Abstract
In the accompanying study, we show how retroviral tropism can be redirected by insertion of short peptide ligands at multiple locations in envelope. Here we use this approach to selectively target and destroy human cancer cells. Many cancer cells overexpress specific cell surface receptors. We have generated Moloney murine leukemia virus (MLV) envelope derivatives bearing short peptide ligands for gastrin-releasing protein (GRP) and human epidermal growth factor receptors. Pseudotyped viruses containing these chimeric envelope derivatives selectively transduce human cancer cell lines that overexpress the cognate receptor. A retrovirus targeting the GRP receptor can deliver the thymidine kinase gene to human melanoma and breast cancer cells, which are killed by the subsequent addition of ganciclovir. Collectively, our results demonstrate that short peptide ligands inserted at appropriate locations in MLV envelope can selectively target retroviruses to human cancer cells and deliver a therapeutically relevant gene.
Retroviral vectors have been used extensively in gene therapy experiments and clinical trials. In the accompanying article (12), we have described a method to alter the tropism of Moloney murine leukemia virus (MLV) ecotropic envelope to target specific receptors on human cells. Gastrin-releasing protein (GRP) is an autocrine and paracrine growth factor (19) belonging to the bombesin family of peptides. The 14-amino-acid bombesin peptide is homologous to the C terminus of GRP and binds with high affinity to the GRP receptor, a member of the G-protein-coupled receptor family (28). The GRP receptor is overexpressed in a variety of carcinomas (11, 18, 20, 25, 27, 31) and is therefore an attractive target for gene therapy.
The epidermal growth factor receptor (EGFR) family consists of at least four members: EGFR (HER1, erbB-1), HER2 (erbB-2), HER3 (erbB-3), and HER4 (erbB-4). Specific members of the epidermal growth factor receptor family are also overexpressed in certain human adenocarcinomas, including breast and ovarian cancers. Overexpression of at least two family members, EGFR and HER2, is associated with poor prognosis and differential response to therapy (15, 16). Heregulin protein (HRG) is a growth factor that has several homologous isoforms that bind directly to HER3 (erbB-3) and HER4 (erbB-4) homodimers and heterodimers containing the HER2 receptor (10, 23, 29). In previous studies, pseudotyped virus has been directed to HER2, HER3, and HER4, by single-chain variable fragments or the HRG protein appended to the N terminus of envelope (8, 13, 26).
Using the method outlined in the accompanying study (12), we examine more than 20 MLV chimeric envelope derivatives that contain the GRP or a modified HRG peptide ligand inserted at various locations within envelope. We demonstrate that this approach can be used to selectively target cancer cells overexpressing GRP or HRG receptors and deliver a therapeutically relevant gene.
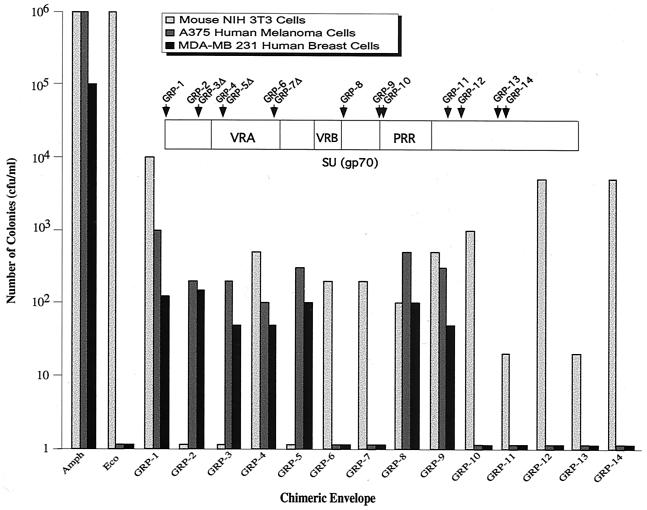
The 21-amino-acid GRP sequence, containing 14 residues of the bombesin protein, was inserted at various locations within the MLV ecotropic envelope to generate 14 GRP chimeric envelope derivatives (Table 1). Pseudotyped virus producer cells were generated for each chimeric envelope derivative, and the resultant GRP viruses initially tested for transduction of host NIH 3T3 cells. Figure 1 shows that all of the GRP viruses transduced NIH 3T3 cells, except when the ligand was inserted within the N-terminal region (GRP-2 and GRP-3) or in one case within the VRA (GRP-5). In general, the GRP viruses transduced NIH 3T3 cells with efficiencies comparable to that observed in the accompanying study (12).
TABLE 1.
Description of GRP and HRG virusesa
| Envelope no. (sequence) | Location (aa)b | Nucleotides deleted from envelope |
|---|---|---|
| GRP (CAAA-EQRLGNQWAVGHLM-TRC) | ||
| GRP-1 | 1 | |
| GRP-2 | 38 | |
| GRP-3 | 38 | 5990-6082 |
| GRP-4 | 68 | |
| GRP-5 | 68 | 6082-6191 |
| GRP-6 | 120 | |
| GRP-7 | 120 | 6238-6281 |
| GRP-8 | 185 | |
| GRP-9 | 230 | |
| GRP-10 | 235 | |
| GRP-11 | 310 | |
| GRP-12 | 321 | |
| GRP-13 | 382 | |
| GRP-14 | 388 | |
| HRG (CAAA-SHLVKCAEKEKTFCVNGGECYRVKTYGYLMCKCPNEFTGDRCQNYVIAS-TRC)c | ||
| HRG-1 | 1 | |
| HRG-2 | 38 | |
| HRG-3 | 38 | 5990-6082 |
| HRG-4 | 68 | |
| HRG-5 | 68 | 6082-6191 |
| HRG-6 | 120 | |
| HRG-7 | 185 | |
| HRG-8 | 230 | |
| HRG-9 | 235 |
The sequences of the GRP and HRG ligands are shown. For the chimeric envelope derivatives, GRP 1 to 14 and HRG 1 to 9, the positions of the ligand insertion and any additional modifications are indicated. GRP chimeric envelope derivatives (GRP 1 to 14) were generated by inserting the 21-amino-acid GRP ligand into the MluI and NotI sites of previously constructed chimeric envelopes (12). The sequence encoding CAAAEQRLGNQWAVGHLMTRC was generated by annealing two oligonucleotides: GRPA (GGCCGAGCAGCGCCTGGGCAACCAGTGGGCCGTCGGCCACCTGATGA) and GRPB (CGCGTCATCAGGTGGCCGACGGCCCACTGGTTGCCCAGGCGCTGCTC). HRG chimeric envelope derivatives (HRG 1 to 9) were generated by inserting a modified 49-amino-acid binding region of the heregulin-β protein (2) into the MluI and NotI sites of previously constructed chimeric envelopes (12). The 49-amino-acid HRG sequence was derived by annealing four oligonucleotides: HRGA (GGCCGCTTCACACCTTGTAAAGTGCGCAGAGAAGGAAAAGACGTTCTGCGTCAACGGCGTGAGTGTTACAG), HRGB(GCCGTAGGTCTTAACCCTGTAACACTCACCGCCGTTGACGCAGAACGTCTTTTCCTTCTCTGCGCACTTTACAAGGTGTGAAGC), HRGC(GGTTAAGACCTACGGCTATCTGATGTGCAAGTGTCCGAACGAGTTCACGGGTGACCGGTGCCAGAACTACGTCATCGCGTCGA), and HRGD (CGCGTCGACGCGATGACGTAGTTCTGGCACCGGTCACCCGTGAACTCGTTCGGACACTTGCACATCAGATA).
aa, amino acid.
Modified HRG sequence (2).
FIG. 1.
Transduction of human cells by GRP virus. NIH 3T3 cells, human A375 melanoma cells, and human MDA-MB-231 breast carcinoma cells were infected with a GRP virus, selected with G418 for 2 weeks, fixed, and stained with Giemsa, and the colonies were counted. Amphotropic (Amph) and ecotropic virus (Eco) was generated by expressing the wild-type amphotropic and ecotropic envelopes, pCAA and pCEE, respectively. Note the log scale. The amphotropic envelope, pCAA, and the LAPNL packaging vectors were generated as described elsewhere (12); the latter expresses the secreted alkaline phosphatase (SEAP) gene and the neomycin resistance gene.
A375 human melanoma and 231 breast carcinoma cells overexpress the GRP receptor (17, 21, 33). GRP viruses with insertions at the N terminus (GRP-1), within the N-terminal region (GRP-2 and GRP-3), within the VRA (GRP-4 and GRP-5), downstream of the VRB (GRP-8), and upstream of the PRR (GRP-9) transduced both human cell lines. In contrast, GRP viruses with insertions within the PRR (GRP-10) or C-terminal region (GRP-11 to GRP-14) failed to transduce human cells.
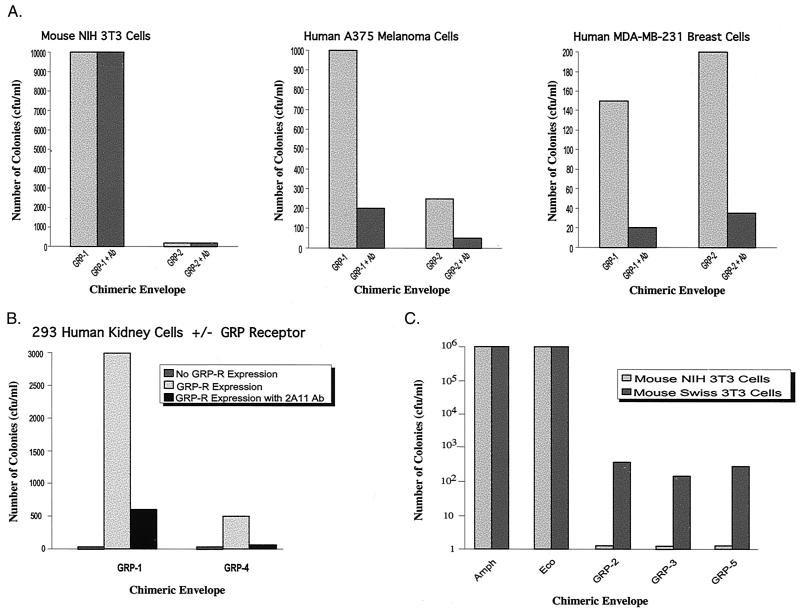
We next performed experiments to confirm that expression of the GRP receptor is required for GRP viruses to transduce human cells. First, we tested whether treatment of GRP viruses with an antibody to the GRP protein would block transduction of human cells. Figure 2A shows that 2A11, an antibody to the C-terminal region of GRP protein, substantially reduced transduction of both human cancer cell lines but not mouse NIH 3T3 cells. Thus, GRP is required for transduction of human cells but not mouse cells.
FIG. 2.
Requirement of the GRP receptor for transduction of human cells by GRP viruses. (A) Antibodies to GRP block transduction of human cells by GRP viruses. GRP-1 or GRP-2 viruses were pretreated with 2A11 antibody (provided by Frank Cuttitta). NIH 3T3, A375 human melanoma cells, or MDA-MB-231 breast carcinoma cells were then infected with 2A11 antibody-treated GRP or untreated virus, and transduction was analyzed as in Fig. 1. The 2A11 antibody was added to pseudotyped virus at a 1:100 dilution, followed by incubation at 4°C for 4 h and then viral infection. (B) Requirement of the GRP receptor for transduction of human 293 cells. 293-GRPR-Zeo cells were infected with the GRP-1 or GRP-4 virus, with or without preincubation with the 2A11 antibody, and transduction was analyzed as in Fig. 1. The GRPR-Zeo construct was generated by insertion of the GRP receptor gene (GRP-R) (provided by James F. Battey, National Institutes of Health) into pcDNA3.1/Zeo+ (Invitrogen). 293-GRPR-Zeo cells were generated by transfection of 293 kidney cells with GRPR-Zeo, selection with zeocin, and verification of GRP receptor expression by reverse transcription-PCR. (C) Requirement of the GRP receptor for transduction of mouse cells by GRP-2, GRP-3, and GRP-5 viruses. NIH 3T3 and Swiss 3T3 cells were infected with a GRP virus, and transduction was analyzed as in Fig. 1.
Second, we sought to determine whether expression of the GRP receptor was required for transduction of human cells by GRP viruses. Human 293 cells do not express the GRP receptor (30). We derived a 293 cell line that constitutively expresses the GRP receptor (293-GRPR cells). Figure 2B shows that 293-GRPR cells, but not the parental 293 cells, were transduced by GRP viruses and that pretreatment with the 2A11 antibody blocked transduction. In a related experiment, Fig. 2C shows that several of the GRP viruses transduced mouse Swiss 3T3 cells, which express the GRP receptor, but not NIH 3T3 cells, which lack the GRP receptor. Collectively, the results of Fig. 2 indicate that transduction of human cells by GRP viruses requires a virus bearing a chimeric GRP envelope derivative and a cell expressing a GRP receptor.
We also constructed a series of chimeric envelope derivatives containing the 56 amino acid heregulin-β peptide sequence (Table 1). A region encompassing residues 177 to 226 of HRG binds to and activates the HER3 and HER4 receptors and was selected as the target ligand (3). This ligand was modified through 11 substitutions known to increase its affinity for the homodimeric HER3 (2) (Table 1). The HRG ligand was inserted in locations that had enabled transduction of human cells by GRP (Fig. 1) and RGD viruses (12).
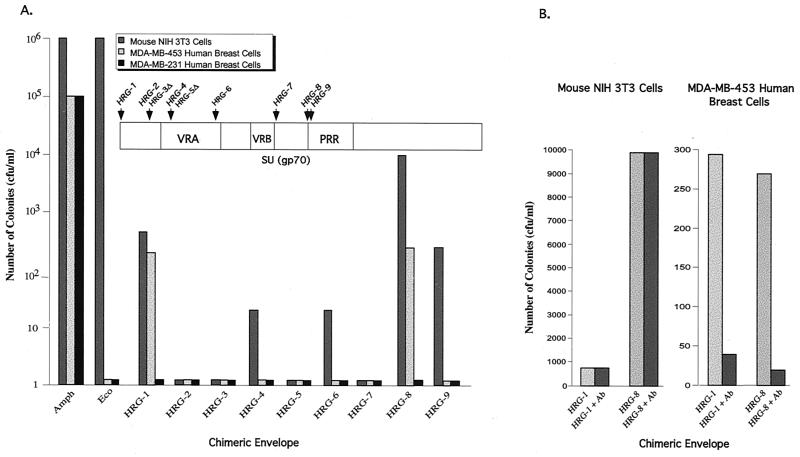
HRG viruses were first tested for their ability to transduce NIH 3T3 cells. Figure 3A shows that transduction efficiencies of the HRG-8 and HRG-9 viruses were comparable to the equivalent GRP viruses (GRP-9 and GRP-10; Fig. 1). In contrast, the transduction efficiencies of the HRG-1, HRG-4, HRG-6, and HRG-7 viruses were significantly lower than the equivalent GRP viruses (GRP-1, GRP-4, GRP-6, and GRP-8).
FIG. 3.
Transduction of NIH 3T3 cells and MDA-MB-453 breast carcinoma cells by HRG viruses. (A) NIH 3T3 cells, MDA-MB-453, and MDA-MB-231 breast carcinoma cells were infected with an HRG virus, and transduction was analyzed as described in the legend to Fig. 1. (B) Antibodies to HER3 and HER4 receptors block transduction of human cells by HRG viruses. NIH 3T3 and MDA-MB-453 breast carcinoma cells were pretreated with antibodies to HER3 and HER4 receptors (Lab Vision Corporation) and infected with the HRG-1 or HRG-8 virus, and transduction was analyzed as in Fig. 1.
MDA-MB-453 breast carcinoma cells overexpress EGFR family members, whereas MDA-MB-231 breast carcinoma cells do not (5, 6, 14). Figure 3A shows that the HRG-1 and HRG-8 viruses transduced MDA-MB-453 cells but not MDA-MB-231 cells. The HRG-1 and HRG-8 viruses also transduced two other human breast cancer cell lines that overexpress EGFR family members: MCF-7 and AU-565 cells (data not shown). In contrast, HRG-2, HRG-3, HRG-4, HRG-5, and HRG-7 failed to transduce MDA-MB-453 cells, which was unexpected based upon the results with the equivalent GRP viruses. Pretreatment of MDA-MB-453 cells with HER3 and HER4 antibodies substantially decreased transduction by HRG-1 and HRG-8 viruses, indicating that viral entry was mediated by the HRG-receptor interaction (Fig. 3B).
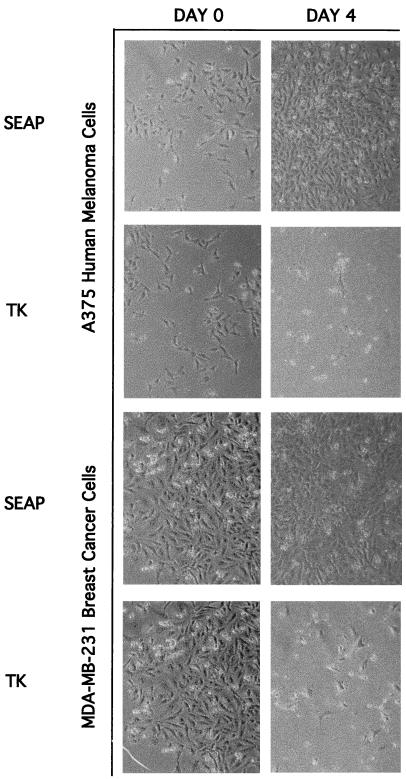
Finally, we tested whether retroviruses bearing an appropriate chimeric envelope derivative could deliver a therapeutically relevant gene to cancer cells. Mammalian cells expressing the herpes simplex virus thymidine kinase (TK) gene are killed by treatment with ganciclovir (7). The GRP-1 virus carrying the herpes simplex virus TK gene was used to transduce A375 human melanoma and MDA-MB-231 breast carcinoma cells. After ganciclovir treatment of transduced melanoma and breast carcinoma cells significant cell death was evident, whereas there was no cytopathic effect in ganciclovir-treated cells transduced by a control GRP-1 virus not expressing the TK gene (Fig. 4).
FIG. 4.
Inducible destruction of human cancer cells by infection with a GRP virus and ganciclovir treatment. A375 human melanoma cells and MDA-MD-231 human breast carcinoma cells were infected with GRP-1 virus expressing either the SEAP or TK gene. Cells were selected with G418 for 2 weeks, followed by isolation of colonies and culture in media containing ganciclovir. The cell densities in the photographs are representative of the entire plate. The packaging vector, LTKNL, was generated by removal of the SEAP gene from LAPNL and insertion of the TK gene (provided by Steve Jones, University of Massachusetts Medical School). GRP virus with the LTKNL packaging construct was generated and used to transduce human cells. Colonies were isolated, grown in 10 μg of ganciclovir (Moravek Biochemicals, Inc.)/ml, and examined by using a Zeiss Axiophot microscope.
This study demonstrates that small ligands inserted into the ecotropic MLV envelope can alter the tropism of pseudotyped virus to target specific receptors on human cancer cells. These results confirm other studies from our laboratory (12) and highlight the importance of the effect of ligand length and position within the envelope on transduction efficiency and tropism. For both GRP and HRG viruses, some insertions within the N-terminal region or VRA interfered with the transduction of mouse cells. Several of these GRP viruses transduced cells expressing the GRP receptor, indicating that tropism had been switched. Thus, for the production of selective targeting retroviral vectors, the N-terminal region and VRA may be the optimal locations for ligand insertion.
Transduction by viruses containing the larger HRG ligand was, in general, decreased relative to their GRP counterparts, and several HRG viruses were unable to transduce mouse or human cells. The HRG ligand is twice the length of the GRP ligand and may have a more disruptive effect on envelope structure and function. It has been reported that certain modified envelope proteins cannot undergo the postbinding conformational change required for transduction (34), and some of the chimeric HRG envelope derivatives may be examples of this phenomenon. The decreased transduction efficiency of HRG viruses may also be related to the HER3 and HER4 receptors that they target. For example, receptors have different rates of internalization into endosomes, and the virus must be in close proximity to the endosomal membrane for fusion to occur. The HER2 receptor is not rapidly endocytosed (4, 24), which may explain the lower transduction efficiency of HRG viruses.
Transduction efficiency also depends on the presentation of the ligand within the envelope. In this and in the accompanying study, cysteine residues flanked the insert and are expected to form a disulfide bond that facilitates ligand presentation. Screening for receptor binding peptides within a constrained disulfide loop structure by using phage display or recombinant peptide libraries (1, 9, 22, 32) in conjunction with the method described here may provide a general approach for targeting retroviral vectors to specific cells.
Acknowledgments
We thank S. Jones and J. F. Battey for plasmids, F. Cuttitta for the 2A11 antibody, and W. S. Pear for Anjou 65 cells.
This work was supported in part by an NIH grant to M.R.G. M.R.G. is an investigator of the Howard Hughes Medical Institute.
REFERENCES
- 1.Arap, W., R. Pasqualini, and E. Ruoslahti. 1998. Cancer treatment by targeted drug delivery to tumor vasculature in a mouse model. Science 279:377-380. [DOI] [PubMed] [Google Scholar]
- 2.Ballinger, M. D., J. T. Jones, J. A. Lofgren, W. J. Fairbrother, R. W. Akita, M. X. Sliwkowski, and J. A. Wells. 1998. Selection of heregulin variants having higher affinity for the ErbB3 receptor by monovalent phage display. J. Biol. Chem. 273:11675-11684. [DOI] [PubMed] [Google Scholar]
- 3.Barbacci, E. G., B. C. Guarino, J. G. Stroh, D. H. Singleton, K. J. Rosnack, J. D. Moyer, and G. C. Andrews. 1995. The structural basis for the specificity of epidermal growth factor and heregulin binding. J. Biol. Chem. 270:9585-9589. [DOI] [PubMed] [Google Scholar]
- 4.Baulida, J., M. H. Kraus, M. Alimandi, P. P. DiFiore, and G. Carpenter. 1996. All ErbB receptors other than the epidermal growth factor receptor are endocytosis impaired. J. Biol. Chem. 271:5251-5257. [DOI] [PubMed] [Google Scholar]
- 5.Baulida, J., and G. Carpenter. 1997. Heregulin degradation in the absence of rapid receptor-mediated internalization. Exp. Cell Res. 232:167-172. [DOI] [PubMed] [Google Scholar]
- 6.Chan, S. D., D. M. Antoniucci, K. S. Fok, M. L. Alajoki, R. N. Harkins, S. A. Thompson, and H. G. Wada. 1995. Heregulin activation of extracellular acidification in mammary carcinoma cells is associated with expression of HER2 and HER3. J. Biol. Chem. 270:22608-22613. [DOI] [PubMed] [Google Scholar]
- 7.Cheng, E. S., Y. C. Huang, J. C. Lin, E. C. Mar, J. S. Pagano, G. E. Dutschman, and S. P. Grill. 1983. Unique spectrum of activity of 9-[(1,3-dihydroxy-2-propoxy)methyl]-guanine against herpesviruses in vitro and its mode of action against herpes simplex virus type 1. Proc. Natl. Acad. Sci. USA 80:2767-2770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cosset, F. L., F. J. Morling, Y. Takeuchi, R. A. Weiss, M. K. Collins, and S. J. Russell. 1995. Retroviral retargeting by envelopes expressing an N-terminal binding domain. J. Virol. 69:6314-6322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cwirla, S. E., P. Balasubramanian, D. J. Duffin, C. R. Wagstrom, C. M. Gates, S. C. Singer, A. M. Davis, R. L. Tansik, L. C. Mattheakis, C. M. Boytos, P. J. Schatz, D. P. Baccanari, N. C. Wrighton, R. W. Barrett, and W. J. Dower. 1997. Peptide agonist of the thrombopoietin receptor as potent as the natural cytokine. Science 276:1696-1699. [DOI] [PubMed] [Google Scholar]
- 10.Dougall, W. C., X. Qian, and M. I. Greene. 1993. Interaction of the neu/p185 and EGF receptor tyrosine kinases: implications for cellular transformation and tumor therapy. J. Cell Biochem. 53:61-73. [DOI] [PubMed] [Google Scholar]
- 11.Fathi, Z., J. W. Way, M. H. Corjay, J. Viallet, E. A. Sausville, and J. F. Battey. 1996. Bombesin receptor structure and expression in human lung carcinoma cell lines. J. Cell Biochem. Suppl. 24:237-246. [DOI] [PubMed] [Google Scholar]
- 12.Gollan, T. J., and M. R. Green. 2002. Redirecting retroviral tropism by insertion of short, nondisruptive peptide ligands into envelope. J. Virol. 76:3558-3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Han, X., N. Kasahara, and Y. W. Kan. 1995. Ligand-directed retroviral targeting of human breast cancer cells. Proc. Natl. Acad. Sci. USA 92:9747-9751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jeschke, M., W. Wels, W. Dengler, R. Imber, E. Stocklin, and B. Groner. 1995. Targeted inhibition of tumor-cell growth by recombinant heregulin-toxin fusion proteins. Int. J. Cancer 60:730-739. [DOI] [PubMed] [Google Scholar]
- 15.Karlan, B. Y., J. Jones, D. J. Slamon, and L. D. Lagasse. 1994. Glucocorticoids stabilize HER-2/neu messenger RNA in human epithelial ovarian carcinoma cells. Gynecol. Oncol. 53:70-77. [DOI] [PubMed] [Google Scholar]
- 16.Kern, J. A., and A. E. Filderman. 1993. Oncogenes and growth factors in human lung cancer. Clin. Chest Med. 14:31-41. [PubMed] [Google Scholar]
- 17.Miyazaki, N. M. Lamharzi, A. V. Schally, G. Halmos, K. Szepeshazi, K. Groot, and R. Z. Cai. 1998. Inhibition of growth of MDA-MB-231 human breast cancer xenografts in nude mice by bombesin/gastrin-releasing peptide (GRP) antagonists RC-3940-II and RC-3095. Eur. J. Cancer 34:710-717. [DOI] [PubMed] [Google Scholar]
- 18.Moody, T. W., C. B. Pert, A. F. Gazdar, D. N. Carney, and J. D. Minna. 1981. High levels of intracellular bombesin characterize human small-cell lung carcinoma. Science 214:1246-1248. [DOI] [PubMed] [Google Scholar]
- 19.Moody, T. W., D. N. Carney, F. Cuttitta, K. Quattrocchi, and J. D. Minna. 1985. High affinity receptors for bombesin/GRP-like peptides on human small cell lung cancer. Life Sci. 37:105-113. [DOI] [PubMed] [Google Scholar]
- 20.Moody, T. W., F. Zia, R. Venugopal, M. Fagarasan, H. Oie, and V. Hu. 1996. GRP receptors are present in non-small cell lung cancer cells. J. Cell Biochem. Suppl. 24:247-256. [DOI] [PubMed] [Google Scholar]
- 21.Pansky, F. A. Peng, M. Eberhard, L. Baselgia, W. Siegrist, J. B. Baumann, A. N. Eberle, C. Beglinger, and P. Hildebrand. 1997. Identification of functional GRP-preferring bombesin receptors on human melanoma cells. Eur. J. Clin. Investig. 27:69-76. [DOI] [PubMed] [Google Scholar]
- 22.Pasqualini, R., and E. Ruoslahti. 1996. Organ targeting in vivo using phage display peptide libraries. Nature 380:364-366. [DOI] [PubMed] [Google Scholar]
- 23.Peles, E., and Y. Yarden. 1993. Neu and its ligands: from an oncogene to neural factors. Bioessays 15:815-824. [DOI] [PubMed] [Google Scholar]
- 24.Pinkas-Kramarski, R., L. Soussan, H. Waterman, G. Levkowitz, I. Alroy, L. Klapper, S. Lavi, R. Seger, B. J. Ratzkin, M. Sela, and Y. Yarden. 1996. Neu differentiation factor and epidermal growth factor signaling by combinatorial receptor interactions. EMBO J. 15:2452-2467. [PMC free article] [PubMed] [Google Scholar]
- 25.Preston, S. R., L. F. Woodhouse, S. Jones-Blackett, G. V. Miller, and J. N. Primrose. 1995. High-affinity binding sites for gastrin-releasing peptide on human colorectal cancer tissue but not uninvolved mucosa. Br. J. Cancer 71:1087-1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schnierle, B. S., D. Moritz, M. Jeschke, and B. Groner. 1996. Expression of chimeric envelope proteins in helper cell lines and integration into Moloney murine leukemia virus particles. Gene Ther. 3:334-342. [PubMed] [Google Scholar]
- 27.Sharif, T. R., W. Luo, and M. Sharif. 1997. Functional expression of bombesin receptor in most adult and pediatric human glioblastoma cell lines; role in mitogenesis and in stimulating the mitogen-activated protein kinase pathway. Mol. Cell Endocrinol. 130:119-130. [DOI] [PubMed] [Google Scholar]
- 28.Spindel, E. R., B. W. Gibson, J. R. Reeve, Jr., and M. Kelly. 1990. Cloning and functional characterization of a complementary DNA encoding the murine fibroblast bombesin/gastrin-releasing peptide receptor. Mol. Endocrinol. 4:1956-1963. [DOI] [PubMed] [Google Scholar]
- 29.Tzahar, E., G. Levkowitz, D. Karunagaran, L. Yi, E. Peles, S. Lavi, D. Chang, N. Liu, A. Yayon, D. Wen, et al. 1994. ErbB-3 and ErbB-4 function as the respective low and high affinity receptors of all. Neu differentiation factor/heregulin isoforms. J. Biol. Chem. 69:25226-25233. [PubMed] [Google Scholar]
- 30.Valdenaire, O., T. Giller, V. Breu, A. Ardati, A. Schweizer, and J. G. Richards. 1998. A new family of orphan G protein-coupled receptors predominantly expressed in the brain. FEBS Lett. 424:193-196. [DOI] [PubMed] [Google Scholar]
- 31.Wasilenko, W. J., J. Cooper, A. J. Palad, K. D. Somers, P. F. Blackmore, J. S. Rhim, G. L. Wright, Jr., and P. F. Schellhammer. 1997. Calcium signaling in prostate cancer cells: evidence for multiple receptors and enhanced sensitivity to bombesin/GRP. Prostate 30:167-173. [DOI] [PubMed] [Google Scholar]
- 32.Wrighton, N. C., F. X. Farrell, R. Chang, A. K. Kashyap, F. P. Barbone, L. S. Mulcahy, D. L. Johnson, R. W. Barrett, L. K. Jolliffe, and W. J. Dower. 1996. Small peptides as potent mimetics of the protein hormone erythropoietin. Science 273:458-464. [DOI] [PubMed] [Google Scholar]
- 33.Yano, T., J. Pinski, K. Groot, and A. V. Schally. 1992. Stimulation by bombesin and inhibition by bombesin/gastrin-releasing peptide antagonist RC-3095 of growth of human breast cancer cell lines. Cancer Res. 52:4545-4547. [PubMed] [Google Scholar]
- 34.Zhao, Y., L. Zhu, S. Lee, L. Li, E. Chang, N. W. Soong, D. Douer, and W. F. Anderson. 1999. Identification of the block in targeted retroviral-mediated gene transfer. Proc. Natl. Acad. Sci. USA 96:4005-4010. [DOI] [PMC free article] [PubMed] [Google Scholar]