Abstract
Objective. The objective of the present study was to investigate the potential of application of growth factor genes to induce chondrogenic differentiation of human-derived mesenchymal stem cells (MSCs). The growth factor genes evaluated in the present study were transforming growth factor 1 (TGF-β1) and insulin-like growth factor 1 (IGF-1).
Methods. Human MSCs were transduced with the adenoviral vectors carrying either TGF-β1 or IGF-1 (AdTGF-β1 and AdIGF-1 respectively) or a combination of both growth factor genes at different multiplicities of infection (MOI) and were then made into pellets. Pellets were also made from nontransduced cells and maintained in culture medium supplemented with 10 ng/mL of TGF-β1. At specified time points, histological analysis, cartilage matrix gene expression, and immunofluorescence were performed to determine the extent of chondrogenic differentiation.
Results. MSCs transduced with the AdTGF-β1 demonstrated robust chondrogenic differentiation, while those made from AdIGF-1 did not. AdTGF-β1 pellets demonstrated aggrecan gene expression as early as day 3 of pellet culture, while type II collagen gene expression was detected by day 10 of culture. The AdIGF-1, alone or in combination with TGF-β1 pellets, did not show any type II collagen gene expression at any time point. By immunofluoresecence, type X collagen was distributed throughout the matrix in TGF-β1 protein pellets while the growth factor gene pellets displayed scant staining.
Conclusion. The results suggest that sustained administration of TGF-β1 may be more effective in suppressing terminal differentiation than intermittent dosing and thus effective for cartilage repair.
Introduction
Articular cartilage is frequently damaged by trauma or degeneration as a result of osteoarthritis. Once damaged, the cartilage has poor regenerative properties. Several different approaches are being investigated as an attempt to repair or regenerate the damaged cartilage. Some of the approaches being used clinically to regenerate cartilage include drilling of articular defects to recruit stem cells from the subchondral bone to participate in repair, mosaic-plasty, and autologous chondrocyte transplantation [1-3]. All these approaches, however, have inherent problems in that they cannot repair cartilage full-thickness defects and also there may be morbidity-associated problems and possible disease transmission [2,4,5].
As an attempt to circumvent the problems associated with the above-mentioned approaches for cartilage repair, novel approaches that include gene and stem cell therapies are being evaluated [6,7]. Adult-derived stem cells that can be harvested from bone, fat, or muscle offer alternative sources for chondrocytes [8-11]. These tissues have been demonstrated to contain stem cells of mesenchymal origin, which have potential to differentiate into various specific tissue cell phenotypes that include tendon, fat, marrow stroma, dermis, muscle, bone, and cartilage [10,12-20]. Numerous studies have demonstrated that bone marrow—derived mesenchymal stem cells (MSCs) can give rise to chondrocytes under appropriate conditions [21-23]. Members of the TGF-β1 (transforming growth factor 1) super-family have been shown to play a role in the induction of chondrogenic differentiation of MSCs in vitro [21,24,25].
Insulin-like growth factor-1 (IGF-1) has been extensively investigated for use in cartilage repair and regeneration [26-33,40]. Most studies on IGF-1 have focused on its effects on mature chondrocytes. Several studies have demonstrated that IGF-1 upregulates matrix synthesis by articular chondrocytes [34,35]. Gene transfer of IGF-1 into cartilage defects has been attempted and the results indicated that the growth factor increased cartilage matrix synthesis as a result of increasing cell number [36]. Some studies have shown that IGF-1 induces chondrogenic differentiation in periosteal cells; its effect on the chondrogenic differentiation of MSCs has not been clearly demonstrated [37,38].
Studies to demonstrate chondrogenic differentiation of MSCs are performed by addition of TGF-β1 in protein form. Differentiation of the stem cells into chondrocytes will require specific growth factors and efficient means to deliver the factors to the cells in vivo. In the present study, we tested the hypothesis that transfer of TGF-β1 or IGF-1 into human MSCs via adenoviral transfer of their candidate genes will induce chondrogenic differentiation of the MSCs. This mode of growth factor delivery would offer sustained growth factor supply to MSCs in vivo, thus circumventing repeated dosing.
Materials and methods
Isolation of MSCs from human bone marrow
Human bone marrow was obtained from the rimings of the femoral heads of the patients undergoing hip surgery under Institutional Review Board (IRB)-approved protocol. The cells were washed 4 times by centrifugation in Dulbecco's Modified Eagle Medium (DMEM; GIBCO, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (FBS; GIBCO) (v/v) and 1% (v/v) penicillin/streptomycin (P/S; Cambrex Bioscience, Baltimore, MD, USA). The washed cells were suspended in 1 mL of DMEM and layered on a ficoll gradient (1.077 g/mL; Amersham Biosciences, Piscataway, NJ, USA) and centrifuged. The cells at the interphase of the gradient were collected, resuspended in DMEM, and centrifuged. The cells were then plated in T-75 flasks at very low density in DMEM supplemented with 20% FBS, 1% P/S, 50 μg/mL ascorbic acid (Sigma, St. Louis, MO, USA), and 10 -7 M dexamethasone (Sigma). After 4 days of incubation, the nonadherent cells were removed and the adherent cells were maintained in culture in presence of 10 -7 M dexamethasone and 50 μg/mL of ascorbic acid with media changes every 3 days.
Adenoviral vector
The first-generation adenovirus, lacking E1 and E3 loci, serotype 5 vectors were used for these studies. The human IGF-1 and TGF-β1 cDNAs were inserted into the E1 region of the virus by crelox recombination [38,39]. In each case, gene expression was driven by the human cytomegalovirus early promoter. The resulting vectors were designated AdIGF-1 and Ad TGF-β1 respectively.
Adenoviral transduction in vitro
The MSCs were plated in T-25 flasks; at confluence, the DMEM was replaced with serum-free medium to which an adenovirus was added carrying either TGF-β1 or IGF-1 cDNA at multiplicities of infection (MOI) of 50, 100, and 150 for AdTGF-β1 and at 150 MOI for AdIGF-1 and AdTGF-β1 in combination. After 3 hours of incubation with the virus, the viral media were removed and the cells were washed in Hank's solution (GIBCO). Regular media (DMEM) supplemented with 10% FBS, 50 μg/mL ascorbic acid were added and the cells were incubated in this media for 24 hours. In some experiments, nontransduced cells were treated with 10 ng/ mL of TGF-β1 every 3 days to induce chondrogenic differentiation [21].
Pellet cultures
The transduced MSCs were trypsinized, counted, and 200,000 cell aliquots were spun down at 500g in 15-mL conical tubes. The nontransduced cells were treated similarly. The FBS-containing medium was then replaced with defined medium, consisting of DMEM supplemented with ITS+ premix (BD Sciences, San Diego, CA,USA)as described previously [21,24].The premix contains 6.25 μg/mL insulin, 6.25 μg/mL transferrin, 6.25 μg/mL selenous acid, 5.35 μg/mL linoleic acid, and 1.25 μg/mL bovine serum albumin (BSA). Medium was also supplemented with 1 mM pyruvate, 37.5 μg/mL of ascorbate-2-phosphate, and 10 -7 M of dexamethasone. The pellets were incubated at 37°C and 5% CO2. Medium changes were carried out at 3-day intervals. At each media change, the media were saved for TGF-β1 and IGF-1 measurements. Pellets made from nontransduced cells were incubated in a medium supplemented with TGF-β1 at 10 ng/mL. All the pellets were harvested at 14 days after incubation.
ELISA for TGF-β1 and IGF-1 production
The media conditioned by AdTGF-β1-or AdIGF-1-transduced cells were used for the determination of TGF-β1 and IGF-1 production. To measure the concentrations of IGF-1 and TGF-β1 in pellet supernatants, each growth factor was quantified using a commercial enzyme-linked immunosorbent assay kit (ELISA; R&D Systems, Minneapolis, MN, USA) following the manufacturer's protocol. The TGF-β1 production was determined either after activation with 0.2 M HCl or without prior activation to determine the active TGF-β1 present in the medium [41]. The mean value concentration of active and inactive TGF-β1 was compared by Student's t-test (n = 4), and p values equal to or less than 0.05 were considered significant.
Histological analysis of the pellets
For histological analysis, the pellets of each group were fixed in 10% buffered formalin for 1 hour and were then embedded in paraffin. Six-μm sections were cut and stained with toluidine blue.
Gene expression analysis for cartilage matrix components
Gene expression for collagen type II or aggrecan by the pellets made from either the AdTGF-β1 or AdIGF-1 was assessed by polymerase chain reaction (PCR). For total RNA isolation, 4 pellets were used from each experimental group. The pellets in all groups were homogenized in 1 mL TRIzol reagent (Invitrogen, Carlsbad, CA, USA). After extraction with chloroform (SIGMA) and precipitation with isopropanol (Fisher Scientific, Pittsburgh, PA, USA), the isolated RNA was suspended in DEPC water (Ambion, Austin, TX, USA) and stored at -80°C. cDNA was reverse transcribed from 1 μg of total cellular RNA using ThermoScript RT-PCR system (Invitrogen). The amplification primer pair for collagen type II was selected as 5′-CTG CTC GCT GCC GCT GTC CTT-3′ and 5′-AAG GGT CCC AGG TTC TCC ATC-3′, aggrecan was 5′-TGA GGA GGG CTG GAA CAA GTA CC-3′ and 5′-GGA GGT GGT AAT TGC AGG GAACA-3 ′, and β-actin was 5′-CAG GTC ATC ACY ATY GGC AAT GAGC-3′ and 5′-CGG ATG TCM ACG TCA CAC TTC ATGA-3′ [7,42]. PCR reactions were performed using aliquots of the cDNA template with PlatinumPCR Supermix kit (Invitrogen). The denaturation temperature was 94°C for 2 minutes, annealing temperature was 58°C for 1 minute, and the extension temperature was 72°C for 1 minute. Thirty-five cycles were performed. PCR products were separated electrophoretically on a 2.0% agarose gel (Sigma) and the products were visualized by staining in ethidium bromide (Invitrogen).
Immunofluorescence
Deparaffinized sections of pellet cultures were blocked in 5% (w/v) BSA, in Dulbecco's phosphate-buffered saline (DPBS; GIBCO), for 30 minutes to minimize nonspecific antibody binding. To permit exposure of type II collagen to the primary antibody, sections were digested with 0.1 U/mL of chondroitinase ABC in 1% (w/v) BSA in DPBS for 45 minutes and were then exposed to 1 mg/mL pronase (Calbiochem, LaJolla, CA, USA) for 30 minutes. Sections were incubated for 1 hour with the type II collagen-specific antibody (II-II6B3) diluted at 1:100 in 1% BSA in DPBS [20,43]. The IIII6B3 antibody was developed by T. Linsenmayer and was obtained from the Developmental Studies Hybridoma Bank maintained by the University of Iowa, Department of Biological Sciences, Iowa City, IA, USA. The sections were then incubated for 45 minutes with fluorescein isothiocyanate (FITC)-conjugated rabbit anti-mouse immunoglobulin diluted to 1:1000 in 1% BSA in DPBS. A drop of mounting medium (Biomeda, Foster City, CA, USA) was added before covering the slide. Slides were rinsed three times with 0.1% BSA in DPBS between each treatment. All incubation procedures were carried out at room temperature in a humidified chamber. For type X collagen immunofluorescence, the same procedure as described above was followed except that Cy3 was used for the visualization.
Results
TGF-β1 production by pellets made from AdTFG-β1
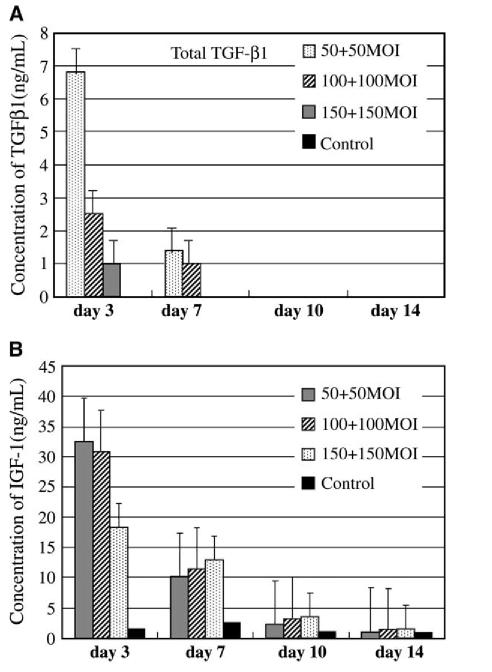
ELISA of TGF-β1 production at different time points demonstrated synthesis of TGF-β1 protein that was secreted in the medium conditioned by the transduced cells. The media used for TGF-β1 determination was collected at 3,7,10, and 14 days of pellet culture. The cells transduced with the AdTGF-β1 at 150 MOI synthesized 155.98 ± 38.5 ng/mL of total TGF-β1 (mean ± SD) at day 7 (Fig. 1A). This concentration was the maximal production of total TGF-β1 at day 7 by the transduced cells. The concentration of total TGF-β1 production in all the groups decreased with time in culture after day 7; however, even by day 14 the cells transduced with AdTGF-β1 at 50 and 100 MOI still synthesized detectable levels of TGF-β1 and these levels were higher than those of the control groups. Most of the TGF-β1 synthesized by the transduced cells was, however, in its inactive form (Fig. 1A and B). The active form of TGF-β1 on day 3 was about 3 ng/mL for cells transduced with AdTGF-β1 at 50 MOI, while at 150 MOI the active TGF-β1 was about 12 ng/mL (Fig. 1B). The concentration of the active TGF-β1 decreased with time and by day 14 there was no detectable active TGF-β1 in all the pellets made from the cells transduced with AdTGF-β1 at different MOIs (Fig. 1B).
Figure 1.
(A): Total TGF-β1 synthesis by pellet cultures made from AdTGF-β1-transduced cells at various MOIs. The highest concentration of TGF-β1 is at day 7 (150 MOI). Values are given as mean of standard error (SE), n = 4. The TGF-β1 was determined after activation with 0.2 M HCl. (B): Active TGF-β1 synthesis by pellets made from cells transduced with AdTGF-β1 at various MOIs. The highest concentration of active TGF-β1 protein is at day 7 (150 MOI). Values are given as mean of SE, n = 4. The TGF-β1 was determined without activation with 0.2 M HCl. Media conditioned by the cells in pellet were collected at 3, 7, 10, and 14 days and used for TGF-β1 determination by ELISA.
IGF-1 production by pellets made from AdIGF-1
The highest level of IGF-1 production by the cells transduced with AdIGF-1 at 100 and 150 MOI was about 77 ng/mL at day 3 (Fig. 2). The IGF-1 production decreased very rapidly after day 3 and the concentration of IGF-1 at day 7 was 31.9 ng/mL for the pellets made from the cells transduced with AdIGF-1 at 100 and at 150 MOI. IGF-1 production, however, was still detectable by day 14 in all the pellets made from AdIGF-1 cells at different MOIs.
Figure 2.
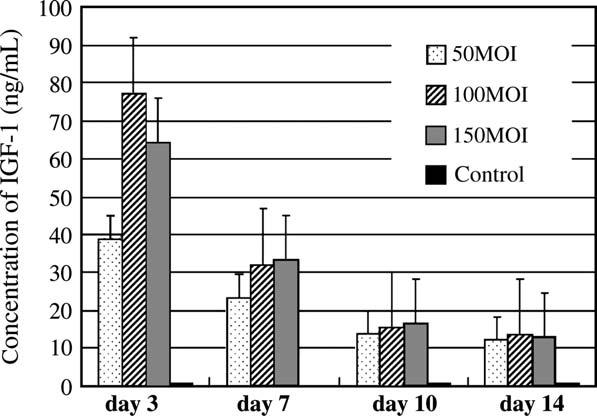
IGF-1 synthesis by pellet cultures made from cells transduced with AdIGF-1 at various MOIs. The highest concentration of IGF-1 was at day 3 (100 MOI). Values are given as mean of SE, n = 4. IGF-1 expression was determined by ELISA.
IGF-1 and TGF-β1 production by cells transduced with both growth factor genes
Cells transduced with a combination of AdTGF-β1 and AdIGF-1 at 150 MOI synthesized very low levels of TGF-β1; by day 3, the cells synthesized about 7 ng/mL of total TGF-β1 ( Fig. 3A). The IGF-1 synthesized by the cells transduced with a combination of AdTGF-β1 and AdIGF-1 was about half of that produced by cells transduced with AdIGF-1 at 150 MOI (Fig. 3B). The production of lower levels of the growth factors by the transduced cells may have been due to cell toxicity as a result of the viral overload of the combined growth factor genes.
Figure 3.
(A): Total TGF-β1 synthesis by pellets made from cells transduced with a combination of equal amounts of AdTGF-β1 and AdIGF-1 at various MOIs. Low levels of TGF-β1 were synthesized presumably as a result of cell toxicity due to the viral load of the combined growth factor genes. Values are given as mean of SE, n = 4. (B): IGF-1 synthesis by pellets made from cells transduced with a combination of AdTGF-β1 and AdIGF-1 at various MOIs. Values are given as mean of SE, n=4. The highest concentration of IGF-1 synthesized by the cells was at day 3.
Histological analysis of AdTGF-β1 pellets
Histological examination of the sections of the pellets made from the cells transduced with AdTGF-β1 at 14 days demonstrated that chondrogenic differentiation was more robust in pellets made from the cells transduced with the viral vector at 50 MOI (Fig. 4A). Morphologically, chondrocytelike cells surrounded by abundant extracellular matrix that stained with toluidine blue were observed in the pellets ( Fig. 4A). Control pellets made from the cells that were not transduced with AdTGF-β1 did not show any chondrocytelike cells and there was no toluidine blue staining of the matrix ( Fig. 4A). The active TGF-β1 synthesized by the cells transduced with AdTGF-1 at 50 MOI was about 3 ng/mL at day 3 and by day 14 there was no detectable active TGF-β1; however, the cells underwent chondrogenic differentiation. These data suggest that sustained production of TGF-β1, although at lower levels, is sufficient for the induction of chondrogenic differentiation of MSCs. Conversion of the inactive TGF-β1 to active TGF-β1 by the transduced cells cannot, however, be ruled out.
Figure 4.

Histological analysis of pellets made from cells transduced with AdTGF-β1 at various MOIs, IGF-1 at 150 MOI, and a combination of AdTGF-β1 and AdIGF-1 at 150 MOI of each growth factor gene. AdTGF-β1 at 50 MOI (a), 100 MOI (b), 150 MOI (c). AdIGF-1 at 150 MOI (d), AdTGF-β1 and AdIGF-1 combination at 150 MOI of each growth factor gene (e), and control (f). All pellets were stained with toluidine blue. (Upper ×40, lower ×200 magnification.) Pellets made from cells transduced with AdTGF-β1 at 50 MOI show robust chondrogenic differentiation. There is no obvious chondrogenic differentiation in either AdIGF-1 or in combination with AdTGF-β1 in any of the pellets.
Histological analysis of AdIGF-1 pellets
Pellets made from AdIGF-1-transduced cells demonstrated very little or no chondrogenesis even in pellets made from cells transduced with AdIGF-1 at 150 MOI (Fig. 4B). There were no morphologically identifiable chondrocyte-like cells in pellets made from the cells transduced with AdIGF-1 at 100 MOI or 150 MOI, as indicated by the absence of the toluidine blue staining. The pellets made from cells transduced with AdIGF-1 at 150 MOI synthesized about 70 ng/mL of IGF-1 and by day 14 the cells were still synthesizing a significant amount of IGF-1. Nevertheless, there was no condrogenic differentiation observed in these pellets. These data clearly demonstrate that sustained production of IGF-1 does not induce chondrogenic differentiation.
Histological analysis of pellets made from the combination of AdTGF-β1 and AdIGF-1
Pellets made from the cells transduced with a combination of AdTGF-β1 and AdIGF-1 at 150 MOI showed minimal staining with toluidine blue (Fig. 4C). These data suggest that there is no synergistic effect between IGF-1 and TGF-β in the induction of chondrogenic differentiation of the MSCs.
Cartilage matrix gene expression
Gene expression analysis for the collagen type II and aggrecan in the pellets made from cells transduced with either AdTGF-β1 or AdIGF-1 growth factor genes at days 3, 10, and 14 are shown in Figure 5. The pellets made from the cells transduced with AdTGF-β1 at 50 MOI demonstrated both type II collagen and aggrecan gene expression (Fig. 5A and B). Aggrecan gene expression was detected as early as day 3 of pellet culture; at this time point type II mRNA was not detected. However, when the pellets were analyzed for type II collagen mRNA at day 10 of culture, type II mRNA was present, although not in high quantities. The expression of type II collagen gene in the pellets increased with time in culture and was very strong by day 14. Aggrecan gene expression also followed a similar trend (Fig. 5B). There was no type II collagen gene expression detected in the pellets made from the cells transduced with AdIGF-1 at any time point (Fig. 5A). Pellets made from the cells transduced with AdIGF-1, however, demonstrated aggrecan gene expression as early as day 3 of pellet culture. This expression was maintained throughout the time of culture up to day 14, the final time at which the pellets were analyzed (Fig. 5B). There was no type II collagen gene expression by pellets made from the combination of both growth factor genes (data not shown). The data suggest that either IGF-1 or culture conditions induce aggrecan gene expression but not type II collagen gene expression.
Figure 5.

Gene expression analysis for collagen type II and aggrecan by pellet cultures at different time points. (C), Control; (I), AdIGF-1 (150 MOI); (T), AdTGF-β1 (50 MOI). (A): Type II collagen gene expression is evident in AdTGF-β1 pellets at 50 MOI by day 10 (T) but not by AdIGF-1 (I). (B): Aggrecan gene expression is evident by day 3 in all the pellets except the control pellet. IGF-1 pellets show aggrecan gene expression but not type II collagen gene expression.
Immunofluorescence
To confirm the expression of type II collagen in the pellets made from the cells transduced with the growth factor genes, immunofluorescence localization for type II collagen in pellets made from AdTGF-β1, AdIGF-1, or a combination of both growth factor genes was performed. Pellets made from AdTGF-β1 at 50 MOI showed robust staining for the type II collagen (Fig. 6A). Pellets made from AdIGF-1 or a combination of both growth factor genes were negative for the type II collagen staining, thus confirming the gene expression analysis results (Fig. 6B and C).
Figure 6.
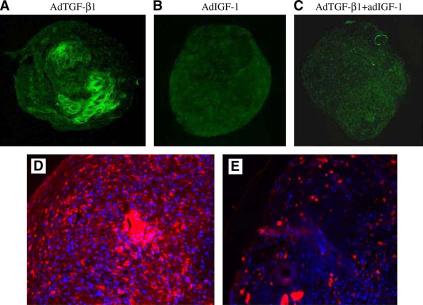
Detection of collagen type II and type X collagen expression in the pellets by immunofluorescence. (A): Pellet with AdTGF-β1-transduced cells at 50 MOI; (B): with AdIGF-1 at 150 MOI; (C): with combination of AdTGF-β1 and AdIGF-1 at 150 MOI (×100 magnification). Type II collagen immunostaining is present in the AdTGF-β1 pellets only. (D): Immunofluorescence for type X collagen in pellets made from nontransduced cells and incubated in a medium supplemented with TGF-β1 protein at 50 ng/mL. (E): Immunofluorescence for type X collagen in pellets made from MSCs transduced with AdTGF-β1 at 50 MOI. There is extensive staining for type X collagen in pellet D than in pellet E. The figure is representative of 3 stained pellets.
Type X collagen expression
Intermittent dosing of TGF-β through every-other-day administration of protein resulted in greater deposition of type X collagen throughout the pellets while sustained administration of TGF-β through adenoviral transduction resulted only in scattered deposition of type X collagen (Fig. 6D and E). These results suggest that the temporal pattern of TGF-β1 administration impacts chondrogenic differentiation of adult human bone marrow mesenchymal stem cells and that sustained administration of TGF-β1 suppresses terminal differentiation of these cells.
Discussion
The present study was initiated to assess the potential of using growth factor genes to induce chondrogenesis in human MSCs and for potential use in cartilage repair and regeneration. The data demonstrated that sustained expression of TGF-β1 is more effective in inducing chondrogenic differentiation of human MSCs than IGF-1. Previous studies using TGF-β1 to induce chondrogenesis in MSC was done by addition of TGF-β1 protein [8,21,24]. The optimal concentration of TGF-β1 used in the protein form to induce chondrogenesis in MSCs was 10 ng/mL [21,24]. Interestingly, the results of ELISA showed that chondrogenic differentiation was more evident in pellets made from cells transduced with AdTGF-β1 at 50 MOI. The active TGF-β1 produced by the cells was as low as 5 ng/mL at day 3 of pellet culture. The concentration of the active TGF-β1 decreased rapidly and by day 14 the active TGF-β1 was no longer detectable. The data therefore suggest that, although the concentration of TGF-β1 was lower in the transduced cells compared to that supplied in the protein form, chondrogenic differentiation still occurred presumably due to the sustained expression of TGF-β1 by the transduced cells. The total TGF-β1 synthesized by the transduced cells was, however, quite high; it is possible that the cells could convert the inactive TGF-β1 to active form when the cells need it.
The combination of TGF-β1 and IGF-1 did not induce chondrogenic differentiation. The data suggest that there is no synergestic effect between TGF-β1 and IGF-1 on MSC differentiation. Substantial levels of IGF-1 were synthesized in the pellets made from the combination of the growth factor genes, lending support to the observation that IGF-1 does not induce chondrogenic differentiation of the human MSCs.
Several studies have shown that exposure of chondrocytes to IGF-1 in vitro enhances chondrocyte metabolism while maintaining the differentiated phenotype. Concentrations of IGF-1 as low as 50 ng/mL are sufficient to exert significant effects on the proliferative and metabolic functions of cultured chondrocytes [6,13,31,34,44]. IGF-1 may therefore be more effective on differentiated chondrocytes than inducing chondrogenic differentiation of MSCs. Although the present findings show that IGF-1 has no effect on chondrogenic differentiation of MSCs, there are some reports that have shown that IGF-1 induces chondrogenic differentiation of periosteal mesenchymal stem cells [37,38]. The difference in response to IGF-1 between the two cell types may be that periosteal cells are characteristically different from the MSCs.
The present findings have shown that IGF-1 induces aggrecan gene expression but does not affect type II collagen gene expression by MSCs. These data are of interest because they still suggest that TGF-β1 and IGF-1 may still be used in combination to augment cartilage repair and regeneration.
Immunofluorescence staining demonstrated that type X collagen was distributed throughout the matrix in pellets incubated in a media supplemented with TGF-β1 protein while the pellets made from cells transduced with AdTGF-β1 demonstrated scattered staining. The importance of TGF-β1 to the prevention of terminal differentiation is of special interest in the use of growth factors to enhance articular cartilage repair. The process of bone formation through endochondral ossification means that bone forms by replacing a cartilage anlagen after the chondrocytes undergo a process of hypertrophy, calcification, and apoptosis known as chondrocyte terminal differentiation. It has been shown that TGF-β1 inhibits the terminal differentiation of chondrocytes and that TGF-β1 acts to preserve the chondrocyte phenotype [45,46]. Our results suggest that sustained administration of TGF-β1 may be more effective in suppressing terminal differentiation than intermittent dosing.
Acknowledgments
This study was supported by PTEI/DAMD (to CRC) and by NIH grant AR049688 (to CN). The authors would like to thank Dr. Kaori Hayashi for the assistance with pellets in which TGF-β1 protein was included, and Helga Georgescu and Lesa Weikermaster for technical assistance.
References
- 1.Hangody L, Kish G, Karpati Z, Szerb I. Arthroscopic autogenous osteochondral mosaicplasty for the treatment of femoral chondyle articular defects. Knee Surg Sports Traumatol Arthrosc. 1997;5:262–267. doi: 10.1007/s001670050061. [DOI] [PubMed] [Google Scholar]
- 2.Chu CR, Convery FR, Akeson WH, Meyer M, Amiel D. Articular cartilage transplantation. Clinical results in the knee. Clin Orthop. 1999;360:159–168. [PubMed] [Google Scholar]
- 3.Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331:889–895. doi: 10.1056/NEJM199410063311401. [DOI] [PubMed] [Google Scholar]
- 4.Shapiro F, Koide S, Glimcher MJ. Cell origin and differentiation in the repair of full thickness defects of articular cartilage. J Bone Joint Surg. 1993;75-A:532–552. doi: 10.2106/00004623-199304000-00009. [DOI] [PubMed] [Google Scholar]
- 5.Furukawa T, Eyre DR, Koide S, Glimcher MJ. Biochemical studies on repair cartilage resurfacing experimental defects in the rabbit knee. J Bone Joint Surg. 1980;62-B:79–89. [PubMed] [Google Scholar]
- 6.Trippel SB, Corvol MT, Dumontier MF, Rappaport R, Hung HH, Mankin HJ. Effect of somatomedin-C/insulin-like growth factor I and growth hormone on cultured growth plate and articular chondrocyte. Pediatr Res. 1989;25:76–82. doi: 10.1203/00006450-198901000-00017. [DOI] [PubMed] [Google Scholar]
- 7.Evans CH, Ghivizzani SC, Smith P, Studer FD, Mi Z, Robbins PD. Using gene therapy to protect and restore cartilage. Clin Orthop Relat Res. 2000;379S:214–219. doi: 10.1097/00003086-200010001-00027. [DOI] [PubMed] [Google Scholar]
- 8.Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;285:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 9.Prockop DJ. Marrow stromal cells as stem cells for non-hematopoietic tissues. Science. 1997;276:71–74. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- 10.Zuk PA, Zhu M, Ashjian P, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jankowski RJ, Deasy BM, Huard J. Muscle-derived stem cells. Gene Ther. 2002;9:642–647. doi: 10.1038/sj.gt.3301719. [DOI] [PubMed] [Google Scholar]
- 12.Murphy JM, Dixon K, Beck S, Fabian D, Feldman A, Barry F. Activity of mesenchymal stem cells from patients with advanced osteoarthritis. Arthritis Rheum. 2002;46(3):704–713. doi: 10.1002/art.10118. [DOI] [PubMed] [Google Scholar]
- 13.Mi Z, Ghivizzani SC, Lechman ER, et al. Adenovirus-mediated gene transfer of insulin-like growth factor I stimulates proteoglycan synthesis in rabbit joints. Arthritis Rheum. 2000;43:2563–2570. doi: 10.1002/1529-0131(200011)43:11<2563::AID-ANR25>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 14.Caplan AI. Mesenchymal stem cells. J Orthop Res. 1991;9:641–665. doi: 10.1002/jor.1100090504. [DOI] [PubMed] [Google Scholar]
- 15.Gronthos S, Zannettino ACW, Hay SJ, et al. Molecular and cellular characterization of highly purified stromal stem cells derived from human bone marrow. J Cell Sci. 2003;116:1827–1835. doi: 10.1242/jcs.00369. [DOI] [PubMed] [Google Scholar]
- 16.Nochi H, Sung JH, Lou J, Adkisson HD, Maloney WJ, Hruska KA. Adenovirus mediated BMP-13 gene transfer induces chondrogenic differentiation of murine mesenchymal progenitor cells. J Bone Miner Res. 2004;19:111–122. doi: 10.1359/jbmr.2004.19.1.111. [DOI] [PubMed] [Google Scholar]
- 17.Snyder EY, Vescovi AL. The possibilities/perplexities of stem cells. Nat Biotechnol. 2000;18:827–828. doi: 10.1038/78428. [DOI] [PubMed] [Google Scholar]
- 18.Ferrari G, Cusella-De Angelis G, Colette M, et al. Muscle regeneration by bone marrow—derived myogenic progenitors. Science. 1998;279:11–16. doi: 10.1126/science.279.5356.1528. [DOI] [PubMed] [Google Scholar]
- 19.Orlic D, Kajstura J, Chimenti S, et al. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–705. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- 20.Theise ND, Nimmakayalu M, Gardner R, et al. Liver from bone marrow in humans. Hepatology. 2000;32:11–16. doi: 10.1053/jhep.2000.9124. [DOI] [PubMed] [Google Scholar]
- 21.Johnstone B, Herring TM, Caplan AI, Goldberg VM, Yoo JU. In vitro chondrogenesis of bone marrow—derived mesenchymal progenitor cells. Exp Cell Res. 1998;38:265–272. doi: 10.1006/excr.1997.3858. [DOI] [PubMed] [Google Scholar]
- 22.Barry F, Raymond E, Boynton E, Liu B, Murphy M. Chondrogenic differentiation of mesenchymal stem cells from bone marrow; Differentiation-dependent gene expression of matrix components. Exp Cell Res. 2001;268:189–200. doi: 10.1006/excr.2001.5278. [DOI] [PubMed] [Google Scholar]
- 23.Oyama M, Tatlock A, Kavalkovich K, et al. Retrovirally transduced bone marrow stromal cells isolated from a mouse model of human osteogenesis imperfecta (oim) persist in bone and retain the ability to form cartilage and bone after extended passaging. Gene Ther. 1999;6:321–329. doi: 10.1038/sj.gt.3300839. [DOI] [PubMed] [Google Scholar]
- 24.Mackay AM, Beck SC, Murphy JM, Barry F, Chichester CO, Pittenger M. Chondrogenic differentiation of cultured human mesenchymal stem cells from marrow. Tissue Eng. 1998;4:415–428. doi: 10.1089/ten.1998.4.415. [DOI] [PubMed] [Google Scholar]
- 25.Pizette S, Niswander L. BMPs are required at two steps of limb chondrogenesis: formation of prechondrogenic condensations and their differentiation into chondrocytes. Dev Biol. 2000;219:237–249. doi: 10.1006/dbio.2000.9610. [DOI] [PubMed] [Google Scholar]
- 26.Nixon AJ, Brower-Toland BD, Bent SJ, et al. Insulin-like growth factor therapy: Applications for cartilage repair. Clin Orthop Res. 2000;379S:201–213. doi: 10.1097/00003086-200010001-00026. [DOI] [PubMed] [Google Scholar]
- 27.Milne M, Quail JM, Barab DT. Dexamethasone stimulated osteogenic differentiation in vertebral and femoral bone marrow cell cultures: Comparison of IGF-1 gene expression. J Cell Biochem. 1998;71:381–391. [PubMed] [Google Scholar]
- 28.Inoue H, Kato Y, Iwamoto M, Hiraki Y, Sakuda M, Suzuki F. Stimulation of cartilage-matrix proteoglycan synthesis by morphologically transformed chondrocytes grown in the presence of fibroblast growth factor and transforming growth factor-β. J Cell Physiol. 1989;138:329–337. doi: 10.1002/jcp.1041380216. [DOI] [PubMed] [Google Scholar]
- 29.Morales TI. Transforming growth factor-β and insulin-like growth factor-1 restore proteoglycan metabolism of bovine articular cartilage after depletion by retinoic acid. Arch Biochem Biophys. 1994;315:190–198. doi: 10.1006/abbi.1994.1489. [DOI] [PubMed] [Google Scholar]
- 30.Nixon AJ, Fortier LA, Villiums J, Mohammed H. Enhanced repair of extensive articular defects by insulin-like growth factor-I—laden fibrin composites. J Orthop Res. 1999;17:475–487. doi: 10.1002/jor.1100170404. [DOI] [PubMed] [Google Scholar]
- 31.Fortier LA, Mohammed OH, Lust G, Nixon AJ. Insulin-like growth factor-1 enhances cell-based repair of articular cartilage. J Bone Joint Surg. 2002;84B:276–288. doi: 10.1302/0301-620x.84b2.11167. [DOI] [PubMed] [Google Scholar]
- 32.Fortier LA, Balkman CE, Sandell LJ, Ratcliffe A, Nixon AJ. Insulin-like growth factor-I gene expression patterns during spontaneous repair of acute articular cartilage injury. J Orthop Res. 2001;19:720–728. doi: 10.1016/S0736-0266(00)00070-X. [DOI] [PubMed] [Google Scholar]
- 33.van Osch GJVM, van den Berg WB, Hunziker EB, Hauselmann HJ. Differential effects of IGF-1 and TGFβ-2 on the assembly of proteoglycans in pericellular and territorial matrix by cultured bovine articular chondrocytes. Osteoarthritis Cartilage. 1998;6:187–195. doi: 10.1053/joca.1998.0111. [DOI] [PubMed] [Google Scholar]
- 34.Smith P, Shuler D, Georgescu I, et al. Genetic enhancement of matrix synthesis by articular chondrocyte. Arthritis Rheum. 2000;43:1156–1164. doi: 10.1002/1529-0131(200005)43:5<1156::AID-ANR26>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 35.Nixon AJ, Lillich JT, Bruton-Wurster N, Lust G, Mohammed HO. Differentiated cellular function in fetal chondrocytes cultured with insulin-like growth factor-I and transforming growth factor-β. J Orthop Res. 1998;16:531–541. doi: 10.1002/jor.1100160503. [DOI] [PubMed] [Google Scholar]
- 36.Madry H, Padera R, Seidel J, et al. Gene transfer of a human insulin-like growth factor I cDNA enhances tissue engineering of cartilage. Hum Gene Ther. 2002;13:1621–1630. doi: 10.1089/10430340260201716. [DOI] [PubMed] [Google Scholar]
- 37.Fukumoto T, Sperling JW, Sanyal A, et al. Combined effects of insulin-like growth factor-1 and transforming growth factor-β1 on periosteal mesenchymal cells during chondrogenesis in vitro. Osteoarthritis and Cartilage. 2003;11:55–64. doi: 10.1053/joca.2002.0869. [DOI] [PubMed] [Google Scholar]
- 38.Mierisch CM, Anderson PC, Balian G, Diduch DR. Treatment with insulin-like growth factor-1 increases chondrogenesis by periosteum in vitro. Connect Tissue Res. 2002;43:559–568. [PubMed] [Google Scholar]
- 39.Robbins P, Ghivizzani SC. Viral vector for gene therapy. Pharmacol Ther. 1998;80:35–47. [PubMed] [Google Scholar]
- 40.Brower-Toland BD, Saxer RA, Goodrich LR, et al. Direct adenovirusmediated insulin-like growth factor I gene transfer enhances transplant chondrocyte function. Hum Gene Ther. 2001;12:117–129. doi: 10.1089/104303401750061186. [DOI] [PubMed] [Google Scholar]
- 41.Annes JP, Munger JS, Rifkin DB. Making sense of latent TGF-β activation. J Cell Sci. 116:217–224. doi: 10.1242/jcs.00229. [DOI] [PubMed] [Google Scholar]
- 42.Ikenoue T, Trindade MCD, Lee MS, et al. Mechanoregulation of human articular chondrocyte aggrecan and type II collagen expression by intermittent hydrostatic pressure in vitro. J Orthop Res. 2003;21:110–116. doi: 10.1016/S0736-0266(02)00091-8. [DOI] [PubMed] [Google Scholar]
- 43.Lennon DP, Haynesworth SE, Young RG, Dennis JE, Caplan IA. A chemically defined medium supports in vitro proliferation and maintains the osteochondral potential of rat marrow—derived mesenchymal stem cells. Exp Cell Res. 1995;219:354–362. doi: 10.1006/excr.1995.1221. [DOI] [PubMed] [Google Scholar]
- 44.Worster AA, Brower-Toland BD, Fortier LA, Bent SJ, Williams J, Nixon AJ. Chondrocytes differentiation of mesenchymal stem cells sequentially exposed to transforming growth factor-β1 in monolayer and insulin-like growth factor-1 in a three-dimensional matrix. J Orthop Res. 2001;19:738–749. doi: 10.1016/S0736-0266(00)00054-1. [DOI] [PubMed] [Google Scholar]
- 45.Ferguson CM, Schwarz EM, Reynolds PR, Puzas JE, Rosier RN, O’Keefe RJ. Smad2 and 3 mediate transforming growth factor-β1-induced inhibition of chondrocyte maturation. Endocrinology. 2000;141:4728–4735. doi: 10.1210/endo.141.12.7848. [DOI] [PubMed] [Google Scholar]
- 46.Kato Y, Iwamoto M, Koike T, Suzuki F, Takano Y. Terminal differentiation and calcification in rabbit chondrocyte cultures grown in centrifuge tubes: regulation by transforming growth factor β and serum factors. Proc Natl Acad Sci U S A. 1988;85:9552–9556. doi: 10.1073/pnas.85.24.9552. [DOI] [PMC free article] [PubMed] [Google Scholar]