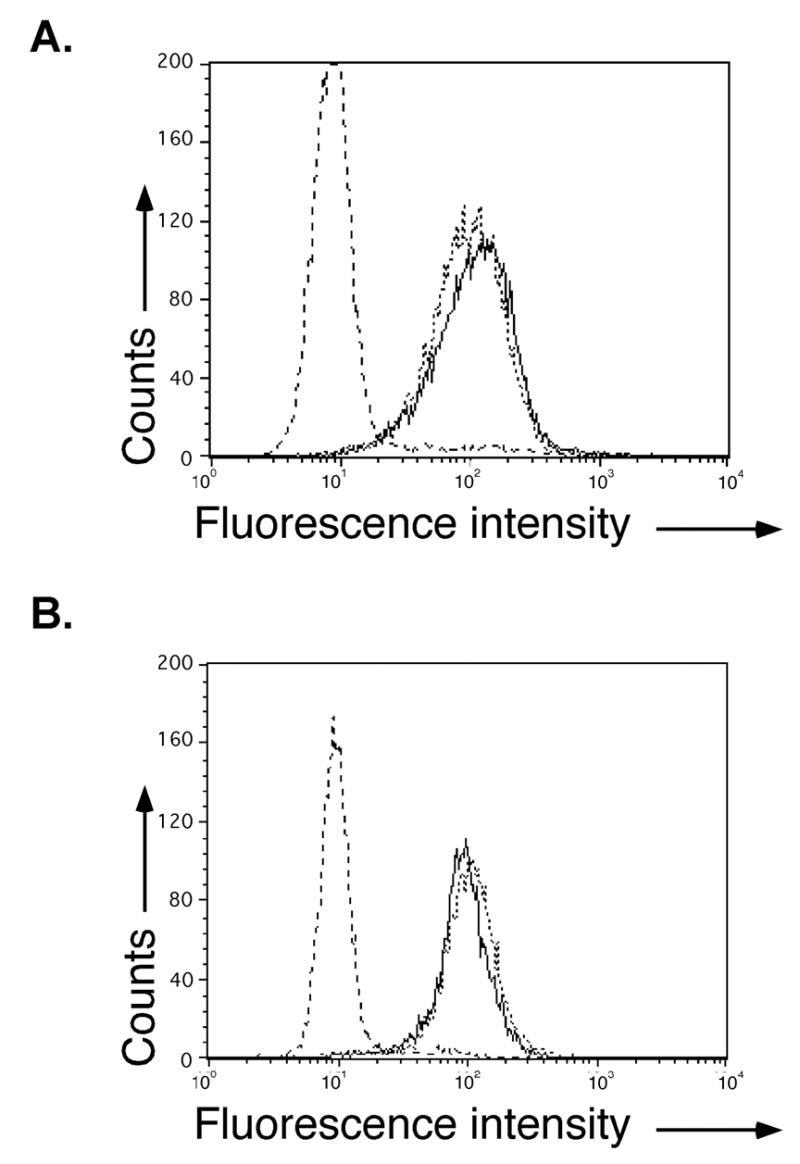
Cell surface expression of nectin-1α and nectin-1α mutant cells lines using flow cytometry analysis. (A) B78H1 (dashed line), B78H1 CJ4E (solid dark line), or B78H1 cyt−A3-2 cells (fine dashed line). (B) B78H1 (dashed line), B78H1 CJ4E (solid dark line), or B78H1n1gpiA cells (fine dashed line). Cell-surface nectin-1 was detected with anti-nectin-1 mAb CK6 and addition of goat anti-mouse FITC conjugated antibody. The experiment was repeated three times and results from a representative experiment are shown.
To generate syncytia, the B78H1-based cell lines were transfected with plasmids expressing gB, gH, gL and either gD, gDgpi, or a control plasmid. Replicate transfections of B78H1, B78H1 CJ4E, B78H1 cyt−A3-2, and B78H1 n1gpiA were fixed at 24, 48, or 72 hour times after transfection and giemsa stained. Representative syncytia are depicted in Figure 9A for the B78H1 CJ4E cells, in Figure 9B for the B78H1 cyt−A3-2 cells, and in Figure 9C for B78H1 n1gpiA cells. No syncytia were observed in any transfections using the parental B78H1 cells (data not shown). The absence of the CT of nectin-1α did not influence the efficiency of syncytium formation but the absence of a TM reduced the size of the syncytia formed over time. The size of syncytia decreased incrementally with the number of GPI anchors in the assay. Syncytia formed when gDgpi, gB, gH, and gL were expressed in B78H1 CJ4E cells were approximately 25-50% smaller over time than those formed by expression of gD, gB, gH, and gL (Fig. 9A), consistent with previous results (Jones and Geraghty 2004). Syncytia induced in nectin-1gpi cells by expression of gD and the other fusion glycoproteins were approximately 25-50% smaller than syncytia formed in cells expressing nectin-1α (Fig. 9A and C). However, when fusion was induced in nectin-1gpi-expressing cells using gDgpi, the syncytia were reduced by approximately 50-75% when compared to the wild-type situation (Fig. 9A and C). These results are in agreement with our results using the cell-mixing assay (Fig. 7A and C) in that two GPI anchors present in the fusion reaction yielded the greatest reduction in cell fusion. Syncytia formed in the cells expressing nectin-1cyt− reached the same approximate size as those observed in wild-type nectin-1α-expressing cells (Fig. 9A and B) indicating that the nectin-1α CT, and the corresponding link to cellular cytoplasmic factors, were not important for cell fusion. However, a membrane spanning domain for both gD and nectin-1 were important for efficient enlargement of syncytia over time.
Figure 9.
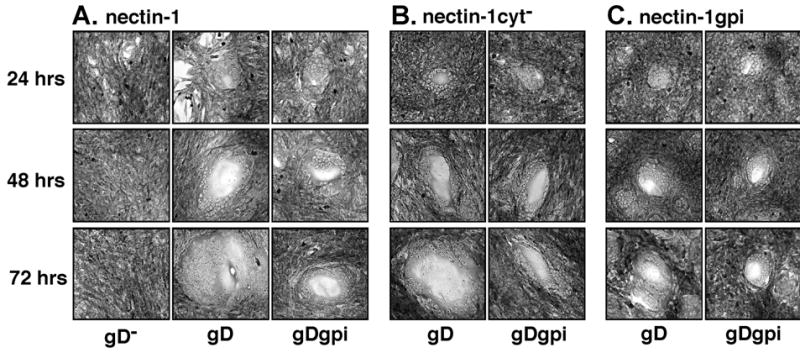
Syncytium formation using wild-type envelope glycoproteins and gpi-linked mutants. Cells were transfected with plasmids expressing gB, gD, gH, and gL (labeled gD), or gB, gDgpi, gH, and gL (labeled gDgpi), or gB, gH, gL, and control plasmid pCAGGS (labeled gD−). At 24, 48, or 72 hours after transfection, the cells were fixed with methanol and stained with giemsa. (A) Cells expressing nectin-1α, B78H1 CJ4E (B) Cells expressing the cytoplasmic tail deletion mutant, nectin-1cyt−, B78H1 cyt−A3-2 (C) Cells expressing the GPI linked mutant of nectin-1, nectin-1gpi, B78H1 n1gpiA. Images were obtained under identical conditions. Transfections with control plasmid alone, pCAGGS, appeared identical to the results with gB, gH, gL and were not included. The results for gB, gH, gL transfections using cells in (B) and (C) were identical to those depicted in (A) and were not included.
DISCUSSION
Based upon the results presented here, two properties of nectin-1α are critical for HSV-1 glycoprotein-induced cell fusion, an intact V-like domain and an attachment to the plasma membrane. The specific TM and CT of nectin-1α were not required for cell fusion because all chimeras that functioned in cell fusion contained either a nectin-2α or CD4 TM and CT. Further, a TM and CT were not required for nectin-1α-mediated cell fusion because the nectin-1cyt− and nectin-1gpi mutants were competent for cell fusion. Our results are in agreement with a recently published report describing receptor activity for a nectin-1 mutant containing a GPI link (Gianni et al. 2004). We further extend the previous results to quantify virus entry, cell fusion, and trans interactions compared to wild-type nectin-1α. Virus entry and trans interactions occurred with wild-type nectin-1α efficiency for both the CT deletion and GPI-linked mutants. However, cell fusion, particularly the size of syncytia formed over time, was reduced in cells expressing nectin-1gpi. This reduction was most evident when a GPI-linked version of gD was used, demonstrating an importance for membrane spanning domains in gD/nectin-1α contributions to HSV-induced cell fusion.
It was not surprising that the CT of nectin-1α was not absolutely required for cell fusion to occur. The other gD receptors, HVEM and 3-O-sulfated heparan sulfate, mediate cell fusion and virus entry. However, nectin-1α has multiple activities linked to its cytoplasmic tail that could influence the efficiency of cell fusion, especially through remodeling of the actin cytoskeleton. The nectin-1cyt− mutant, missing the nectin-1α CT and unlikely to interact with cytoplasmic factors, had approximately wild-type fusion activity in the cell-mixing, syncytium formation, and virus entry assays. Therefore, the absence of an interaction with actin through afadin or possible actin remodeling through Rac and Cdc42 activation did not influence HSV-1 glycoprotein-induced cell fusion. It is formally possible that the cells used in this report, mouse B78H1 and hamster CHO K1, could not support human nectin-1-based Rac and Cdc42 activation. We think this is unlikely because the activation has been demonstrated in a variety of cell lines and also in mouse L cells expressing human nectin-1 (Kawakatsu et al. 2002; Fukuhara et al. 2003; Honda et al. 2003; Fukuhara et al. 2004; Fukuyama et al. 2004; Hoshino et al. 2004; Kawakatsu et al. 2004). Previous reports describing an inhibition of HSV-1 syncytium formation by the actin filament-disrupting drug, cytochalasin D, suggest a role for actin in fusion (Heeg et al. 1986; Walev et al. 1991; Yura et al. 2000). The potential pleiotropic effects of cytochalasin D on cellular physiology and virus replication, however, make it difficult to attribute the observed reduction in syncytium formation to a nectin-1-dependent influence on the actin cytoskeleton. Similar results have been seen with HIV fusion in that cytochalasin B and D inhibit fusion (Frey et al. 1995; Iyengar et al. 1998; Gallo et al. 2001; Pontow et al. 2004), but cytoplasmic tail mutants of chemokine co-receptors, predicted to reduce or abolish signaling, mediate fusion like wild-type co-receptor (Lu et al. 1997; Amara et al. 2003). Thus, rearrangement of the actin cytoskeleton is most likely important for fusion, but the genesis of the signal for rearrangement does not appear to result from signaling through the CT of viral receptors.
Our analysis of cell fusion activity of the chimeric and mutant receptors revealed similarities for receptor requirements between virus entry and cell fusion. An intact V-like domain attached to the surface of the cell was required for both virus entry and cell fusion. The 1*/2/2 chimera was deficient in mediating cell fusion consistent with a defect in virus entry (Geraghty et al. 2001). The 1*/2/2 chimera differs from the 1/2/2 chimera, which displayed near wild-type nectin-1α receptor activity (Geraghty et al. 2001) (Fig. 2), by 10 amino acid substitutions and the absence of an N-linked glycosylation site near the end of the V-like domain (Geraghty et al. 2001). Both 1*/2/2 and 1/2/2 bind gD equivalently to nectin-1α (Geraghty et al. 2001), emphasizing the idea that mutations outside of the gD binding domain can impact receptor function. These findings further indicate that cell fusion and virus entry require gD-receptor events that are not entirely conveyed by binding of a secreted form of gD. In determining the importance of the TM and CT to nectin-1-dependent fusion, the cell mixing fusion assay appeared to more closely delineate fusion during virus entry than did the syncytium formation assay. The nectin-1cyt− and nectin-1gpi mutants mediated entry of HSV-1(KOS) with wild-type nectin-1α efficiency. Both mutants also mediated cell fusion in the cell mixing fusion assay with approximately nectin-1α efficiency when wild-type gD was expressed. The nectin-1gpi mutant, however, displayed reduced ability to support syncytium formation when either gD or gDgpi was used. Syncytium formation requires the fusion of many cells and may amplify subtle differences in fusion especially over time.
Receptor requirements also differed for virus entry and cell fusion in the experiments with the nectin-1/CD4 chimeras. Previous results demonstrated that 1/1/1/4/4 was unable to mediate HSV-1 entry despite binding a secreted form of HSV-1 gD efficiently (Geraghty et al. 2001). The V-like domain of the 1/1/1/4/4 chimera was postulated to extend too far from the plasma membrane to mediate HSV-1 entry, even though the chimera mediated pseudorabies virus entry efficiently (Geraghty et al. 2001). In contrast to virus entry, the 1/1/1/4/4 chimera mediated HSV-1 glycoprotein-induced cell fusion efficiently. Therefore, if there are distance requirements for the V-like domain in relation to the plasma membrane during virus entry, those distance requirements are not important for cell fusion. Unlike the results obtained previously for virus entry (Geraghty et al. 2001), the ratio of relative cell fusion activity to relative cell surface expression was higher for 1/4/4 and 1/1/4/4 than nectin-1α, suggesting the chimeras have increased cell fusion activity. The chimeras may be more efficient at mediating cell fusion due to the addition of CD4 sequences or the removal of nectin-1α sequences. However, because nectin-1cyt− displays fusion activity comparable to nectin-1α, it is unlikely that cytoplasmic factors binding the nectin-1α CT negatively influence fusion. Elements in the two C-like extracellular domains of nectin-1 could influence cell fusion or alternatively, the extracellular domain, TM, or CT of CD4 may have a positive influence on fusion via a mechanism not yet clear. Our interpretation of the chimera results is based upon the assumption of a linear relationship between nectin-1α cell surface expression and fusion activity. If no such relationship exists, fusion activity for nectin-1α and the 1/4/4 and 1/1/4/4 proteins may be equivalent if there is excess nectin-1α expressed on the cell surface not involved in fusion. However, higher surface expression of nectin-1cyt− and nectin-1gpi yields higher fusion activity (Fig. 7A and C). Also, greater cell surface expression of nectin-1α yields larger and more numerous syncytia when the four fusogenic glycoproteins are expressed (unpublished results). The mAbs used to detect cell surface expression of nectin-1α and the chimeras may also affect the determination of fusion efficiency. CK6 recognizes a linear epitope in the V-like domain (Krummenacher et al. 2000) and would be expected to equivalently recognize nectin-1α and chimeras containing an intact V-like domain. Similar results were obtained with the mAb CK8 which also recognizes a linear epitope in the V-like domain, although the epitope at least partially overlaps that of CK6 (Krummenacher et al. 2000).
Another difference between cell fusion and virus entry was observed in experiments using nectin-2α. Nectin-2α mediates entry of HSV-1 isolates with mutations in the amino terminus of gD and not HSV-1(KOS) (Warner et al. 1998; Lopez et al. 2000). Despite the inability of nectin-2α to mediate HSV-1(KOS) entry, reproducibly low but significant levels of cell fusion occurred when cells expressing HSV-1(KOS) glycoproteins were mixed with cells expressing nectin-2α, in agreement with previously published results (Yoon et al. 2003; Zago and Spear 2003). The cell fusion assay could simply be more sensitive in detecting HSV-1(KOS) gD and nectin-2α interactions and resulting fusion. Alternatively, the requirements for interactions between gD and receptor for cell fusion could be less stringent compared to requirements for virus entry, such that nectin-2α bound HSV-1(KOS) gD efficiently enough to mediate cell fusion but not virus entry.
The fusion perpetrated by viral fusion proteins is characterized by many common steps such as binding receptor, bringing membranes into close proximity, and forming and enlarging fusion pores. These steps have primarily been characterized during fusion with the class I fusion proteins. The mechanism of fusion conducted by HSV glycoproteins is highly likely to involve equivalent steps to those taken by the class I fusion proteins. However, whereas class I fusion proteins are often single proteins, HSV requires four distinct proteins to conduct the steps to fusion. Thus, the functionality present in class I fusion proteins may be spread among all four HSV fusion proteins such that one or more proteins is involved in each step towards fusion. It has been proposed that gD serves largely a receptor binding role in fusion because, unlike class I fusion proteins, gD does not require a membrane spanning domain to function in fusion (Cocchi et al. 2004; Jones and Geraghty 2004). The results presented here indicate that the TM of gD and the TM of nectin-1α influence fusion beyond gD/receptor binding and beyond simply attaching the respective protein to the membrane. The binding of gD to nectin-1 occurs equivalently regardless of how the proteins are attached to the membrane. It is likely that a post-receptor-binding event is affected when both gD and nectin-1α are GPI linked. A conformational change is thought to occur after gD binds receptor, enabling the gD pro-fusion domain to interact with gB and/or gH-gL to form a “tri-partite complex” (receptor/gD/gBgH-gL) that mediates fusion (Cocchi et al. 2004; Zago et al. 2004). When both gD and nectin-1 are GPI linked, the gD conformational change may occur less efficiently, thereby reducing the level of fusion. Alternatively, the conformational change may be unaffected but the GPI anchors could cause improper localization of gDgpi/nectin-1gpi in the membrane, thereby hindering interactions necessary for complex formation and reducing fusion efficiency. The localization of gDgpi/nectin-1gpi may differ from gD/nectin-1 either because GPI-anchored proteins can be sequestered in lipid rafts or because of the enhanced membrane mobility of GPI anchored proteins. Regardless, if tri-partite complex formation is necessary for HSV fusion, understanding how gD/receptor interacts with gB/gH-gL will be key to designing strategies to block fusion.
MATERIALS AND METHODS
Cell lines and antibodies
CHO-K1 cells were provided by P. Spear (Northwestern Univ.) and were grown in F12 media supplemented with 7% fetal bovine serum and pen/strep. B78H1 cells (provided by P. Spear, Northwestern Univ.) were grown in DMEM supplemented with 7% fetal bovine serum and pen/strep.
B78H1 cells constitutively expressing nectin-1 (CJ4E), nectin-1cyt− (cyt−A3-2), and nectin-1gpi (n1gpiA) were created by transfecting B78H1 cells with 1.5ug of the corresponding expression plasmid, selecting the cells with 500μg/ml G418, and sorting via flow cytometry for a population of cells expressing high levels of nectin-1 or a mutant. The cells were sorted three to four times until greater than 95% of the cells expressed the appropriate protein.
The antibodies against nectin-1 (all bind the V-like domain) were the mouse monoclonals CK5, CK6, and CK41 (provided by G. Cohen and R. Eisenberg, Univ. of Pennsylvania) (Krummenacher et al. 2000), and also Prr1-PE (Immunotech, Beckman Coulter). CK5 and CK6 recognize linear epitopes while CK41 and Prr1-PE (also known as R1.302) recognize conformation dependent epitopes (Cocchi et al. 1998a; Krummenacher et al. 2000). HSV-1 gD antibody was a polyclonal rabbit anti-HSV-1 gD serum, R7 (G. Cohen and R. Eisenberg, Univ. of Pennsylvania). The biotin- and FITC-conjugated secondary antibodies were α-mouse biotin, α-rabbit biotin, α-mouse FITC, and α-rabbit FITC (Sigma).
Construction of expression vectors
Plasmids expressing HSV-1 gB (pPEP98), HSV-1 gD (pPEP99), HSV-1 gH (pPEP100), and HSV-1 gL (pPEP101) were provided by P. Pertel (Bayer Pharmaceuticals, West Haven, Connecticut) and previously described (Pertel et al. 2001). Other previously described plasmids include the nectin-1α expression plasmid pCJ4 (Geraghty et al. 2000), CD4 expression plasmid pBG53 (Geraghty et al. 2001), T7 RNA polymerase plasmid pT7pol (provided by P. Pertel, Northwestern Univ.) (Pertel et al. 2001), the plasmid expressing β-gal under the control of the T7 promoter, pG1NT7β-gal (provided by E. Berger, National Institutes of Health) (Nussbaum et al. 1994), and the gDgpi expression plasmid pgDgpi (Jones and Geraghty 2004). The plasmid expressing gD:Fc was pBG64 (Geraghty et al. 2001) and the plasmid expressing the nectin-3:Fc protein was pCFR3.a2 (provided by M. Lopez, Institut De Cancerologie Et D’Immunmunologie Marseille, France) (Fabre et al. 2002).
The nectin-1cyt− plasmid was created by first amplifying the nectin-1α expression plasmid, pBG38, (Geraghty et al. 1998) with the primers CD3prim (5′CACTGCTTACTGGCTTATCG) and Prr1cyt− (5′GCTCTAGACCGGCGCCGACGCAGGGCGACCAC). The PCR product was digested with HindIII and BamHI and ligated into pcDNA3 digested with HindIII and BamHI. The nectin-1 gpi mutant was created by amplifying the extracellular domain of the glycoprotein, amplifying the gpi-addition sequence of DAF, combining the two purified PCR products, and conducting PCR using the most 5′ and 3′ primers to yield the final full-length product. The nectin-1 gpi expression plasmid was constructed by amplifying pCJ4 with the primers CD3prim and Nec1gpc (5′CGTGGGCACCGGCCCGGCGC). The plasmid pDAF-12 (provided by J. White, Univ. of Virginia) (Kemble et al. 1993) was amplified with the primers Necgpi (5′GCGCCGGGCCGGTGCCCACGCCAAATAAAGGAAGTGGAACC) and DAFC (5′CCAACCGAAGGAAAGATG). The two PCR products were gel purified, combined, and amplified with the primers CD3prim and DAFC. The final product was digested with restriction enzymes BstEII and BglII. This product was ligated into pCJ4 digested with BstEII and BglII. The plasmid expressing nectin-1tmcyt−, pBG41, was constructed by amplifying pBG38 with the primers Prr113 (5′CGGGATCCGAATTCTGTGATATTGACCTCCACC) and BG1-2a (5′GCTCTAGAATGGCTCGGATGGGGCTTGCG), digestion of the resulting product with XbaI and BamHI, and ligation into pcDNA3.1mychisA (Invitrogen) digested with XbaI and BamHI. To ensure appropriate construction, all newly created expression plasmids were verified by determining the DNA sequence (Davis Sequencing, Davis, CA).
Transfections
In each well of a 6-well plate, approximately 80% confluent CHO-K1 or B78H1-derived cells were incubated with 1.5μg of plasmid DNA and 5 μl of LipofectAMINE (GibcoBRL), according to the manufacturer’s instructions. The cells were incubated with the transfection reagents for 6-8 hours and the transfection media was replaced with F12 or DMEM media/20% fetal bovine serum.
CELISA
The CELISA technique has been previously described (Geraghty et al. 2000; Geraghty et al. 2001; Jones and Geraghty 2004). The anti-nectin-1 mouse monoclonal CK6 was used at 1:500 dilution of ascites fluid; Prr1-PE at 1:10 dilution, and anti-gD R7 at 1:2000. The CELISA experiments using PIPLC were performed as described (Jones and Geraghty 2004). The primary anti-nectin-1 mAb was added at dilution described above.
To produce the gD:Fc and nectin-3:Fc protein, CHO-K1 cells were transfected with pBG64 and pCFR3.a2 respectively. Cells expressing the hybrid proteins secreted gD:Fc or nectin-3:Fc into the culture medium. The cells were incubated in Opti-MEM containing 4% Ultra-low Ig fetal bovine serum (Invitrogen), and 48 hrs later the culture supernatant was collected. The culture supernatant was clarified by low speed centrifugation prior to use. The supernatant was used neat and after a 30 minute incubation with nectin-1- or nectin-1 mutant-expressing cells, CELISA analysis was conducted as described above.
Flow cytometry
To remove the cells from the tissue culture dishes, the cells were rocked in a 37°C incubator in a solution of PBS/4 mM EDTA. The cells were washed in FACS buffer (PBS/2% heat-inactivated fetal bovine serum) and then incubated for 10 minutes on ice in 100 μl of primary antibody diluted in FACS buffer. The cells were again washed in FACS buffer and then incubated for 10 minutes on ice in 100 μl of FITC-conjugated secondary antibody diluted in FACS buffer. The cells were washed in FACS buffer and stored on ice in a solution of FACS buffer/1.25 μg/ml propidium iodide prior to flow cytometry analysis. The antibodies used to detect nectin-1 expression were CK6, at a 1:100 dilution of ascites fluid, and FITC-conjugated anti-mouse, at a 1:100 dilution.
Virus entry assay.
CHO-K1 cells were transfected as described above and replated in 96-well plates. The next day the cells were exposed to various dilutions of HSV-1 in PBS containing calcium and magnesium. After 6 hrs, the cells were lysed and β-gal activity determined, as previously described (Montgomery 96), as a measure of viral entry. The β-gal-reporter virus used has been described previously, HSV-1(KOS)tk12 (Warner et al. 1998).
Cell mixing fusion assay
The assay conditions used were as previously described (Jones and Geraghty 2004). CHO-K1 effector cells were transfected with the plasmids expressing the HSV-1 fusion glycoproteins (gB, gD, gH and gL) and T7 RNA polymerase. Where appropriate, the gDgpi expression plasmid was substituted for the gD plasmid. Target CHO-K1 cells were transfected with the plasmids expressing nectin-1 (pCJ4), nectin-1gpi (pNectin-1gpi), nectin-1 deleted for the cytoplasmic tail (pNectin-1cyt−), or CD4 expression plasmid (pBG53) and β-gal under control of the T7 promoter (pG1NT7β-gal). Twenty-four hours later, effector and target cells were mixed in a 1:1 ratio and co-cultivated for 18 hr. β-gal activity was quantitated using the substrate CPRG (0.7 mg/ml in PBS with 0.5% NP40) and spectrometry.
Syncytium formation assay
B78H1 CJ4E, B78H1 n1gpiA, B78H1 cyt−A3-2, and B78H1 cells were transfected with plasmids expressing gB (pPEP98), gD (pPEP99), gH (pPEP100), and gL (pPEP101), or transfected with a control plasmid or pgDgpi substituted for pPEP99. At 24, 48, and 72 hours, the cells were fixed with methanol and stained with giemsa. The cells were examined on an Axiovert S100 inverted microscope at the same magnification and photographs taken using Axiovision 3 software (Zeiss) at the same exposure. Each independent experiment was blinded, 12-15 different syncytia imaged, and images depicted were deemed representative of syncytia for a particular cell line.
Indirect Immunofluorescence
B78H1 cells expressing nectin-1 (CJ4E), nectin-1gpi (n1gpiA), or control B78H1 cells were plated onto cover slips. The next day, the cells were fixed in −20°C methanol, blocked in PBS/10% normal goat serum, incubated in primary anti-nectin-1 antibody, CK41 (1:300 dilution of ascites fluid). The cells were then incubated with goat anti-mouse FITC-conjugated secondary antibody (1:100 dilution), mounted in mounting media (40mM Tris pH 8, 75% glycerol, and 1mg/ml p-phenylenediamine). Pictures were taken on a Zeiss Axioscope.
Acknowledgments
We thank P. Spear, G. Cohen, R. Eisenberg, R. Dutch, E. Berger, M. Lopez, and J. White for reagents. We also thank Natasha Jones, and Mary Margaret Collins for contributions to this work, Tony Sinai for helpful discussions, and Cheryl Jogger for critical reading of the manuscript. Some materials used in this report were created while R.J.G. was a postdoctoral fellow in P. Spear’s laboratory at Northwestern University.
This investigation was funded by a National Institutes of Health Grant #AI51476.
References
- Amara A, Vidy A, Boulla G, Mollier K, Garcia-Perez J, Alcami J, Blanpain C, Parmentier M, Virelizier JL, Charneau P, Arenzana-Seisdedos F. G protein-dependent CCR5 signaling is not required for efficient infection of primary T lymphocytes and macrophages by R5 human immunodeficiency virus type 1 isolates. J Virol. 2003;77:2550–8. doi: 10.1128/JVI.77.4.2550-2558.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balan P, Davis-Poynter N, Bell S, Atkinson H, Browne H, Minson T. An analysis of the in vitro and in vivo phenotypes of mutants of herpes simplex virus type 1 lacking glycoproteins gG, gE, gI or the putative gJ. J Gen Virol. 1994;75 ( Pt 6):1245–58. doi: 10.1099/0022-1317-75-6-1245. [DOI] [PubMed] [Google Scholar]
- Cai WH, Gu B, Person S. Role of glycoprotein B of herpes simplex virus type 1 in viral entry and cell fusion. J Virol. 1988a;62:2596–604. doi: 10.1128/jvi.62.8.2596-2604.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai WZ, Person S, DebRoy C, Gu BH. Functional regions and structural features of the gB glycoprotein of herpes simplex virus type 1. An analysis of linker insertion mutants. J Mol Biol. 1988b;201:575–88. doi: 10.1016/0022-2836(88)90639-0. [DOI] [PubMed] [Google Scholar]
- Cocchi F, Fusco D, Menotti L, Gianni T, Eisenberg RJ, Cohen GH, Campadelli-Fiume G. The soluble ectodomain of herpes simplex virus gD contains a membrane-proximal pro-fusion domain and suffices to mediate virus entry. Proc Natl Acad Sci U S A. 2004;101:7445–50. doi: 10.1073/pnas.0401883101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocchi F, Lopez M, Dubreuil P, Campadelli Fiume G, Menotti L. Chimeric nectin1-poliovirus receptor molecules identify a nectin1 region functional in herpes simplex virus entry. J Virol. 2001;75:7987–94. doi: 10.1128/JVI.75.17.7987-7994.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocchi F, Lopez M, Menotti L, Aoubala M, Dubreuil P, Campadelli-Fiume G. The V domain of herpesvirus Ig-like receptor (HIgR) contains a major functional region in herpes simplex virus-1 entry into cells and interacts physically with the viral glycoprotein D. Proc Natl Acad Sci U S A. 1998a;95:15700–5. doi: 10.1073/pnas.95.26.15700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocchi F, Menotti L, Mirandola P, Lopez M, Campadelli-Fiume G. The ectodomain of a novel member of the immunoglobulin subfamily related to the poliovirus receptor has the attributes of a bona fide receptor for herpes simplex virus types 1 and 2 in human cells. J Virol. 1998b;72:9992–10002. doi: 10.1128/jvi.72.12.9992-10002.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly SA, Landsburg DJ, Carfi A, Wiley DC, Cohen GH, Eisenberg RJ. Structure-based mutagenesis of herpes simplex virus glycoprotein D defines three critical regions at the gD-HveA/HVEM binding interface. J Virol. 2003;77:8127–40. doi: 10.1128/JVI.77.14.8127-8140.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis-Poynter N, Bell S, Minson T, Browne H. Analysis of the contributions of herpes simplex virus type 1 membrane proteins to the induction of cell-cell fusion. J Virol. 1994;68:7586–90. doi: 10.1128/jvi.68.11.7586-7590.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eitzen G. Actin remodeling to facilitate membrane fusion. Biochim Biophys Acta. 2003;1641:175–81. doi: 10.1016/s0167-4889(03)00087-9. [DOI] [PubMed] [Google Scholar]
- Fabre S, Reymond N, Cocchi F, Menotti L, Dubreuil P, Campadelli-Fiume G, Lopez M. Prominent role of the Ig-like V domain in trans-interactions of nectins. Nectin3 and nectin 4 bind to the predicted C-C′-C″-D beta-strands of the nectin1 V domain. J Biol Chem. 2002;277:27006–13. doi: 10.1074/jbc.M203228200. [DOI] [PubMed] [Google Scholar]
- Feng Y, Broder CC, Kennedy PE, Berger EA. HIV-1 entry cofactor: Functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science. 1996;272:872–877. doi: 10.1126/science.272.5263.872. [DOI] [PubMed] [Google Scholar]
- Forrester A, Farrell H, Wilkinson G, Kaye J, Davis-Poynter N, Minson T. Construction and properties of a mutant of herpes simplex virus type 1 with glycoprotein H coding sequences deleted. J Virol. 1992;66:341–8. doi: 10.1128/jvi.66.1.341-348.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey S, Marsh M, Gunther S, Pelchen-Matthews A, Stephens P, Ortlepp S, Stegmann T. Temperature dependence of cell-cell fusion induced by the envelope glycoprotein of human immunodeficiency virus type 1. J Virol. 1995;69:1462–72. doi: 10.1128/jvi.69.3.1462-1472.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuhara A, Shimizu K, Kawakatsu T, Fukuhara T, Takai Y. Involvement of nectin-activated Cdc42 small G protein in organization of adherens and tight junctions in Madin-Darby canine kidney cells. J Biol Chem. 2003;278:51885–93. doi: 10.1074/jbc.M308015200. [DOI] [PubMed] [Google Scholar]
- Fukuhara T, Shimizu K, Kawakatsu T, Fukuyama T, Minami Y, Honda T, Hoshino T, Yamada T, Ogita H, Okada M, Takai Y. Activation of Cdc42 by trans interactions of the cell adhesion molecules nectins through c-Src and Cdc42-GEF FRG. J Cell Biol. 2004;166:393–405. doi: 10.1083/jcb.200401093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuyama, T., Ogita, H., Kawakatsu, T., Fukuhara, T., Yamada, T., Sato, T., Shimizu, K., Nakamura, T., Matsuda, M., Takai, Y., 2004. Involvement of the c-Src-Crk-C3G-Rap1 signaling in the nectin-induced activation of Cdc42 and formation of adherens junctions. J Biol Chem. [DOI] [PubMed]
- Gallo SA, Puri A, Blumenthal R. HIV-1 gp41 six-helix bundle formation occurs rapidly after the engagement of gp120 by CXCR4 in the HIV-1 Env-mediated fusion process. Biochemistry. 2001;40:12231–6. doi: 10.1021/bi0155596. [DOI] [PubMed] [Google Scholar]
- Geraghty RJ, Fridberg A, Krummenacher C, Cohen G, Eisenberg R, Spear PG. Use of chimeric nectin-1 (HveC)-related receptors to demonstrate that ability to bind gD is not necessarily sufficient for alphaherpesvirus entry. Virology. 2001;285:366–75. doi: 10.1006/viro.2001.0989. [DOI] [PubMed] [Google Scholar]
- Geraghty RJ, Jogger CR, Spear PG. Cellular expression of alphaherpesvirus gD interferes with entry of homologous and heterologous alphaherpesviruses by blocking access to a shared gD receptor. Virology. 2000;268:147–158. doi: 10.1006/viro.1999.0157. [DOI] [PubMed] [Google Scholar]
- Geraghty RJ, Krummenacher C, Cohen GH, Eisenberg RJ, Spear PG. Entry of alphaherpesviruses mediated by poliovirus receptor-related protein 1 and poliovirus receptor. Science. 1998;280:1618–1620. doi: 10.1126/science.280.5369.1618. [DOI] [PubMed] [Google Scholar]
- Gianni T, Campadelli-Fiume G, Menotti L. Entry of herpes simplex virus mediated by chimeric forms of nectin1 retargeted to endosomes or to lipid rafts occurs through acidic endosomes. J Virol. 2004;78:12268–76. doi: 10.1128/JVI.78.22.12268-12276.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heeg U, Dienes HP, Muller S, Falke D. Involvement of actin-containing microfilaments in HSV-induced cytopathology and the influence of inhibitors of glycosylation. Arch Virol. 1986;91:257–70. doi: 10.1007/BF01314285. [DOI] [PubMed] [Google Scholar]
- Highlander SL, W Cai, S Person, M Levine, JC Glorioso. Monoclonal antibodies define a domain on herpes simplex virus glycoprotein B involved in virus penetration. J Virol. 1988;62:1881–1888. doi: 10.1128/jvi.62.6.1881-1888.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda T, Shimizu K, Kawakatsu T, Fukuhara A, Irie K, Nakamura T, Matsuda M, Takai Y. Cdc42 and Rac small G proteins activated by trans-interactions of nectins are involved in activation of c-Jun N-terminal kinase, but not in association of nectins and cadherin to form adherens junctions, in fibroblasts. Genes Cells. 2003;8:481–91. doi: 10.1046/j.1365-2443.2003.00649.x. [DOI] [PubMed] [Google Scholar]
- Hoshino T, Shimizu K, Honda T, Kawakatsu T, Fukuyama T, Nakamura T, Matsuda M, Takai Y. A novel role of nectins in inhibition of the E-cadherin-induced activation of Rac and formation of cell-cell adherens junctions. Mol Biol Cell. 2004;15:1077–88. doi: 10.1091/mbc.E03-05-0321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huff V, Cai W, Glorioso JC, Levine M. The carboxy-terminal 41 amino acids of herpes simplex virus type 1 glycoprotein B are not essential for production of infectious virus particles. J Virol. 1988;62:4403–4406. doi: 10.1128/jvi.62.11.4403-4406.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson L, Browne H, Wargent V, Davis-Poynter N, Primorac S, Goldsmith K, Minson AC, Johnson DC. A novel herpes simplex virus glycoprotein, gL, forms a complex with glycoprotein H (gH) and affects normal folding and surface expression of gH. J Virol. 1992;66:2240–50. doi: 10.1128/jvi.66.4.2240-2250.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyengar S, Hildreth JE, Schwartz DH. Actin-dependent receptor colocalization required for human immunodeficiency virus entry into host cells. J Virol. 1998;72:5251–5. doi: 10.1128/jvi.72.6.5251-5255.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DC, Ligas MW. Herpes simplex viruses lacking glycoprotein D are unable to inhibit virus penetration: quantitative evidence for virus-specific cell surface receptors. J Virol. 1988;62:4605–4612. doi: 10.1128/jvi.62.12.4605-4612.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones NA, Geraghty RJ. Fusion activity of lipid-anchored envelope glycoproteins of herpes simplex virus type 1. Virology. 2004;324:213–28. doi: 10.1016/j.virol.2004.03.024. [DOI] [PubMed] [Google Scholar]
- Kawakatsu, T., Ogita, H., Fukuhara, T., Fukuyama, T., Minami, Y., Shimizu, K., Takai, Y., 2004. Vav2 as a Rac-GEF responsible for the nectin-induced, c-Src- and Cdc42-mediated activation of Rac. J Biol Chem. [DOI] [PubMed]
- Kawakatsu T, Shimizu K, Honda T, Fukuhara T, Hoshino T, Takai Y. Trans-interactions of nectins induce formation of filopodia and Lamellipodia through the respective activation of Cdc42 and Rac small G proteins. J Biol Chem. 2002;277:50749–55. doi: 10.1074/jbc.M209846200. [DOI] [PubMed] [Google Scholar]
- Kemble GW, Henis YI, White JM. GPI- and transmembrane-anchored influenza hemagglutinin differ in structure and receptor binding activity. J Cell Biol. 1993;122:1253–65. doi: 10.1083/jcb.122.6.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krummenacher C, Baribaud F, Ponce de Leon M, Baribaud I, Whitbeck JC, Xu R, Cohen GH, Eisenberg RJ. Comparative usage of herpesvirus entry mediator A and nectin-1 by laboratory strains and clinical isolates of herpes simplex virus. Virology. 2004;322:286–99. doi: 10.1016/j.virol.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Krummenacher C, Baribaud I, Ponce de Leon M, Whitbeck JC, Lou H, Cohen GH, Eisenberg RJ. Localization of a binding site for herpes simplex virus glycoprotein D on herpesvirus entry mediator C by using antireceptor monoclonal antibodies. J Virol. 2000;74:10863–72. doi: 10.1128/jvi.74.23.10863-10872.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krummenacher C, Baribaud I, Sanzo JF, Cohen GH, Eisenberg RJ. Effects of herpes simplex virus on structure and function of nectin-1/HveC. J Virol. 2002;76:2424–33. doi: 10.1128/jvi.76.5.2424-2433.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krummenacher C, Rux AH, Whitbeck JC, Ponce de Leon M, Lou H, Baribaud I, Hou W, Zou C, Geraghty RJ, Spear PG, Eisenberg RJ, Cohen GH. The first Ig-like domain of HveC is sufficient to bind HSV gD with full affinity while the third domain is involved in oligomerization of HveC. J Virol. 1999;73:8127–8137. doi: 10.1128/jvi.73.10.8127-8137.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon BS, Tan KB, Ni J, Oh KO, Lee ZH, Kim KK, Kim YJ, Wang S, Gentz R, Yu GL, Harrop J, Lyn SD, Silverman C, Porter TG, Truneh A, Young PR. A newly identified member of the tumor necrosis factor receptor superfamily with a wide tissue distribution and involvement in lymphocyte activation. J Biol Chem. 1997;272:14272–6. doi: 10.1074/jbc.272.22.14272. [DOI] [PubMed] [Google Scholar]
- Ligas MW, Johnson DC. A herpes simplex virus mutant in which glycoprotein D sequences are replaced by beta-galactosidase sequences binds to but is unable to penetrate into cells. J Virol. 1988;62:1486–94. doi: 10.1128/jvi.62.5.1486-1494.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez M, Aoubala M, Jordier F, Isnardon D, Gomez S, Dubreuil P. The human poliovirus receptor related 2 protein is a new hematopoietic/endothelial homophilic adhesion molecule. Blood. 1998;92:4602–4611. [PubMed] [Google Scholar]
- Lopez M, Cocchi F, Menotti L, Avitabile E, Dubreuil P, Campadelli-Fiume G. Nectin2alpha (PRR2alpha or HveB) and nectin2delta are low-efficiency mediators for entry of herpes simplex virus mutants carrying the Leu25Pro substitution in glycoprotein D. J Virol. 2000;74:1267–74. doi: 10.1128/jvi.74.3.1267-1274.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Z, Berson JF, Chen Y, Turner JD, Zhang T, Sharron M, Jenks MH, Wang Z, Kim J, Rucker J, Hoxie JA, Peiper SC, Doms RW. Evolution of HIV-1 coreceptor usage through interactions with distinct CCR5 and CXCR4 domains. Proc Natl Acad Sci U S A. 1997;94:6426–31. doi: 10.1073/pnas.94.12.6426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandai K, Nakanishi H, Satoh A, Obaishi H, Wada M, Nishioka H, Itoh M, Mizoguchi A, Aoki T, Fujimoto T, Matsuda Y, Tsukita S, Takai Y. Afadin: a novel actin filament-binding protein with one PDZ domain localized at cadherin-based cell-cell adherens junction. J Cell Biol. 1997;139:517–528. doi: 10.1083/jcb.139.2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez WM, Spear PG. Amino acid substitutions in the V domain of nectin-1 (HveC) that impair entry activity for herpes simplex virus types 1 and 2 but not for Pseudorabies virus or bovine herpesvirus 1. J Virol. 2002;76:7255–62. doi: 10.1128/JVI.76.14.7255-7262.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CG, Krummenacher C, Eisenberg RJ, Cohen GH, Fraser NW. Development of a syngenic murine B16 cell line-derived melanoma susceptible to destruction by neuroattenuated HSV-1. Mol Ther. 2001;3:160–8. doi: 10.1006/mthe.2000.0240. [DOI] [PubMed] [Google Scholar]
- Miyahara M, Nakanishi H, Takahashi K, Satoh-Horikawa K, Tachibana K, Takai Y. Interaction of nectin with afadin is necessary for its clustering at cell-cell contact sites but not for its cis dimerization or trans interaction. J Biol Chem. 2000;275:613–8. doi: 10.1074/jbc.275.1.613. [DOI] [PubMed] [Google Scholar]
- Momose Y, Honda T, Inagaki M, Shimizu K, Irie K, Nakanishi H, Takai Y. Role of the second immunoglobulin-like loop of nectin in cell-cell adhesion. Biochem Biophys Res Commun. 2002;293:45–9. doi: 10.1016/S0006-291X(02)00183-3. [DOI] [PubMed] [Google Scholar]
- Montgomery RI, Warner MS, Lum BJ, Spear PG. Herpes simplex virus-1 entry into cells mediated by a novel member of the TNF/NGF receptor family. Cell. 1996;87:427–436. doi: 10.1016/s0092-8674(00)81363-x. [DOI] [PubMed] [Google Scholar]
- Nicola AV, McEvoy AM, Straus SE. Roles for endocytosis and low pH in herpes simplex virus entry into HeLa and Chinese hamster ovary cells. J Virol. 2003;77:5324–32. doi: 10.1128/JVI.77.9.5324-5332.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicola AV, Straus SE. Cellular and viral requirements for rapid endocytic entry of herpes simplex virus. J Virol. 2004;78:7508–17. doi: 10.1128/JVI.78.14.7508-7517.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nussbaum O, Broder CC, Berger EA. Fusogenic mechanisms of enveloped-virus glycoproteins analyzed by a novel recombinant vaccinia virus-based assay quantitating cell fusion-dependent reporter gene activation. J Virol. 1994;68:5411–5422. doi: 10.1128/jvi.68.9.5411-5422.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertel PE, Fridberg A, Parish ML, Spear PG. Cell fusion induced by herpes simplex virus glycoproteins gB, gD, and gH-gL requires a gD receptor but not necessarily heparan sulfate. Virology. 2001;279:313–24. doi: 10.1006/viro.2000.0713. [DOI] [PubMed] [Google Scholar]
- Pertel, P. E., Spear, P. G. (1998). Biology of herpesviruses. Sexually Transmitted Diseases, 3rd Edition New York, McGraw-Hill.
- Pontow SE, Heyden NV, Wei S, Ratner L. Actin cytoskeletal reorganizations and coreceptor-mediated activation of rac during human immunodeficiency virus-induced cell fusion. J Virol. 2004;78:7138–47. doi: 10.1128/JVI.78.13.7138-7147.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reymond N, Fabre S, Lecocq E, Adelaide J, Dubreuil P, Lopez M. Nectin4/PRR4, a new afadin-associated member of the nectin family that trans-interacts with nectin1/PRR1 through V domain interaction. J Biol Chem. 2001;276:43205–15. doi: 10.1074/jbc.M103810200. [DOI] [PubMed] [Google Scholar]
- Roop C, Hutchinson L, Johnson DC. A mutant herpes simplex virus type 1 unable to express glycoprotein L cannot enter cells, and its particles lack glycoprotein H. J Virol. 1993;67:2285–97. doi: 10.1128/jvi.67.4.2285-2297.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakisaka T, Taniguchi T, Nakanishi H, Takahashi K, Miyahara M, Ikeda W, Yokoyama S, Peng YF, Yamanishi K, Takai Y. Requirement of Interaction of Nectin-1alpha/HveC with Afadin for Efficient Cell-Cell Spread of Herpes Simplex Virus Type 1. J Virol. 2001;75:4734–4743. doi: 10.1128/JVI.75.10.4734-4743.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh-Horikawa K, Nakanishi H, Takahashi K, Miyahara M, Nishimura M, Tachibana K, Mizoguchi A, Takai Y. Nectin-3, a new member of immunoglobulin-like cell adhesion molecules that shows homophilic and heterophilic cell-cell adhesion activities. J Biol Chem. 2000;275:10291–9. doi: 10.1074/jbc.275.14.10291. [DOI] [PubMed] [Google Scholar]
- Shukla D, Liu J, Blaiklock P, Shworak NW, Bai X, Esko JD, Cohen GH, Eisenberg RJDRR, Spear PG. A novel role for 3-O-sulfated heparan sulfate in herpes simplex virus 1 entry. Cell. 1999;99:13–22. doi: 10.1016/s0092-8674(00)80058-6. [DOI] [PubMed] [Google Scholar]
- Spear, P. G. (1993). Membrane fusion induced by herpes simplex virus. Viral Fusion Mechanisms J. Bentz. Boca Raton, CRC Press, Inc.: 201–232.
- Spear PG, Eisenberg RJ, Cohen GH. Three classes of cell surface receptors for alphaherpesvirus entry. Virology. 2000;275:1–8. doi: 10.1006/viro.2000.0529. [DOI] [PubMed] [Google Scholar]
- Struyf F, Martinez WM, Spear PG. Mutations in the N-terminal domains of nectin-1 and nectin-2 reveal differences in requirements for entry of various alphaherpesviruses and for nectin-nectin interactions. J Virol. 2002a;76:12940–50. doi: 10.1128/JVI.76.24.12940-12950.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struyf F, Posavad CM, Keyaerts E, Van Ranst M, Corey L, Spear PG. Search for polymorphisms in the genes for herpesvirus entry mediator, nectin-1, and nectin-2 in immune seronegative individuals. J Infect Dis. 2002b;185:36–44. doi: 10.1086/338116. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Nakanishi H, Miyahara M, Mandai K, Satoh K, Satoh A, Nishioka H, Aoki J, Nomoto A, Mizoguchi A, Takai Y. Nectin/PRR: an immunoglobulin-like cell adhesion molecule recruited to cadherin-based adherens junctions through interaction with afadin, a PDZ domain-containing protein. J Cell Biol. 1999;145:539–549. doi: 10.1083/jcb.145.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takekuni K, Ikeda W, Fujito T, Morimoto K, Takeuchi M, Monden M, Takai Y. Direct binding of cell polarity protein PAR-3 to cell-cell adhesion molecule nectin at neuroepithelial cells of developing mouse. J Biol Chem. 2003;278:5497–500. doi: 10.1074/jbc.C200707200. [DOI] [PubMed] [Google Scholar]
- Tiwari V, Clement C, Duncan MB, Chen J, Liu J, Shukla D. A role for 3-O-sulfated heparan sulfate in cell fusion induced by herpes simplex virus type 1. J Gen Virol. 2004;85:805–9. doi: 10.1099/vir.0.19641-0. [DOI] [PubMed] [Google Scholar]
- Turner A, Bruun B, Minson T, Browne H. Glycoproteins gB, gD, and gHgL of herpes simplex virus type 1 are necessary and sufficient to mediate membrane fusion in a Cos cell transfection system. J Virol. 1998;72:873–5. doi: 10.1128/jvi.72.1.873-875.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walev I, Wollert KC, Weise K, Falke D. Characterization of fusion from without induced by herpes simplex virus. Arch Virol. 1991;117:29–44. doi: 10.1007/BF01310490. [DOI] [PubMed] [Google Scholar]
- Warner MS, Geraghty RJ, Martinez WM, Montgomery RI, Whitbeck JC, Xu R, Eisenberg RJ, Cohen GH, Spear PG. A cell surface protein with herpesvirus entry activity (HveB) confers susceptibility to infection by mutants of herpes simplex virus type 1, herpes simplex virus type 2 and pseudorabies virus. Virology. 1998;246:179–189. doi: 10.1006/viro.1998.9218. [DOI] [PubMed] [Google Scholar]
- Wilson DW, Davis-Poynter N, Minson AC. Mutations in the cytoplasmic tail of herpes simplex virus glycoprotein H suppress cell fusion by a syncytial strain. J Virol. 1994;68:6985–6993. doi: 10.1128/jvi.68.11.6985-6993.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittels M, Spear PG. Penetration of cells by herpes simplex virus does not require a low pH-dependent endocytic pathway. Virus Res. 1991;18:271–290. doi: 10.1016/0168-1702(91)90024-p. [DOI] [PubMed] [Google Scholar]
- Yoon M, Spear PG. Disruption of adherens junctions liberates nectin-1 to serve as receptor for herpes simplex virus and pseudorabies virus entry. J Virol. 2002;76:7203–8. doi: 10.1128/JVI.76.14.7203-7208.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon, M., Spear, P. G., 2004. Random mutagenesis of the gene encoding a viral ligand for multiple cell entry receptors to obtain viral mutants altered for receptor usage. Proc Natl Acad Sci U S A. [DOI] [PMC free article] [PubMed]
- Yoon M, Zago A, Shukla D, Spear PG. Mutations in the N termini of herpes simplex virus type 1 and 2 gDs alter functional interactions with the entry/fusion receptors HVEM, nectin-2, and 3-O-sulfated heparan sulfate but not with nectin-1. J Virol. 2003;77:9221–31. doi: 10.1128/JVI.77.17.9221-9231.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yura Y, Kusaka J, Bando T, Yamamoto S, Yoshida H, Sato M. Enhancement of herpes simplex virus-induced polykaryocyte formation by 12-O-tetradecanoyl phorbol 13-acetate: association with the reorganization of actin filaments and cell motility. Intervirology. 2000;43:129–38. doi: 10.1159/000025038. [DOI] [PubMed] [Google Scholar]
- Zago A, Jogger CR, Spear PG. Use of herpes simplex virus and pseudorabies virus chimeric glycoprotein D molecules to identify regions critical for membrane fusion. Proc Natl Acad Sci U S A. 2004;101:17498–503. doi: 10.1073/pnas.0408186101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zago A, Spear PG. Differences in the N termini of herpes simplex virus type 1 and 2 gDs that influence functional interactions with the human entry receptor Nectin-2 and an entry receptor expressed in Chinese hamster ovary cells. J Virol. 2003;77:9695–9. doi: 10.1128/JVI.77.17.9695-9699.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Dutch RE, Lamb RA. Proper spacing between heptad repeat B and the transmembrane domain boundary of the paramyxovirus SV5 F protein is critical for biological activity. Virology. 1997;239:327–39. doi: 10.1006/viro.1997.8917. [DOI] [PubMed] [Google Scholar]


