Abstract
Products of arachidonic acid (AA) metabolism by cyclooxygenase (COX) are important in regulation of neonatal cerebral circulation. The brain and cerebral microvessels also express heme oxygenase (HO) that metabolizes heme to carbon monoxide (CO), biliverdin, and iron. The purpose of this study in newborn pig cerebral microvessels was to address the hypothesis that COX products affect HO activity and HO products affect COX activity. AA (2.0-20μM) increased PGE2 measured by RIA and also CO measured by gas chromatography/mass spectrometry (GC-MS). Further, indomethacin (10-4M), that inhibited COX, reduced both AA and heme-induced CO production. Conversely, neither exogenous heme (2×10-6M), that markedly increased CO production, nor the inhibitor of HO, chromium mesoporphyrin, altered PGE2 synthesis. Because AA metabolism by COX generates both prostanoids and superoxides, we determined the effects of the predominant prostanoid and superoxide on CO production. While PGE2 caused a small increase in CO production, xanthine oxidase plus hypoxanthine that produces superoxide strongly stimulated the production of CO by cerebral microvessels. This increase was mildly attenuated by catalase. These data suggest that COX catalyzed AA metabolite(s), most likely superoxide, H2O2, and / or a subsequent reactive oxygen species increases cerebrovascular CO production. This increase appears to be due, at least in part, to the elevation of HO-2 catalytic activity. Conversely, COX activity is not affected by HO-catalyzed heme metabolites. These data suggest that some cerebrovascular functions attributable to COX activity could be mediated by CO.
Keywords: cerebrovascular circulation, heme oxygenase, reactive oxygen species
INTRODUCTION
The gaseous molecule, carbon monoxide (CO), is a neurotransmitter in the brain and peripheral nervous system and is an important regulator of vascular tone (1). In the cerebral microvasculature, CO causes vasodilation via activation of large conductance calcium-activated potassium channels (2). Also in some conditions, CO can modulate intracellular cGMP levels in both autocrine and paracrine fashions (3).
CO is endogenously produced in the brain via enzymatic degradation of heme by heme oxygenase (HO) to CO, biliverdin IXa, and free iron (1). All heme-degradation products are potentially toxic, but may also provide strong cytoprotection, depending on the generated amounts and the microenvironment. Besides its function as prosthetic moiety in heme proteins, heme affects a wide spectrum of biochemical processes including gene expression by regulating transcription, mRNA stability, protein synthesis, splicing and post-translational modification (4).
Heme oxygenase (HO) is expressed as three known isoforms which are products of different genes and differ markedly in their tissue distribution as well as their molecular properties. Expression of HO-1 (heat shock protein-32) is easily induced by numerous stimuli (1), while HO-2 is constitutively expressed and is known to be upregulated only by steroids (1). HO-3, a third isoform has much lower heme degrading activity (1). HO generates CO from cellular heme, which is produced in cells from glycine and succinyl CoA (5). Of the vascular tissues examined, among the greatest producers of CO are the cerebral microvessels (6). The cellular mechanisms of regulation of CO production by the constitutive HO-2 enzyme include control of catalytic activity and substrate delivery (7)
The prostanoids are also important autocrine/paracrine dilators in the control of cerebral circulation in the newborn pig. The cells that produce CO and can respond to CO also produce and respond to prostanoids (8). Cyclooxygenase (COX) products are synthesized by the catalytic conversion of arachidonic acid (AA) into prostaglandin H2 that is subsequently processed by different enzymes into various prostanoids (9). In addition, COX metabolism of AA produces superoxide anion (10, 11). The similarity of the cellular locations of HO and COX, suggests that these paracrine mediators could be part of a coordinated system (12). COX-1 and COX-2 are the two predominant isoforms of COX (13). The pattern of COX-1 and COX-2 expression is cell and tissue specific. COX-1 is constitutively expressed in a variety of cell types (14). COX-2 is inducible and rapidly and transiently upregulated upon stimulation of cells with serum, growth factors, inflammatory mediators, and tumor promoters (15). However, in some tissues, COX -2 also is expressed under non stimulated conditions (16).
This study, that uses freshly isolated piglet cerebral microvessels, was designed to address the hypothesis that COX products affect HO activity and CO production and that HO products affect COX activity and prostanoid production.
METHODS
The animal protocols were performed in adherence to the guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were reviewed and approved by the Animal Care and Use Committee of the University of Tennessee Health Science Center. Newborn piglets of either sex (1-3 days old, 1-2.5 kg) were used for these experiments. Animals were anaesthetized with ketamine hydrochloride (33mg/kg i.m.) and acepromazine (3.3mg/kg i.m.).
Isolation of cerebral microvessels. The brain was removed, placed in ice-cold Krebs solution (in mM): 120 NaCl, 5KCl, 0.62 MgSO4 × 7H2O, 1.8 CaCl, 10 HEPES, and 10 glucose (pH 7.4 with NaOH). The dura mater and attached vessels were removed. The brain cortex tissue was minced and gently homogenized in a Douce homogenizer with a loose pestle. The homogenate was passed through a 300-μm nylon mesh screen, and the passage was then refiltered through a 60-μm nylon mesh screen. The microvessels collected are those that pass through the 300μm mesh but are stopped by the 60μm mesh. The definition of “microvessel” remains nebulous. While some investigators contend that only capillaries are microvessels, we employ the more common definition that includes arterioles, capillaries and venules. Although our cut-off may include small arteries and veins, 200-300μm in diameter, observation of the actual sizes collected shows the predominant vessels to be less than 100μm. Although the mesh is 300μm, intact vessels of that diameter are unlikely to pass because the segment lengths cause the vessel to drape over the mesh. Experiments on freshly isolated cerebral microvessels began immediately after vessel collection with resuspension of the microvessels in Krebs solution.
Experimental treatments. Treatments were started by replacement of the Krebs solution in the vials with fresh Krebs solution that contained the experimental treatment. Heme prepared as heme-L-lysinate (HLL), indomethacin, and chromium mesoporphyrin (CrMP) were dissolved in Krebs solution. AA and PGE2 were dissolved in ethanol and diluted a minimum of 100-fold in Krebs solution. The light sensitive HLL and CrMP were protected from light exposure. The catalytic activity of HO in intact cerebral microvessels was determined by providing exogenous heme (HLL), so that endogenous substrate delivery would not affect CO production. To investigate COX products that may increase CO production, endogenous COX was blocked with indomethacin and PGE2 or a superoxide generator was exogenously added and CO production was measured. PGE2 was used as the prostanoid because it is produced in greatest quantity from exogenous AA. Two concentrations of PGE2 were used that were equimolar to the exogenous AA. To investigate the effect of superoxide anion and subsequent reactive oxygen species (ROS) on CO production in piglet cerebral microvessels, the activated oxygen-generating system xanthine oxidase (1U/ml) and hypoxanthine (0.2 mM) was administered to the microvessels for incubation(17). These concentrations maximally produced 13μM ·O2-(17). To evaluate the potential role of H2O2 and ROS produced from H2O2, H2O2 was removed with catalase (1,200 U/ml), beginning 10 min before addition of xanthine oxidase and hypoxanthine.
Measurements of CO production. Freshly isolated microvessels were placed inside amber vials (2.0 ml) containing Krebs solution. For the experiments in which CrMP (2x 10-5M) was used, the vessels were pretreated with CrMP for 30 min before the experiment was started and the inhibitor was maintained throughout. The internal standard (see below) was injected into the bottom of the vial and the vial was immediately sealed with a rubberized Teflon-lined cap. Cerebral microvessels were incubated for 30 min at 37°C. Incubations were terminated by placing the samples in hot water (75°C) and CO production was determined immediately.
A saturated solution of the isotopically labeled CO (13C16O; isotopic purity >99%) was used as an internal standard for quantitative measurements by gas chromatography/mass spectrometry (GC/MS)(6).
GC/MS analysis of the headspace gas was performed on a Hewlett-Packard 5970 mass-selective ion detector interfaced to a Hewlett-Packard 5890A gas chromatograph. The separation of CO from other gases was carried out on a Varian 5A mole sieve capillary column (30 m; 0.32 mm ID) with a linear temperature gradient from 35 to 65°C at 5 degrees/min. Helium was the carrier gas at a column head pressure of 4.0 psi. Aliquots (100-μl) of the headspace gas were injected by using a gas-tight syringe into the splitless injector having a temperature of 120°C. Mass-to-charge ratios (m/z) 28 and 29 corresponding to 12C16O and 13C16O, respectively, were recorded via selective ion monitoring. The amount of CO in samples was calculated from the ratio of peak areas of m/z 28 and 29. The results are expressed as picomol of CO released into the headspace gas per 100-μg protein in 30 min. Protein was measured by the Lowery method.
Measurements of PGE2 production. Concentrations of PGE2 in the cell incubation medium were determined by radioimmunoassay (18). Antibodies to the prostanoid were produced in rabbits immunized with PGE2 coupled to thyroglobulin. Our antibodies cross-react minimally (<1%) with other biologically relevant prostanoids. Moreover, PGE2 was not displaced from its antibodies by AA (20μg/ml); 5-hydroxyeicosateraenoic acid (HETE) or 15-HETE (1μg/ml); leukotriene (LT) B4, LTC4, LTD4, or LTE4 (5μg/ml); or lipoxin A4 or B4 (10 ng/ml). The free tracer fraction was separated from the fraction bound to antibodies using dextran-coated charcoal. Concentrations were calculated from the second-order-regression of tracer bound to the antibody vs. unlabeled prostanoid.
PGE2 production and COX activity, detected as PGE2 production from exogenous AA (2.0-20μM), were determined. After 30 min of incubation at 37°C, the medium was aspirated and stored at -20°C for PGE2 determination.
Materials. CO was purchased as compressed gas (99.5%) and saturated solutions (10-3M) produced in Krebs. The HO substrate, HLL, was prepared using methods described by Tenhunen et al. (19). The HO inhibitor, CrMP, was purchased from Frontier Scientific (Logan, UT). AA and PGE2 were purchased from Cayman Chemical Co. (Ann Arbor, MI). Water soluble indomethacin, (Indomethacin trihydrate) was a gift from Merck Sharp & Dohme Research Laboratories, Rahway, NJ. Xanthine oxidase, hypoxanthine, catalase and all other chemicals were of analytical grade and purchased from Sigma Chemical (St. Louis, MO, USA).
Statistical analysis. Values are presented as means ± SE. Results were subjected to a one-way ANOVA for repeated measures with Tukey's post hoc test to isolate differences between groups. Determination of difference from (0) zero was by Student t. A level of P<0.05 was considered significant.
RESULTS
Effects of AA on CO production in piglet cerebral microvessels. As expected, AA increased PGE2 production and indomethacin inhibited basal PGE2 production, as well as that produced from exogenous AA (2 μm - 20 μM)(Fig. 1). Treatment with AA also dose-dependently increased CO and this increase was inhibited by indomethacin (Fig. 2). CO production from exogenous heme also was reduced by indomethacin (Fig. 3).
Figure 1.
Effect of arachidonic acid and indomethacin (10-4M) on prostaglandin E2 (PGE2) production by piglet cerebral microvessels. Means ± SE of 5 experiments * P< 0.05 compared with zero arachidonic acid, † P<0.05 compared with corresponding values without indomethacin.
Figure 2.
Effect of arachidonic acid and indomethacin (10-4M) on CO production by cerebral microvessels of piglets. Means ± SE of 5 experiments. *p < 0.05 compared with zero arachidonic acid. † P < 0.05 Indomethacin compared to corresponding values without indomethacin.
Figure 3.
Effect of HLL and indomethacin (10-4M) on CO production in piglet cerebral microvessels. Means ± SE of 5 experiments * P < 0.05 compared with no HLL. † P < 0.05 compared to no indomethacin.
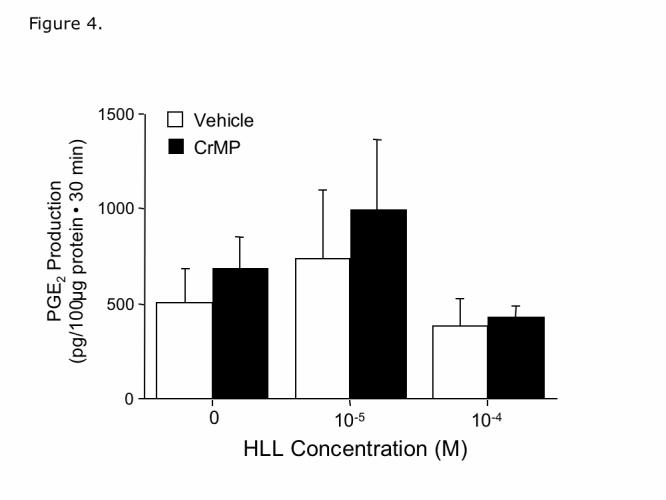
Effect of HO-2 metabolites on PGE2 production. As shown in Fig. 3, the HO substrate, HLL, increases CO production by freshly isolated microvessels that express HO-2, but not HO-1 (6, 20). However, neither HLL nor CrMP affected PGE2 production (Fig. 4). The dose of CrMP (2×10-5M) was selected because this concentration blocked dilation of pial arterioles in vivo caused by HLL, but not dilation caused by CO (6). These data suggest that products of HO-2 metabolism of heme, CO, bilirubin and iron, do not affect either free AA levels or COX catalytic activity.
Figure 4.
Effect of HLL and Chromium mesoporphyrin (CrMP) (2×10-5M) on prostaglandin E2 (PGE2) production by piglet cerebral microvessels. Means ± SE of 5 experiments.
Effect of exogenous PGE2 in indomethacin pretreated cerebral microvessels. In piglet cerebral microvessels pretreated with indomethacin (10-4 M), PGE2 (2μM and 20μM) caused small, dose dependent increases in CO production (Fig. 5).
Figure 5.

Effects of PGE2 and the superoxide generating system of xanthine oxidase (XO) (1U/ml) plus hypoxanthine (HX) (0.2mM), with and without catalase (CAT) (1200U/ml) on CO production by indomethacin (10-4M) treated piglet cerebral microvessels. n =11, 10, 10, and 7. Values are percentage change from vessels without either PGE2 or XO/HX. Means ± SE. * P < 0.05 compared to zero (no change) † P < 0.05 compared to no XO and HX.
Effects of the superoxide generating system of xanthine oxide and hypoxanthine. Xanthine oxidase with hypoxanthine, that produces superoxide, stimulated the production of CO about three-fold (Fig. 5), which is similar to the increase caused by 2μM and 20 μM AA (Fig. 2) as compared to PGE2. The increase in CO production was mildly attenuated by catalase.
DISCUSSION
This study demonstrates that COX products, apparently ROS, increase CO production by freshly isolated newborn pig cerebral microvessels. This conclusion is based on our new findings that: 1) AA, which increases COX activity, increases CO production from both endogenous and exogenous heme, 2) indomethacin, which decreases COX activity, decreases CO production. 3) xanthine oxidase with hypoxanthine, that produces superoxide, stimulates CO production in cerebral microvessels similarly to AA, and 4) PGE2 does not increase CO production nearly as much as AA or xanthine oxidase plus hypoxanthine. Conversely, since increasing HO activity with heme and inhibiting it with CrMP failed to affect PGE2 production, it appears that neither CO nor other heme metabolites affect phospholipase or COX activity.
COX (PGG/H synthases) has both cyclooxygenase and peroxidase activity. COX oxidizes AA to the cyclic endoperoxide intermediate PGG2, which is then converted to PGH2 from which subsequent prostanoids are synthesized. AA metabolism can also be a source of ROS production (10, 11). In particular, COX isoforms and 5-lipoxygenase contain heme iron and generate superoxide anion (10). PGE2, even at equimolar concentration to AA, did not increase CO production nearly as much as AA. Conversely, the ·O2- increased CO production similarly to AA. These results suggest that COX metabolism of AA increases CO production via production of ROS.
The reaction of hypoxanthine plus xanthine oxidase generates ·O2- and H2O2 (17,21). Superoxide was generated by xanthine oxidase acting on hypoxanthine to study the effect of ·O2- on cerebral microvessels. Because this reaction produces both ·O2- and H2O2, catalase (100 U/ml) was used to eliminate the effect of H2O2 produced via dismutation of ·O2-. When catalase was added to the xanthine oxidase plus hypoxanthine, CO production was reduced, but was still over 2.5 times basal. The maximal ·O2-concentration produced, 13 μM (17), is similar to the AA concentration used in these experiments.
Heme oxygenases are the main producers of CO although small amounts of CO can be derived from other sources such as lipid peroxidation (22). CO production can be controlled either by regulation of substrate (heme) delivery or of HO-2 catalytic activity. HO-2 catalytic activity may be altered by co-factor availability, cellular localization, and/or posttranslational modifications of the enzyme. Necessary co-factors for heme metabolism by HO are oxygen, NADPH, and NADPH-cytochrome c reductase (23). Under the experimental conditions used in the present experiments it is highly unlikely, although not impossible, that any of these co-factors would be low and thus limiting. Since indomethacin decreased and AA increased CO production from exogenous heme, a COX product increases HO-2 catalytic activity. However, the possibility that COX activity also can increase cellular heme cannot be excluded.
Recent studies suggested that low levels of ROS such as ·O2- and H2O2 modulate signal transduction pathways in mammalian cells (24). H2O2 stimulates protein phosphorylation (25), activates protein kinases (PKs) (26) inhibits tyrosine phosphatases (27), alters intracellularCa2+(Cai2+)(28) and stimulates phospholipases. In piglet cerebral microvessels tyrosine phosphorylation appears critical (29). Conversely, in rat neurons serine phosphorylation increases HO-2 activity(30).
In contrast to the present results on cerebral microvessels, elevation of HO increases COX activity (31) in the rat hypothalamus. In the present study, increasing heme up to 10-4M did not modify PGE2 production. Furthermore, the HO inhibitor, CrMP, did not affect prostanoid production either. Since increasing HO activity with heme and inhibiting it with CrMP failed to affect PGE2 production, it appears that neither CO nor the other heme metabolites change phospholipase or COX activity in newborn pig cerebral microvessels.
In conclusion, these data suggest that one or more products of AA metabolism by COX, apparently ROS, increase cerebrovascular CO production. This increase appears to include an increase in HO-2 catalytic activity, but a concomitant increase in free heme cannot be excluded. Conversely, neither CO nor other heme metabolites appear to markedly affect the activity of COX.
Acknowledgements:
This research was supported by the National Heart, Lung, and Blood Institute/ National Institutes of Health (NHLBI/NIH). Dr. Kanu is supported by a postdoctoral training grant from NHLBI/NIH. We thank D. Morse for preparing the final figures and M. Lester for clerical assistance.
References
- 1.Maines MD. The heme oxygenase system: a regulator of second messenger gases. Annu Rev Pharmacol Toxicol. 1997;37:517–554. doi: 10.1146/annurev.pharmtox.37.1.517. [DOI] [PubMed] [Google Scholar]
- 2.Jaggar JH, Leffler CW, Cheranov SY, Tcheranov D, Cheng X. Carbon monoxide dilates cerebral arterioles by enhancing the coupling of Ca2+ sparks to Ca2+-activated K+ channel. Circ. Res. 2002;91:610–617. doi: 10.1161/01.res.0000036900.76780.95. [DOI] [PubMed] [Google Scholar]
- 3.Cao L, Blute TA, Eldred WD. Localization of heme oxygenase-2 and modulation of cGMP levels by carbon monoxide and / or nitric oxide in the retina. Vis Neurosci. 2000;17:319–329. doi: 10.1017/s0952523800173018. [DOI] [PubMed] [Google Scholar]
- 4.Zhu Y, Hon T, Zhang L. Heme initiates changes in the expression of a wide array of genes during the early erythroid differentiation stage. Biochem Biophys Res Commun. 1999;258:87–93. doi: 10.1006/bbrc.1999.0586. [DOI] [PubMed] [Google Scholar]
- 5.Maines MD. Carbon monoxide, an emerging regulator of cGMP in the brain. Mol Cell Neurosciencs. 1996;4:389–397. doi: 10.1006/mcne.1993.1049. [DOI] [PubMed] [Google Scholar]
- 6.Leffler CW, Nasjletti A, Yu C, Johnson RA, Fedinec AL. Carbon monoxide and cerebral microvascular tone in newborn pigs. Am J Physiol Heart Circ Physiol. 1999;276:H1641–H1646. doi: 10.1152/ajpheart.1999.276.5.H1641. [DOI] [PubMed] [Google Scholar]
- 7.Leffler CW, Balabanova L, Sullivan D, Wang X, Fedinec AL, Parfenova H. Regulation of CO production in cerebral microvessels of newborn pigs. Am J Physiol Heart Circ Physiol. 2003;285:H292–H297. doi: 10.1152/ajpheart.01059.2002. [DOI] [PubMed] [Google Scholar]
- 8.Leffler CW, Jaggar JH, Fan Z. CO and Neonatal Cerebral Circulation. In: Abraham NG, editor. In Heme Oxygenase in Biology and Medicine. Kluwer Academic/Plenum Publisher; New York; 2002. [Google Scholar]
- 9.Vane JR, Bakhle YS, Botting RM. Cyclooxygenases 1 and 2. Annu Rev Pharmacol Toxicol. 1998;38:97–120. doi: 10.1146/annurev.pharmtox.38.1.97. [DOI] [PubMed] [Google Scholar]
- 10.Schreiber J, Eling TE, Mason RP. The oxidation of arachidonic acid by the cyclooxygenase activity of purified prostaglandin H synthase: Spin trapping a carbon-centered free radical intermediate. Arch Biochem Biophys. 1986;249:126–136. doi: 10.1016/0003-9861(86)90567-9. [DOI] [PubMed] [Google Scholar]
- 11.Kontos HA. Oxygen radicals from arachidonate metabolism in abnormal vascular responses. Am Rev Respir Dis. 1987;136:474–477. doi: 10.1164/ajrccm/136.2.474. [DOI] [PubMed] [Google Scholar]
- 12.Zakhary R, Gaine JL, Dinerman M, Ruat M, Snyder SH. Heme oxygenase-2: endothelial and neuronal localization and role in endothelium-dependent relaxation. Proc Natl Acad Sci U.S.A. 1996;798 doi: 10.1073/pnas.93.2.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tatsuguchi A, Sakamoto C, Wada K, Akamatsu T, Fukuda Y, Yamanaka N, Kobayashi M. Localization of cyclooxygenase-1 and cyclooxygenase-2 in Helicobacter pylori: related gastric ulcer tissues in humans. Gut. 2000;46:782–789. doi: 10.1136/gut.46.6.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Akarasereenont PJA, Mitchell YS, Thiemermann C. Bakhle, Vane JR. Comparison of the induction of cyclooxygenase and nitric oxide synthase by endotoxin in endothelial cells and macrophages. Eur J Pharmacol. 1995;273:121–128. doi: 10.1016/0014-2999(94)00680-6. [DOI] [PubMed] [Google Scholar]
- 15.Kujubu DA, Hershman Dexamethason inhibits mitogenic induction of the TIS10 prostaglandin synthase/cyclooxygenase gene. J Biol Chem. 1992;267:7991–7994. [PubMed] [Google Scholar]
- 16.Parfenova H, Eidson TH, Leffler CW. Upregulation of COX-2 in cerebral microvascular endothelial cells by smooth muscle cell signals. Am J Physiol. 1997;273:C277–C288. doi: 10.1152/ajpcell.1997.273.1.C277. [DOI] [PubMed] [Google Scholar]
- 17.Leffler CW, Mirro R, Thompson C, Shibata M, Armstead WM, Pourcyrous M, Thelin O. Activated oxygen species do not mediate hypercapnia-induced cerebral vasodilation in newborn pigs. Am J Physiol Heart Circ Physiol. 1991;261:H335–H342. doi: 10.1152/ajpheart.1991.261.2.H335. [DOI] [PubMed] [Google Scholar]
- 18.Leffler CW, Busija DW. Prostanoids in cortical subarachnoid cerebral hemodynamics. Cirs Res. 1985;57:689–694. doi: 10.1161/01.res.57.5.689. [DOI] [PubMed] [Google Scholar]
- 19.Tenhunen R, Tokola O, Linden IB. Haem arginate: a new stable haem compound. J Pharm Pharmacol. 1987;39:780–786. doi: 10.1111/j.2042-7158.1987.tb05119.x. [DOI] [PubMed] [Google Scholar]
- 20.Parfenova H, Neff RA, 3rd, Alonso JS, Shlopov BV, Jamal CN, Sarkisova SA, Leffler CW. Cerebral vascular endothelial heme oxygenase: expression, localization, and activation by glutamate. Am J Physiol Cell Physiol. 2001;281:C1954–1963. doi: 10.1152/ajpcell.2001.281.6.C1954. [DOI] [PubMed] [Google Scholar]
- 21.Fridovich I. Quantitative aspects of the production of peroxide anion radical by milk xanthine oxidase. J Biol Chem. 1970;245:4053–4057. [PubMed] [Google Scholar]
- 22.Piantadosi CA. Biological chemistry of carbon monoxide. Antioxid redox sifgnal. 2002;4:259–270. doi: 10.1089/152308602753666316. [DOI] [PubMed] [Google Scholar]
- 23.Maines MD. The heme oxygenase system and its functions in the brain. Cell Mol Biol. 2000;46:573–585. [PubMed] [Google Scholar]
- 24.Suzuki YH, Forman HJ, Sevanian A. Oxidants as stimulators of signal transduction. Free Radic Biol Med. 1997;22:269–285. doi: 10.1016/s0891-5849(96)00275-4. [DOI] [PubMed] [Google Scholar]
- 25.Schieven GL. Tyrosine phosphorylationin oxidative stress. Chapman and Hall; New York: 1997. pp. 181–199. [Google Scholar]
- 26.Abe JI, Kusuhara M, Ulevitch BJ, Berk BC, Lee JD. Big mitogen-activated protein kinase 1 (BMK1) is redox-sensitie kinase. J Biol Chem. 1996;271:16586–16590. doi: 10.1074/jbc.271.28.16586. [DOI] [PubMed] [Google Scholar]
- 27.Caselli AR, Marzocchini G, Camici G, Manao G, Moneti G, Pierraccini G, Ramponi G. The inactivation mechanism of low molecular weight phosphotyrosine protein phosphatase by H2O2. J Biol Chem. 1998;273:32554–32560. doi: 10.1074/jbc.273.49.32554. [DOI] [PubMed] [Google Scholar]
- 28.Schilling WP, Elliott SJ. Ca2+ signaling mechanisms of vascular endothelial cells and their role in oxidant-induced endothelial cell dysfunction. Am J Physiol Heart Circ Physiol. 1992;262:H1617–H1630. doi: 10.1152/ajpheart.1992.262.6.H1617. [DOI] [PubMed] [Google Scholar]
- 29.Leffler CW, Balabanova L, Sullivan D, Wang X, Fedinec AL, Parfenova H. Regulation of CO production in cerebral microvessels of newborn pigs. Am J Physiol Heart Circ Physiol. 2003;285:H292–H297. doi: 10.1152/ajpheart.01059.2002. [DOI] [PubMed] [Google Scholar]
- 30.Boehning D, Sedaghat L, Sedlak TW, Snyder SH. Heme Oxygenase-2 is activated by Calcium-Calmodulin. J Biol Chem. 2004;279:30927–30930. doi: 10.1074/jbc.C400222200. [DOI] [PubMed] [Google Scholar]
- 31.Mancuso C, Pistritto G, Tringali G, Grossman AB, Preziosi P, Navarra P. Evidence that carbon monoxide stimulates prostaglandin endoperoxide synthase activity in rat hypothalamic explants and in primary cultures of rat hypothalamic astrocytes. Brain Res Mol Brain Res. 1997:294–300. doi: 10.1016/s0169-328x(96)00258-6. [DOI] [PubMed] [Google Scholar]