Abstract
Activation of NKT cells using the glycolipid α-galactosylceramide (α-GalCer4) has availed many investigations into their immunoregulatory and therapeutic potential. However, it remains unclear how NKT cells respond to stimulation in vivo, which co-stimulatory pathways are important, and what factors (eg. antigen availability and activation-induced cell death) limit their response. We have explored these questions in the context of anin vivo model of NKT cell dynamics spanning activation, population expansion and subsequent contraction. Neither the B7/CD28 nor the CD40/CD40-L co-stimulatory pathways were necessary for cytokine production by activated NKT cells, either early (2 hours) or late (3 days) following initial stimulation, but both pathways were necessary for normal proliferative expansion of NKT cells in vivo. The pro-apoptotic Bcl-2 family member Bim was necessary for normal contraction of the NKT cell population between days 3-9 after stimulation, suggesting the pool size is regulated by apoptotic cell death in a manner similar to that of conventional T cells. Antigen availability was not the limiting factor for NKT cell expansion in vivo, and a second injection of α-GalCer induced a very blunted response, whereby cytokine production was reduced and further expansion did not occur. This appeared to be a form of anergy that was intrinsic to the NKT cells and not associated with up-regulation of inhibitory NK cell receptors such as NKG2A or Ly49 family members. Furthermore, NKT cells from mice pre-challenged with α-GalCer in vivoshowed little cytokine production and reduced proliferation in vitro. In summary, this study significantly enhances our understanding of how NKT cells respond to α-GalCer in vivo, revealing that the full primary response depends on costimulation via the CD28 and CD40 pathways, with subsequent Bim-dependent contraction. After contraction, the NKT cells are hypo-responsive to further antigenic induced expansion.
Keywords: T cells, Rodent, Natural Killer cells, Cell proliferation
Introduction
NKT cells are a unique population of thymus-derived T cells that express a CD1d-restricted, invariant T cell receptor (TCR) α chain, Vα14-Jα18, coupled to TCR β chains containing either Vβ8.2, 7 or 2 (TCR Vα24-Jα18 and Vβ11 in humans) (1). NKT cells recognise and respond to glycolipid antigens presented by the CD1d molecule and, upon primary stimulation, produce large amounts of cytokines, such as IL-4 and IFN-γ. The production of these cytokines appears critical for the involvement of these cells in a diverse range of immune responses, including autoimmune disease, allergy, infection and tumor rejection/destruction (2-4).
The best-defined and most commonly used agonist ligand for NKT cells is α-GalCer, a glycolipid originally isolated from a marine sponge (4, 5). α-GalCer has very potent and specific effects on NKT cells, including the induction of rapid cytokine production and proliferation and as such, can affect a large number of experimental diseases in an NKT cell-dependent fashion, including autoimmunity and cancer (2-4). Based on these studies, the therapeutic potential of α-GalCer is being studied in clinical trials with cancer patients (6, 7).
Within 3-12 hours of stimulation with α-GalCer in vivo, TCR+NK1.1+ NKT cells are no longer detectable in peripheral lymphoid organs, reappearing 24-48 hours later. Their (apparent) disappearance was originally attributed to activation-induced cell death (AICD), although this also occurred in mice lacking the pro-apoptotic Bcl-2 family member Bim (8), which controls death of conventional T cells and contraction of the T cell pool following antigen-induced expansion (9, 10). At least three separate studies have shown that this ‘disappearance’ is mostly due to transient TCR and NK1.1 down-regulation (8, 11, 12). By day 3, NKT cells expand to up to ten times their normal number in vivo, with ongoing production of IFN-γ, before contracting to normal levels between 6 and 9 days later. The factors that regulate the cytokine production, expansion and subsequent contraction of the NKT cell population are unknown, but several studies have suggested a role for CD28 and CD40 pathways in this process (13-16). This remains controversial, with at least one paper showing that the initial burst of cytokines from NKT cells in response to α-GalCer is independent of these co-stimulatory factors (17). The mechanisms underlying the contraction phase that occurs between days 3 and 9 are unknown, but antigen depletion and/or programmed antigen-induced cell death are two likely possibilities.
Therapeutic use of α-GalCer in mouse models typically involves 2 or more treatments with this reagent, although the effect of secondary treatments on NKT cell expansion and NKT cell-derived cytokine production in vivo is unclear. At least two studies have examined cytokine production by NKT cells following α-GalCer re-challenge, however these provided conflicting results (17, 18). One study found that despite a bias towards increased IL-4 production with no detectable IFN-γ in serum, NKT cells were producing both IFN-γ and IL-4 following re-challenge in vivo (17) while the other found NKT cells were anergic upon re-stimulation, based on in vitro rechallenge after primary stimulation with soluble α-GalCer in vivo (18). When chronic α-GalCer stimulation was used (weekly injections for 8 weeks), this depleted peripheral NKT cells, which were gradually replaced in a thymus dependent manner, however, the new NKT cells were severely hypo-responsive to further stimulation, due to increased expression of inhibitory receptors that appeared to be imprinted during their intrathymic development (19). A more recent study (20) showed that upregulation of the inhibitory receptor CD94/NKG2A on NKT cells, and Qa1b on antigen presenting cells, occurred even after only a single injection of α-GalCer in vivo, and provided data showing that this interaction was responsible for impaired responsiveness to a second challenge with α-GalCer in vivo.
Stimulation of NKT cells is known to induce bystander cytokine production and proliferation of other cells including NK, T, B and dendritic cells (DC) (14, 21-25). NK cells appear to be the main source of serum IFN-γ following α-GalCer stimulation in vivo (26), and IFN-γ production by NK cells downstream of NKT cell activation is essential for α-GalCer-mediated tumour rejection (27). For this reason, studies that have examined the NKT cell response via cytokine levels or proliferation in unfractionated cell cultures, or serum cytokine levels, cannot clearly distinguish between direct and indirect consequences of NKT cell stimulation. The aim of this study was to understand the factors that regulate the NKT cell response to α-GalCer in vivo, using CD1d/α-GalCer tetramers to specifically examine NKT cells by flow cytometry and directly assess the response of these cells to primary and secondary challenge and the importance of the CD28 and CD40 co-stimulatory pathways in NKT cell activation, early and late stage cytokine production, and expansion. We also examined whether NKT cell activation and expansion is limited by antigen availability, whether contraction of the NKT cell pool to pre-stimulation levels is Bim-dependent, as it is for conventional T cells, and whether the expansion or cytokine response from previously challenged NKT cells is different from naïve NKT cells upon re-stimulation.
Materials and Methods
Mice
Inbred C57BL/6J (B6), B6.TAP-1-deficient (TAP-1-/-), B6.CD40L-deficient (CD40L-/-) (28), B6.CTLA-4Ig transgenic (CTLA-4IgTg) (29) and B6.Bim-deficient (Bim-/-) mice (30) were obtained from The University of Melbourne, Department of Microbiology and Immunology Animal House, or the Walter and Eliza Hall Institute (WEHI) Central Animal House. Gene targeted mice were generated on the 129sv background, and backcrossed ≥ 10 generations to B6 before use. CTLA-4IgTg mice were generated on the Bm1 background and backcrossed 8 times to B6. All mice were aged between 6-9 weeks, and were housed under SPF conditions. Techniques performed in this study received approval from the University of Melbourne Animal Ethics Committee.
Culture media
Culture media contained RPMI-1640 (Gibco, Grand Island, NY) supplemented with 10% FCS (JRH, Melbourne, Australia), 100 U/ml Penicillin (Gibco), 100ug/ml Streptomycin (Gibco), 2mM Glutamax (Gibco), 1mM Sodium Pyruvate (Gibco), 5x10-5M 2-mercaptoethanol (Sigma), 0.1mM non-essential amino acids (Gibco) and 15mM HEPES buffer (Gibco).
Antibodies and flow cytometry
Thymus as well as spleen cell suspensions and liver lymphocyte isolation were performed as previously described (31). Cells were counted using an automated cell counter (Z Series Dual; Coulter Electronics, Luton, England) or a hemocytometer. To avoid non-specific binding of Abs to FcRγ, hybridoma supernatant containing anti-mouse CD16/32 mAb (2.4G2) (grown in-house) was including in cell labelling experiments. Cells were stained with FITC-conjugated NK1.1 (PK136), CD4 (RM4-5), Ly49C/I (5E6), NKG2A/C/E (20D5) or TCR-β (H57-597), phycoerythrin (PE)-conjugated CD4 (RM4-5), or NKG2D (CX5 eBioscience), PerCP-conjugated CD4 (RM4-5), allophycocyanin (APC)-conjugated TCR-β (H57-597) or Ly49G2 (4D11), biotin-conjugated CD28 (37.51), CD94 (18d3), Fas-L/CD178 (MFL3), Ly49A (A1), NK1.1 (PK136) or NKG2A () (all from BD Pharmingen; San Diego, CA unless otherwise specified), and/or PE, PE-Cy5 or PE-Cy7-conjugated mouse α-GalCer-loaded CD1d tetramers (produced in house as previously described (32), using recombinant baculovirus encoding his-tagged mouse CD1d and mouse β-2 microglobulin, kindly provided by Prof. M. Kronenberg's laboratory). Biotinylated Abs were detected using streptavidin-Alexa Fluor®488 (Molecular Probes, Eugene, OR), or streptavidin conjugated to PerCP, PE-Cy5 or PE-Cy7 (BD Pharmingen). Stained cells were analyzed using a FACSCalibur, LSRII, or FACSAria flow cytometers (BD Biosciences; San Jose, CA), and data were processed with either CellQuest™ (Becton Dickinson) or FlowJo (Treestar Inc. Ashland, OR) software. For B cell depletion, single cell suspensions were stained with anti-B220 (RA3.6B2, grown in-house), washed, and incubated with sheep anti-rat IgG conjugated immunomagnetic beads (Dynal Pty Ltd, Carlton, VIC, Australia). Bead-labeled cells were magnetically removed according to supplier's instructions, and the remaining cells surface labeled for flow cytometric sorting. Multi-color sorting was performed using a FACSAria, with purities consistently greater than 95%. Where necessary, digital data was presented using biexponential scaling to resolve on-axis events.
Intracellular cytokine staining
Following isolation from the liver or spleen, lymphocytes were cultured for 2 hours in 2.0 μM Monensin (GolgiStop™, BD Biosciences) or 10μg/mL Brefeldin-A, withoutin vitro stimulation. After cell-surface Ab labeling, lymphocytes were washed once before fixing in 0.5% paraformaldehyde (BDH Chemicals; Poole, U.K.) in the dark for 30 min at room temperature (RT). Cells were then washed twice in FACS buffer before incubation with PE-conjugated antibodies to IFN-γ (XMG1.2) or IL-4 (11B11), or with an IgG1 isotype control (R3-34). Intracellular staining was performed in FACS buffer containing 0.05% saponin (Sigma-Aldrich) for 1 h at RT in the dark. In some experiments, the BD intracellular staining kit, with GolgiStop, was used as per instructions.
In vivo treatment with α-galactosylceramide
α-GalCer was kindly provided by the Pharmaceutical Research Division (Kirin Brewery; Gunma, Japan) and was prepared in saline supplemented with 0.5% w/v polysorbate-20. Control vehicle saline was also supplemented with 0.5% w/v polysorbate-20. Mice were injected i.p. with 2 μg α-GalCer or control vehicle.
In vitro α-GalCer-induced NKT cell proliferation
Following isolation, 4 × 107 spleen lymphocytes from naïve or α-GalCer pre-treated mice (2 μg/mouse injected i.p. 2 months, 1 month or 2 weeks prior) were CFSE-labeled in 1 ml PBS with 16 μl of CFSE at 0.1 mM in 2 ml of PBS with 0.1% BSA, for 10 minutes at 37C with gentle mixing. Cells were washed twice with 5 ml culture media containing 20% FCS, resuspended in culture media and cultured in the presence of α-GalCer (ranging from 1 to 500 ng/ml in different experiments) with or without IL-2 (50 U/ml). At the end of the first day, cells were washed by spinning through an underlay of fetal calf serum and resuspended in fresh culture media without a-GalCer but with IL-2 in appropriate wells. This was designed to allow TCR re-expression in the absence of continued antigen exposure over the next 2.5 days of culture, thus permitting identification of NKT cells by CD1d tetramer staining. After the total 3.5-day culture, cells were harvested, counted and analysed by flow cytometry.
Results
CD28 and CD40 signalling pathways are essential for expansion of the NKT cell pool but not for cytokine production
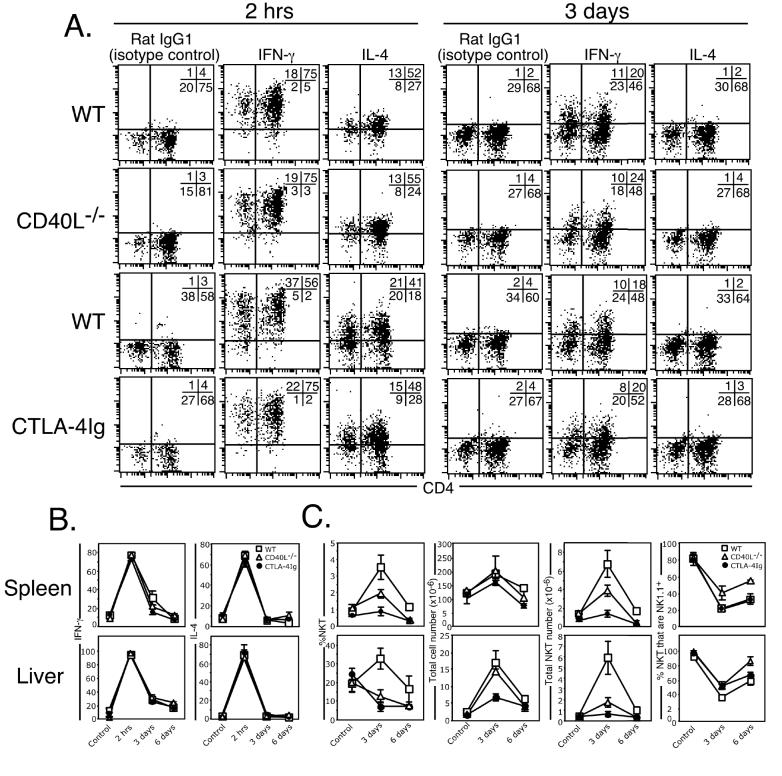
The influence of CD28 and CD40 signalling on NKT cell stimulation is controversial, with one study (13) showing that both factors are necessary for NKT cell activation while another (17) showed that NKT cell activation proceeds normally in the absence of these factors. These studies were performed prior to the realisation that NKT cells do not die following α-GalCer stimulation in vivo, but instead, transiently down-regulate their TCR and NK1.1 markers, undergo significant expansion and display sustained cytokine production for up to three days following treatment (8, 11, 12). It was therefore important to revisit these questions, and investigate the involvement of CD40 and CD28 signalling pathways in early (2 hours) and late (3-6 days) phases of α-GalCer-induced NKT cell activation in vivo. CD40L-/- and CTLA-4IgTg mice (that secrete soluble CTLA-4-Ig and effectively inhibit the B7/CD28 signalling pathway in vivo (29, 33)) were injected i.p. with α-GalCer and examined between 0 and 6 days after treatment for NKT cell-derived cytokine production and expansion. NKT cells from CD40L-/- and CTLA-4IgTg mice were indistinguishable from WT mice in their ability to produce IFN-γ and IL-4, labelled at both 2 hours and 3 days after treatment (Figures 1A and B). This demonstrates that these signalling pathways are not critical for either the early or late phases of NKT cell-derived cytokine production. In contrast, both groups of mice, particularly CTLA-4IgTg mice, displayed reduced numbers and frequency of NKT cells in both the liver and spleen when compared with WT mice (Figure 1C), demonstrating an important role for CD28 and CD40 signalling in NKT cell expansion. CTLA-4IgTg mice also had lower total liver cell counts when compared with WT and CD40L-/- mice (Figure 1C), suggesting a CD28-dependent process is necessary for intra-hepatic recruitment or proliferation of bystander cells, however this effect was not observed in the spleen. The transient down-regulation of NK1.1 on activated NKT cells that has been previously reported (8, 11) was observed in each group of mice (Figure 1C). Taken together, these data suggest that some aspects of NKT cell activation, such as cytokine production, can occur independently of the CD28/CD40 pathway, but both co-stimulatory factors are required for optimal NKT cell expansion after stimulation.
Figure 1.
CD40 and CD28 signalling are both essential for NKT cell expansion but dispensable for cytokine production. Mice (WT, CD40L-/- and CTLA4-Ig transgenic) were injected i.p. with 2 μg α-GalCer and examined between 2 hrs and 6 days later. Liver and spleen-derived lymphocytes were isolated and cultured for 2 hrs in GolgiStop, surface-labelled with CD1d/α-GalCer tetramer or anti-αβTCR, CD4 or NK1.1 mAb, and then fixed and permeabilized for intracellular staining with antibodies to IL-4 or IFN-γ. NKT cells were electronically gated and analysed for their expression of IFN-γ or IL-4 (A and B). A. displays representative flow cytometry data from each group at each time point. Quadrants were set based on staining with the rat IgG1 isotype control antibody. B. Pooled data of IFN-γ and IL-4 production and is representative of (WT: C = control (PBS injected) 8 mice; 2 hr = 8 mice; 3 days = 6 mice; 6 days = 3 mice; CD40L-/-: C = control (PBS injected) 2 mice; 2 hr = 2 mice; 3 days = 2 mice; 6 days = 2 mice; CTLA-4IgTg: C = control (PBS injected) = 5 mice; 2 hr = 5 mice; 3 days = 6 mice; 6 days = 3 mice; between 1-3 experiments/strain/time-point. Control (PBS injected) mice were harvested at each time-point to control for unstimulated NKT cell status. (C) Graphs from left to right show the percentage CD1d/α-GalCer-tetramer-binding NKT cells, total number of lymphocytes per organ, total number of NKT cells per organ and percentage of NKT cells that were NK1.1+. For B and C, the top row shows spleen and bottom row shows liver. Error bars represent the standard error of the mean.
Contraction of the NKT cell pool requires the pro-apoptotic Bcl-2 family member Bim
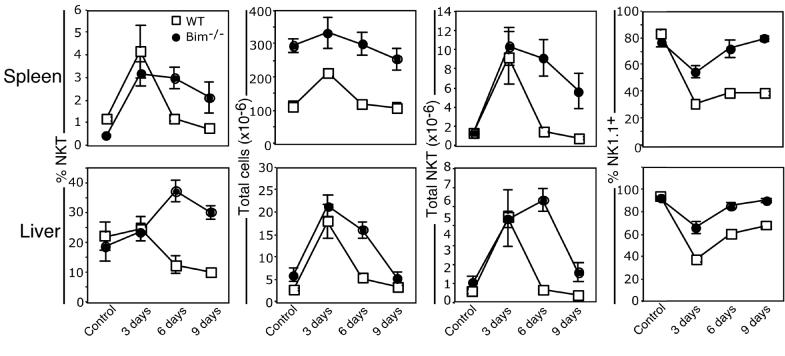
Following α-GalCer-induced expansion (8, 11, 12), NKT cell numbers return to pre-treatment levels within 6-9 days after stimulation (8, 12). Upon immunization or challenge with a pathogen, conventional antigen-specific T cells also undergo a period of expansion prior to contracting to pre-stimulation levels. The death of conventional T cells during shut-down of an immune response is known to be under the control of the BH3-only protein, Bim (9, 10), a pro-apoptotic factor important in the mitochondrial pathway of apoptosis (30). To investigate whether contraction of the NKT cell pool was the result of a similar cell-death pathway, Bim-deficient mice were injected with α-GalCer and examined for expansion and contraction of the NKT cell pool. NKT cells expanded similarly in WT and Bim-/- mice, but contraction of the NKT cell pool was significantly delayed, in both spleen and liver, until at least 9 days after treatment in Bim-/- mice (Figure 2).
Figure 2.
Contraction of the NKT cell pool is bim-dependent Mice (WT and bim-/-) were injected i.p. with 2 μg α-GalCer and examined between 3 and 9 days later. Liver and spleen-derived lymphocytes were isolated and stained with CD1d/α-GalCer tetramer, anti-αβTCR and NK1.1 mAbs and analysed for the percentage of CD1d/α-GalCer tetramer-binding αβTCR+ NKT cells, total lymphocyte number and total NKT cell number in both the spleen (top row) and liver (bottom row). Data are derived from two independent experiments with a total of n = (WT: = 5 mice; 3 days = 5 mice. 6 days = 5 mice; 9 days = 5 mice; Bim-/-: C (Control, PBS injected) = 5 mice, 3 days = 5 mice. 6 days = 6 mice; 9 days = 5 mice). Control (PBS injected) mice were harvested at each time-point to control for unstimulated NKT cell status. Error bars represent the standard error of the mean.
α-GalCer re-challenge induces NKT cell-derived cytokines but not NKT cell expansion
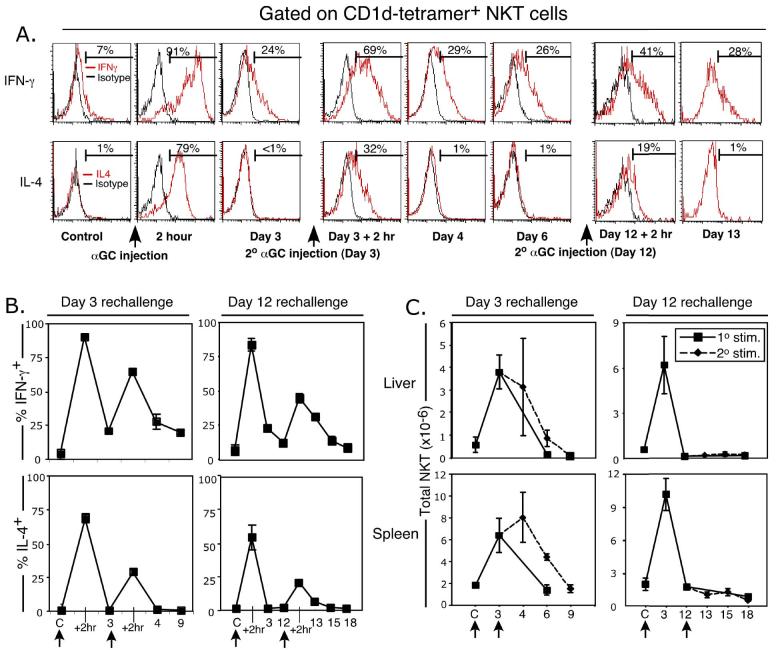
To assess the ability of antigen-experienced NKT cells to respond to further TCR-mediated stimuli, α-GalCer-challenged mice were re-injected with α-GalCer on day 3, at the peak of NKT cell expansion when they are still producing IFN-γ, but no detectable IL-4, or on day 12, when NKT cell numbers have contracted and cytokine production has ceased. Re-challenge on either day 3 or 12 induced a second, albeit reduced, burst of cytokine production within 2 hours (Figure 3A and B) and the response at 12 days was similar (Figure 3B). Re-challenge on day 3 appeared to transiently sustain NKT cell numbers in the spleen, but re-challenge on day 12, after the NKT cell pool had contracted to normal size, failed to induce any additional expansion of the NKT cell pool in either the spleen or liver (Figure 3C), demonstrating that antigen-experienced NKT cells are refractory to secondary α-GalCer-induced expansion. In contrast to primary stimulation, re-challenge on either day 3 or day 12 also caused less TCR down-regulation (data not shown). Collectively, these data demonstrate that antigen-experienced NKT cells, and/or their progeny, are less responsive to antigenic stimulation with α-GalCer than naïve NKT cells in vivo, despite expressing apparently normal TCR levels. This is the first study to directly examine NKT cell numbers after antigenic rechallenge in vivo.
Figure 3.
The NKT cell response to α-GalCer re-challenge in vivo. Mice (WT) were injected i.p. with 2 μg α-GalCer and re-injected on day 3 or day12 after primary challenge. Liver and spleen-derived lymphocytes were isolated and cultured for 2 hrs in Brefeldin-A, surface-labelled with CD1d/α-GalCer tetramer or anti-αβTCR, CD4 or NK1.1 mAbs, and then fixed and permeabilized for intracellular staining with mAbs to IL-4 or IFN-γ. CD1d/α-GalCer tetramer αβTCR+ NKT cells were gated and analysed for their expression of IFN-γ or IL-4, as shown in (A) with representative data from liver NKT cells after a 3 day or 12 day rechallenge experiment. Accumulated cytokine data from day 3 and day 12 rechallenge experiments are shown in B. Total NKT cell numbers at each time-point are shown in C. Data were derived from 3-5 mice per group. Control (PBS injected) mice were harvested at each timepoint to control for unstimulated NKT cell status. Error bars represent the standard error of the mean. Arrows indicate the time of α-GalCer injection.
Reduced NKT cell responsiveness is not associated with up-regulation of NK cell inhibitory receptors
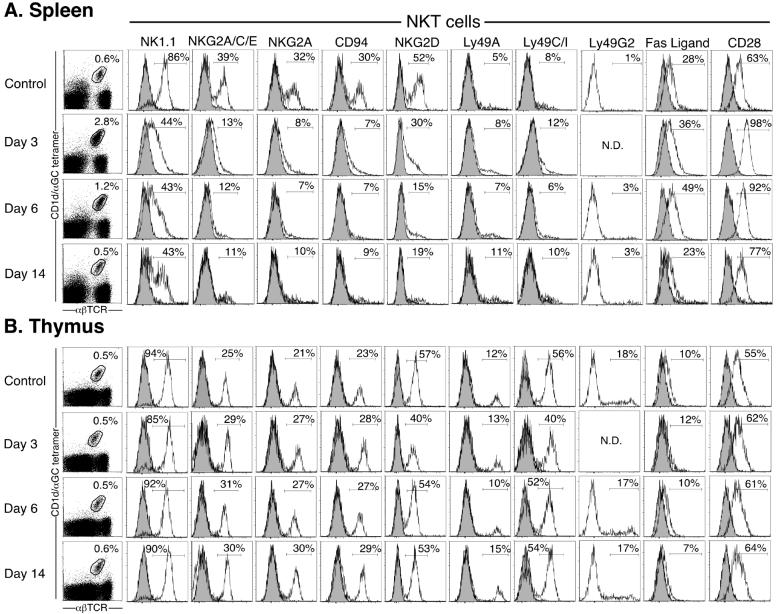
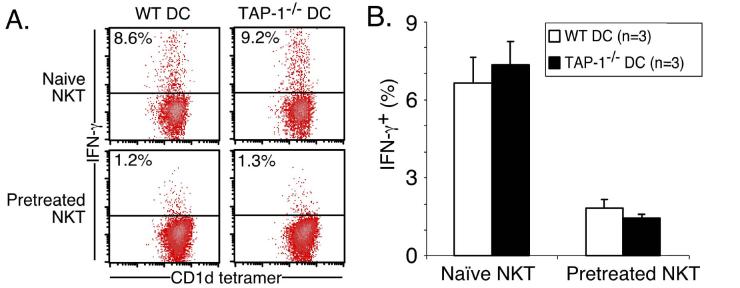
Chronic α-GalCer stimulation (2 μg i.p. weekly for 8 weeks), followed by 8 weeks rest without additional stimulation, results in depletion and thymus-dependent repopulation of the NKT cell pool by NKT cells with increased expression of inhibitory receptors (19). Indeed, this appeared to be the main reason underlying the non-responsiveness (‘anergy’) of chronically challenged NKT cells, because TAP-1-/- antigen presenting cells (which lack the MHC-I molecules that are ligands for these inhibitory receptors but retain CD1d expression) were able to stimulate a full response from the ‘anergized’ NKT cells. A recent report also suggested that NKG2A upregulation occurred following even a single injection of α-GalCer, and that ligation of this inhibitory receptor was responsible for hypo-responsiveness to a second α-GalCer challenge (20). Therefore, we examined a range of stimulatory and inhibitory NK receptors as well as other relevant receptors on splenic and thymic NKT cells at various times after a single injection of α-GalCer in vivo. Surprisingly, we found little/no evidence of inhibitory receptor up-regulation at any time-point tested (including days 3, 6, 14 (Figure 4) and 18 not shown). Moreover, NKG2A expression was undetectable on spleen NKT cells (supported by staining with three different antibodies: NKG2A, NKG2A/C/E and CD94) at each of these time-points, and likewise, the stimulatory receptor NKG2D was also down-regulated on these NKT cells. Expression of other receptors, including Ly49D, Ly49F, CD152 (CTLA-4) and CD154 (CD40 Ligand) by NKT cells was unaffected by α-GalCer challenge, and none of these markers showed altered expression on conventional (CD1d tetramer-negative) αβTCR+ T cells following α-GalCer stimulation (data not shown). To test the involvement of inhibitory NKRs in NKT cell anergy, splenic NKT cells were purified from naïve or 7 day α-GalCer pre-treated WT mice, and co-cultured in vitro with WT or TAP-1-/--derived DCs, for 12 h ± 200 ng/ml α-GalCer. As TAP-1-/- mice lack the ligands for Ly49 receptors and NKG2A/C/E (MHC-I (34) and the Qa1b-associated nonameric peptide Qdm (35, 36) respectively), but not CD1d, they serve as an ideal tool for investigating the involvement of these receptors in NKT cell anergy. Cultures of pre-treated splenic NKT cells and WT DCs produced lower levels of IFN-γ when compared with naïve NKT cells, and this effect was not reversed by the use of TAP-1-/- DCs (Figure 5). Equivalent data were obtained from sorted liver-derived NKT cells (data not shown). These data confirm that inhibitory receptor up-regulation is not the cause for systemic NKT cell hypo-responsiveness in response to secondary challenge with α-GalCer. This also indicates that the observed anergy is intrinsic to the NKT cells, rather than reflecting an alteration in APC function, since the APC came from naïve mice.
Figure 4.
Modulation of cell surface receptors on NKT cells following α-GalCer stimulation in vivo. Mice (WT) were injected i.p. with 2 μg α-GalCer on day 0, and examined 3, 6, or 14 days later. Spleen and thymus-derived lymphocytes were isolated and stained with CD1d/α-GalCer tetramer plus anti-αβTCR (first column), and NKT cells were electronically gated as shown and examined for expression of NK1.1, NKG2A/C/D/E, CD94, Ly49A/C/G2/I, Fas-Ligand (CD178) and CD28 (open histograms), overlayed onto relevant isotype controls (greyed histograms). Controls (no injection), n = 3 mice; day 3, n = 2 mice; day 6, n = 3 mice; day 14, n = 3 mice.
Figure 5.
NKT cell anergy is autonomous and independent of NK inhibitory receptor ligation. Mice (WT) were injected i.p. with 2 μg α-GalCer or PBS, and spleen-derived lymphocytes were isolated 7 days later, B cell depleted using magnetic beads, and NKT cells sorted based on CD1d/α-GalCer tetramer-binding and αβTCR expression. CD11c+ DCs were also sorted from WT and TAP-1-/- mice. Purified NKT cells from naïve or α-GalCer-pretreated mice were cocultured with WT or TAP-1-/- DCs, at a ratio of 5:1 for 12 hin vitro ± 200 ng/ml α-GalCer, and subsequently stained for anti-IFN-γ-PE, or rat IgG1-PE isotype control. (A) Representative profiles of IFN-γ production by gated CD1d/α-GalCer tetramer+ αβTCR+ NKT cells and (B) Graph depicts mean and standard error for each group. IFN-γ staining regions were set based on the isotype control, and data is representative of 3 mice per group.
Reduced NKT cell proliferation after in vitro re-stimulation following in vivo challenge.
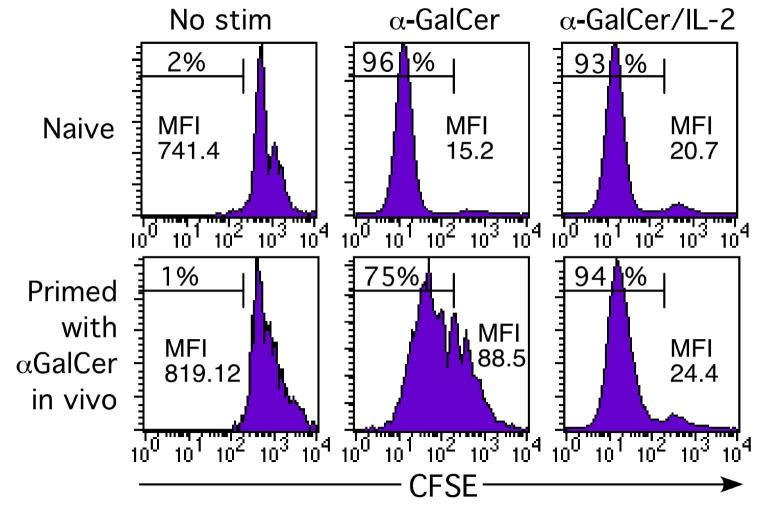
Unfractionated splenocytes were isolated from naïve or α-GalCer pre-treated mice (either 2 weeks, 1 month or 2 months prior), labeled with CFSE, and cultured for 3-4 days to test their proliferative response to α-GalCer-mediated stimulation in vitro. Data in Figure 6 show a comparison of NKT cells from naïve and 2 week pre-treated mice. NKT cells from naïve mice, cultured in the presence of α-GalCer, proliferated more extensively (as indicated by CFSE dilution) than NKT cells from mice pre-challenged in vivo. A previous study suggested that IL-2 in combination with α-GalCerin vitro could enhance the number of IFN-γ producing splenocytes from mice pre-treated with α-GalCer in vivo (18), however it was not clear from this study exactly how this affected the specific NKT cell response, for example, did this reflect an increase in NKT cell number, NKT cell-derived IFN-γ production, or IFN-γ production from bystander cells? Using the CD1d/α-GalCer tetramer to specifically identify NKT cells, we found that IL-2 overcame the impaired proliferative response of NKT cells pre-challenged in vivo, resulting in a similar level of CFSE dilution to that seen for naïve NKT cells (Figure 6).
Figure 6.
Previously challenged NKT cells show reduced in vitro proliferation following re-challenge with α-GalCer. Spleen-derived lymphocytes were labelled with CFSE and cultured for 1 day with α-GalCer with or without 50 units/ml IL-2, washed and the culture continued for an additional 2.5 days without α-GalCer (to allow T cell receptor re-expression), but with IL-2 replenished in appropriate cultures. Following culture, NKT cells were stained with CD1d/α-GalCer tetramer and anti-αβTCR mAb and examined for evidence of cell division as indicated by dilution of CFSE-labeling. The percentage of cells having undergone one or more divisions is shown, as well as the geometric mean fluorescence intensity (MFI) for the entire NKT cell population. This data represents results from 1 of 4 similar experiments carried out between 2 weeks and 2 months after primary in vivo α-GalCer stimulation.
Reduced NKT cell cytokine production following α-GalCer rechallenge in vitro
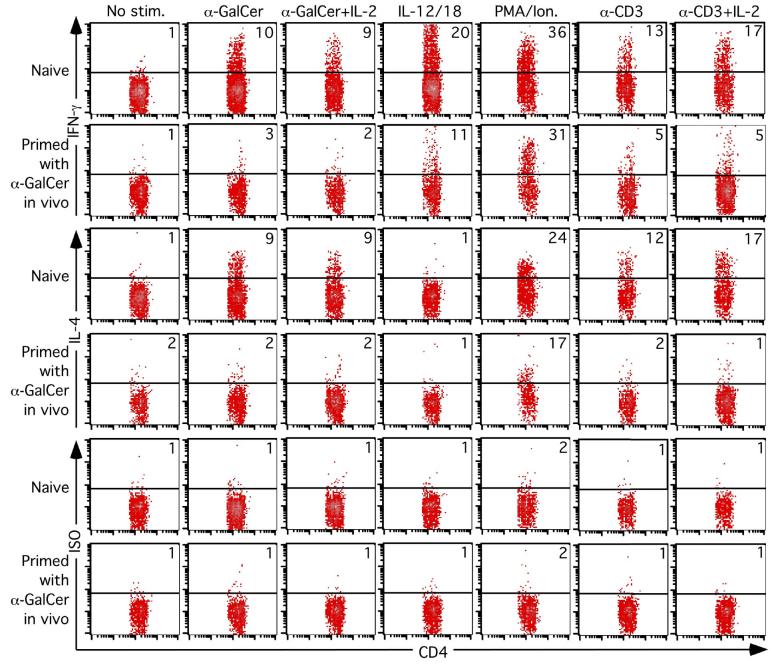
Lymphocytes were harvested from livers of naïve or α-GalCer pre-treated mice (either 2 weeks, 1 month and 2 months prior), and stimulated in vitro in the presence of α-GalCer with or without IL-2, IL-12 plus IL-18, PMA/ionomycin or anti-CD3 with or without IL-2, to examine cytokine production by intracellular cytokine staining. Data from a one-month pre-challenge experiment is shown in Figure 7, and provided similar data to that observed from the 2 week and 2 month pre-challenge groups (not shown). As expected, naïve NKT cells produced both IFN-γ and IL-4 in response to α-GalCer stimulation, whereas the response of NKT cells from α-GalCer pre-challenged mice was barely detectable. While IL-2 enhanced the proliferative response of pre-challenged NKT cells (Figure 6), it had no apparent effect on the cytokine response by these NKT cells in the presence of α-GalCer. In contrast, simultaneous stimulation with IL-12 plus IL-18 in the absence of α-GalCer, a combination that drives potent IFN-γ production by NKT cells (Figure 7 and (37)) triggered a partial, but still reduced, IFN-γ response from these in vivo pre-challenged NKT cells. PMA/ionomycin stimulation drove strong production of IFN-γ and IL-4, regardless of whether the NKT cells were from naïve or pre-challenged mice. Thisin vitro hypo-responsiveness was unlikely to be the result of impaired APC function in pre-challenged mice, as similar hypo-responsiveness was observed when plate bound anti-CD3 was used to stimulate the NKT cells (Figure 7). This supports our data shown in figure 5 demonstrating that anergy is intrinsic to the NKT cells from α-GalCer pre-challenged mice and cannot be overcome when DC from naïve mice are used as stimulators.
Figure 7.
Impaired in vitro cytokine production by NKT cells from mice previously challenged with α-GalCer. Mice were injected with 2 μg α-GalCer i.p. and killed 1 month later. Liver-derived lymphocytes were isolated and cultured for 6 hours with no further stimulation, 200ng/mL α-GalCer ± 50 units/mL IL-2, 100ng/mL each IL-12 and IL-18, 10ng/mL PMA and 3μM ionomycin, or 10μg/mL plate-bound anti-CD3 ± 50 units/ml IL-2. GolgiStop was added for the final 5 hours of culture before cells were surface-stained with the CD1d/α-GalCer tetramer and anti-CD4, and then fixed and permeabilized for intracellular staining with anti-IFN-γ, anti-IL-4 or rat IgG1 isotype control intracellular mAb. CD1d/α-GalCer tetramer+ CD4+ cells were selected by electronic gating and analysed for their expression of IFN-γ, for specific staining by the isotype control. Data is representative of 4 mice per group and this is experiment is representative of 4 experiments carried out between 2 weeks and 2 months after primary in vivo α-GalCer treatment.
Discussion
Until recently, NKT cells were thought to respond very rapidly to antigen-mediated stimulation in vivo, releasing a burst of cytokines, and dying within hours due to activation-induced cell death (38). We and others recently published a revised model of NKT cell dynamics following activation in vivo, which postulates that these cells do not die rapidly upon stimulation, but instead proliferate within 3 days to reach levels that are up to 10 times their steady state numbers, before contracting to pre-stimulation levels by 6-9 days (8, 11, 12). With this new model in mind, it was necessary to revisit the question of what factors regulate the response of NKT cells to antigenic challenge in vivo. Specifically, what role do co-stimulatory factors play in the initial activation and subsequent expansion of the NKT cell pool? Is NKT cell expansion limited by antigen availability? Is the subsequent contraction of the NKT cell pool due to Bim-mediated apoptosis, as is the case for conventional T cells (9, 10).
Our data showed that both the initial burst, and longer term (3 days), cytokine production by NKT cells was independent of CD28/CD40 co-stimulatory pathways. This is consistent with, and expands upon, one previous study that showed the cytokine response at 2 hours did not require co-stimulation (17) and contrasts with some earlier studies suggesting that the CD40 and CD28 pathways were important for NKT cell-derived cytokine production (13-15). This discrepancy is likely to reflect the importance of co-stimulatory molecules in activation of cells downstream of NKT cells, since these studies typically relied on cytokine measurements from heterogeneous cell culture supernatants or serum, and were therefore unable to determine whether the cytokines assayed were coming directly from the NKT cells. CD40 is known to be important in this capacity, as has been clearly demonstrated with NKT cell-mediated DC activation, resulting in IL-12 production and activation of other bystander cells including T cells and NK cells (14, 17, 21, 23, 25, 39). While CD28 and CD40 signalling were unnecessary for NKT cell-derived cytokine production, we showed that they were required for the characteristic rapid and extensive expansion of the NKT cell pool in vivo. As CD80 (CD28-ligand) up-regulation on APCs follows CD40 ligation, it is reassuring that both CD40L-/- mice and CTLA-4IgTg groups of mice displayed similar phenotypes with impaired NKT cell expansion following α-GalCer stimulation in each. The results from these experiments are consistent with the idea that NKT cells themselves can be activated without requiring co-stimulation via CD28, while the production of downstream factors (eg. IL-12), from bystander cells (eg. DC), may be necessary for a fully developed NKT cell response (14, 40). We cannot exclude the possibility however, that other NKT cell intrinsic factors not measured in this study may depend on signalling via the CD40/CD28 pathways, and these may be directly involved in the NKT cell proliferative response, or that some residual B7 function might exist in these mice, despite the excess of CTLA-4Ig protein present in the serum. It should be added that while CD28 signalling may not be necessary for the normal α-GalCer-induced, NKT cell-derived, cytokine response in vivo, it does not necessarily mean that NKT cells are unresponsive to CD28. Indeed, CD28 signalling appears to override MHC class I-mediated inhibition of constitutive NKT cell cytokine production (in the absence of exogenously added antigen)in vitro (16). Moreover, CD28 co-stimulation was found to enhance NKT cell-derived cytokine production in response to in vitro treatment with anti-CD3 antibodies (41). Regardless of which stage of the NKT cell response that CD28 and CD40 pathways influence, these factors are likely to play an important role in many α-GalCer-induced responses where disease resolution depends upon increased NKT cell numbers and/or downstream activation and cytokine production by effector cells (13).
An interesting problem was whether antigen depletion was a key factor that limited NKT cell expansion and/or whether this was responsible for the subsequent contraction of the NKT cell pool to pre-stimulation levels. The observation that re-injection of α-GalCer three and twelve days after primary challenge failed to trigger a second wave of expansion, strongly suggested that the antigen availability was not a key limiting factor, but rather, that the expansion/contraction dynamics of the NKT cell pool was a programmed response. Our results also show that the contraction phase of NKT cells involves a Bim-dependent apoptotic process, which aligned this stage of the NKT cell response with that of conventional T cells that also undergo Bim-dependent apoptosis during shut-down of an immune response (9, 10), however the partial and delayed decline in NKT cell number in Bim-/- mice suggests that other factors may also contribute to this stage. It is noteworthy that this contrasts with the early stage of NKT cell activation, when they transiently disappear within a few hours of antigenic challenge in vivo, which we previously demonstrated was independent of Bim (8).
The finding that NKT cells are hypo-responsive to rechallenge with α-GalCer is partly consistent with results from a previous study that suggested NKT cells become anergic following stimulation with free α-GalCer in vivo (18). It must be pointed out however, that the approach and conclusions from this previous study were significantly different from ours, in that it examined the ability of α-GalCer-pulsed DC to induce an NKT-dependent response, in vitro or in vivo, and concluded that when NKT cells were isolated from mice previously challenged with free α-GalCer in vivo, they did not respond to α-GalCer-pulsed DCin vitro. However, the assays employed in that study failed to demonstrate a clear response to free α-GalCer, regardless of whether the cells were isolated from naïve or free α-GalCer pre-treated mice, and were therefore unable to compare the specific NKT cell response to primary and secondary challenge with free α-GalCer, which to date, is the most common form of treatment in therapeutic models. It is also important to note that in our study, although cytokine production from re-challenged NKT cells was reduced, it was still clearly detectable in vivo, and is therefore likely to have significant biological consequences. This is important when considering the regimen for α-GalCer therapy that usually involves more than one injection in vivo. Earlier studies have illustrated that α-GalCer recall responses are biased toward IL-4 production in the serum andin vitro (17, 42), and that multiple α-GalCer treatment regimes can be used to treat Th1 disorders such as type 1 diabetes (43, 44) and experimental autoimmune encephalomyelitis (EAE) (45, 46). Our data reveal that, consistent with an earlier report (17), NKT cells themselves remain unpolarised following secondary α-GalCer challenge, suggesting that the systemic loss of IFN-γ following multiple α-GalCer treatment regimes might be primarily due to a downstream effect on other IFN-γ-producing cells, such as NK cells, as originally suggested by Matsudaet al. (17). This is also consistent with an earlier report that NK cells act as the main source of serum IFN-γ (26), highlighting the importance of analysing cytokine production at a single cell level, and the requirement for a greater understanding of the interactive biology between NKT, NK and DCs. Why NKT cells failed to expand in response to a secondary challenge with α-GalCer in vivo, but still produced moderate amounts of cytokines, remains unclear, but this might be determined by the nature of the antigen presenting cell, with DCs providing optimal stimulation (18, 47), although this may vary in a tissue-dependent manner, since Kupffer cells appear to be more important for liver NKT cell activation (47). An additional factor may be the affinity of the TCR-antigen interaction on primary challenge, since the lower affinity α-GalCer analogue, known as OCH, appears to permit a more vigorous secondary response to α-GalCer in vivo (20). However, we were unable to verify part of this study that suggested the mechanism for hypo-responsiveness by NKT cells involved an interaction between up-regulated NKG2A on NKT cells, and Qa1b on antigen presenting cells. In contrast, we found NKG2A and it's co-receptor CD94 were down regulated to undetectable levels on splenic NKT cells for at least 18 days after challenge with free α-GalCer in vivo. We favour the hypothesis that NKT cell hypo-responsiveness to α-GalCer re-challenge is intrinsic to the NKT cells themselves, since plate bound anti-CD3 stimulation was unable to enhance cytokine production from pre-challenged NKT cellsex vivo, and DCs isolated from naïve WT or TAP-1-/- mice (which lack the Qdm peptide that associates with Qa1b and is required for NKG2A binding (35, 36)) were unable to overcome this anergy. While we do not see up-regulation of inhibitory receptors on NKT cells after a single stimulation with α-GalCer in vivo, our results are not at odds with an earlier study from our group (19). That study involved chronic α-GalCer-stimulation over 8 weeks in vivo that gradually depleted peripheral NKT cells, followed by thymus-dependent NKT cell repopulation over the next 8 weeks. In that study, the repopulating NKT cells in the periphery and thymus expressed higher levels of inhibitory receptors, apparently as a result of altered thymic education. This is obviously very different from the approach used in the current study, where NKT cells were not depleted and repopulated by the thymus over a long period, and thymic NKT cells did not alter their inhibitory receptor profile. Taken together, it appears that multiple mechanisms may exist leading to NKT cell unresponsiveness, the physiological significance for which may be to protect against NKT cell-mediated damage resulting from sustained NKT cell activation, which would otherwise generate high levels of systemic cytokine production and activation of a broad range of bystander cells.
In summary, we have demonstrated that injection of free α-GalCer, the most commonly used approach for experimental NKT cell activation/therapy in vivo, leads to a potent primary NKT cell response that depends on both CD40 and CD28 pathways for maximal NKT cell expansion but not NKT cell cytokine production. The subsequent contraction phase involved Bim-dependent apoptotic cell death and surviving NKT cells were shown to be hypo-responsive to further activation by free α-GalCer injection, exhibiting reduced cytokine production and expansion potential in vivo. It remains to be determined whether the primary and secondary response to recently identified self and other foreign CD1d restricted antigens, including iGb3 (48,49) and bacteria-derived glfycolipids (50-52) are regulated in a similar manner.
Acknowledgements
The authors wish to thank: Kirin Breweries for kindly supplying α-Galactosylceramide; David Taylor and animal house staff at University of Melbourne, Dept of Microbiology and Immunology for their care and maintenance of the mice used in these studies; Mr Ken Field for assistance with flow cytometry and Drs Stuart Berzins and Yoshihiro Hayakawa for helpful discussions.
Footnotes
This research has been supported by grants and fellowships from the National Health and Medical Research Council (NHMRC) of Australia, The National Institutes of Health, the Juvenile Diabetes Research Foundation, the Leukemia and Lymphoma Society of America (LLS) and the Association for International Cancer Research. APU was supported by an Australian Postgraduate Research Award. NYC was supported by a Cancer Council Victoria Postdoctoral Fellowship. YZ was supported by a career development award from the NHMRC. DIG, AML and MJS were supported by Research Fellowships from NHMRC.
Abbreviations: α-GalCer, alpha galactosylceramide; AICD, antigen induced cell death; CFSE, carboxyfluorescein di-acetate succinimidyl ester.
References:
- 1.Godfrey DI, MacDonald HR, Kronenberg M, Smyth MJ, Van Kaer L. NKT cells: what's in a name? Nat Rev Immunol. 2004;4:231. doi: 10.1038/nri1309. [DOI] [PubMed] [Google Scholar]
- 2.Godfrey DI, Kronenberg M. Going both ways: immune regulation via CD1d-dependent NKT cells. J Clin Invest. 2004;114:1379. doi: 10.1172/JCI23594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brigl M, Brenner MB. CD1: Antigen Presentation and T Cell Function. Annu Rev Immunol. 2004;22:817. doi: 10.1146/annurev.immunol.22.012703.104608. [DOI] [PubMed] [Google Scholar]
- 4.Kronenberg M. Toward an understanding of NKT cell biology: Progress and Paradoxes. Annu Rev Immunol. 2005;23:877. doi: 10.1146/annurev.immunol.23.021704.115742. [DOI] [PubMed] [Google Scholar]
- 5.Hayakawa Y, Godfrey DI, Smyth MJ. Alpha-galactosylceramide: potential immunomodulatory activity and future application. Curr Med Chem. 2004;11:241. doi: 10.2174/0929867043456115. [DOI] [PubMed] [Google Scholar]
- 6.Giaccone G, Punt CJA, Ando Y, Ruijter R, Nishi N, Peters M, von Blomberg BME, Scheper RJ, van der Vliet HJJ, van den Eertwegh AJM, Roelvink M, Beijnen J, Zwierzina H, Pinedo HM. A phase I study of the natural killer T-cell ligand alpha-Galactosylceramide (KRN7000) in patients with solid tumors. Clinical Cancer Research. 2002;8:3702. [PubMed] [Google Scholar]
- 7.Nieda M, Okai M, Tazbirkova A, Lin H, Yamaura A, Ide K, Abraham R, Juji T, Macfarlane DJ, Nicol AJ. Therapeutic activation of V{alpha}24+V{beta}11+ NKT cells in human subjects results in highly coordinated secondary activation of acquired and innate immunity. Blood. 2004;103:383. doi: 10.1182/blood-2003-04-1155. [DOI] [PubMed] [Google Scholar]
- 8.Crowe NY, Uldrich AP, Kyparissoudis K, Hammond KJ, Hayakawa Y, Sidobre S, Keating R, Kronenberg M, Smyth MJ, Godfrey DI. Glycolipid Antigen Drives Rapid Expansion and Sustained Cytokine Production by NK T Cells. J Immunol. 2003;171:4020. doi: 10.4049/jimmunol.171.8.4020. [DOI] [PubMed] [Google Scholar]
- 9.Hildeman DA, Zhu Y, Mitchell TC, Bouillet P, Strasser A, Kappler J, Marrack P. Activated T cell death in vivo mediated by proapoptotic bcl-2 family member bim. Immunity. 2002;16:759. doi: 10.1016/s1074-7613(02)00322-9. [DOI] [PubMed] [Google Scholar]
- 10.Pellegrini M, Belz G, Bouillet P, Strasser A. Shutdown of an acute T cell immune response to viral infection is mediated by the proapoptotic Bcl-2 homology 3-only protein Bim. Proc Natl Acad Sci U S A. 2003;100:14175. doi: 10.1073/pnas.2336198100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilson MT, Johansson C, Olivares-Villagomez D, Singh AK, Stanic AK, Wang CR, Joyce S, Wick MJ, Van Kaer L. The response of natural killer T cells to glycolipid antigens is characterized by surface receptor down-modulation and expansion. Proc Natl Acad Sci U S A. 2003;100:10913. doi: 10.1073/pnas.1833166100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harada M, Seino K, Wakao H, Sakata S, Ishizuka Y, Ito T, Kojo S, Nakayama T, Taniguchi M. Down-regulation of the invariant Valpha14 antigen receptor in NKT cells upon activation. Int Immunol. 2004;16:241. doi: 10.1093/intimm/dxh023. [DOI] [PubMed] [Google Scholar]
- 13.Hayakawa Y, Takeda K, Yagita H, Van Kaer L, Saiki I, Okumura K. Differential regulation of Th1 and Th2 functions of NKT cells by CD28 and CD40 costimulatory pathways. Journal of Immunology. 2001;166:6012. doi: 10.4049/jimmunol.166.10.6012. [DOI] [PubMed] [Google Scholar]
- 14.Kitamura H, Iwakabe K, Yahata T, Nishimura S, Ohta A, Ohmi Y, Sato M, Takeda K, Okumura K, van Kaer L, Kawano T, Taniguchi M, Nishimura T. The natural killer T (NKT) cell ligand alpha-galactosylceramide demonstrates its immunopotentiating effect by inducing interleukin (IL)-12 production by dendritic cells and IL-12 receptor expression on NKT cells. Journal of Experimental Medicine. 1999;189:1121. doi: 10.1084/jem.189.7.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nishimura T, Kitamura H, Iwakabe K, Yahata T, Ohta A, Sato M, Takeda K, Okumura K, Van Kaer L, Kawano T, Taniguchi M, Nakui M, Sekimoto M, Koda T. The interface between innate and acquired immunity: glycolipid antigen presentation by CD1d-expressing dendritic cells to NKT cells induces the differentiation of antigen-specific cytotoxic T lymphocytes. International Immunology. 2000;12:987. doi: 10.1093/intimm/12.7.987. [DOI] [PubMed] [Google Scholar]
- 16.Ikarashi Y, Mikami R, Bendelac A, Terme M, Chaput N, Terada M, Tursz T, Angevin E, Lemonnier FA, Wakasugi H, Zitovogel L. Dendritic cell maturation overrules H-2D-mediated natural killer T (NKT) cell inhibition: Critical role for B7 in CD1d-dependent NKT cell interferon gamma production. Journal of Experimental Medicine. 2001;194:1179. doi: 10.1084/jem.194.8.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsuda JL, Gapin L, Baron JL, Sidobre S, Stetson DB, Mohrs M, Locksley RM, Kronenberg M. Mouse V alpha 14i natural killer T cells are resistant to cytokine polarization in vivo. Proc Natl Acad Sci U S A. 2003;100:8395. doi: 10.1073/pnas.1332805100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fujii S, Shimizu K, Kronenberg M, Steinman RM. Prolonged IFN-gamma-producing NKT response induced with alpha-galactosylceramide-loaded DCs. Nature Immunology. 2002;3:867. doi: 10.1038/ni827. [DOI] [PubMed] [Google Scholar]
- 19.Hayakawa Y, Berzins SP, Crowe NY, Godfrey DI, Smyth MJ. Antigen-induced tolerance by intrathymic modulation of self-recognizing inhibitory receptors. Nat Immunol. 2004;5:590. doi: 10.1038/ni1069. [DOI] [PubMed] [Google Scholar]
- 20.Ota T, Takeda K, Akiba H, Hayakawa Y, Ogasawara K, Ikarashi Y, Miyake S, Wakasugi H, Yamamura T, Kronenberg M, Raulet DH, Kinoshita K, Yagita H, Smyth MJ, Okumura K. Blood. 2005. IFN-{gamma}-mediated negative feedback regulation of NKT cell function by CD94/NKG2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carnaud C, Lee D, Donnars O, Park SH, Beavis A, Koezuka Y, Bendelac A. Cutting edge: Cross-talk between cells of the innate immune system: NKT cells rapidly activate NK cells. Journal of Immunology. 1999;163:4647. [PubMed] [Google Scholar]
- 22.Eberl G, Brawand P, MacDonald HR. Selective bystander proliferation of memory CD4(+) and CD8(+) T cells upon NK T or T cell activation. Journal of Immunology. 2000;165:4305. doi: 10.4049/jimmunol.165.8.4305. [DOI] [PubMed] [Google Scholar]
- 23.Eberl G, MacDonald HR. Selective induction of NK cell proliferation and cytotoxicity by activated NKT cells. European Journal of Immunology. 2000;30:985. doi: 10.1002/(SICI)1521-4141(200004)30:4<985::AID-IMMU985>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 24.Fujii S, Shimizu K, Smith C, Bonifaz L, Steinman RM. Activation of natural killer T cells by alpha-galactosylceramide rapidly induces the full maturation of dendritic cells in vivo and thereby acts as an adjuvant for combined CD4 and CD8 T cell immunity to a coadministered protein. J Exp Med. 2003;198:267. doi: 10.1084/jem.20030324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fujii S, Liu K, Smith C, Bonito AJ, Steinman RM. The Linkage of Innate to Adaptive Immunity via Maturing Dendritic Cells In Vivo Requires CD40 Ligation in Addition to Antigen Presentation and CD80/86 Costimulation. J Exp Med. 2004;199:1607. doi: 10.1084/jem.20040317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hayakawa Y, Takeda K, Yagita H, Kakuta S, Iwakura Y, Van Kaer L, Saiki I, Okumura K. Critical contribution of IFN-gamma and NK cells, but not perforin-mediated cytotoxicity, to anti-metastatic effect of alpha-galactosylceramide. European Journal of Immunology. 2001;31:1720. [PubMed] [Google Scholar]
- 27.Smyth MJ, Crowe NY, Pellicci DG, Kyparissoudis K, Kelly JM, Takeda K, Yagita H, Godfrey DI. Sequential production of interferon-gamma by NK1.1(+) T cells and natural killer cells is essential for the antimetastatic effect of alpha-galactosylceramide. Blood. 2002;99:1259. doi: 10.1182/blood.v99.4.1259. [DOI] [PubMed] [Google Scholar]
- 28.Xu J, Foy TM, Laman JD, Elliott EA, Dunn JJ, Waldschmidt TJ, Elsemore J, Noelle RJ, Flavell RA. Mice deficient for the CD40 ligand. Immunity. 1994;1:423. doi: 10.1016/1074-7613(94)90073-6. [DOI] [PubMed] [Google Scholar]
- 29.Sutherland RM, Brady JL, Georgiou HM, Thomas HE, Lew AM. Protective effect of CTLA4Ig secreted by transgenic fetal pancreas allografts. Transplantation. 2000;69:1806. doi: 10.1097/00007890-200005150-00013. [DOI] [PubMed] [Google Scholar]
- 30.Bouillet P, Metcalf D, Huang DC, Tarlinton DM, Kay TW, Kontgen F, Adams JM, Strasser A. Proapoptotic Bcl-2 relative Bim required for certain apoptotic responses, leukocyte homeostasis, and to preclude autoimmunity. Science. 1999;286:1735. doi: 10.1126/science.286.5445.1735. [DOI] [PubMed] [Google Scholar]
- 31.Hammond KJL, Pelikan SB, Crowe NY, Randle-Barrett E, Nakayama T, Taniguchi M, Smyth MJ, van Driel IR, Scollay R, Baxter AG, Godfrey DI. NKT cells are phenotypically and functionally diverse. European Journal of Immunology. 1999;29:3768. doi: 10.1002/(SICI)1521-4141(199911)29:11<3768::AID-IMMU3768>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 32.Matsuda JL, Naidenko OV, Gapin L, Nakayama T, Taniguchi M, Wang CR, Koezuka Y, Kronenberg M. Tracking the response of natural killer T cells to a glycolipid antigen using CD1d tetramers. Journal of Experimental Medicine. 2000;192:741. doi: 10.1084/jem.192.5.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhan Y, Corbett AJ, Brady JL, Sutherland RM, Lew AM. CD4 help-independent induction of cytotoxic CD8 cells to allogeneic P815 tumor cells is absolutely dependent on costimulation. J Immunol. 2000;165:3612. doi: 10.4049/jimmunol.165.7.3612. [DOI] [PubMed] [Google Scholar]
- 34.Van Kaer L, Ashton-Rickardt PG, Ploegh HL, Tonegawa S. TAP1 mutant mice are deficient in antigen presentation, surface class I molecules, and CD4-8+ T cells. Cell. 1992;71:1205. doi: 10.1016/s0092-8674(05)80068-6. [DOI] [PubMed] [Google Scholar]
- 35.Vance RE, Kraft JR, Altman JD, Jensen PE, Raulet DH. Mouse CD94/NKG2A is a natural killer cell receptor for the nonclassical major histocompatibility complex (MHC) class I molecule Qa-1(b) J Exp Med. 1998;188:1841. doi: 10.1084/jem.188.10.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kraft JR, Vance RE, Pohl J, Martin AM, Raulet DH, Jensen PE. Analysis of Qa-1(b) peptide binding specificity and the capacity of CD94/NKG2A to discriminate between Qa-1-peptide complexes. J Exp Med. 2000;192:613. doi: 10.1084/jem.192.5.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leite-De-Moraes MC, Hameg A, Arnould A, Machavoine F, Koezuka Y, Schneider E, Herbelin A, Dy M. A distinct IL-18-induced pathway to fully activate NK T lymphocytes independently from TCR engagement. Journal of Immunology. 1999;163:5871. [PubMed] [Google Scholar]
- 38.Leite-De-Moraes MC, Herbelin A, Gouarin C, Koezuka Y, Schneider E, Dy M. Fas/Fas ligand interactions promote activation-induced cell death of NK T lymphocytes. Journal of Immunology. 2000;165:4367. doi: 10.4049/jimmunol.165.8.4367. [DOI] [PubMed] [Google Scholar]
- 39.Yang YF, Tomura M, Ono S, Hamaoka T, Fujiwara H. Requirement for IFN-gamma in IL-12 production induced by collaboration between V(alpha)14(+)NKT cells and antigen-presenting cells. International Immunology. 2000;12:1669. doi: 10.1093/intimm/12.12.1669. [DOI] [PubMed] [Google Scholar]
- 40.Brigl M, Bry L, Kent SC, Gumperz JE, Brenner MB. Nat Immunol. 2003. Mechanism of CD1d-restricted natural killer T cell activation during microbial infection. [DOI] [PubMed] [Google Scholar]
- 41.Yang Y, Ueno A, Bao M, Wang Z, Im JS, Porcelli S, Yoon JW. Control of NKT cell differentiation by tissue-specific microenvironments. J Immunol. 2003;171:5913. doi: 10.4049/jimmunol.171.11.5913. [DOI] [PubMed] [Google Scholar]
- 42.Burdin N, Brossay L, Kronenberg M. Immunization with alpha-galactosylceramide polarizes CD1-reactive NK T cells towards Th2 cytokine synthesis. Eur J Immunol. 1999;29:2014. doi: 10.1002/(SICI)1521-4141(199906)29:06<2014::AID-IMMU2014>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 43.Hong S, Wilson MT, Serizawa I, Wu L, Singh N, Naidenko OV, Miura T, Haba T, Scherer DC, Wei J, Kronenberg M, Koezuka Y, Van Kaer L. The natural killer T-cell ligand alpha-galactosylceramide prevents autoimmune diabetes in non-obese diabetic mice. Nat Med. 2001;7:1052. doi: 10.1038/nm0901-1052. [DOI] [PubMed] [Google Scholar]
- 44.Wang B, Geng YB, Wang CR. CD1-restricted NK T cells protect nonobese diabetic mice from developing diabetes. J Exp Med. 2001;194:313. doi: 10.1084/jem.194.3.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Singh AK, Wilson MT, Hong S, Olivares-Villagomez D, Du C, Stanic AK, Joyce S, Sriram S, Koezuka Y, Van Kaer L. Natural killer T cell activation protects mice against experimental autoimmune encephalomyelitis. J Exp Med. 2001;194:1801. doi: 10.1084/jem.194.12.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miyamoto K, Miyake S, Yamamura T. A synthetic glycolipid prevents autoimmune encephalomyelitis by inducing TH2 bias of natural killer T cells. Nature. 2001;413:531. doi: 10.1038/35097097. [DOI] [PubMed] [Google Scholar]
- 47.Schmieg J, Yang G, Franck RW, Van Rooijen N, Tsuji M. Glycolipid presentation to natural killer T cells differs in an organ-dependent fashion. Proc Natl Acad Sci U S A. 2005;102:1127. doi: 10.1073/pnas.0408288102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhou D, Mattner J, Cantu C, 3rd, Schrantz N, Yin N, Gao Y, Sagiv Y, Hudspeth K, Wu YP, Yamashita T, Teneberg S, Wang D, Proia RL, Levery SB, Savage PB, Teyton L, Bendelac A. Lysosomal glycosphingolipid recognition by NKT cells. Science. 2004;306:1786. doi: 10.1126/science.1103440. [DOI] [PubMed] [Google Scholar]
- 49.Godfrey DI, Pellicci DG, Smyth MJ. The elusive NKT cell antigen--is the search over? Science. 2004;306:1687. doi: 10.1126/science.1106932. [DOI] [PubMed] [Google Scholar]
- 50.Wu D, Xing GW, Poles MA, Horowitz A, Kinjo Y, Sullivan B, Bodmer-Narkevitch V, Plettenburg O, Kronenberg M, Tsuji M, Ho DD, Wong CH. Proc Natl Acad Sci U S A. 2005. Bacterial glycolipids and analogs as antigens for CD1d-restricted NKT cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kinjo Y, Wu D, Kim G, Xing GW, Poles MA, Ho DD, Tsuji M, Kawahara K, Wong CH, Kronenberg M. Recognition of bacterial glycosphingolipids by natural killer T cells. Nature. 2005;434:520. doi: 10.1038/nature03407. [DOI] [PubMed] [Google Scholar]
- 52.Mattner J, Debord KL, Ismail N, Goff RD, Cantu C, 3rd, Zhou D, Saint-Mezard P, Wang V, Gao Y, Yin N, Hoebe K, Schneewind O, Walker D, Beutler B, Teyton L, Savage PB, Bendelac A. Exogenous and endogenous glycolipid antigens activate NKT cells during microbial infections. Nature. 2005;434:525. doi: 10.1038/nature03408. [DOI] [PubMed] [Google Scholar]