Abstract
The orbitofrontal cortex (OFC) has been characterized as a higher-order, multimodal sensory cortex. Evidence from electrophysiological and behavioral studies in the rat has suggested that OFC plays a role in modulating olfactory guided behavior, and a significant projection to OFC arises from piriform cortex, the traditional primary olfactory cortex. To discern how OFC interacts with primary olfactory structures, the anterograde tracer Phaseolus vulgaris leucoagglutinin was injected into orbitofrontal cortical areas in adult male rats. Labeled fibers were found in the piriform cortex and olfactory bulb on the side ipsilateral to the injection. Notably, the projection to piriform cortex was predominantly from ventrolateral orbital cortex, and was not uniform; rostrally, the projection to the ventral portion of the anterior piriform cortex (APC) was substantial, while the dorsal APC was virtually free of labeled fibers. Labeled fibers were found in both the dorsal and ventral portions in more caudal regions of APC. Most labeled fibers were found in layer III, although a substantial number of fibers were observed in layers Ib and II. Labeled fibers in posterior piriform cortex also were seen after injection into orbitofrontal areas. Taken together with previous reports, these findings suggest that piriform cortex includes multiple subdivisions, which may perform separate, parallel functions in olfactory information processing. Further, these results suggest that the OFC, in addition to its putative role in encoding information about the significance of olfactory stimuli, may play a role in modulating odor response properties of neurons in piriform cortex.
Keywords: olfaction, olfactory cortex, cortical processing, multimodal association
Piriform cortex, the traditional primary olfactory cortex and the largest cortical recipient of afferent fibers from the olfactory bulb (OB), is thought to serve an important role in olfactory learning tasks (Schoenbaum and Eichenbaum, 1995a; Wilson and Stevenson, 2003). Supporting this view is evidence that cells in rat piriform cortex display experience-dependent functional plasticity: single cells can exhibit a high degree of specificity for individual odorants (Wilson, 1998, 2000), but their specificity can change with olfactory experience (McCollum et al., 1991; Wilson, 2003; Wilson and Stevenson, 2003). In addition, cells in piriform cortex can discriminate learned odors based on differences in incentive or predictive values, and can respond to nonolfactory contextual cues in a behavioral task (Schoenbaum and Eichenbaum, 1995a). Together, these results suggest that the piriform cortex encodes odors based in part on associations with available nonolfactory information.
The integrative circuitry of piriform cortex makes it well-suited to perform associative tasks. In addition to signals from the OB, cells in piriform cortex receive input from the anterior olfactory cortex (AOC; also known as the anterior olfactory nucleus), from an extensive autoassociative network, and from many neocortical and subcortical areas (Haberly and Price, 1978a,b; Luskin and Price, 1982; see Haberly, 1998, for a review; Johnson et al., 2000; Bouret and Sara, 2002). Of particular interest are the connections between the rat piriform cortex and the orbitofrontal neocortical areas (OFC), which appear to be involved in odor information processing (Schoenbaum and Eichenbaum, 1995a; Datiche et al., 1996; Johnson et al., 2000). Single-unit recording studies indicate that odor responses in OFC are related to the incentive or reward value of odors in behavioral tasks (Schoenbaum and Eichenbaum, 1995b; Schoenbaum et al., 1999), and together with the amygdala the OFC plays an important role in encoding the predictive value of odor stimuli (Schoenbaum et al., 1998, 1999, 2003a,b; Ramus and Eichenbaum, 2000; Setlow et al., 2002).
The purpose of this study was to characterize the projections to piriform cortex from the OFC to elucidate whether these areas may play a role in shaping neuronal response properties. Injections of anterograde tracer were made in three different orbitofrontal areas: ventrolateral orbital cortex (VLO), lateral orbital cortex (LO), and the ventral agranular insular cortex (AIV). Results demonstrate that these areas have distinct projection patterns to olfactory areas, and features of the projections suggest that OFC may play a role in shaping the odor response characteristics of cells in piriform cortex. These results further support a role for piriform cortex, in conjunction with the amygdala and OFC, in the formation and recall of associations between olfactory stimuli, contextual cues, and behavioral outcomes.
MATERIALS AND METHODS
Anterograde tract tracing
All surgical procedures were carried out under sterile conditions in accordance with NIH guidelines under protocols approved by the University of Virginia Animal Care and Use Committee. Results are based on injections of Phaseolus vulgaris leucoagglutinin (PHA-L; Vector Laboratories, Burlingame, CA) into 27 adult male hooded rats (250-350 g); the injections from 23 of these rats were selected for detailed analyses. Rats were anesthetized with a ketamine/xylazine mixture (ketamine 80 mg/kg; xylazine 8 mg/kg) and placed in a stereotaxic frame (Kopf Instruments, Tujunga, CA). An incision was made in the scalp, skull landmarks (bregma and lambda) were located, and the head leveled to conform with the atlas of Paxinos and Watson (1986). A small hole was drilled in the skull overlying the intended injection site, through which a glass pipette (tip diameter 10-15 μm) that had been back-filled from the tip with a 2.5% PHA-L solution was positioned using a micromanipulator. PHA-L was iontophoretically injected by delivering 2.5-5 μA of positive DC current passed through the pipette using a current generator (Grass Instruments, Quincy, MA) on a 50% duty cycle (7 seconds on/off) for 5-15 minutes. Current flow was monitored using an in-line ammeter (Fluka, Buchs, Switzerland). Seven to 10 days following the injection, animals were deeply anesthetized with an overdose of sodium pentobarbital (>50 mg/kg) and perfused through the aorta with a rapid wash of phosphate-buffered saline (PBS, pH 7.4) containing heparin followed by 4% formaldehyde freshly depolymerized from paraformaldehyde. The brain was removed from the skull and placed in an increasing concentration series of cryoprotectant solutions (10%, 15%, and 20% sucrose) over a 48-hour period. Sections (30-50 μm) were cut on a cryostat and placed in wells of PBS for free-floating immunocytochemistry.
Immunocytochemistry for PHA-L
Sections were stained using a modification of the protocol of Gerfen and Sawchenko (1984). Briefly, sections were washed five times in PBS containing 2% bovine serum albumin (BSA) and 0.5% Triton X-100 (Sigma, St. Louis, MO), followed by incubation in PBS + BSA + Triton with the primary antibody (biotinylated goat anti-PHA-L, 1:800 dilution, Vector) overnight at room temperature. Sections were then washed five times in PBS and reacted with avidin using an ABC kit (Vector Standard Elite Kit). Staining was visualized using 0.04% 3,3′-diaminobenzadine (DAB; Sigma) with 0.01% H2O2 in PB. Sections were mounted on gelatin-coated slides and coverslipped.
Light microscopy and photomicrography
Tracings were made of PHA-L labeled axons with the aid of a camera lucida (Lucivid) and a computer running Neurolucida (MicroBrightField, Colchester, VT) at a final magnification of 200× or 400×. Only those axons which were clearly discernable and which displayed varicosities, (i.e., presumed synaptic boutons) were included in the tracing. Tracings were exported, corrected for variable magnification, and compiled using CorelDraw (v. 10) to allow for the addition of labels and other markers. Tracings of cortical features (layers, fiber bundles)—made using adjacent Nissl-stained sections, or using traced sections under darkfield illumination—facilitated the demarcation of cortical divisions and features. Contrast and brightness levels for photomicrographs appearing in figures were matched using PhotoShop 6.0 (Adobe, San Jose, CA).
RESULTS
Nomenclature
The terminology in this article describing orbitofrontal and insular cortical regions conforms to that of Ray and Price (1992). The term anterior olfactory cortex (AOC) has been proposed for the structure commonly known as the anterior olfactory nucleus (Haberly, 2001) and is used here. Subdivisions of the AOC conform to previous descriptions of the structure (Haberly and Price, 1978a).
The piriform cortex, although long recognized to consist of several subdivisions (Rose, 1912, 1929), is typically divided into anterior (APC) and posterior (PPC) divisions, with the division made where the lateral olfactory tract (LOT) disappears on the ventral surface of the brain (∼0.5 mm posterior to bregma; see Fig. 1). In addition, where appropriate, this study follows the terminology of Ekstrand et al. (2001), who identified a ventrorostral subdivision of APC (termed APCVR) based on immunocytochemical and cytoarchitectural features. The term dorsal APC (APCD) was applied to that portion of APC dorsal to the APCVR, which forms part of the ventral wall of the rhinal sulcus and continues ventrally to the border with APCVR (about halfway between the dorsal and ventral extent of the LOT; see Ekstrand et al., 2001). Descriptions of structures outside the OFC and olfactory areas described above conform to boundaries defined by Paxinos and Watson (1986).
Fig. 1.
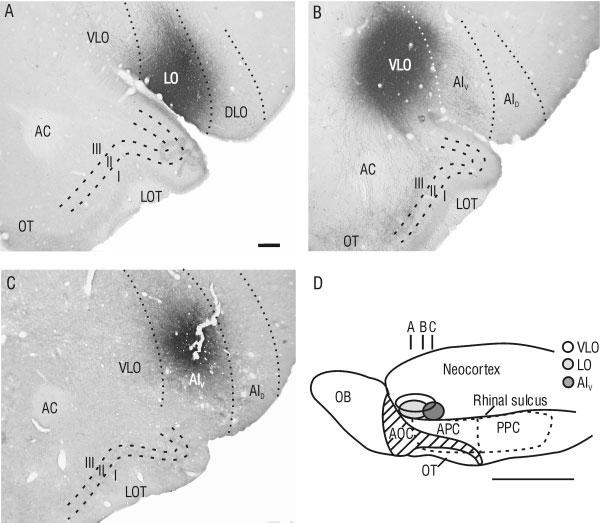
Representative injection sites in orbitofrontal cortex (OFC). Injections are shown in coronal sections for lateral orbital (LO; A), ventrolateral orbital (VLO; B), and ventral agranular insular (AIV; C) cortices, according to the terminology of Ray and Price (1992). D: Schematic ventrolateral view of the rat brain showing the approximate location of injection sites in OFC (ovals), and the approximate location of coronal slices shown in A-C. Hatching denotes the LOT. I-III, layers of piriform cortex; AC, anterior commissure; APC, anterior piriform cortex; AID, dorsal agranular insular cortex; AOC; anterior olfactory cortex; DLO, dorsolateral orbital cortex; LOT, lateral olfactory tract; OB, olfactory bulb; OT, olfactory tubercle; PPC, posterior piriform cortex. Scale bar = 250 μm in A (applies to A-C); 5 mm in D.
Injection sites
A total of seven injections were made in the VLO, centered between 2.7 and 3.7 mm anterior to bregma (see plot in Fig. 1). Eight injections were made in the LO, centered between 2.9 and 4.2 mm anterior to bregma (see plot in Fig. 1), and 8 injections were made in the AIV, centered between 1.7 and 2.2 mm anterior to bregma (Fig. 1). Cell bodies immunopositive for PHA-L were typically found within an ∼300 μm radius from the center of the injection site. Injections chosen for detailed analyses displayed immunopositive cell bodies only inside the borders of the structure of interest as defined by Ray and Price (1992).
Projections to the piriform cortex
From VLO. Labeled fibers were found throughout the APC after an injection in VLO, and these were restricted to the ipsilateral hemisphere. A striking pattern was observed in the rostral portion of piriform cortex; PHA-L-labeled processes were found in the ventral portion of APC (APCV), largely coinciding with the recently identified ventrorostral subdivision APCVR, but continuing beyond its caudal extent (Figs. 2, 3; compare with figs. 3 and 5 in Ekstrand et al., 2001). The density of labeled axons in APCV was highest in layer III (association fiber layer), but a large number of fibers were found in layer II (compact cell body layer) and in layer Ib (association fiber layer). Fibers in layer Ib could often be identified as extensions of fibers in layer III that traveled through layer II, then turned parallel with the cortical surface and traveled up to several hundred microns within layer Ib. PHA-L-labeled fibers displayed many stalked processes and varicosities within all layers (Fig. 3).
Fig. 2.
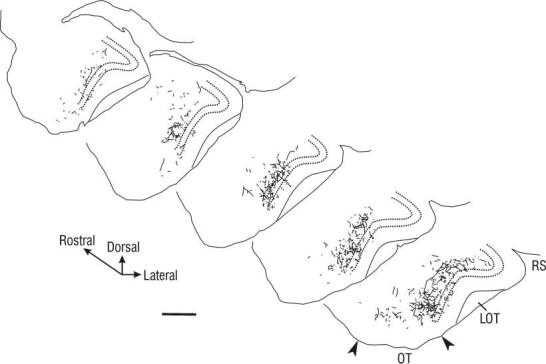
Tracings of PHA-L-labeled axonal arbors in the rostral APC following injection into VLO. Diagrammed sections are separated by 600 μm, and represent approximately the rostral two-thirds of the APC, with the rostralmost section taken near the border with the AOC (∼3.7 mm anterior to bregma; see Fig. 1). Note that most labeled fibers were found in the ventral half of rostral APC. Labeled fibers were found predominantly in layer III, with some fibers visible in layer II (between dashed lines) and layer Ib. More caudally, an increase in labeled fibers in the dorsal half of APC was found (data not shown). LOT, lateral olfactory tract; RS, rhinal sulcus; OT, olfactory tubercle. Scale bar = 250 μm.
Fig. 3.
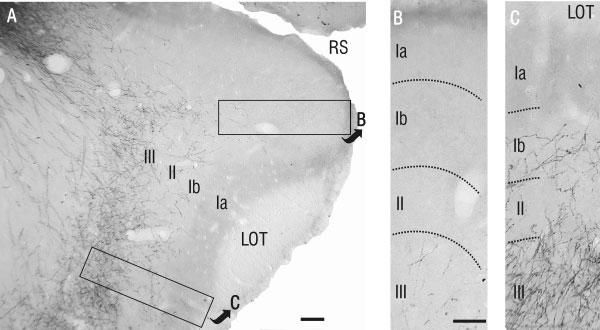
Details of PHA-L-labeled axons in rostral APC. A: Low-power photomicrograph showing PHA-L-labeled axons predominantly within layer III in the ventral half of rostral APC. B,C: High-power photomicrographs of the areas outlined in A. B: In dorsal APC, note the relatively small number of labeled fibers in layer III, and the absence of fibers in layers I and II. This contrasts remarkably with ventral APC (C), where PHA-L-labeled fibers were found in layers Ib, II, and III. Scale bars = 100 μm in A; 50 μm in B (applies to B,C).
In contrast, at this rostral level the dorsal region of APC (i.e., APCD)— beginning along the ventral bank of the rhinal sulcus and continuing around to the dorsal border of the lateral olfactory tract (LOT)—was almost completely devoid of PHA-L-labeled processes (Figs. 2, 3). In all cases, this fiber-free region encompassed the rostral third of APCD. More caudally, fibers were seen entering the dorsal portion of APC; however, the density of labeling was far greater in APCV (Fig. 3). Even at the caudalmost levels of APC, labeled axons in APCD were far less numerous than in the ventral half of APC.
The rostrocaudal level at which fibers began to innervate the dorsal part of APC followed a broad topography, with fibers labeled by more caudal injections entering the dorsal section of APC more caudally. However, this topography was limited. For the rostralmost injections (3.7-4.2 mm anterior to bregma), the rostral limit for labeled fibers in APCD was ∼3.5 mm anterior to bregma; labeled processes originating in VLO were only found in APCD caudal to this point.
Only a very few labeled fibers were seen in PPC following PHA-L injections in VLO. This finding is in agreement with a previous retrograde tracing experiment which showed low levels of cellular label in VLO following injections of cholera toxin B into PPC (Datiche et al., 1996).
From LO. Overall, injections in LO yielded far fewer labeled processes in the APC than injections in VLO, and were almost entirely found within layer III (Fig. 4). Al-though the labeled axons from LO were more homogeneously distributed than from VLO, more labeled fibers consistently were found in the dorsal APC than in its ventral portion. In PPC many labeled processes were found, and these were restricted to layer III.
Fig. 4.
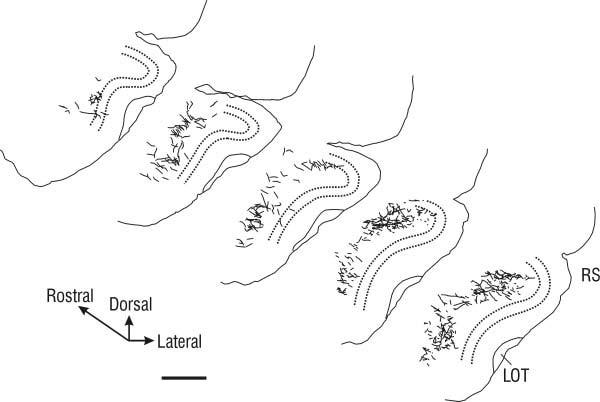
Tracings of PHA-L-labeled axonal arbors in the APC following injection into LO. Diagrammed sections are taken from the caudal two-thirds of the APC, with the rostralmost section ∼2.4 mm anterior to bregma. Sections are separated by 600 μm. Note that most labeled fibers were found in layer III, and that labeled fibers are more numerous in dorsal APC. Dashed line, outline of layer II; LOT, lateral olfactory tract; RS, rhinal sulcus. Scale bar = 250 μm.
From AIV. Injections in AIV revealed only a very light projection to APCD, and no fibers were found in APCV. In contrast, a moderate projection was found in PPC, restricted to layer III.
Projections to rostral olfactory structures
From VLO. Only a very small number of fibers were found in the OB following injection of PHA-L into the VLO. The few labeled projections seen were located in the ipsilateral OB, in the superficial portion of the granule cell layer. In contrast, a substantial number of labeled fibers were found in the AOC. The heaviest labeling here was found in the pars lateralis, with some projections visible in the adjacent portion of the pars dorsalis. Most labeled axons within pars lateralis were found in the deep fiber layer of the AOC, with only a few axons visible in the cellular and superficial fiber layers just deep to the LOT. There were very few fibers in the pars ventralis or pars medialis. The projection to the AOC was almost exclusively ipsilateral to the injection site, with very few fibers found in the contralateral AOC. There was no observed topographic organization of the projection from VLO to the AOC that correlated with the location of the PHA-L injection.
From LO and AIV. The projection from LO and AIV to the OB and AOC was very weak, with only a few axons visible, and these were restricted to the central fiber zone in both structures.
Projections to other cortical structures
Injection in VLO. Within the neocortex, the largest concentration of anterogradely labeled fibers was found within the VLO itself, and to the contralateral VLO. Projections extended to the rostral pole of the structure, and were most prominent in the superficial layers of the cortex. A high concentration of fibers was seen in layer I, while layer II/III just deep to it appeared relatively devoid of fibers, perhaps most visible in the medial VLO. Fiber density in layer II/III gradually increased laterally toward the border with LO/AIV. More caudally, nearer the injection site, this fiber-poor layer was not visible.
A large number of fibers also were observed within LO and AIV ipsilateral to the VLO injection site, primarily in deep layers. The density of the projection to LO was higher in more caudal sections. Labeled fibers also were found in medial (MO) and ventral orbital (VO) cortices, both ipsilateral and contralateral to the injection site, beginning at the rostral pole of the neocortex. Only a very few labeled fibers could be found in the dorsolateral orbital cortex (DLO) and the dorsal agranular insular cortex (AID). More caudally, labeled fibers were found in infralimbic cortex. Labeled fibers also were seen ipsilaterally in a strip of cortex ventral to the rhinal sulcus, situated ventrolateral to the boundary of VLO and medial to rostral APCD (this strip of cortex, between piriform cortex and VLO, is included as part of piriform cortex in some atlases).
Injection in LO. The largest concentration of anterogradely labeled fibers in the neocortex arising from an injection in LO was a bilateral projection within the LO. The projection extended to the rostral pole of the structure in both hemispheres, and was heaviest in the deep layers of the cortex.
A large number of fibers also were observed within VLO ipsilateral to the injection site, primarily in layers II/III and the deep layers. Rostrally, fibers in VLO were less numerous than within LO, but this projection became stronger more caudally. Labeled fibers also were found in MO and VO cortices, both ipsilateral and contralateral to the injection, beginning at the rostral pole of the neocortex. These projections appeared similar to projections to these structures from the VLO in number and distribution. Only a very few labeled fibers could be found in AIV. More caudally, fibers also were found in DLO and in cingulate and infralimbic cortices.
As with injections from VLO, a moderate projection to a strip of cortex ventral to the rhinal sulcus situated between the boundaries of VLO and rostral APCD was found arising from LO ipsilaterally, but this projection did not enter the lateral portion of APCD.
Injection in AIV. Like the other structures, the greatest density of PHA-L immunolabeled fibers following an injection in AIV were in AIV both ipsilateral and contralateral to the injection site. A small projection to the rostral portion of LO was present in deep layers on the ipsilateral side, but this projection was less dense than the projection from LO and VLO. This projection became stronger more caudally, near the AIV injection site. The heaviest projection outside AIV was found in AID on the ipsilateral side, primarily in the deep layers.
Other projections
From VLO. There was a moderate projection to the olfactory tubercle, with many labeled fibers visible throughout the plexiform, pyramidal, and polymorph layers. Labeled fibers were not found within the Islands of Calleja. As was the case for the AOC, there did not appear to be any topographic organization of fibers innervating the tubercle related to the location of the injection site in VLO. There was also a moderate projection from VLO to the dorsal endopiriform nucleus.
From AIV. A substantial number of fibers were found in the dorsal endopiriform nucleus deep to the APC and PPC following injections in the AIV. There were no fibers found within the olfactory tubercle.
DISCUSSION
This study characterized projections from OFC to the piriform cortex, using injections of PHA-L to anterogradely label axons. The results provide evidence that the OFC, a region of the brain implicated in associating environmental cues with predicted outcomes, has heavy projections to the piriform cortex. These projections are heterogeneous, with the predominant portion arising from the ipsilateral VLO and innervating the ventral segment of APC. These results suggest that subregions of piriform cortex are functionally separate and, taken together with previous studies, indicate that the piriform cortex, OFC, and amygdala may comprise a network in which olfactory, motivational, and predictive cues are associated. The implications for olfactory function are discussed below following a consideration of methodological limitations.
Limitations
The effective PHA-L injection site is defined by the intensely stained neurons that have taken up the tracer at the injection site (Gerfen and Sawchenko, 1984). However, there is a potential for PHA-L to be transported retrogradely, particularly where fibers of passage are damaged by the pipette tip during the injection. If such labeling occurred in the present study, retrograde transport of PHA-L in axons of piriform cortical cells that project to OFC could mark branches of these axons that project within piriform cortex. While evidence for such an artifact was uncommon, two experimental animals displayed PHA-L immunolabeled somata in layer II of piriform cortex, well away from the injection sites in OFC. These animals were excluded from analyses.
A further limitation, common to all tracing studies, is that the functional significance of labeled projections cannot be assessed. We have been careful to include in our analyses only those projections that display varicosities (i.e., presumed synaptic boutons); however, studies utilizing physiological or electron microscopy methods must follow to determine whether projections from OFC to piriform cortex are functional.
Comparison with previous studies
Connections between the olfactory and orbitofrontal cortices in the rat have been investigated by a number of previous studies utilizing tracer injections (e.g., Luskin and Price, 1983; Ray and Price, 1992; Datiche et al., 1996; Johnson et al., 2000; Ekstrand et al., 2001) and physiological techniques (e.g., Cinelli et al., 1987). Using field responses to ortho- and antidromic stimulation, Cinelli et al. (1987) described heavy reciprocal connections between OFC and the APC, and noted a functional connection from VLO/LO to the olfactory bulb. Further, this study noted that the projections from dorsal and ventral AI to APC were much lighter than those from VLO/LO. The results of the present study using PHA-L injections are consistent with these physiological findings, and extend them to detail the particular relationships that exist between structures within OFC and APC.
In a retrograde tracing study using injections of the B subunit of cholera toxin, Datiche et al. (1996) reported that VLO, LO, and AIV each extended projections to APC; however, two findings in the present study contradict those results. First, Datiche et al. reported a large number of retrogradely labeled cells in VLO following injection of cholera toxin in the dorsal part of APC; this contrasts with the paucity of anterogradely labeled fibers to this part of piriform cortex with a PHA-L injection in the present study. This discrepancy may be due to a difference in the level of detail undertaken by our analyses; figures in the article by Datiche et al. (1996) do not show the location of labeled cells for injections in APCD, nor is there any discussion of differences between injections in the dorsal and ventral parts of APC. It is possible that more detailed analyses of their material would reveal differences in staining between dorsal and ventral subdivisions of APC. The present anterograde tracing results suggest that APCD, especially in the rostral extent, receives only a limited projection from cells in VLO, a small projection from LO, and a very small projection from AIV.
The second discrepancy relates to Datiche et al. (1996) report of a large number of retrogradely labeled cells in LO and AIV after injections in APC. As noted above, injections of anterograde tracer in either LO or AIV in the present study suggested only a limited projection from these structures to APC. Again, this discrepancy may be attributed to the scope of analyses, as Datiche et al. do not discriminate between LO and VLO (see fig. 4 and table 1 in Datiche et al., 1996). Further, their analyses used the definitions of orbitofrontal cortex provided by Paxinos and Watson (1986) rather than Ray and Price (1992), which could compound the difference. When the Ray and Price (1992) terminology is applied to figure 4 from Datiche et al. (1996), it appears that most of the retrogradely labeled neurons found following injection into APC in that study were in VLO, with only a very few cells in either LO or AIV, which is consistent with results from the present study. Another possibility is that retrograde labeling of cells in LO and AIV may have resulted from damage to fibers of passage during the injection of cholera toxin (Datiche et al., 1996).
Functional circuitry
An intriguing finding in the present study was that VLO does not project homogeneously to all areas of piriform cortex, but has its most prominent projection to APCV. This finding is particularly noteworthy in light of a recent article identifying part of this region as a distinct subdivision of piriform cortex (APCVR) which may be the only cortical recipient of input from tufted cells in the olfactory bulb (Ekstrand et al., 2001). Notably, that study found a substantial, selective projection from APCVR to VLO (Ekstrand et al., 2001). Taken together with those findings, the present results demonstrating a heavy projection to ventral APC from VLO provide further support for APCVR as a unique subdivision of piriform cortex, and suggest that VLO and APCVR may function as a largely separate, parallel processing network for olfactory information arising from tufted cells.
The APC also receives projections from the amygdaloid complex (Krettek and Price, 1977; Luskin and Price, 1983; Santiago and Shammah-Lagnado, 2004; Majak et al., 2004). Using small anterograde injections of PHA-L into various amygdaloid structures, Majak et al. (2004) found that projections terminating in APC originated in several nuclei, including the lateral, basal, and accessory basal nuclei; however, these projections were relatively light, and were only found in the dorsal part of APC (Majak et al., 2004). In light of the present results, which indicate that the projection from VLO is primarily in APCV, and does not include a significant projection to PPC, it may appear that the projections from these amygdaloid structures and those from OFC may target largely distinct subsets of cells in piriform cortex. However, other findings suggest areas in piriform cortex where convergence of projections from OFC and some amygdaloid structures could occur: the smaller projections from LO and VLO to caudal APCD may overlap with the projection from the accessory basal nucleus of the amygdala (Majak et al., 2004), and the heavy projection from VLO to APCV may overlap with the projection from the nucleus of the lateral olfactory tract (Luskin and Price, 1983; Santiago and Shammah-Lagnado, 2004).
A broad topography has been reported in the projections from the piriform cortex to the orbitofrontal areas, such that as injections progress caudally from APCVR to APCD to PPC, the region displaying the heaviest labeling shifts from VLO to LO to AIV, respectively (see Fig. 5; Ray and Price, 1992; Ekstrand et al., 2001). The present results suggest that this broad topography is reciprocal, with injections in VLO, LO, and AIV resulting in the heaviest labeling in APCV, APCD and PPC, respectively.
Fig. 5.
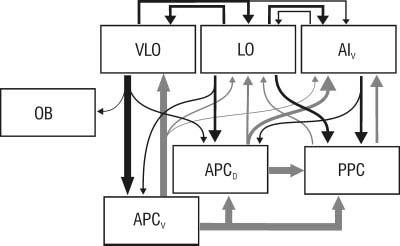
Schematic diagram and summary of results. Projections within OFC and from OFC to piriform cortex revealed by PHA-L injections in the present study are shown in black, with the width of the arrow representing the relative size of the projection. Projections among areas within piriform cortex, and from piriform cortex to OFC, are shown in gray and represent previously reported findings. For simplicity, projections from the OB are not shown.
Functional implications
The orbitofrontal cortex, along with the amygdala, is involved in learning and representing information pertaining to the behavioral significance of odors (Schoenbaum et al., 1998, 1999, 2003a; Ramus and Eichenbaum, 2000). The present study provides evidence that these orbitofrontal areas, especially VLO and LO, project to layer III of piriform cortex, where they may converge with autoassociative (Haberly, 1998) and amygdaloid projections (Majak et al., 2004). Taken together, these results suggest that the piriform cortex, the OFC, and the amygdala may constitute a highly interconnected network to encode and integrate information about the behavioral significance of olfactory stimuli. The finding that inputs from VLO also project to layer Ib, proximal to the afferent input on the apical dendritic tree of pyramidal cells in ventral APC (Haberly, 1998), is particularly intriguing in this regard. The location of these projections suggests that the VLO may actively modulate afferent fiber-induced activity in piriform cortex, perhaps to ensure that cells responsive to a particular odor fire in an appropriate behavioral context. This hypothesis seems plausible in light of the finding that cells in piriform cortex can display robust responses to nonolfactory cues during a behavioral task (Schoenbaum and Eichenbaum, 1995a).
The finding that the projections from OFC to piriform cortex terminate in the association fiber layers within APC also raises the possibility that OFC may play a role in initiating activity within piriform cortex in the absence of an odor stimulus during recall of multimodal memories (so-called “retrograde reconstruction”; Haberly, 2001). In particular, the heavy projection onto the proximal apical dendritic tree of cells in piriform cortex (i.e., layer Ib) from VLO could serve to mimic the effect of afferent fibers during memory retrieval. Subsequent activation of cells in piriform cortex may then allow recall of odors or odor-related associations. Evidence for such activity has been found in other sensory systems (Crick and Koch, 1998; Zeki and Bartels, 1998; O’Craven and Kanwisher, 2000).
The piriform cortex not only receives significant projections from both the OFC and the amygdaloid complex, it also has heavy projections back to these areas (Fig. 5; Johnson et al., 2000; Ekstrand et al., 2001; Majak et al., 2004). Thus, while the OFC may modulate cellular activity in piriform cortex, it must be considered that cells in piriform cortex may perform a reciprocal function by shaping responses to olfactory cues in the orbitofrontal areas.
ACKNOWLEDGMENTS
The author thanks Ms. Dixie Shurling, Ms. Meghan Mayhood, and Ms. Alexandra Ortiz for excellent technical work, and Dr. Peter C. Brunjes for support and critical reading of the article.
Footnotes
Grant sponsor: National Institutes on Deafness and Other Communicative Disorders; Grant number: DC00338; Grant number: DC005557.
Published online in Wiley InterScience (www.interscience.wiley.com).
LITERATURE CITED
- Bouret S, Sara SJ. Locus coeruleus activation modulates firing rate and temporal organization of odour-induces single-cell responses in rat piriform cortex. Eur J Neurosci. 2002;16:2371–2382. doi: 10.1046/j.1460-9568.2002.02413.x. [DOI] [PubMed] [Google Scholar]
- Cinelli AR, Ferreyra-Moyano H, Barragan E. Reciprocal functional connections of the olfactory bulbs and other olfactory related areas with the prefrontal cortex. Brain Res Bull. 1987;19:651–661. doi: 10.1016/0361-9230(87)90051-7. [DOI] [PubMed] [Google Scholar]
- Crick F, Koch C. Consciousness and neuroscience. Cereb Cortex. 1998;8:97–107. doi: 10.1093/cercor/8.2.97. [DOI] [PubMed] [Google Scholar]
- Datiche F, Litaudon P, Cattarelli M. Intrinsic association fiber system of the piriform cortex: a quantitative study based on a cholera toxin B subunit tracing in the rat. J Comp Neurol. 1996;376:265–277. doi: 10.1002/(SICI)1096-9861(19961209)376:2<265::AID-CNE8>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Ekstrand JJ, Domroese ME, Johnson DMG, Feig SL, Knodel SM, Behan M, Haberly LB. A new subdivision of anterior piriform cortex and associated deep nucleus with novel features of interest for olfaction and epilepsy. J Comp Neurol. 2001;434:289–307. doi: 10.1002/cne.1178. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Sawchenko PE. An anterograde neuroanatomical tracing method that shows the detailed morphology of neurons, their axons and terminals: immunohistochemical localization of an axonally transported plant lectin, Phaseolus vulgaris leucoagglutinin (PHA-L) Brain Res. 1984;290:219–238. doi: 10.1016/0006-8993(84)90940-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberly LB. Olfactory cortex. In: Shepherd GM, editor. The synaptic organization of the brain. Oxford University Press; New York: 1998. pp. 377–416. [Google Scholar]
- Haberly LB. Parallel-distributed processing in olfactory cortex: new insights from morphological and physiological analysis of neuronal circuitry. Chem Senses. 2001;26:551–576. doi: 10.1093/chemse/26.5.551. [DOI] [PubMed] [Google Scholar]
- Haberly LB, Price JL. Association and commissural fiber systems of the olfactory cortex of the rat. I. Systems originating in the piriform cortex and adjacent areas. J Comp Neurol. 1978a;178:711–740. doi: 10.1002/cne.901780408. [DOI] [PubMed] [Google Scholar]
- Haberly LB, Price JL. Association and commissural fiber systems of the olfactory cortex of the rat. II. Systems originating in the olfactory peduncle. J Comp Neurol. 1978b;181:781–808. doi: 10.1002/cne.901810407. [DOI] [PubMed] [Google Scholar]
- Johnson DMG, Illig KR, Behan M, Haberly LB. New features of connectivity in piriform cortex visualized by intracellular injection of pyramidal cells suggest that “primary” olfactory cortex functions like “association” cortex in other sensory systems. J Neurosci. 2000;20:6974–6982. doi: 10.1523/JNEUROSCI.20-18-06974.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krettek JE, Price JL. Projections from the amygdaloid complex to the cerebral cortex and thalamus in the rat and cat. J Comp Neurol. 1977;172:687–722. doi: 10.1002/cne.901720408. [DOI] [PubMed] [Google Scholar]
- Luskin MB, Price JL. The distribution of axon collaterals from the olfactory bulb and the nucleus of the horizontal limb of the diagonal band to the olfactory cortex, demonstrated by double retrograde labeling techniques. J Comp Neurol. 1982;209:249–263. doi: 10.1002/cne.902090304. [DOI] [PubMed] [Google Scholar]
- Luskin MB, Price JL. The topographic organization of associational fibers of the olfactory system in the rat, including centrifugal fibers to the olfactory bulb. J Comp Neurol. 1983;216:264–291. doi: 10.1002/cne.902160305. [DOI] [PubMed] [Google Scholar]
- Majak K, Rönnkö S, Kemppainen S, Pitkänen A. Projections from the amygdaloid complex to the piriform cortex: a PHA-L study in the rat. J Comp Neurol. 2004;476:414–428. doi: 10.1002/cne.20233. [DOI] [PubMed] [Google Scholar]
- McCollum J, Larson J, Otto T, Schottler F, Granger R, Lynch G. Short-latency single unit processing in olfactory cortex. J Cogn Neurosci. 1991;3:293–299. doi: 10.1162/jocn.1991.3.3.293. [DOI] [PubMed] [Google Scholar]
- O’Craven KM, Kanwisher N. Mental imagery of faces and places activates corresponding stimulus-specific brain regions. J Cogn Neurosci. 2000;12:1013–1023. doi: 10.1162/08989290051137549. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press; San Diego: 1986. [DOI] [PubMed] [Google Scholar]
- Ramus SJ, Eichenbaum H. Neural correlates of olfactory recognition memory in the rat orbitofrontal cortex. J Neurosci. 2000;20:8199–8208. doi: 10.1523/JNEUROSCI.20-21-08199.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray JP, Price JL. The organization of the thalamocortical connections of the mediodorsal thalmic nucleus in the rat, related to the ventral forebrain-prefrontal cortex topography. J Comp Neurol. 1992;323:167–197. doi: 10.1002/cne.903230204. [DOI] [PubMed] [Google Scholar]
- Rose M. Histologische lokaalisation der Grobhirnrinde bei kleinen Säugetieren (Rodentia, Insectivora, Chiroptera) J Psychol Neurol. 1912;19:391–479. [Google Scholar]
- Rose M. Cytoarchitektonischer Atlas der Grobhirnrinde der Maus. J Psychol Neurol. 1929;40:1–51. [Google Scholar]
- Santiago AC, Shammah-Lagnado SJ. Efferent connections of the nucleus of the lateral olfactory tract in the rat. J Comp Neurol. 2004;471:314–332. doi: 10.1002/cne.20028. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Eichenbaum H. Information coding in the rodent prefrontal cortex. I. Single-neuron activity in orbitofrontal cortex compared with that in pyriform cortex. J Neurophysiol. 1995a;74:733–750. doi: 10.1152/jn.1995.74.2.733. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Eichenbaum H. Information coding in the rodent prefrontal cortex. II. Ensemble activity in orbitofrontal cortex. J Neurophysiol. 1995b;74:751–762. doi: 10.1152/jn.1995.74.2.751. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Chiba AA, Gallagher M. Orbitofrontal cortex and basolateral amygdala encode expected outcomes during learning. Nat Neurosci. 1998;1:155–159. doi: 10.1038/407. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Chiba AA, Gallagher M. Neural encoding in orbitofrontal cortex and basolateral amygdala during olfactory discrimination learning. J Neurosci. 1999;19:1876–1884. doi: 10.1523/JNEUROSCI.19-05-01876.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum G, Setlow B, Nugent SL, Saddoris MP, Gallagher M. Lesions of orbitofrontal cortex and basolateral amygdala complex disrupt acquisition of odor-guided discriminations and reversals. Learn Mem. 2003a;10:129–140. doi: 10.1101/lm.55203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum G, Setlow B, Ramus SJ. A systems approach to orbitofrontal cortex function: recordings in rat orbitofrontal cortex reveal interactions with different learning systems. Behav Brain Res. 2003b;146:19–29. doi: 10.1016/j.bbr.2003.09.013. [DOI] [PubMed] [Google Scholar]
- Setlow B, Gallagher M, Holland P. The basolateral complex of the amygdala is necessary for acquisition but not expression of CS motivational value in appetitive Pavlovian second-order conditioning. Eur J Neurosci. 2002;15:1841–1853. doi: 10.1046/j.1460-9568.2002.02010.x. [DOI] [PubMed] [Google Scholar]
- Wilson DA. Habituation of odor responses in the rat anterior piriform cortex. J Neurophysiol. 1998;79:1425–1440. doi: 10.1152/jn.1998.79.3.1425. [DOI] [PubMed] [Google Scholar]
- Wilson DA. Odor specificity of habituation in the rat anterior piriform cortex. J Neurophysiol. 2000;83:139–145. doi: 10.1152/jn.2000.83.1.139. [DOI] [PubMed] [Google Scholar]
- Wilson DA. Rapid, experience-induced enhancement in odorant discrimination by anterior piriform cortex neurons. J Neurophysiol. 2003;90:65–72. doi: 10.1152/jn.00133.2003. [DOI] [PubMed] [Google Scholar]
- Wilson DA, Stevenson RJ. The fundamental role of memory in olfactory perception. Trends Neurosci. 2003;26:243–247. doi: 10.1016/S0166-2236(03)00076-6. [DOI] [PubMed] [Google Scholar]
- Zeki S, Bartels A. The autonomy of the visual systems and the modularity of conscious vision. Philos Trans R Soc Lond B Biol Sci. 1998;353:1911–1914. doi: 10.1098/rstb.1998.0343. [DOI] [PMC free article] [PubMed] [Google Scholar]


